Abstract
Atherosclerosis has been considered a syndrome of dysregulated lipid storage until recent evidence has emphasized the critical contribution of the immune system. Dendritic cells (DC) are positioned at the interface of the innate and adaptive immune system. Recognition of danger signals in atheromas leads to DC activation. Activated DC regulate effector T cells which can kill plaque-resident cells and damage the plaque structure. Two types of DC have been identified in atherosclerotic lesions; classical myeloid DC (mDC) which mainly recognize bacterial signatures and plasmacytoid DC (pDC) which specialize in sensing viral fragments and have the unique potential of producing large amounts of type I interferon (IFN). In human atheromas, type I IFN upregulates expression of the cytotoxic molecule TRAIL which leads to apoptosis of plaque resident cells. This review will elucidate the role of DC in atherogenesis and particularly in plaque rupture, the underlying pathophysiologic cause of myocardial infarction.
Keywords: dendritic cells, T cells, atherosclerosis, plasmacytoid dendritic cells, apoptosis, type I interferon
Dendritic Cells in the Vessel Wall – From Healthy Arteries to Rupture-Prone Atherosclerotic Plaques
Dendritic cells (DC) are indigenous residents of healthy arteries and are typically localized in the sub-endothelial space as well as at the media-adventitia junction [1, 2]. It has now been proposed that such wall-embedded DC play an important role in the surveillance of the arterial wall and in tolerization against autoantigens by silencing T-cell responses [3]. However, once activated sufficiently, vascular DC may also present autoantigens to T cells and initiate inflammatory responses directly in the arterial wall. Modification of autoantigens and molecular mimicry may lead to recognition of self-determinants in this unique tissue niche. DC are primarily activated by sensing potential dangers in an antigen-independent way via scavenger receptors recognizing typical damage-associated molecular patterns. Localization of DC adjacent to vasa vasorum allows them to monitor the most important access pathways to the vessel wall and screen the tissue environment for the appearance of exogenous and indigenous stressors. Millonig et al. described that already at early stages of atherosclerosis, DC appear in the subendothelial layer, particularly in areas exposed to turbulent flow conditions [4]. More than 10 years ago, Bobryshev et al. reported for the first time that DC accumulate in atherosclerotic lesions and concluded that DC may play an important role in the disease process [5]. Yilmaz et al. located DC mainly in rupture-prone areas of the atherosclerotic plaque where they exhibit a mature phenotype [6]. In accordance, we found the presence of DC to be associated with features of plaque instability [7]. Additionally, higher DC densities have been reported in carotid plaques from symptomatic patients compared with those from asymptomatic patients [8]. In addition to visualizing dendrites, typical morphologic features of DC, the presence of DC in the atherosclerotic plaque has been confirmed by using a variety of antibodies recognizing DC markers in humans and mice (CD11c, CD1a, S-100, CD83, and DC-SIGN). Recent reviews have given comprehensive insight into the diversity of immune cells in atherosclerosis [9, 10]. This review aims to further elucidate the functional role of DC in atherogenesis and, in particular, in plaque destabilization ultimately leading to plaque rupture and acute coronary syndrome (ACS). DC in the vessel wall almost certainly participate in other clinical circumstances such as aortic aneurysms [11] and in-stent restenosis and balloon injury [12, 13], and much can be learned from comparing their immunoregulatory functions in different settings of vascular inflammation.
The Circulating Pool of Dendritic Cells
In healthy individuals DC constitute about 0.3% of circulating peripheral blood mononuclear cells [14]. Circulating DC encompass immature and mature forms trafficking to different organ destinations. There are conflicting data about whether circulating DC are increased or decreased in patients with stable coronary artery disease compared to healthy controls [15, 16]. During ACS, circulating DC are markedly decreased. At the same time, DC accumulate in vulnerable atherosclerotic tissue [14]. One may speculate that circulating DC evade into inflamed tissue sites attracted by chemokines produced by the inflammatory infiltrate occupying the plaque. However, accumulation of DC into a single tissue site cannot be responsible for the major changes in the number of circulating DC reported so far. More likely, DC may also migrate into lymphoid tissues in response to systemic inflammatory activation which redirects trafficking and compartmentalization of antigen-presenting DC as well as lymphocytes. The process of redistribution of DC and their accumulation in tissue niches, such as atherosclerotic plaques, may be affected by changes in the lipid profile which are prototypic for atherosclerotic disease [17].
Recruitment of Dendritic Cells into the Atherosclerotic Lesion
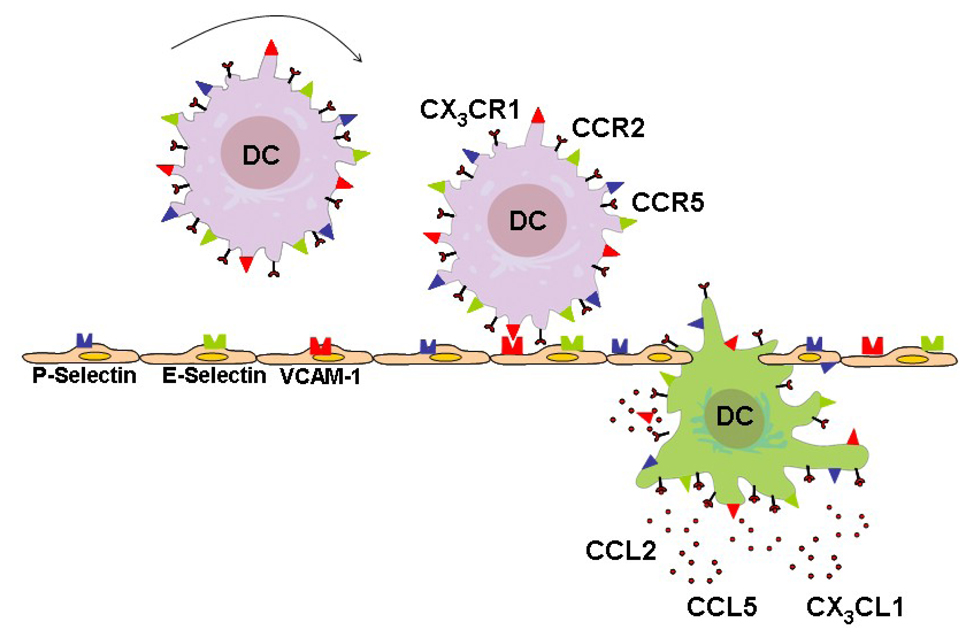
Adhesion and chemotaxis are requisites for invasion of DC into the inflamed atheroma (Figure 1). Adhesion molecules such as P- and E-selectin, and VCAM-1 are responsible for tethering DC to the microvascular bed (Figure 1)[18, 19]. Hypoxia, oxidized low-density lipoprotein, tumor necrosis factor-α, and inhibition of endothelial NO synthase may all augment adhesion of DC to the endothelium [20]. In addition, adhesion of DC to injured vessels may be mediated by platelets covering the lesion [21]. Conversely, statin treatment may decrease adhesion of DC [22].
