Abstract
The subperitoneal space is a large, unifying, anatomically continuous potential space that connects the peritoneal cavity with the retroperitoneum. This space is formed by the subserosal areolar tissue that lines the inner surfaces of the peritoneum and the musculature of the abdomen and pelvis. It contains the branches of the vascular, lymphatic, and nervous systems that supply the viscera. The subperitoneal space extends into the peritoneal cavity and is invested between the layers of the mesenteries and ligaments that support and interconnect the abdominal and pelvic organs. As such, it provides one large continuous space in which infectious, neoplastic, inflammatory, and hemorrhagic disease may spread in many directions.
Keyword: Subperitoneal space, pathways of disease spread, carcinomatosis
Introduction
Traditionally, the abdominal cavity has been divided into a number of peritoneal, retroperitoneal, and extraperitoneal spaces[1]. Although it is useful for learning abdominal anatomy and appreciating the confinement of disease in a particular space, this classic approach affords limited understanding of the intra-abdominal spread of disease. Dissemination of disease between the retroperitoneum and the peritoneal cavity, between the subdivisions of the retroperitoneum, and within the subperitoneal spaces is difficult to conceptualize if the various spaces and compartments of the abdomen and pelvis are considered fixed, immutable, and isolated delineators of anatomy. Meyers and colleagues have perceived that the abdominal cavity should be viewed rather as a continuous space that is punctuated by the abdominal mesenteries, ligaments, and fascia, which may either confine disease or actually provide an avenue for its spread[2–7]. Blood vessels, lymphatics, and the biliary system also contribute to the spread of a number of benign and malignant processes. This holistic schema provides a rationale for understanding the dissemination of intra-abdominal disease, both focally and to areas distant from the site of origin.
The subperitoneal space is a large, unifying, anatomically continuous potential space that connects the peritoneal cavity with the retroperitoneum (Fig. 1). This space is formed by the subserosal areolar tissue that lines the inner surfaces of the peritoneum and the musculature of the abdomen and pelvis. It contains the branches of the vascular, lymphatic, and nervous systems that supply the viscera. The subperitoneal space extends into the peritoneal cavity and is invested between the layers of the mesenteries and ligaments that support and interconnect the abdominal and pelvic organs. As such, it provides one large continuous space in which infectious, neoplastic, inflammatory, and hemorrhagic disease may spread in many directions[2–4,6,8–13].
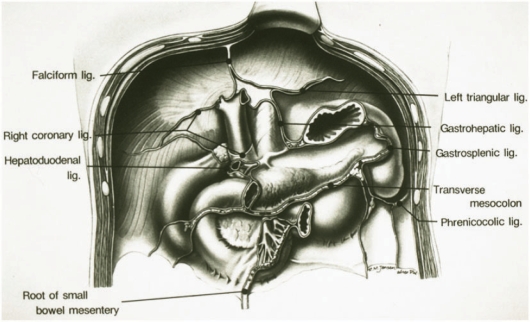
Figure 1.
Subperitoneal spaces. Frontal diagram of the posterior parietal wall of the upper abdomen shows the planes of peritoneal reflections that constitute the major ligaments and mesenteries of the subperitoneal space. Anatomic continuity between intraperitoneal structures and between extraperitoneal and intraperitoneal sites is established along the bare areas at the roots of origin of the supporting ligaments and mesenteries. FL, falciform ligament; RCL, right coronary ligament; BA, bare area of liver; HDL, hepatoduodenal ligament; LTL, left triangular ligament; GHL, gastrohepatic ligament; LS, lesser sac; GSL, gastrosplenic ligament; PL, phrenicocolic ligament; TM, transverse mesocolon; SBM, root of small bowel mesentery; DC, bare area of descending colon; AC, bare area of ascending colon. (From Meyers MA, Oliphant M, Berne AS, et al. The peritoneal ligaments and mesenteries: pathways of abdominal disease spread. Radiology 1987; 63: 594; with permission.)
