Abstract
P-glycoprotein (P-gp/ABCB1), multidrug resistance protein 1 (MRP1/ABCC1), and breast cancer resistance protein (BCRP/ABCG2) are plasma membrane efflux pumps that limit the intracellular uptake and retention of numerous lipophilic, amphipathic xeno- and endobiotics. Little is known about the neonatal and developmental expression of P-gp/ABCB1, MRP1/ABCC1, and BCRP/ABCG2 in the human central nervous system (CNS), therefore postmortem CNS tissue from infants born 220/7- 420/7 week gestation and adults was immunostained to determine their ontogeny and cellular localization. P-gp/ABCB1 imunostaining was observed in microvessel endothelial cells as early as 220/7 weeks, increasing in prevalence and intensity with maturation, and later in gestation in large pyramidal neurons. MRP1/ABCC1 immunostaining was prominent early in the choroid plexus and ventricular ependyma, and noted later in large pyramidal neurons. BCRP/ABCG2 expression was limited to microvessel endothelial cells. P-gp/ABCB1, MRP1/ABCC1 and BCRP/ABCG2 in adult brain matched term newborn CNS but with more intense immunostaining. We conclude that P-gp/ABCB1, MRP1/ABCC1, and BCRP/ABCG2 are expressed in a developmental, cell specific, fashion in the human CNS. The complementary pattern of P-gp/ABCB1 and BCRP/ABCG2 at the blood-brain with MRP1/ABCC1 at the blood-CSF barriers may limit CNS uptake and retention of drugs and toxins in neonates.
INTRODUCTION
The ATP-binding cassette (ABC) transporter superfamily members: i) P-glycoprotein (P-gp; gene symbol ABCB1), ii) multidrug resistance-associated protein 1 (MRP1; gene symbol ABCC1), and iii) breast cancer resistance protein (BCRP; gene symbol ABCG2, also known as mitoxantrone resistance protein) are integral plasma membrane efflux pumps [25]. They transport a vast array of lipophilic, amphipathic (containing both hydrophobic and hydrophilic domains) substrates against their concentration gradients by hydrolyisis of adenosine triphosphate, limiting substrate cellular influx and retention, thereby conferring a multidrug resistance phenotype [25]. Notably, these transporters share common protein motifs and a degree of substrate overlap [22,25]. Numerous xeno- and endobiotics, some therapeutic (e.g. anticonvulsants), others potentially toxic (e.g. unconjugated bilirubin), are among P-gp/ABCB1, MRP1/ABCC1 and/or BCRP/ABCG2 substrates [22,25,28,38,46,47].
The reported localization of i) P-gp/ABCB1 and BCRP/ABCG2 on the apical, i.e., luminal (blood-side) aspect of brain capillary endothelial cells of the blood-brain barrier (BBB) [5,8,25,40,48], and ii) MRP1/ABCC1 on the basolateral aspect of choroid plexus epithelium of the blood-cerebrospinal fluid (CSF) barrier in adult subjects [31] point to a strategic role for these transporters in limiting the CNS uptake and retention of a variety of compounds. Indeed, P-gp/ABCB1, MRP1/ABCC1 and BCRP/ABCG2 are postulated to contribute to antiepileptic drug resistance [38] and P-gp/ABCB1 and MRP1/ABCC1 to neuroprotection against bilirubin-induced brain injury [28,46,47].
There is, however, a paucity of data on P-gp/ABCB1, MRP1/ABCC1 and BCRP/ABCG2 expression in the developing CNS [26,33,36,41,42,44], most limited to murine models [26,33,41], and none that characterize their ontogeny, regional expression and cellular localization in human newborns. Previous immunohistochemical findings suggest that P-gp/ABCB1 may be expressed during embyrogenesis in human brain microvessels and serve as an early marker of BBB development [36,42,44]. Reports in murine models demonstrate a marked developmental modulation of CNS P-gp/ABCB1 expression [26,33,41]. The objective of the current study was to determine the developmental expression of P-gp/ABCB1, MRP1/ABCC1, and BCRP/ABCG2 in the human CNS and to assess the similarities and differences across the three transporter proteins in terms of their regional and cellular localization using immunohistochemical techniques.
METHODS
Autopsies of neonates delivered at Magee-Womens Hospital were reviewed to identify those live born infants who had: 1) a neuropathologic exam; 2) formalin fixed, paraffin-embedded postmortem CNS tissue blocks available for study; 3) lived ≤ 8 days, and 4) were free of identifiable congenital CNS malformations or chromosomal anomalies. Twenty-eight such infants of 220/7 to 420/7 weeks gestation were identified between 1984 and 1995 and grouped into post-menstrual age ranges of 220/7-266/7, 270/7-326/7, and 330/7-420/7 weeks representing the late 2nd trimester, 1st half and 2nd half of the third trimester, and coupled with three adult counterparts comprised the study cohort. Gestational age assigned by the attending neonatologist reflected a best estimate based on menstrual history, obstetrical ultrasound dating and assessments of neonatal neuromuscular and physical maturity. The table summarizes the newborn study cohort including neuropathologic lesions observed at postmortem. Two subjects had a history of clinical seizures and all newborns succumbed to complications of extreme prematurity and/or respiratory insufficiency. The study was approved by the Magee-Womens Hospital Institutional Human Subjects Review Board.
Table.
Newborn Demographics
| Gestation (wks) | N | Sex | Age at Death# (hrs) | Cause of Death (n**) | Death-Autopsy Interval# (hrs) | Neuropathologic Lesions Observed (n++) |
|---|---|---|---|---|---|---|
|
220/7-266/7 |
9 |
3M:6F |
2 hr (0.5-57) |
Extreme prematurity (9) RDS (9) Pulmonary hemorrhage (1) Pulmonary hypoplasia (1) |
20 hr (9-65) |
Grade I IVH (1) Grade II IVH (3) Subarachnoid hemorrhage (3) Acute cerebellar hemorrhage (1) Gliosis and microglial proliferation (1) |
|
270/7-326/7 |
10 |
8M:2F |
13 hr (0.1-168) |
Prematurity (7) RDS (8) Pulmonary hemorrhage (4) Pulmonary hypoplasia (2) Sepsis-pneumonia (2) |
19 hr (3-71) |
Grade I IVH (1) Grade II IVH (2) Diffuse astrocytosis (1) Microinfarcts in germinal matrix and white matter (1) Periventricular leukomalacia (2) Pontosubicular necrosis (1) |
|
330/7-426/7 |
9 |
5M:4F |
9 hr (1-192) |
RDS (3) Pulmonary hypoplasia (4) Sepsis-pneumonia (3) Asphyxia (2) Meconium Aspiration (1) |
18 hr (3-71) |
Dysmature CNS (1) Grade II IVH (1) Meningoencephalitis (1) Pontosubicular necrosis (1) Neuronal necrosis (2) |
median and range
number of infants with this contributing cause of death; may be more than one contributing cause per infant
number of infants demonstrating this neuropathologic finding
Immunohistochemistry was performed using an immunoperoxidase staining method for amplified antigen detection [40]. Briefly, 5-8 um thick sections of 1) cortex, 2) thalamus, 3) hippocampus, 4) cerebellum, 5) brain stem (medulla, pons, midbrain), and/or 6) choroid plexus were mounted on glass slides, heated at 60°C for 30 min, deparaffinized in xylene, and rehydrated with a series of graded ethanols. Endogenous peroxidase activity was quenched by incubating slides in 3% H2O2 for 30 min at room temperature followed by a rinse in phosphate buffered saline (PBS). Enhanced antigen retrieval [40] was performed by treating slides in 10 mM sodium citrate, pH 6.0, for 40 min at 97°C [17]. After cooling at room temperature for 20 min, slides were rinsed in PBS (pH 7.6) with 0.05% Tween 20 and treated with PBS (pH 7.4) containing 0.03% casein and 0.05% Tween-20 for 30 min to prevent nonspecific protein binding.
