Abstract
Melanotic neuroectodermal tumor of infancy (MNTI) is an uncommon melanin-containing mesenchymal tumor of neural crest origin. What make this tumor unique and interesting is its characteristic predilection for anterior maxilla (premaxilla) and the presence of pigment melanin which gives the tumor distinct clinicopathological, immunohistochemical, ultrastructural and imaging features. Although first described almost a century ago, to the authors’ knowledge, only a few hundred cases of MNTI have been reported worldwide in the English medical literature. The pool of documented radiological findings is even more sparse as not more than a dozen cases could be abstracted from an Internet search of the radiology literature. We document a case of MNTI and describe the imaging findings with intent to contribute to its small but accruing radiological data.
Keywords: Melanotic neuroectodermal tumor of infancy, neuroectoderm, melanin, premaxilla
Case report
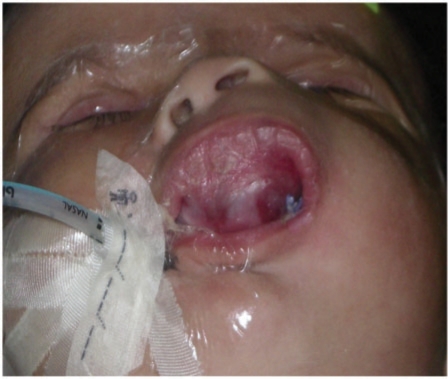
A 10-week-old boy presented with a history of rapidly progressive swelling over the left upper alveolus of a few weeks duration. The past medical and obstetric history was unremarkable and there was no history of trauma. Physical examination revealed a healthy infant with a reddish-bluish, firm, fixed and non-tender orofacial swelling involving the left upper vestibule and anterior hard palate, crossing the midline towards the right side (Fig. 1). The overlying skin and mucosa were normal. No developmental delay or craniofacial dysmorphism was noted. Laboratory tests were normal.
Figure 1.
Orofacial swelling involving the left upper vestibule, alveolar margin and anterior hard palate. Nose is elevated. (Reproduced with parental permission.)
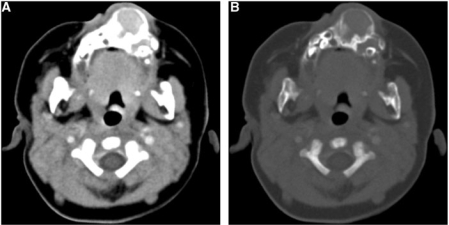
Plain digital radiograph showed an expansile lytic bony lesion in the anterior maxilla region. Computed tomography (CT) and magnetic resonance (MR) scans were done to evaluate the lesion. A CT examination of the face was done on a multi-detector computed tomography (MDCT) scanner (Emotion Duo; Siemens Medical Systems, Erlangen, Germany). Contiguous axial 5-mm thick sections were taken. Contrast-enhanced scans were obtained after manual intravenous injection of 20 ml of nonionic Iomeprol (Iomeron-300, Bracco, Ferentino, Italy) containing 300 mg/ml iodine. The CT scan revealed a bilobular, expansile bone lesion with homogenous soft-tissue density content, involving the anterior maxilla and adjacent hard palate, and displacing the developing dentition (Fig. 2). The bone margins were thin, lobulated and continuous with areas of hyperostosis. No calcification was noted. Moderate homogenous enhancement was seen on contrast study.
Figure 2.
Axial contrast-enhanced CT scan images in (A) soft-tissue window and (B) bone window settings show an expansile, bilobed, well-circumscribed, solid lesion with epicenter in the anterior maxillary alveolar ridge. Bone margins are continuous, thin, lobulated with areas of sclerosis and hyperostosis. Moderate soft-tissue enhancement is present. Note the displaced developing teeth.
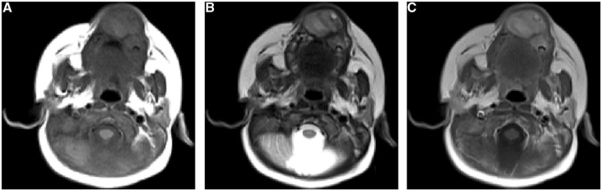
An MR scan of the face was done on a 1.5-Tesla scanner (Avento; Siemens Medical Systems, Erlangen, Germany). T1-weighted (T1W) and T2-weighted (T2W) turbo spin echo sequences were obtained in multiple planes with 3-mm-slice thickness. Contrast-enhanced T1-weighted turbo spin echo scans were obtained after intravenous injection of 0.1 ml/kg of gadobenate dimeglumine (MultiHance, Bracco, Milan, Italy). The MRI scan revealed a bilobular bone lesion with lobulated margins and internal soft-tissue content involving the anterior maxillary alveolar margin and adjacent hard palate. The lesion showed hyperintense signals on T1W images and mildly hyperintense signals on T2W images (Fig. 3). Moderate contrast enhancement was seen on intravenous contrast.