Transverse mesocolon
The transverse mesocolon is the linchpin that unites the various subperitoneal spaces and is a major conduit for both focal and distant spread of disease. On the right, the transverse mesocolon is continuous with the duodenocolic ligament; on the left, it is continuous with the phrenicocolic ligament; and centrally, it communicates with the small bowel mesentery[2–4,7] (Fig. 2).
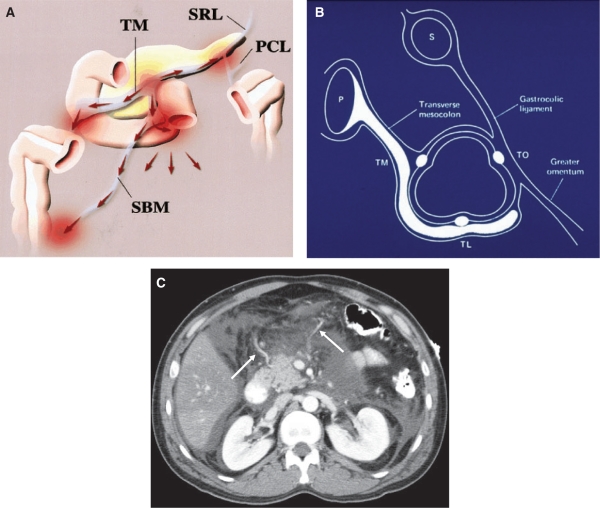
Figure 2.
Transverse mesocolon: anatomic relationships and planes of disease spread. (A) Frontal diagram shows the relationships of the transverse mesocolon (TM). The transverse mesocolon is continuous with the root of the small bowel mesentery (SBM), the splenorenal ligament (SRL), and the phrenicocolic ligament (PCL). (From Okino Y, Kiyosue H, Mori H, et al. Root of the small-bowel mesentery: correlative anatomy and CT features of pathologic conditions. Radiographics 2001; 21: 1480; with permission.) (B) Sagittal diagram through the transverse colon demonstrates preferential spread of pancreatic (P) disease through the transverse mesocolon, inferiorly along the taenia mesocolica–taenia libera (TL) haustra towards the TL–taenia omentalis (TO) row. This constitutes the inferior border of the transverse mesocolon. LS, lesser sac; S, stomach. (C) Fluid surrounds the middle colic vessels of the transverse mesocolon in this patient with pancreatitis. (From Meyers MA, Volberg R, Katzen B, et al. Haustral anatomy and pathology: a new look. II. Roentgen interpretation of pathologic alterations. Radiology 1973; 108: 505–12; with permission.)
The dissemination of fluid and enzymes in pancreatitis typifies the role of this space in disease spread. The inflammatory changes of pancreatitis can spread to the transverse colon and stomach (both intraperitoneal organs) by the gastrosplenic ligament, the lesser sac, and gastrocolic ligament; to the left kidney (a retroperitoneal organ) via the splenorenal ligament; to the liver and gallbladder (intraperitoneal organs) via the hepatoduodenal ligament; and to the right lower quadrant via the root of the small bowel mesentery[2–4,10–13].
Because the transverse mesocolon inserts on the taenia mesocolica, pancreatic processes carried by this ligament preferentially affect the inferior border of the transverse colon[12,13]. Fixation of the inferior margin and pseudosacculation of the superior (uninvolved) margin of the transverse colon may be seen on barium enema studies in patients with pancreatic disease. Colon neoplasms and diverticulitis can invade the transverse mesocolon and subsequently the pancreas[2–4].
Gastrocolic ligament–greater omentum
The gastrocolic ligament connects the greater curvature of the stomach to the transverse colon (Fig. 3). It is formed by fusion of the four layers of peritoneum that invest the stomach. These layers descend a variable distance to form the greater omentum and then fuse at the level of the colon.
