Abstract
P50, N100, and P200 auditory sensory gating could reflect mechanisms involved in protecting higher-order cognitive functions, suggesting relationships between sensory gating and cognition. This hypothesis was tested in 56 healthy adults who were administered the paired-click paradigm and two adaptations of the continuous performance test (Immediate/Delayed Memory Task, IMT/DMT). Stronger P50 gating correlated with fewer commission errors and prolonged reaction times on the DMT. Stronger N100 and P200 gating correlated with better discriminability on the DMT. Finally, prolonged P200 latency related to better discriminability on the IMT. These findings suggest that P50, N100, and P200 gating could be involved in protecting cognition by affecting response bias, behavioral inhibition, working memory, or attention.
Descriptors: Sensory gating, Executive functions, Evoked potentials, Attention, Working memory, Impulsivity
Cognitive functions may be protected from potentially interfering information by brain mechanisms that filter stimuli before information is transferred for more elaborate processing by higher-order mechanisms (Venables, 1964).P50, N100, and P200 sensory gating could reflect distinct biological substrates (Brockhaus-Dumke, Mueller, Faigle, & Klosterkoetter, 2008; Hanlon et al., 2005; Kisley, Noecker, & Guinther, 2004; Oranje, Geyer, Böcker, Kenemans,& Verbaten, 2006;Wan, Crawford,& Boutros, 2007) that preserve the integrity of cognitive functioning (Boutros, Korzyukov, Jansen, Feingold, & Bell, 2004; Wan, Friedman, Boutros, & Crawford, 2008) by preventing irrelevant information from reaching higher-order functions (Freedman, Waldo, Bickford-Wimer, & Nagamoto, 1991; Hsieh, Liu, Chiu, Hwu, & Chen, 2004; Kisley et al., 2004). This suggests that P50, N100, and P200 gating represent strategically important protective mechanisms whose impairment could lead to diminished cognitive functioning.
P50, N100, and P200 sensory gating can be studied with the paired-click paradigm. In the paired-click paradigm a simple auditory stimulus elicits a P50-N100-P200 complex that is attenuated in amplitude if the initial stimulus (S1) is followed shortly in time by a second, identical stimulus (S2) (Fruhstorfer, Soveri, & Järvilehto, 1970). The reduction in P50, N100, and P200 amplitude from S1 to S2 can be expressed either as the ratio between the amplitude evoked by S2 and the amplitude evoked by S1 or as the absolute difference between the amplitude of S1 and S2 (Smith, Boutros,&Schwarzkopf, 1994).A higher ratio or a smaller difference score reflects weaker gating, which may relate to diminished cognitive functioning.
P50 Sensory Gating
P50 sensory gating reflects a predominantly preattentional (Freedman et al., 1987; Jerger, Biggins, & Fein, 1992; White & Yee, 1997, 2006) inhibitory filter mechanism that could protect the integrity of higher-order functions (Freedman et al., 1991; Jerger et al., 1992; Wan et al., 2008), suggesting that impaired P50 gating in subjects with Alzheimer dementia (Thomas et al., 2009), antisocial personality disorder (Lijffijt, Moeller, Boutros, Burroughs, et al., 2009), panic disorder (Ghisolfi et al., 2006), post-traumatic stress disorder (Karl, Malta, & Maercker, 2006), schizotypal personality disorder (Cadenhead, Light, Geyer, & Braff, 2000; Wan et al., 2007; Wang, Miyazato, Hokama, Hiramatsu, & Kondo, 2004), cocaine abuse (Boutros, Gooding, Sundaresan, Burroughs, & Johanson, 2006; Fein, Biggins, & MacKay, 1996), schizophrenia (Adler et al., 1982; Bramon, Rabe-Hesketh, Sham, Murray, & Frangou, 2004; De Wilde, Bour, Dingemans, Koelman, & Linszen, 2007; Patterson et al., 2008), and bipolar I disorder (Franks, Adler, Waldo, Alpert, & Freedman, 1983; Lijffijt, Moeller, Boutros, Steinberg, et al., 2009; Olincy & Martin, 2005; Schulze et al., 2007) could relate to diminished cognitive functioning commonly found in these disorders.
The studies that have tested the potential relationship between P50 gating and cognitive functioning have reported inconsistent outcomes. In samples limited to 10 to 19 subjects with schizophrenia (Cullum et al., 1993; Erwin, Turetsky, Moberg, Gur, & Gur, 1998; Hsieh et al., 2004), Alzheimer disease, or healthy elderly controls (Thomas et al., 2009), associations were found between stronger P50 gating and better performance on computer tasks measuring attention, motor speed, and learning. These findings are consistent with a report relating stronger P50 gating to faster reaction times, better orienting of attention, and better inhibition of conflicting information in 39 healthy subjects (Wan et al., 2008). However, others did not find these relationships (Cullum et al., 1993; Hsieh et al., 2004; Thoma et al., 2006). These differences in findings could be due to a lack of power in most studies (n = 10–19) or due to the use of the P50 ratio, which has a lower reliability than P50 difference score, as the gating measure of interest (Fuerst, Gallinat, & Boutros, 2007; Lu et al., 2007; Rentzsch, Jockers-Scherübl, Boutros, & Gallinat, 2008; Smith et al., 1994).
N100 and P200 Sensory Gating
N100 and P200 sensory gating are impaired in subjects with schizophrenia (Boutros, Korzyuko, Oliwa, et al., 2004), cocaine abuse (Boutros et al., 2006), and bipolar I disorder (Lijffijt, Moeller, Boutros, Steinberg, et al., 2009) and potentially relate to diminished cognitive performance in these disorders. N100 and P200 sensory gating could reflect different mechanisms than those reflected by P50 gating (Boutros, Korzyukov, Jansen, et al., 2004; Wan et al., 2008) and could thus relate to different functions. N100 and P200 auditory evoked potentials measured in passive listening tasks could, respectively, reflect a trigger to allocate attention (Näätänen, 1992, pp. 113–135; Näätänen & Picton, 1987), and early allocation of attention (Näätäaen, 1992, p. 222). Based on these considerations, N100 gating could relate to filter mechanisms involved in triggering of attention, whereas P200 sensory gating could relate to filter mechanisms involved in allocation of attention. As these functions differ from those hypothesized for P50 gating, N100 and P200 gating could affect different aspects of cognitive functioning (Boutros, Korzyukov, Jansen, et al., 2004; Kisley et al., 2004).
However, only two studies have addressed potential relationships between cognitive functioning and N100 and P200 gating directly. N100 gating correlated with performance on an implicit learning task in healthy subjects (Hsieh et al., 2004), and N100 and P200 sensory gating correlated with latency and peak amplitude of the P300 potential obtained in an oddball task (Boutros, Korzyukov, Jansen, et al., 2004), suggesting relationships between N100 and P200 gating and learning and attention. We know of no other studies on potential associations between N100 and P200 sensory gating and cognitive functioning.
Aims
Despite the theoretical importance of P50, N100, and P200 sensory gating in protecting the integrity of higher-order cognitive functions and the widespread impairment in these measures across a wide range of psychiatric disorders, previous studies have only sporadically focused on the potential relationships between gating and cognitive functioning. Moreover, the few available studies have focused almost exclusively on P50 gating rather than on gating of the N100 and P200. Finally, outcomes have been inconsistent, possibly because of inadequate sample sizes or because of the low reliability of ratio measures.
