Abstract
Since an immuno-inhibitory environment exists within tumors, successful vaccines will likely require additional approaches to alter the tumor microenvironment. Monocyte chemoattractant proteins (such as CCL2) are produced by many tumors and have both direct and indirect immuno-inhibitory effects. We hypothesized that CCL2 blockade would reduce immunosuppression and augment vaccine immunotherapy. Anti-murine-CCL2/CCL12 monoclonal antibodies were administered in three immunotherapy models: one aimed at the HPV-E7 antigen expressed by a non-small cell lung cancer line, one targeted to mesothelin expressed by a mesothelioma cell line, and one using an adenovirus expressing Interferon-α to treat a non-immunogenic, non-small cell lung cancer line. We evaluated the effect of the combination treatment on tumor growth and assessed the mechanism of these changes by evaluating cytotoxic T cells, immunosuppressive cells, and the tumor microenvironment. Administration of anti-CCL2/CCL12 antibodies along with the vaccines markedly augmented efficacy with enhanced reduction in tumor volume and cures of approximately half of the tumors. The combined treatment generated more total intra-tumoral CD8+ T-cells that were more activated and more anti-tumor antigen specific, as measured by tetramer evaluation. Another important potential mechanism was reduction in intratumoral T-regulatory (T-reg) cells. CCL2 appears to be a key proximal cytokine mediating immunosuppression in tumors. Its blockade augments CD8+ T cell immune response to tumors elicited by vaccines via multifactorial mechanisms. These observations suggest that combining CCL2 neutralization with vaccines should be considered in future immunotherapy trials.
Keywords: CCL2, Cancer immunotherapy, Lung Cancer, Mesothelioma, T-lymphocytes
Introduction
Current immunotherapies are primarily aimed at initiating or boosting T cell responses to tumors and their antigens. However, the effectiveness of these therapies may be limited by systemic and local tumor-induced immunosuppression (1). It is therefore becoming more widely accepted that successful immunotherapy will require a second approach to alter tumor microenvironment and/or decrease immune-suppression (2). Several approaches have been used such as blockade of TGF-β or TGF-β signaling (3), use of Cox-2 inhibitors (4), depletion of T-regulatory cells (5), or blocking CTLA-4 (6).
Another immunomodulatory factor secreted from tumor cells and the associated tumor stromal cells is monocyte chemoattractant protein 1 (MCP-1, CCL2), a CC (β) member of the cytokine/chemokine superfamily. Although first identified as a chemokine that could induce the migration of monocytes (7), CCL2 has a number of other chemotactic properties that include attraction of subsets of lymphocytes (including T-regulatory cells) and endothelial cells into sites of inflammation (7–9). Importantly, it has also been observed to directly affect T-cell function, specifically inhibiting CD8+ T cell effector functions (10–12).
Because of these immunosuppressive properties, we hypothesized that CCL2 was acting as an inhibitor of the effect of cancer immunotherapy, and its blockade might thus be benficial. In the mouse, there are two human CCL2 orthologues – CCL2 (MCP-1) and CCL12 (MCP-5). We initially evaluated the effect of blocking either one of these orthologues with antibodies that specifically neutralize these chemokines (8, 13), and found that mAb against each one of them had a modest effect alone on tumor growth. We therefore used a mixture of the two mAb in further clinical and mechanistic experiments (which we will heretofore refer to as “α-CCL2”). Our data suggest that CCL2/12 is an endogenous barrier to cancer immunotherapy, and that blockade could be a promising approach to augment CD8+ T-cell-mediated immunotherapy.
Materials and Methods
Animals
Female C57BL/6 mice were purchased from Charles River Laboratories (Wilmington, MA). Female C57BL/6J X 129P3/J hybrids (B6-129/J1) were purchased from Jackson Labs (Bar Harbor, ME). The Animal Use Committee of the University of Pennsylvania approved all protocols in compliance with the Guide for the Care and Use of Laboratory Animals.
Cell lines
TC1 cells were derived from mouse lung epithelial cells of a C57B6 mouse, immortalized with HPV-16 E6 and E7 and transformed with the c-Ha-ras oncogene (14). The murine lung cancer line LKR was derived from an explant of a pulmonary tumor from an activated Kras G12D mutant mouse that had been induced in an F1 hybrid of 129Sv.J and C57BL/6 (15). The murine malignant mesothelioma cell line AE17 was derived from the peritoneal cavity of C57BL/6J mice injected with asbestos (crocidolite) fibers, and given to us by Dr. Delia Nelson (16). Human mesothelin was transfected into the AE17 cell line using a lentiviral construct (AE17-hmeso).
Anti-CCL2/CCL12 monoclonal antibodies
C1142 is a rat/mouse chimeric monoclonal antibody (mAb) that neutralizes mouse CCL2/JE (MCP-1) and C1450 is a human/mouse chimeric mAb that neutralizes the second mouse homolog CCL12 (MCP-5) (8, 13, 17). Both mAb were produced at Centocor Inc. (Malvern, PA). In most experiments mice were treated with a mixture of 250 μg per mouse of each mAb (α-CCL2),, in a total volume of 200 μl normal saline intra-peritoneally (IP), twice per week. Control mice were treated with an equal volume of normal saline.
Immunotherapy models
We used three different immunotherapy models. For the TC1 tumor model, we used an E1/E3-deleted type 5 adenoviral vector expressing the HPV-E7 protein under control of a cytomegalovirus promoter as previously described (Ad.E7) (4). Animals bearing TC1 tumors were vaccinated subcutaneously (S.Q.) with 1×109 pfu of Ad.E7 vector, followed by a booster after seven days. For the LKR cell line, we used an Adenovirus (Ad) expressing a hybrid Interferon-α2α1 (Ad.IFNα) with activity in mice, received from Schering-Plough Inc. (17). One dose of 1×109 pfu of virus was injected intratumorally. For the AE17.hmeso tumor model, we used a modified, live attenuated Listeria monocytogenes vector expressing human mesothelin (Lm.Meso) provided by Drs. Dirk Brockstedt and Thomas Dubinsky of Anza Corporation. Mesothelin is a tumor-associated antigen highly expressed in human malignant mesotheliomas (18). Lm.Meso was constructed by inserting a mesothelin expression cassette integrated at the inlB locus.
Animal flank tumor models
Mice were injected on the right flank with 1×106 TC1, LKR or AE17-h.meso tumor cells in the appropriate mouse strain. The flank tumors were allowed to reach an average size of 200–250 mm3 (approximately 12–15 days). Mice were treated in one of 4 groups: 1) control-untreated, 2) α-CCL2/CCL12 mAbs 3) Immunotherapy alone, or 4) Combination of immunotherapy and α-CCL2/CCL12 mAbs. All experiments had at least 5 mice per group and were repeated at least once. When needed for analysis (i.e. for FACS, RNA, cell subsets isolation, etc.), flank tumorswere harvested from the mice, and digested with 2 mg/mL DNase I (Sigma, St. Louis, MO) and 4 mg/mL collagenase type IV (Sigma) at 37°C for 1 hour.