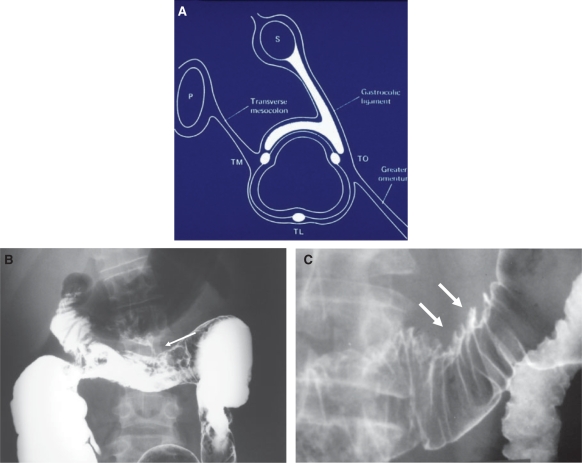
Figure 3.
Gastrocolic ligament: normal anatomy and pathology. (A) Sagittal diagram through the transverse colon demonstrates preferential spread of disease from the stomach (S), through the gastrocolic ligament, along the taenia omentalis (TO)–taenia mesocolica (TM) haustral row. This constitutes the superior border of the transverse colon. P, pancreas; TL, taenia libera. (From Meyers MA, Volberg R, Katzen B, et al. Haustral anatomy and pathology: a new look. II. Roentgen interpretation of pathologic alterations. Radiology 1973; 108: 505–12; with permission.) (B) Barium enema in a patient with Crohn disease demonstrates a fistula (arrow)from the transverse colon to the greater curvature aspect of the stomach via the gastrocolic ligament. (C) Direct invasion (arrows) of the superior aspect of the transverse colon along the gastrocolic ligament from a scirrhous carcinoma of the stomach.
The potential space between the middle layers of the greater omentum forms the inferior recess of the lesser sac. In most adults, partial fusion of the middle layers prevents the lesser sac from extending below the transverse colon. The greater omentum consists of a trabecular framework of vessels with various amounts of adipose tissue, lymphatics, or macrophages. It has been called ‘the abdominal policeman’ and is frequently involved by infectious and neoplastic processes[14–17].
On the left, the gastrocolic ligament is continuous with the gastrosplenic ligament. On the right, it ends at the gastroduodenal junction, near the hepatoduodenal ligament. Carcinoma of the transverse colon can invade to the greater curvature of the stomach, and gastric neoplasms can spread to the superior aspect of the transverse colon via the gastrocolic ligament[12,13,17].
Gastrohepatic ligament
The gastrohepatic ligament is part of the lesser omentum. It joins the gastro-oesophageal junction and lesser curvature of the stomach to the liver at the fissure of the ligamentum venosum superiorly and the porta hepatis inferiorly. Beneath the diaphragm, the oesophagus is invested by the gastrohepatic ligament on the right and the gastrophrenic ligament on the left. The gastrohepatic ligament contains the left gastric artery, coronary vein, and left gastric lymph node chain. The subperitoneal fat of the gastrohepatic ligament continues into the liver as Glisson capsule. Lymph nodes and blood vessels seen in the gastrohepatic ligament by computed tomography should be smaller than 8 mm in diameter. Non-enhancing structures larger than 8 mm are suggestive of adenopathy; if they enhance, varices should be considered[18].
Gastric cancers often spread first to the lymph nodes in the gastrohepatic ligament (Fig. 4). The left gastric nodes also receive direct lymphatic drainage from the distal oesophagus, so neoplasms in this region may produce gastrohepatic ligament adenopathy. Because the gastrohepatic ligament is contiguous with the hepatoduodenal ligament, adenopathy may occur in the porta hepatis and the peripancreatic area secondary to spread of a gastric neoplasm. Direct spread of fundal gastric malignancies into the left hepatic lobe occurs via this ligament and Glisson capsule[4]. Infectious, inflammatory, and autoimmune disorders of the liver often cause adenopathy in the gastrohepatic and gastroduodenal ligaments. Lymphoma and carcinoma of the breast, lung, and oesophagus may spread to the same ligament.
