Abstract
Aims
We investigated the feasibility of real-time magnetic resonance imaging (RTMRI) guided ablation of the cavotricuspid isthmus (CTI) by using a MRI-compatible ablation catheter.
Methods and results
Cavotricuspid isthmus ablation was performed in an interventional RTMRI suite by using a novel 7 French, steerable, non-ferromagnetic ablation catheter in a porcine in vivo model (n = 20). The catheter was introduced and navigated by RTMRI visualization only. Catheter position and movement during manipulation were continuously visualized during the entire intervention. Two porcine prematurely died due to VT/VF. Anatomical completion of the CTI ablation line could be achieved after a mean of 6.3±3 RF pulses (RF energy: 1807±1016.4 Ws/RF pulse, temperature: 55.9±5.9°C) in n = 18 animals. In 15 of 18 procedures (83.3%) a complete CTI block was proven by conventional mapping in the electrophysiological (EP) lab.
Conclusion
Completely non-fluoroscopic ablation guided by RTMRI using a steerable and non-ferromagnetic catheter is a promising novel technology in interventional electrophysiology.
Keywords: Interventional magnetic resonance imaging, Atrial flutter, Catheter ablation, Non-ferromagnetic ablation catheter, Interactive real time
Introduction
Over the last two decades, RF ablation mainly guided by conventional fluoroscopy has become a first-line therapy for many cardiac arrhythmias,1 e.g. atrioventricular nodal re-entrant tachycardia, atrioventricular re-entrant tachycardia, and atrial flutter (AFL). Ablation is currently guided by conventional fluoroscopy, with the disadvantage of limited imaging capabilities of the heart and adjacent structures, poor tissue contrast, lack of three-dimensional projections, spatial information, and the involvement of ionizing radiation.1–4
Magnetic resonance imaging (MRI) provides tissue imaging with unrivalled contrast, in any user-defined plane, without exposure of patient and investigator to ionizing radiation. Magnetic resonance imaging has been proposed as an alternative imaging modality for guiding and monitoring electrophysiological (EP) procedures.5,6 In order to overcome limitations of fluoroscopy, recent technical advances permit real-time MRI (RTMRI) to guide catheter-based procedures.7 Despite the obvious advantages, interventional MRI raises several problems. One of them is the lack of MRI-compatible ablation catheters with comparable characteristics as conventional ablation catheters.
Thus, we investigated a novel, steerable, non-ferromagnetic ablation catheter with a 7-French diameter and evaluated the feasibility, safety, and efficacy of catheter ablation in interactive RTMRI for cavotricuspid isthmus (CTI) ablation in a porcine animal model.
Methods
Magnetic resonance imaging compatible ablation catheter
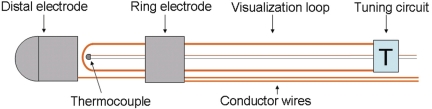
We developed a novel ablation catheter with a 7-French diameter and a shaft length of 110 cm. The catheter has a deflectable tip at the distal end and a steering handle at the proximal end. The handle includes an electrical connector that links the electrical wires to both ablation electrodes and to the thermocouple wire. The round-shaped, 4 mm distal electrode is made of a platinum–iridium alloy. The temperature of the ablation electrodes is monitored through distally fixed thermocouple wires, to permit control over the ablation temperatures as well as control over any passive heating of the catheter tip during MRI. For improved visualization, a passive coil is integrated in the distal catheter, isolated from the electrical conductors yielding hyperintensity in RTMRI. The coil is based on a conductor wire loop which is tuned with a circuit to resonance frequency of the MR tomography (63.8 MHz). The loop-tuning circuit is embedded between the distal electrode and shaft (Figure 1). All catheter materials were non-ferromagnetic and bio-compatible to reduce susceptibility artefacts, critical temperature rises, and interaction with blood and tissue.
Figure 1.
Schematic of the distal catheter. A distally fixed Cu/Ni-thermocouple monitors the ablation temperature. The loop-tuning circuit is embedded between the distal and proximal shaft. The loop is isolated from the electrical conductor wires and tuned with a circuit to magnetic resonance imaging resonance frequency.
The ablation catheter was connected to a standard RF ablation generator (Stockert EP Shuttletm, Cordis Webster, Freiburg, Germany), which was placed outside the MR scanner room. To reject noise from the generator entering into the scanner room, the signal was filtered by a custom-made, hermetically shielded low-pass filter (Butterworth characteristic, seventh degree, cut-off frequency 750 kHz).