For P-gp/ABCB1 detection, two different murine derived anti-human monoclonal antibodies were used including: 1) C219 (Signet Labs, Dedham MA, 1:10 dilution) directed to an internal, highly conserved epitope adjacent to the nucleotide binding sites in the amino and carboxyl terminal halves [16] and 2) JSB1 (Signet Labs, Dedham, MA, 1:50 dilution) directed towards a conserved cytoplasmic epitope [29]. For MRP1/ABCC1 detection, MRPr1, a rat monoclonal antibody directed to an internal epitope (Kamiya Biomedical, Seattle, WA, 1:20 dilution [35]) was employed and confirmed in several cases using A23, a rabbit polyclonal antibody to a synthetic peptide corresponding to amino acids 1509-1531 of human MRP1/ABCC1 (Alexis Biochemicals, San Diego, CA, 1:50 dilution [14]). For BCRP/ABCG2 detection, BXP-21, a monoclonal antibody directed to an internal epitope that does not cross-react with P-gp/ABCB1 or MRP1/ABCC1 (Alexis Biochemicals, San Diego, CA, 1:40 dilution [10]) was utilized.
Slides were incubated with the primary antibody overnight in a humidified chamber at 4°C, then rinsed with PBS, pH 7.4 containing 0.03% casein and 0.05% Tween-20 after this and all subsequent antibody incubations. A rabbit anti-mouse peroxidase labeled or a rabbit anti-rat peroxidase labeled antibody (Jackson ImmunoResearch, West Grove, PA) at dilutions of 1:75 was then applied for 45 min at 4°C, followed by a 45 min incubation with either mouse monoclonal peroxidase-anti-peroxidase or rat monoclonal peroxidase-anti-peroxicdase (Sternberger Immunochemicals, Lutherville, MD) at dilutions of 1:150. After rinsing, the 1st secondary antibody was applied a second time as above. Slides were developed in 3,3’-diaminobenzidine (DAB) chromogen substrate containing 0.015% hydrogen peroxide for 20 min at room temperature. Sections were counter-stained with hematoxylin and mounted with Permount (Fisher Scientific). Adult brain sections stained with primary antibody served as both fully mature counterpart and positive control, whereas parallel tissue and age specific sections incubated with diluent and no primary antibody was used as negative control.
In addition, GFAP staining of astrocytes was performed using GA5 [10] mouse monoclonal antibody (Chemicon, Temecula, CA) applied overnight at 4°C. Rabbit anti-mouse peroxidase labeled secondary antibody was applied for 1.5 hrs and DAB substrate applied as above. Fluorescent double-immunostaining was performed using primary antibodies to calbindin, which preferentially stains Purkinje cells [20]. Briefly, after deparaffinizing and rehydrating sections as above slides were incubated in 0.1% protease at 37°C for 15 min, washed in PBS and antigen retrieval carried out as above. Following antigen retrieval, slides were incubated in 1% sodium borohydride for 10 min to reduce autofluorescence. Sections were incubated in 4% normal goat serum for 1 hr before incubating with P-gp/ABCB1, MRP1/ABCC1, or BCRP/ABCG2 antibodies at a 1:50 dilution overnight at 4°C. The next morning, a rabbit anti-rat alkaline phosphatase–linked secondary antibody was applied for 1 hr and Vector Red Substrate (Vector Labs, Burlingame, CA) used as the chromagen according to the manufacturer's protocol. After washing, rabbit anti-calbindin antibody (Sigma, St. Louis, MO) was applied at a 1:50 dilution for 2 hrs at 37°C. FITC-labeled goat anti-rabbit secondary antibody was applied for 1 hr, sections washed and mounted in Crystal Mount (Fisher Scientific).