Figure 1. Recruitment of Dendritic Cells into the Atherosclerotic Plaque.
DC are tethered to activated endothelium covering atherosclerotic plaques with the help of adhesion molecules including P-Selectin, E-Selectin, and VCAM-1. Production of chemokines in the atherosclerotic lesion determines which cells enter the lesion. The chemokines CCL2, CCL5, and CX3CL1 are abundantly expressed in the lesion and build a gradient towards the lesion in the vessel wall. DC express the corresponding receptors CX3CR1, CCR2, and CCR5 and follow the gradient towards the atheroma. Transmigration through the endothelium is associated with phenotypic changes of DC.
DC = dendritic cells
Upon fixation to the vessel wall, a chemotactic stimulus is required for invasion of DC into the tissue microenvironment. CCL2 and CCL5 are potential chemotactic candidates abundantly expressed in the inflamed atheroma (Figure 1)[23, 24]. These chemokines activate DC via binding to the respective G protein-coupled receptors and thereby recruit DC fixed to the vessel wall by building a gradient towards the inflamed tissue site. A recent publication of Liu et al. has shown that fractalkine may be another important chemokine for accumulation of DC in the atherosclerotic plaque [25]. Deficiency of the fractalkine receptor CX3CR1 resulted in decreased atherosclerosis and a decreased number of DC in atheromas. Transformed circulating monocytes are an additional source of DC. Monocytes may transform into DC under inflammatory conditions [26] with a potential role for granulocyte/macrophage colony-stimulating factor (GM-CSF) facilitating this transformation [26]. Knocking out GM-CSF resulted in a significant reduction of DC in murine atherosclerotic lesions [27]. Particularly, Ly-6Clow monocytes may differentiate into CD11c+ DC [28]. These cells rely on CCR5 to enter the atheroma.
Activation of Dendritic Cells
Recognition of damage-associated molecular patterns including endogenous alarm signals as well as pathogen-associated molecular patterns has checkpoint function in initiating the cascade of DC activation [29]. In turn, DC start to produce mediators of the innate immune system and express costimulatory molecules such as CD40, CD80 and CD86, which are crucial for induction of adaptive immune responses. The most thoroughly investigated receptors recognizing danger signals are Toll-like receptors (TLR, Table 1). Among them, TLR4 plays a central role in initiation and progression of atherosclerosis. This receptor has been shown to activate and mature DC in patients with ACS [30]. Markers of activation are spontaneously expressed on circulating DC from ACS patients, raising the possibility that they have been exposed to stimulatory ligands [31]. Fragments of bacteria such as lipopolysaccharides (LPS), modified autoantigens such as oxidized LDL, and heat-shock proteins are recognized by TLR4 and activate the subsequent signaling cascade [32, 33]. However, a recent publication indicates that oxidized lipoproteins may also inhibit TLR4 signaling [34]. TLR2 may also play an important role in atherogenesis, possibly due to activation of DC, e.g. by Chlamydia pneumoniae [35]. Furthermore, TLR7-, TLR8-, and TLR9-recognizing motifs of nucleic acids deriving from infectious pathogens may be involved in plaque destabilization. Vessel-specific TLR expression patterns inducing distinct types of vascular inflammation may explain the selective susceptibility of different vascular beds to atherosclerosis [2, 36, 37]. Disturbed blood flow may determine TLR expression patterns [38]. Activation of DC may lead to loss of tolerance and may fuel a local immune response [1]. While dyslipidemia favors aggravation of local inflammation and may break tolerance against autoantigens [17], severe dyslipidemia can lead to inhibition of the production of effector cytokines via TLR [39]. High concentrations of oxidized low density lipoprotein may also cause decreased activity of DC due to increased apoptosis of DC [32]. Further, nicotine has been shown to be a strong inducer of DC [40]. However, there are contradictory data indicating immunosuppressive effects of nicotine [41]. Hypoxia and hypoxia inducible factor 1α are emerging as alternate triggers of DC activation [42]. While hypoxia specifically induces cytokine production of DC, DC maturation and the capacity to stimulate T cells are impaired during hypoxic conditions preventing self reactivity [43]. Thereby the net effect of hypoxia on the contribution of DC to atherogenesis remains to be elucidated. Also, platelets have shown to induce DC maturation thereby enhancing DC-mediated lymphocyte proliferation [21]. Finally, C-reactive protein has been implicated in activating DC, but the responsible molecular mechanisms are unknown [44]. There is some evidence that statins may prevent accumulation and function of DC [6, 45, 46]. Also, diltiazem has been shown to delay DC maturation in cell culture studies [47].