Flow cytometric analysis of tumors and spleens
Splenocytes, lymph nodes and tumor cells were studied by FACS analysis as previously described (4). All fluorescently labeled antibodies used were purchased from BD Biosciences (San Jose, CA) except for: CD206-PE, obtained from Serotec (Oxford, UK); 4-1BB (CD137)-PE, obtained from Abcam (Cambridge, UK); and GR-1-FITC, obtained from eBioscience (San Diego, CA). Flow cytometry was done using a Becton Dickinson FACS Calibur flow cytometer (San Jose, CA). Data analysis was done using FlowJo software (Ashland, OR). The allophycocyanin-labeled H-2Db tetramer loaded with E7 peptide (RAHYNIVTF) was obtained fromthe National Institute of Allergy and Infectious Diseases tetramercore. Intracellular staining for FoxP3 was done using the PE anti mouse/rat FoxP3 staining set (eBioscience).
RNA isolation and real-time, reverse transcription-PCR
Mice with tumors were treated with either one of the four treatments detailed above, removed 2 days after the Ad.E7 boost vaccine, flash frozen, and the RNA from each tumor isolated. For each treatment condition, a pool of RNA was created by adding the same amount of RNA from each of the tumors within the group. cDNA was made from each pool, RNA levels were normalized to β-actin levels, and quantification of tumor mRNA levels was performed as previously described (19). Relative levels of expression of each of the selected genes (fold change versus control) were determined. Each sample was run in quadruplicate and the experiment was repeated at least once. Primer sequences are given in supplemental Table 1.
Immunohistochemical staining of tumors
Animals bearing flanktumors, treated with each of the treatments as above, were euthanized 2–3 days after the booster Ad.E7 vaccine. The tumors were immediately placedin Tissue-Tek OCT compound (Sakura Finetek USA, Inc., Torrance,CA) to be stored at 80°C. Staining was done as previously described (4).
Evaluation of secretion of cell products (TNF-α) from explants
Mice with tumors were treated with one of the four treatment options as above. Tumors were removed 2 days after the boost vaccine, cut into pieces of about 5 × 5 mm, weighed and placed in a 24 well plate with 800 μl of culture media. After 24 hours, the media was collected, and spun to remove cellular debris (5 min, 1500 rpm). The amountof TNF-α secreted by tumors (corrected for weight) was quantifiedusing an ELISA kit according to the instructionsof the manufacturer (BD OptEIA ELISA set, BD Biosciences).
Statistical analyses
For the RT-PCR, FACS studies, and flank tumor studies comparing differences between two groups, we used unpaired Student t-tests. For FACS and flank tumor studies comparing more than two groups, we used one sided ANOVA with appropriate post hoc testing. Differences were considered significant when P<0.05. Data are presented as mean ± SEM.
Results
CCL2 blockade has a modest effect as monotherapy, but significantly augments the effect of immunotherapy
We initially evaluated the effect of blocking each of the two murine CCL2 orthologues – CCL2 and CCL12 on tumor growth. Using mAb blocking either of the 2 orthologues alone there was a non-significant trend for slower tumor growth (Fig. 1A). However, the combination of mAb against both CCL2 and CCL12 consistently inhibited tumor growth by 30–50% (p<0.05) in the TC-1 NSCLC cell line (Fig. 1A), as well as in several other flank and orthotopic mouse lung cancer and mesothelioma models (manuscript in preparation). Thus, for all remaining experiments, unless otherwise specified, we used the combination of CCL2 and CCL12. For brevity, we heretofore refer to the antibody as “CCL2”.
Figure 1. CCL2 blockade significantly augments tumor immunotherapy.
Panel A – Effects of α-CCL2 and α-CCL12 blockade. Mice bearing large TC1 tumors were treated in one of four ways: 1) No treatment (control); 2) α-CCL2 mAb twice per week starting at day 13 (a-CCL2); 3) α-CCL12 mAb twice per week starting at day 13 (a-CCL12); and 4) Combination of α-CCL2 and α-CCL12 mAb (a-CCL2/12). α-CCL2 or α-CCL12 had a trend to slow tumor growth, but only the combination of both mAbs significantly reduced tumor growth (* = p<0.05 vs. control).
Panels B–D – Combination of Immunotherapy and CCL2 blockade. Mice bearing large flank tumors, were treated in one of four ways: 1) No treatment (control); 2) I.P. α-CCL2/CCL12 mAb twice per week starting at day 13 (a-CCL2); 3) Immunotherapy; and 4) Combination of immunotherapy and α-CCL2 mAb (Combo). In all 3 models only combination therapies led to clear tumor regression and cures. Three different models of immunotherapy are shown: AdE7 in the NSCLC cell line TC-1 (Panel B, *=p<0.01 for Combo vs. Ad.E7); A listeria-mesothelin vaccine (Lm-meso) in the mesothelin-expressing mesothelioma cell line AE17 (Panel C, *=p<0.05 vs. control for each); and Ad.IFNα in the NSCLC cell line LKR (Panel D, *=p<0.05 for combo vs. each group).
Figure 1B shows the effects of α-CCL2 alone, the Ad.E7 vaccine alone, or the combination of treatments on TC1 tumors. Both the antibodies alone and Ad.E7 alone significantly slowed tumor growth (p<0.05 vs. control for each), without causing regressions. However, combining these agents led to clear tumor regressions and an approximate 50% cure rate in two independent experiments. Although some effect of the combination with immunotherapy was noted when we used either anti-CCL2 alone or anti-CCL12 alone (Supplemental Figure 1), the effect was less than when the two mAb were combined (Figure 1B), suggesting that blocking both CCL2 and CCL12 is important in augmenting immunotherapy. Further mechanistic studies were therefore done with the combination of both mAbs.
Figure 1C shows similar results when we combined α-CCL2 mAb with Lm.Meso in the AE17.hmeso mesothelioma model. Again, either antibodies alone or Lm.Meso alone significantly slowed tumor growth (p<0.05 vs. control for each), without regressions. However, combining these agents led to tumor regressions and two thirds of the animals were cured (Fig. 1C). In order to examine the effect of blocking CCL2 in a non-immunogenic tumor, we used the NSCLC cell line LKR, treated with Ad.IFNα (Figure 1D). As in the other two models examined, the combination of the vaccine with CCL2 blockade significantly slowed tumor growth (p<0.05 versus all other groups).
