Abstract
Oxygen and nitrogen centered reactive species can cause specific structural modifications in amino acids and proteins, such as the addition of a nitro group onto aromatic residues. Heretofore, studies on protein nitration have mainly focused on the in vitro and in vivo nitro addition to tyrosine residues (3-nitrotyrosine or 3NT), whereas the formation of nitrotryptophan in proteins in vivo and/or its functional significance has remained quite obscure. A novel structural modification, involving the addition of nitro and hydroxy groups to tryptophan, has been detected in the mitochondrial protein succinyl-CoA:3-oxoacid CoA transferase (SCOT) in rat heart. Modified SCOT accumulated progressively with age, which was associated with an elevation of its activity. The specific biochemical properties of this novel amino acid were characterized by a combination of HPLC-electrochemical detection and mass spectrometric analysis. This chapter describes the experimental steps involved in the characterizations and a procedure for the synthesis of nitrohydroxytryptophan. Similar methodology can be applied to the identification of nitrohydroxytryptophan in other proteins.
1. Introduction
Certain amino acids within proteins are particularly sensitive to oxidation by reactive oxygen and nitrogen species (ROS and RNS), which, depending on the nature and the location of the modified residue, can cause perturbations in the properties of the protein, such as conformation, catalytic activity, susceptibility to proteolysis, intracellular location, and immunogenicity (Sohal, 2002; Stadtman and Berlett, 1998). One instance of a posttranslational modification induced by the combined action of ROS and RNS is the nitration of proteins, that is, the addition of a nitro group (NO2) onto aromatic residues. Currently, 3-nitrotyrosine (3NT) containing proteins are the main focus of interest, perhaps because of the commercial availability of anti-3NT antibodies (Ye et al., 1996). Thus, a considerable amount of data have accrued, showing, among others, that 3-NT containing proteins accumulate during normal aging (Hong et al., 2007; Kanski et al., 2003, 2005a,b; Sharov et al., 2006; Viner et al., 1999), and in association with pathologies, including neurodegenerative diseases (Beckman et al., 1993; Duda et al., 2000), thereby raising the possibility of a causal association (for review, see Greenacre and Ischiropoulos, 2001).
Despite its relatively lower content in proteins than tyrosine, tryptophan has also been shown to be a specific target of nitration upon in vitro exposure to nitrating agents, such as peroxynitrite or the peroxidase/hydrogen peroxide/nitrite system (Herold et al., 2002; Thiagarajan et al., 2004). Using an antibody against 6-nitrotryptophan, Ikeda et al. (2007) identified several tryptophan-nitrated proteins following in vitro exposure to peroxynitrite. Other authors have reported the presence of nitrotryptophan containing proteins in the liver of mice, treated with acetaminophen, an analgesic that can cause hepatoxicity, in part via RNS-mediated damage. Nevertheless, the nitrated proteins were not individually identified, and no nitrotryptophan containing proteins were observed in the liver of untreated mice (Ishii et al., 2007). Thus far, with the exception of the identification of a bacterial peptide (Kers et al., 2004), nitrotryptophan containing proteins have not been found in mammalian cells in vivo. Physiological relevance of such a modification has obviously also remained unknown.
Occurrence of a cross-reaction between a monoclonal anti-3NT antibody and the mitochondrial succinyl-CoA:3-ketoacid transferase (SCOT) protein has been described (Rebrin et al., 2007). Amino acid analysis by HPLC combined with mass spectrometric studies failed to detect the presence of the nitro derivatives, 3-nitrotyrosine and 4- and 5-nitrotryptophan. Instead, a novel amino acid derivative, namely nitrohydroxytryptophan, was detected and identified using HPLC-electrochemical detection (EC) and matrix-assisted laser desorption/ionization (MALDI) and electrospray ionization (ESI) mass spectrometric studies. The amount of the modified SCOT, as well as SCOT catalytic activity, showed an increase with age.
The procedures used for the identification and characterization of nitrohydroxytryptophan in the SCOT protein in rat heart mitochondria are described. Mitochondria are particularly relevant organelles for the search of nitrated proteins because they are the primary intracellular site of ROS production, and an isoform of nitric oxide synthase may exist in this organelle (Kato and Giulivi, 2006). Thus, peroxynitrite, which arises from a reaction between nitric oxide (NO) and superoxide anion, might be generated in mitochondria.