Figure 3.
MR axial (A) T1-weighted, turbo spin echo, 630/17, (B) T2-weighted, turbo spin echo 4400/72, (C) gadolinium-enhanced T1-weighted turbo spin echo images. Images demonstrate a well-defined bilobed, solid, homogenous mass centered at the anterior maxillary ridge. The mass is hyperintense to muscles and tongue on both T1W (A) and T2W (B) images, contrary to the expected findings of melanin pigment. No soft-tissue infiltration is noted. Following administration of contrast, moderate homogenous enhancement is identified (C).
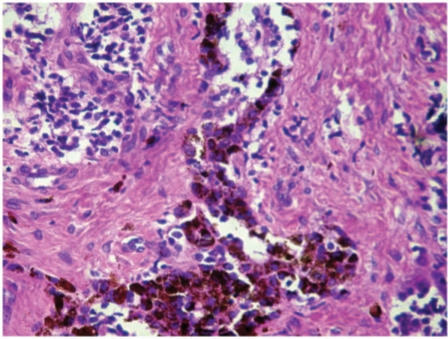
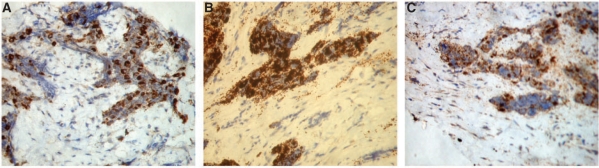
A core needle biopsy revealed small round cells with peripherally arranged larger pigment-containing cells in alveolar distribution lying in fibrous stroma (Fig. 4). A full panel of immunohistochemistry markers were run on the biopsy tissue, which was strongly reactive for cytokeratin (CK), HMB45 and neuron-specific enolase (NSE) and negative for S-100, CD99 and leukocyte common antigen (LCA), confirming the diagnosis of melanotic neuroectodermal tumor of infancy (MNTI) (Fig. 5).
Figure 4.
Histopathological image (magnification 300×; hematoxylin and eosin stain) of the excised mass showing a nest of small cells (small arrow) and larger melanin-containing cells (large arrow) in a fibrous stroma (open arrow).
Figure 5.
Immunohistochemical assay images showing marked uptake of (A) CK, (B) HMB45 and (C) NSE.
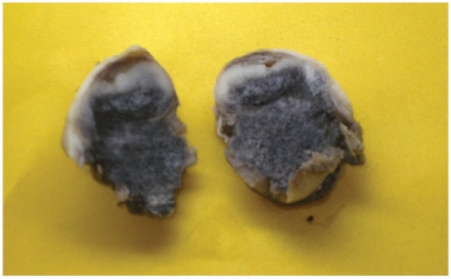
The patient underwent uneventful wide local excision. The excised specimen was a bluish-black oval mass consistent with a pigment-containing tumor (Fig. 6).
Figure 6.
Image of the extirpated specimen showing an oval mass with bluish-black pigmented areas.
Discussion
MNTI was first described by Krompecker in 1918; he called it congenital melanocarcinoma. Since then, there has been uncertainty about its cellular origin. The origin presumed earlier was from odontogenic or retinal rests, and this led to a plethora of names such as retinal anlage tumor, pigmented ameloblastoma, melanotic adamantinoma, retinal choristoma, melanotic epithelial odontoma, melanotic progonoma, pigmented teratoma, atypical melanoblastoma, pigmented epulis and retinoblastic teratoma. However, based on immunohistochemical, ultrastructural, and electron microscope studies and on occasional high urinary excretion of vanillylmandelic acid (VMA)[1], it is now agreed that the tumor originates from the neural crest[2]. Therefore, the tumor is most commonly referred to as a melanotic (melanocytic) neuroectodermal tumor of infancy, a term first coined by Borello and Gorlin in 1966[1].
MTNI is a fast growing but benign tumor. It may be locally aggressive in 15–36% of cases; 3–7% of tumors have an overtly malignant behavior with distant metastases[2–5]. The average recurrence rate after surgery is about 20%[4].