Blocking CCL2 in mice treated with immunotherapy increases the number of activated splenic CD8+ T cells, reduces the number of splenic T-regs, but has no effect on myeloid derived suppressor cells (MDSC)
For mechanistic studies, we focused on the TC1/Ad.E7 system. We first studied systemic effects by evaluating splenocytes.
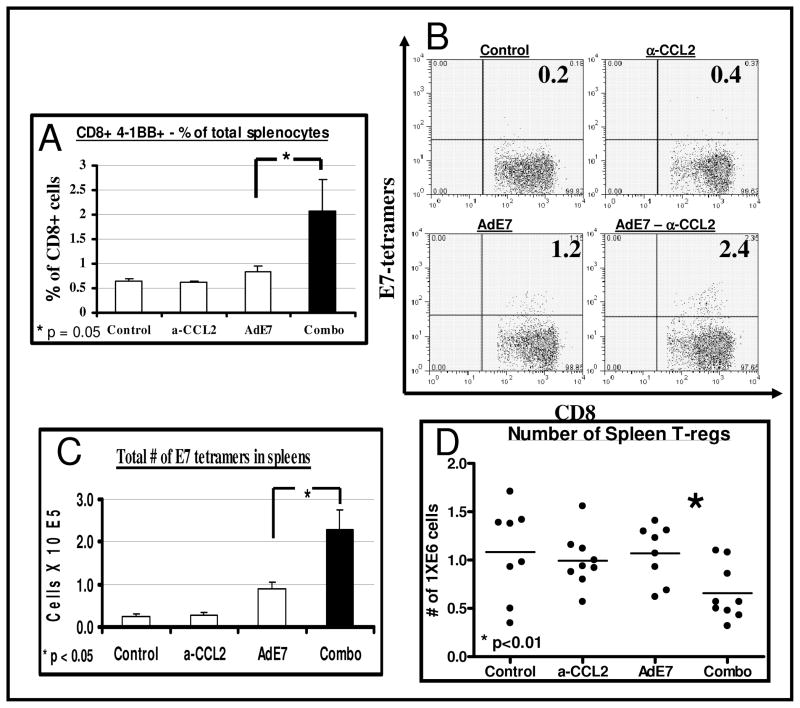
To assess the effect of the combination therapy on CD8+ T cell activation, we measured the expression of the surface activation marker, 4-1BB (CD137) (3, 20) in CD8+ cells from spleens harvested from mice 2 days after the boost Ad.E7 vaccine (Fig. 2A). The percentage of CD8+ T cells expressing 4-1BB in the spleens of mice treated with α-CCL2 mAb or Ad.E7 alone was not increased compared to control mice. However, in tumor-bearing mice treated with the combination therapy, we found a 2-fold increase in the percentage of activated CD8+ T cells (CD8+/4-1BB+) out of total splenocytes (p=0.05, Fig. 2A).
Figure 2. CCL2 blockade increases the activity and antigen-specificity of splenic CD8.
+ T-cells and reduces splenic T-regs.
Flow cytometry was performed on spleens from control tumor-bearing mice (TC1), and mice treated with α-CCL2 mAb, Ad.E7 or the combination of both, 2 days after the second (booster) Ad.E7 vaccine (n=5 per group).
Panel A summarizes the percentage of CD8+ T cells expressing the activation marker 4-1BB out of all splenocytes, showing increased activity in the combination therapy (*=p<0.05).
Panels B–C show splenic CD8+/tetramer-E7+. Panel B shows representative FACS tracings of splenic CD8 versus E7 tetramer+ cells in each group. The number in each quadrant is the percentage of the CD8+ cells. Panel C summarizes the mean number of positive cells per spleen in the four groups, showing increased numbers in the combination group (n=5, *=p<0.05).
Panel D shows the mean number of splenic T-regs (defined as CD4+/CD25+) in each group. T-regs were significantly lower in the combination group (n=5, *=p<0.01).
The murine NSCLC line TC1, which expresses the HPV-E7 peptide, enabled us to directly evaluate the reactivity of CD8+ T cells to a specific tumor antigen (HPV-E7) by flow cytometry using tetramers (4). CCL2 blockade by itself did not change the percentage of antigen-specific CD8+ cells (0.4 ± 0.1 %) in the spleen compared to control tumor-bearing mice (0.3 ± 0.1 %). Ad.E7 immunotherapy increased the percentage of splenic E7-specific CD8+ cells almost 4-fold to 1.1 ± 0.2 % of CD8+ cells. Addition of CCL2 blockade to Ad.E7 significantly increased the percentage of E7-reactive CD8+ cells to 2.3 ± 0.5 % of CD8+ cells (p<0.05, representative tracings in Fig, 2B). The total calculated number of specific CTLs showed the same pattern (p<0.02, Fig. 2C).
We next evaluated the changes in T-regulatory (T-reg) cells in the spleen, defined by flow cytometry as CD4+/FoxP3+. The percentage of FoxP3+ cells out of CD4+ cells in the spleen was slightly, but significantly, reduced from 19.2 % ± 0.6 in the AdE7-treated mice to 17.4 % ± 0.6 in the combination-treated mice (n=12, p<0.05). When the total number of T-reg cells in the spleens was calculated, we did not see significant changes in the number of splenic T-regs among control, α-CCL2-treated, and AdE7-treated mice. However, the combination treatment resulted in a reduction in their number in about 2-fold (p<0.01, Fig. 2D).
Finally, we evaluated splenic CD11b+/GR1+ cells, generally accepted to be myeloid derived suppressor cells (MDSC). CD11b+/GR1+ cells made up to 15% of the splenocytes. However we saw no differences between the four treatment groups in the number of these cells in the spleens. We further evaluated if there were changes in the numbers of the two subsets of CD11b+/GR1+ as described recently (21). We saw no changes in the CD11b+/Ly6G+ granulocytic population, nor in the CD11b+/Ly6C+ monocytic population (data not shown).