All studied tissues were histologically well preserved and only tissue blocks and sections away from sites of neuropathological lesions (e.g. hemorrhage, infarct or leukomalacia) were examined. Cells were visualized using an Olympus BH-2 microscope fitted with the appropriate fluorescent filters and images photographed with a Sony CCD camera. Staining was characterized according to cell type (endothelial, astroglial, ependymal, neuronal, and leptomeningeal) by a pediatric neuropathologist (MAB) across the different brain regions outlined above, the three gestational age ranges, and in adults. For the analysis of staining intensity, a semi-quantitative scoring system was used, graded by two observers (CT, MAB) who independently confirmed cell staining intensity as negative (0), detectable (+1), low (+2), intermediate (+3), or high (+4).
RESULTS
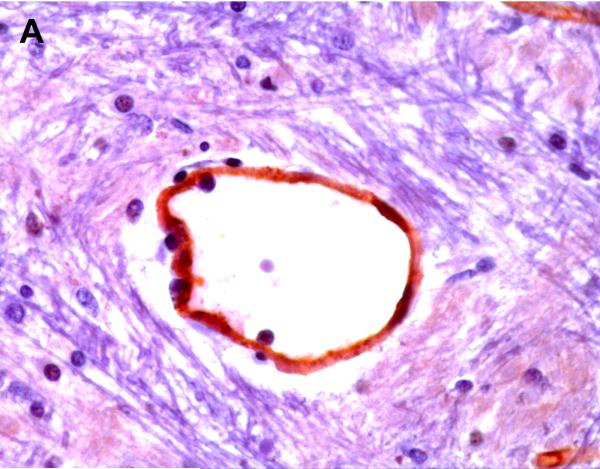
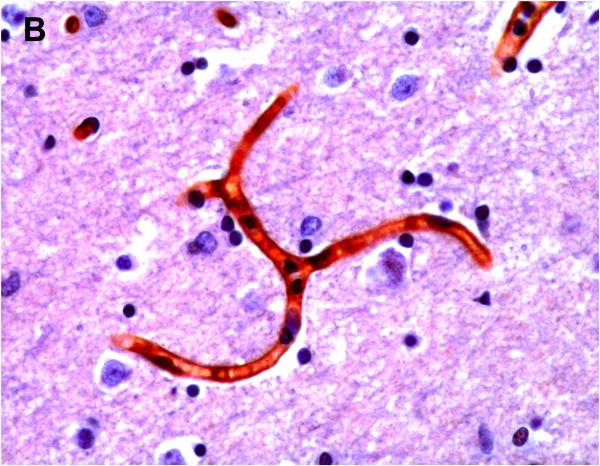
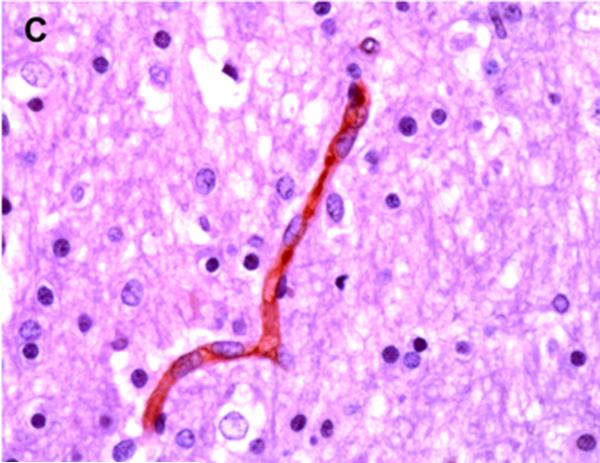
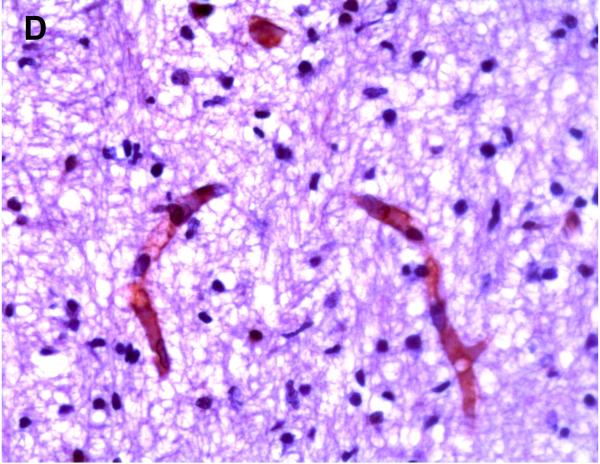
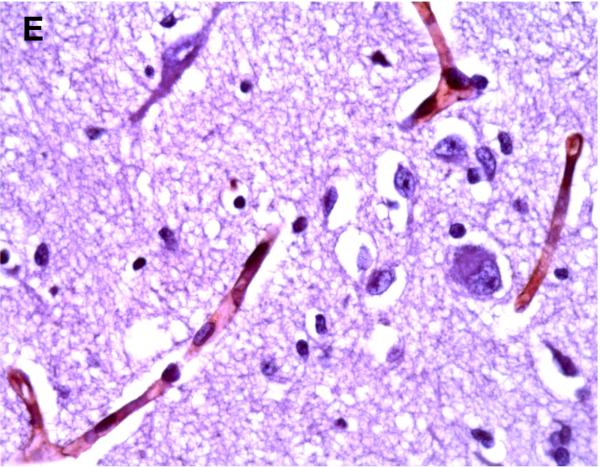
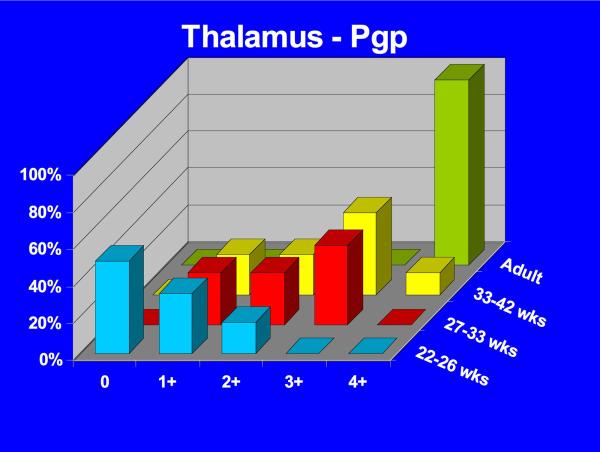
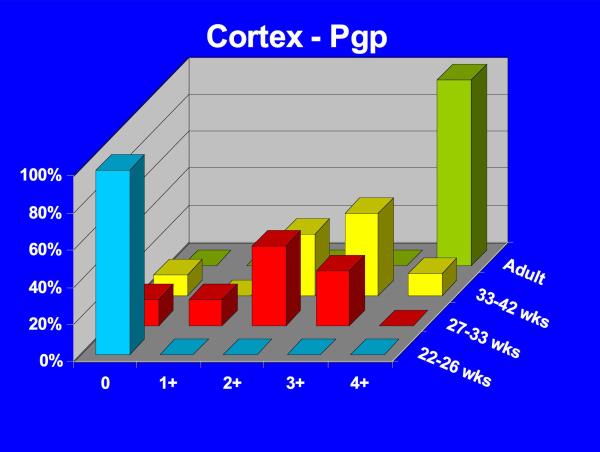
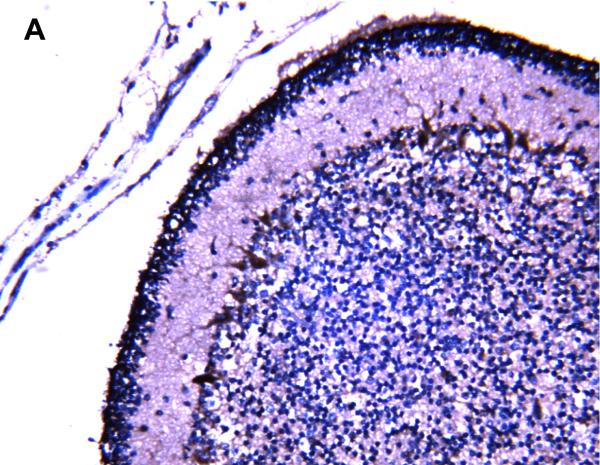
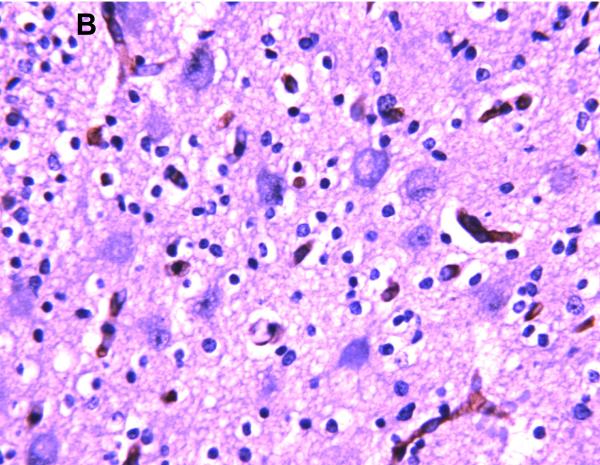
Positive P-gp/ABCB1 immunostaining of microvessel endothelial cells was detected as early as 220/7-266/7 week gestation in ~ 1/3 to 1/2 of newborns and at this gestational age range confined to the brainstem (Figure 1), other regions of the hindbrain, and thalamus. No endothelial cell P-gp/ABCB1 immunostaining in the cerebral cortex was detected in the 220/7-266/7 week gestation age range cohort. Neonatal endothelial cell P-gp/ABCB1 immunostaining was noted uniformly in the hindbrain and thalamus and first identified in other regions of the forebrain after 270/7weeks gestation including both allo- and neocortex in which P-gp/ABCB1 immunostaining was most prevalent at 33 weeks gestation and beyond. The intensity of microvessel endothelial cell immunostaining increased with gestational age as shown in Figure 1 and well defined in all brain regions in term infants and in the adult CNS. Figure 2 summarizes the developmental pattern of microvessel endothelial cell P-gp/ABCB1 antibody staining intensity for the thalamus (posterior forebrain) as contrasted with cerebral cortex showing i) earlier Pgp/ABCB1 expression in posterior forebrain and ii) increasing P-gp/ABCB1 immunostaining intensity with maturation in both CNS regions. Perivascular astrocyte P-gp/ABCB1 immunostaining was not observed.
Figure 1.
P-gp/ABCB1 immunostaining in sections of human brainstem as a function of age, using 3,3’-diaminobenzidine as chromogen: (A) adult vessel cross-section demonstrates staining of endothelium; (B) adult microvessels; (C) 42 week gestation; (D) 35 week gestation; (E) 30 week gestation; and (F) 26 week gestation newborns. Magnification is 400 X.
Figure 2.
3-D bar graph of Pgp/ABCB1 microvessel endothelial cell immunostaining prevalence and intensity as a function of age for the thalamus (A) and cerebral cortex (B) demonstrating earlier Pgp/ABCB1 expression in posterior forebrain and increasing P-gp/ABCB1 immunostaining intensity with maturation in both CNS regions. Immunostaining intensity was scored as negative (0), detectable (+1), low (+2), intermediate (+3), or high (+4).
