Abstract
Flow cytometric analyses were performed to evaluate HLA-DR+ activated T lymphocytes (Tact; CD3+/CD4+/CD25medium) and T regulatory cells (Treg; CD3+/CD4+/CD25high) in the circulation of children 8–10 years of age living in an area endemic for both Plasmodium falciparum and Schistosoma mansoni in western Kenya. Those children with only S. mansoni had a higher mean percentage of HLA-DR+ Tact than those who were co-infected with these two intravascular parasites. The proportion of circulating Treg was comparable in children with only schistosomiasis and both schistosomiasis and malaria. However, the mean level of memory Treg (Treg expressing CD45RO+) in those with dual infections was lower than in children with schistosomiasis alone. These imbalances in Tact and Treg memory subsets in children infected with both schistosomiasis and malaria may be related to the differential morbidity or course of infection attributed to coinfections with these parasites.
The parasites causing Schistosomiasis mansoni and falciparum malaria are co-endemic in parts of Africa. 1,2 Also, the prevalence of these diseases is often highest in school children. 1,3–9 Simultaneous infections with schistosome and Plasmodium species may impact the immunologic responses, the clinical manifestations, and possibly the development of acquired resistance to either parasite. 4,10 Schistosomiasis is often acquired early in life (1–4 years of age) in Lake Victoria fishing villages, 11 and if not treated, develops into a chronic infection. 12,13 Malaria is acquired very early in life in holoendemic areas and, if a child survives beyond the fifth year, relative acquired immunity is established, although repeated sub-clinical and clinical infections can reoccur periodically. Although both of these infections are intravascular, schistosomiasis provides chronic antigen exposure, whereas malaria results in more episodic antigenic exposure. Because of the holoendemic nature of the area in which we did this study, the subjects may have had fairly frequent malarial episodes and thus somewhat more frequent exposure to malarial antigens. However, among 8- to 10-yearold children, natural worm death of their initial infections is not likely to have occurred frequently, if at all. This means their exposure to schistosome antigens will have been largely a result of chronic egg antigen exposure. Each of these parasites is known to stimulate both lymphocyte activation and T regulatory cell (Treg) involvement. 14,15 In humans infected with P. falciparum, removal of Treg can enhance peripheral blood mononuclear cell proliferation and interferon (IFN)-γ responses to malaria antigen, 16 which could indicate that Treg may play an important role in the control of immunopathology.
We studied immune profiles in children with schistosomiasis and malaria recruited from 8 primary schools in Bondo District, western Kenya, which is endemic for both P. falciparum and S. mansoni. The schools are all located within 3 km of the shores of Lake Victoria. A previous study indicated a prevalence of S. mansoni greater than 50% among 9- to 12-year-old children attending these schools.17 Data were collected between June and December 2006.
The Scientific Steering Committee of the Kenya Medical Research Institute, the National Ethics Review Board of Kenya, and the Institutional Review Boards of the University of Georgia and the Centers for Diseases Control and Prevention approved the investigation. After obtaining written informed consent from parents and assent from children, 2 slides from 1 stool specimen were examined for S. mansoni eggs and other helminth ova by the modified Kato-Katz method (Vestergaard-Frandsen, Denmark). Data were recorded as eggs per gram (EPG) of stool. Thick and thin blood smears of finger prick blood were examined for malaria parasites. Those few children with malaria caused by Plasmodium spp. other than P. falciparum were not included in this study. Participants positive for S. mansoni were further consented to take part in immunologic studies. When diagnosed with any of the soil-transmitted helminths or schistosomiasis, the children were offered treatment with albendazole (400 mg) and/or praziquantel (PZQ; 40 mg/kg; Cosmos, Ltd., Nairobi, Kenya), respectively. Participants who were blood smear-positive for malaria were offered appropriate therapy with CoArtem (Beijing Novartis Pharma, Ltd., Beijing, China). None of the children had been previously treated with PZQ. These current findings are initial baseline observations from an ongoing longitudinal protocol to evaluate the immunologic impact of multiple treatments for schistosome reinfections acquired during childhood.