Blocking CCL2 in mice treated with immunotherapy increases the number and activity of intra-tumoral CD8+ T Cells
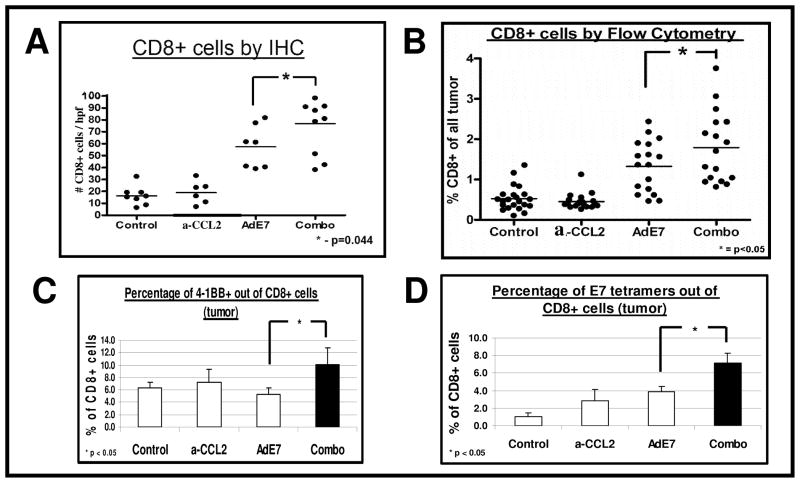
We next evaluated the total number of CD8+ cells infiltrating the tumors. We evaluated the tumors two days following the boost vaccination with Ad.E7, a time point at which there was no significant change in tumor size. CCL2 blockade as monotherapy had no significant effect on the number of intra-tumoral CD8+ cells. In contrast the Ad.E7 vaccine induced a significant influx of CD8+ cells into the tumor. However, the combination of vaccine with CCL2 blockade significantly (p<0.05) increased intra-tumoral CD8+ cells compared to vaccine alone, as seen by immunohistochemistry (Fig. 3A), flow cytometry (Fig. 3B) and by evaluating the expression levels of CD8 mRNA in the tumors using real-time RT-PCR (Table 1).
Figure 3. CCL2 blockade in mice treated with immunotherapy increases the number and activity of intra-tumoral CD8.
+ T Cells
Mice (n = 4–6 for each group) bearing large TC-1 tumors, were treated in one of four ways: 1) No treatment (Control); 2) I.P. α-CCL2 mAb (a-CCL2); 3) S.Q. vaccine with Ad.E7 (Ad.E7); and 4) combination of Ad.E7 and α-CCL2. Two days after the second (booster) Ad.E7 vaccine, tumors were harvested.
Panels A–B summarize the number of intratumoral CD8+ cells by IHC (Panel A), and their percentage of total tumor by flow cytometry (Panel B). The combination of vaccine plus CCL2/CCL12 blockade significantly increased intra-tumoral CD8+ cells (*=p<0.05). Each dot represents one mouse.
Panel C summarizes the percentage of intratumoral CD8+ T cells expressing the activation marker 4-1BB, showing increased activity in the combination therapy (*=p<0.05).
Panel D summarizes the percentage of intratumoral CD8+/tetramer-E7+ cells, showing increased antigen-specific cells in the combination therapy (*=p<0.05).
Table 1.
Real time RT-PCR and Protein level in whole tumors
| PCR | Control | α-CCL2 | AdE7 | AdE7 + α-CCL2 | p-value (Ade7 Vs. combination) |
|---|---|---|---|---|---|
| TNF-α | 1 | 0.8 | 1.8 | 2.8 | < 0.01 |
| TGF-β | 1 | 0.6 | 0.6 | 0.9 | < 0.01 |
| IFN-γ | 1 | 0.8 | 4.1 | 7 | < 0.01 |
| IL10 | 1 | 1 | 0.95 | 1.8 | 0.01 |
| IL-12 | 1 | 1.3 | 0.8 | 1.5 | < 0.01 |
| CXCL-10 (IP-10) | 1 | 2.3 | 1.7 | 3 | < 0.01 |
| CCL2 (MCP-1) | 1 | 1.2 | 2.2 | 2.2 | NS |
| CCL12 | 1 | 0.6 | 1.1 | 1 | NS |
| CCL5 (RANTES) | 1 | 0.9 | 1 | 1 | NS |
| ICAM-1 | 1 | 0.7 | 0.8 | 1.6 | < 0.01 |
| CD8 | 1 | 0.7 | 2.4 | 4.7 | < 0.01 |
| Protein | |||||
| TNF (pg/ml/g) | 331 | 180 | 373 | 999 | 0.02 |
Mice (n = 4–5 for each group) bearing large (average size of 200–250 mm3) TC1 tumors, were treated in one of four ways: 1) control no treatment (Control); 2) I.P. α-CCL2 mAb twice per week starting at day 13 and throughout the experiment (α-CCL2); 3) S.Q. vaccine with Ad.E7, and a booster vaccine after a week (Ad.E7); and 4) combination of Ad.E7 and α-CCL2 mAb (Combo). Two days after the second (booster) Ad.E7 vaccine, tumors were harvested, digested, and RNA was extracted. Equal amounts of RNA from each tumor in each group were pooled, cDNA generated, and subjected to real time RT-PCR analysis. RNA was normalized using β-actin levels. Each assay was run in at least quadruplicate. Fold change with each treatment compared to control is shown. Major changes between immunotherapy alone and the combination with α-CCL2 mAb are highlighted.
For the evaluation of TNF-α protein levels (bottom line), explants from individual tumors of each treatment (n=8 of each subgroup) were plated in medium. After 24 hours, the level of TNF-α was evaluated using an ELISA kit. Mean levels are shown for each treatment, adjusted to tumor weight and medium volume.
The percentage of activated intra-tumoral CD8+ T cells (4-1BB+) out of CD8+ cells was 2-fold higher following combination therapy compared with mice treated only with mAbs or immunotherapy (p<0.05, Fig. 3C and Supplemental Fig. 2A).
Immunotherapy or α-CCL2 mAb alone increased the percentage of intratumoral E7-specific CD8+ cells by 3–4 fold as shown by tetramer staining (Fig. 3D). However, combining α-CCL2 mAb with Ad.E7 vaccine significantly increased the percentage of tetramer-positive CD8+ cells, up to 7.2 fold compared to control (p<0.05 compared to all other groups). Representative tracings are shown in Supplemental Figure 2B.
