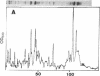
Abstract
High levels of phosphocreatine, a compound known to serve as an intracellular energy reserve, were found in the fluid contained in seminal vesicle glands. The concentrations of phosphocreatine in the extracellular fluid in the mouse and rat were found to be 5.6 +/- 1.6 and 2.2 +/- 0.8 mumol/g, respectively, which are higher than the intracellular levels reported for smooth muscles. The creatine concentrations in the seminal vesicular fluid from these two species were 22.8 +/- 3.1 and 13.0 +/- 5.3 mumol/g, respectively. These creatine levels are approximately 100 and 65 times higher than the creatine levels in mammalian blood. Smaller amounts of ATP (phosphocreatine/ATP ratio of 20-40) and traces of ADP were also found. Comparison of the pattern of distribution of macromolecules (proteins and DNA) with the distribution of phosphocreatine between the cells and the fluid of the seminal vesicle indicates that cell lysis did not account for the phosphocreatine in the seminal vesicle fluid. Rather, the available evidence strongly suggests that this high-energy compound is actively secreted. We found that in the testes, the sperm are exposed to the highest known creatine concentration in any mammalian tissue studied. Based on these results and other recent reports, we propose that the extracellular phosphocreatine, ATP, and creatine are involved in sperm metabolism.
Full text
PDF

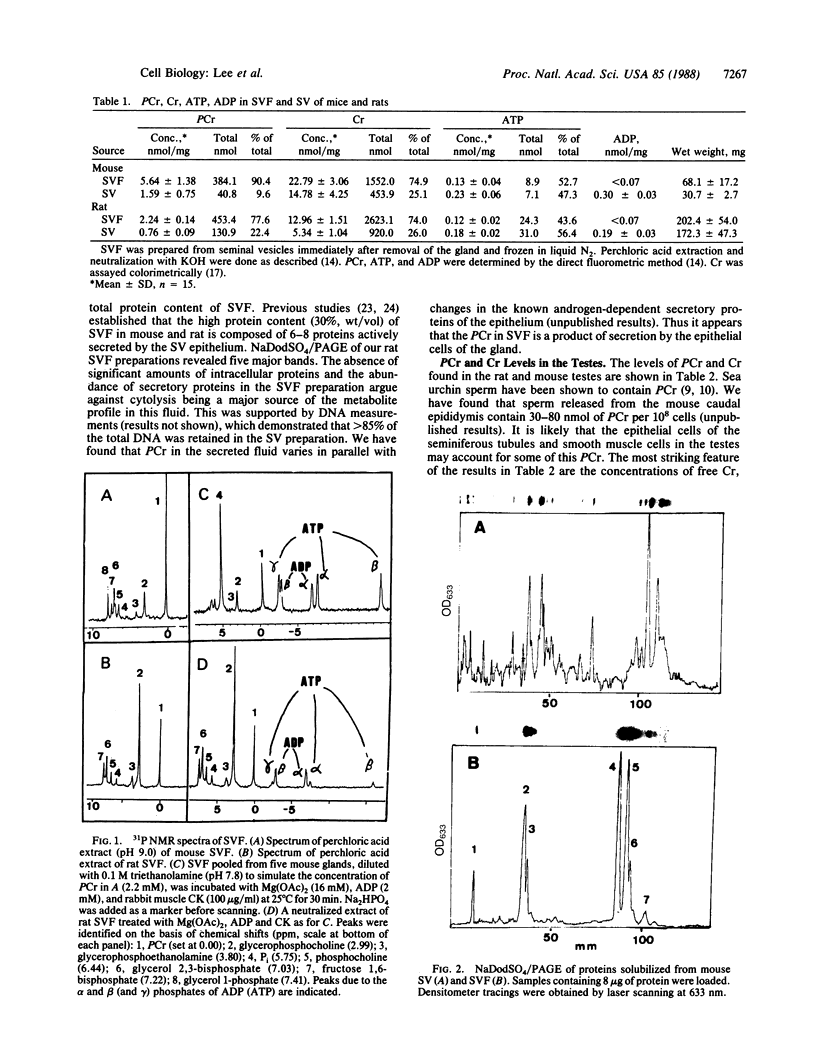
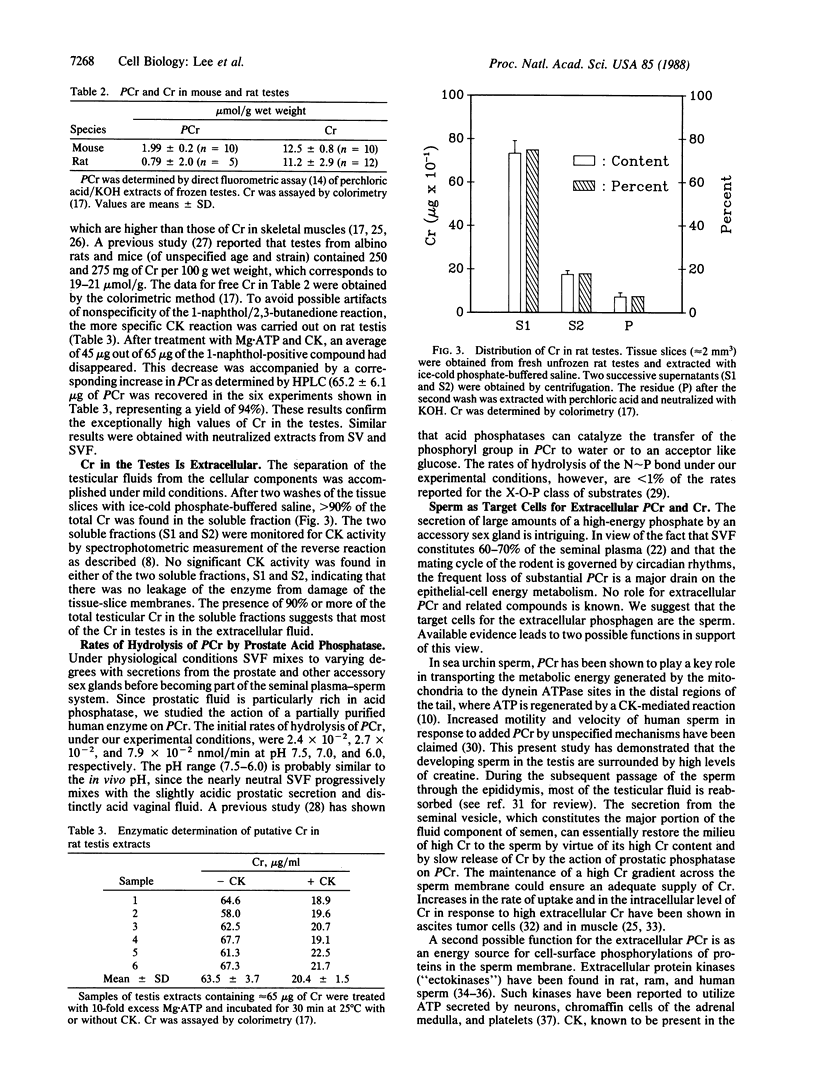

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALEKSEEVA A. M., TIMOFEEVA N. M. Kreatin i kreatinfosfat v semennikakh razlichnykh zhivotnykh. Biokhimiia. 1957 Nov-Dec;22(6):976–980. [PubMed] [Google Scholar]
- Allen G. W., Haake P. Mechanism of phosphorylation by N'-phosphorocreatine. Concurrent formation of creatine and creatinine. J Am Chem Soc. 1976 Aug 4;98(16):4990–4996. doi: 10.1021/ja00432a046. [DOI] [PubMed] [Google Scholar]
- Asseo P. P., Panidis D. K., Papadimas J. S., Ikkos D. G. Creatine kinase in seminal plasma of infertile men: activity and isoenzymes. Int J Androl. 1981 Aug;4(4):431–439. doi: 10.1111/j.1365-2605.1981.tb00727.x. [DOI] [PubMed] [Google Scholar]
- Bessman S. P., Geiger P. J. Transport of energy in muscle: the phosphorylcreatine shuttle. Science. 1981 Jan 30;211(4481):448–452. doi: 10.1126/science.6450446. [DOI] [PubMed] [Google Scholar]