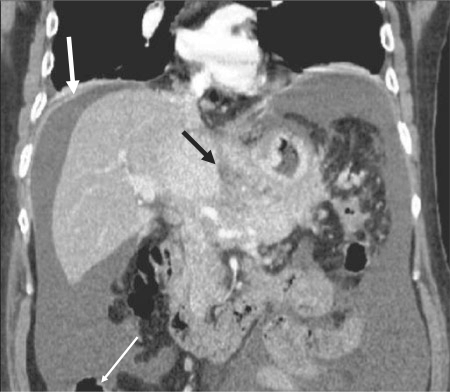
Figure 4.
Spread of gastric cancer into the gastrohepatic ligament. Coronal reformatted CT image shows tumour invasion of the gastrohepatic ligament (black arrow). Peritoneal tumour implants (white arrows) are identified as well.
Hepatoduodenal ligament
The hepatoduodenal ligament is formed at the free edge of the gastrohepatic ligament and connects this portion of the peritoneal cavity with the right anterior pararenal space. It extends from the junction of the first and second portions of the duodenum to the porta hepatis and contains the blood vessels and lymphatics that supply the liver, gallbladder, and biliary tree as well as the common bile duct to its insertion into the ampulla of Vater. Any hepatic or biliary process adjacent to the liver hilum, including the anterior portion of the caudate lobe, can spread via the hepatoduodenal ligament and subsequently extend to the gastrohepatic ligament, gastrocolic ligament, duodenocolic ligament, transverse mesocolon, and falciform ligament. Pancreatic neoplasms invade the porta hepatis via this ligament[19–24].
Duodenocolic ligament
The duodenocolic ligament is the right margin of the transverse mesocolon and provides a pathway for disease spread between the descending duodenum and the junction of the ascending and transverse colon[19,25].
Gastrosplenic ligament
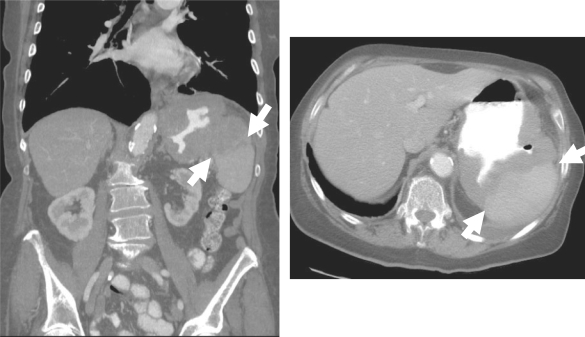
The gastrosplenic ligament is formed by the left lateral extension of the peritoneal layers of the greater omentum and connects the greater curvature of the stomach with the splenic hilum. It contains the left gastroepiploic and short gastric vessels. The gastrosplenic ligament provides a pathway for spread of disease between the pancreatic tail, the spleen, and the stomach. Carcinoma of the stomach may spread to the splenic hilum along this ligament. Similarly, carcinoma of the pancreatic tail can spread to the stomach by invading first the splenic hilum and subsequently the stomach via the gastrosplenic ligament. The pancreatic tail borders this ligament, so this is a fairly frequent site of pseudocyst formation (Fig. 5). Benign gastric ulcers may penetrate into the spleen via this ligament as well[2–4,6,26].
Figure 5.
Gastric cancer spread to the spleen via the gastrosplenic ligament. The subperitoneal space of the gastrosplenic ligament (arrows) is serving as a conduit of tumour spread in this patient with adenocarcinoma of the greater curvature of the stomach.