The example of SCOT is meant here to illustrate the experimental approaches that can be used as a guide to identify and characterize other nitrohydroxytryptophan containing proteins in vivo.
2. Methods
2.1. Isolation of mitochondria and preparation of soluble proteins
Heart tissue, pooled from about 100 rats, is kept in ice-cold antioxidant buffer, consisting of 150 mM potassium phosphate, 2 mM EDTA, and 0.1 mM butylated hydroxytoluene, pH 7.4. Hearts are chopped thinly and homogenized in 5% (w/v) isolation buffer containing 0.3 M sucrose, 0.03 M nicotinamide, and 0.02 M EDTA, pH 7.4, at a low speed using a Polytron homogenizer. To inhibit proteolysis, the homogenization buffer is supplemented with 0.2 mM of freshly prepared phenylmethylsulfonyl fluoride (PMSF) and a complete protease inhibitor cocktail, at the concentration suggested by the manufacturer (Boehringer). Low- and high-speed differential centrifugations for heart mitochondria isolation are, respectively, 700g for 10 min and 10,000g for 5 min. Heart mitochondria are thus isolated within 1 to 2 h after tissue dissection.
Mitochondrial soluble proteins, located in the matrix and intermembrane space compartments, can be obtained as follows: mitochondria (≈5 mg/ml protein) are placed on ice, sonicated for 30 s, and centrifuged at 100,000g for 60 min to separate soluble and membranous proteins. Pellets are resuspended in a buffer containing 50 mM imidazole, pH 7.0, 50 mM sodium chloride, and 5 mM ε-amino-caproic acid, followed by another sonication and an ultracentrifugation, as described earlier. Supernatants from both ultracentrifugation steps are combined and stored in aliquots at −80 °C.
2.2. Purification of the SCOT protein
Mitochondrial soluble proteins (100 mg) are then dialyzed against 25 mM imidazol buffer containing 0.2 mM PMSF, pH 7.85, for 2 h, and applied onto a chromatofocusing column (6 ×100 mm), equilibrated with the same buffer, and eluted with 100 ml of Polybuffer 74 (dilution 1:10), pH 3.9. The flow rate is 6 ml/h and fractions of 2 ml each are collected. Measurements of pH are made in every fifth fraction. Each consecutive fraction is subjected to one-dimensional SDS-PAGE electrophoresis. Gels are electrotransferred onto a PVDF membrane for immunodetection with the polyclonal anti-SCOT antibody (BioSource). SCOT containing fractions are then pooled and concentrated in a volume of 200 μl, using Centricon 30 concentrators. Gel filtration is performed at room temperature using a Shimadzu class VP HPLC system and BioSep-SEC-S 3000 gel permeation column (5 μm, 7.5 × 300 mm) obtained from Phenomenex (Torrance, CA). The column is equilibrated with 25 mM Tris buffer, pH 7.4, containing 75 mM NaCl at a flow rate of 0.5 ml/min. Fifty microliters of concentrated sample containing SCOT is injected onto the column, and absorbance (200–600 nm) is monitored with a diode-array ultraviolet (UV) detector. Fractions (0.5 ml) of the eluate are collected from the gel filtration column between 12 and 20 min. Three consecutive injections are made. The purity of the SCOT protein can be assessed by SDS-PAGE. This purification procedure yields ≈200 μg of electrophoretically pure (>85%) SCOT protein.
