Abstract
Objective
Public health policies for physical activity presume that the greatest health benefits are achieved by increasing physical activity among the least active. This presumption is based largely on studies of cardiorespiratory fitness. To assess whether studies of cardiorespiratory fitness are germane to physical activity guidelines, we compared the dose-response relationships between cardiovascular disease endpoints with leisure-time physical activity and fitness from published studies.
Data Sources
Twenty-three sex-specific cohorts of physical activity or fitness (representing 1,325,004 person-years of follow-up), cited in Tables 4-1 and 4-2 of the Surgeon General's Report.
Data Synthesis
Relative risks were plotted as a function of the cumulative percentages of the samples when ranked from least fit or active, to most fit or active. To combine study results, a weighted average of the relative risks over the 16 physical activity or seven fitness cohorts was computed at every 5th percentile between the 5% and 100%. The analyses show that the risks of coronary heart disease or cardiovascular disease decrease linearly in association with increasing percentiles of physical activity. In contrast, there is a precipitous drop in risk occurring before the 25th percentile of the fitness distribution. As a consequence of this drop, there is a significant difference in the risk reduction associated with being more physically active or physically fit (P ≤ 0.04).
Conclusions
Being unfit warrants consideration as a risk factor, distinctly from inactivity, and worthy of screening and intervention. Formulating physical activity recommendations on the basis of fitness studies may inappropriately demote the status of physical fitness as a risk factor while exaggerating the public health benefits of moderate amounts of physical activity.
Introduction
Physical activity is defined as voluntary movement produced by skeletal muscles that results in energy expenditure, whereas cardiorespiratory fitness relates to the ability of circulation and respiration to supply oxygen during sustained physical activity [6,57]. In the formulation of current physical activity guidelines, cardiorespiratory fitness is often treated as a surrogate (purportedly more accurate) measure of physical activity [13,38,41,57]. The pooling of prospective epidemiological studies of cardiorespiratory fitness and physical activity have led to current government guidelines which presume that the greatest health benefits will be achieved by increasing physical activity among the least active members of society [57]. The National Institutes of Health consensus development conference stated that: “The most active individuals have lower cardiovascular morbidity and mortality rates than do those who are least active; however, much of the benefit appears to be accounted for by comparing the least active individuals to those who are moderately active” [41]. A special report by the American Heart Association repeats these conclusions, agreeing that “The greatest potential for reduced mortality is in the sedentary who become more active.” [13]. In fact, the relationship between physical activity dose and health published by the Centers for Disease Control and Prevention purports nine-fold greater health benefit from increasing physical activity status in sedentary, compared with physically active, individuals [38,58].
The above-mentioned policies for promoting physical activity depend upon showing that either: 1) there is a pronounced nonlinear dose-response relationship between disease risk and physical activity, or that 2) fitness-based studies are relevant to the formulation of physical activity recommendations. To this end, the dose-response relationships between leisure-time physical activity, or fitness, and cardiovascular disease endpoints were assessed for the studies cited in the Surgeon General's report on physical activity and health [57].
Methods
The Surgeon General's report cites forty population-based studies associating self-reported physical activity to coronary heart disease (CHD) or cardiovascular diseases (CVD, including both CHD and stroke). Twelve studies were excluded from the analyses for examining only occupational, rather than leisure, or total, physical activity; one for its case-control design [34]; three for presenting only two activity categories [26,39,45,46]; and three for not reporting the percentage of men within each activity category [21,30,44]. Also excluded were two early reports from the Honolulu Heart Program [8,56], two early reports on British Civil Servants [7,36], and one report from the Harvard Alumni Study [36a] because later reports supersede them. A 1991 paper on the British Regional Heart Study [48] was selected over a 1994 paper [49] because the earlier paper reported more person-years of follow-up. The remaining sixteen cohorts represent a total of 1,012,809 person-years of follow-up (Table 1). Of these, nine of the studies report rates for CHD [16,18,20,27,35,42,48,51,53] and five report rates for CVD [1,22,24,37,50]. CVD rates were used when both CHD and CVD were reported.
TABLE 1.
Studies used in dose-response relationship between percentiles of physical activity or fitness and the relative risk of CHD or CVD.†
| Study | Description |
|---|---|
| Physical Activity Studies | |
| Canadian Health Survey Mortality Follow-up Study [1] | 7 yr follow-up of 9491 men and women |
| Primary Prevention Study in Goteborg, Sweden [20] | 11.8 yr follow-up of 7395 men |
| Multiple Risk Factor Intervention Trial [27] | 7 yr follow-up of 12,138 men |
| Framingham Study [22,50] | 14 yr follow-up of 1909 men and 16 yr follow-up of 1404 women |
| British Civil Servants [35] | 9.34 yr follow-up of 9376 men |
| Harvard Alumni Study [37] | 12.62 yr follow-up of 16,936 men |
| Honolulu Heart Program [42] | 23 yr follow-up of 7074 men |
| British Regional Heart Study [48] | 8 yr follow-up of 5714 men |
| Puerto Rico Heart Health Program [16] | 8.25 yr follow-up of 5802 urban and 2419 rural men |
| U.S. Railroad Study [51] | 17 to 20 yr follow-up of 2562 men |
| Belgian Physical Fitness Study [53] | 5 yr follow-up of 2106 men |
| Copenhagen Male Study [18] | 17 yr follow-up of 4859 men |
| Individuals 65≤ years in HMO [24] | 4.2 yr follow-up of 615 men and 1030 women |
| Physical Fitness Studies | |
| Aerobic Center Longitudinal Study [4] | 8.32 yr follow-up of 10,224 men and 8.15 yr follow-up of 3120 women |
| Lipid Research Clinics Mortality Follow-up Study [11] | 8.5 yr follow-up of 3106 men |
| Copenhagen Male Study [18] | 17 yr follow-up of 4999 men |
| U.S. Railroad Study [52] | 20 yr follow-up of 2431 men |
| Belgian Physical Fitness Study [53] | 5 yr follow-up of 2109 men |
| Middle-aged Norwegian men [47] | 15.9 yr follow-up of 1960 men |
The total combined person years of follow-up for physical activity (1,012,809) and fitness studies (312,195) differ slightly from the numbers reported here due to rounding.
The physical activity questionnaires differ by recall period (2 wk [1,24], 4 wk [35], 12 months [20,27,51,53], or usual activity [16,18,22,37,42,48,50]) and whether they rank physical activity by total expenditure or intensity. Four reports rank subjects by total energy expenditure from hours spent at different intensities of physical activity over a 24-h period and thus include both occupational and leisure time [16,22,42,50]. Five studies rank subjects by total estimated caloric expenditure from the intensity, duration, and frequency of specific activities [1,27,48,51,53]. Exercise amount is used to rank Harvard alumni by calculating energy expenditure from reported stairs climbed, city blocks walked, and time and type of sports activity [37], and senior HMO members by calculating hours walked [24]. British civil servants were analyzed by exercise amount exclusive of vigorous exercise [35]. Swedish and Copenhagen men were ranked by intensity from almost completely inactive, having some weekly physical activity totaling 4 h or more, regularly active at more vigorous activity, or partaking in hard physical competitive training [18,20].