MTNI usually (95%) occurs in children less then 1 year of age peaking between 2 and 6 months, although occasionally it has been described in adults. There is no distinct sex predilection[4]. The tumor usually occurs in the head and neck region because of its origin from the neuroectoderm. Anterior maxilla (premaxilla) is the most common site of origin, occurring in almost 71% of cases[4,6–8]. Rarely, it can arise in the skull, mandible, brain, meninges, transverse sinus, retina, mediastinum, bones, soft tissue, uterus, and epididymis[4,8–13]. In the skull, the anterior fontanelle is the most common site[12]; the epididymis is the most common extracranial site[10].
The classic clinical presentation is a sessile, firm swelling involving the upper anterior alveolar ridge and anterior hard palate. The presence of melanin gives it a bluish hue, often mimicking a vascular malformation. It may result in facial asymmetry, dentition displacement and difficulty in feeding.
On gross examination, the tumor appears dark blue because of its pigment content. There is a suggestion of pseudo-encapsulation due to reactive bone formation. Microscopically, a biphasic pattern of cellular proliferation is characteristic. Two groups of cells are noted with larger pigment-containing melanocyte-like cells arranged in an alveolar pattern between the fibrovascular septae and smaller non-pigmented neuroblast-like cells dispersed in the center of nests. Histopathologic examination is usually unreliable to differentiate benign from malignant changes as cellular atypia and sparse mitosis may be seen even in benign lesions[3,14]. Immunohistochemistry is characteristic, being strongly reactive to cytokeratin, HMB45, synaptophysin, NSE, epithelial membrane antigen, glial fibrillary acidic protein and Leu-7, but negative for S-100 protein[15–19]. Electron microscopy shows ultrastructural findings typical of neural, epithelial and melanocytic cells, such as the presence of intracellular melanosomes in various stages of development, numerous intercellular junctions, neurofibrils and desmosomes[20]. Pathological differential diagnosis would include other small round cell tumors such as neuroblastoma, Ewing sarcoma, rhabdomyosarcoma, lymphoma, primitive neuroectodermal tumor, etc. Some patients may show increased VMA excretion in urine, particularly with aggressive tumors[4].
The plain radiograph of MNTI shows a well-circumscribed, radiolucent, expansile lesion involving the anterior maxilla. Developing dentitions are often displaced. Spiculation with sunburst pattern may be seen[11,12,21,22]. In aggressive tumors, the cortex may be breached in places, mimicking a malignant process.
The CT scan better delineates osseous involvement, which helps in surgical planning[21]. The mass appears as an expansile bone lesion centered at the anterior maxilla, with a thin continuous bone shell and internal soft-tissue density content that enhances on intravenous contrast[11]. Bone expansion, erosion, hyperostosis and spiculations can all be seen in the same lesion[11,21–23]. Calcification is uncommon.
MR imaging findings should follow the paramagnetic effect of its melanin content, that is, the characteristic hyperintense signals on T1W images and hypo- to iso-intense signals on T2W images[12,20,24]. The enhanced T1 relaxation (shortened) of melanin is probably due to chelation of paramagnetic metal ions by melanin. The enhanced (shortened) T2 relaxation is explained by melanin-induced heterogeneity in the magnetic susceptibility of the surrounding environment, resulting in rapid T2 decay[11]. However, this typical MR signal pattern of melanin is only seen in very densely pigmented tumors, and when present can often clinch the diagnosis in an appropriate clinical setting. More often than not and as in our case, the MR signals are unlike a melanin signal, because of the variable proportion of melanin in the predominantly non-melanin content[11,21]. Multiple foci with variable signals representative of melanin, bone and soft tissue can be seen within the same tumor. Melanin signals may be mimicked by a paramagnetic effect of methemoglobin or a highly cellular tumor[12].
The differential diagnosis of MNTI includes: (1) developmental cysts (nasopalatine cyst, globulomaxillary cyst); (2) odontogenic lesions (odontoma, ameloblastoma, ameloblastic fibroma, odontogenic myxoma, adenooameloblastoma, odontogenic keratocyst); (3) non-odontogenic non-neoplastic lesions (central giant cell granuloma, fibrous dysplasia and arteriovenous malformation); and (4) non-odontogenic neoplastic lesions (rhabdomyosarcoma, Burkitt lymphoma, Langerhans cell histiocytosis, Ewing sarcoma). However, this long list can be reduced to just a few based on the clinical and radiological features.
Management is usually by wide surgical excision with at least 5 mm of healthy margins. In the absence of free margins, the recurrence rate is increased by five times, most of which occur within 4 months and local radiotherapy with combination chemotherapy is then recommended[22,25]. Delayed recurrences can be seen[17] which makes close post-operative follow-up essential in all cases.