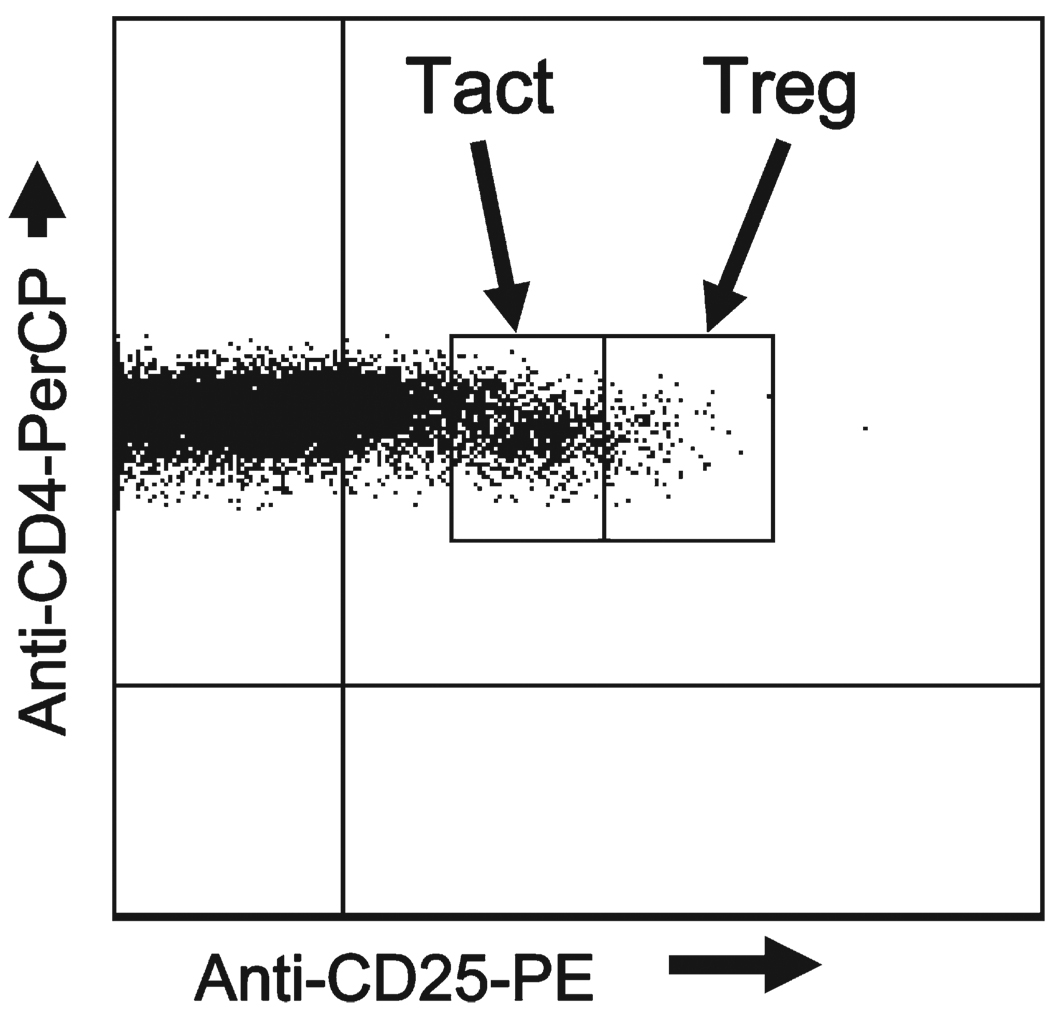
Blood for flow cytometric analyses was collected by venipuncture into EDTA-coated glass tubes (BD Vacutainer System, Franklin Lakes, NJ). To identify Treg in peripheral blood, anti-CD3, anti-CD4, and anti-CD25 (e-Bioscience, San Diego, CA) were used. In our previous study of Treg in adults with schistosomiasis, 15 we defined Treg as the CD3+/CD4+/CD25high population. 18–24 These longitudinal studies were started before the commercial availability of appropriate FoxP3 reagents that identify CD+/CD25high functional Treg. 25 Activated T cells (Tact) were defined as the CD3+/CD4+/CD25medium cells and those that also expressed HLA-DR+ (e-Bioscience, San Diego, CA) were considered highly activated. 15 We first gated to separate CD25negative cells from CD25positive cells based on fluorescence minus one (FMO) stained samples. 26,27 To distinguish CD25high from CD25medium cells, we examined the CD4/ CD25 dot plots from all patients studied and set thresholds (Figure 1) that most often differentiated these subpopulations (CD25low, CD25medium, CD25high). These thresholds were then used for all data analyses. To examine phenotypic subsets of Treg, anti-PD-1 (CD279), and anti-CD45RO (BD Pharmingen, San Diego, CA) were used and gating performed using FMO-stained samples. For statistical analyses the Kruskal-Wallis non-parametric post-test was used following analysis of variance (ANOVA) analysis when 3 or more groups were compared. The Mann-Whitney non-parametric t test was used when 2 groups were compared. Comparisons with P values ≤ 0.05 were considered statistically significant.