The Surgeon General's report cites 11 population-based papers relating cardiorespiratory fitness with total CVD [1,3,4,11,47] or CHD [11,12,18,29,40,52,53]. Two reports [12,29] were excluded for representing earlier reports from the same study [47], one report [3] for representing a subset of an earlier report [4], and two studies for providing insufficient data for computing the dose-response relationship [1,40]. The remaining seven sex-specific cohorts represent a total of 312,195 person-years of follow-up (Table 1). The subjects were ranked into fitness categories by cycle ergometers or treadmill tests. Studies that used cycle ergometers measured fitness from indirect estimates of VO2max [18], differences between observed and expected work capacity according to body weight [47], or interpolated physical working capacities at a heart rate of 150 bpm [53]. Those that used treadmill tests measured fitness from total treadmill test times [4], submaximal heart rates [11], or heart rates after 3 min of walking at 4.8 km/h at 5% grade [52].
Subsequent Reports Since the Surgeon General's
Since the Surgeon General's report, physical activity has been associated with myocardial infarction risk in the Kuopio Ischemic Heart Disease Risk Factor Study (4.9 yr of follow-up of 1166 men [25]), with cardiovascular disease mortality in the Zutphen Elderly Study (10-yr follow-up of 802 Dutch men [2]), the German Cardiovascular Prevention Study (6,658 and 7,993 person-years of experience for males and females, respectively [33]), postmenopausal Iowa women (4 yr follow-up of 32,763 women [23]), and the Canadian Survey Fitness cohort (7 yr follow-up of 6,620 women [55]), and with CHD mortality in Israeli Ischemic Heart Disease Study (160,414 person-years of experience in 8463 men [9]), and CHD incident events in the Atherosclerosis Risk in Communities Study (4–7 yr follow-up of 6188 men and 7852 women [15]), the Scottish Heart Health Cohort Study (7.6 yr follow-up of 5754 men and 5875 women [54]), in three northern Finnish municipalities (10 yr follow-up of 842 men and 953 women [17]), and in the Nurse's Health Study (7.72 yr follow-up of 72,488 women [32]). Additional years of follow-up were reported by the Goteborg Primary Prevention Study (from 11.8 to 20 yr follow-up [43]), and the Multiple Risk Factor Intervention Trial (from 7 to 16 yr follow-up [28]). Cardiorespiratory fitness (maximal oxygen uptake during a cycle ergometer test) was also examined in relation to myocardial infarction in participants of the Kuopio Ischemic Heart Disease Risk Factor Study [25].
Meta-Analysis of Fitness and Physical Activity
The diversity of activity and fitness measurements preclude conversion to a common scale of energy expenditure or fitness. Accordingly, relative risks were plotted as a function of the cumulative percentages of the samples when ranked from least fit or active to most fit or active across 20 categories. The least fit or least active category was used as the referent to calculate relative risk. To combine study results, a weighted average was computed for the relative risks of the 16 physical activity or seven fitness cohorts at every 5th percentile between 5 and 100%,
where the summation over “i” is from 1 to 7 in case of fitness. To show that the findings were relatively robust to the choice of weights, three approaches were used to pool the relative risks across studies: person-years of follow-up, reciprocal of the squared standard error (the meta-analysis choice of weights), and reciprocal of the squared standard error with interpolation. By establishing consistent results across all three choices of weights, our objective was to make moot arguments concerning which weightings are most appropriate.
Person-year weights
Each cohort was represented by a step function having a relative risk value of 1.0 at the 5th percentile and relative risk values at each 5% increment between 0% and 100% determined by the relative risk for the interval, which were then weighted across cohorts by the person-years of experience. For example, in the Lipid Research Clinics (LRC) Mortality Follow-up Study, we assigned a relative risk of 1.0 to all percentiles between 5% and 25%, 0.71 between 30% and 50%, 0.59 between 55% and 75%, and 0.12 between 80% and 100%. Each point received a weight of 0.085 (26,401 person-years divided by 312,195 total person-years). Reported person-years of experience were used to compute the weights, and if these were not presented in the paper, then person-years was computed as the product of sample size and follow-up years.
Meta-analysis weights
The natural logarithms of the relative risks {i.e., ln (relative risk)} were weighted by the reciprocal of their squared standard errors, divided by the sum of the weights over all studies. Specifically, for cohort i, we calculated the standard error for the log(relative risks) from the formula
in which pi1 and ni1 refer to the event rate and sample size for the lowest fitness or activity category (referent), and pij and nij refer to the event rate and sample size for the interval containing the jth percentile [14]. Although technically zero, for the purposes of creating a weighted sum, the “standard error” for the referent was calculated as
Therefore, for the LRC study the log (relative risk) we assigned log relative risks of 0.0, -0.35, -0.53, and -2.14 to the first, second, third, and fourth quartiles, with corresponding “standard errors” of 0.34, 0.37, 0.39, and 0.74. In one study, it was necessary to calculate the standard error from published 95% confidence intervals [47] and the weight for the referent group from the average standard error of the relative risks of the other fitness categories.
Meta-analysis weights with interpolation
The relative risks and their standard errors were assigned to the midpoint of each interval, and linear interpolation was used to estimate the intermediate values. Although this author favors weighting by person-years of experience, all of the above procedures should yield good estimates.
Bootstrap resampling was used to estimate the standard errors for the pooled relative risks and significance levels for the differences in relative risk [10]. Specifically: 1) sampling was done with replacement to create a bootstrap data set of 7 fitness and 16 physical activity cohorts; 2) the weighted average of the relative risk for fitness studies, physical activity studies, and the difference between fitness and physical activity studies was determined at each 5% percentile between 0 and 100; and 3) these steps were repeated 1,000,000 times to estimate the standard error for relative risks and their differences. The number of bootstrap iterations will not affect the estimated significance level other than to increase the precision of its estimate. Two-tailed significance levels were computed as 2*minimum [p, 1-p], for which p is the proportion of times that the relative risks were less than one or relative risk differences were less than zero.
Results
Figure 1 presents the relative risks of the 7 fitness and 16 physical activity cohorts. Their analyses appear in Table 2 and Figure 2. Focusing first on the analyses that weights each cohort by person-years of experience:
Figure 1.