2.3. SCOT autolytic fragmentation
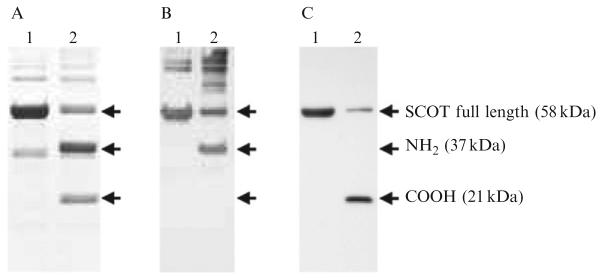
The catalytic properties of SCOT allow, under specific conditions, cleavage of the protein at its active site, glutamate 303 (Howard et al., 1986). Two fragments, the amino- and carboxy-terminal fragments, are thus generated, thereby facilitating identification of the nitrated residue. Briefly, the purified SCOT protein is incubated in the absence or presence of 1 mM acetoacetylcoenzyme A for 5 min at room temperature and then for 1 h at 70 °C. Alternatively, 1 mM succinyl-CoA can also be used. The buffer consists of 50 mM sodium phosphate, pH 7.4. The protein mixtures are separated by one-dimensional SDS-PAGE electrophoresis, following which the gels are either stained with Coomassie blue or electrotransferred to PVDF membranes for immunodetection with the monoclonal anti-3NT antibody (clone 1A6, Upstate) and the polyclonal anti-SCOT antibody. The expected results are shown in Fig. 19.1. The purified full-length SCOT protein (58 kDa), as well as the amino (37 kDa)- and carboxy (21 kDa)-terminal fragments, obtained after substrate-induced cleavage, are visible on the Coomassie-stained gel (Fig. 19.1A). Due to the presence of the peptide recognized by the anti-SCOT serum, the immunoblot using the anti-SCOT serum showed a positive cross-reaction with the amino-terminal fragment (Fig. 19.1B). In contrast, the anti-3NT antibody showed a positive reaction only with the carboxy-terminal fraSgment (Fig. 19.1C), suggesting that the nitrated residue was located exclusively in this particular portion of the protein, which contains only one tyrosine and two tryptophan residues.
Figure 19.1.
(A) Substrate-induced cleavage of the SCOT protein. Lanes 1 and 2 contain the purified SCOT protein incubated in the absence or presence of acetoacetyl-CoA, respectively. The protein bands, namely full-length SCOTand the fragments generated after fragmentation, are shown in a Coomassie-stained gel. (B) An immunoblot using the anti-SCOT serum showing positive reaction with the amino-terminal fragment after cleavage of the protein. (C) An immunoblot with the anti-3NT antibody showing positive cross-reaction with the carboxy-terminal fragment only. The positions of the SCOT full-length protein (58 kDa) and carboxy (COOH, 21 kDa)- and amino (NH2, 37 kDa)-terminal fragments are indicated by arrows.
2.4. Analysis of nitrated amino acids
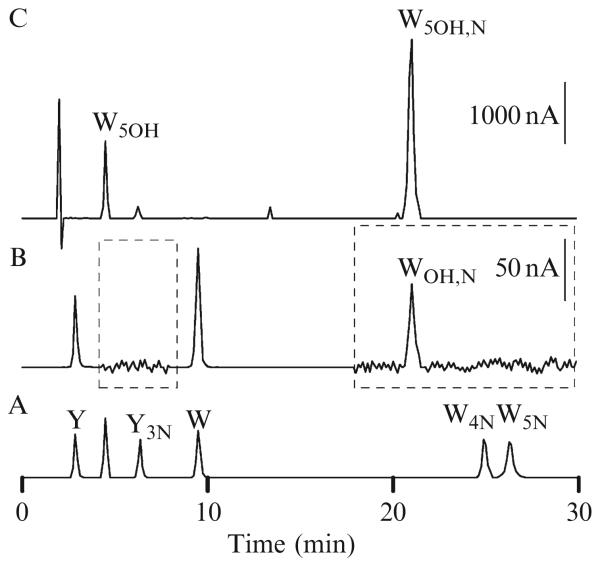
The purified SCOT protein can thus be fragmented by the procedure described earlier and the reaction mixtures separated by one-dimensional SDS-PAGE electrophoresis. The gels can then be electrotransferred onto polyvinylidene fluoride (PVDF) membranes to be stained with Coomassie blue. To isolate the protein, bands (10–100 μg) corresponding to the carboxy-terminal fragment are cut and placed in 0.5-ml plastic tubes containing 100 μl of 0.1 M sodium acetate buffer, pH 7.4, mixed with 5% (w/w) pronase from Staphylococcus griseus (Boehringer) to hydrolyze the proteins into amino acids. Hydrolysis is carried out overnight at 50 °C. [It can be stopped by the addition of 100 μl 10% (w/v) metaphosphoric acid.] Samples are then centrifuged at 18,000g for 