Table 1.
Immune Triggers Activating DC in Atherosclerotic Disease
| Receptor | Ligand | Source |
|---|---|---|
| TLR2 | Lipoproteins, Peptidoglycans, Lipoteichoic acid |
Gram-positive bacteria, Mycoplasma, and other pathogens |
| entry-mediating envelope gp, gp B (gB) and gp H (gH)[62] |
CMV | |
| ? | HSV | |
| Heat shock protein 60 | Human and chlamydial | |
| Lipopolysaccharides | Porphyromonas gingivalis | |
| TLR3 | Double-stranded RNA | Viruses |
| ? | CMV | |
| TLR4 | Lipopolysaccharides | Outer membrane of gram-negative bacteria |
| Lipoteichoic acids | Gram-positive bacteria | |
| Protein F | Respiratory syncytial virus | |
| Heat shock protein 60 | Human and chlamydial | |
| Outer membrane protein? | Chlamydia | |
| Oxidized LDL (inhibitory role?), minimally modified LDL |
Human | |
| Fibronectin Extra Domain A | Human | |
| TLR5 | Flagellin | Bacteria with flagella, e.g. Salmonella |
| TLR7 | single stranded RNAs | Virus |
| TLR9 | Unmethylated CpG motifs | Bacterial DNA |
| DNA from CMV, HSV, Hepatitis B Virus |
Virus | |
| Human DNA? | Dying cells? |
modified from de Kleijn et al. [63]
Plaque Destabilizing Effector Functions of Dendritic Cells
Inclusion of DC in (bioengineered) vessels leads to infiltration of CD4 T cells [48]. DC-derived CCL19 and CCL21 have been implicated in orchestrating T-cell attraction. Further, DC also produce IL-12. This cytokine modifies the function of T cells by upregulation of the chemokine receptor CCR5, which in turn leads to accumulation of T cells into the atherosclerotic plaque [23]. Diltiazem has been shown to inhibit IL-12 production of DC, resulting in a decreased DC-dependent T-cell activation [49]. In the atherosclerotic plaque, T cells are positioned in close vicinity to DC [50]. However, DC–T-cell interaction may also take place in adjacent lymphoid organs. DC as professional antigen-presenting cells are crucial for priming of T cells as measured by production of IFN-γ [48]. DC present processed antigens complexed with HLA molecules to ligate the T-cell receptor (TCR). Biologic consequences include clonal expansion of T cells expressing a TCR specifically recognizing the presented antigen. In support of the concept that antigen recognition occurs in the plaque microenvironment, clonally expanded T cells have been found in human plaques [51]. Costimulatory signals provided by activated DC are crucial for full-blown activation of T cells. Nicotine augments the DC-mediated capacity of T-cell activation [40]. Thus, multiple factors acting within the plaque will shape the ultimate outcome of antigen recognition and T-cell activation. Statins may suppress the ability of DC to activate T cells [45].
Activated cytotoxic CD4 T cells have the ability to destabilize the atherosclerotic plaque by killing plaque-resident cells, such as endothelial cells and vascular smooth muscle cells forming the protective inner layer (Figure 2). These cells are equipped with cytoplasmic granules containing perforin and granzyme B. Perforin forms pores that enable granzyme B to enter the target cell. Granzyme B activates caspases within the target cells thereby inducing its apoptosis. Moreover, T-cell derived cytokines such as IFN-γ induce macrophage-mediated tissue damage, e. g. by metalloproteinase-induced digestion of the extracellular matrix. In addition to regulating the effector functions of T cells, DC have also been implicated in shaping the functional activity of natural killer cells thereby further enhancing the cytotoxic potential in the atherosclerotic plaque [52]. DC may also affect activation of CD8 T cells, another important fraction of cytotoxic T cells regularly found in advanced atheromas in close vicinity to DC [11, 53].
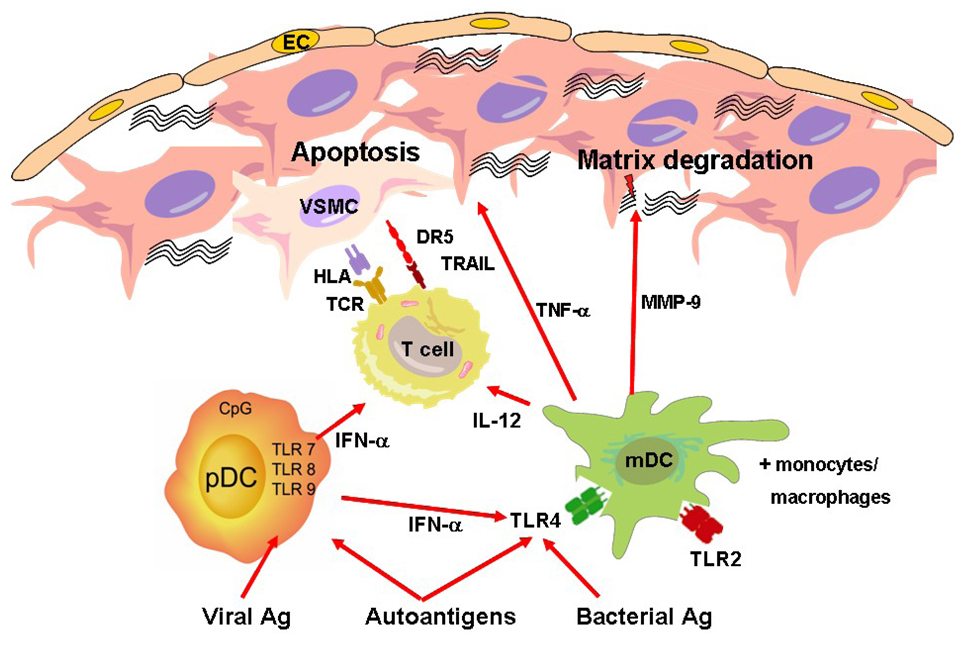
Figure 2. The Role of Dendritic Cells in the Atherosclerotic Plaque.