The aim of this study was to study the potential protective function of P50, N100, and P200 sensory gating on cognition. The first hypothesis was based on previous findings, expecting that stronger P50, N100, and P200 sensory gating would relate to better performance on computer tasks measuring cognitive functions. The second hypothesis was based on the theoretical difference in preattentional and attentional bases of P50, N100, and P200 gating, expecting that P50 gating would differ from N100 or P200 gating in their specific relationships on measures of cognition.
To address potential pitfalls, a sample size was included that should provide adequate power to detect relationships between sensory gating and task performance (n = 56). Furthermore, sensory gating difference scores were included that could be more reliable than ratio measures (Fuerst et al., 2007; Rentzsch et al., 2008; Smith et al., 1994). Higher-order functions were addressed with two adaptations of the continuous performance test (the immediate memory task [IMT] and the delayed memory task [DMT]; Dougherty, Bjork, Huckabee, Moeller, & Swann, 1999; Dougherty, Marsh, Moeller, Chokshi,& Rosen, 2000), assessing behavioral measures of attention, response inhibition, reaction time, and working memory.
Methods
Participants
The study complied with the Declaration of Helsinki and was approved by the Committee for the Protection of Human Subjects, Institutional Review Board of the University of Texas Health Science Center at Houston. Participants received thorough descriptions of the study, with a full opportunity for questions, and signed informed consent before any research-related procedures were conducted. Participants were recruited from the general population through advertisements in the local press. Fifty-six participants (age: 31.41 ± 10.09 years; highest level of completed education: 14.39 ± 2.17 years; 32 women; 10 current smokers) were included in the study. Three other participants were excluded from this study because of outcomes that were more than 3 SD above the mean for P50 latency for S1 (114 ms), N100 S1–S2 latency difference (82 ms), and P200 amplitude for S1 (36 µV). Although 1 participant in the current sample had a P50 ratio more than 3 SD above the mean (178%), that participant was not excluded due to evidence than approximately 3% of subjects in healthy samples could have a P50 gating ratio exceeding 180% (Patterson et al., 2008).
Participants were required to have good hearing by self-report, normal or corrected-to-normal vision, no history of head trauma or epilepsy, no criminal history, and no history of alcohol and drug abuse. Participants had no current and past axis I or II disorders assessed by the Structured Clinical Interview for DSM-IV axis I and axis II disorders (SCID-I and SCID-II; First, Spitzer, Gibbon, & Williams, 1996), which were administered by fully trained staff members and confirmed by consensus meeting.
Measures
Paired-click paradigm
The paired-click paradigm consisted of two blocks, each containing 50 pairs of two identical 40-ms 90-dB clicks (1000 Hz, 4 ms raise-fall) presented binaurally through headphones using STIM software (Neuroscan, Inc., El Paso, TX). The interval between the first (S1) and second (S2) click was 500 ms; the interval between two consecutive pairs was randomized between 8 and 10 s, allowing full recovery of the P50 component (Freedman, Adler, Waldo, Pachtman, & Franks, 1983; Fruhstorfer et al., 1970; Zouridakis & Boutros, 1992).
Immediate Memory Task and Delayed Memory Task
The IMT and DMT are adaptations of the continuous performance test (Dougherty et al., 1999). In both the IMT and DMT stimuli were black digits randomly combined into five-digit numbers. The numbers were randomized for each new presentation of the task. Stimuli were presented for 500 ms on a white computer screen with an inter stimulus interval of 500 ms. Subjects performed two 5-min blocks containing 300 trials. After a training session, subjects performed 1 block of the IMT followed by 1 block of the DMT. After a 30-s break the IMT and DMT were performed a second time. Each block lasted 5 min; total testing time was 20 min.
For the IMT, subjects were instructed to press the left mouse button as quickly as possible if they perceived a repetition of a five-digit number (e.g., 3 1 7 4 8) and to refrain from responding with any other succeeding number (e.g., 3 1 7 5 8 or 9 3 5 2 1).
The DMT is exactly the same as the IMT except that each five-digit number was succeeded by three distracter trials consisting of the number 1 2 3 4 5. Subjects were instructed to ignore the distracters and to respond as quickly as possible when a five-digit number preceding the distracters matched the five-digit number following the distracters.
Stimuli were designated as target (33%; repetition stimulus with all digits the same and on the same location as a previous stimulus), off-target (33%; nonrepetitive stimulus with four out of five digits identical to the previous stimulus; location of off-target digit was randomized across the five positions), and filler (34%; nonrepetitive stimulus with all digits differing from the previous stimulus).
Dependent variables for both the IMT and DMT included (a) correct detections (hits) reflecting the ability to sustain attention, (b) commission errors (false alarms to off-target stimuli) reflecting the ability to inhibit a response generated before a stimulus is fully analyzed, and (c) reaction times for correct detections or commission errors assessing response speed (Dougherty et al., 2000). Two summary variables were used: (a) the signal detection measures A′ (the non-parametric equivalent of d′); 2) the signal detection measure B″ (the nonparametric equivalent of response bias). A′ reflects the sensitivity to discriminate between signal – target– and noise –off-target–, which is affected by attentional and perceptional processes; scores range from 0.5 (chance level) to 1 (perfect discrimination). B″ reflects response strategy for a preference for many correct detections that may also produce many commission errors (liberal reporting) or for a preference for fewer commission errors, which may also produce fewer correct detections (conservative reporting); scores range from −1 to 1, with lower scores reflecting more liberal reporting (Dougherty et al., 2000). Finally, functional magnetic resonance imaging (MRI) studies contrasting performance on the IMT and DMT revealed the involvement of brain areas also associated with working memory (Moeller et al., 2004; Valdes et al., 2006). In the current study the differences between DMT and IMT (DMT minus IMT) performance on rate of correct detections and commission errors and A′ were used as potential measures of working memory.
Procedure
On the day of testing, subjects had to have negative screens for drugs (RediCup, Redwood Biotech, Santa Rosa, CA) and alcohol (Alco-Sensor III, Intoximeters Inc., St. Louis, MO), and were asked to refrain from consuming caffeinated products for at least 8 h and from smoking for at least 1 h prior to testing. For psychophysiologic testing subjects were seated in a chair in a hospital room with no additional safeguards against noise and electrical interference. Subjects were instructed to listen passively to the clicks, to relax, and sit quietly with their eyes fixated on a fixation cross mounted on the wall. Blinking was allowed. Subjects performed the IMT and DMT in a sound-reducing cabin with a fan creating constant background noise. For most subjects, IMT/DMT and gating measures were obtained on the same day.
Data Recording and Analysis
Raw electroencephalogram (EEG) was recorded from 32 electrodes attached in a Quik-cap (Neuromedics Neuroscan, El Paso, TX), arranged according to the international 10–20 system (Jasper, 1958). Data were recorded using Acquire 4.3 (NeuroScan, El Paso, TX). Signals were sampled at 1000 Hz, filtered between 0.1 and 100 Hz (AC mode), and amplified × 10,000 through SynAmps amplifiers. Signals were referenced to linked electrodes attached to the mastoids. Vertical and horizontal electrooculograms were assessed with electrodes placed above and below the right eye and at the right and left canthi, respectively. The ground electrode was attached in the cap anteriorly to F3 and F4, extending the midline. Impedances were kept below 5 kΩ.
Signals were filtered off-line between 1 and 50 Hz (48 dB/oct rolloff) and corrected for eyeblinks with a regression algorithm (cf. Semlitsch, Anderer, Schuster, & Presslich, 1986). The data were epoched from 100 ms preceding (baseline) to 400 ms following S1 and S2 and corrected for baseline activity. Trials with artifacts were detected manually and rejected from further analysis. Trials were always rejected as a pair; that is, if S1 was rejected, the accompanying S2 trial was rejected also. Signals for S1 and S2 were averaged separately per block. Next, averages were made for each stimulus across the two blocks. On average, 79 pairs (SD ± 12.59) were retained across blocks. Prior to averaging, signals were filtered with a 10-Hz high-pass filter to optimize scoring of the P50 or with a 20 Hz low-pass filter to optimize scoring of the N100 and P200 (Jerger et al., 1992).