20 min, and supernatants are transferred to autosample microvials for injection onto a HPLC column. Amino acids (tyrosine, 3-nitrotyrosine, tryptophan, 4-nitrotryptophan, 5-nitrotryptophan, 5-hydroxytryptophan, and kynurenine) are separated by HPLC, fitted with a Shimadzu class VP solvent delivery system using a reversed-phase C18 Gemini column (4.6 × 150 mm, 5 μm, Phenomenex). The mobile phase for isocratic elution consists of 25 mM monobasic sodium phosphate, 12.5% methanol, pH 2.7, adjusted with 85% phosphoric acid. The flow rate is 1 ml/min. Under these conditions, separation is completed in 30 min; 5-nitrotryptophan is the last eluted peak, with a retention time of approximately 27 min. For the analysis of tyrosine and 3NT, omit the methanol from the solvent. The elution of 3NT occurs toward the end, with a retention time of approximately 25 min. The amino acids used as calibration standard are prepared in 5% metaphosphoric acid. Amino acids can be detected with a Model 5600 CoulArray electrochemical detector (ESA, Chelmsford, MA), equipped with a four-channel analytical cell, using potentials of +600, +700, +800, and +900 mV. With a signal-to-noise ratio of 4:1, the lower limit for electrochemical detection is 300 fmol for 3-nitrotyrosine and 4- and 5-nitrotryptophan and 200 fmol for tyrosine, tryptophan, 5-hydroxytryptophan, and kynurenine. For quantification, inject each sample twice and average the peak areas. Representative HPLC chromatograms are shown Fig. 19.2. The separation of standards (tyrosine, 3NT, tryptophan, 4-and 5-nitrotryptophan) is shown in trace A (Fig. 19.2). Amino acid analysis of the SCOT carboxy-terminal fragment (trace B, Fig. 19.2) indicates the absence of 3NT and 4- and 5-tryptophan. The amino acid nitrohydroxytryptophan (WOH,N with a retention time of 21 min) occurs exclusively in the full-length protein and the C-terminal fragment. The UV absorption spectrum of nitrohydroxytryptophan, detected with a diode array, is similar to those of nitrated aromatic amino acids, with a characteristic emission peak at 340 to 360 nm. The retention time and UV spectrum of nitrohydroxytryptophan observed during HPLC analysis of the digest of the SCOT protein should be identical to the tryptophan derivative produced in vitro (trace C, Fig. 19.2).
Figure 19.2.
Characteristic HPLC-EC chromatographic profile of nitrohydroxytryptophan. The amino acids tyrosine (Y), 3-nitrotyrosine (Y3N), tryptophan (W), and 4-and 5-nitrotryptophan (W4N and W5N) were used as calibration standards (trace A). The elution profile resulting from the amino acid analysis of the SCOT carboxy-terminal fragment shows the absence of nitrotyrosine and nitrotryptophan and the presence of a peak identified as nitrohydroxytryptophan (WOH,N) with a retention time of 21 min (trace B). The elution profile obtained after mixing 5-hydroxytryptophan (W5OH, retention time of 5 min) shows an intense peak corresponding to nitrohydroxytryptophan (W5OH,N) with an identical retention time to the residue observed in the carboxy-terminal portion of SCOT in vivo (trace C). The electrochemical signal intensities represented by the vertical bars are 1000 nA outside and 50 nA inside the dotted box.