mDC are activated by exogenous and endogenous danger signals binding to TLR2 and TLR4. Activated mDC produce effector molecules such as metalloproteinases degrading the extracellular matrix. Further, they trigger the recruitment of cytotoxic T cells via production of IL-12. pDC are mainly activated by viral antigens binding to intracellular receptors such as TLR9. Activated pDC produce vast amounts of IFN-α. This cytokine enhances the sensitivity of other antigen-presenting cells by upregulation of TLR4. Furthermore, it upregulates the expression of the proapoptotic molecule TRAIL on T cells thereby multiplying their cytotoxic potential. These TRAIL-expressing T cells have the ability to kill plaque-resident cells such as activated VSMC and EC expressing the death receptor DR5.
VSMC = vascular smooth muscle cells, EC = endothelial cells, mDC = myeloid dendritic cells, pDC = plasmacytoid dendritic cells, MMP = metalloproteinase, DR5 = death receptor 5, TNF-α = tumor necrosis factor-alpha
Plasmacytoid Dendritic Cells in the Atherosclerotic Plaque
Recently, DC have been further divided into subsets, including conventional myeloid (m) and plasmacytoid (p)DC. pDC received their name because their shape resembles that of plasma cells [54], and about one third of circulating DC are classified as pDC. Various markers are used for identification of these cells. While BDCA-2 appears on immature pDC [55], CD123, the receptor for IL-3, is constitutively expressed on pDC. Typically, pDC show only low expression of CD11c while this marker is abundantly expressed by mDC. Apart from differences in morphology and expression of surface molecules, there are important functional differences between mDC and pDC (Table 2). pDC have a different TLR expression profile with abundant expression of TLR7, TLR8, and TLR9. These TLR are expressed intracellularly and recognize RNA and DNA deriving from pathogens, particularly viruses. In the atherosclerotic plaque, they may recognize viruses but also nucleotides deriving form dying cells. However, pDC may also recognize bacterial signatures [56]. It has been shown that, similar to mDC, circulating pDC are significantly reduced in patients with troponin-positive ACS [57].
Table 2.
Comparison of Myeloid and Plasmacytoid Dendritic Cells
| Dendritic Cell Subtype | Myeloid | Plasmacytoid |
|---|---|---|
| Circulating numbers* | 0.2% | 0.1% |
| Preferentially-expressed Toll- receptor profile |
TLR2, TLR4, (TLR5) | TLR7-TLR9 |
| Site of TLR expression | Cell surface | Cytoplasm |
| Recognition of | ||
| Pathogens | Bacterial fragments, e.g. LPS | Viral fragments, e.g. RNA |
| Autoantigens | Oxidized LDL, HSP60 | DNA from dying cells? |
| Typical effector cytokines | IL-12, TNF-α, IL-6 | Type I interferon |
| Effector function | Activation of T cells via HLA-antigen complexes and costimulatory molecules |
Regulation of cellular functions via type I interferon, e.g. induction of TRAIL on T cells |
| Crosstalk | Sensitized to TLR4 ligands by pDC- derived type I interferon |
Upregulation of TLR4 expression on mDC |
% of peripheral blood mononuclear cells
pDC have the unique function of producing large amounts of type I IFN. This cytokine exerts strong antiviral effects. Furthermore, it induces marked upregulaton of the molecule TRAIL on CD4 T cells [7]. TRAIL-expressing CD4 T cells effectively kill plaque-resident cells, potentially weakening the scaffold of the lesion and rendering the plaque vulnerable (Figure 2) [58]. Moreover, type I IFN produced by pDC also sensitizes mDC by upregulating TLR4 on their surface [59]. This interaction leads to a major amplification of immune responses as mDC and pDC are concomitantly triggered with different danger signals. This may for instance happen when a viral infection activates pDC to produce type I IFN while mDC are chronically stimulated by modified lipoproteins via TLR4. In accordance, type I IFN has been found to be associated with plaque instability in human atheromas [7]. In essence, interactions between distinct types of DC emerge as mechanisms of setting inflammatory thresholds in the atheroma, assigning a critical role to innate sensing tools in modulating the fate of the atherosclerotic lesion.
Outlook and Potential Therapeutic Immunomodulation
This review summarizes the crucial pathogenic role of DC in plaque inflammation, contributing to all stages of the atherosclerotic process. DC seem to be of particular importance in advanced vulnerable lesions. However, exploring their contribution in early stages of atherosclerosis is complicated by the extended time span through which this process proceeds. Capturing early steps in human atherosclerosis would literally require studying teenagers. DC are activated by recognition of damage-associated molecular patterns via scavenger receptors. While receptors on mDC mainly recognize bacterial signatures, pDC are specialized in recognizing viral particles. Also, modified autoantigens have the ability to stimulate DC in the atherosclerotic plaque. Activated DC participate in destabilizing the atherosclerotic plaque in two different ways. First, they are highly efficient antigen-presenting cells, determining the differentiation of T cells. A critical effector pathway exposing the plaque to risk of rupture is the activation of cytotoxic T cells. Secondly, DC induce production of proteases such as metalloproteinases which disintegrate the extracellular matrix. A close interplay among mDC, pDC, and other immune cells results in full-blown immune activation, paving the way for the detrimental rupture of the atherosclerotic plaque.