Magnetic resonance imaging in the interventional suite
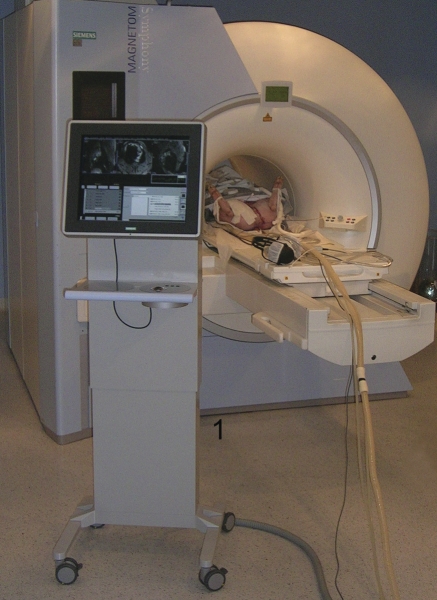
The RTMRI-guided CTI ablation was performed in a clinical interventional MRI suite (1.5 T, Symphony™, Siemens Medical Solutions, Erlangen, Germany) (Figure 2). The images were displayed on an in-room monitor console (IMC, Siemens) for continuous imaging control of the movement and positioning of the catheter. Eight-channel phased-array body surface coils were used and ECG electrodes were connected for ECG gating during MR tomography.
Figure 2.
Interventional magnetic resonance imaging suite with 1.5 T scanner and in-room monitor console (1).
Balanced steady-state free precession (SSFP) pulse sequence with a variable cartesian k-space filling scheme was used for interactive real-time imaging for basic catheter manipulation. Radial sampling strategy was applied for more detailed imaging of the catheter tip. Typical parameters were a repetition time (TR) of 4.3 ms, echo time (TE) of 2.0 ms, a 50° flip angle, a bandwidth of 558 Hz/pixel, field of view (FOV) 300 × 300 mm, matrix 128 × 128, and slice thickness 6.0 mm, generating 2.3 × 2.3 × 8.0 mm3 voxels with a frame rate of five to six images per second.
For verification of final catheter positions, an additional cine SSFP pulse sequence technique was applied. During breath hold by apnoea ventilation setting for a minimum of 10 s, a retrospectively ECG-gated sequence was used to acquire two-dimensional images in three orthogonal multiplanar orientations of the catheter tip before ablation. For this purpose, the following parameters were used: TR 45.8 ms, TE 1.4 ms, flip angle 45°, bandwidth 965 Hz/pixel, FOV 244 × 300 mm, matrix 156 × 192, slice thickness 6.0 mm, resulting in a reconstructed voxel size of 1.6 × 1.6 × 6.0 mm3, allowing us to reconstruct cine imaging with up to 20 frames per second.
To detect ablation lesion focal oedema after ablation, an additional T2-weighted turbo spin echo (TSE) pulse sequence was applied before and after ablation at the cavotricuspid. This single-slice imaging was performed during breath hold with prospective ECG gating with the following parameters: TR 1000 ms, TE 66 ms, bandwidth 235 Hz/pixel, FOV 228 × 280 mm, matrix 208 × 256, and slice thickness 5.0 mm, giving a reconstructed voxel size of 1.4 × 1.1 × 5.0 mm3.
In vitro evaluation of magnetic resonance imaging-related heating of the catheter
The non-ferromagnetic ablation catheter, equipped with a distal coil for active visualization, was evaluated in torso phantoms filled with (i) 45 L of PAA gel (10 g/L polyacrylic acid, 2.5 g/L NaCl) and (ii) highly viscous HEC gel (30 g/L hydroxyl ethyl cellulose, 1.5 g/L NaCl), with the catheter positioned in the isocentre of the gantry and phantom, as well as gradually off-centre in 4 cm steps. The steerable distal catheter ending was also tested in different configurations (straight, bending of 90°). Additional measurements were obtained inside a flow model of a 10 mm tube, filled with PAA gel, at 0.5, 0.1, and 0.05 L/min. All testing was performed using the interventional MRI suite. The catheter was connected to the RF ablation generator. Magnetic resonance imaging was conducted using the body coil with a modified SSFP sequence for applying a standardized energy of a whole-body-averaged SAR of 4.0 W/kg. A fibre optic thermometry system (FOTEMP4, 4-Channel-Thermometer, OPTOcon, Dresden, Germany) was used to record temperatures at eight catheter locations before and during 15 min of MRI.