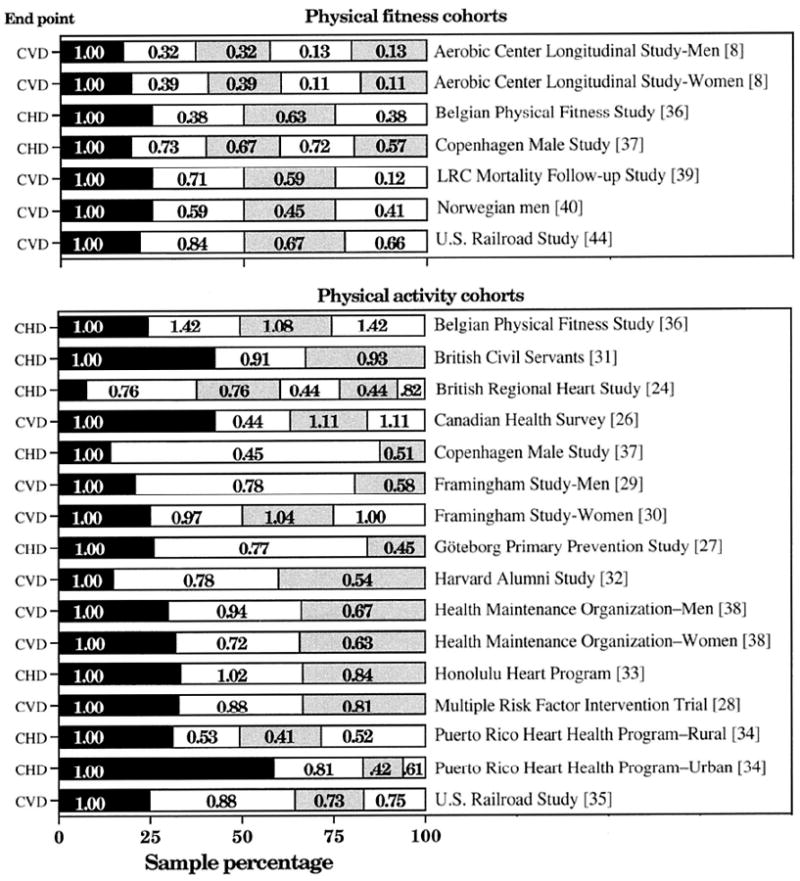
Relative risk for CHD or CVD in 7 physical fitness and 16 physical activity cohorts cited in the Report of the Surgeon General [57]. Sample percentages sorted from least to most fit or active, with the lowest category used as the referent. Several studies report CHD in addition to CVD endpoints (relative risk in order of lowest to highest percentage): Framingham Men [22] (1.00, 0.69, 0.60); Harvard Alumni Study [37] (1.00, 0.82, 0.64); LRC Mortality Follow-up Study [36] (1.00, 0.54, 0.54, 0.15); and U.S. Railroad study cohorts [52] for fitness (1.00, 0.88, 0.66, 0.69) and activity (1.00, 0.84, 0.74, 0.80).
TABLE 2.
Relative risk (±SE) of CHD or CVD associated with increasing percentiles of physical fitness or physical activity in studies reported in the Surgeon General's report on physical activity.
| Weighted by Person Years | Weighted by 1/SE2—No Interpolation | Weighted by 1/SE2—With Interpolation | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Percentile | Fitness (rr) | Physical Activity (rr) | Difference (P) | Fitness ln(rr) | Physical Activity ln(rr) | Difference (P) | Fitness ln(rr) | Physical Activity ln(rr) | Difference (P) |
| 1% | 1.00±0.00 | 1.00±0.00 | - | 0.00±0.00 | 0.00±0.00 | - | 0.00±0.00 | 0.21±0.11 | 0.864 |
| 5% | 1.00±0.00 | 1.00±0.00 | - | 0.00±0.00 | 0.00±0.00 | - | 0.10±0.04 | 0.12±0.06 | 0.835 |
| 10% | 1.00±0.00 | 0.99±0.01 | 0.711 | 0.00±0.00 | -0.01±0.01 | 0.713 | 0.00±0.02 | 0.06±0.03 | 0.051 |
| 15% | 1.00±0.00 | 0.90±0.05 | 0.072 | 0.08±0.04 | -0.07±0.05 | 0.000 | -0.02±0.05 | 0.01±0.02 | 0.417 |
| 20% | 0.76±0.13 | 0.90±0.05 | 0.367 | -0.21±0.15* | -0.07±0.05 | 0.314 | -0.19±0.09§ | -0.02±0.01 | 0.000 |
| 25% | 0.61±0.09§ | 0.89±0.05* | 0.003 | -0.39±0.16§ | -0.08±0.05† | 0.003 | -0.32±0.13§ | -0.05±0.02§ | 0.000 |
| 30% | 0.58±0.09§ | 0.87±0.05† | 0.002 | -0.39±0.16§ | -0.11±0.05§ | 0.007 | -0.35±0.15§ | -0.08±0.02§ | 0.000 |
| 35% | 0.58±0.09§ | 0.85±0.05§ | 0.003 | -0.39±0.16§ | -0.14±0.05§ | 0.020 | -0.39±0.15§ | -0.10±0.03§ | 0.000 |
| 40% | 0.57±0.08§ | 0.85±0.05§ | 0.002 | -0.42±0.16§ | -0.14±0.05§ | 0.015 | -0.42±0.15§ | -0.13±0.04§ | 0.000 |
| 45% | 0.57±0.08§ | 0.81±0.06§ | 0.013 | -0.42±0.16§ | -0.17±0.05§ | 0.039 | -0.45±0.14§ | -0.15±0.04§ | 0.000 |
| 50% | 0.54±0.08§ | 0.80±0.06§ | 0.008 | -0.44±0.17§ | -0.17±0.05§ | 0.033 | -0.48±0.15§ | -0.17±0.05§ | 0.000 |
| 55% | 0.52±0.07§ | 0.80±0.06§ | 0.001 | -0.53±0.12§ | -0.17±0.06§ | 0.000 | -0.51±0.16§ | -0.20±0.05§ | 0.001 |
| 60% | 0.49±0.11§ | 0.75±0.07§ | 0.036 | -0.54±0.18§ | -0.21±0.07§ | 0.009 | -0.53±0.17§ | -0.22±0.06§ | 0.002 |
| 65% | 0.46±0.12§ | 0.77±0.08§ | 0.016 | -0.54±0.19§ | -0.22±0.07§ | 0.010 | -0.56±0.19§ | -0.23±0.06§ | 0.004 |
| 70% | 0.46±0.12§ | 0.74±0.06§ | 0.024 | -0.54±0.19§ | -0.28±0.06§ | 0.022 | -0.59±0.23§ | -0.25±0.06§ | 0.007 |
| 75% | 0.41±0.12§ | 0.74±0.06§ | 0.008 | -0.63±0.30§ | -0.28±0.06§ | 0.010 | -0.62±0.25§ | -0.26±0.07§ | 0.007 |
| 80% | 0.40±0.12§ | 0.74±0.06§ | 0.005 | -0.64±0.30§ | -0.28±0.06§ | 0.009 | -0.63±0.27§ | -0.28±0.07§ | 0.017 |
| 85% | 0.36±0.10§ | 0.69±0.07§ | 0.002 | -0.73±0.28§ | -0.33±0.08§ | 0.014 | -0.64±0.29§ | -0.30±0.07§ | 0.031 |
| 90% | 0.36±0.10§ | 0.69±0.06§ | 0.001 | -0.73±0.28§ | -0.31±0.07§ | 0.007 | -0.64±0.30§ | -0.31±0.07§ | 0.044 |
| 95% | 0.36±0.10§ | 0.72±0.06§ | 0.001 | -0.73±0.28§ | -0.29±0.07§ | 0.003 | -0.76±0.30§ | -0.32±0.08§ | 0.007 |
| 100% | 0.36±0.10§ | 0.72±0.06§ | 0.001 | -0.73±0.28§ | -0.29±0.07§ | 0.003 | -0.79±0.31§ | -0.32±0.08§ | 0.006 |
Significance levels for relative risk different from one or log(relative risk) different than zero are coded;
P <0.05;
P <0.01;
P < 0.001.
