Abstract
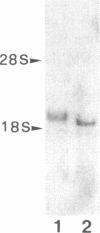
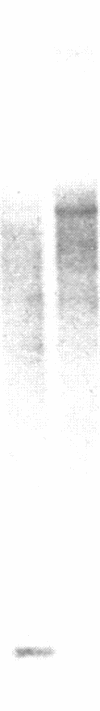
A cDNA expression library in lambda gt11 was screened with affinity-purified polyclonal anti-rat cytochrome b5 reductase antibodies. One positive clone out of 450,000 clones was isolated and found to be incomplete. This clone was used to rescreen the library, and a second, overlapping clone that contained the entire coding sequence was isolated. RNA gel blots showed that the two overlapping clones contained approximately 90% of the reductase mRNA sequence. Sequencing data showed (i) that rat reductase has a 93% sequence similarity with bovine and human reductase and (ii) that reductase is not synthesized as a high molecular weight precursor. Results of Southern blot analysis were consistent with the hypothesis that a single gene codes for the soluble and membrane-bound (microsomal and mitochondrial) forms of the reductase, present in erythrocytes and liver, respectively. The cloned cDNA was used to study reductase transcripts in liver and reticulocytes. Two antisense RNA probes that together covered the entire coding region and part of the noncoding region of reductase mRNA were used in RNase A protection experiments. These probes detected only one transcript in liver, suggesting that endoplasmic reticulum and mitochondrial reductase are translated from the same mRNA. In contrast, two transcripts were detected in reticulocytes, one of which mismatched the liver probe approximately 30 nucleotides downstream from the initiation codon. Since the soluble and membrane form of the reductase are known to differ at the N terminus, we suggest that this second transcript encodes soluble reductase.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beutler E., West C., Blume K. G. The removal of leukocytes and platelets from whole blood. J Lab Clin Med. 1976 Aug;88(2):328–333. [PubMed] [Google Scholar]
- Borgese N., Gaetani S. In vitro synthesis and post-translational insertion into microsomes of the integral membrane protein, NADH-cytochrome b5 oxidoreductase. EMBO J. 1983;2(8):1263–1269. doi: 10.1002/j.1460-2075.1983.tb01579.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgese N., Gaetani S. Site of synthesis of rat liver NADH--cytochrome b5 reductase, an integral membrane protein. FEBS Lett. 1980 Apr 7;112(2):216–220. doi: 10.1016/0014-5793(80)80183-9. [DOI] [PubMed] [Google Scholar]
- Borgese N., Macconi D., Parola L., Pietrini G. Rat erythrocyte NADH-cytochrome b5 reductase. Quantitation and comparison between the membrane-bound and soluble forms using an antibody against the rat liver enzyme. J Biol Chem. 1982 Nov 25;257(22):13854–13861. [PubMed] [Google Scholar]
- Borgese N., Pietrini G. Distribution of the integral membrane protein NADH-cytochrome b5 reductase in rat liver cells, studied with a quantitative radioimmunoblotting assay. Biochem J. 1986 Oct 15;239(2):393–403. doi: 10.1042/bj2390393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgese N., Pietrini G., Gaetani S. Concentration of NADH-cytochrome b5 reductase in erythrocytes of normal and methemoglobinemic individuals measured with a quantitative radioimmunoblotting assay. J Clin Invest. 1987 Nov;80(5):1296–1302. doi: 10.1172/JCI113205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgese N., Pietrini G., Meldolesi J. Localization and biosynthesis of NADH-cytochrome b5 reductase, an iontegral membrane protein, in rat liver cells. III. Evidence for the independent insertion and turnover the enzyme in various subcellular compartments. J Cell Biol. 1980 Jul;86(1):38–45. doi: 10.1083/jcb.86.1.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Green M. R., Maniatis T., Melton D. A. Human beta-globin pre-mRNA synthesized in vitro is accurately spliced in Xenopus oocyte nuclei. Cell. 1983 Mar;32(3):681–694. doi: 10.1016/0092-8674(83)90054-5. [DOI] [PubMed] [Google Scholar]