FIGURE 1.
Flow cytometry analysis of whole blood cells gated on CD3+/CD4 + cells within the lymphocyte gate, stained with anti-CD25 and used to define CD3+/CD4+/CD25low, CD3+CD4+CD25medium, and CD3 + CD4 + CD25high cells. Staining gates for CD3, CD4, and CD25 were determined using thresholds based on the fluorescence minus one (FMO) “no stain” controls. After examination of the scatter plots of all patients, a threshold was set between CD25medium and CD25high cells (line between the blocks denoting Tact and Treg, respectively). These thresholds, and those provided by the FMO controls, were then used throughout for all analyses.
A total of 153 school children (8–10 years of age) with S. mansoni infection were included in the study. Group sample sizes differ for different tests because of the unavailability of some samples from some children. Children were stratified into three levels of infection intensity based on S. mansoni: low (< 100; N = 89), medium (100–399; N = 41), and high (≥ 400; N = 23). The demographic, clinical, and laboratory characteristics of the study participants are presented in Table 1. Malaria prevalence was comparable in the low and medium intensity S. mansoni groups (29%, and 30%, respectively) and lower in the high intensity group (10.5%), although this difference was not significant compared with the other 2 groups. There were no significant differences in age, hemoglobin concentrations, height, weight, or prevalence of other helminth infections (Ascaris, hookworm, and Trichuris) between the groups (Table 1).
TABLE 1.
Demographic, clinical, and laboratory characteristics of the study participants categorized based on levels of Schistosoma mansoni eggs per gram of feces
| Low intensity* | Medium intensity* | High intensity* | |
|---|---|---|---|
| Number of participants | 89 | 41 | 23 |
| Gender (n, % Male) | 46 (51.7) | 21 (51.2) | 14 (60.9) |
| Age (year) (mean ± SD) | 9.2 ± 0.9 | 9.3 ± 0.9 | 9.6 ± 0.6 |
| Hemoglobin (g/dL) (mean ± SD) | 11.2 ± 1.6 | 11.6 ± 1.4 | 11.1 ± 2.1 |
| Plasmodium falciparum (%) | 29.0 | 30.3 | 10.5 |
| Other helminths (%) | 38.2 | 43.9 | 43.5 |
| Height (cm) (mean ± SD) | 130.3 ± 8.6 | 131.5 ± 8.6 | 133.7 ± 1.2 |
| Weight (kg) (mean ± SD) | 27.8 ± 5.2 | 28.8 ± 5.4 | 29.7 ± 4.2 |
Low (1–99 eggs per gram [EPG]), Medium (100–399 EPG), and High (≥ 400 EPG).
We found no difference in the percentage of circulating CD3+/CD4+ cells in children with schistosomiasis only when compared with those in children with both schistosomiasis and malaria (55.4 ± 1.0 versus 55.4 ± 1.7; mean ± SEM, respectively).