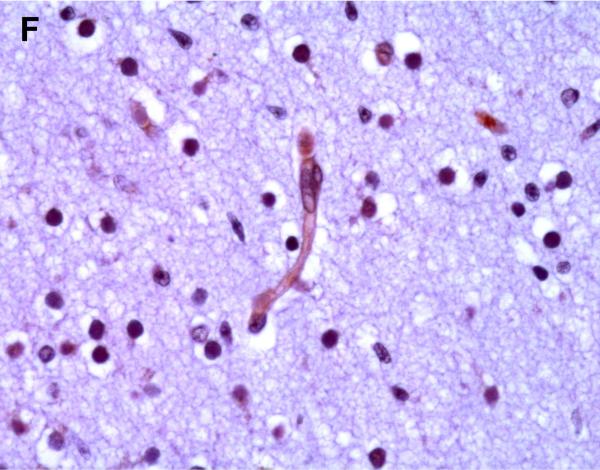
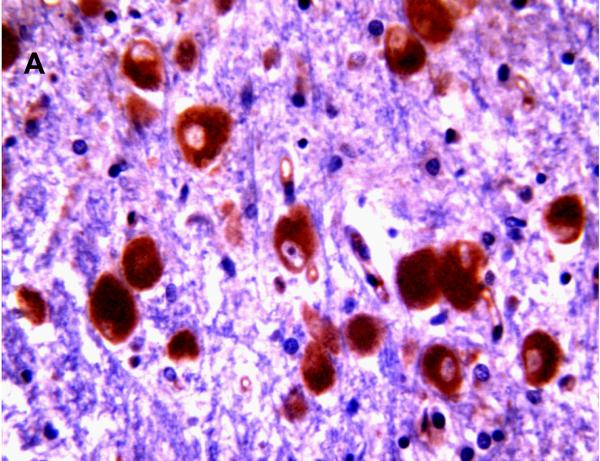
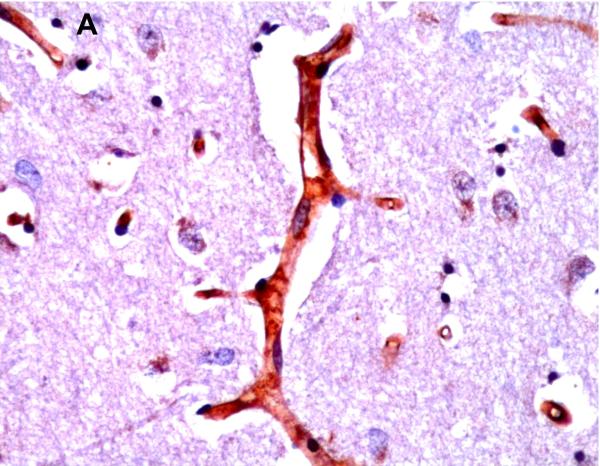
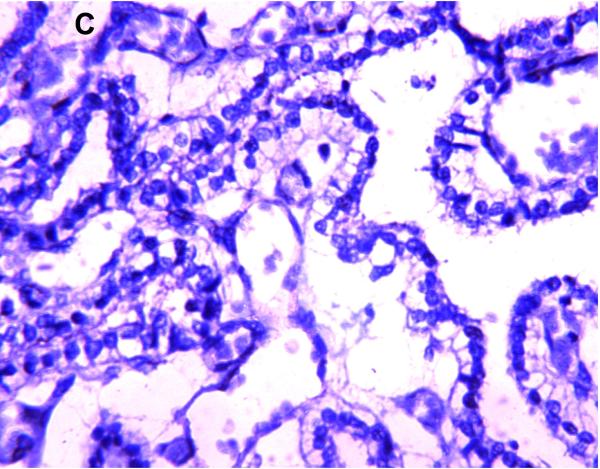
Selected neuronal cells also demonstrated positive immunostaining for P-gp/ABCB1 as shown in Figure 3. More specifically, large pyramidal neurons of the brainstem and thalamus, and Purkinje cells of the cerebellum were stained in ~20% of subjects at 220/7-266/7 weeks gestation but in all subjects after 30 weeks gestation. Pyramidal cells evidenced both membranous and cytoplasmic P-gp/ABCB1 immunostaining (Figure 3). Choroid plexus epithelium also demonstrated positive P-gp/ABCB1 immunostaining as early as 23 weeks gestation (Figure 3). The intensity of choroid plexus epithelial cell P-gp/ABCB1 immunostaining increased with gestational age and was well defined in term infants and in the adult CNS.
Figure 3.
P-gp/ABCB1 immunostaining of large pyramidal neurons in brainstem (36 week gestation, magnification is 400 X) (A), and choroid plexus (36 week gestation, magnification is 200 X) (B), using 3,3’-diaminobenzidine as chromogen. Pyramidal cells evidenced both membranous and cytoplasmic P-gp/ABCB1 immunostaining.
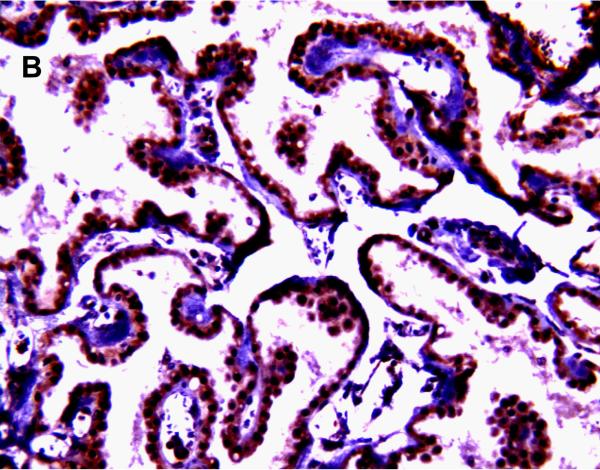
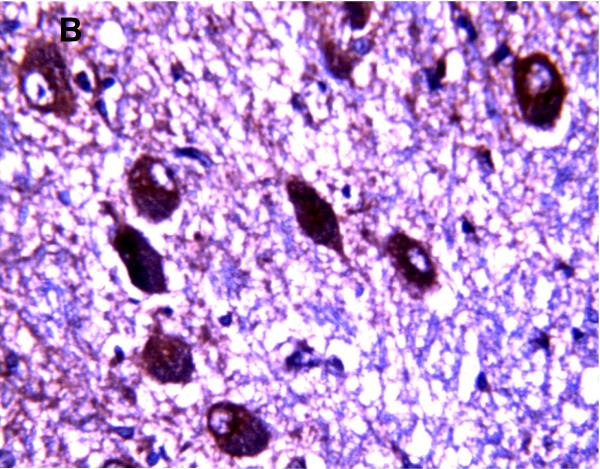
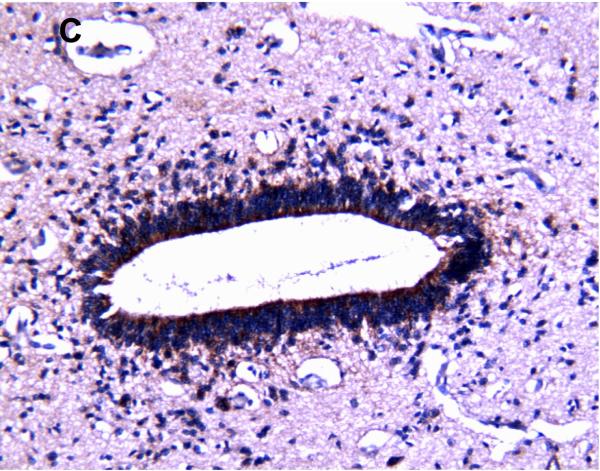
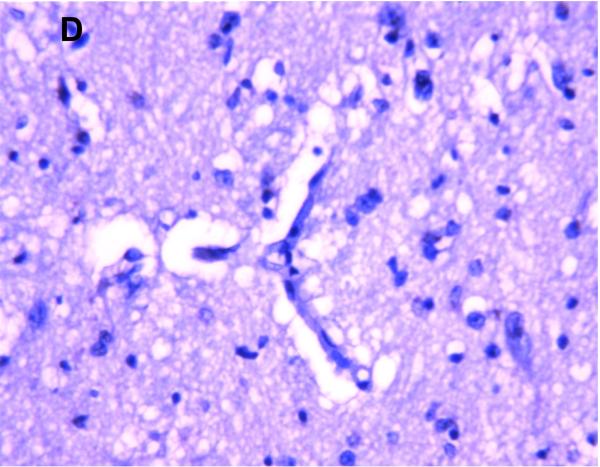
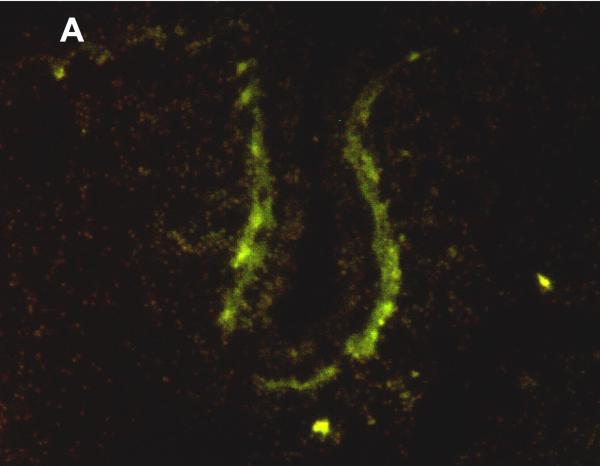
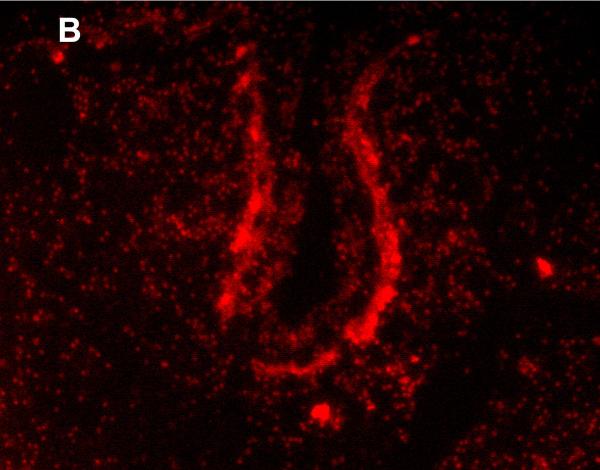
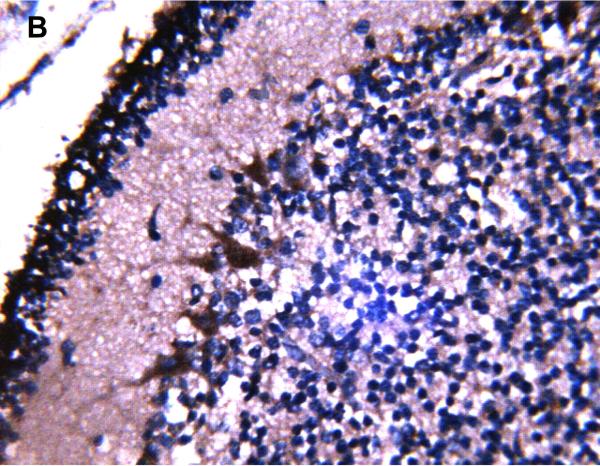
MRP1/ABCC1 immunostaining was most prominent in the choroid plexus epithelium and ependymal cells of the cerebral ventricles (Figure 4 A and 4C respectively), noted consistently early in gestation (220/7-266/7 weeks) and with its intensity relatively constant across the gestational age groups studied. MRP1/ABCC1 immunostaining was also observed in large pyramidal cells of the cerebellum (Figure 4B) of selected newborns as early as 26 weeks gestation becoming increasingly prevalent after 30 weeks gestation. Figure 5 demonstrates MRP1/ABCC1 co-localization with the Purkinje cell marker calbindin while Figure 6 shows MRP1/ABCC1 immunostaining localization to the Purkinje cell layer of the cerebellum. MRP1/ABCC1 immunostaining was not observed in brain microvessel endothelial cells or perivascular astrocytes at any gestational age or in adults (Figure 4D).