Splenorenal ligament
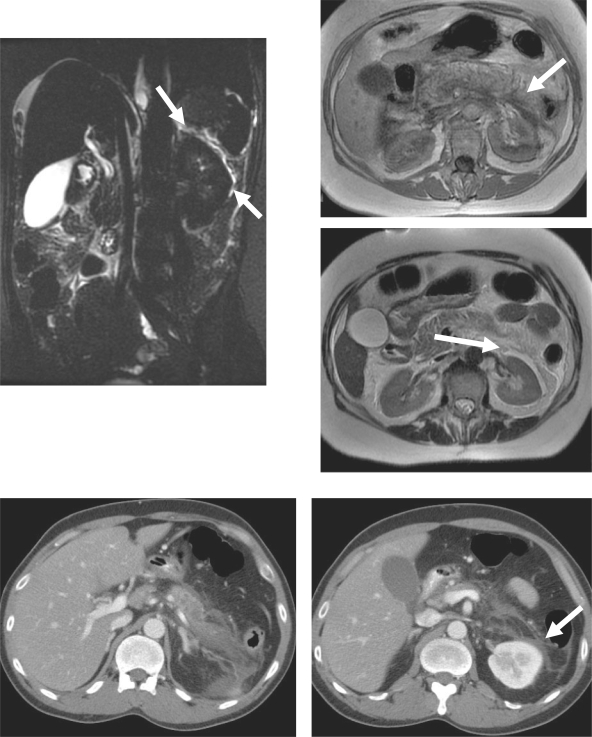
The splenorenal ligament invests the pancreatic tail and affords communication between the anterior pararenal space, which contains the pancreas, and the perirenal space, which contains the kidney. Although pancreatitis (Fig. 6) and carcinoma of the pancreas can commonly spread through this ligament, renal disease seldom involves the pancreatic tail[2–4,6].
Figure 6.
Inflammation of pancreatitis spreading from pancreas to left kidney via the splenorenal ligament: MR and CT findings in the same patient. (A) Coronal image from a magnetic resonance cholangiopancreatography examination shows high signal intensity fluid (arrow) in the perinephric space highlighting the lateral aspect of the left kidney. (B) Axial T1-weighted image shows low signal intensity between the pancreatic tail and left kidney (arrow). (C) Fat-suppressed T2-weighted axial image shows high signal intensity fluid lateral to the left kidney in the perinephric space. (D) CT scan shows low density necrosis in the pancreatic tail. (E) Scan obtained caudal to (D) shows fluid in the left perinephric space (arrow).
Phrenicocolic ligament
The phrenicocolic ligament is the left lateral extension of the transverse mesocolon. It acts as the suspensory ligament of the spleen, reflects the anatomic splenic flexure of the colon, and is directly continuous with the splenorenal ligament and transverse mesocolon. Thus, it can transmit neoplastic and inflammatory disease between the pancreas and the colon, the left kidney, and the spleen[2–6,10,11].
Small bowel mesentery
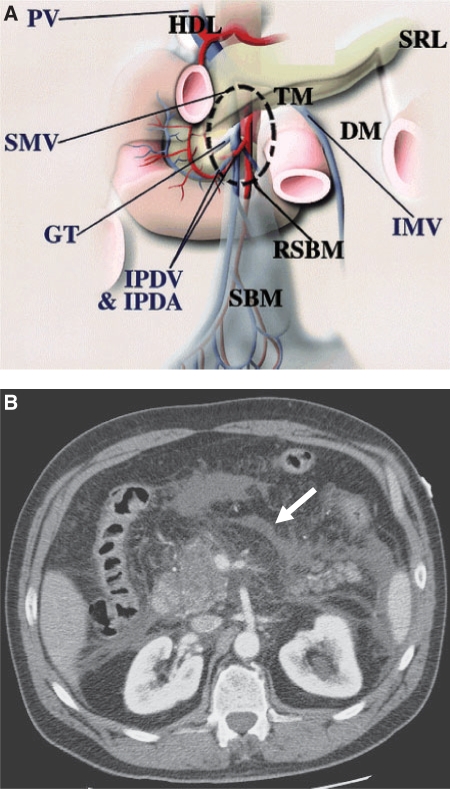
The small bowel mesentery occupies a major portion of the peritoneal cavity, as it suspends the small bowel, and it represents an enormous potential space for disease spread[2–4,10,11]. Seeded neoplastic or infectious material associated with free intraperitoneal fluid cascades along the mesenteric leaves in the inframesocolic space and is deposited on the medial aspect of the caecum on the right and on the superior aspect of the sigmoid mesocolon on the left[5,6].