2.5. Synthesis of nitrated 5-hydroxytryptophan
5-Hydroxynitrotryptophan can be synthesized by the reaction between 5-hydroxytryptophan and tetranitromethane. The procedure should be carried out on ice in the dark (amber glass vial). Dissolve 10 mg of solid 5-hydroxytryptophan in 50 μl of tetranitromethane, overlay the solution with 450 μl of 70% acetonitrile, and stir gently. It should be noted that, because of the insolubility of tetranitromethane (bottom) in acetonitrile (top), the solution in the vial separates into two phases. In due course, the dark red tetranitromethane phase migrates into the upper acetonitrile containing phase. Progress of the reaction can be monitored by examining a 50-μl aliquot (100-fold dilution of the upper phase of the reaction mixture in 5% metaphosphoric acid) by HPLC, as described previously. The highest yield (≈70%) of 5-hydroxynitrotryptophan is obtained after a prolonged period of reaction (up to 6 h). Further incubation will result in the appearance of additional minor peaks with retention times of more than 30 min, probably due to the occurrence of multiple nitration reactions on the indole ring of tryptophan. In contrast, if the reaction is carried out at pH 8.0, using 50 mM phosphate buffer, the formation of 5-hydroxynitrotryptophan is accompanied by relatively high amounts of multiple nitration by-products, and a rapid decay of 5-hydroxynitrotryptophan is detected within several minutes in phosphate buffer in the pH range of 7.0 to 8.0. Therefore, to obtain high yield and purity, as well as prolonged stability, the buffer should be excluded. No significant decomposition of 5-hydroxynitrotryptophan is observed after storage at 4 °C for up to 5 days if the reaction is acidified with 0.1% trifluoroacetic and 5% metaphosphoric acid.
2.6. Matrix-assisted laser desorption ionization mass spectrometric analysis
Matrix-assisted laser desorption ionization time-of-flight (MALDI-TOF) mass spectra can be acquired on AXIMA CFR (Shimadzu, MD) operated in the positive ion linear mode using α-cyano-4-hydroxycinnamic acid (10 mg/ml in 70% acetonitrile) as a matrix compound. Spectra should represent the average of at least 100 laser shots. To optimize fragmentation spectra, examine various settings of laser power (60, 90, 120, and 150) in order. External mass calibration can be achieved using methionine, tryptophan, 5-hydroxytryptophan, and 5-nitrotryptophan, whose protonated ions [M+H]+ masses are 150.1, 205.1, 221.09, and 250.08, respectively. One milligram of dried sample of amino acids is dissolved in 100 μl of 0.1% trifluoroacetic acid (TFA). One microliter of the sample solution is then mixed with 1 μl of matrix solution, and the resulting mixture is deposited on a stainless-steel sample holder and let dry for several minutes on air. The HPLC fraction containing the putative peak corresponding to nitrohydroxytryptophan is collected between 20 and 22 min during HPLC separation. The eluate is then concentrated using a Speed-Vac (Thermo Savant) and dissolved in 10 μl of 0.1% TFA. Three independent MALDI measurements should be made for each sample to evaluate the reproducibility of the ion peaks.
The theoretical mass of the protonated ion for nitrohydroxytryptophan is 266.08. Under MALDI conditions, the nitro group undergoes photodeoxygenation and yields fragmented ions, which correspond to the loss of one oxygen or two oxygen atoms. The peak abundance of these fragmented ions varies depending on the initial concentration of the compound. Thus, if picomolar amounts of 5-nitrotryptophan are used, the most intense ions observed correspond to 5-nitrosotryptophan. Such a concentration effect has been described previously (Sarver et al., 2001). The expected masses of these ions are indicated in Table 19.1 and can be considered a fingerprint of the presence of a nitrohydroxytryptophan. The presence of such a nitrotryptophan derivative should also be confirmed by treating the sample with dithionite, a reducing agent that converts nitro (NO2) to amino (NH2) group. Thus, under MALDI, a new peak corresponding to amino-hydroxytryptophan can be observed at m/z 236.38. Altogether, MALDI mass spectrometric studies can ascertain the presence of such a tryptophan derivative. Further characterization of nitrohydroxytryptophan can be performed by ESI tandem mass spectrometry after tryptic digestion of the SCOT full-length protein or carboxy-terminal fragment, using the methodology described in the chapter sixteen.
Table 19.1.
