Abstract
Despite the paradigm that the innate immune system uses nucleic acid-specific receptors to detect viruses due to a lack of other conserved features, a number of viruses are recognized by TLR2 and TLR4. The relevance of this recognition for antiviral immunity remains largely unexplained. Here we report that TLR2 activation by viruses leads to production of type I interferon (IFN). TLR2-dependent induction of type I IFN only occurs in response to viral ligands, indicating that TLR2 is capable of discriminating between pathogen classes. We demonstrate that this specialized response is mediated by Ly6Chigh inflammatory monocytes. Thus, the innate immune system can detect certain non-nucleic acid features of viruses and links this recognition to specific antiviral gene induction.
Introduction
Receptors of the innate immune system have evolved to recognize conserved microbial features that represent broad pathogen classes1. This strategy ensures that diverse pathogen species can be quickly recognized by the host, as long as these microbial features are sufficiently constrained that they remain invariant. Examples of such features are the bacterial cell wall components lipopolysaccharide (LPS) and peptidoglycan. Members of the Toll-like receptor (TLR) family recognize these and other microbial ligands and induce signals important for initiation of both innate and adaptive immunity1. Accordingly, mice lacking TLR function show increased susceptibility to infection.
Viral recognition by the innate immune system is more challenging than recognition of other pathogen classes because of the relative paucity of conserved features2. Viruses replicate within host cells, and they do not generate any of the unique biochemical products present in bacterial and fungal cell walls. It has been argued that this lack of conserved viral features has forced the innate immune system to use nucleic acid as a means of detecting viral infection. Indeed, several members of the TLR family recognize nucleic acids: TLR3 recognizes dsRNA, TLR7 and TLR8 recognize ssRNA, and TLR9 recognizes CpG motifs in DNA2. In addition, a family of cytosolic receptors, including RIG-I, MDA-5, and DAI, recognize various nucleic acid species in the cytosol2. Targeting nucleic acids allows for the recognition of highly diverse viral species with only a few innate receptors.
One of the key components of antiviral immunity is induction of the type I interferon (IFN) family of cytokines, hereafter referred to as type I IFN3. Type I IFN induces hundreds of genes that promote an antiviral state in cells. The importance of this signaling network is illustrated by the extreme susceptibility of mice lacking the type I IFN receptor4. All of the nucleic acid sensing TLRs induce type I IFN, underscoring the importance of the cytokine family in antiviral immunity. For TLR7 and TLR9, though, induction of type I IFN only occurs in plasmacytoid dendritic cells (pDCs) via the common signaling adaptor MyD88. In other cell types, activation of TLR7 and TLR9 does not lead to type I IFN production5. Similarly, most TLRs involved in bacterial or fungal recognition (TLR2 and TLR5) are not expressed in pDCs6 and do not induce type I IFN in other cell types. The notable exception is TLR4, which can induce type I IFN in macrophages and conventional DCs via the signaling adaptor Trif7. Nevertheless, type I IFN clearly plays a less critical role for antibacterial immunity than for antiviral immunity8, 9.
By multiple criteria, viral proteins would seem poor choices as targets for innate receptors relative to nucleic acids. First, any given viral protein is unlikely to be shared among diverse viruses. Second, innate recognition of a viral protein would likely select for mutants that escape recognition yet retain function, if at all possible. Nevertheless, several viruses do encode proteins that are capable of stimulating TLR2, a receptor known to recognize multiple bacterial and fungal cell wall components. The best-characterized example is stimulation of TLR2 by glycoprotein B from human cytomegalovirus (HCMV)10, 11, but mouse cytomegalovirus (MCMV)12, Herpes simplex virus 1 and 2 (HSV-1 and HSV-2)13, 14, Hepatitis C virus15, Lymphocytic choriomeningitis virus16, measles virus17, and vaccinia virus18 are also capable of stimulating TLR2. In some of these cases, it seems that viruses benefit in some way from the stimulation of TLRs. For instance, measles virus may have evolved the ability to activate TLR2 as a means of upregulating the viral entry receptor, CD15017. In other examples, however, there is evidence that TLR2 activation contributes to protection. Most notably, mice lacking TLR2 are impaired in their ability to mount an innate or adaptive immune response against vaccinia virus18. One problematic aspect of any general role for TLR2 in antiviral immunity, however, is the apparent inability of this receptor to induce type I IFN19-21.
In this work, we describe a specialized role for TLR2 in innate recognition of several viruses. In contrast to the well-documented transcriptional response induced by bacterial ligands, we show that TLR2 induces type I IFN when activated by viruses. This novel signaling pathway is unique to inflammatory monocytes. The functional specialization of these cells is conceptually analogous to the role played by pDCs in TLR7 and TLR9 signaling and likely represents a general strategy to achieve specificity within innate immune signaling.
Results
TLR2-mediated recognition of multiple DNA viruses
TLR2 has been implicated in the recognition of several DNA viruses, including vaccinia virus, HCMV, MCMV, HSV-1, and HSV-2. These viruses contain ligands that can activate additional TLRs (e.g., TLR9), so we first sought to determine the relative contribution made by TLR2 for viral recognition. To minimize potential viral interference with innate immune signaling, we UV-inactivated each virus prior to stimulating cells. Both vaccinia virus and MCMV induced NF-κB activation in HEK293 cells stably expressing murine TLR2 but not in control cells (Fig. 1a), supporting a role for TLR2 in recognition of these viruses. We directly tested the relative contributions of TLR2 and TLR9 by stimulating bone marrow derived dendritic cells (DCs) from TLR2-deficient, TLR9-deficient, Myd88-deficient, and control mice. Surprisingly, TNFα production by DCs was entirely TLR2-dependent (Fig. 1b). TLR9 appeared to play little role in these cells. Similar results were obtained with bone marrow derived macrophages (Fig. 1c).