Figure 4.
MRP1/ABCC1 immunostaining using 3,3’-diaminobenzidine as chromogen of choroid plexus (35 week gestation; magnification is 200 X) (A); large pyramidal neurons in thalamus (35 week gestation; magnification is 400 X) (B); and ventricular ependyma (35 week gestation; magnification is 200 X) (C). Pyramidal cells evidenced both membranous and cytoplasmic MRP1/ABCC1 immunostaining. MRP1/ABCC1 immunostaining was not observed in brain microvessel endothelial cells at any gestational age or in adults (adult, magnification is 400X) (D).
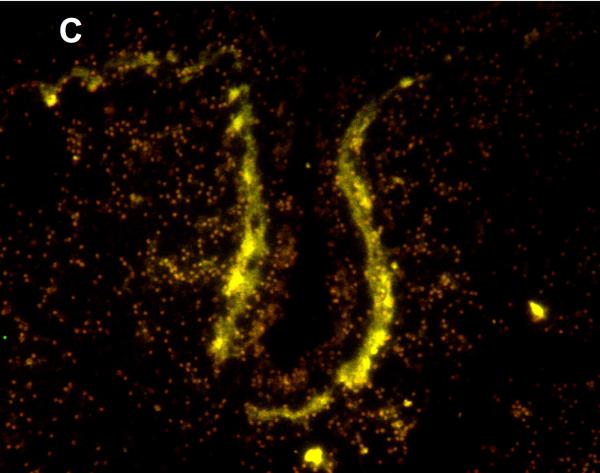
Figure 5.
Immunofluoresence of MRP1/ABCC1 (A), the Purkinje cell marker calbinin (B), and their co-localization (C) in Purkinje cells of the thalamus (36 week gestation; magnification is 100 X).
Figure 6.
MRP1/ABCC1 immunostaining using 3,3’-diaminobenzidine as chromogen demonstrating localization to the Purkinje cell layer of the cerebellum (35 week gestation; magnification is 200 X (A); magnification is 400 X (B).
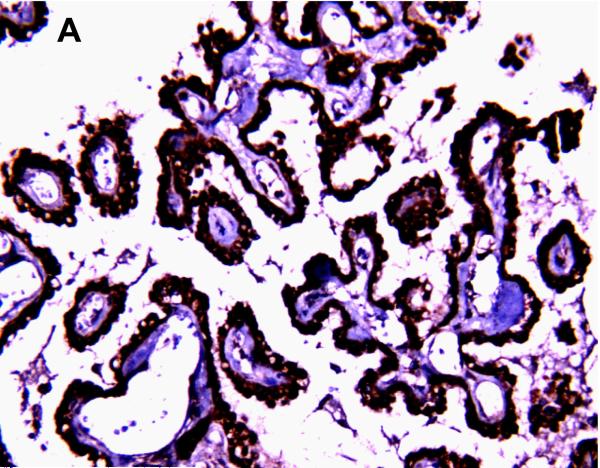
BCRP/ABCG2 immunostaining was observed in brain microvessel endothelial cells (Figure 7) in all age groups and brain regions. The intensity of BCRP/ABCG2 immunostaining did not vary notably with maturation. There was no evidence of BCRP/ABCG2 immunostaining in choroid plexus epithelium, perivascular astrocytes or neurons (Figure 7).
Figure 7.
BCRP/ABCG2 immunostaining using 3,3’-diaminobenzidine as chromogen was observed in brain microvessel endothelial cells (A) in all age groups and brain regions (adult thalamus; magnification is 400 X). There was no evidence of BCRP/ABCG2 immunostaining in neurons (B) (adult thalamus; magnification is 400 X) or choroid plexus epithelium (C) (adult; magnification is 200 X).
DISCUSSION
P-gp/ABCB1, MRP1/ABCC1 and BCRP/ABCG2 are expressed in a cell specific pattern in the developing human CNS: i) P-gp/ABCB1 and BCRP/ABCG2 most prominently in microvessel endothelial cells, ii) MRP1/ABCC1 in the choroid plexus and ependymal cells, and iii) both P-gp/ABCB1 and MRP1/ABCC1 in large pyramidal neurons. Once detected, P-gp/ABCB1 immunostaining intensity increased during gestational maturation, whereas MRP1/ABCC1 and BCRP/ABCG2 cellular staining intensity remained constant.