Chemical structures, properties, and theoretical m/z values of nitrohydroxytryptophan and its derivatives by MALDI analysis
| Structures | Comments | Theoretical m/z of the protonated ions |
|---|---|---|
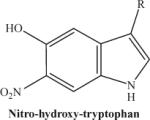 |
The protonated molecular ion is abundantly formed if the synthetic nitrohydroxytryptophan is used. In contrast, the peak intensity will be much lower if using an HPLC eluate from a protein digest. | 266.23 |
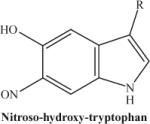 |
Photodecomposition product of nitrohydroxytryptophan following laser exposure: there is a loss of one oxygen atom from the nitro group. The fragmented ion will be predominant if the initial concentration of nitrohydroxytryptophan is low. | 250.23 |
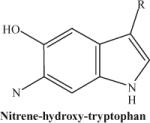 |
Photodecompostion product of nitrohydroxytryptophan following laser exposure: note the loss of two oxygen atoms from the nitro group. This fragmented ion is not observed consistently. | 234.23 |
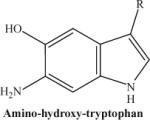 |
The compound obtained after treatment with dithionite. | 236.25 |
3. Conclusions
The experimental procedures described in this chapter can be used for the detection of nitrohydroxytryptophan in proteins in vivo. Briefly, a preliminary screen for nitrated targets can be made in any organelle of choice using the monoclonal anti-3NT antibody. Purification steps are a prerequisite for identification of the nitrated protein and HPLC-EC amino acid analysis. Identification of the nitrated residues is made using various nitrated amino acids as standards. Nitrohydroxytryptophan is generated by the reaction between 5-hydroxytryptophan and tetranitromethane. The presence of a nitrated amino acid is confirmed using MALDI-TOF mass spectrometric analysis, and the lability of the nitro group is used as a fingerprint of nitration. Treatment with dithionite can provide additional evidence of the presence of a nitrated aromatic amino acid derivative. Altogether, these experimental approaches should facilitate the identification and characterization of nitrohydroxytryptophan containing proteins in vivo as well as in vitro.
ACKNOWLEDGMENT
This research was supported by Grant RO1 AG 13563 from National institute on Aging- National Institutes of Health.
REFERENCES
- Beckman JS, Carson M, Smith CD, Koppenol WH. ALS, SOD and peroxynitrite. Nature. 1993;364:584. doi: 10.1038/364584a0. [DOI] [PubMed] [Google Scholar]
- Duda JE, Giasson BI, Chen Q, Gur TL, Hurtig HI, Stern MB, Gollomp SM, Ischiropoulos H, Lee VM, Trojanowski JQ. Widespread nitration of pathological inclusions in neurodegenerative synucleinopathies. Am. J. Pathol. 2000;157:1439–1445. doi: 10.1016/S0002-9440(10)64781-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenacre SA, Ischiropoulos H. Tyrosine nitration: Localisation, quantification, consequences for protein function and signal transduction. Free Radic. Res. 2001;34:541–581. doi: 10.1080/10715760100300471. [DOI] [PubMed] [Google Scholar]
- Herold S, Shivashankar K, Mehl M. Myoglobin scavenges peroxynitrite without being significantly nitrated. Biochemistry. 2002;41:13460–13472. doi: 10.1021/bi026046h. [DOI] [PubMed] [Google Scholar]
- Hong SJ, Gokulrangan G, Schoneich C. Proteomic analysis of age dependent nitration of rat cardiac proteins by solution isoelectric focusing coupled to nanoHPLC tandem mass spectrometry. Exp. Gerontol. 2007;42:639–651. doi: 10.1016/j.exger.2007.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard JB, Zieske L, Clarkson J, Rathe L. Mechanism-based fragmentation of coenzyme A transferase: Comparison of alpha 2-macroglobulin and coenzyme A transferase thiol ester reactions. J. Biol. Chem. 1986;261:60–65. [PubMed] [Google Scholar]