P50, N100, and P200 peaks were scored at Cz relative to their preceding troughs. This could be the P50 for the N100 and was always the N100 for the P200 component. All peaks and preceding troughs were detected automatically (Neuroscan, El Paso, TX) using preset intervals (cf. Boutros, Korzyukov, Oliwa, et al., 2004). The N100 was identified first as the most pronounced peak between 80 and 150ms. If no N100 peak could be identified within that time window, the time window between 0 and 300 ms was taken into account in which the most pronounced negativity with a clear fronto-central scalp distribution was considered the N100. The N100 had to be distinguishable from baseline activity and visible on more than one lead for subjects to be included in the analysis. P50 components were scored by two investigators (N.N.B. and S.B.) unaware of the purpose of the study. The P50 was defined as the most pronounced negativity between 35 and 85 ms. If no P50 was detected in that window, the P50 was identified as the most positive peak preceding the N100. The P200 was detected last and defined as the most pronounced positivity between 150 and 250 ms. If no P200 peak was found in that window, the P200 was identified as the most positive peak following the N100 as long as it peaked before 300 ms. In this study, P50 peaks were detected between 38 and 86 ms, N100 peaks were detected between 79 and 156 ms, and P200 peaks were detected between 142 and 273 ms. Scalp distributions were taken into account to identify components that were ambiguous due to multiple peaks or small amplitudes, in which case peaks were selected with a central or fronto-central scalp distribution. For S2 evoked components, additional constraints included S2 P50 activity peaking within 10 ms of the S1 P50 (Nagamoto, Adler, Waldo, Griffith, & Freedman, 1991) and S2 N100 and P200 activity peaking within 40 and 80 ms of the S1 N100 and P200, respectively. If no peaks occurred within those windows, the component was considered completely attenuated unless S2 elicited clear components falling outside the windows as revealed by iso-potential maps (P50: n = 4, window to 15 ms; N100: n = 3, window to 58 ms; P200: n = 1, window to 98 ms).
Statistical Analysis
Electrophysiological variables included P50, N100, and P200 peak amplitudes and latencies for S1 and S2, and gating ratio ((S2amplitude/S1amplitude) × 100) and difference score (S1amplitude– S2amplitude). A lower ratio or higher difference score indicated stronger sensory gating. Distributions were tested for normality using z tests with p =.01 (Tabachnick & Fidell, 1989, pp. 72–73). Nonnormal distributions (all variables, except P50 ratio, N100 ratio and P50 latencies; for IMT/DMT only correct detections had to be corrected) were transformed with a natural logarithm (Tabachnick & Fidell, 1989, pp. 83–86). If this failed to yield a normal distribution (N100 and P200 latencies), nonparametric statistics were used to express relationships (Kendal’s tau instead of Pearson’s r). Finally, if a performance variable correlated with more than one evoked potential variable, we conducted a multiple regression analysis with variables entered in the order of strength of association. We did not perform regression analyses including N100 and P200 difference score or S1 amplitude measures into one model or explore effects of IMT and DMT performance on gating measures, because high correlations between N100 and P200 (difference score r =.74, S1 amplitude r =.82) and between similar IMT and DMT measures (e.g., IMT and DMT A′) (r >.41) would violate the collinearity assumption for regression analysis.
Results
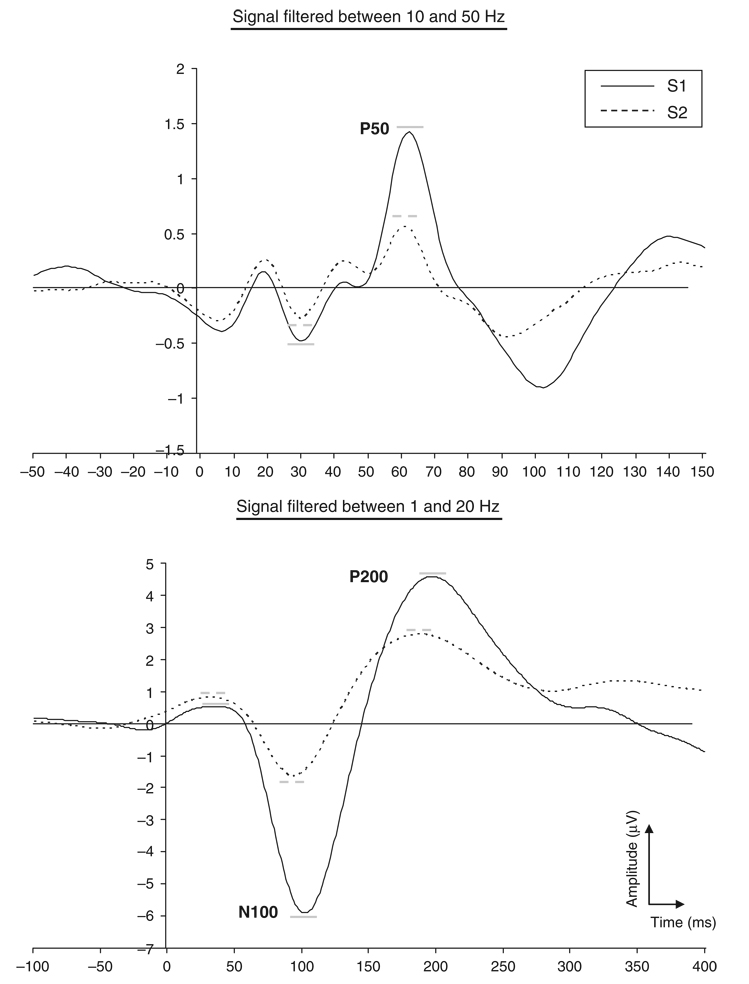
Figure 1 presents the grand average for the P50, N100, and P200 components. Table 1 presents descriptive statistics for P50, N100, and P200 variables. Amplitudes for S2 were significantly attenuated compared to S1 for the P50, F(1,55) = 61.59, p <.001, N100, F(1,55) = 154.72, p <.001, and P200, F(1,55) = 218.40, p <.001, indicating P50, N100, and P200 sensory gating. S2 peaked significantly earlier than S1 for the N100, Z = −4.12, p <.001, df = 55, and P200, Z = −4.10, p <.001, df = 55, but not for the P50 component, F(1,49) = 2.41, p =.13 (subjects with optimal gating were not included). Table 2 presents the outcomes of IMT and DMT performance and task-dependent differences. Reaction time for correct detections and commission errors within tasks were not different, t <1.69, p >.10, df = 55.
Figure 1.
Grand averages of the auditory evoked potential at Cz for S1 and S2. The top average was filtered between 10 and 50 Hz to optimize scoring of the P50 component, whereas the bottom average was filtered between 1 and 20 Hz to optimize scoring of the N100 and P200
Table 1.