Depletion and repletion experiments in animal models of complex atherosclerotic lesions are necessary to explore which steps of the inflammatory cascade are regulated by these innate immune cells. It is important to keep in mind that DC may also be helpful in inducing tolerance against modified autoantigens in the microenvironment of the arterial wall and that this particular immune function could prove beneficial in novel therapeutic approaches to atherosclerosis. Particularly, DC may expand CD4+Foxp3+ T regulatory cells [60]. This T cell subtype is crucial for balancing immune responses and inhibits atherogenesis by secretion of transforming growth factor-beta and interleukin-10. There are ongoing efforts to develop a vaccination against autoantigens found in the atherosclerotic plaque to induce immune tolerance and avoid tissue-damaging immune responses [61]. The state of DC presenting such antigens to lymphocytes may be crucial for inducing tolerance. As the induction of immune memory may be irreversible, considerably more research on the safety of vaccination against epitopes found in atherosclerotic lesions is required. Developing experimental approaches to assign selected DC functions to certain stages of the atherosclerotic process seems particularly promising as it may be necessary to harness DC functions through diverse means. Both inhibiting unwanted immune responses and fostering protective immune responses may converge on the level of modulating DC function.
Acknowledgments
This work was funded in part by grants from the National Institutes of Health (RO1 AR42527, RO1 AR41974, R01 AI44142, R01 AI57266, RO1 EY11916, and R01 AG15043). The authors thank Tamela Yeargin for editing the manuscript and Sergey Pryshchep for his support in developing the figures.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Bibliography
- 1.Ma-Krupa W, Jeon MS, Spoerl S, Tedder TF, Goronzy JJ, Weyand CM. Activation of arterial wall dendritic cells and breakdown of self-tolerance in giant cell arteritis. J Exp Med. 2004;199:173–183. doi: 10.1084/jem.20030850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pryshchep O, Ma-Krupa W, Younge BR, Goronzy JJ, Weyand CM. Vessel-specific Toll-like receptor profiles in human medium and large arteries. Circulation. 2008;118:1276–1284. doi: 10.1161/CIRCULATIONAHA.108.789172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Steinman RM, Hawiger D, Liu K, Bonifaz L, Bonnyay D, Mahnke K, Iyoda T, Ravetch J, Dhodapkar M, Inaba K, Nussenzweig M. Dendritic cell function in vivo during the steady state: a role in peripheral tolerance. Ann N Y Acad Sci. 2003;987:15–25. doi: 10.1111/j.1749-6632.2003.tb06029.x. [DOI] [PubMed] [Google Scholar]
- 4.Millonig G, Niederegger H, Rabl W, Hochleitner BW, Hoefer D, Romani N, Wick G. Network of vascular-associated dendritic cells in intima of healthy young individuals. Arterioscler Thromb Vasc Biol. 2001;21:503–508. doi: 10.1161/01.atv.21.4.503. [DOI] [PubMed] [Google Scholar]
- 5.Bobryshev YV, Lord RS. S-100 positive cells in human arterial intima and in atherosclerotic lesions. Cardiovasc Res. 1995;29:689–696. [PubMed] [Google Scholar]
- 6.Yilmaz A, Lochno M, Traeg F, Cicha I, Reiss C, Stumpf C, Raaz D, Anger T, Amann K, Probst T, Ludwig J, Daniel WG, Garlichs CD. Emergence of dendritic cells in rupture-prone regions of vulnerable carotid plaques. Atherosclerosis. 2004;176:101–110. doi: 10.1016/j.atherosclerosis.2004.04.027. [DOI] [PubMed] [Google Scholar]
- 7.Niessner A, Sato K, Chaikof EL, Colmegna I, Goronzy JJ, Weyand CM. Pathogen-sensing plasmacytoid dendritic cells stimulate cytotoxic T-cell function in the atherosclerotic plaque through interferon-alpha. Circulation. 2006;114:2482–2489. doi: 10.1161/CIRCULATIONAHA.106.642801. [DOI] [PubMed] [Google Scholar]
- 8.Kawahara I, Kitagawa N, Tsutsumi K, Nagata I, Hayashi T, Koji T. The expression of vascular dendritic cells in human atherosclerotic carotid plaques. Hum Pathol. 2007;38:1378–1385. doi: 10.1016/j.humpath.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 9.Hansson GK, Libby P. The immune response in atherosclerosis: a double-edged sword. Nat Rev Immunol. 2006;6:508–519. doi: 10.1038/nri1882. [DOI] [PubMed] [Google Scholar]
- 10.Bobryshev YV. Dendritic cells in atherosclerosis: current status of the problem and clinical relevance. Eur Heart J. 2005;26:1700–1704. doi: 10.1093/eurheartj/ehi282. [DOI] [PubMed] [Google Scholar]
- 11.Bobryshev YV, Lord RS, Parsson H. Immunophenotypic analysis of the aortic aneurysm wall suggests that vascular dendritic cells are involved in immune responses. Cardiovasc Surg. 1998;6:240–249. doi: 10.1016/s0967-2109(97)00168-3. [DOI] [PubMed] [Google Scholar]