In summary, MNTI, although rare, has a distinctive clinicopathological and imaging features and it should be considered in the differential diagnosis of all patients presenting with head and neck masses particularly if present in the maxillary region.
References
- 1.Borello ED, Gorlin RJ. Melanotic neuroectodermal tumor of infancy – a neoplasm of neural crest origin. Report of a case associated with high urinary excretion of vanillylmandelic acid. Cancer. 1966;19:196–206. doi: 10.1002/1097-0142(196602)19:2<196::aid-cncr2820190210>3.0.co;2-6. doi:10.1002/1097-0142(196602)19:2<196::AID-CNCR2820190210>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 2.Cutler LS, Chaudhry AP, Topazian R. Melanotic neuroectodermal tumor of infancy: an ultrastructural study, literature review, and reevaluation. Cancer. 1981;48:257–70. doi: 10.1002/1097-0142(19810715)48:2<257::aid-cncr2820480209>3.0.co;2-1. doi:10.1002/1097-0142(19810715)48:2<257::AID-CNCR2820480209>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 3.Fowler DJ, Chisholm J, Roebuck D, Newman L, Malone M, Sebire NJ. Melanotic neuroectodermal tumor of infancy: clinical, radiological, and pathological features. Fetal Pediatr Pathol. 2006;25:59–72. doi: 10.1080/15513810600788715. doi:10.1080/15513810600788715. PMid:16908456. [DOI] [PubMed] [Google Scholar]
- 4.Kruse-Lösler B, Gaertner C, Bürger H, Seper L, Joos U, Kleinheinz J. Melanotic neuroectodermal tumor of infancy: systematic review of the literature and presentation of a case. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;102:204–16. doi: 10.1016/j.tripleo.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 5.Block JC, Waite DE, Dehner LP, Leonard AS, Ogle RG, Gatto DJ. Pigmented neuroectodermal tumor of infancy. An example of rarely expressed malignant behavior. Oral Surg Oral Med Oral Pathol. 1980;49:279–85. doi: 10.1016/0030-4220(80)90133-4. doi:10.1016/0030-4220(80)90133-4. [DOI] [PubMed] [Google Scholar]
- 6.Som MS, Margaret SB. Tumor and tumor like conditions. In: Som PM, Curtin HD, editors. Head and neck imaging. 4th. St Louis, MO: Mosby; 2003. p. 290. [Google Scholar]
- 7.Tan O, Atik B, Ugras S. Melanotic neuroectodermal tumor in a newborn. Int J Pediatr Otorhinolaryngol. 2005;69:1441–4. doi: 10.1016/j.ijporl.2005.04.024. doi:10.1016/j.ijporl.2005.04.024. PMid:15963575. [DOI] [PubMed] [Google Scholar]
- 8.Russell DS, Rubinstein LJ. Pathology of tumours of the nervous system. 5th ed. Baltimore, MD: Williams and Wilkins; 1989. pp. 720–1. [Google Scholar]
- 9.Johnson RE, Scheithauer BW, Dahlin DC. Melanotic neuroectodermal tumor of infancy: a review of seven cases. Cancer. 1983;52:661–6. doi: 10.1002/1097-0142(19830815)52:4<661::aid-cncr2820520416>3.0.co;2-x. doi:10.1002/1097-0142(19830815)52:4<661::AID-CNCR2820520416>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 10.El-Saggan A, Bang G, Olofsson J. Melanotic neuroectodermal tumour of infancy arising in the maxilla. J Laryngol Otol. 1998;112:61–4. doi: 10.1017/s0022215100139908. [DOI] [PubMed] [Google Scholar]
- 11.George JC, Edwards MK, Jakacki RI, Kho-Duffin J. Melanotic neuroectodermal tumor of infancy. AJNR. 1995;16:1273–5. [PMC free article] [PubMed] [Google Scholar]
- 12.Atkinson GO, Davis PC, Patrick LE, Winn KJ, Ball TI, Wyly JB. Melanotic neuroectodermal tumor of infancy: MR findings and review of literature. Pediatr Radiol. 1989;20:20–2. doi: 10.1007/BF02010627. doi:10.1007/BF02010627. PMid:2557574. [DOI] [PubMed] [Google Scholar]
- 13.Calabrese F, Danieli D, Valente M. Melanotic neuroectodermal tumor of the epididymis in infancy: case report and review of the literature. Urology. 1995;46:415–8. doi: 10.1016/S0090-4295(99)80234-9. doi:10.1016/S0090-4295(99)80234-9. [DOI] [PubMed] [Google Scholar]