Descriptive Statistics of Mean (SD) for P50, N100, and P200 Ratio, Difference Score, Amplitudes, and Latencies
| P50 | N100 | P200 | |
|---|---|---|---|
| Gating measure | |||
| Ratio | 55.23 (40.84) | 48.03 (22.86) | 46.90 (19.78) |
| ΔS1S2 (µV) | 1.37 (1.34) | −4.06 (2.92) | 6.68 (4.66) |
| Amplitude (µV) | |||
| S1 | 2.68 (1.41) | −7.26 (3.53) | 11.64 (5.50) |
| S2 | 1.31 (1.01) | −3.20 (1.72) | 4.96 (1.99) |
| Latency (ms) | |||
| S1 | 61.21 (11.24) | 104.64 (12.72) | 200.96 (27.09) |
| S2 | 58.76 (10.81)a | 95.13 (13.99) | 183.84 (29.43) |
| ΔS1S2 (ms) | 1.12 (5.50)a | 8.86 (18.01) | 17.88 (27.31) |
Note: ΔS1S2 difference score for amplitude (µV) and latency (ms).
n = 50 due to absence of P50 latency for S2 with optimal P50 gating.
Table 2.
Descriptive Statistics of IMT and DMT Performance
| IMT | DMT | Difference | |
|---|---|---|---|
| CD (%)a | 86.45 (9.92) | 89.58 (11.06) | t = 2.73, p = .008, df = 55 |
| CE (%) | 25.52 (16.04) | 26.85 (16.99) | t = −0.92, p = .36, df = 55 |
| Filler errors (%) | 0.42 (0.70) | 0.82 (2.52) | Z = −0.61, p = .54, df = 55 |
| RT CD (ms) | 450.21 (70.57) | 504.74 (92.67) | Z = −6.06, p < .001, df = 55 |
| RT CE (ms)b | 444.17 (68.58) | 499.65 (106.16) | Z = −4.61, p < .001, df = 53 |
| A′ | 0.88 (0.06) | 0.89 (0.08) | Z = −0.73, p = .45, df = 55 |
| B″ | −0.30 (0.50) | −0.51 (0.51) | Z = −3.07, p = .002, df = 55 |
Note: Differences between IMT and DMT performance were calculated with paired t tests or Wilcoxon signed ranks tests depending on normality of the data. CD: correct detections (hits); CE: commission errors (false alarms); RT CD: reaction time for correct detections; RT CE: reaction time for commission errors.
Data were transformed prior to statistical analysis.
n = 54 because 2 subjects made no commission errors.
IMT and Sensory Gating
IMT A′ correlated with P200 latency for S1=.23, p =.02, df = 55, suggesting that a prolonged P200 latency relates to better discrimination between signal and noise on the IMT. P50 and N100 measures did not correlate significantly with IMT performance.
DMT and Sensory Gating
Table 3 summarizes correlations between DMT performance and P50, N100, and P200 variables. P50 gating ratio and P50 amplitude for S2 correlated significantly with commission errors and reaction time for correct detections. DMT reaction time for correct detections correlated significantly with rates of correct detections, r =.34, p =.01, df = 55, and commission errors, r = −.55, p<.001, df = 55. However, a Fisher’s r-to-Z test to test differences between two dependent correlations (cf. Kirk, 1990, p. 361) showed that the latter relationship (as an absolute value) was significantly higher than the former relationship, z = 1.91, p <.05, indicating that the relationship between stronger P50 gating and a prolonged reaction time for DMT correct detections could mediate the relationship between stronger P50 gating and fewer commission errors.
Table 3.
Correlation (p Value) between DMT Performance Variables and Selective P50, N100, and P200 Variables
| Reaction Time | |||||
|---|---|---|---|---|---|
| A′a | B″a | CDb | CE | CD | |
| P50 | |||||
| Ratio | — | — | — | .27 (.05) | −.36 (.007) |
| Amplitude S2b | — | −.20 (.03) | — | .27 (.05) | −.35 (.008) |
| N100 | |||||
| ΔS1S2 (µV)b | .23 (.01) | — | .28 (.04) | — | — |
| Amplitude S1b | .27 (.005) | — | — | −.27 (.04) | — |
| Amplitude S2b | — | — | — | −.26 (.05) | |
| Latency S1b | — | −.20 (.02)a | — | — | — |
| P200 | |||||
| ΔS1S2 (µV)b | .26 (.006) | — | .35 (.008) | — | — |
| Amplitude S1b | .32 (.001) | — | .40 (.002) | — | — |
| Amplitude S2b | .29 (.002) | — | — | −.34 (.01) | — |
Note: CD: correct detections; CE: commission errors. ΔS1S2: gating difference score.
Kendall’s tau instead of Pearson’s r.
Data were transformed prior to statistical analysis.
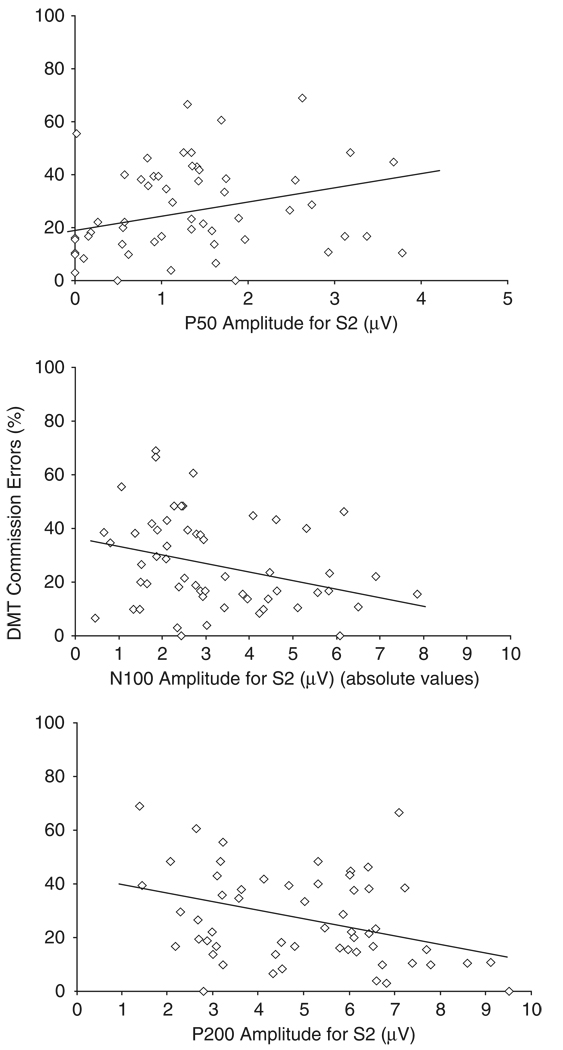
In addition, DMT commission errors, but not reaction times, correlated significantly with N100 amplitudes for S1 and S2 and with the P200 amplitude for S2, although the relationship was reversed for N100 and P200 amplitudes compared to the P50 amplitude (Figure 2). To specify what component could account for most of the variation in DMT commission errors, regression analyses were conducted. When predictor variables were entered based on the strength of association with DMT commission errors, P200 amplitude for S2 was entered first, followed by N100 amplitude for S2 and P50 amplitude for S2. The results revealed that only P200 amplitude for S2 contributed significantly to the model (Table 4).
Figure 2.
Linear relationships between P50 (top panel), N100 (middle panel), and P200 (lower panel) amplitudes for S2 DMT commission errors. The scales in the figure are not logarithmically transformed.
Table 4.