- Ikeda K, Yukihiro Hiraoka B, Iwai H, Matsumoto T, Mineki R, Taka H, Takamori K, Ogawa H, Yamakura F. Detection of 6-nitrotryptophan in proteins by Western blot analysis and its application for peroxynitrite-treated PC12 cells. Nitric Oxide. 2007;16:18–28. doi: 10.1016/j.niox.2006.04.263. [DOI] [PubMed] [Google Scholar]
- Ishii Y, Ogara A, Katsumata T, Umemura T, Nishikawa A, Iwasaki Y, Ito R, Saito K, Hirose M, Nakazawa H. Quantification of nitrated tryptophan in proteins and tissues by high-performance liquid chromatography with electrospray ionization tandem mass spectrometry. J. Pharm. Biomed. Anal. 2007;44:150–159. doi: 10.1016/j.jpba.2007.01.012. [DOI] [PubMed] [Google Scholar]
- Kanski J, Alterman MA, Schoneich C. Proteomic identification of age-dependent protein nitration in rat skeletal muscle. Free Radic. Biol. Med. 2003;35:1229–1239. doi: 10.1016/s0891-5849(03)00500-8. [DOI] [PubMed] [Google Scholar]
- Kanski J, Behring A, Pelling J, Schoneich C. Proteomic identification of 3-nitrotyrosine-containing rat cardiac proteins: Effects of biological aging. Am. J. Physiol. Heart Circ. Physiol. 2005a;288:H371–H381. doi: 10.1152/ajpheart.01030.2003. [DOI] [PubMed] [Google Scholar]
- Kanski J, Hong SJ, Schoneich C. Proteomic analysis of protein nitration in aging skeletal muscle and identification of nitrotyrosine-containing sequences in vivo by nanoelectrospray ionization tandem mass spectrometry. J. Biol. Chem. 2005b;280:24261–24266. doi: 10.1074/jbc.M501773200. [DOI] [PubMed] [Google Scholar]
- Kato K, Giulivi C. Critical overview of mitochondrial nitric-oxide synthase. Front. Biosci. 2006;11:2725–2738. doi: 10.2741/2002. [DOI] [PubMed] [Google Scholar]
- Kers JA, Wach MJ, Krasnoff SB, Widom J, Cameron KD, Bukhalid RA, Gibson DM, Crane BR, Loria R. Nitration of a peptide phytotoxin by bacterial nitric oxide synthase. Nature. 2004;429:79–82. doi: 10.1038/nature02504. [DOI] [PubMed] [Google Scholar]
- Rebrin I, Bregere C, Kamzalov S, Gallaher TK, Sohal RS. Nitration of tryptophan 372 in succinyl-CoA:3-ketoacid CoA transferase during aging in rat heart mitochondria. Biochemistry. 2007;46:10130–10144. doi: 10.1021/bi7001482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarver A, Scheffler NK, Shetlar MD, Gibson BW. Analysis of peptides and proteins containing nitrotyrosine by matrix-assisted laser desorption/ionization mass spectrometry. J. Am. Soc. Mass Spectrom. 2001;12:439–448. doi: 10.1016/S1044-0305(01)00213-6. [DOI] [PubMed] [Google Scholar]
- Sharov VS, Galeva NA, Kanski J, Williams TD, Schoneich C. Age-associated tyrosine nitration of rat skeletal muscle glycogen phosphorylase b: Characterization by HPLC-nanoelectrospray-tandem mass spectrometry. Exp. Gerontol. 2006;41:407–416. doi: 10.1016/j.exger.2006.02.012. [DOI] [PubMed] [Google Scholar]
- Sohal RS. Role of oxidative stress and protein oxidation in the aging process. Free Radic. Biol. Med. 2002;33:37–44. doi: 10.1016/s0891-5849(02)00856-0. [DOI] [PubMed] [Google Scholar]
- Stadtman ER, Berlett BS. Reactive oxygen-mediated protein oxidation in aging and disease. Drug Metab. Rev. 1998;30:225–243. doi: 10.3109/03602539808996310. [DOI] [PubMed] [Google Scholar]
- Thiagarajan G, Lakshmanan J, Chalasani M, Balasubramanian D. Peroxynitrite reaction with eye lens proteins: Alpha-crystallin retains its activity despite modification. Invest. Ophthalmol. Vis. Sci. 2004;45:2115–2121. doi: 10.1167/iovs.03-0929. [DOI] [PubMed] [Google Scholar]
- Viner RI, Ferrington DA, Williams TD, Bigelow DJ, Schoneich C. Protein modification during biological aging: Selective tyrosine nitration of the SER-CA2a isoform of the sarcoplasmic reticulum Ca2+-ATPase in skeletal muscle. Biochem. J. 1999;340:657–669. [PMC free article] [PubMed] [Google Scholar]
- Ye YZ, Strong M, Huang ZQ, Beckman JS. Antibodies that recognize nitrotyrosine. Methods Enzymol. 1996;269:201–209. doi: 10.1016/s0076-6879(96)69022-3. [DOI] [PubMed] [Google Scholar]