- Butler T. M., Siegman M. J., Mooers S. U., Davies R. E. Chemical energetics of single isometric tetani in mammalian smooth muscle. Am J Physiol. 1978 Jul;235(1):C1–C7. doi: 10.1152/ajpcell.1978.235.1.C1. [DOI] [PubMed] [Google Scholar]
- Chen Y. H., Pentecost B. T., McLachlan J. A., Teng C. T. The androgen-dependent mouse seminal vesicle secretory protein IV: characterization and complementary deoxyribonucleic acid cloning. Mol Endocrinol. 1987 Oct;1(10):707–716. doi: 10.1210/mend-1-10-707. [DOI] [PubMed] [Google Scholar]
- DAWSON R. M., MANN T., WHITE I. G. Glycerylphosphorylcholine and phosphorylcholine in semen, and their relation to choline. Biochem J. 1957 Apr;65(4):627–634. doi: 10.1042/bj0650627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggleton P., Eggleton G. P. The Inorganic Phosphate and a Labile Form of Organic Phosphate in the Gastrocnemius of the Frog. Biochem J. 1927;21(1):190–195. doi: 10.1042/bj0210190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggleton P., Elsden S. R., Gough N. The estimation of creatine and of diacetyl. Biochem J. 1943;37(5):526–529. doi: 10.1042/bj0370526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fakih H., MacLusky N., DeCherney A., Wallimann T., Huszar G. Enhancement of human sperm motility and velocity in vitro: effects of calcium and creatine phosphate. Fertil Steril. 1986 Nov;46(5):938–944. doi: 10.1016/s0015-0282(16)49839-0. [DOI] [PubMed] [Google Scholar]
- Fiske C. H., Subbarow Y. THE NATURE OF THE "INORGANIC PHOSPHATE" IN VOLUNTARY MUSCLE. Science. 1927 Apr 22;65(1686):401–403. doi: 10.1126/science.65.1686.401. [DOI] [PubMed] [Google Scholar]
- Fitch C. D., Shields R. P. Creatine metabolism in skeletal muscle. I. Creatine movement across muscle membranes. J Biol Chem. 1966 Aug 10;241(15):3611–3614. [PubMed] [Google Scholar]
- Glonek T., Kopp S. J., Kot E., Pettegrew J. W., Harrison W. H., Cohen M. M. P-31 nuclear magnetic resonance analysis of brain: the perchloric acid extract spectrum. J Neurochem. 1982 Nov;39(5):1210–1219. doi: 10.1111/j.1471-4159.1982.tb12557.x. [DOI] [PubMed] [Google Scholar]
- Haldar S., Majumder G. C. Phosphorylation of external cell-surface proteins by an endogenous ecto-protein kinase of goat epididymal intact spermatozoa. Biochim Biophys Acta. 1986 Aug 1;887(3):291–303. doi: 10.1016/0167-4889(86)90157-6. [DOI] [PubMed] [Google Scholar]