Stepwise Regression Analyses for DMT Commission Errors and P50, N100, and P200 Amplitude for S2
| Contribution | Model | |||||||
|---|---|---|---|---|---|---|---|---|
| Predictorsa | B | tvalue | p value | rpart | F value | p value | ||
| Model 1 | ||||||||
| 1P200 S2 amplitude | −28.94 | −2.64 | .011 | −.34 | .10 | 6.99 | .011 | |
| 2P200 S2 amplitude | −27.06 | −2.52 | .015 | −.32 | .14 | 5.47 | .007 | |
| N100 S2 amplitude | 20.91 | 1.90 | .063 | .24 | ||||
| 3P200 S2 amplitude | −22.12 | −1.91 | .062 | −.24 | .14 | 4.09 | .011 | |
| P50 S2 amplitude | 20.62 | 1.88 | .066 | .23 | ||||
| N100 S2 amplitude | −14.42 | −1.12 | .267 | −.14 | ||||
| Model 2 | ||||||||
| 1P50 S2 amplitude | 23.47 | 2.04 | .046 | .27 | .06 | 4.17 | .046 | |
| 2P50 S2 amplitude | 22.22 | 1.98 | .053 | .25 | .10 | 4.10 | .022 | |
| N100 S2 amplitudea | −23.76 | −1.95 | .056 | −.25 | ||||
| 3P50 S2 amplitude | 20.62 | 1.88 | .066 | .23 | .14 | 4.09 | .011 | |
| N100 S2 amplitude | −14.42 | −1.12 | .267 | −.14 | ||||
| P200 S2 amplitude | −22.12 | −1.91 | .062 | −.24 | ||||
Data were transformed prior to statistical analysis.
However, if predictor variables were entered based on the timing of the components, thus accounting for potential effects of P50 amplitudes on N100 and P200 amplitudes, regression analyses revealed that only S2 amplitude for P50 resulted in a significant change of the model, F(1,54) = 4.17, p =.046 (R2 adjusted =.06, B = 23.47). This suggests that P50, N100 and P200 gating could be interrelated in their involvement in DMT commission error rate, although the specific contributions seemed to be reversed for P50 compared to N100 and P200 gating: Fewer commission errors were related to a smaller P50 for S2 (i.e., better P50 gating), whereas more commission errors were related to smaller N100 and P200 amplitudes for S2.
N100 and P200 difference scores further correlated with DMT A′, suggesting a relationship between N100 and P200 sensory gating and discriminability. These relationships were mediated by a relationship between higher A′ and more pronounced amplitudes for N100 and P200 for S1 rather than gating mechanisms per se. The increase in discriminability with stronger N100 and P200 gating and increased N100 and P200 amplitudes could subsequently be related to more correct detections (difference scores) or fewer commission errors (amplitudes for S1 or S2). Thus, these outcomes suggest better DMT performance with stronger N100 and P200 gating, potentially related to more pronounced amplitudes for S1.
Contrasting IMT and DMT Performance
Finally, as P50, N100, and P200 variables related more strongly and consistently with DMT than with IMT measures, the question arises whether these outcomes could reflect relationships with working memory. To investigate this question IMT performance was subtracted from DMT performance. The difference in commission errors correlated with N100, r = −.44, p = .001, df = 55, and P200, r = −.27, p =.05, df = 55, amplitudes for S2, but not with P50 amplitude for S2. This suggests that more pronounced amplitudes for S2 (potentially reflecting diminished N100 and P200 gating) relate to fewer commission errors on the DMT than the IMT, possibly reflecting better working memory. Again, the absence for such a relationship with P50 variables suggests that the relationship between P50 gating and DMT commission errors may reflect a different mechanism.
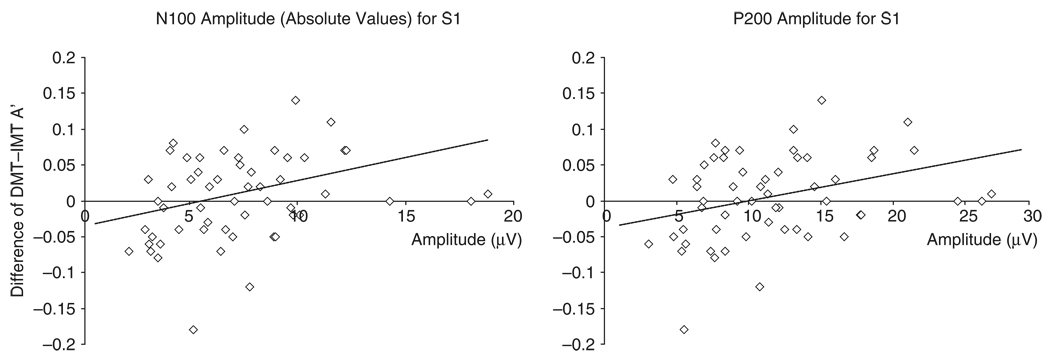
Finally, the difference in DMT and IMT A′ correlated with N100, r = .33, p = .01, df = 55, and P200, r = .36, p = .007, df = 55, amplitudes for S1 (Figure 3), suggesting that discriminability between signal and noise is better on the DMT than the IMT as a function of more pronounced S1 amplitudes. This could be due to relations with increases in correct detections on the DMT but not on the IMT, although it was only significant for S1 amplitude for P200, r = .32, p = .02, df = 55, and not N100, r = .23, p = .08, df = 55.
Figure 3.
Linear relationships between N100 (left panel) and P200 (right panel) amplitudes evoked by S1 and the difference score of the discriminability (A′) for DMT and IMT. The amplitudes for N100 are in absolute values to enhance interpretation of the results. The scales in the figure are not logarithmically transformed.
Discussion
The current study tested the hypotheses that sensory gating protects higher-order cognitive functioning (Freedman et al., 1991; Venables, 1964) measured by performance on the IMT and DMT, and that P50 gating would relate to different mechanisms than N100 and P200 gating.
P50 Gating
P50 sensory gating reflects a preattentional (Jerger et al., 1992) mechanism that could protect the integrity of higher-order functions (Boutros, Korzyukov, Jansen, et al., 2004; Freedman et al., 1991; Jerger et al., 1992; Wan et al., 2008). The current study showed a significant relationship between stronger P50 sensory gating and fewer commission errors and slower reaction times on the DMT. The stronger relationship between reaction time and commission errors than between reaction time and correct detections further suggests that the benefit of slower responses lies more in reduced commission errors than in increased correct detections. Although the overlap in variance (R2 adjusted = .06) is very low, the outcomes suggests than P50 gating could be involved in optimizing response inhibition through prolonged reaction times, apparently corroborating the protective function of P50 gating on cognitive functioning.
These findings are partially consistent with those of previous studies in healthy controls that showed stronger P50 sensory gating with better inhibition of interfering stimuli (Wan et al., 2008). However, in contrast to the current findings, Wan et al. (2008) reported a relationship between stronger P50 gating and faster response times on cuing and Stroop tasks. Furthermore, they found a relation between P50 gating and measures of attention.
Differences in outcomes may be related to the different tasks that were used: The IMT and DMT do not distinguish between separate mechanisms associated with attention as did the task used by Wan et al. (2008). A second explanation of differences across studies in relationships between P50 gating and cognitive functioning could be the low reliability of P50 gating measures (Fuerst et al., 2007; Rentzsch et al., 2008), especially for P50 ratio (Smith et al., 1994), which could attenuate the chance to find consistent outcomes between studies and which might additionally account for a lack of association between P50 gating and IMT performance.
N100 Gating
The N100 evoked by auditory stimuli in passive listening tasks could reflect a trigger to allocate attention to the stimulus (Näätänen, 1992, pp. 113–135; Näätänen & Picton, 1987). N100 sensory gating may thus relate to filter mechanisms involved in triggering of attention.
The N100 difference score correlated with DMT A′ and correct detections, suggesting a relationship between N100 gating and the sensitivity of discriminating between signal (on target) and noise (off target). Further analysis revealed a relationship between higher DMT A′ and a more pronounced N100 for S1, which may be interpreted as a relationship between a stronger signal for triggering the allocation of attention and improved discrimination between signal and noise, possibly through biased processing of the signal relative to noise. This outcome seems to confirm the hypothesized relationship between N100 gating and cognitive functioning.