The cellular localization of P-gp/ABCB1 to microvessel endothelial cells in newborns is consistent with prior observations in adults [5,9,21,25]. A few previous studies of human fetal CNS demonstrate distinct P-gp/ABCB1 brain microvessel immunoreactivity at crown-rump lengths corresponding to 17-24 weeks gestation positing P-gp/ABCB1 as an early marker of BBB development [36,42,44]. One of these investigations detected a diffuse cytoplasmic P-gp/ABCB1 labeling of endothelium as early as 12 weeks that became linear and continuous on endothelial cell membranes at 22 weeks gestation [44]. The latter is consistent with the detection of Pgp/ABCB1 immunostaining as early as 220/7 weeks gestation in the current study. None of these prior investigations, however, examined human CNS P-gp/ABCB1 expression at later gestational or perinatal ages. The marked developmental increase in human microvessel endothelial cell P-gp/ABCB1 staining intensity between 22 and 42 weeks gestation is analogous to murine studies that showed a marked perinatal [41], early postnatal [26] or late postnatal [33] increase of CNS P-gp microvessel expression. Moreover, Pgp/ABCB1 immunostaining was observed earlier in human brain microvessel endothelium than in large pyramidal cells, a pattern consistent with CNS cellular maturation, i.e., earlier in mesenchymal cells (e.g., endothelial cells) followed later by expression in neuronal cells. We did not detect P-gp/ABCB1 immunostaining of perivascular astrocytes or astrocytes not associated with vessels, findings consistent with several prior reports [5,39,43,44] but not others [18].
Given P-gp's efflux action at the BBB, limited P-gp/ABCB1 microvessel expression in immature humans might enhance the CNS passage and/or retention of P-gp/ABCB1 substrates. Indeed, Goralski et al., recently confirmed that the marked postnatal maturational increase in murine brain P-gp is associated with less CNS cyclosporin A and 3H-digoxin accumulation in the mature as contrasted with immature mouse, i.e. the CNS accumulation of these two well characterized Pgp/ABCB1 substrates was highly dependent on, and inversely related to, the developmental increase in brain P-gp expression [19]). Whether a similar predisposition to increased CNS Pgp substrate (therapeutic and toxic alike) accumulation exists in immature humans is unknown but merits investigation.
P-gp/ABCB1 immunostaining was also observed in the choroid plexus of neonates consistent with prior immunohistochemical observations of Rao et al., in adult humans [31]. As observed in brain microvessel endothelium, choroid plexus P-gp/ABCB1 immunostaining was developmentally regulated, first detected at 23 weeks gestation and most clearly observed in the term infant and adult. P-gp/ABCB1 is reportedly localized to the apical membrane of the choroid plexus where it would be expected to mediate transport of substrates from blood into the CSF [29]; the pharmacotoxicologic relevance of such P-gp/ABCB1 expression is unclear [25].
P-gp/ABCB1 immunostaining was noted in large pyramidal cells of the brainstem and thalamus and Purkinje cells of the cerebellum as early as 22 weeks gestation becoming uniform at 30 weeks gestation and beyond, cells formerly believed to be P-gp/ABCB1 non-expressive and a here-to-fore unreported finding in human neonates. Such immunostaining was noted to be both cytoplasmic and membranous in nature. The former may reflect active P-gp/ABCB1 synthesis and its intracellular trafficking as recently demonstrated by Bendayan and colleagues [6]. The finding of pyramidal and Purkinje cell P-gp/ABCB1 expression is consistent with recent reports of i) neuronal P-p/ABCB1 expression in pathologic human CNS conditions, e.g., seizures, focal cortical dysplasia, glioneuronal tumors, and tuberous sclerosis [2,3,23,30] and ii) neuronal P-gp expression after induced seizures [45] and experimental brain hypoxia or ischemia [24] in murine models. Such neuronal expression has been confined to lesional or perilesional CNS tissue (e.g., ischemic penumbra or seizure focus, dysplastic brain, tuber cells) [2,3,23,24,30,45]. In this regard, two neonates in the current patient cohort evidenced clinically overt seizures and two others difficult perinatal transitions consistent with acute hypoxic–ischemic encephalopathy (HIE). These infants showed neuropathological findings, i.e., pontosubicular necrosis or neuronal necrosis, classically linked to HIE. Although we were careful to examine only tissue sections away from gross neuropathological lesions, HIE could have had more global CNS effects that may have contributed to remote sites of neuronal P-gp/ABCB1 expression. Moreover, as a group these were catastrophically ill neonates who did succumb to a range of severe postnatal conditions, most commonly prematurity coupled with respiratory failure and may have experienced an adverse intrauterine milieu prior to delivery as well. It is plausible that these antenatal, perinatal, or postnatal events contributed to neuronal P-gp/ABCB1 expression. The current findings suggest that neuronal P-gp/ABCB1 expression may occur in critically ill patients and determining the prevalence of P-gp/ABCB1 in neurons from other patient populations is warranted.
The functional consequence(s) of neuronal P-gp/ABCB1 expression is unclear but has been hypothesized to include: i) neuroprotection and ii) limited transport and efficacy of drugs with intraneuronal targets [24,45]. That Purkinje cells should be damaged in kernicteric humans [1] and in the Gunn rat model of kernicterus [7,34] would appear to contradict the former. However, substrate-induced up-regulation of protein expression is characteristic of ABC transporters [25]. Purkinje cell injury could be explained by prolonged and/or markedly elevated levels of bilirubin that exceed the cytoprotective capacity of Pgp/ABCB1 (or MRP1/ABCC1 - see below). Indeed, in Gunn rat pups only prolonged hyperbilirubinemia or sulfadimethoxine induced elevations in unbound bilirubin result in Purkinje cell damage [7,34].
Prominent MRP1/ABCC1 immunostaining in the choroid plexus of human neonates is consistent with prior cellular localization studies of the CNS in adults [31]. The intensity of MRP1/ABCC1 choroid plexus immunostaining did not change appreciably with gestational maturation a finding that corresponds with the lack of developmental modulation of murine MRP1 brain expression [33]. MRP1/ABCC1 localization to the basolateral choroid plexus membrane is thought to facilitate substrate transport from CSF to blood, i.e. enhance their CSF clearance [33].
MRP1/ABCC1 immunostaining of large pyramidal neurons of the brainstem and cerebellum was also observed, cells formerly believed to be MRP1/ABCC1 non-expressive. Previous studies of neuronal MRP1 expression have been confined to subjects with focal cortical dysplasia or tuberous sclerosis [2,3,23]. The nature and scope of neuronal MRP1/ABCC1 expression, like that of neuronal P-gp/ABCB1, are unclear as are its etiologic antecedents and functional consequences; matters that merit further study.
No evidence of MRP1/ABCC1 immunostaining was observed in human brain microvessel endothelial cells or perivascular astrocytes in any age group. This finding is consistent with a preponderance of studies reporting an absence of detectable MRP1/ABCC1 expression in human brain endothelium in situ or in isolated human brain microvessels [3,15,37-39]. Collectively these in vivo observations contrast with i) a recent report of weak MRP1/ABCC1 staining in some endothelial cells in perilesional brain tissue samples obtained at neurosurgery in human adults [27] and ii) in vitro evidence of MRP1/ABCC1 expression in cultured human brain capillary endothelial cells [11,27,32]. The latter, in all likelihood, represents an in vitro artifact, a result of de-differentiation of cultured or immortalized brain microvessel cells [11,27,32] possibly related to the removal of CNS regulatory signals [37]; and likely not representative of the BBB in vivo. The former is consistent with prior reports of MRP1/ABCC1 expression in human brain endothelial cells of lesional or perilesional tumor regions (e.g., glioblastoma, meningioma) and sites of focal cortical dysplasia [3,38]. A report of MRP1/ABCC1 expression in protein extracts of the entire brain from patients with Down syndrome likely reflects constitutive expression in the choroid plexus [13].