Animal experiments
Twenty healthy juvenile pigs (German landrace) with a mean age of 99 ± 29 days and a bodyweight of 29.5–77.0 kg (mean 43.5 ± 14.5 kg) were held during a day-and-night cycle of 12 h for at least 7 days before the interventional procedure.
General anaesthesia was induced with an intramuscular injection of ketamin and azaperon and maintained with 0.6 mg/kg bodyweight propofol continuously and 30–50 µg/kg bodyweight fentanyl every 30 min. After orotracheal intubation, ventilation was performed by a compact transport ventilator (Oxylog 2000, Draeger Inc., Luebeck, Germany). Heart frequency and oxygen saturation (SpO2) were monitored. The animal protocol was approved by the local institutional animal care and use committee.
A 8-French introducer sheath (Maximum™, Daig Inc., Minnetonka, MN, USA) and a 8-French long introducer sheath (Fast-Cath SR0™, Daig Inc., Minnetonka, MN, USA; modified by deletion of the distal ring marker) were inserted in the right iliac vein. Heparin was administered intravenously and supplemented during the procedure. The animals were placed head first in a supine position on the scanner table, with the legs and the ventilation tube secured by a gauze. The ablation catheter was passed through the introducer sheath and guided through the right atrium, which was constantly visualized by RTMRI. While the interventionalist performed the catheter placement into the right atrium, another in-room operator adjusted the imaging planes to follow the course of the catheter through the venous system and the right atrium.
Cavotricuspid isthmus ablation
After MRI ensured proper positioning of the ablation catheter at the tricuspid valve annulus, RF current was applied between the distal electrode of the catheter and an adhesive patch on the back of the animal as an external neutral electrode. The ablation along the CTI required several energy applications during a stepwise catheter pullback (‘point-by-point’ ablation), starting at the ventricular aspect of the CTI and ending at the caval insertion. The target temperature for RF application was set to 65°C and the power limit to 30 W, with each application lasting 60 s. Throughout each energy application, continuous RTMRI was used to verify the ablation catheter in appropriate contact with the CTI. The primary endpoint was a continuous ablation line between the inferior aspect of the tricuspid annulus and the insertion of the inferior caval vein (ICV) represented as a signal-enhanced line in the T2-weighted TSE sequence. The ablation procedure was performed by an experienced electrophysiologist (B.A.H.) under guidance of an interventional radiologist (A.K.).
Electrophysiological study in the electrophysiological laboratory
After RTMRI-guided ablation, the animals were brought to the EP laboratory. A 6-French quadripolar catheter (XTrem™, ELA Medical, Montrouge, France) was placed in the coronary sinus (CS) under conventional fluoroscopical guidance. Intracardiac electrograms were filtered in a range from 30 to 250 Hz and stored using a computer-based recording system (LabSystem™ Pro, BARD Electrophysiology, Murray Hill, NJ, USA). The EP endpoint was defined as bidirectional block of the CTI. Verification of conduction block was performed by pacing from the proximal CS. The mapping catheter was placed septally to the line of block, confirming a short delay from the pacing site. Subsequently, the catheter was placed on the ablated CTI, documenting a long delay from the pacing site. By moving the mapping catheter lateral to the ablation line, the delay shortens, consistent with the cranio-caudal activation of the lateral right atrium and the unidirectional (septal to lateral) block of the CTI. The bidirectional block was confirmed by pacing from the mapping catheter at the line and more laterally. The EP endpoint was compared with the final macroscopic and microscopic anatomical results.
Macroscopical examination
After the experiments, the animals were sacrificed with a lethal bolus injection of 80 mg/kg bodyweight of embutramid/mebezonium/tetracaine (T61™, Intervet, Wiesbaden, Germany). Through a mid-sternal thoracotomy, incidental pericardial and mediastinal injury by virtue of the ablation were validated. Heart and lung were excised and examined macroscopically. The right atrium was dissected and opened at the lateral wall, between the superior and the ICV to evaluate the ablation lesions regarding size and anatomical position at the CTI. Transmurality and lesion size were evaluated by an experienced pathologist (S.K.) macroscopically and microscopically (5 µm paraffin-embedded slices, haematoxylin/eosin stain).