CCL2 blockade decreases intra-tumoral T-reg cells in the combined therapy, but does not change tumor-associated macrophages (TAMs) compared to immunotherapy alone
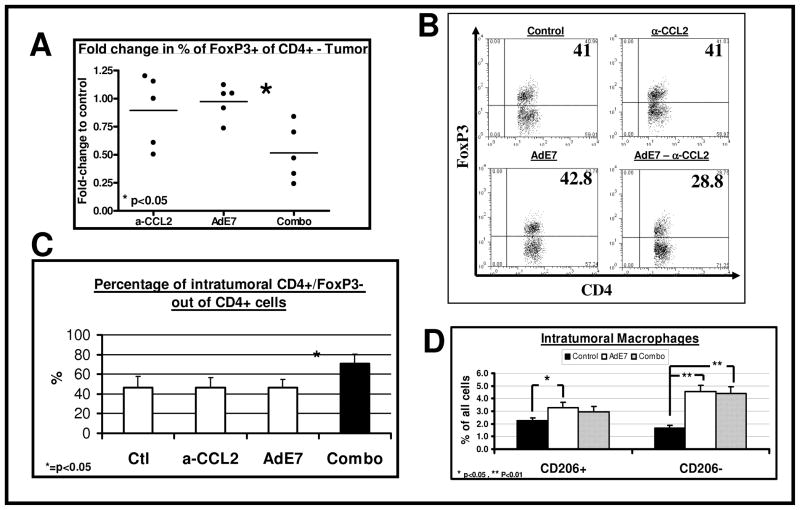
There was only a small, non-significant reduction in the percentage of total CD4+ cells in the tumors with the combined treatment (data not shown). By normalizing the percentage of T-reg cells out of CD4+ cells to those of control tumors, we found a small, non-significant reduction of T-regs in the α-CCL2 treated mice, and no change with AdE7. However, the combination treatment significantly reduced the percentage of intra-tumoral T-regs with to about a half of control (p<0.01 compared to AdE7). The data on intratumoral T-regs is summarized in Figure 4A, with examples of FACS tracings shown in Figure 4B. We also compared the percentage of the CD4+ cells within the tumors that were negative for FoxP3, suggesting that these are activated CD4+ T-cells (Fig. 4C). AdE7 or CCL2-blockade alone did not change the percentage of these cells. However the combination of AdE7 with α-CCL2 mAb significantly increased the percentage of these CD4+/FOXP3− cells by about 50% (p<0.05 for the combination treatment vs. AdE7 alone).
Figure 4. CCL2 blockade in mice treated with immunotherapy decreases the percentage of intratumoral T-regs, but does not change macrophages phenotype.
Mice (n = 4–5 for each group) bearing large TC-1 tumors, were treated as in Figure 3. Two days after the booster vaccine, tumors were and subjected to flow cytometry.
Panel A summarizes the fold-change in the percentage of FoxP3+ cells out of intratumoral CD4+ T-cells, normalized to control, in 5 different experiments (3–5 mice pooled in each subgroup), showing a significant decrease in T-regs in the combination group. (*=p<0.05).
Panel B shows representative FACS tracings of CD4 versus FoxP3 in each group. The number in each quadrant is the percentage of FoxP3+ cells out of CD4+ cells.
Panel C summarizes the percentage of CD4+/FoxP3− cells out of intratumoral CD4+ T-cells, in 5 different experiments (3–5 mice pooled in each subgroup), showing a significant increase in activated CD4+ T cells in the combination group. (*=p<0.05 vs. AdE7).
Panel D summarizes the percentage of classically and alternatively activated macrophages (defined as CD11b+/F480+ and CD206− or CD206+, respectively). Immunotherapy mildly increased CD206+ macrophages, but induced a stronger increase in CD206− macrophages. CCL2 blockade did not further alter these changes (n=15–20, *=p<0.05).
We next sought to look for major changes in the phenotype of tumor-associated macrophages (TAM). Treatment with Ad.E7 increased the total number of TAM (data not shown) and increased the percentage of alternatively activated macrophages (defined as CD11b+/F4-80+/CD206+) out of total tumor cells, by about 50% compared to control tumors (p<0.05) (Fig. 4D). However, Ad.E7 also increased the percentage of the CD206− macrophages (defined as CD11b+/F4-80+/CD206−), which likely represent classically activated “M1” macrophages, by about 3-fold (Fig. 4D). Although treatment with mAbs alone did reduce the percentage of CD206+ macrophages, suggesting polarization of the macrophages towards an M1 phenotype compared to control (data not shown), we did not find a difference in the level of either CD206+ or CD206− macrophages in the combined therapy compared to immunotherapy alone (Fig. 4D). We further calculated the ratio of mRNA of typical cytokines secreted from classically and alternatively activated macrophages. The ratio of IL12/IL10 was reduced by 16% compared to control in the tumors from mice treated with AdE7 alone, and by 17% in the mice treated with the combination therapy. The lack of changes in the percentage of CD206+ cells and the similar small changes in IL12/IL10 ratio, suggest that changes in TAM phenotypes were not the main mechanism of the augmentation of immunotherapy by α-CCL2 mAb.
CCL2 blockade changes tumor microenvironment to be more pro-inflammatory
Finally, we evaluated changes in the tumor microenvironment induced by CCL2 blockade that could explain the increased numbers and activation of the CD8+ CTLs.
We used real-time RT-PCR of tumor extracts to profile a set of relevant cytokines, chemokines, and cell adhesion molecules (Table 1). CCL2 blockade induced only minor changes in our panel, with the exception of a 2.3 fold increase in CXCL-10 (IP-10). In Ad.E7 treated tumors, the mRNA expression levels of TNF-α, CCL5, and CXCL-10 all increased about 2 fold and the IFNγ level increased 4-fold. However, expression levels of TNF-α, IFNγ, IL-10, IL-12, ICAM, and CXCL-10 were about 2 fold higher in the combination group compared to the Ad.E7 alone group (p’s <0.01) IL-12 and ICAM-1 were only increased in the combination therapy group.
We then measured the amount of TNF-α secreted from whole tumor explants. Although no significant change in the secretion of TNF-α was noted in tumors from mice treated with α-CCL2 mAbs or Ad.E7 alone, tumors from mice treated with the combination secreted more than 3 fold the amount of TNF-α (p=0.017, Table 1, bottom) compared to the other treatments.
Discussion
In recent years, immunotherapy strategies have been aggressively pursued to enhance anti-tumor immune responses and many phase II and III clinical trials have been conducted (22). Although a variety of immunotherapeutic approaches have been shown to generate active cytotoxic T-lymphocytes (CTLs), success in patients has been limited. It has become increasingly clear that the generation of CTL is necessary, but not sufficient for an effective response (22, 23). There may be a number of reasons for this. First, in addition to inducing immune stimulation, cancer immunotherapies also appear to trigger counter-regulatory immune-suppressive mechanisms such as upregulation of inhibitory surface molecules on T-cells (like CTLA-4 or PD1) or production of T-regulatory cells (24, 25). Second, tumors are known to produce inhibitory cytokines and chemokines (26, 27), as well as induce populations of suppressor cells (28). Thus, it is becoming increasingly apparent that in addition to the generation of CTL, successful immunotherapy will also require “inhibiting the inhibitors”. The studies presented here, using specific anti-murine CCL2 and CCL12 mAb in three different models of immunotherapy, suggest that MCPs may be additional unrecognized key proximal cytokines able to block the immune responses elicited by immunotherapy.
Human CCL2 has two murine orthologues – CCL2 (MCP-1) and CCL12 (MCP-5). Both bind to the CCR2 receptor, although CCL2 is a better agonist of murine CCR2 (29). Most functions described for CCL12 are similar to those found for CCL2 (13). We found that each of these mAbs had some effect on tumor growth, but saw significantly more growth inhibition when the two mAb were combined by themselves (Figure 1A) or in combination with immunotherapy (Supplemental Fig. 1). To most accurately model potential effects in humans, we therefore used a mixture of both mAbs for all of our experiments.