Figure 2.
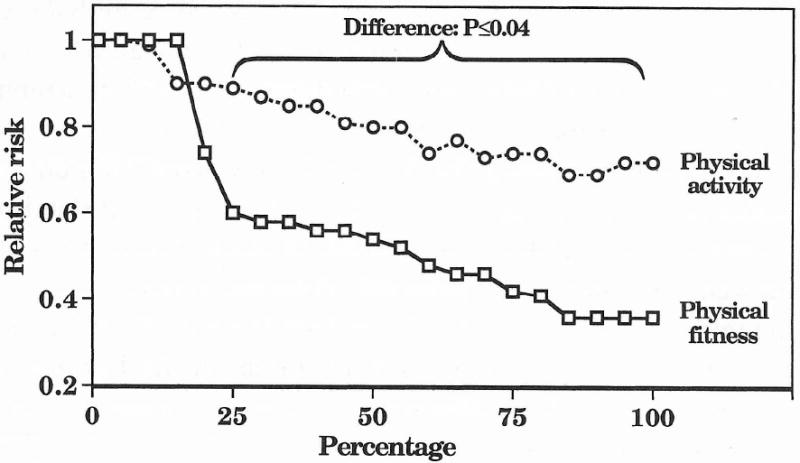
Estimated dose-response curve for the relative risk of either CHD or CVD by sample percentages of fitness and physical activity. Studies weighted by person-years of experience.
Result 1: The risks of CHD or CVD decreased linearly in association with increasing percentiles of physical activity
The pooled results from the 16 cohorts are displayed in Figure 2. Each 1% increase between the first and 100th percentile was associated with a -0.0031 ± 0.0006 reduction in relative risk (±SE), which was significant at P<0.001. Table 2 shows that each percentile of physical activity ≥25th percentile has significantly lower risk of CHD or CVD risk, relative to the least active percentile.
Result 2: In contrast to the linear reduction in CHD or CVD risk associated with increasing percentiles of physical activity, there is a precipitous decrease in risk occurring before the 25th percentile of the fitness distribution
Five of the seven fitness cohorts show their greatest decrease in relative risk occurring between the lowest- and second-lowest fitness categories [4,4,18,47,53]. The pooled results, displayed in Figure 2, indicate that all percentiles above the 20th are at significantly less risk than the percentiles of least fit individuals. Between the 30th and 100th percentile of the distribution, each percent increase in fitness was associated with a -0.0038 ± 0.0009 reduction in relative risk [P <0.001].
Result 3: There is a significant difference in the risk reduction associated with being more physically active or more physically fit
Relative to the least fit or active percentiles, the relative risk reduction is significantly greater for fitness than physical activity at all percentiles ≥25th (Table 2, Fig. 2). After the initial sharp decline in the fitness curve, the risk reduction associated with increasing levels of fitness and increasing levels of physical activity are parallel {i.e., the regression line for fitness between the 30th and 100th percentile (-0.0038 ± 0.0009) has a different intercept but similar slope to the regression line for physical activity (i.e., -0.0024 ± 0.0009 between the 1st and 100th percentile, P = 0.28 for difference, and -0.0031 ± 0.0006 when restricted to within the 30th and 100th percentile, P = 0.51). The significant differences between the physical activity and cardiorespiratory fitness curves persist when the analyses are restricted to CVD endpoints (Fig. 3).
Figure 3.
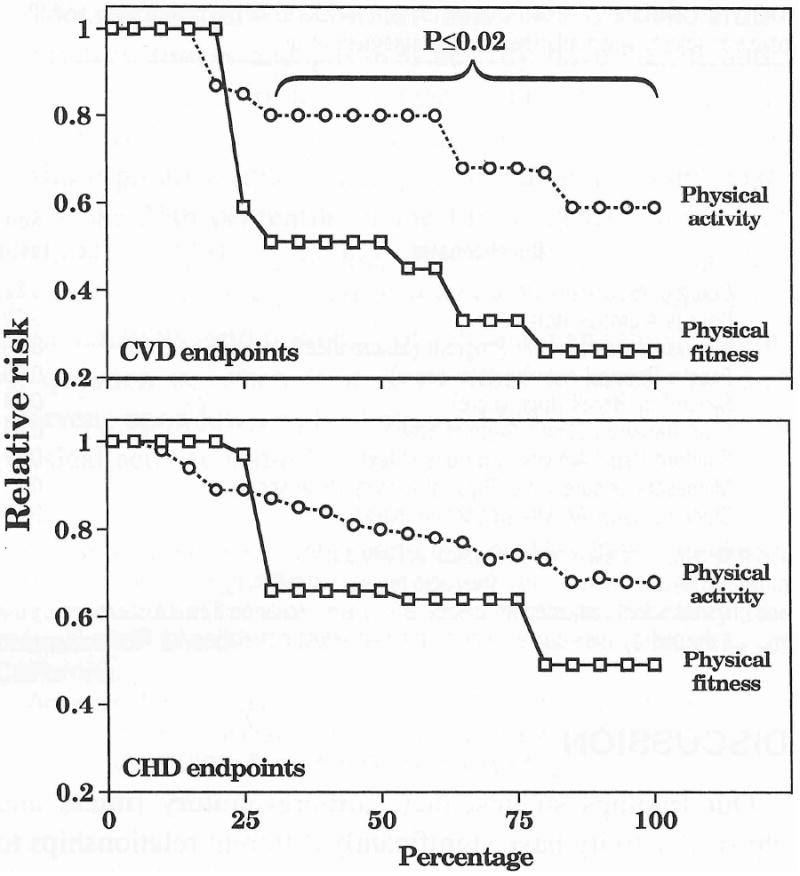
Estimated dose-response curve for the relative risk of CHD or CVD separately by sample percentages of fitness and physical activity. The differences between the fitness and physical activity were greater when CVD was the endpoint rather than CHD. Studies weighted by person-years of experience.