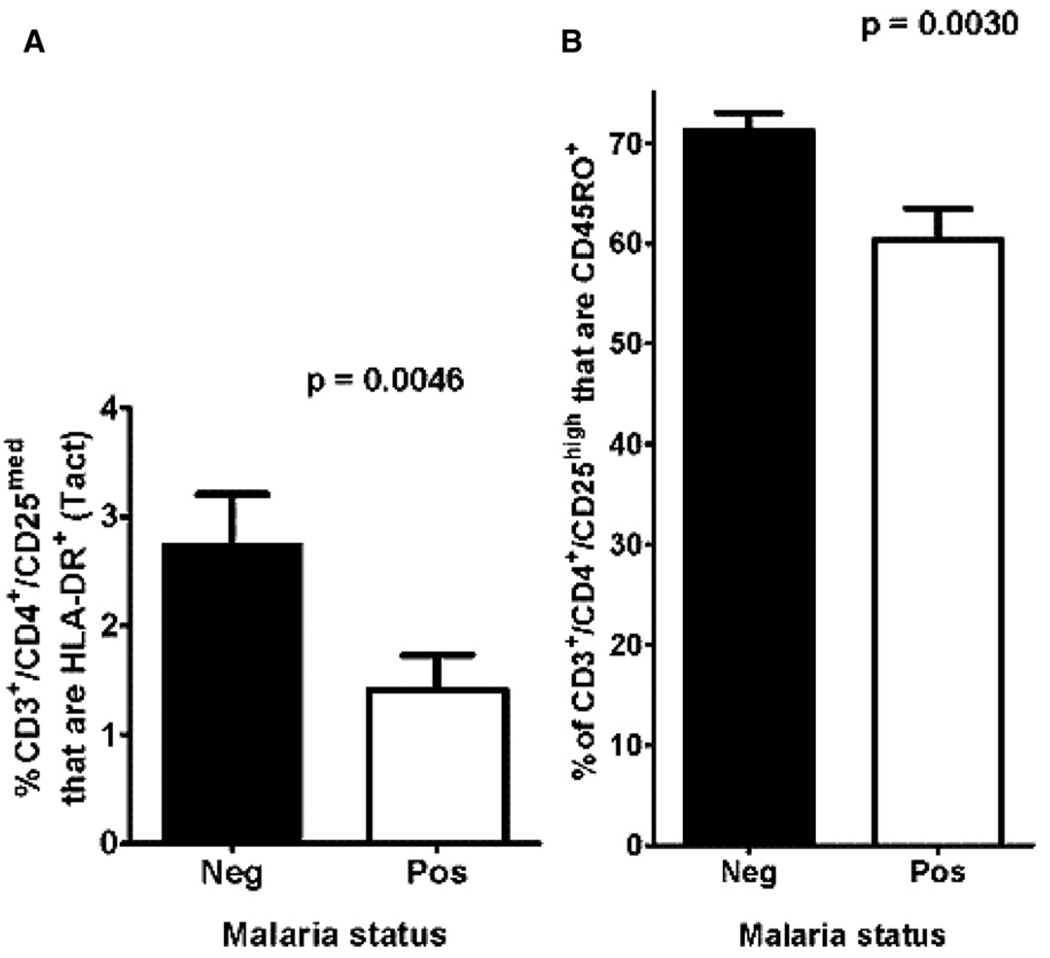
We examined the level of circulating Tact cells in the children with only S. mansoni infections (N = 90) and found that the mean percentage of these cells that also expressed HLA-DR+, indicating highly activated T cells, 28 was 16.1%. However, when this Tact (HLA-DR+) subset was analyzed in the circulation of children with S. mansoni and P. falciparum coinfections (N = 32), the level was significantly lower (5.2%;P = 0.0173; Figure 2A). This significantly lower level of circulating HLA-DR+, highly activated T lymphocytes in children co-infected with schistosomes and P. falciparum, compared with schistosomes alone, may indicate that responses to coinfections led to either decreased T cell activation or immune sequestration of HLA-DR+ Tact from the circulating lymphocyte pool.
FIGURE 2.
A, Comparison of the circulating level of HLA-DR+ highly activated T lymphocytes and B, Treg that expressed the T cell memory marker CD45RO between children with Schistosomiasis mansoni (Neg; filled columns) or co-infected with S. mansoni and Plasmodium falciparum (Pos; open columns).
Erratum
In Am J Trop Med Hyg 80: 475–478 by Muok et al, the wrong data set was used in Figure 2A. The data shown in the original Figure 2A are those obtained by measuring CD3+/CD4+/CD25high/HLA-DR+ cells, not CD3+/CD4+/CD25med/HLA-DR+ cells as indicated on the Y axis of the original figure. The corrected Figure 2A does not alter the conclusions of the paper because the statistical differences reported remain the same. However, the actual percentages of the population represented differ and corrected statements concerning these percentages are now substituted in the appropriate text referring to Figure 2A. With the correct substitutions the text (2nd paragraph, right hand column, page 476) would read as follows: We examined the level of circulating Tact cells in the children with only S. mansoni infections (N = 82) and found that the mean percentage of these cells that also expressed HLA-DR+, indicating highly activated T cells, (28) was 2.7%. However when this Tact (HLA-DR+) subset was analyzed in the circulation of children with S. mansoni and P. falciparum coinfections (N = 34), the level was significantly lower (1.4%; P = 0.0046; Figure 2A). All the text statements in the Abstract and the body of the paper that draw conclusions from this Figure are still valid, i.e., they are the same as the authors drew originally from the incorrect Figure. The correct figure appears below. The authors and the journal regret this error.