However, relationships between N100 gating and amplitude for S1 and A′ and correct detections were found only for the DMT. Studies using functional MRI showed that the brain area involved in performing the DMT relative to IMT overlapped a brain area associated with working memory (Moeller et al., 2004; Valdes et al., 2006). This suggests a relationship between N100 gating and working memory. This conclusion is consistent with a report of enhanced auditory N100 amplitudes in subjects with a high compared to a low working memory span (Brumback, Low, Gratton, & Fabiani, 2004), although differences in scoring of the N100 (P50–N100 in the current study and baseline-to-peak in most other studies) argues for caution for this interpretation.
Thus, our findings suggest that N100 gating could protect the integrity of cognitive functioning. Second, our results seem to relate N100 sensory gating not primarily to attention, but to working memory. Finally, the relationship between N100 gating and working memory relates more directly to stimulus processing properties (S1) than to N100 sensory gating per se.
P200 Gating
The P200 evoked by auditory stimuli in passive listening tasks could reflect early allocation of attention and initial conscious experience of the stimulus (Näätänen, 1992, p. 222). P200 gating may thus relate to allocation of attention and the initial conscious awareness of a stimulus.
Similar to findings with N100 variables, P200 difference score and amplitude for S1 correlated with DMT A′ and correct detection, as well as with the difference in A′ and correct detections between IMT and DMT. This suggests that P200 gating, associated with P200 amplitude for S1, is related more to working memory than to attention. On the other hand, the association between a prolonged P200 latency for S1 and to higher A′ on the IMT suggested that P200 latency could relate to attention. These outcomes confirm the hypothesis that P200 gating has a protective function and that it relates to different functions from P50 gating.
Despite evidence from functional MRI showing involvement of brain areas associated with working memory studies in performance on the DMT but not the IMT (Moeller et al., 2004; Valdes et al., 2006), the significantly higher correct detection rate for the DMT compared to the IMT at group level (see Table 2) may raise the question of whether the DMT appropriately measures working memory.
At a group level, the increase in correct detections for the DMT was accompanied with significantly longer reaction times and a more conservative response bias (B″). This suggest that the increase in correct detections is a consequence or an artifact of a more conservative bias associated with slower reaction times, which could result from difference in task design of the DMT relative to the IMT (e.g., waiting between potential targets in the DMT). This could indicate that any correlation between N100 and P200 gating with correct detections on the DMT may reflect an indirect association with changes in response bias rather than better working memory.
However, this interpretation seems more appropriate for P50 gating that related to both commission errors and reaction times. In contrast, N100 and P200 gating, mediated by the more pronounced N100 and P200 amplitudes for S1, related to discriminability (A′) but not to reaction times or response bias (B″). These outcomes suggest that the relationship between N100 and P200 with DMT hit rate could be explained at least partially as a relationship with working memory.
An alternative interpretation is that the DMT measures interference control. The memory set (stimulus before the distracters) of the DMT, in contrast to the memory set of the IMT, has to be stored in working memory and protected from potential interference of the three distracters in between potential targets. Better interference control may be reflected by more correct detections or fewer commission errors on the DMT than the IMT and could be associated with slower reaction times. Relationships between N100 and P200 gating measures and DMT variables therefore suggest that N100 and P200 measures are partially related to interference control, potentially in combination with an association with working memory. A future study should address this possibility.
Potential Overlap in Functional Significance of P50, N100, and P200 Gating
Interestingly, commission errors related to gating of the P50, N100, and P200, although the relationships with N100 and P200 gating were reversed compared to the relation with P50. This reversed pattern of associations between P50 and commission errors and between N100 and P200 and commission errors in addition to the correlation between P50 gating and reaction times suggest that the relationship between commission errors and P50 gating could reflect a fundamentally different underlying mechanism than the relationship between commission errors and the N100 and P200 components. This conclusion further corroborates the hypothesis that P50 and N100 and P200 gating may relate to different cognitive functions reflecting different underlying mechanisms involved in P50 gating and N100 and P200 gating.
Limitations
This study has several limitations that need to be addressed in future studies. First, the influence of N100 and P200 variables on task performance could not be separated adequately because of high overlap between both components due to the commonly applied procedure in sensory gating studies to measure components peak to peak rather than baseline to peak. The inability to separate unique contributions of N100 and P200 limits conclusions on individual involvement of N100 and P200 gating in protection of cognitive functioning.
Second, we scored P50 components after the signals were filtered between 10 and 50 Hz, following the convention that has been most generally used in studies of P50 gating. Recent studies, however, have reported P50 gating in frequency bands lower than 10 Hz (Jansen, Agarwal, Hegde, & Boutros, 2003; Jansen, Hedge, & Boutros, 2004), which may have functionally significant contributions in information processing. However, to optimize generalizability with previous studies, we decided to use the 10–50-Hz filter instead of filter settings that additionally capture the lower-frequency components. Future studies could take the lower frequency bands into account.
Third, gating was assessed in the auditory domain, whereas cognitive functions were assessed in the visual domain. Although this is a general limitation across most studies examining sensory gating in relation to cognition, it limits generalizability of our findings.
Summary
Within the limitations of our study design and analyses, the current study provides evidence for a relation between the role of stronger P50, N100, and P200 gating and higher task performance, which is consistent with the theory that sensory gating mechanisms are involved in protecting higher-order cognitive functions (Venables, 1964). More specifically, P50 gating might relate to protecting cognitive functioning by affecting response strategies, resulting in fewer commission errors, whereas N100 and P200 gating might relate to protecting cognitive functioning by affecting working memory, resulting in better target discrimination. Additionally, P200 latency might relate to attention.
Acknowledgments
Thanks, in alphabetical order, are due to Hima Bodagala, Sherine Kurian, Lorena Maili, Leslie M. Paith, Irshad N. Prasla, and Tony G. Zamudio for their help with recruitment of subjects and the General Clinical Research Center for providing research facilities and excellent nursing support. This study was supported in part by the Pat R. Rutherford, Jr., Chair in Psychiatry (A.C.S.) and by NIH grants RO1-MH 69944 (A.C.S.), RO1-DA08425 (F.G.M.), KO2-DA00403 (F.G.M.), RO1-MH58784 (N.N.B.), and UL1-RR024148 (CCTS/CRU).