Statistical analysis
Statistical analysis was performed using custom-designed software (SigmaStat, Version 3.5, Systat Inc., Chicago, IL, USA). Continuous parameters were described as mean ± SD. The difference between procedure times was tested with a Mann–Whitney rank-sum test. The differences were considered significant by error probability P < 0.05.
Results
In vitro evaluation of magnetic resonance imaging-related heating of the catheter
Among all sites of temperature measurement, the highest temperature increases were measured in a straight catheter, at the isocentre and +4, +8, and +12 cm off-centre positions (0.7, 1.6, 4.5, 8.9°C for the PAA and 0.2, 6.4, 11.4, and 15.2°C for HEC gel). With the catheter in isocentre position and the distal portion bending 90° (consecutively up to 8 cm off-centred tip), a maximal temperature increase of 1.8°C was found, compared with 2.4°C of an isocentred tip with similar bending and a maximal 8 cm off-centred shaft portion. All measurements in the flow model showed a maximum temperature increase of no more than 0.2°C.
Temperature control during real-time magnetic resonance imaging
Continuous temperature control throughout the procedure did not show any MRI-related heating of more than 1.0°C at the distal electrode, recorded by the integrated thermocouple. During all stages of the experiment in the interventional MRI suite, except while ablation was performed, the integrated thermometer did not exceed 38.1°C. The thermocouple probe itself was validated in a phantom study with a fibre-optic thermometer (FOTEMP4).
Catheter ablation during real-time magnetic resonance imaging
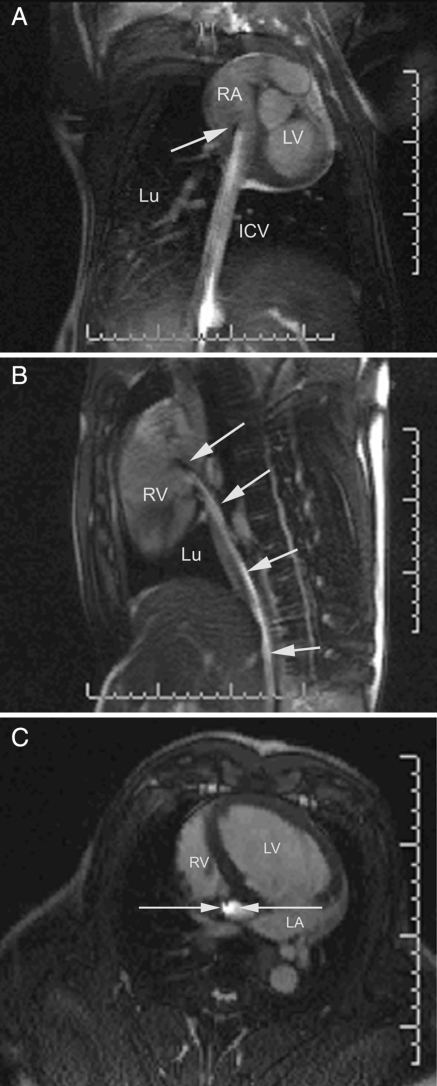
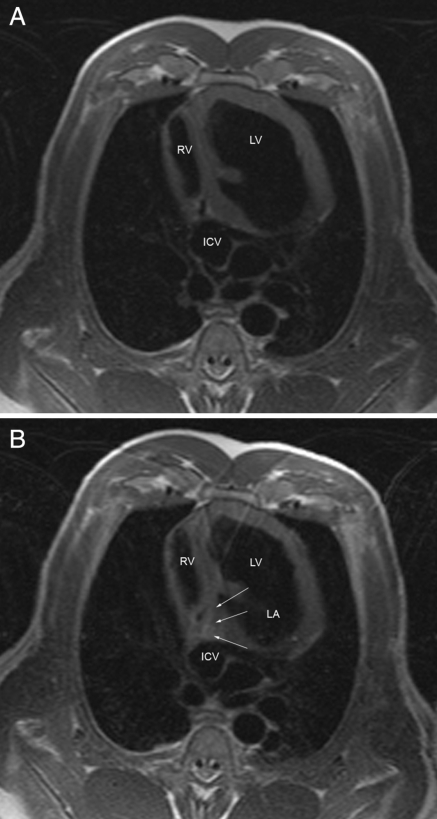
The ablation procedure duration within the MRI was 154.8 ± 24.2 min. There was a significant difference between the first and the last 10 ablation procedures (174.0 ± 17.6 vs. 135.7 ± 10.7 min, P < 0.001). The ablation catheter was safely visualized throughout the steering manoeuvre in the interventional procedure, yielding hyperintensity at its distal portion owing to the integrated visualization coil (Figure 3A–C). CTI and adjacent cardiac structures were reliably identified by MRI in all cases. The catheter was successfully placed in the inferior region of the tricuspid valve annulus in all animals. Continuous real-time imaging during each 60 s period of RF ablation allowed constant monitoring of the actual ablation electrode position. An anatomically oriented lesion line (T2-weighted TSE sequence) was achieved after a mean number of 6.3 ± 3 RF pulses (energy: 1807 ± 1016.4 Ws/RF pulse, temperature: 55.9 ± 5.9°C, impedance: 106.9 ± 13.3 Ohm). The TSE sequences for the depiction of focal oedema at the target regions did show a relative swelling and focal hyperintensity; however, not every single focal ablation site could be visualized in detail (Figure 4A and B).