The percentages of Treg did not differ statistically when compared on the basis of children’s ages or when children with schistosomiasis alone (N = 122) were compared with those co-infected with both S. mansoni and P. falciparum (N = 32) (data not shown). The mean percentage of Treg in the circulation of children with schistosomiasis only was 2.1% (SD ± 1.3). We have previously reported the mean Treg percentage in adults with chronic patent S. mansoni infections is 4.7%, and approximately 31% of adult S. mansoni patients have 5–10% circulating Treg and another 9% have very high Treg percentages (> 10%).15 The percent of circulating Treg in uninfected adults is usually 1–3%,24 Thus, 8- to 10-year-old children with S. mansoni infection exhibit circulating Treg levels that are comparable to those of uninfected adults and appear lower than adults with long-standing patent S. mansoni. The percentages of Treg in the peripheral blood were analyzed based on low, medium, or high numbers of EPG of feces, but as with infected adults,15 there were no significant differences in Treg percentages between the three groups (data not shown). Likewise, the intensity of a child’s schistosomiasis infection did not lead to significant differences in either the percentage of Treg that expressed the immunosuppressive marker PD-1, or mean fluorescence intensity PD-1 expression levels (data not shown).
There was, however, a significant difference (P = 0.0030; Figure 2B ) between the mean percentage of Treg that expressed the T cell memory marker CD45RO,29 based on whether children had only schistosomiasis or coinfection with both S. mansoni and falciparum malaria (Figure 2B). The mean percentage of CD45RO+ Treg (memory Treg) in children with S. mansoni infection alone was 71.2%, whereas it was 60.3% in those with both infections. This lower level of circulating Treg memory cells suggests that coinfection of schistosome-infected children with falciparum malaria may lead to decreased immunoregulatory capacity, or differential margination of memory Treg.
Analyses of T lymphocyte subsets based on patent infections with any of the three soil-transmitted helminths failed to yield statistically significant differences in terms of coinfections with schistosomiasis (data not shown).
Children in rural western Kenya and many other parts of sub-Saharan Africa are at risk of simultaneously harboring multiple infectious diseases. The goal of this baseline study was to determine whether coinfection of primary school children with S. mansoni and P. falciparum alters the levels of certain T lymphocyte subsets, as compared with children with S. mansoni alone. We have found that children with coinfections of S. mansoni and falciparum malaria present with alterations in their circulating highly Tact and Treg memory subsets. Others have reported on children with coinfections with both parasites,6,30 and have found differences in morbidity,3,31 and possibly resistance to reinfections with either of the parasites compared with infection with only one parasite.18 In fact, Wilson, and others 32 have shown that these coinfections lead to decreased Th2 cytokine responses to egg antigens, whereas the reverse was true for TNF-α production. Furthermore, these coinfection-mediated, immunologic alterations correlated with the degree of hepatomegaly. It is plausible that a stabilized heightened immune system resulting from chronic antigenic exposure from patent schistosomiasis might be influenced by the episodic immune disturbances characterized by bouts of clinical or sub-clinical falciparum malaria. These studies begin to evaluate those immune parameters often associated with over-activation or immunoregulation because of these infections, and we anticipate our ongoing, longitudinal studies of these and other immune markers will allow increased understanding of both protective and morbidity-related immune responses caused by both schistosomiasis and malaria in children living in co-endemic areas.
Acknowledgments
We thank Erick Livaha, Esther Wanjala, Keziah Odhiambo, Hassan Jimale, and Henry Karanja for technical assistance. We are grateful to all the children and their parents/guardians for their participation.
Financial support: This work was supported by PHS grants AI 053695 (DGC) and T32 AI 060546 (CLB) from the NIAID and D43 TW007123 (PNMM) from the FIC of the National Institutes of Health, the PHS, the Centers for Disease Control and Prevention, and the Kenya Medical Research Institute.
Footnotes
Disclaimer: This work is published with the permission of the Director, Kenya Medical Research Institute. The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the CDC. The authors have no commercial or other association that might pose a conflict of interest.