REFERENCES
- Adler LE, Pachtman E, Franks RD, Pecevich MC, Waldo M, Freedman R. Neurophysiological evidence for a defect in neuronal mechanisms involved in sensory gating in schizophrenia. Biological Psychiatry. 1982;17:639–655. [PubMed] [Google Scholar]
- Boutros NN, Gooding D, Sundaresan K, Burroughs S, Johanson C. Cocaine-dependence and cocaine-induced paranoia and mid-latency auditory evoked responses and sensory gating. Psychiatry Research. 2006;145:147–154. doi: 10.1016/j.psychres.2006.02.005. [DOI] [PubMed] [Google Scholar]
- Boutros NN, Korzyukov O, Jansen B, Feingold A, Bell M. Sensory gating deficits during the mid-latency phase of information processing in medicated schizophrenic patients. Psychiatry Research. 2004;126:203–215. doi: 10.1016/j.psychres.2004.01.007. [DOI] [PubMed] [Google Scholar]
- Boutros NN, Korzyuko O, Oliwa G, Feingold A, Campbell D, McClain-Furmanski D, et al. Morphological and latency abnormalities of the mid-latency auditory evoked responses in schizophrenia: A preliminary report. Schizophrenia Research. 2004;70:303–313. doi: 10.1016/j.schres.2003.12.009. [DOI] [PubMed] [Google Scholar]
- Bramon E, Rabe-Hesketh S, Sham P, Murray RM, Frangou S. Meta-analysis of the P300 and P50 waveforms in schizophrenia. Schizophrenia Research. 2004;70:315–329. doi: 10.1016/j.schres.2004.01.004. [DOI] [PubMed] [Google Scholar]
- Brockhaus-Dumke A, Mueller R, Faigle U, Klosterkoetter J. Sensory gating revisited: Relation between brain oscillations and auditory evoked potentials in schizophrenia. Schizophrenia Research. 2008;99:238–249. doi: 10.1016/j.schres.2007.10.034. [DOI] [PubMed] [Google Scholar]
- Brumback CR, Low KA, Gratton G, Fabiani M. Sensory ERPs predict differences in working memory span and fluid intelligence. Cognitive Neuroscience and Neurophysiology. 2004;15:373–376. doi: 10.1097/00001756-200402090-00032. [DOI] [PubMed] [Google Scholar]
- Cadenhead KS, Light GA, Geyer MA, Braff DL. Sensory gating deficits assessed by the P50 event-related potential in subjects with schizotypal personality disorder. American Journal of Psychiatry. 2000;157:55–59. doi: 10.1176/ajp.157.1.55. [DOI] [PubMed] [Google Scholar]
- Cullum CM, Harris JG, Waldo MC, Smernoff E, Madison A, Nagamoto HT, et al. Neurophysiological and neuropsychological evidence for attentional dysfunction in schizophrenia. Schizophrenia Research. 1993;10:131–141. doi: 10.1016/0920-9964(93)90048-n. [DOI] [PubMed] [Google Scholar]
- De Wilde OM, Bour LJ, Dingemans PM, Koelman JHTM, Linszen DH. A meta-analysis of P50 studies in patients with schizophrenia and relatives: Differences in methodology between research groups. Schizophrenia Research. 2007;97:137–151. doi: 10.1016/j.schres.2007.04.028. [DOI] [PubMed] [Google Scholar]
- Dougherty DM, Bjork JM, Huckabee HCG, Moeller FG, Swann AC. Laboratory measures of aggression and impulsivity in women with borderline personality disorder. Psychiatry Research. 1999;85:315–326. doi: 10.1016/s0165-1781(99)00011-6. [DOI] [PubMed] [Google Scholar]
- Dougherty DM, Marsh DM, Moeller FG, Chokshi RV, Rosen VC. Effects of moderate and high doses of alcohol on attention, impulsivity, discriminability, and response bias in immediate and delayed memory task performance. Alcoholism: Clinical and Experimental Research. 2000;24:1702–1711. [PubMed] [Google Scholar]
- Erwin RJ, Turetsky BI, Moberg P, Gur RC, Gur RE. P50 abnormalities in schizophrenia: Relationship to clinical and neurophysiological indices of attention. Schizophrenia Research. 1998;33:157–167. doi: 10.1016/s0920-9964(98)00075-9. [DOI] [PubMed] [Google Scholar]
- Fein G, Biggins C, MacKay S. Cocaine abusers have reduced auditory P50 amplitude and suppression compared to both normal controls and alcoholics. Biological Psychiatry. 1996;39:955–965. doi: 10.1016/0006-3223(95)00299-5. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JB. Structured clinical interview for DSM-IV axis I disorders—patient edition. New York: Biometrics Research Institute, New York State Psychiatric Institute; 1996. [Google Scholar]
- Franks R, Adler L, Waldo M, Alpert J, Freedman R. Neurophysiological studies of sensory gating in mania: Comparison with schizophrenia. Biological Psychiatry. 1983;18:989–1005. [PubMed] [Google Scholar]
- Freedman R, Adler LE, Gerhardt GA, Waldo MC, Baker N, Rose GM, et al. Neurobiological studies of sensory gating in schizophrenia. Schizophrenia Bulletin. 1987;13:669–678. doi: 10.1093/schbul/13.4.669. [DOI] [PubMed] [Google Scholar]
- Freedman R, Adler LE, Waldo MC, Pachtman E, Franks RD. Neurophysiological evidence for a defect in inhibitory pathways in schizophrenia: Comparison of medicated and drug-free patients. Biological Psychiatry. 1983;18:537–551. [PubMed] [Google Scholar]
- Freedman R, Waldo M, Bickford-Wimer P, Nagamoto H. Elementary neuronal dysfunction in schizophrenia. Schizophrenia Research. 1991;4:233–243. doi: 10.1016/0920-9964(91)90035-p. [DOI] [PubMed] [Google Scholar]
- Fruhstorfer H, Soveri P, Järvilehto T. Short-term habituation of the auditory evoked response in man. Electroencephalography and Clinical Neurophysiology. 1970;28:153–161. doi: 10.1016/0013-4694(70)90183-5. [DOI] [PubMed] [Google Scholar]
- Fuerst DR, Gallinat J, Boutros NN. Range of sensory gating values and test–retest reliability in normal subjects. Psychophysiology. 2007;44:620–626. doi: 10.1111/j.1469-8986.2007.00524.x. [DOI] [PubMed] [Google Scholar]
- Ghisolfi ES, Heldt E, Zanardo AP, Strimitzer IM, Jr, Prokopiuk AS, Becker J, et al. P50 sensory gating in panic disorder. Psychiatry Research. 2006;40:535–540. doi: 10.1016/j.jpsychires.2006.02.006. [DOI] [PubMed] [Google Scholar]
- Hanlon FM, Miller GA, Thoma RJ, Irwin J, Jones A, Moses SN, et al. Distinct M50 and M100 auditory gating deficits in schizophrenia. Psychophysiology. 2005;42:417–427. doi: 10.1111/j.1469-8986.2005.00299.x. [DOI] [PubMed] [Google Scholar]
- Hsieh MH, Liu S, Chiu M, Hwu H, Chen ACN. Memory impairment and auditory evoked potential gating deficit in schizophrenia. Psychiatry Research: Neuroimaging. 2004;130:161–169. doi: 10.1016/j.pscychresns.2002.12.001. [DOI] [PubMed] [Google Scholar]
- Jansen BH, Agarwal G, Hegde A, Boutros NN. Phase synchronization of the ongoing EEG and auditory EP generation. Clinical Neurophysiology. 2003;114:79–85. doi: 10.1016/s1388-2457(02)00327-9. [DOI] [PubMed] [Google Scholar]
- Jansen BH, Hegde A, Boutros NN. Contribution of different EEG frequencies to auditory evoked potential abnormalities in schizophrenia. Clinical Neurophysiology. 2004;115:523–533. doi: 10.1016/j.clinph.2003.10.016. [DOI] [PubMed] [Google Scholar]
- Jasper HH. Report of the committee on methods of clinical examination in electroencephalography. Electroencephalography and Clinical Neurophysiology. 1958;10:370–371. [Google Scholar]