The subperitoneal space of the small bowel mesentery is continuous with the bare area of the ascending colon and the transverse mesocolon (Fig. 7). The leaves of the small bowel mesentery continue as the posterior peritoneum, overlying the posterior abdominal wall. The connective tissue in the small bowel mesentery merges with the subperitoneal tissue of the retroperitoneum. The subperitoneal tissue continues without interruption inferiorly, from the right lower abdomen over the pelvic musculature and along the lateral pelvic side walls. Because this space potentially communicates with the broad ligament, it provides a pathway for bidirectional disease spread between the abdomen and the pelvis[2–4].
Figure 7.
Small bowel mesentery: conduit of disease. (A) The anatomy near the root of the SBM (RSBM). The root of the SBM (area within dashed circle) is contiguous superiorly to the hepatoduodenal ligament (HDL) along the SMV, anteriorly to the transverse mesocolon (TM), and posterolaterally to the ascending mesocolon and descending mesocolon (DM). The gastrocolic trunk (GT) is a landmark of the junction between the transverse mesocolon and the root of the SBM. The inferior mesenteric vein (IMV) is a landmark of the descending mesocolon and joins the SMV or splenic vein on the left side of the root of the SBM. IPDA, inferior pancreaticoduodenal artery; IPDV, inferior pancreaticoduodenal vein; PV, portal vein; SRL, splenorenal ligament. (From Okino Y, Kiyosue H, Mori H, et al. Root of the small-bowel mesentery: correlative anatomy and CT features of pathologic conditions. Radiographics 2001; 21: 1476; with permission.) (B) CT scan shows fluid (arrow) in the subperitoneal space of the small bowel mesentery in this patient with pancreatitis.
Sigmoid mesocolon
The mesocolon or mesentery of the sigmoid colon provides a major avenue for spread of disease between the abdominal cavity and the pelvis. It is directly continuous with the posterior bare area of the colon, the bare area of the rectum, and in females, the broad ligament. Diverticulitis usually spreads into and is confined by the sigmoid mesocolon. Carcinoma of the sigmoid colon may spread to the ovary, either haematogenously or via the mesocolon, and then along the broad ligament[2–4,7]. Similarly, ovarian neoplasms or tubo-ovarian abscesses may spread directly via the broad ligament to the sigmoid mesocolon and subsequently involve the sigmoid colon[2–4,7].
Broad ligament
The broad ligaments pass from the margins of the uterus to the lateral walls of the pelvis and, together with the uterus, form a septum across the lesser pelvis, dividing it into two parts. The anterior part contains the bladder and uterocystic recess; the posterior part includes the rectum, cul-de-sac, terminal ileum, and part of the sigmoid colon. These ligaments enclose the subperitoneal space, which includes the uterus, ovaries, fallopian tubes, arteries, nerves, lymphatics, and distal ureters as they enter the bladder. On the right side, communication with the base of the caecum and right inferior-lateral termination of the small bowel mesentery provides subperitoneal continuity for bidirectional spread of disease between the female pelvic organs and the retroperitoneal and peritoneal organs of the abdomen. On the left, the broad ligament communicates with the sigmoid mesocolon. Tumours of the caecum, appendicitis, Crohn disease abscesses, and drop metastases can all spread to the right ovary via this pathway. Similarly, an ovarian neoplasm or tubo-ovarian abscess may spread to the caecum and terminal ileum[7].
Falciform ligament and ligamentum teres
The ligamentum teres is located in the free edge of the falciform ligament and anchors the liver anterio-superiorly to the abdominal wall. Infections and malignancies in the porta hepatis can penetrate the falciform ligament when they extend along the fissure of the ligamentum teres. The superficial lymphatics of the liver also traverse the falciform ligament, providing additional pathways of disease spread. The ligamentum teres, gastrohepatic ligament, and hepatoduodenal ligament are continuous, deep in the porta hepatis near the left portal vein[2–4,27].
Coronary ligament and bare area
The right coronary ligament suspends the right lobe of the liver from behind and, together with the left coronary ligament, defines the margins of the bare area of the liver. The bare area of the liver is continuous with the anterior pararenal space[4,8]. The inferior vena cava lies within the bare area of the liver, and fluid in the anterior pararenal space can extend ventrally to the inferior vena cava, close to the epiploic foramen[2–4,27].