Reprint requests: Daniel G. Colley, Center for Tropical and Emerging Global Diseases, Coverdell Center, Room 330B, University of Georgia, 500 D.W. Brooks Drive, Athens, GA 30602, E-mail: dcolley@uga.edu.
Contributor Information
Erick M. O. Muok, Centre for Global Health Research, Kenya Medical Research Institute, PO Box 1578, Kisumu, Kenya, E-mail: emuok@kisian.mimcom.net.
Pauline N. M. Mwinzi, Centre for Global Health Research, Kenya Medical Research Institute, PO Box 1578, Kisumu, Kenya, E-mail: pmwinzi@kisian.mimcom.net
Carla L. Black, Center for Tropical and Emerging Global Diseases, Room 145 Coverdell Center, University of Georgia, Athens, GA 30602-7399, E-mail: blackc@uga.edu
Jennifer M. Carter, Center for Tropical and Emerging Global Diseases, Room 145 Coverdell Center, University of Georgia, Athens, GA 30602-7399, E-mail: ginaphur@uga.edu
Zipporah W. Ng’ang’a, Department of Medical Laboratory Sciences, Jomo Kenyatta University of Agriculture and Technology, Nairobi, Kenya, E-mail: Zipnganga@gmail.com
Michael M. Gicheru, Department of Zoological Sciences, Kenyatta University, Nairobi, Kenya, E-mail: mgicheru@africaonline.co.ke
W. Evan Secor, Division of Parasitic Diseases, Centers for Disease Control and Prevention, 4770 Buford Highway, N.E., Mail-stop F-13, Atlanta, GA 30341, E-mail: was4@cdc.gov.
Diana M. S. Karanja, Centre for Global Health Research, Kenya Medical Research Institute, PO Box 1578, Kisumu, Kenya, E-mail: dkaranja@kisian.mimcom.net
Daniel G. Colley, Center for Tropical and Emerging Global Diseases, Room 145 Coverdell Center, University of Georgia, Athens, GA 30602-7399, E-mail: dcolley@uga.edu
REFERENCES
- 1.Brooker S, Akwale W, Pullan R, Estambale B, Clarke SE, Snow RW, Hotez JP. Epidemiology of Plasmodium-helminth co-infection in Africa: populations at risk, potential impact on anemia, and prospects for combining control. Am J Trop Med Hyg. 2007;77:88–98. [PMC free article] [PubMed] [Google Scholar]
- 2.Pierrot C, Wilson MS, Lallet H, Laffite S, Jones MF, Daher W, Capron M, Dunne DW, Khalife J. Identification of a novel antigen of Schistosoma mansoni shared with Plasmodium falciparum and evaluation of different cross-reactive antibody subclasses induced by human schistosomiasis and malaria. Infect Immun. 2006;74:3347–3354. doi: 10.1128/IAI.01724-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Booth M, Vennervald BJ, Kenty L, Butterworth AE, Kariuki HC, Kadzo H, Ireri E, Amaganga C, Kimani G, Mwatha JK, Otedo A, Ouma JH, Muchiri E, Dunne DW. Micro-geographical variation in exposure to Schistosoma mansoni and malaria, and exacerbation of splenomegaly in Kenyan school-aged children. BMC Infect Dis. 2004;4:13. doi: 10.1186/1471-2334-4-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Briand S, Watier L, Jay LEH, Garcia A, Cot M. Coinfection with Plasmodium falciparum and Schistosoma haematobium: protective effect of schistosomiasis on malaria in senegaleese children? Am J Trop Med Hyg. 2005;72:702–707. [PubMed] [Google Scholar]
- 5.Le Hesran JY, Akaina JN, Diaye EHM, Dia M, Sengor P, Konate L. Severe malaria attack associated with high prevalence of Ascaris lumbricoides infection among children in rural Senegal. Am J Trop Med Hyg. 2004;98:397–399. doi: 10.1016/j.trstmh.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 6.Lyke KE, Dicko A, Dabo A, Sangare L, Kone A, Coulibaly D, Guido A, Traore K, Daou M, Diarra I, Sztein MB, Plowe CB, Doumbo KO. Association of Schistosoma haematobium infection with protection against acute Plasmodium falciparum malaria in Malian children. Am J Trop Med Hyg. 2005;73:1124–1130. [PMC free article] [PubMed] [Google Scholar]