Figure 3.
(A) Coronal, (B) sagittal and (C) transversal magnetic resonance imaging images (steady-state free precession sequence) of the distal ablation catheter yielding a hyperintense signal in its distal portion. The catheter tip is close to the region of cavotricuspid isthmus (RV, right ventricle; LA, left atrium; LV, left ventricle; ICV, inferior caval vein; Lu, lung; arrow, ablation catheter).
Figure 4.
(A) Pre- and (B) post-ablational T2-weighted turbo spin echo sequence showing focal hyperintensity (arrows) in the CTI region (RV, right ventricle; LA, left atrium; LV, left ventricle; ICV, inferior caval vein).
During the entire MRI-guided ablation, no cardiac perforation, pericardial effusion or tamponade was observed.
Animal studies
Ventricular tachycardia or ventricular fibrillation occurred in two pigs (10%) during RF current delivery in the MRI suite. Immediate direct current cardioversion/defibrillation failed in both cases. In these animals, the autopsy found no ablation lesions in the right ventricle. In all other animals (n = 18), no further complications, e.g. pericardial effusion or tamponade, occurred during or after the interventional MRI procedure.
Primary endpoint
An anatomically oriented RTMRI ablation of the CTI was successful in 18 animals. All animals were transferred to the EP laboratory where a complete isthmus block was confirmed in 15 animals (83.3%). No complications occurred during the additional EP mapping.
Macroscopical examination
Gross examination during autopsy revealed no signs of myocardial perforation, pericardial effusion, or mediastinal damage. The dissection of the heart found no thrombus formation or valve damage. The macroscopic examination consistently confirmed positioning of the ablation line in all 18 animals within the inferior cavotricuspid isthmus. Transmural lesions at the CTI appeared epicardially as white-red areas of varying sizes with an irregular outline. In 50.7%, we found a haemorrhagic border. The mean length of the inferior cavotricuspid isthmus lesion set was 20.2 ± 7.5 mm. The mean length of a single lesion was 4.2 ± 2.4 mm, with a mean width of 3.3 ± 1.7 mm and a maximum thickness at the lesion site of 5.3 ± 0.6 mm. A complete ablation line without lesion gaps was found in 16 of the 18 surviving animals (88.9%). Contiguous transmural lesions were demonstrated in 15 animals (83.3%). In all animals with incomplete block, histological non-transmural lesions and gaps with intact atrial myocardium were seen.
Discussion
In the present study, we developed and evaluated an interventional MRI EP procedure with real-time multiplanar imaging and tissue visualization. To the best of our knowledge, we demonstrated for the first time CTI ablation. Ablation was exclusively supported by interactive RTMRI using a novel, non-ferromagnetic ablation catheter with a thin outer diameter and excellent steering capabilities. After a short learning curve, the ablation procedure was performed within a moderate time. We furthermore demonstrated that RTMRI permits an anatomically guided ablation of the cavotricuspid isthmus exclusively based on this non-fluoroscopic imaging modality.
Device safety
Substantial passive heating of the EP catheter can occur in the MR environment in a clinically unlikely worst-case scenario. As the catheter has so far been tested for right atrial ablation with an inferior transcaval introduction, no tip position of >4–8 cm off-centre position to the isocentre of the gantry is necessary. In consideration of blood flow, no critical temperature increase could be simulated in the tested in vitro flow model.