Results 1–3 appear robust with respect to the particular choice of weights
Similar results were obtained whether the analyses were based on the person-years of experience (null hypothesis of relative risk = 1) or the standard error of the relative risk estimates (null hypothesis of ln [relative risk] =0). Table 2 presents the significance levels for ln(relative risk) at increasing levels of physical activity or cardiorespiratory fitness. Cardiorespiratory fitness is again associated with a precipitous drop in risk below the 25th percentile of fitness and a gradual, graded risk reduction thereafter. Physical activity shows a gradual, graded reduction in ln(relative risk) from the least to most active individuals. Also as previously demonstrated, the risk reduction associated with fitness after the initial drop stabilized (i.e., after the 25th percentile) parallels that observed with physical activity. Specifically without interpolation, ln(relative risk) is reduced -0.0058 ± 0.0029 per fitness percentile (P < 0.001) between the 30th and 100th percentiles, which is not significantly different (P = 0.11) from the reduction per percentile of activity between 1% and 100% (-0.0034 ± 0.0007, P < 0.001). The corresponding regression slopes for the analyses of interpolated values were -0.0057 ± 0.0033 (P <0.001) per fitness percentile and -0.0048±0.0008 (P<0.001) per physical activity percentile, which again are not significantly different from each other (P = 0.38).
These conclusions are unaffected by the addition of subsequent reports since the Surgeon General's
Figure 4 shows that the relationships remain essentially unaltered with the addition of recent reports, which increased the follow-up to 317,908 person-years for studies of fitness and 2,286,806 person-years for studies of physical activity.
Figure 4.
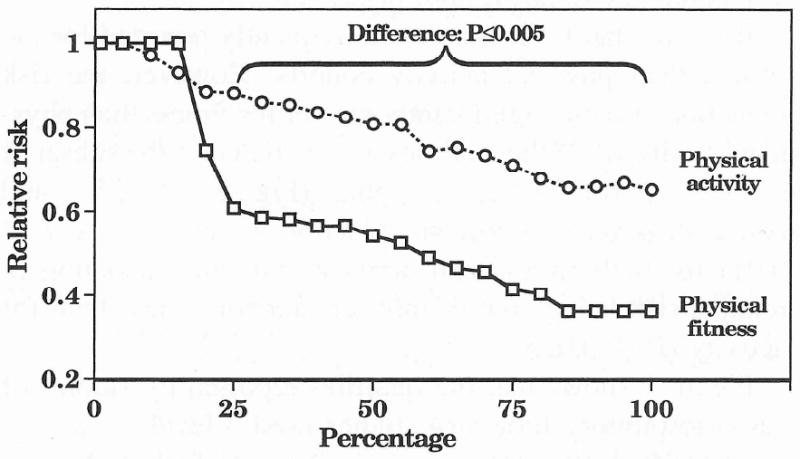
Relative risk for CHD or CVD in 8 physical fitness (317,908 person-years of follow-up) and 30 physical activity cohorts (2,286,806 person-years of follow-up) for studies cited in, and subsequent to, the Report of the Surgeon General [57].
Discussion
Our findings suggest that cardiorespiratory fitness and physical activity have significantly different relationships to CVD (or CHD) risk. Although physical activity increases fitness and may be an appropriate therapy for the unfit, inactivity may not be the principal cause for being unfit when subclinical disease or genetics may be involved. In sedentary subjects, maximal heritability estimates of at least 50% are reported for cardiorespiratory fitness, which may be an underestimate because the estimate includes the attenuating effects of measurement error [5]. Formulating physical activity recommendations on the basis of fitness studies may inappropriately demote the status of cardiorespiratory fitness as a risk factor while exaggerating the public health benefits of moderate amounts of physical activity.
The differences observed between the cardiorespiratory fitness and physical activity curves could not be ascribed to either age or disease endpoint. Although the mean age during the midpoint of follow-up was about 5 yr older in the physical activity cohorts than the fitness cohorts, within the 16 physical activity cohorts, there was no significant association between midpoint follow-up age and relative risk at any percentile (analyses not displayed).
It is true that CVD was more frequently reported for the fitness than physical activity cohorts. However, the risk reduction remains significantly greater for fitness than physical activity when the analyses are restricted to the subset of studies reporting CVD endpoints (Fig. 3). At 25% and above, there were significant reductions in relative risk (P<0.01) for both fitness and activity, with the reduction in relative risk being significantly greater for fitness than for activity (P<0.025).
Figure 1 shows that the quartiles reported by studies of cardiorespiratory fitness (4 studies used 4 levels, 3 used 5 levels) differed from those reported by studies of physical activity [9 studies used 3 levels, 6 used 4 levels, and 1 used 6 levels]. By choosing the first cut point too high, the activity curve could conceal a sharp drop in risk because the drop was lost within the referent class. However, when CVD is considered separate from CHD, choice of cutpoints appears unlikely to bias the comparison between cardiorespiratory fitness and physical activity because their means for the first cutpoint (19.6% vs 17.8%) are not different.
This is not to say that some of the difference in shape between the fitness and activity curves couldn't arise by the way the samples were partitioned into intervals. However, this assumes that the cutoff points used to define the lowest interval (i.e., the referent group) for the physical activity studies were not result driven. Specifically, it assumes that the investigators would obscure, by accident or design, a reduction in risk among the least active individuals. It is more reasonable to assume that several of the physical activity studies defined broadly the interval for the referent group because there was no trend within the interval. Moreover, a broad referent group would increase statistical power to detect significant reductions in risk for other categories.