- 7.Mwangi TW, Bethony JM, Brooker S. Malaria and helminthic interactions in humans: an epidemiological viewpoint. Ann Trop Med Parasitol. 2006;100:551–570. doi: 10.1179/136485906X118468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nacher M, Gay F, Sighhasivanon P, Krudsood S, Treeprasertsuk S, Mazier D, Vouldoukis I, Looareesuwan S. Ascaris lumricoides infection is associated with protection from cerebral malaria. Parasite Immunol. 2000;22:107–113. doi: 10.1046/j.1365-3024.2000.00284.x. [DOI] [PubMed] [Google Scholar]
- 9.Petney TN, Andrews RH. Multiparasite communities in animals and humans: frequency, structure and pathogenic significance. Int J Parasitol. 1998;28:377–393. doi: 10.1016/s0020-7519(97)00189-6. [DOI] [PubMed] [Google Scholar]
- 10.Druilhe P, Tall A. Worms can worsen malaria: towards a new means to roll back malaria. Trends Parasitol. 2005;21:359–362. doi: 10.1016/j.pt.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 11.Stothaard JR, Gabrieli AF. Schistosomiasis in African infants and preschool children: to treat or not to treat. Trends Immunol. 2007;23:83–88. doi: 10.1016/j.pt.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 12.Engels D, Chitsulo L, Montresor A, Savioli L. The global epidemiological situation of schistosomiaisis and new approaches to control and research. Act Trop. 2002;82:139–146. doi: 10.1016/s0001-706x(02)00045-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gryseels B, Polman K, Clerinx J, Kestens L. Human schistosomiasis. Lancet. 2006;368:1106–1118. doi: 10.1016/S0140-6736(06)69440-3. [DOI] [PubMed] [Google Scholar]
- 14.Millington OR, Gibson BV, Rush CM, Zinselmeyer R, Stephen P, Garside P, Brewer MJ. Malaria impairs T cell clustering and immune priming despite normal signal 1 from dendritic cells. PLoS Pathog. 2007;3:143. doi: 10.1371/journal.ppat.0030143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Watanabe K, Mwinzi PMN, Black CL, Muok EOM, Karanja DMS, Secor WE, Colley DG. T regulatory cells decrease in people infected with Schistosoma mansoni upon effective treatment. Am J Trop Med Hyg. 2007;77:676–682. [PMC free article] [PubMed] [Google Scholar]
- 16.Walther M, Tongren JE, Andrews L, Korbel D, King E, Fletcher H, Andersen RF, Bejon P, Thompson F, Dunachie SJ, Edele F, de Souza JB, Sinden RE, Gilbert SC, Riley EM, Hill AV. Upregulation of TGF beta, FOXP3 and CD4+CD25+ regulatory T cell correlates with more rapid parasite growth in human malaria infection. Immunity. 2005;23:287–296. doi: 10.1016/j.immuni.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 17.Handzel T, Karanja DMS, Addis DG, Allen WH, Rosen DH, Colley DG, Andove J, Slutsker L, Secor WE. Geographic distribution of schistosomiasis and soil transmitted helminths in Western Kenya: implication of antihelminthic mass treatment. Am J Trop Med Hyg. 2003;69:318–323. [PubMed] [Google Scholar]
- 18.Bayry J, Triebel F, Kaveri SV, Tough DF. Human dendritic cells acquire a semimature phenotype and lymph node homing potential through interaction with CD4+CD25+ regulatory T cells. J Immunol. 2007;178:4184–4193. doi: 10.4049/jimmunol.178.7.4184. [DOI] [PubMed] [Google Scholar]
- 19.Duggleby RC, Shaw TN, Jarvis LB, Gaston JS. CD27 expression discriminates between regulatory and non-regulatory cells after expansion of human peripheral blood CD4+ CD25+ cells. Immunol. 2007;121:129–139. doi: 10.1111/j.1365-2567.2006.02550.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Keever-Taylor CA, Browning MB, Johnson BD, Truitt RL, Bredeson CN, Behn B, Tsao A. Rapamycin enriches for CD4 (+) CD25 (+) CD27 (+) Foxp3 (+) regulatory T cells in ex vivo-expanded CD25-enriched products from healthy donors and patients’ with multiple sclerosis. Cytotherapy. 2007;9:144–157. doi: 10.1080/14653240601145223. [DOI] [PubMed] [Google Scholar]