Cavotricuspid isthmus ablation using real-time magnetic resonance imaging
The concept of CTI ablation is an example of an EP procedure with a well-defined substrate. Anatomically controlled ablation by virtue of superior visualization of complex anatomy with simultaneous device and soft tissue imaging by RTMRI may be an optimal non-fluoroscopical tool in this setting. With its excellent tissue contrast and the possibility of multiplanar orientation, RTMRI offers the advantage of catheter guidance as well as monitoring of adjacent structures during the entire ablation procedure with the potential to eliminate ionizing radiation. One of the important potential advantages of RTMRI might be the recognition and prevention of potential complications during interventional EP procedures. However, it was not aim of our study to show reduction of procedural complications.
In our experimental study setting, ablation could be reliably performed with continuous online visualization of both, tracking of the non-ferromagnetic ablation catheter and the depiction of adjacent anatomical structures. Although the applied real-time SSFP imaging technique resulted in a spatial and temporal resolution significantly lower than the conventional cine SSFP sequence, a high accuracy in lesion application was demonstrated. Only 5.3% of the ablation lesions were found more than 10 mm deviant to the CTI line. A decrease in spatial resolution in the real-time imaging planes (2.3 × 2.3 mm vs. 1.6 × 1.6 mm in the regular SSFP sequence) can be better tolerated than a further decrease in the imaging frame rate (about 6 frames per second vs. 20 frames per second), which would result in the temporarily loss of perceptibility of the moving ablation catheter during the cardiac cycle.
Furthermore, the desired procedural endpoint of the bidirectional block was reached in more than 80% of cases without concomitant complications typically associated with CTI ablation in humans, thus underscoring the promising expectations of RTMRI-guided ablation in terms of efficacy and safety issues.
Previous studies
In two previous studies,5,8 the feasibility of ablation within the MRI has been demonstrated. However, no specific potentially arrhythmogenic substrate was targeted. Further limitations in the previous studies were the large diameter and non-steerability of the used catheter. Additionally, a suitable clinically established RTMRI environment combining in-room monitor control and interactive multiplanar imaging was not available at this time. A recently published paper of Nazarian et al.7 has shown the feasibility of diagnostic EP procedures in RTMRI. A restriction of all previous studies was the lack of ablation catheters with an appropriate diameter and steering capabilities, which enabled exact ablation of a small arrhythmogenic target in the current study.
Study complications
Two animals died due to ventricular tachycardia and fibrillation whereas the exact mechanism is unclear. As Anfinsen et al.9 described, porcines are generally very prone to ventricular arrhythmia during RF current delivery. Induction of ventricular arrhythmia can occur even though the ablation was performed in the atrium (Supplementary material online, Video 2). We did not observe any arrhythmic events during MRI scanning alone. This observation makes a magnetic field induction of current into the catheter rather unlikely. In general the induction of ventricular arrhythmia in human EP studies is a very rare complication. However, no complications rarely associated with CTI ablation in humans have been observed.
Study limitations
Intracardiac electrogram
The strong magnetic field during MRI scanning interferes with the surface ECG signal as well as with the intracardiac electrogram. The adequate recording of intracardiac electrograms during the MRI scan requires an extensive hardware setting consisting of passive filter coils and digital filter algorithms that were not implemented in our filter box.
Lesion mapping
Pre- and post-ablation imaging at the target region of anatomical ablation did demonstrate that the substrate of CTI ablation can be depicted in MRI by focal oedema (Figure 4B). However, the lesions in the TSE imaging were not regularly detectable in all cases and hyperintensity was often weak, which can be explained by (i) the high motion at the tricuspid annulus in swine, (ii) the thin myocardial wall thickness, resulting in significant artefacts during MRI sequences with a long TE, and (iii) the relatively low resolution in 1.5 T MRI scanner. Lesion mapping was published recently in different publications.10,11 Most of the publications focus on lesion mapping in relatively thick myocardium, e.g. in the right ventricle, whereas the precise evaluation of lesions in the atrium is rather difficult. Peters et al.11 showed that the evaluation of atrial scar tissue after RFC ablation is feasible. However, this group failed to show a precise evaluation of the completeness of the ablation lines. Only 62% of all patients demonstrated an at least 90% circumferential late enhancement. Our own experience is that the resolution with a 1.5 T MRI scanner is too low to adequately evaluate the lesion size or completeness of lines in the atrium.
Human translation
The human translation of our results will require further safety testing of the used ablation catheter. Especially, a non-anatomical EP ablation approach would require the insertion of multiple MRI-compatible diagnostic and ablation catheters. This can result in a more increased heating than evaluated in our single-catheter setting.