Table 3 presents test-retest correlations for physical activity questionnaires and correlations between physical activity and fitness reported by Jacobs et al. [19]. We interpret these correlations as showing that cardiorespiratory fitness may be related to physical activity but that cardiorespiratory fitness seems unlikely to be a more accurate, less biased estimate of physical activity than the activity measures themselves for two reasons. First, if fitness is a more accurate measure of physical activity scores than the activity scores themselves, then there are mathematical constraints on the correlations of Table 3 that must apply. Specifically, the physical activity scores should be more strongly correlated with cardiorespiratory fitness than with the scores from a second physical activity questionnaire (test-retest correlations), which they are not (i.e., the nine test-retest correlations for physical activity are all greater than the correlation physical activity versus fitness in Table 3). Second, if fitness is principally a measure of physical activity, then most of the variation in fitness should be explained by physical activity after removing the effects of measurement error (i.e., a correlation near one). The median adjusted correlation for the nine questionnaire scores combined was r = 0.59, or 35% of the variance in fitness explained by physical activity. This leaves a majority of the fitness variance (65%) unexplained. Moreover, the least active individuals may not be the ones characterized by low fitness because fitness correlates poorly with the amounts of light or moderate intensity activity [19]. Fitness measures are not necessarily more precisely measured than reported physical activity either. Test-retest correlations for fitness measurements taken 1 month apart were reported by Jacobs et al. [19] as (r = 0.82) for workload at 160 bpm and r = 0.77 for VO2max. These values tend to be less than or equal to the test-retest correlations for questionnaires that assess usual physical activity {e.g., Minnesota Leisure Time, r = 0.92; Lipid Research Clinic, r = 0.93; Coronary Artery Risk Development in Young Adults (CARDIA) study, r = 0.88; Baecke, r = 0.93; College Alumnus, r = 0.72; Minnesota Heart Health Program Leisure Index, r = 0.86}.
TABLE 3.
Correlations between questionnaire measures of physical activity and fitness as measured by VO2max, and the percentage of the physical fitness variance explained by physical activity when adjusted for measurement error
| Questionnaire | Correlation of Physical Activity With: | Variance in Fitness Explained By Physical Activity (%) | ||
|---|---|---|---|---|
| Self (i.e., Test-Retest) | Fitness (No Correction) | Fitness Corrected for Measurement Error | ||
| Godin questionnaire (leisure score) | 0.62 | 0.56 | 0.81 | 66 |
| College Alumnus (total score) | 0.72 | 0.52 | 0.70 | 49 |
| Minnesota Heart Health Program (leisure index) | 0.86 | 0.56 | 0.69 | 47 |
| Baecke Physical Activity (total score) | 0.93 | 0.54 | 0.64 | 41 |
| Seven Day Recall (total score) | 0.34 | 0.30 | 0.59 | 34 |
| Lipid Research Clinics (4-point scale) | 0.93 | 0.49 | 0.58 | 34 |
| Stanford Usual Activity (vigorous index) | 0.67 | 0.38 | 0.53 | 28 |
| Minnesota Leisure Time Physical Activity (total score) | 0.92 | 0.43 | 0.51 | 26 |
| Stanford Usual Activity (moderate index) | 0.77 | 0.27 | 0.35 | 12 |
The test-retest correlations for physical activity questionnaires measured 1 month apart (column 1) and correlations between physical activity and fitness (i.e.,VO2max, column 2) were published by Jacobs et al. [19]. They also reported that VO2max measured one month apart had a test retest correlation of r = 0.77. The theoretical correlations between physical fitness and physical activity adjusted for measurement error (column 3) were calculated using the attenuation formula [31], which is the correlation between physical activity and fitness (rPA,Fit) divided by the square roots of the test-retest correlation for fitness (rFit,Fit) and the test-retest correlation for physical activity (rPA,PA), i.e., rPA,Fit/(rPA,PA*rFit,Fit)1/2.
The analyses presented in this paper suggest that cardiorespiratory fitness and physical activity have significantly different relationships to combined CVD and CHD risk. The reductions in relative risk are nearly twice as great for cardiorespiratory fitness than physical activity. Individuals below the 25th percentile of the fitness distribution are at substantially higher risk than those at higher percentiles. Being unfit warrants consideration as a risk factor, distinctly from inactivity, and is worthy of screening and intervention. At question is: when is it appropriate to screen for and intervene upon low levels of physical fitness, irrespective of physical activity status?
Acknowledgments
This work was supported in part by grants HL-58621, HL-45652, and HL-55640 from the National Heart Lung and Blood Institute and was conducted at the Lawrence Berkeley National Laboratory (Department of Energy DE-AC03-76SF00098 to the University of California).
References
- 1.Arraiz GA, Wiggle DT, Mao Y. Risk assessment of physical activity and physical fitness in the Canada Health Survey mortality follow-up study. J Clin Epidemiol. 1992;45:419–428. doi: 10.1016/0895-4356(92)90043-m. [DOI] [PubMed] [Google Scholar]
- 2.Bijnen FC, Caspersen CJ, Feskens EJ, et al. Physical activity and 10-year mortality from cardiovascular diseases and all causes: the Zutphen Elderly Study. Arch Intern Med. 1998;27:1499–1505. doi: 10.1001/archinte.158.14.1499. [DOI] [PubMed] [Google Scholar]
- 3.Blair SN, Kohl HW, II, Barlow CE, et al. Changes in physical fitness and all-cause mortality: a prospective study of healthy and unhealthy men. JAMA. 1995;273:1093–1098. [PubMed] [Google Scholar]
- 4.Blair SN, Kohl HW, III, Paffenbarger RS, Jr, et al. Physical fitness and all-cause mortality: a prospective study of healthy men and women. JAMA. 1989;262:2395–2401. doi: 10.1001/jama.262.17.2395. [DOI] [PubMed] [Google Scholar]
- 5.Bouchard C, Daw EW, Rice T, et al. Familial resemblance for VO2max in the sedentary state: the HERITAGE family study. Med Sci Sports Exerc. 1998;30:252–258. doi: 10.1097/00005768-199802000-00013. [DOI] [PubMed] [Google Scholar]
- 6.Caspersen CJ, Powell KE, Christenson GM. Physical activity, exercise and physical fitness: definitions and distinctions for health-related research. Public Health Rep. 1985;100:126–130. [PMC free article] [PubMed] [Google Scholar]