- 12.Bauriedel G, Jabs A, Skowasch D, Hutter R, Badimon JJ, Fuster V, Welsch U, Luderitz B. Dendritic cells in neointima formation after rat carotid balloon injury: coordinated expression withanti-apoptotic Bcl-2 and HSP47 in arterial repair. J Am Coll Cardiol. 2003;42:930–938. doi: 10.1016/s0735-1097(03)00828-3. [DOI] [PubMed] [Google Scholar]
- 13.Skowasch D, Jabs A, Andrie R, Dinkelbach S, Luderitz B, Bauriedel G. Presence of bone-marrow- and neural-crest-derived cells in intimal hyperplasia at the time of clinical in-stent restenosis. Cardiovasc Res. 2003;60:684–691. doi: 10.1016/j.cardiores.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 14.Yilmaz A, Weber J, Cicha I, Stumpf C, Klein M, Raithel D, Daniel WG, Garlichs CD. Decrease in circulating myeloid dendritic cell precursors in coronary artery disease. J Am Coll Cardiol. 2006;48:70–80. doi: 10.1016/j.jacc.2006.01.078. [DOI] [PubMed] [Google Scholar]
- 15.Shi H, Ge J, Fang W, Yao K, Sun A, Huang R, Jia Q, Wang K, Zou Y, Cao X. Peripheral-blood dendritic cells in men with coronary heart disease. Am J Cardiol. 2007;100:593–597. doi: 10.1016/j.amjcard.2007.03.067. [DOI] [PubMed] [Google Scholar]
- 16.Yilmaz A, Schaller T, Cicha I, Altendorf R, Stumpf C, Klinghammer L, Ludwig J, Daniel WG, Garlichs CD. Predictive value of the decrease in circulating dendritic cell precursors in stable coronary artery disease. Clin Sci (Lond) 2008 doi: 10.1042/CS20080392. [DOI] [PubMed] [Google Scholar]
- 17.Angeli V, Llodra J, Rong JX, Satoh K, Ishii S, Shimizu T, Fisher EA, Randolph GJ. Dyslipidemia associated with atherosclerotic disease systemically alters dendritic cell mobilization. Immunity. 2004;21:561–574. doi: 10.1016/j.immuni.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 18.Bobryshev YV, Lord RS, Rainer SP, Munro VF. VCAM-1 expression and network of VCAM-1 positive vascular dendritic cells in advanced atherosclerotic lesions of carotid arteries and aortas. Acta Histochem. 1996;98:185–194. doi: 10.1016/S0065-1281(96)80037-7. [DOI] [PubMed] [Google Scholar]
- 19.Alvarez D, Vollmann EH, von Andrian UH. Mechanisms and consequences of dendritic cell migration. Immunity. 2008;29:325–342. doi: 10.1016/j.immuni.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weis M, Schlichting CL, Engleman EG, Cooke JP. Endothelial determinants of dendritic cell adhesion and migration: new implications for vascular diseases. Arterioscler Thromb Vasc Biol. 2002;22:1817–1823. doi: 10.1161/01.atv.0000036418.04998.d5. [DOI] [PubMed] [Google Scholar]
- 21.Langer HF, Daub K, Braun G, Schonberger T, May AE, Schaller M, Stein GM, Stellos K, Bueltmann A, Siegel-Axel D, Wendel HP, Aebert H, Roecken M, Seizer P, Santoso S, Wesselborg S, Brossart P, Gawaz M. Platelets recruit human dendritic cells via Mac-1/JAM-C interaction and modulate dendritic cell function in vitro. Arterioscler Thromb Vasc Biol. 2007;27:1463–1470. doi: 10.1161/ATVBAHA.107.141515. [DOI] [PubMed] [Google Scholar]
- 22.Kofler S, Schlichting C, Jankl S, Nickel T, Weis M. Dual mode of HMG-CoA reductase inhibition on dendritic cell invasion. Atherosclerosis. 2008;197:105–110. doi: 10.1016/j.atherosclerosis.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 23.Zhang X, Niessner A, Nakajima T, Ma-Krupa W, Kopecky SL, Frye RL, Goronzy JJ, Weyand CM. Interleukin 12 induces T-cell recruitment into the atherosclerotic plaque. Circ Res. 2006;98:524–531. doi: 10.1161/01.RES.0000204452.46568.57. [DOI] [PubMed] [Google Scholar]
- 24.Charo IF, Taubman MB. Chemokines in the pathogenesis of vascular disease. Circ Res. 2004;95:858–866. doi: 10.1161/01.RES.0000146672.10582.17. [DOI] [PubMed] [Google Scholar]
- 25.Liu P, Yu YR, Spencer JA, Johnson AE, Vallanat CT, Fong AM, Patterson C, Patel DD. CX3CR1 deficiency impairs dendritic cell accumulation in arterial intima and reduces atherosclerotic burden. Arterioscler Thromb Vasc Biol. 2008;28:243–250. doi: 10.1161/ATVBAHA.107.158675. [DOI] [PubMed] [Google Scholar]
- 26.Shortman K, Naik SH. Steady-state and inflammatory dendritic-cell development. Nat Rev Immunol. 2007;7:19–30. doi: 10.1038/nri1996. [DOI] [PubMed] [Google Scholar]
- 27.Shaposhnik Z, Wang X, Weinstein M, Bennett BJ, Lusis AJ. Granulocyte macrophage colony-stimulating factor regulates dendritic cell content of atherosclerotic lesions. Arterioscler Thromb Vasc Biol. 2007;27:621–627. doi: 10.1161/01.ATV.0000254673.55431.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tacke F, Alvarez D, Kaplan TJ, Jakubzick C, Spanbroek R, Llodra J, Garin A, Liu J, Mack M, van Rooijen N, Lira SA, Habenicht AJ, Randolph GJ. Monocyte subsets differentially employ CCR2, CCR5, and CX3CR1 to accumulate within atherosclerotic plaques. J Clin Invest. 2007;117:185–194. doi: 10.1172/JCI28549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Matzinger P. Friendly and dangerous signals: is the tissue in control? Nat Immunol. 2007;8:11–13. doi: 10.1038/ni0107-11. [DOI] [PubMed] [Google Scholar]