Conclusions
From this discussion, it is apparent that the abdominal and pelvic organs and their supporting ligaments, peritoneal reflections, and mesenteries form a complex interconnecting network. This unifying concept explains the presence of abdominal disease at sites distant from its origin. Knowledge of the various pathways of spread provides a clearer understanding of many disease processes and can help tailor the imaging approach to abdominal and pelvic disease.
References
- 1.Borley NR. In: Gray's anatomy. 40th. Philadelphia: WB Saunders; 2009. Peritoneum and peritoneal cavity; pp. 1347–1501. [Google Scholar]
- 2.Oliphant M, Berne AS, Meyers MA. Subperitoneal spread of intra-abdominal disease. In: Meyers MA, editor. Computed tomography of the gastrointestinal tract. New York: Springer-Verlag; 1986. pp. 95–138. [Google Scholar]
- 3.Oliphant M, Berne AS. Computed tomography of the subperitoneal space: demonstration of direct spread of intraabdominal disease. J Comput Assist Tomogr. 1982;6:1127–37. doi: 10.1097/00004728-198212000-00014. [DOI] [PubMed] [Google Scholar]
- 4.Meyers MA, Oliphant M, Berne AS, et al. The peritoneal ligaments and mesenteries: pathways of intraabdominal spread of disease. Radiology. 1987;163:593–604. doi: 10.1148/radiology.163.3.3575702. [DOI] [PubMed] [Google Scholar]
- 5.Meyers MA. Intraperitoneal spread of infections. In: Meyers MA, editor. Dynamic radiology of the abdomen: normal and pathologic anatomy. 4th. New York: Springer-Verlag; 1993. pp. 55–114. [Google Scholar]
- 6.Gore RM, Meyers MA. Pathways of abdominal and pelvic disease spread. In: Gore RM., Levine MS, editors. Textbook of gastrointestinal radiology. 3rd. Philadelphia: Saunders; 2008. pp. 2099–118. [Google Scholar]
- 7.Oliphant M, Berne AS, Meyers MA. Imaging the direct bidirectional spread of disease between the abdomen and the female pelvis via the subperitoneal space. Gastrointest Radiol. 1988;13:285–98. doi: 10.1007/BF01889084. doi:10.1007/BF01889084. PMid:3049207. [DOI] [PubMed] [Google Scholar]
- 8.Meyers MA. In: Dynamic radiology of the abdomen: normal and pathologic anatomy. 3rd. Meyers MA, editor. New York: Springer-Verlag; 1988. pp. 219–342. [Google Scholar]
- 9.Meyers MA, Mindelzun RE. Peritoneal reflections, ligaments, recesses, and mesenteries. In: Margulis AR, editor. Modern imaging of the alimentary tube. Berlin: Springer-Verlag; 1998. pp. 159–84. [Google Scholar]
- 10.Meyers MA, Evans JA. Effects of pancreatitis on the small bowel and colon: spread along mesenteric planes. Am J Roentgenol Radium Ther Nucl Med. 1973;119:151–65. doi: 10.2214/ajr.119.1.151. [DOI] [PubMed] [Google Scholar]
- 11.Oliphant M, Berne AS, Meyers MA. The subperitoneal space: normal and pathologic anatomy. In: Meyers MA, editor. Dynamic radiology of the abdomen: normal and pathologic anatomy. 4th. New York: Springer-Verlag; 1993. pp. 431–54. [Google Scholar]
- 12.Meyers MA, Volberg F, Katzen B, et al. Haustral anatomy and pathology: a new look. I. Roentgen identification of normal patterns and relationships . Radiology. 1973;108:497–504. doi: 10.1148/108.3.497. [DOI] [PubMed] [Google Scholar]