- 7.Chave SP, Morris JN, Moss S, Semmence AM. Vigorous exercise in leisure time and the death rate: a study of male civil servants. J Epidemiol Community Health. 1978;32:239–243. doi: 10.1136/jech.32.4.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Donahue RP, Abbott RD, Reed DM, Yano K. Physical activity and coronary heart disease in middle-aged and elderly men: the Honolulu Heart Program. Am J Public Health. 1988;78:683–685. doi: 10.2105/ajph.78.6.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eaton CB, Medalie JH, Flocke SA, et al. Self-reported physical activity predicts long-term coronary heart disease and all-cause mortalities: twentyone- year follow-up of the Israeli Ischemic Heart Disease Study. Arch Fam Med. 1995;4:323–329. doi: 10.1001/archfami.4.4.323. [DOI] [PubMed] [Google Scholar]
- 10.Efron B. The Jackknife, the Bootstrap and Other Resampling Plans. Philadelphia: Society for Industrial and Applied Mathematics; 1982. pp. 1–92. [Google Scholar]
- 11.Ekelund LG, Haskell WL, Johnson JL, et al. Physical fitness as a predictor of cardiovascular mortality in asymptomatic North American men: the Lipid Research Clinics Mortality Follow-up Study. N Engl J Med. 1988;319:1379–1384. doi: 10.1056/NEJM198811243192104. [DOI] [PubMed] [Google Scholar]
- 12.Erikssen J. Physical fitness and coronary heart disease morbidity and mortality: a prospective study in apparently healthy, middleaged men. Acta Med Scand Suppl. 1986;711:189–192. [PubMed] [Google Scholar]
- 13.Fletcher GF, Balady G, Blair SN, et al. Statement on exercise: benefits and recommendations for physical activity programs for all Americans: a statement for health professionals by the Committee on Exercise and Cardiac Rehabilitation of the Council on Clinical Cardiology, American Heart Association. Circulation. 1996;94:857–862. doi: 10.1161/01.cir.94.4.857. [DOI] [PubMed] [Google Scholar]
- 14.Fleiss JL. The statistical basis of meta-analysis. Stat Methods Med Res. 1993;2:121–145. doi: 10.1177/096228029300200202. [DOI] [PubMed] [Google Scholar]
- 15.Folsom AR, Arnett DK, Hutchinson RG, et al. Physical activity and incidence of coronary heart disease in middle-aged women and men. Med Sci Sports Exerc. 1997;29:901–909. doi: 10.1097/00005768-199707000-00009. [DOI] [PubMed] [Google Scholar]
- 16.Garcis-Palmieri MR, Costas R, Jr, Cruz-Vidal M, et al. Increased physical activity: a protective factor against heart attacks in Puerto Rico. Am J Cardiol. 1982;50:749–755. doi: 10.1016/0002-9149(82)91229-2. [DOI] [PubMed] [Google Scholar]
- 17.Haapanen N, Miilunpalo S, Vuori I, et al. Association of leisure time physical activity with the risk of coronary heart disease, hypertension and diabetes in middle-aged men and women. Int J Epidemiol. 1997;26:739–747. doi: 10.1093/ije/26.4.739. [DOI] [PubMed] [Google Scholar]
- 18.Hein HO, Suadicani P, Gyntelberg F. Physical fitness or physical activity as a predictor of ischaemic heart disease? A 17-year follow-up in the Copenhagen Male Study. J Intern Med. 1992;232:471–479. doi: 10.1111/j.1365-2796.1992.tb00619.x. [DOI] [PubMed] [Google Scholar]
- 19.Jacobs DR, Jr, Ainsworth BE, Hartman TJ, Leon AS. A simultaneous evaluation of 10 commonly used physical activity questionnaires. Med Sci Sports Exerc. 1993;25:81–91. doi: 10.1249/00005768-199301000-00012. [DOI] [PubMed] [Google Scholar]
- 20.Johansson S, Rosengren A, Tsipogianni A, et al. Physical inactivity as a risk factor for primary and secondary coronary events in Goteborg, Sweden. Eur Heart J. 1988;9(Suppl. L):8–19. doi: 10.1093/eurheartj/9.suppl_l.8. [DOI] [PubMed] [Google Scholar]
- 21.Kannel WB, Belanger A, D'Agostino R, Israel I. Physical activity and physical demand on the job and risk of cardiovascular disease and death: the Framingham Study. Am Heart J. 1986;112:820–825. doi: 10.1016/0002-8703(86)90480-1. [DOI] [PubMed] [Google Scholar]
- 22.Kannel WB, Sorlie P. Some health benefits of physical activity: the Framingham Study. Arch Intern Med. 1979;139:857–861. [PubMed] [Google Scholar]
- 23.Kushi LH, Fee RM, Fulsom R, et al. Physical activity and mortality in postmenopausal women. JAMA. 1997;277:1287–1292. [PubMed] [Google Scholar]
- 24.Lacroix AZ, Leveille SG, Hecht JA, et al. Does walking decrease the risk of cardiovascular disease hospitalizations and death in older adults? J Am Geriatr Soc. 1996;44:113–120. doi: 10.1111/j.1532-5415.1996.tb02425.x. [DOI] [PubMed] [Google Scholar]
- 25.Lakka TA, Venalainen JM, Rauramaa R, et al. Relation of leisure-time physical activity and cardiorespiratory fitness to the risk of acute myocardial infarction. N Engl J Med. 1994;330:1549–1554. doi: 10.1056/NEJM199406023302201. [DOI] [PubMed] [Google Scholar]
- 26.Lapidus L, Bengtsson C. Socioeconomic factors and physical activity in relation to cardiovascular disease and death: a 12 year follow up of participants in a population study of women in Gothenburg, Sweden. Br Heart J. 1986;55:295–301. doi: 10.1136/hrt.55.3.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leon AS, Connett J, Jacobs DR, Jr, Rauramaa R. Leisure-time physical activity levels and risk of coronary heart disease and death: the Multiple Risk Factor Intervention Trial. JAMA. 1987;258:2388–2395. [PubMed] [Google Scholar]
- 28.Leon AS, Myers MJ, Connett J. Leisure time physical activity and the 16-year risks of mortality from coronary heart disease and all-causes in the Multiple Risk Factor Intervention Trial [MRFIT] Int J Sports Med. 1997;18(Suppl 3):S208–S115. doi: 10.1055/s-2007-972717. [DOI] [PubMed] [Google Scholar]
- 29.Lie H, Mundal R, Erikssen J. Coronary risk factors and incidence of coronary death in relation to physical fitness: seven year follow-up study of middle-aged and elderly men. Eur Heart J. 1985;6:147–157. doi: 10.1093/oxfordjournals.eurheartj.a061829. [DOI] [PubMed] [Google Scholar]
- 30.Lindsted KD, Tonstad S, Kuzma JW. Self-report of physical activity and patterns of mortality in Seventh-Day Adventist men. J Clin Epidemiol. 1991;44:355–364. doi: 10.1016/0895-4356(91)90074-j. [DOI] [PubMed] [Google Scholar]
- 31.Lord FM, Novick MR. Statistical Theory of Mental Test Scores. Reading, MA: Addison-Wesley; 1968. pp. 69–74. [Google Scholar]
- 32.Manson JE, Hu FB, Rich-Edwards JW, et al. A prospective study of walking as compared with vigorous exercise in the prevention of coronary heart disease in women. N Engl J Med. 1999;341:650–658. doi: 10.1056/NEJM199908263410904. [DOI] [PubMed] [Google Scholar]