There has been some controversy in the literature about the role of CCL2 in tumor development. CCL2, originally identified as a potent chemoattractant for monocytes (7, 8), can also function as a T-cell chemoattractant and induce T-cell tumor tropism, including memory T-cells (30–32). It seemed reasonable that CCL2 would thus function to inhibit tumor growth. Indeed, early work showed that transfection of tumor cells that secreted high levels of CCL2 resulted in massive monocyte/macrophage infiltration into the tumor mass, leading to its destruction (33). However, in patients, CCL2 has been found at high levels in multiple tumor types, including NSCLC (7, 34, 35) and high levels usually correlate with poor clinical outcome (36). Studies, such as those by Loberg et al., showed that systemic administration of anti-CCL2 neutralizing antibodies significantly retarded tumor growth (8). The use of α-CCL2 mAb in mice has been recently shown to reduce tumorigenesis and metastasis in prostate cancer xenograft models (37).
These observations support mounting evidence suggesting that most of the effects of CCL2 in non-transduced tumors are actually pro-tumorigenic (7). First, it is now recognized that most monocytes recruited into tumors do not kill tumor cells, but are subverted to an M2 phenotype where they actually support tumor growth (38). Second, CCL2 appears to directly augment the growth and invasiveness of certain tumor cells, that express the CCR2 receptor (7, 39). Third, CCR2 is expressed by endothelial cells and CCL2 appears to promote angiogenesis (40). Fourth, it has been observed that CCL2 can also serve as a chemoattractant for T-regs (41, 42). Finally, it is now recognized that CCL2 also has direct immunoinhibitory (pro-tumorigenic) effects on T-cell function (10, 11), such as inhibiting T cell effector functions and switching T cell differentiation towards Th2-like cells (12).
We noted that vaccines induced an influx of macrophages, compared to control, that was not prevented with CCL2 blockade. It was somewhat surprising that, neutralization of a major chemokine attracting monocytes to the tumor did not reduce the total number of monocytes. We have no definitive answer to this question, but we speculate that in tumors there are many other agents (including CCL5, CCL7, CCL8, CXCL8, CXCL12, CXCL1, M-CSF, and VEGF) that can replace MCP-1 in terms of chemoattraction of monocytes (38). Another possibility is that inducing CD8+ activity and reducing T-regs changed the total balance of chemokines in tumor microenvironment, allowing for the influx of monocytes to continue based on other chemokines.
Our mechanistic studies in the TC1 model show that CCL2 blockade in combination with the Ad.E7 vaccine clearly resulted in increased numbers, activity, and antigen-specificity of CD8+ T cells, and in the percentage of activated CD4+ T cells in the spleens (Figure 2), and even more importantly, intra-tumorally (Figure 3). These data are consistent with those of Peng et al. in adoptive transfer studies who used neutralizing α-CCL2 antibodies and showed that this led to the generation of T-cells that were substantially more active and more vigorous at eliminating tumor suggesting increased tumor specificity (10), and later studies, showing that prevention of tumor-secretion of CCL2 had a positive effect on CTLs that were more active in both spleen and draining lymph nodes (43, 44).
It is likely that some of these activating effects on CD8+ T-cells were due to blockade of the direct effects of CCL2 on the T-cells; however, we also explored other factors that might be involved. First, we did not note any changes in the levels of CD11b+/GR1+ cells, generally accepted as MDSC. In our studies, we did find some effects of α-CCL2 mAb alone on TAM phenotype (manuscript in preparation), however, we could not implicate these changes in explaining the augmented effects we saw in combination therapy, since there were no obvious differences in macrophages phenotype populations when we compared Ad.E7 treatment alone with combination treatment (Fig. 4D).
In contrast, we found significant reductions in T-regs in the spleens (Fig. 2D) and tumors of mice treated with the combination therapy versus vaccine alone (Fig. 4A–B), suggesting an important possible mechanism for reduction of immune suppression. The idea that T-regs are important inhibitors of anti-tumor immune responses is well established (45) and their presence correlates with poor prognosis of cancer patients (46). The finding that CCL2 blockade could inhibit T-reg recruitment is consistent with previous studies showing that: 1) CD4+ T-regs selectively over-express the CCL2/CCL12 receptors CCR2 (47), and CCR4 (48, 49), 2) CCL2 has been shown to specifically chemoattract T-regs in vitro (41, 42), and 3) blocking CCR2 in vivo reduced the influx of T-regs to disease sites in a model of arthritis (47). Interestingly, in the TC1 tumor model, depletion of CD4 cells using a specific mAb leads to slower growth (data not shown), suggesting that these tumors do induce T-regs that then augment their growth.
Given their strong immuno-inhibitory properties, reduction of T-regs has been a goal of many groups. To date, most attempts to reduce T-regs have used non-specific agents such as low dose cyclophosphamide or antibodies/antibody-toxins directed toward the IL2-receptor (CD25). Targeting CD25 may have disadvantages, however, since it is also expressed on activated CD8+ CTLs (45, 46). Our data suggest that a novel, and possibly safer way to prevent the influx of T-regs into the tumor microenvironment may be via CCL2 blockade. This may be particularly important when the strong immune reaction induced by vaccines is also accompanied by a strong induction of T-regs.
Finally, we also found that the tumor microenvironment was altered in the combination-treated tumors with increased mRNA levels of Th1 type mediators such as TNF-α, IFNγ, CXCL10, and ICAM-1 (Table 1), and protein levels of TNF-α. It is currently uncertain if this is a direct result of CCL2 blockade leading to enhanced T-cell activation or whether increased numbers of activated CD8+ T cells results in a more immunostimulatory microenvironment.
In summary, we demonstrated here that blocking CCL2 dramatically augmented the effect of immunotherapy for NSCLC and mesothelioma in a multifactorial immunologic mechanism. Our observations suggest that combining CCL2 neutralization with vaccines should be considered in future immunotherapy trials.
Supplementary Material
Acknowledgments
Grant support: NCI PO1 CA 66726
We thank Luana Pereira and Aaron Blouin for technical assistance in the experiments.
Footnotes
Potential conflicts of interest: Dr. Snyder is an employee of Centocor, Inc. This study was partially funded by a research grant from Centocor, Inc.