BCRP/ABCG2 immunostaining was consistently observed in brain microvessel endothelial cells in all gestational age groups and in adults, the latter consistent with prior investigations demonstrating abundant constitutive BCRP/ABCG2 expression at the human BBB [4,8,48]. Indeed, immunofluorescence studies of adult human CNS tissue demonstrate BCRP/ABCG2 localization to the microvessel luminal surface [8], a finding that when coupled with enhanced substrate efflux by human BCRP/ABCG2 transfected cerebrovascular endothelial cells [48] evidence a potentially important functional barrier role for BCRP/ABCG2 at the BBB [48]. We did not detect BCRP/ABCG2 expression in other CNS cells consistent with the absence of neuronal or astroglial cell BCRP/ABCG2 expression observed by others [4].
The current observations where made on post-mortem CNS tissue samples. Whereas the pattern and level of transporter immunostaining were likely modulated and possibly induced by the conditions that preceded and/or led to the patient's demise including antenatal, perinatal and postnatal events, perimortem factors and the interval between death and postmortem, if anything, may have diminished and thereby masked transporter protein expression. Recognizing these possibilities, it is nevertheless of interest that cell specific patterns of CNS transporter expression were consistent across gestational age groups, adult subjects, and with those of prior observations in humans.
Acknowledgements
Supported by the National Institutes of Health NINDS 38993, Irene McClenahan Young Investigator Fund of the Magee-Womens Health Foundation (CT), Mario Lemieux Centers for Patient Care and Research of the Mario Lemieux Foundation, and 25 Club of Magee-Womens Hospital.
REFERENCES
- 1.Ahdab-Barmada M, Moossy J. The neuropathology of kernicterus in the premature neonate: diagnostic problems. J Neuropathol Exp Neurol. 1984;43:45–56. doi: 10.1097/00005072-198401000-00004. [DOI] [PubMed] [Google Scholar]
- 2.Ak H, Ay B, Tanriverdi T, et al. Expression and cellular distribution of multidrug resistance-related proteins in patients with focal cortical dysplasia. Seizure. 2007;16:493–503. doi: 10.1016/j.seizure.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 3.Aronica E, Gorter JA, Jansen GH, et al. Expression and cellular distribution of multidrug transporter proteins in two major causes of medically intractable epilepsy: focal cortical dysplasia and glioneuronal tumors. Neuroscience. 2003;118:417–429. doi: 10.1016/s0306-4522(02)00992-2. [DOI] [PubMed] [Google Scholar]
- 4.Aronica E, Gorter JA, Redeker S, et al. Localization of breast cancer resistance protein (BCRP) in microvessel endothelium of human control and epileptic brain. Epilepsia. 2005;46:849–857. doi: 10.1111/j.1528-1167.2005.66604.x. [DOI] [PubMed] [Google Scholar]
- 5.Beaulieu E, Demeule M, Ghitescu L, et al. P-glycoprotein is strongly expressed in the luminal membranes of the endothelium of blood vessels in the brain. Biochem J. 1997;326:539–544. doi: 10.1042/bj3260539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bendayan R, Ronaldson PT, Gingras D, et al. In situ localization of P-gycoprotein (ABCB1) in human and rat brain. J Histochem Cytochem. 2006;54:1159–1167. doi: 10.1369/jhc.5A6870.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Conlee JW, Shapiro SM. Development of cerebellar hypoplasia in jaundiced Gunn rats: a quantitative light microscopic analysis. Acta Neuropathol. 1997;93:450–460. doi: 10.1007/s004010050639. [DOI] [PubMed] [Google Scholar]
- 8.Cooray HC, Blackmore CG, Maskell L, et al. Localization of breast cancer resistance protein in microvessel endothelium of human brain. NeuroReport. 2002;13:2059–2063. doi: 10.1097/00001756-200211150-00014. [DOI] [PubMed] [Google Scholar]
- 9.Cordon-Cardo C, O'Brien JP, Casals D, et al. Multidrug-resistance gene (P-glycoprotein) is expressed by endothelial cells at blood-brain barrier sites. Proc Natl Acad Sci USA. 1989;86:695–698. doi: 10.1073/pnas.86.2.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Debus E, Weber K, Osborn M. Monoclonal antibodies specific for glial fibrillary acidic (GFA) protein and for each of the neurofilament triplet polypeptides. Differentiation. 1983;25:193–203. doi: 10.1111/j.1432-0436.1984.tb01355.x. [DOI] [PubMed] [Google Scholar]
- 11.Decleves X, Regina A, Laplanche JL, et al. Functional expression of P-glycoprotein and multidrug resistance-associated protein (Mrp1) in primary cultures of rat astrocytes. J Neurosci Res. 2000;60:594–601. doi: 10.1002/(SICI)1097-4547(20000601)60:5<594::AID-JNR4>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 12.Diestra JE, Scheffer GL, Catala I, et al. Frequent expression of the multi-drug resistance-associated protein BCRP/MXR/ABCP/ABCG2 in human tumours detected by the BXP-21 monoclonal antibody in paraffin-embedded material. J Pathol. 2002;198:213–219. doi: 10.1002/path.1203. [DOI] [PubMed] [Google Scholar]
- 13.Engidawork E, Roberts JC, Hardmeier R, et al. Expression of the multidrug resistance associated protein (MRP1) in Down syndrome brains. J Neurol Transm Suppl. 2001;61:35–45. doi: 10.1007/978-3-7091-6262-0_3. [DOI] [PubMed] [Google Scholar]
- 14.Fernetti C, Pascolo L, Podda E, et al. Preparation of an antibody recognizing both human and rodent MRP1. Biochem Biophys Res Comm. 2001;288:1064–1068. doi: 10.1006/bbrc.2001.5885. [DOI] [PubMed] [Google Scholar]
- 15.Flens MJ, Zaman GJ, van der Valk P, et al. Tissue distribution of the multidrug resistance protein. Am J Pathol. 1996;148:1237–1247. [PMC free article] [PubMed] [Google Scholar]
- 16.Georges E, Bradley G, Gariepy J, et al. Detection of P-glycoprotein isoforms by gene-specific monoclonal antibodies. Proc Natl Acad Sci USA. 1990;87:152–156. doi: 10.1073/pnas.87.1.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ginn PE. Immunohistochemical detection of P-glycoprotein in formalin-fixed paraffin-embedded normal and neoplastic canine tissues. Vet pathol. 1996;33:533–541. doi: 10.1177/030098589603300508. [DOI] [PubMed] [Google Scholar]
- 18.Golden PL, Partridge WM. P-glycoprotein on astrocyte foot processes of unfixed isolated human brain capillaries. Brain Res. 1999;819:143–146. doi: 10.1016/s0006-8993(98)01305-5. [DOI] [PubMed] [Google Scholar]
- 19.Goralski KB, Acott PD, Frasere AD, et al. Brain cyclosporin A levels are determined by ontogenic regulation of mdr1a expression. Drug Metab Dispos. 2006;34:288–295. doi: 10.1124/dmd.105.007427. [DOI] [PubMed] [Google Scholar]
- 20.Hockberger PE, Yousif L, Nam SC. Identification of acutely isolated cells from developing rat cerebellum. Neuroimage. 1994;1:276–287. doi: 10.1006/nimg.1994.1012. [DOI] [PubMed] [Google Scholar]
- 21.Jette L, Tetu B, Beliveau R. High levels of P-glycoprotein detected in isolated brain capillaries. Biochim Biophys Acta. 1993;1150:147–154. doi: 10.1016/0005-2736(93)90083-c. [DOI] [PubMed] [Google Scholar]