- 33.Mensink GB, Deketh M, Mul MD, et al. Physical activity and its association with cardiovascular risk factors and mortality. Epidemiology. 1996;7:391–397. doi: 10.1097/00001648-199607000-00009. [DOI] [PubMed] [Google Scholar]
- 34.Morris JN, Chave SP, Adam C, et al. Vigorous exercise in leisure-time and the incidence of coronary heart-disease. Lancet. 1973;1(799):333–339. doi: 10.1016/s0140-6736(73)90128-1. [DOI] [PubMed] [Google Scholar]
- 35.Morris JN, Clayton DG, Everitt MG, et al. Exercise in leisure time: coronary attack and death rates. Br Heart J. 1990;63:325–334. doi: 10.1136/hrt.63.6.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Morris JN, Everitt MG, Pollard R, et al. Vigorous exercise in leisure-time: protection against coronary heart disease. Lancet. 1980;2(8206):1207–1210. doi: 10.1016/s0140-6736(80)92476-9. [DOI] [PubMed] [Google Scholar]
- 36a.Paffenbarger RS, Wing AL, Hyde PT. Physical activity as an index of heart attack risk in college alumni. Am J Epidemiol. 1978;108:161–175. doi: 10.1093/oxfordjournals.aje.a112608. [DOI] [PubMed] [Google Scholar]
- 37.Paffenbarger RS, Jr, Hyde RT, Wing AL, Steinmetz CH. A natural history of athleticism and cardiovascular health. JAMA. 1984;252:491–495. [PubMed] [Google Scholar]
- 38.Pate RR, Pratt M, Blair SN, et al. Physical activity and public health: a recommendation from the Centers for Disease Control and Prevention and the American College of Sports Medicine. JAMA. 1995;273:402–407. doi: 10.1001/jama.273.5.402. [DOI] [PubMed] [Google Scholar]
- 39.Pekkanen J, Marti B, Nissinen A, et al. Reduction of premature mortality by high physical activity: a 20-year follow-up of middle-aged Finnish men. Lancet. 1987;1(8548):1473–1477. doi: 10.1016/s0140-6736(87)92218-5. [DOI] [PubMed] [Google Scholar]
- 40.Peters RK, Cady LD, Jr, Bischoff DP, et al. Physical fitness and subsequent myocardial infarction in healthy workers. JAMA. 1983;249:3052–3056. [PubMed] [Google Scholar]
- 41.Physical activity and cardiovascular health. NIH Consensus Development Panel on Physical Activity and Cardiovascular Health. JAMA. 1996;276:241–246. [PubMed] [Google Scholar]
- 42.Rodriguez BL, Curb JD, Burchfiel CM, et al. Physical activity and 23-year incidence of coronary heart disease morbidity and mortality among middle-aged men: the Honolulu Heart Program. Circulation. 1994;89:2540–2544. doi: 10.1161/01.cir.89.6.2540. [DOI] [PubMed] [Google Scholar]
- 43.Rosengren A, Wilhelmsen L. Physical activity protects against coronary death and deaths from all causes in middle-aged men: evidence from a 20-year follow-up of the primary prevention study in Goteborg. Ann Epidemiol. 1997;7:69–75. doi: 10.1016/s1047-2797(96)00106-8. [DOI] [PubMed] [Google Scholar]
- 44.Roseman RH, Bawol RD, Oscherwitz M. A 4-year prospective study of the relationship of different habitual vocational physical activity to risk and incidence of ischemic heart disease in volunteer male federal employees. Ann NY Acad Sci. 1977;301:627–641. doi: 10.1111/j.1749-6632.1977.tb38234.x. [DOI] [PubMed] [Google Scholar]
- 45.Salonen JT, Puska P, Tuomilehto J. Physical activity and risk of myocardial infarction, cerebral stroke and death: a longitudinal study in Eastern Finland. Am J Epidemiol. 1982;115:526–537. doi: 10.1093/oxfordjournals.aje.a113334. [DOI] [PubMed] [Google Scholar]
- 46.Salonen JT, Slater JS, Tuomilehto J, Rauramaa R. Leisure time and occupational physical activity: risk of death from ischemic heart disease. Am J Epidemiol. 1988;127:87–94. doi: 10.1093/oxfordjournals.aje.a114794. [DOI] [PubMed] [Google Scholar]
- 47.Sandvik L, Erikssen J, Thaulow E, et al. Physical fitness as a predictor of mortality among healthy, middle-aged Norwegian men. N Engl J Med. 1993;328:533–537. doi: 10.1056/NEJM199302253280803. [DOI] [PubMed] [Google Scholar]
- 48.Sharper AG, Wannamethee G, Weatherall R. Physical activity and ischaemic heart disease in middle-aged British men. Br Heart J. 1991;66:384–394. doi: 10.1136/hrt.66.5.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sharper AG, Wannamethee G, Walker M. Physical activity, hypertension and risk of heart attack in men without evidence of ischaemic heart disease. J Hum Hypertens. 1994;8:3–10. [PubMed] [Google Scholar]
- 50.Sherman SE, D'Agostino RB, Cobb JL, Kannel WB. Physical activity and mortality in women in the Framingham Heart Study. Am Heart J. 1994;128:879–884. doi: 10.1016/0002-8703(94)90583-5. [DOI] [PubMed] [Google Scholar]
- 51.Slattery ML, Jacobs DR, Jr, Nichaman MZ. Leisure time physical activity and coronary heart disease death: the US Railroad Study. Circulation. 1989;79:304–311. doi: 10.1161/01.cir.79.2.304. [DOI] [PubMed] [Google Scholar]
- 52.Slattery ML, Jacobs DR., Jr Physical fitness and cardiovascular disease mortality: the US Railroad Study. Am J Epidemiol. 1988;127:571–580. doi: 10.1093/oxfordjournals.aje.a114832. [DOI] [PubMed] [Google Scholar]
- 53.Sobolski J, Kornitzer M, De Backer G, et al. Protection against ischemic heart disease in the Belgian Physical Fitness Study: physical fitness rather than physical activity? Am J Epidemiol. 1987;125:601–610. doi: 10.1093/oxfordjournals.aje.a114573. [DOI] [PubMed] [Google Scholar]
- 54.Tunstall-Pedoe H, Woodward M, Tavendale R, et al. Comparison of the prediction by 27 different factors of coronary heart disease and death in men and women of the Scottish Heart Health Study: cohort study. Br Med J. 1997;315:722–729. doi: 10.1136/bmj.315.7110.722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Weller I, Corey P. The impact of excluding non-leisure energy expenditure on the relation between physical activity and mortality in women. Epidemiology. 1998;9:632–635. [PubMed] [Google Scholar]
- 56.Yano K, Reed DM, McGee DL. Ten-year incidence of coronary heart disease in the Honolulu Heart Program: relationship to biologic and lifestyle characteristics. Am J Epidemiol. 1984;119:653–666. doi: 10.1093/oxfordjournals.aje.a113787. [DOI] [PubMed] [Google Scholar]
- 57.U. S. Department of Health and Human Services. Physical Activity and Health: A report of the Surgeon General. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion; 1996. [Google Scholar]
- 58.Williams PT. Physical activity and public health [Letter] JAMA. 1995;274:533–534. doi: 10.1001/jama.1995.03530070031016. [DOI] [PubMed] [Google Scholar]