- Jerger K, Biggins C, Fein G. P50 suppression is not affected by attentional manipulations. Biological Psychiatry. 1992;31:365–377. doi: 10.1016/0006-3223(92)90230-w. [DOI] [PubMed] [Google Scholar]
- Karl A, Malta LS, Maercker A. Meta-analytic review of event-related potential studies in post-traumatic stress disorder. Biological Psychology. 2006;71:123–147. doi: 10.1016/j.biopsycho.2005.03.004. [DOI] [PubMed] [Google Scholar]
- Kirk RE. Statistics: An introduction. 3rd ed. Fort Worth, TX: Holt, Rinehart and Winston, Inc.; 1990. [Google Scholar]
- Kisley MA, Noecker TL, Guinther PM. Comparison of sensory gating to mismatch negativity and self-reported perceptual phenomena in healthy adults. Psychophysiology. 2004;41:604–612. doi: 10.1111/j.1469-8986.2004.00191.x. [DOI] [PubMed] [Google Scholar]
- Lijffijt M, Moeller FG, Boutros NN, Burroughs S, Steinberg JL, Lane SD, et al. A pilot study revealing impaired P50 gating in antisocial personality disorder. Journal of Neuropsychiatry and Clinical Neurosciences. 2009 doi: 10.1176/appi.neuropsych.21.3.328. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lijffijt M, Moeller FG, Boutros NN, Steinberg JL, Meier SL, Lane SD, et al. Diminished P50, N100 and P200 auditory sensory gating in bipolar I disorder. Psychiatry Research. 2009 doi: 10.1016/j.psychres.2008.04.001. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu B, Edgar JC, Jones AP, Smith AK, Huang MX, Miller GA, et al. Improved test–retest reliability of 50 ms paired-click auditory gating using magnetoencephalopgaphy source modeling. Psychophysiology. 2007;44:86–90. doi: 10.1111/j.1469-8986.2006.00478.x. [DOI] [PubMed] [Google Scholar]
- Moeller FG, Steinberg JL, Dougherty DM, Narayana PA, Kramer LA, Renshaw PF. Functional MRI study of working memory in MDMA users. Psychopharmacology. 2004;177:185–194. doi: 10.1007/s00213-004-1908-5. [DOI] [PubMed] [Google Scholar]
- Näätänen R. Attention and brain function. Hillsdale, NJ: Erlbaum; 1992. [Google Scholar]
- Näätänen R, Picton TW. The N1 wave of the human electric and magnetic response to sound: A review and an analysis of the component structure. Psychophysiology. 1987;24:375–425. doi: 10.1111/j.1469-8986.1987.tb00311.x. [DOI] [PubMed] [Google Scholar]
- Nagamoto HT, Adler LE, Waldo MC, Griffith J, Freedman R. Gating of the auditory response in schizophrenics and normal controls. Schizophrenia Research. 1991;4:31–40. doi: 10.1016/0920-9964(91)90007-e. [DOI] [PubMed] [Google Scholar]
- Olincy v, Martin L. Diminished suppression of the P50 auditory evoked potential in bipolar disorder subjects with a history of psychosis. American Journal of Psychiatry. 2005;162:43–49. doi: 10.1176/appi.ajp.162.1.43. [DOI] [PubMed] [Google Scholar]
- Oranje B, Geyer MA, Böcker KBE, Kenemans JL, Verbaten MN. Prepulse inhibition and P50 suppression: Commonalities and dissociations. Psychiatry Research. 2006;143:147–158. doi: 10.1016/j.psychres.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Patterson JV, Hetrick WP, Boutros NN, Jin Y, Sandman C, Stern H, et al. P50 sensory gating ratios in schizophrenics and controls: A review and data analysis. Psychiatry Research. 2008;158:226–247. doi: 10.1016/j.psychres.2007.02.009. [DOI] [PubMed] [Google Scholar]
- Rentzsch J, Jockers-Scherübl MC, Boutros NN, Gallinat J. Test-retest reliability of P50, N100, and P200 auditory sensory gating in healthy controls. International Journal of Psychophysiology. 2008;67:81–90. doi: 10.1016/j.ijpsycho.2007.10.006. [DOI] [PubMed] [Google Scholar]
- Schulze KK, Hall M, McDonald C, Marshall N, Walshe M, Murray RM, et al. P50 auditory evoked potential suppression in bipolar disorder patients with psychotic features and their unaffected relatives. Biological Psychiatry. 2007;62:121–128. doi: 10.1016/j.biopsych.2006.08.006. [DOI] [PubMed] [Google Scholar]
- Semlitsch HV, Anderer P, Schuster P, Presslich O. A solution for reliability and valid reduction of ocular artifacts applied to the P300 ERP. Psychophysiology. 1986;23:695–703. doi: 10.1111/j.1469-8986.1986.tb00696.x. [DOI] [PubMed] [Google Scholar]
- Smith DA, Boutros NN, Schwarzkopf SB. Reliability of the P50 auditory event-related potential indices of sensory gating. Psychophysiology. 1994;31:495–502. doi: 10.1111/j.1469-8986.1994.tb01053.x. [DOI] [PubMed] [Google Scholar]
- Tabachnick BG, Fidell LS. Using multivariate statistics. 2nd ed. New York: Harper & Row; 1989. [Google Scholar]
- Thoma RJ, Hanlon FM, Miller GA, Huang M, Weisend MP, Sanchez FP, et al. Neuropsychological and sensory gating deficits related to remote alcohol abuse history in schizophrenia. Journal of the International Neuropsychological Society. 2006;12:34–44. doi: 10.1017/S1355617706060097. [DOI] [PubMed] [Google Scholar]
- Thomas C, VomBerg I, Rupp A, Seidl U, Schröder J, Roesch-Ely D, et al. P50 gating deficit in Alzheimer dementia correlates to frontal neuropsychological function. Neurobiology of Aging. 2009 doi: 10.1016/j.neurobiolaging.2008.05.002. (in press) [DOI] [PubMed] [Google Scholar]
- Valdes IH, Steinberg JL, Narayana PA, Kramer LA, Dougherty DM, Swann AC, et al. Impulsivity and BOLD fMRI activation in MDMA users and healthy control subjects. Psychiatry Research: Neuroimaging. 2006;147:239–242. doi: 10.1016/j.pscychresns.2006.01.014. [DOI] [PubMed] [Google Scholar]
- Venables PH. Input dysfunction in schizophrenia. Progress in Experimental and Personality Research. 1964;72:1–47. [PubMed] [Google Scholar]
- Wan L, Crawford HJ, Boutros N. Early and late auditory sensory gating: Moderating influences from schizotypal personality, tobacco smoking status, and acute smoking. Psychiatry Research. 2007;151:11–20. doi: 10.1016/j.psychres.2006.01.020. [DOI] [PubMed] [Google Scholar]
- Wan L, Friedman BH, Boutros NN, Crawford HJ. P50 sensory gating and attentional performance. International Journal of Psychophysiology. 2008;67:91–100. doi: 10.1016/j.ijpsycho.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Miyazato H, Hokama H, Hiramatsu K, Kondo T. Correlation between P50 suppression and psychometric schizotypy among non-clinical Japanese subjects. International Journal of Psychophysiology. 2004;52:147–157. doi: 10.1016/j.ijpsycho.2003.06.001. [DOI] [PubMed] [Google Scholar]
- White PM, Yee CM. Effects of attentional and stressor manipulations on the P50 gating response. Psychophysiology. 1997;34:703–771. doi: 10.1111/j.1469-8986.1997.tb02145.x. [DOI] [PubMed] [Google Scholar]
- White PM, Yee CM. P50 sensitivity to physical and psychological state influences. Psychophysiology. 2006;43:320–328. doi: 10.1111/j.1469-8986.2006.00408.x. [DOI] [PubMed] [Google Scholar]
- Zouridakis G, Boutros NN. Stimulus parameter effects on the P50 evoked response. Biological Psychiatry. 1992;32:839–841. doi: 10.1016/0006-3223(92)90088-h. [DOI] [PubMed] [Google Scholar]