- 22.Johnson DR, Finch RA, Lin ZP, et al. The pharmacological phenotype of combined multidrug-resistance mdr1a/1b- and mrp1-deficient mice. Cancer Res. 2001;61:1469–1476. [PubMed] [Google Scholar]
- 23.Lazarowski A, Lubieniecki F, Camarero HT, et al. Multidrug resistance proteins in tuberous sclerosis and refractory epilepsy. Pediatr Neurol. 2004;30:102–106. doi: 10.1016/S0887-8994(03)00407-7. [DOI] [PubMed] [Google Scholar]
- 24.Lazarowski A, Caltana L, Merelli A, et al. Neuronal mdr-1 gene expression after experimental focal hypoxia: a new obstacle for neuroprotection. J Neurol Sci. 2007;258:84–92. doi: 10.1016/j.jns.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 25.Leslie EM, Deeley RG, Cole SPC. Multidrug resistance proteins: role of P-glycoprotein, MRP1, MRP2 and BCRP (ABCG2) in tissue defense. Toxicol Appl Pharmacol. 2005;204:216–237. doi: 10.1016/j.taap.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 26.Matsuoka Y, Okazaki M, Kitamura Y, et al. Developmental expression of P-glycoprotein (multidrug resistance gene product) in the rat brain. J Neurobiol. 1999;39:383–392. doi: 10.1002/(sici)1097-4695(19990605)39:3<383::aid-neu5>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 27.Nies AT, Jedlitschky, Konig J, et al. Expression and immunolocalization of the multidrug resistance proteins, MRP1-MRP6 (ABCC1-ABCC6), in human brain. Neuroscience. 2004;129:349–360. doi: 10.1016/j.neuroscience.2004.07.051. [DOI] [PubMed] [Google Scholar]
- 28.Ostrow JD, Pascolo L, Brites D, et al. Molecular basis of bilirubin-induced neurotoxicity. Trends Mol Med. 2004;10:65–70. doi: 10.1016/j.molmed.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 29.Pavelic ZP, Sever Z, Fontaine RN, et al. Detection of P-glycoprotein with JSB-1 monoclonal antibody in B-5 fixed and paraffin-embedded cell lines and tissues. Sel Cancer Ther. 1989;7:49–58, 1989. doi: 10.1089/sct.1991.7.49. [DOI] [PubMed] [Google Scholar]
- 30.Ramos AJ, Lazarowski A, Villar MJ, et al. Transient expression of MDR-1/P-glycoprotein in a model of partial cortical devascularization. Cell Mol Neurobiol. 2004;24:101–107. doi: 10.1023/B:CEMN.0000012728.19117.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rao VV, Dahlheimer JL, Bardgett ME, et al. Choroid plexus epithelial expression of MDR1 P-glycoprotein and multidrug resistance-associated protein contribute to the blood-cerebrospinal fluid drug-permeability barrier. Proc Natl Acad Sci USA. 1999;96:3900–3905. doi: 10.1073/pnas.96.7.3900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Regina A, Koman A, Piciotti M, et al. Mrp1 multidrug resistance-associated protein and P-glycoprotein expression in rat brain microvessel endothelial cells. J Neurochem. 1998;71:705–715. doi: 10.1046/j.1471-4159.1998.71020705.x. [DOI] [PubMed] [Google Scholar]
- 33.Rosati A, Maniori S, Decorti G, et al. Physiological regulation of P-glycoprotein, MRP1, MRP2 and cytochrome P450 3A2 during rat ontogeny. Develop Growth Differ. 2003;45:377–387. doi: 10.1046/j.1440-169x.2003.00699.x. [DOI] [PubMed] [Google Scholar]
- 34.Sawasaki Y, Yamada N, Nakajima H. Developmental features of cerebellar hypoplasia and brain bilirubin levels in a mutant (Gunn) rat with hereditary hyperbilirubinemia. J Neurochem. 1976;27:577–583. doi: 10.1111/j.1471-4159.1976.tb12285.x. [DOI] [PubMed] [Google Scholar]
- 35.Scheffer GL, Kool M, Heijn M, et al. Specific detection of multidrug resistance proteins MRP1, MRP2, MRP3, MRP5, and P-Glycoprotein with a panel of monoclonal antibodies. Cancer Res. 2000;60:5269–5277. [PubMed] [Google Scholar]
- 36.Schumacher U, Mollgard K. The multidrug-resistance P-glycoprotein (Pgp, MDR1) is an early marker of blood-brain barrier development in the microvessels of the developing human brain. Histochem Cell Biol. 1997;108:179–182. doi: 10.1007/s004180050159. [DOI] [PubMed] [Google Scholar]
- 37.Seetharaman S, Barrand MA, Maskell L, et al. Multidrug resistance-related transport proteins in isolated human brain microvessels and in cells cultured from these isolates. J Neurochem. 1998;70:1151–1159. doi: 10.1046/j.1471-4159.1998.70031151.x. [DOI] [PubMed] [Google Scholar]
- 38.Sisodiya SM, Lin WR, Harding BN, et al. Drug resistance in epilepsy: expression of drug resistance proteins in common causes of refractory epilepsy. Brain. 2002;125:22–31. doi: 10.1093/brain/awf002. [DOI] [PubMed] [Google Scholar]
- 39.Sisodiya SM, Martinian L, Scheffer GL, et al. Vascular colocalization of P-glycoprotein, multidrug-resistance associated protein 1, breast cancer resistance protein and major vault protein in human epileptogenic pathologies. Neuropathol Appl Neurobiol. 2006;32:51–63. doi: 10.1111/j.1365-2990.2005.00699.x. [DOI] [PubMed] [Google Scholar]
- 40.Toth K, Vaughn MM, Slocum HK, et al. New immunohistochemical “sandwich” staining method for mdr1 P-glycoprotein detection with JSB-1 monoclonal antibody in formalin-fixed, paraffin-embedded human tissues. Am J Pathol. 1994;144:227–236. [PMC free article] [PubMed] [Google Scholar]
- 41.Tsai C, Daood MJ, Lane RH, et al. P-glycoprotein expression in mouse brain increases with maturation. Biol Neonate. 2002;81:58–64. doi: 10.1159/000047185. [DOI] [PubMed] [Google Scholar]
- 42.van Kalken CK, Giaccone G, van der Valk P, et al. Multidrug resistance gene (P-glycoprotein) expression in the human fetus. Am J Pathol. 1992;141:1063–1072. [PMC free article] [PubMed] [Google Scholar]
- 43.Virgintino D, Robertson D, Errede M, et al. Expression of P-glycoprotein in human cerebral cortex microvessels. J Histochem Cytochem. 2002;501:1671–1676. doi: 10.1177/002215540205001212. [DOI] [PubMed] [Google Scholar]
- 44.Virgintino D, Errede M, Girolamo F, et al. Fetal blood-brain barrier P-glycoprotein contributes to brain protection during human development. J Neuropathol Exp Neurol. 2008;67:50–61. doi: 10.1097/nen.0b013e31815f65d9. [DOI] [PubMed] [Google Scholar]
- 45.Volk HA, Burkhardt K, Potschka H, et al. Neuronal expression of the drug efflux transporter P-glycoprotein in the rat hippocampus after limbic seizures. Neuroscience. 123:751–759. doi: 10.1016/j.neuroscience.2003.10.012. 204. [DOI] [PubMed] [Google Scholar]
- 46.Watchko JF, Daood MJ, Hansen TWR. Brain bilirubin content is increased in P-glycoprotein deficient transgenic null mutant mice. Pediatr Res. 1998;44:763–766. doi: 10.1203/00006450-199811000-00020. [DOI] [PubMed] [Google Scholar]
- 47.Watchko JF, Daood MJ, Mahmood B, et al. P-glycoprotein and bilirubin disposition in the neonate. J Perinatol. 2001;21:S43–S47. doi: 10.1038/sj.jp.7210633. [DOI] [PubMed] [Google Scholar]
- 48.Zhang W, Mojsilovic-Petrovic J, Andrade MF, et al. The expression and functional characterization of ABCG2 in brain endothelial cells and vessels. FASEB J. 2003;17:2085–2087. doi: 10.1096/fj.02-1131fje. [DOI] [PubMed] [Google Scholar]