Future perspective and clinical implications
Three-dimensional mapping and catheter tracking
Combined three-dimensional mapping and MR-based catheter tracking in EP procedures might be a feasible modality. Initial work on MRI-guided cardiac EP procedures is encouraging. Ector et al.6 described the methodology of manual contouring in differently oriented imaging planes. They used balanced fast-field-echo MRI sequences with 6 mm slice thickness to construct three-dimensional models and fused them with conventional fluoroscopy. With these sequences, a three-dimensional reconstruction without losing the ability to include fine anatomic structures such as the eustachian valve and CS was feasible. The accuracy of MRI, including these SSFP sequences, with regard to quantitative assessment of cardiac dimensions and function has been validated in different reports.12–15 Despite three-dimensional MRI reconstruction, the exact localization of the ablation catheter is necessary. Nazarian et al.7 recently described the feasibility RTMRI guidance of active catheters. They found that active catheters were easier to localize on MRI and required a median of two initial real-time imaging planes for catheter tip localization.
Lesion mapping and preventions of complications
A future aspect for interventional MRI in EP procedures comprises the possibility of scar, lesion,10 and temperature mapping,11 respectively. This might be a crucial aspect for the prevention of a major complication, e.g. atrioesophageal fistula in PVI. Several methods to avoid this complication are available, e.g. oesophageal temperature probe and oesophageal tagging. However, so far, there is no technique that combines anatomical orientation, tissue imaging, and temperature monitoring. This might be an advantage of temperature-sensitive MRI, which is able to guide thermal procedures, as well as for target definition.16 Further technical development and the development of an irrigated MRI-compatible ablation catheter may enable other novel clinical applications, e.g. MRI-guided pulmonary vein ablation.
Supplementary material
Supplementary material is available at European Heart Journal online.
Funding
Our study received support from Biotronik GmbH & Co. KG (Berlin, Germany), which provided continuous intellectual support concerning the catheter design and from Siemens Medical Solutions (Erlangen, Germany), which provided continuous support concerning MR technology. None of the sponsors had any involvement in the design, collection, management, or analysis of the study or in manuscript preparation. None of the authors are employees of or consultants for the organizations that provided support. Funding to pay the Open Access publication charges for this article was provided by Biotronik GmbH & Co. KG.
Conflict of interest: none declared.
Supplementary Material
Acknowledgements
We would like to thank Wolfgang Geistert, Michelle Maxfield, and Sabine Hoffmeister of Biotronik GmbH & Co. KG (Berlin, Germany) and Joachim Graessner of Siemens Medical Solutions (Erlangen, Germany) for their help and assistance during development and conduction of the study.
References
- 1.Calkins H, Canby R, Weiss R, Taylor G, Wells P, Chinitz L, Milstein S, Compton S, Oleson K, Sherfesee L, Onufer J. Results of catheter ablation of typical atrial flutter. Am J Cardiol. 2004;94:437–442. doi: 10.1016/j.amjcard.2004.04.058. [DOI] [PubMed] [Google Scholar]
- 2.Ventura R, Klemm H, Lutomsky B, Demir C, Rostock T, Weiss C, Meinertz T, Willems S. Pattern of isthmus conduction recovery using open cooled and solid large-tip catheters for radiofrequency ablation of typical atrial flutter. J Cardiovasc Electrophysiol. 2004;15:1126–1130. doi: 10.1046/j.1540-8167.2004.04125.x. [DOI] [PubMed] [Google Scholar]
- 3.Feld GK. Radiofrequency catheter ablation of Type 1 atrial flutter using a large-tip electrode catheter and high-power radiofrequency energy generator. Expert Rev Med Devices. 2004;1:187–192. doi: 10.1586/17434440.1.2.187. [DOI] [PubMed] [Google Scholar]