- 21.Longhi MS, Hussain MJ, Mitry RR, Arora SK, Mieli-Vergani G, Vergano D, Ma Y. Functional study of CD4+CD25+ regulatory T cells in health and autoimmune hepatitis. J Immunol. 2006;176:4484–4491. doi: 10.4049/jimmunol.176.7.4484. [DOI] [PubMed] [Google Scholar]
- 22.Strauss L, Whiteside TL, Knights A, Bergmann C, Knuth A, Zippelius A. Selective survival of naturally occurring human CD4+CD25+Foxp3+ regulatory T cells cultured with rapamycin. J Immunol. 2007;178:320–329. doi: 10.4049/jimmunol.178.1.320. [DOI] [PubMed] [Google Scholar]
- 23.Valencia X, Yarboro C, Illei G, Lipsky PE. Deficient CD4+CD25high T regulatory cell function in patients with active systemic lupus erythematosus. J Immunol. 2007;178:25–88. doi: 10.4049/jimmunol.178.4.2579. [DOI] [PubMed] [Google Scholar]
- 24.Baecher-Allan C, Brown JA, Freeman GL, Hafler DA. CD4+CD25 high regulatory cells in human peripheral blood. J Immunol. 2001;167:1245–1253. doi: 10.4049/jimmunol.167.3.1245. [DOI] [PubMed] [Google Scholar]
- 25.Sumida Y, Nakamura K, Kanayama K, Akiho H, Teshima T, Takayanagi R. Preparation of functionally preserved CD4+ CD25high regulatory TT cells from leukapheresis products from ulcerative colitis patients, applicable to regulatory T-cell transfer therapy. Cytotherapy. 2008;10:698–710. doi: 10.1080/14653240802345812. [DOI] [PubMed] [Google Scholar]
- 26.Roederer M. Spectral compensation for flow cytometry: visualization artifacts, limitations and caveats. Cytometry. 2001;45:194–205. doi: 10.1002/1097-0320(20011101)45:3<194::aid-cyto1163>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 27.Tung JW, Parks DR, Moore A, Herzenberg LA, Herzenberg AL. New approaches to fluorescence compensation and visualization of FACS data. Clin Immunol. 2004;110:277–283. doi: 10.1016/j.clim.2003.11.016. [DOI] [PubMed] [Google Scholar]
- 28.Kaushik S, Vajpayee M, Sreenivas V, Pradeep S. Correlation of T-lymphocyte subpopulations with immunological markers in HIV-1-infected Indian patients. Clin Immunol. 2006;119:330–338. doi: 10.1016/j.clim.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 29.Machura E, Mazur B, Pieniazel W, Karczewska K. Expression of naive/memory (CD45RA/CD45RO) markers by peripheral blood CD4+ and CD8+ T cells in children with asthma. Arc Immuno Therap Eep. 2008;56:55–62. doi: 10.1007/s00005-008-0005-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Remoue F, Diallo TO, Angeli V, Herve M, deClercq D, Scharcht AM, Charrier N, Capron M, Vercruysse A, Ly A, Capron A, Riveau G. Malaria coinfection in children influences antibodies response to schistosome antigens and inflammatory markers associated with morbidity. Trans R Soc Trop Med Hyg. 2003;97:361–364. doi: 10.1016/s0035-9203(03)90170-2. [DOI] [PubMed] [Google Scholar]
- 31.Wilson S, Vennervald BJ, Kadzo H, Ireri E, Amaganga C, Booth M, Kariuki HC, Mwatha JK, Kimani G, Ouma JH, Muchiri E, Dunne DW. Hepatosplenomegaly in Kenyan schoolchildren: exacerbation by concurrent chronic exposure to malaria and Schistosoma mansoni infection. Trop Med Int Health. 2007;12:1442–1449. doi: 10.1111/j.1365-3156.2007.01950.x. [DOI] [PubMed] [Google Scholar]
- 32.Wilson S, Jones FM, Mwatha JK, Kimani G, Booth M, Kariuki HC, Vennervald BJ, Ouma JH, Muchiri E, Dunne DW. Hepatosplenomegaly is associated with low regulatory and Th2 responses to schistosome antigens in childhood schistosomiasiss and malaria coinfection. Infect Immun. 2008;76:2212–2218. doi: 10.1128/IAI.01433-07. [DOI] [PMC free article] [PubMed] [Google Scholar]