- Hultquist D. E., Slaughter S. R., Douglas R. H., Sannes L. J., Sahagian G. G. Erythrocyte cytochrome b5; structure, role in methemoglobin reduction, and solubilization from endoplasmic reticulum. Prog Clin Biol Res. 1978;21:199–216. [PubMed] [Google Scholar]
- Kozak M. Comparison of initiation of protein synthesis in procaryotes, eucaryotes, and organelles. Microbiol Rev. 1983 Mar;47(1):1–45. doi: 10.1128/mr.47.1.1-45.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lederer F., Ghrir R., Guiard B., Cortial S., Ito A. Two homologous cytochromes b5 in a single cell. Eur J Biochem. 1983 Apr 15;132(1):95–102. doi: 10.1111/j.1432-1033.1983.tb07330.x. [DOI] [PubMed] [Google Scholar]
- Leroux A., Junien C., Kaplan J., Bamberger J. Generalised deficiency of cytochrome b5 reductase in congenital methaemoglobinaemia with mental retardation. Nature. 1975 Dec 18;258(5536):619–620. doi: 10.1038/258619a0. [DOI] [PubMed] [Google Scholar]
- Meldolesi J., Corte G., Pietrini G., Borgese N. Localization and biosynthesis of NADH-cytochrome b5 reductase, an integral membrane protein, in rat liver cells. II. Evidence that a single enzyme accounts for the activity in its various subcellular locations. J Cell Biol. 1980 Jun;85(3):516–526. doi: 10.1083/jcb.85.3.516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueckler M. M., Pitot H. C. Sequence of the precursor to rat ornithine aminotransferase deduced from a cDNA clone. J Biol Chem. 1985 Oct 25;260(24):12993–12997. [PubMed] [Google Scholar]
- Okada Y., Frey A. B., Guenthner T. M., Oesch F., Sabatini D. D., Kreibich G. Studies on the biosynthesis of microsomal membrane proteins. Site of synthesis and mode of insertion of cytochrome b5, cytochrome b5 reductase, cytochrome P-450 reductase and epoxide hydrolase. Eur J Biochem. 1982 Feb;122(2):393–402. doi: 10.1111/j.1432-1033.1982.tb05894.x. [DOI] [PubMed] [Google Scholar]
- Ozols J., Korza G., Heinemann F. S., Hediger M. A., Strittmatter P. Complete amino acid sequence of steer liver microsomal NADH-cytochrome b5 reductase. J Biol Chem. 1985 Oct 5;260(22):11953–11961. [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slaughter S. R., Hultquist D. E. Membrane-bound redox proteins of the murine Friend virus-induced erythroleukemia cell. J Cell Biol. 1979 Oct;83(1):231–239. doi: 10.1083/jcb.83.1.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sottocasa G. L., Kuylenstierna B., Ernster L., Bergstrand A. An electron-transport system associated with the outer membrane of liver mitochondria. A biochemical and morphological study. J Cell Biol. 1967 Feb;32(2):415–438. doi: 10.1083/jcb.32.2.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura M., Yubisui T., Takeshita M., Kawabata S., Miyata T., Iwanaga S. Structural comparison of bovine erythrocyte, brain, and liver NADH-cytochrome b5 reductase by HPLC mapping. J Biochem. 1987 May;101(5):1147–1159. doi: 10.1093/oxfordjournals.jbchem.a121979. [DOI] [PubMed] [Google Scholar]
- Werner D., Chemla Y., Herzberg M. Isolation of poly(A)+ RNA by paper affinity chromatography. Anal Biochem. 1984 Sep;141(2):329–336. doi: 10.1016/0003-2697(84)90050-2. [DOI] [PubMed] [Google Scholar]
- Yubisui T., Miyata T., Iwanaga S., Tamura M., Takeshita M. Complete amino acid sequence of NADH-cytochrome b5 reductase purified from human erythrocytes. J Biochem. 1986 Feb;99(2):407–422. doi: 10.1093/oxfordjournals.jbchem.a135495. [DOI] [PubMed] [Google Scholar]
- Yubisui T., Naitoh Y., Zenno S., Tamura M., Takeshita M., Sakaki Y. Molecular cloning of cDNAs of human liver and placenta NADH-cytochrome b5 reductase. Proc Natl Acad Sci U S A. 1987 Jun;84(11):3609–3613. doi: 10.1073/pnas.84.11.3609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinn K., DiMaio D., Maniatis T. Identification of two distinct regulatory regions adjacent to the human beta-interferon gene. Cell. 1983 Oct;34(3):865–879. doi: 10.1016/0092-8674(83)90544-5. [DOI] [PubMed] [Google Scholar]