- 4.Heidbüchel H, Willems R, van Rensburg H, Adams J, Ector H, Van de Werf F. Right atrial angiographic evaluation of the posterior isthmus: relevance for ablation of typical atrial flutter. Circulation. 2000;101:2178–2184. doi: 10.1161/01.cir.101.18.2178. [DOI] [PubMed] [Google Scholar]
- 5.Susil RC, Yeung CJ, Halperin HR, Lardo AC, Atalar E. Multifunctional interventional devices for MRI: a combined electrophysiology/MRI catheter. Magn Reson Med. 2002;47:594–600. doi: 10.1002/mrm.10088. [DOI] [PubMed] [Google Scholar]
- 6.Ector J, De Buck S, Adams J, Dymarkowski S, Bogaert J, Maes F, Heidbüchel H. Cardiac three-dimensional magnetic resonance imaging and fluoroscopy merging: a new approach for electroanatomic mapping to assist catheter ablation. Circulation. 2005;112:3769–3776. doi: 10.1161/CIRCULATIONAHA.105.565002. [DOI] [PubMed] [Google Scholar]
- 7.Nazarian S, Kolandaivelu A, Zviman MM, Meininger GR, Kato R, Susil RC, Roguin A, Dickfeld TM, Ashikaga H, Calkins H, Berger RD, Bluemke DA, Lardo AC, Halperin HR. Feasibility of real-time magnetic resonance imaging for catheter guidance in electrophysiology studies. Circulation. 2008;118:223–229. doi: 10.1161/CIRCULATIONAHA.107.742452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lardo AC, McVeigh ER, Jumrussirikul P, Berger RD, Calkins H, Lima J, Halperin HR. Visualization and temporal/spatial characterization of cardiac radiofrequency ablation lesions using magnetic resonance imaging. Circulation. 2000;102:698–705. doi: 10.1161/01.cir.102.6.698. [DOI] [PubMed] [Google Scholar]
- 9.Anfinsen OG, Kongsgaard E, Foerster A, Amlie JP, Aass H. Bipolar radiofrequency catheter ablation creates confluent lesions at larger interelectrode spacing than does unipolar ablation from two electrodes in the porcine heart. Eur Heart J. 1998;19:1075–1084. doi: 10.1053/euhj.1998.1015. [DOI] [PubMed] [Google Scholar]
- 10.Dickfeld T, Kato R, Zviman M, Nazarian S, Dong J, Ashikaga H, Lardo AC, Berger RD, Calkins H, Halperin H. Characterization of acute and subacute radiofrequency ablation lesions with nonenhanced magnetic resonance imaging. Heart Rhythm. 2007;4:208–214. doi: 10.1016/j.hrthm.2006.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peters DC, Wylie JV, Hauser TH, Kissinger KV, Botnar RM, Essebag V, Josephson ME, Manning WJ. Detection of pulmonary vein and left atrial scar after catheter ablation with three-dimensional navigator-gated delayed enhancement MR imaging: initial experience. Radiology. 2007;243:690–695. doi: 10.1148/radiol.2433060417. [DOI] [PubMed] [Google Scholar]
- 12.Miquel ME, Hill DL, Baker EJ, Qureshi SA, Simon RD, Keevil SF, Razavi RS. Three- and four-dimensional reconstruction of intra-cardiac anatomy from two-dimensional magnetic resonance images. Int J Cardiovasc Imaging. 2003;19:239–254. doi: 10.1023/a:1023671031207. discussion 255–6. [DOI] [PubMed] [Google Scholar]
- 13.Sorensen TS, Korperich H, Greil GF, Eichhorn J, Barth P, Meyer H, Pedersen EM, Beerbaum P. Operator-independent isotropic three-dimensional magnetic resonance imaging for morphology in congenital heart disease: a validation study. Circulation. 2004;110:163–169. doi: 10.1161/01.CIR.0000134282.35183.AD. [DOI] [PubMed] [Google Scholar]
- 14.Shors SM, Fung CW, Francois CJ, Finn JP, Fieno DS. Accurate quantification of right ventricular mass at MR imaging by using cine true fast imaging with steady-state precession: study in dogs. Radiology. 2004;230:383–388. doi: 10.1148/radiol.2302021309. [DOI] [PubMed] [Google Scholar]
- 15.Beygui F, Furber A, Delepine S, Helft G, Metzger JP, Geslin P, Le Jeune JJ. Routine breath-hold gradient echo MRI-derived right ventricular mass, volumes and function: accuracy, reproducibility and coherence study. Int J Cardiovasc Imaging. 2004;20:509–516. doi: 10.1007/s10554-004-1097-7. [DOI] [PubMed] [Google Scholar]
- 16.de Senneville BD, Mougenot C, Quesson B, Dragonu I, Grenier N, Moonen CT. MR thermometry for monitoring tumor ablation. Eur Radiol. 2007;17:2401–2410. doi: 10.1007/s00330-007-0646-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.