- 30.Wang L, Li D, Yang K, Hu Y, Zeng Q. Toll-like receptor-4 and mitogen-activated protein kinase signal system are involved in activation of dendritic cells in patients with acute coronary syndrome. Immunology. 2008;125:122–130. doi: 10.1111/j.1365-2567.2008.02827.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Erbel C, Sato K, Meyer FB, Kopecky SL, Frye RL, Goronzy JJ, Weyand CM. Functional profile of activated dendritic cells in unstable atherosclerotic plaque. Basic Res Cardiol. 2007;102:123–132. doi: 10.1007/s00395-006-0636-x. [DOI] [PubMed] [Google Scholar]
- 32.Alderman CJ, Bunyard PR, Chain BM, Foreman JC, Leake DS, Katz DR. Effects of oxidised low density lipoprotein on dendritic cells: a possible immunoregulatory component of the atherogenic micro-environment? Cardiovasc Res. 2002;55:806–819. doi: 10.1016/s0008-6363(02)00447-9. [DOI] [PubMed] [Google Scholar]
- 33.Shen LH, Zhou L, Wang BY, Pu J, Hu LH, Chai DJ, Wang L, Zeng JZ, He B. Oxidized low-density lipoprotein induces differentiation of RAW264.7 murine macrophage cell line into dendritic-like cells. Atherosclerosis. 2008;199:257–264. doi: 10.1016/j.atherosclerosis.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 34.von Schlieffen E, Oskolkova OV, Schabbauer G, Gruber F, Bluml S, Genest M, Kadl A, Marsik C, Knapp S, Chow J, Leitinger N, Binder BR, Bochkov VN. Multi-Hit Inhibition of Circulating and Cell-Associated Components of the Toll-Like Receptor 4 Pathway by Oxidized Phospholipids. Arterioscler Thromb Vasc Biol. 2008 doi: 10.1161/ATVBAHA.108.173799. [DOI] [PubMed] [Google Scholar]
- 35.Naiki Y, Sorrentino R, Wong MH, Michelsen KS, Shimada K, Chen S, Yilmaz A, Slepenkin A, Schroder NW, Crother TR, Bulut Y, Doherty TM, Bradley M, Shaposhnik Z, Peterson EM, Tontonoz P, Shah PK, Arditi M. TLR/MyD88 and liver X receptor alpha signaling pathways reciprocally control Chlamydia pneumoniae-induced acceleration of atherosclerosis. J Immunol. 2008;181:7176–7185. doi: 10.4049/jimmunol.181.10.7176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Deng J, Ma-Krupa W, Gewirtz AT, Younge BR, Goronzy JJ, Weyand CM. Toll-like receptors 4 and 5 induce distinct types of vasculitis. Circ Res. 2009;104:488–495. doi: 10.1161/CIRCRESAHA.108.185777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yilmaz A, Arditi M. Giant cell arteritis: dendritic cells take two T's to tango. Circ Res. 2009;104:425–427. doi: 10.1161/CIRCRESAHA.109.194266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mullick AE, Soldau K, Kiosses WB, Bell TA, 3rd, Tobias PS, Curtiss LK. Increased endothelial expression of Toll-like receptor 2 at sites of disturbed blood flow exacerbates early atherogenic events. J Exp Med. 2008;205:373–383. doi: 10.1084/jem.20071096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shamshiev AT, Ampenberger F, Ernst B, Rohrer L, Marsland BJ, Kopf M. Dyslipidemia inhibits Toll-like receptor-induced activation of CD8alpha-negative dendritic cells and protective Th1 type immunity. J Exp Med. 2007;204:441–452. doi: 10.1084/jem.20061737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Aicher A, Heeschen C, Mohaupt M, Cooke JP, Zeiher AM, Dimmeler S. Nicotine strongly activates dendritic cell-mediated adaptive immunity: potential role for progression of atherosclerotic lesions. Circulation. 2003;107:604–611. doi: 10.1161/01.cir.0000047279.42427.6d. [DOI] [PubMed] [Google Scholar]
- 41.Nouri-Shirazi M, Guinet E. Evidence for the immunosuppressive role of nicotine on human dendritic cell functions. Immunology. 2003;109:365–373. doi: 10.1046/j.1365-2567.2003.01655.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jantsch J, Chakravortty D, Turza N, Prechtel AT, Buchholz B, Gerlach RG, Volke M, Glasner J, Warnecke C, Wiesener MS, Eckardt KU, Steinkasserer A, Hensel M, Willam C. Hypoxia and hypoxia-inducible factor-1 alpha modulate lipopolysaccharide-induced dendritic cell activation and function. J Immunol. 2008;180:4697–4705. doi: 10.4049/jimmunol.180.7.4697. [DOI] [PubMed] [Google Scholar]
- 43.Mancino A, Schioppa T, Larghi P, Pasqualini F, Nebuloni M, Chen IH, Sozzani S, Austyn JM, Mantovani A, Sica A. Divergent effects of hypoxia on dendritic cell functions. Blood. 2008;112:3723–3734. doi: 10.1182/blood-2008-02-142091. [DOI] [PubMed] [Google Scholar]
- 44.Van Vre EA, Bult H, Hoymans VY, Van Tendeloo VF, Vrints CJ, Bosmans JM. Human C-reactive protein activates monocyte-derived dendritic cells and induces dendritic cell-mediated T-cell activation. Arterioscler Thromb Vasc Biol. 2008;28:511–518. doi: 10.1161/ATVBAHA.107.157016. [DOI] [PubMed] [Google Scholar]