- 14.Kapadia SB, Frisman DM, Hitchcock CL, Ellis GL, Popek EJ. Melanotic neuroectodermal tumor of infancy. Clinicopathological, immunohistochemical, and flow cytometric study. Am J Surg Pathol. 1993;17:566–73. doi: 10.1097/00000478-199306000-00004. [DOI] [PubMed] [Google Scholar]
- 15.Barrett AW, Morgan M, Ramsay AD, Farthing PM, Newman L, Speight PM. A clinicopathologic and immunohistochemical analysis of melanotic neuroectodermal tumor of infancy. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2002;93:688–98. doi: 10.1067/moe.2002.124000. doi:10.1067/moe.2002.124000. [DOI] [PubMed] [Google Scholar]
- 16.Pettinato G, Manivel JC, d'Amore ES, Jaszez W, Gorlin RJ. Melanotic neuroectodermal tumor of infancy. A reexamination of a histogenetic problem based on immunohistochemical, flow cytometric, and ultrastructural study of 10 cases. Am J Surg Pathol. 1991;15:233–45. [PubMed] [Google Scholar]
- 17.Argenyi ZB, Schelper RL, Balogh K. Pigmented neuroectodermal tumor of infancy. A light microscopic and immunohistochemical study. J Cutan Pathol. 1991;18:40–5. doi: 10.1111/j.1600-0560.1991.tb00600.x. doi:10.1111/j.1600-0560.1991.tb00600.x. PMid:1708790. [DOI] [PubMed] [Google Scholar]
- 18.Raju U, Zarbo R, Regezi J, Krutchkoff D, Perrin E. Melanotic neuroectodermal tumors of infancy. Intermediate filament-, neuroendocrine-, and melanoma-associated antigen profiles. Appl Immunohistochem. 1993;1:69–76. [Google Scholar]
- 19.Stirling RW, Powell G, Fletcher CD. Pigmented neuroectodermal tumour of infancy. An immunohistochemical study. Histopathology. 1988;12:425–35. doi: 10.1111/j.1365-2559.1988.tb01957.x. doi:10.1111/j.1365-2559.1988.tb01957.x. PMid:2453438. [DOI] [PubMed] [Google Scholar]
- 20.Dehner LP, Sibley RK, Sauk JJ, Jr, et al. Malignant melanotic neuroectodermal tumor of infancy: a clinical, pathologic, ultrastructural and tissue culture study. Cancer. 1979;43:1389–410. doi: 10.1002/1097-0142(197904)43:4<1389::aid-cncr2820430429>3.0.co;2-v. doi:10.1002/1097-0142(197904)43:4<1389::AID-CNCR2820430429>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 21.Mirich DR, Blaser SI, Harwood-Nash DC, Armstrong DC, Becker LE, Posnick JC. Melanotic neuroectodrmal tumor of infancy: clinical, radiologic, and pathologic findings in five cases. AJNR. 1991;12:689–97. [PMC free article] [PubMed] [Google Scholar]
- 22.Neven J, Hulsbergen-van, der Kaa C, Groot-Loonen J, de Wilde PCM, Merkx MAW. Recurrent melanotic neuroectodermal tumor of infancy: a proposal for treatment protocol with surgery and adjuvant chemotherapy. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008;106:493–6. doi: 10.1016/j.tripleo.2008.02.001. doi:10.1016/j.tripleo.2008.02.001. PMid:18602297. [DOI] [PubMed] [Google Scholar]
- 23.Suzuki C, Maeda M, Matsushima N, Takamura M, Matsubara T, Taki W. Melanotic neuroectodermal tumor of infancy in the skull: CT and MRI features. J Neuroradiol. 2007;34:212–15. doi: 10.1016/j.neurad.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 24.Mosby EL, Lowe MW, Cobb CM, Ennis RL. Melanotic neuroectodermal tumor of infancy: review of the literature and report of a case. J Oral Maxillofac Surg. 1992;50:886–94. doi: 10.1016/0278-2391(92)90285-8. doi:10.1016/0278-2391(92)90285-8. [DOI] [PubMed] [Google Scholar]
- 25.Woessmann W, Neugebauer M, Gossen R, Blütters-Sawatzki R, Reiter A. Successful chemotherapy for melanotic neuroectodermal tumor of infancy in a baby. Med Pediatr Oncol. 2003;40:198–9. doi: 10.1002/mpo.10135. doi:10.1002/mpo.10135. PMid:12518354. [DOI] [PubMed] [Google Scholar]