- Iyengar M. R., Coleman D. W., Butler T. M. Phosphocreatinine, a high-energy phosphate in muscle, spontaneously forms phosphocreatine and creatinine under physiological conditions. J Biol Chem. 1985 Jun 25;260(12):7562–7567. [PubMed] [Google Scholar]
- Iyengar M. R., Iyengar C. W., Chen H. Y., Brinster R. L., Bornslaeger E., Schultz R. M. Expression of creatine kinase isoenzyme during oogenesis and embryogenesis in the mouse. Dev Biol. 1983 Mar;96(1):263–268. doi: 10.1016/0012-1606(83)90327-5. [DOI] [PubMed] [Google Scholar]
- Koons S. J., Eckert B. S., Zobel C. R. Immunofluorescence and inhibitor studies on creatine kinase and mitosis. Exp Cell Res. 1982 Aug;140(2):401–409. doi: 10.1016/0014-4827(82)90130-6. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Loike J. D., Zalutsky D. L., Kaback E., Miranda A. F., Silverstein S. C. Extracellular creatine regulates creatine transport in rat and human muscle cells. Proc Natl Acad Sci U S A. 1988 Feb;85(3):807–811. doi: 10.1073/pnas.85.3.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majumder G. C. Enzymic characteristics of an ecto-cyclic AMP-dependent protein kinase in rat epididymal spermatozoa. Biochem J. 1981 Apr 1;195(1):111–117. doi: 10.1042/bj1950111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newsholme E. A., Beis I., Leech A. R., Zammit V. A. The role of creatine kinase and arginine kinase in muscle. Biochem J. 1978 Jun 15;172(3):533–537. doi: 10.1042/bj1720533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrowski M. C., Kistler M. K., Kistler W. S. Purification and cell-free synthesis of a major protein from rat seminal vesicle secretion. A potential marker for androgen action. J Biol Chem. 1979 Jan 25;254(2):383–390. [PubMed] [Google Scholar]
- Schoff P. K., Forrester I. T., Haley B. E., Atherton R. W. A study of cAMP binding proteins on intact and disrupted sperm cells using 8-azidoadenosine 3',5'-cyclic monophosphate. J Cell Biochem. 1982;19(1):1–15. doi: 10.1002/jcb.240190102. [DOI] [PubMed] [Google Scholar]
- Tombes R. M., Shapiro B. M. Metabolite channeling: a phosphorylcreatine shuttle to mediate high energy phosphate transport between sperm mitochondrion and tail. Cell. 1985 May;41(1):325–334. doi: 10.1016/0092-8674(85)90085-6. [DOI] [PubMed] [Google Scholar]
- Winkler M. M., Matson G. B., Hershey J. W., Bradbury E. M. 31P-NMR study of the activation of the sea urchin egg. Exp Cell Res. 1982 May;139(1):217–222. doi: 10.1016/0014-4827(82)90335-4. [DOI] [PubMed] [Google Scholar]