- 13.Meyers MA, Volberg F, Katzen B, et al. Haustral anatomy and pathology: a new look. II. Roentgen interpretation of pathologic alterations. Radiology. 1973;108:505–12. doi: 10.1148/108.3.505. [DOI] [PubMed] [Google Scholar]
- 14.Okino Y, Kiyosue H, Mori H, et al. Root of the small-bowel mesentery: correlative anatomy and CT features of pathologic conditions. Radiographics. 2001;21:1475–90. doi: 10.1148/radiographics.21.6.g01nv121475. [DOI] [PubMed] [Google Scholar]
- 15.Sompayrac SW, Mindelzun RE, Silverman PM, et al. Greater omentum. AJR Am J Roentgenol. 1997;168:683–8. doi: 10.2214/ajr.168.3.9057515. [DOI] [PubMed] [Google Scholar]
- 16.Sheth S, Horton KM, Garland MR, et al. Mesenteric neoplasms: CT appearances of primary and secondary tumors and differential diagnosis. RadioGraphics. 2003;23:457–73. doi: 10.1148/rg.232025081. [DOI] [PubMed] [Google Scholar]
- 17.Pickhardt PJ, Bhalla S. Primary neoplasms of peritoneal and subperitoneal origin: CT findings. Radiographics. 2005;25:983–95. doi: 10.1148/rg.254045140. doi:10.1148/rg.254045140. PMid:16009819. [DOI] [PubMed] [Google Scholar]
- 18.Balfe DM, Mauro MA, Koehler RE, et al. Gastrohepatic ligament: normal and pathologic CT anatomy. Radiology. 1984;150:485–99. doi: 10.1148/radiology.150.2.6691106. [DOI] [PubMed] [Google Scholar]
- 19.Meyers MA. Dynamic radiology of the abdomen: normal and pathologic anatomy. 4th. New York: Springer-Verlag; 1993. pp. 319–40. [Google Scholar]
- 20.Engels JT, Balfe DM, Lee JKT. Biliary carcinoma: CT evaluation of extrahepatic spread. Radiology. 1989;172:35–40. doi: 10.1148/radiology.172.1.2544924. [DOI] [PubMed] [Google Scholar]
- 21.Mori H, Aikawa H, Hirao K, et al. Exophytic spread of hepatobiliary disease via perihepatic ligaments: demonstration with CT and US. Radiology. 1989;172:41–6. doi: 10.1148/radiology.172.1.2662255. [DOI] [PubMed] [Google Scholar]
- 22.Baker ME, Silverman PM, Halvorsen RA, et al. Computed tomography of masses in periportal hepatoduodenal ligament. J Comput Assist Tomogr. 1987;11:258–63. doi: 10.1097/00004728-198703000-00012. [DOI] [PubMed] [Google Scholar]
- 23.Weil FS, Costa R, Racle A, et al. Ultrasound study of adenopathies within the hepatoduodenal ligament: the “rose-bud” pattern. Gastrointest Radiol. 1986;11:140–2. doi: 10.1007/BF02035056. [DOI] [PubMed] [Google Scholar]
- 24.Zeman RK, Schiebler M, Clark LR, et al. The clinical and imaging spectrum of pancreaticoduodenal lymph node enlargement. AJR Am J Roentgenol. 1985;144:1223–8. doi: 10.2214/ajr.144.6.1223. [DOI] [PubMed] [Google Scholar]
- 25.Meyers MA, Whalen JP. Roentgen significance of the duodenocolic relationships: an anatomic approach. Am J Roentgenol Radium Ther Nucl Med. 1973;117:263–74. doi: 10.2214/ajr.117.2.263. [DOI] [PubMed] [Google Scholar]
- 26.Glick SN, Levine MS, Teplick SJ, et al. Splenic penetration by benign gastric ulcer: preoperative recognition with CT. Radiology. 1987;163:637–9. doi: 10.1148/radiology.163.3.3575707. [DOI] [PubMed] [Google Scholar]
- 27.Pannu HK, Bristow RE, Montz FJ, et al. Multidetector CT of peritoneal carcinomatosis from ovarian cancer. Radiographics. 2003;23:687–701. doi: 10.1148/rg.233025105. doi:10.1148/rg.233025105. PMid:12740470. [DOI] [PubMed] [Google Scholar]