References
- 1.Rosenberg SA, Yang JC, Restifo NP. Cancer immunotherapy: moving beyond current vaccines. Nature Medicine. 2004;10:909–15. doi: 10.1038/nm1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pardoll D. Does the immune system see tumor as foreign or self? Annual Review of Immunology. 2003;21:807. doi: 10.1146/annurev.immunol.21.120601.141135. [DOI] [PubMed] [Google Scholar]
- 3.Kim S, Buchlis G, Fridlender ZG, et al. Systemic Blockade of Transforming Growth Factor-beta Signaling Augments the Efficacy of Immunogene Therapy. Cancer Res. 2008;68:10247–56. doi: 10.1158/0008-5472.CAN-08-1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Haas AR, Sun J, Vachani A, et al. Cycloxygenase-2 Inhibition Augments the Efficacy of a Cancer Vaccine. Clin Cancer Res. 2006;12:214–22. doi: 10.1158/1078-0432.CCR-05-1178. [DOI] [PubMed] [Google Scholar]
- 5.Comes A, Rosso O, Orengo AM, et al. CD25+ Regulatory T Cell Depletion Augments Immunotherapy of Micrometastases by an IL-21-Secreting Cellular Vaccine. J Immunol. 2006;176:1750–8. doi: 10.4049/jimmunol.176.3.1750. [DOI] [PubMed] [Google Scholar]
- 6.Egen JG, Kuhns MS, Allison JP. CTLA-4: new insights into its biological function and use in tumor immunotherapy. Nature Immunology. 2002;3:611–8. doi: 10.1038/ni0702-611. [DOI] [PubMed] [Google Scholar]
- 7.Conti I, Rollins BJ. CCL2 (monocyte chemoattractant protein-1) and cancer. Seminars in Cancer Biology. 2004;14:149–54. doi: 10.1016/j.semcancer.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 8.Loberg RD, Ying C, Craig MJ, Yan L, Snyder L, Pienta KJ. CCL2 is an important mediator of prostate cancer growth in vivo via regulation of macrophage infiltration. Neoplasia. 2007;9:556–62. doi: 10.1593/neo.07307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hasegawa H, Inoue A, Muraoka M, Yamanouchi J, Miyazaki T, Yasukawa M. Therapy for pneumonitis and sialadenitis by accumulation of CCR2-expressing CD4+CD25+ regulatory T cells in MRL/lpr mice. Arthritis Research & Therapy. 2007;9:R15. doi: 10.1186/ar2122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peng L, Shu S, Krauss JC. Monocyte Chemoattractant Protein Inhibits the Generation of Tumor-reactive T Cells. Cancer Res. 1997;57:4849–54. [PubMed] [Google Scholar]
- 11.Terwey TH, Kim TD, Kochman AA, et al. CCR2 is required for CD8-induced graft-versus-host disease. Blood. 2005;106:3322–30. doi: 10.1182/blood-2005-05-1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hu K, Xiong J, Ji K, Sun H, Wang J, Liu H. Recombined CC chemokine ligand 2 into B16 cells induces production of Th2-dominanted cytokines and inhibits melanoma metastasis. Immunology Letters. 2007;113:19–28. doi: 10.1016/j.imlet.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 13.Tsui P, Das A, Whitaker B, et al. Generation, characterization and biological activity of CCL2 (MCP-1/JE) and CCL12 (MCP-5) specific antibodies. Human Antibodies. 2007;16:117–25. [PubMed] [Google Scholar]
- 14.Lin K-Y, Guarnieri FG, Staveley-O’Carroll KF, et al. Treatment of Established Tumors with a Novel Vaccine That Enhances Major Histocompatibility Class II Presentation of Tumor Antigen. Cancer Res. 1996;56:21–6. [PubMed] [Google Scholar]
- 15.Wilderman MJ, Sun J, Jassar AS, et al. Intrapulmonary IFN-beta gene therapy using an adenoviral vector is highly effective in a murine orthotopic model of bronchogenic adenocarcinoma of the lung. Cancer Res. 2005;65:8379–87. doi: 10.1158/0008-5472.CAN-05-0920. [DOI] [PubMed] [Google Scholar]
- 16.Jackaman C, Bundell CS, Kinnear BF, et al. IL-2 Intratumoral Immunotherapy Enhances CD8+ T Cells That Mediate Destruction of Tumor Cells and Tumor-Associated Vasculature: A Novel Mechanism for IL-2. J Immunol. 2003;171:5051–63. doi: 10.4049/jimmunol.171.10.5051. [DOI] [PubMed] [Google Scholar]
- 17.Brin E, Atencio I, Helmich BK, Maneval D, Laface D. Adenovirus delivery provides extended interferon-alpha exposure and augments treatment of metastatic carcinoma. Cancer Gene Ther. 2006;13:664–75. doi: 10.1038/sj.cgt.7700942. [DOI] [PubMed] [Google Scholar]
- 18.Hassan R, Ho M. Mesothelin targeted cancer immunotherapy. European Journal of Cancer. 2008;44:46–53. doi: 10.1016/j.ejca.2007.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Suzuki E, Kapoor V, Jassar AS, Kaiser LR, Albelda SM. Gemcitabine Selectively Eliminates Splenic Gr-1+/CD11b+ Myeloid Suppressor Cells in Tumor-Bearing Animals and Enhances Antitumor Immune Activity. Clin Cancer Res. 2005;11:6713–21. doi: 10.1158/1078-0432.CCR-05-0883. [DOI] [PubMed] [Google Scholar]
- 20.Dawicki W, Tania HW. Expression and function of 4-1BB during CD4 versus CD8 T cell responses in vivo. European Journal of Immunology. 2004;34:743–51. doi: 10.1002/eji.200324278. [DOI] [PubMed] [Google Scholar]
- 21.Youn JI, Nagaraj S, Collazo M, Gabrilovich DI. Subsets of myeloid-derived suppressor cells in tumor-bearing mice. J Immunol. 2008;181:5791–802. doi: 10.4049/jimmunol.181.8.5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Emens LA. Cancer vaccines: on the threshold of success. Expert Opinion on Emerging Drugs. 2008;13:295–308. doi: 10.1517/14728214.13.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Finn O. Cancer immunology. N Engl J Med. 2008;358:2704–15. doi: 10.1056/NEJMra072739. [DOI] [PubMed] [Google Scholar]
- 24.Parmiani G, Castelli C, Pilla L, Santinami M, Colombo MP, Rivoltini L. Opposite immune functions of GM-CSF administered as vaccine adjuvant in cancer patients. Ann Oncol. 2007;18:226–32. doi: 10.1093/annonc/mdl158. [DOI] [PubMed] [Google Scholar]
- 25.Gajewski TF. Failure at the Effector Phase: Immune Barriers at the Level of the Melanoma Tumor Microenvironment. Clin Cancer Res. 2007;13:5256–61. doi: 10.1158/1078-0432.CCR-07-0892. [DOI] [PubMed] [Google Scholar]