- 45.Yilmaz A, Reiss C, Weng A, Cicha I, Stumpf C, Steinkasserer A, Daniel WG, Garlichs CD. Differential effects of statins on relevant functions of human monocyte-derived dendritic cells. J Leukoc Biol. 2006;79:529–538. doi: 10.1189/jlb.0205064. [DOI] [PubMed] [Google Scholar]
- 46.Yilmaz A, Reiss C, Tantawi O, Weng A, Stumpf C, Raaz D, Ludwig J, Berger T, Steinkasserer A, Daniel WG, Garlichs CD. HMG-CoA reductase inhibitors suppress maturation of human dendritic cells: new implications for atherosclerosis. Atherosclerosis. 2004;172:85–93. doi: 10.1016/j.atherosclerosis.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 47.Bachetoni A, D'Ambrosio A, Mariani P, Cortesini R, Quintieri F. Diltiazem affects human dendritic cell maturation. Transplant Proc. 2001;33:231–233. doi: 10.1016/s0041-1345(00)01989-8. [DOI] [PubMed] [Google Scholar]
- 48.Han JW, Shimada K, Ma-Krupa W, Johnson TL, Nerem RM, Goronzy JJ, Weyand CM. Vessel wall-embedded dendritic cells induce T-cell autoreactivity and initiate vascular inflammation. Circ Res. 2008;102:546–553. doi: 10.1161/CIRCRESAHA.107.161653. [DOI] [PubMed] [Google Scholar]
- 49.Bachetoni A, D'Ambrosio A, Mariani P, Cortesini R, Quintieri F. Diltiazem impairs maturation and functions of human dendritic cells. Hum Immunol. 2002;63:524–533. doi: 10.1016/s0198-8859(02)00407-x. [DOI] [PubMed] [Google Scholar]
- 50.Bobryshev YV, Lord RS. Mapping of vascular dendritic cells in atherosclerotic arteries suggests their involvement in local immune-inflammatory reactions. Cardiovasc Res. 1998;37:799–810. doi: 10.1016/s0008-6363(97)00229-0. [DOI] [PubMed] [Google Scholar]
- 51.Stemme S, Rymo L, Hansson GK. Polyclonal origin of T lymphocytes in human atherosclerotic plaques. Lab Invest. 1991;65:654–660. [PubMed] [Google Scholar]
- 52.Bobryshev YV, Lord RS. Co-accumulation of dendritic cells and natural killer T cells within rupture-prone regions in human atherosclerotic plaques. J Histochem Cytochem. 2005;53:781–785. doi: 10.1369/jhc.4B6570.2005. [DOI] [PubMed] [Google Scholar]
- 53.Gewaltig J, Kummer M, Koella C, Cathomas G, Biedermann BC. Requirements for CD8 T-cell migration into the human arterial wall. Hum Pathol. 2008;39:1756–1762. doi: 10.1016/j.humpath.2008.04.018. [DOI] [PubMed] [Google Scholar]
- 54.Asselin-Paturel C, Trinchieri G. Production of type I interferons: plasmacytoid dendritic cells and beyond. J Exp Med. 2005;202:461–465. doi: 10.1084/jem.20051395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dzionek A, Fuchs A, Schmidt P, Cremer S, Zysk M, Miltenyi S, Buck DW, Schmitz J. BDCA-2, BDCA-3, and BDCA-4: three markers for distinct subsets of dendritic cells in human peripheral blood. J Immunol. 2000;165:6037–6046. doi: 10.4049/jimmunol.165.11.6037. [DOI] [PubMed] [Google Scholar]
- 56.Decker T, Muller M, Stockinger S. The yin and yang of type I interferon activity in bacterial infection. Nat Rev Immunol. 2005;5:675–687. doi: 10.1038/nri1684. [DOI] [PubMed] [Google Scholar]
- 57.Van Vre EA, Hoymans VY, Bult H, Lenjou M, Van Bockstaele DR, Vrints CJ, Bosmans JM. Decreased number of circulating plasmacytoid dendritic cells in patients with atherosclerotic coronary artery disease. Coron Artery Dis. 2006;17:243–248. doi: 10.1097/00019501-200605000-00007. [DOI] [PubMed] [Google Scholar]
- 58.Sato K, Niessner A, Kopecky SL, Frye RL, Goronzy JJ, Weyand CM. TRAIL-expressing T cells induce apoptosis of vascular smooth muscle cells in the atherosclerotic plaque. J Exp Med. 2006;203:239–250. doi: 10.1084/jem.20051062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Niessner A, Shin MS, Pryshchep O, Goronzy JJ, Chaikof EL, Weyand CM. Synergistic proinflammatory effects of the antiviral cytokine interferon-alpha and Toll-like receptor 4 ligands in the atherosclerotic plaque. Circulation. 2007;116:2043–2052. doi: 10.1161/CIRCULATIONAHA.107.697789. [DOI] [PubMed] [Google Scholar]
- 60.Lan YY, Wang Z, Raimondi G, Wu W, Colvin BL, de Creus A, Thomson AW. "Alternatively activated" dendritic cells preferentially secrete IL-10, expand Foxp3+CD4+ T cells, and induce long-term organ allograft survival in combination with CTLA4-Ig. J Immunol. 2006;177:5868–5877. doi: 10.4049/jimmunol.177.9.5868. [DOI] [PubMed] [Google Scholar]
- 61.Shah PK, Chyu KY, Fredrikson GN, Nilsson J. Vaccination for atherosclerosis: a novel therapeutic paradigm. Expert Rev Vaccines. 2004;3:711–716. doi: 10.1586/14760584.3.6.711. [DOI] [PubMed] [Google Scholar]
- 62.Boehme KW, Guerrero M, Compton T. Human cytomegalovirus envelope glycoproteins B and H are necessary for TLR2 activation in permissive cells. J Immunol. 2006;177:7094–7102. doi: 10.4049/jimmunol.177.10.7094. [DOI] [PubMed] [Google Scholar]
- 63.de Kleijn D, Pasterkamp G. Toll-like receptors in cardiovascular diseases. Cardiovasc Res. 2003;60:58–67. doi: 10.1016/s0008-6363(03)00348-1. [DOI] [PubMed] [Google Scholar]