- 26.Finn O. Cancer vaccines: between the idea and the reality. Nat Rev Immunol. 2003;3:630–41. doi: 10.1038/nri1150. [DOI] [PubMed] [Google Scholar]
- 27.Finke J, Ferrone S, Frey A, Mufson A, Ochoa A. Where have all the T cells gone? Mechanisms of immune evasion by tumors. Immunol Today. 1999;20:158–60. doi: 10.1016/s0167-5699(98)01435-2. [DOI] [PubMed] [Google Scholar]
- 28.Gallina G, Dolcetti L, Serafini P, et al. Tumors induce a subset of inflammatory monocytes with immunosuppressive activity on CD8+ T cells. J Clin Invest. 2006;116:2777–90. doi: 10.1172/JCI28828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sarafi MN, Garcia-Zepeda EA, MacLean JA, Charo IF, Luster AD. Murine Monocyte Chemoattractant Protein (MCP)-5: A Novel CC Chemokine That Is a Structural and Functional Homologue of Human MCP-1. J Exp Med. 1997;185:99–110. doi: 10.1084/jem.185.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brown CE, Vishwanath RP, Aguilar B, et al. Tumor-Derived Chemokine MCP-1/CCL2 Is Sufficient for Mediating Tumor Tropism of Adoptively Transferred T Cells. J Immunol. 2007;179:3332–41. doi: 10.4049/jimmunol.179.5.3332. [DOI] [PubMed] [Google Scholar]
- 31.Carr M, Roth S, Luther E, Rose S, Springer T. Monocyte chemoattractant protein 1 acts as a T-lymphocyte chemoattractant. Proc Natl Acad Sci U S A. 1994;91:3652–6. doi: 10.1073/pnas.91.9.3652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang T, Dai H, Wan N, Moore Y, Dai Z. The Role for Monocyte Chemoattractant Protein-1 in the Generation and Function of Memory CD8+ T Cells. J Immunol. 2008;180:2886–93. doi: 10.4049/jimmunol.180.5.2886. [DOI] [PubMed] [Google Scholar]
- 33.Nesbit M, Schaider H, Miller TH, Herlyn M. Low-Level Monocyte Chemoattractant Protein-1 Stimulation of Monocytes Leads to Tumor Formation in Nontumorigenic Melanoma Cells. J Immunol. 2001;166:6483–90. doi: 10.4049/jimmunol.166.11.6483. [DOI] [PubMed] [Google Scholar]
- 34.Negus R, Stamp G, Relf M, et al. The detection and localization of monocyte chemoattractant protein-1 (MCP-1) in human ovarian cancer. J Clin Invest. 1995;95:2391–6. doi: 10.1172/JCI117933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Arenberg D, Keane M, DiGiovine B, et al. Macrophage infiltration in human non-small-cell lung cancer: the role of CC chemokines. Cancer Immunol Immunother. 2000;49:63–70. doi: 10.1007/s002620050603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ueno T, Toi M, Saji H, et al. Significance of Macrophage Chemoattractant Protein-1 in Macrophage Recruitment, Angiogenesis, and Survival in Human Breast Cancer. Clin Cancer Res. 2000;6:3282–9. [PubMed] [Google Scholar]
- 37.Loberg RD, Ying C, Craig M, et al. Targeting CCL2 with Systemic Delivery of Neutralizing Antibodies Induces Prostate Cancer Tumor Regression In vivo. Cancer Res. 2007;67:9417–24. doi: 10.1158/0008-5472.CAN-07-1286. [DOI] [PubMed] [Google Scholar]
- 38.Allavena P, Sica A, Garlanda C, Mantovani A. The Yin-Yang of tumor-associated macrophages in neoplastic progression and immune surveillance. Immunological Reviews. 2008;222:155–61. doi: 10.1111/j.1600-065X.2008.00607.x. [DOI] [PubMed] [Google Scholar]
- 39.Loberg RD, Day LL, Harwood J, et al. CCL2 is a potent regulator of prostate cancer cell migration and proliferation. Neoplasia. 2006;8:578–86. doi: 10.1593/neo.06280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Salcedo R, Ponce M, Young H, et al. Human endothelial cells express CCR2 and respond to MCP-1: direct role of MCP-1 in angiogenesis and tumor progression. Blood. 2000;96:34–40. [PubMed] [Google Scholar]
- 41.Fu S, Yopp A, Mao X, et al. CD4+ CD25+ CD62+ T-Regulatory Cell Subset Has Optimal Suppressive and Proliferative Potential. American Journal of Transplantation. 2004;4:65–78. doi: 10.1046/j.1600-6143.2003.00293.x. [DOI] [PubMed] [Google Scholar]
- 42.Jordan J, Sun W, Hussain S, DeAngulo G, Prabhu S, Heimberger A. Preferential migration of regulatory T cells mediated by glioma-secreted chemokines can be blocked with chemotherapy. Cancer Immunology, Immunotherapy. 2008;57:123–31. doi: 10.1007/s00262-007-0336-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vitiello PF, Shainheit MG, Allison EM, Adler EP, Kurt RA. Impact of tumor-derived CCL2 on T cell effector function. Immunology Letters. 2004;91:239–45. doi: 10.1016/j.imlet.2003.12.009. [DOI] [PubMed] [Google Scholar]
- 44.Owen JL, Lopez DM, Grosso JF, et al. The expression of CCL2 by T lymphocytes of mammary tumor bearers: Role of tumor-derived factors. Cellular Immunology. 2005;235:122–35. doi: 10.1016/j.cellimm.2005.08.032. [DOI] [PubMed] [Google Scholar]
- 45.Curiel TJ. Tregs and rethinking cancer immunotherapy. J Clin Invest. 2007;117:1167–74. doi: 10.1172/JCI31202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Piersma SJ, Welters MJP, Van der Burg SH. Tumor-specific regulatory T cells in cancer patients. Human Immunology. 2008;69:241–9. doi: 10.1016/j.humimm.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 47.Bruhl H, Cihak J, Schneider MA, et al. Dual Role of CCR2 during Initiation and Progression of Collagen-Induced Arthritis: Evidence for Regulatory Activity of CCR2+ T Cells. J Immunol. 2004;172:890–8. doi: 10.4049/jimmunol.172.2.890. [DOI] [PubMed] [Google Scholar]
- 48.Iellem A, Mariani M, Lang R, et al. Unique Chemotactic Response Profile and Specific Expression of Chemokine Receptors CCR4 and CCR8 by CD4+CD25+ Regulatory T Cells. J Exp Med. 2001;194:847–54. doi: 10.1084/jem.194.6.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Giaccia A, Siim BG, Johnson RS. HIF-1 as a target for drug development. Nat Rev Drug Discov. 2003;2:803–11. doi: 10.1038/nrd1199. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.