Figure 1. TLR2-mediated recognition of vaccinia virus and MCMV.
a) HEK293 cells stably transfected with a NF-κB luciferase reporter (HEK293) or the reporter together with plasmids encoding human TLR2 and human CD14 (HEK293-TLR2) were stimulated with UV-inactivated MCMV or vaccinia virus (VV) at the indicated MOIs. Pam3SK4 (Pam3) served as a positive control. Luciferase activity was measured 10 hours after activation. Fold activation was calculated relative to unstimulated cells. b & c) Bone marrow-derived DCs (b) or macrophages (c) were derived from the indicated mouse strains, stimulated with the indicated TLR ligands or viruses, and intracellular TNFα was measured by flow cytometry. Plots of stimulated cells (black lines) overlaid on plots of unstimulated cells (shaded) are shown. Plots of DCs are gated on CD11c-positive cells. The data presented are representative of at least 3 experiments.
TLR2 induces type I interferon in response to virus
The lack of TLR9 activation in DCs and macrophages treated with vaccinia virus or MCMV is at odds with the well-documented role played by TLR9 in recognition of DNA viruses22. One potential explanation for this discrepancy is that TLR9-mediated virus recognition is best observed in pDCs. To address this possibility, we used bone marrow cells as a source of ex vivo pDCs and measured the production of type I IFN in response to MCMV and vaccinia virus. As previously described, MCMV induced potent production of type I IFN and this response was reduced in TLR9-deficient cells (Fig. 2a). Surprisingly, type I IFN production in response to MCMV was also partially TLR2-dependent. Moreover, the production of type I IFN in response to vaccinia virus was entirely TLR2-dependent (Fig. 2a). A similar dependence on TLR2 was observed in splenocytes (data not shown).
Figure 2. TLR2 induces type I IFN production in response to virus.
a) TLR2-dependent type I IFN production requires MyD88 but not Trif. Bone marrow cells from the indicated mice were stimulated as indicated and type I IFN was measured in the supernatants 24 hours after stimulation by bioassay. b & c) TLR2-dependent type I IFN production requires IRF3 and IRF7 but not TLR3, TLR7, or TLR9. Bone marrow cells from the indicated mice were stimulated as indicated and type I IFN production was measured by bioassay. d) TLR2 and TLR9 induce type I IFN with different kinetics. Transcripts for IFNβ and IFNα4 were measured by real-time PCR in bone marrow cells stimulated with vaccinia virus (VV) or CpG oligos. The data presented are representative of at least 2 experiments.
There is no known pathway by which TLR2 can induce type I IFN, so a requirement for TLR2 in IFN production by MCMV and vaccinia virus is quite unexpected. There are two known mechanisms of type I IFN induction by TLR family members. TLR3 and TLR4 induce IRF3 activation via the signaling adaptor Trif7. TLR7 and TLR9 can induce IRF7 activation downstream of MyD88, but this interaction only occurs in pDCs, which do not express TLR26. To define the signaling pathway responsible for TLR2-dependent type I IFN production, we examined the requirement for a number of known TLR signaling components. Type I IFN production in response to vaccinia virus or MCMV was markedly reduced in MyD88-deficient and MyD88×Trif-deficient cells but not Trif-deficient cells (Fig. 2a). Both IRF3 and IRF7 appeared to contribute to type I IFN production by TLR2, and IRF3×IRF7-deficient cells were completely impaired in type I IFN production (Fig. 2b). In contrast, the response to vaccinia virus was unaffected in IRF1-deficient cells (Sup. Fig. 1a). As expected, production of type I IFN was also impaired in cells lacking the type I IFN receptor (Sup. Fig. 1b). Finally, to rule out any contribution of the nucleic acid sensing TLRs we tested the response to vaccinia virus in “3d” mice containing a non-functional allele of the Unc93b1 gene. These mice respond normally to TLR2 and TLR4 ligands but are unable to respond to TLR3, TLR7, and TLR9 ligands23. Importantly, the response to vaccinia virus in cells from 3D mice was equivalent to wildtype controls, ruling out any role for nucleic acid recognition in the type I IFN production. Collectively, these data indicate that in response to virus a MyD88-dependent pathway downstream of TLR2 leads to activation of IRF3, IRF7 and transcription of type I IFN genes.
We next measured which specific type I IFN genes were induced by TLR2 in response to viruses. We focused on the response to vaccinia virus because, as opposed to MCMV, recognition was entirely dependent on TLR2. Both IFNβ and IFNα4 were induced in bone marrow treated with vaccinia virus in a TLR2-dependent manner (Fig. 2c). Interestingly, the induction of each cytokine peaked at 12 hours while the response to CpG oligonucleotides peaked at 4 hours. PDCs are responsible for the rapid type I IFN induction in response to CpG ODN24. The delayed response to VV suggests that a cell type other than pDCs may be responsible for the TLR2 response.
Differential TLR2 response to viral and bacterial ligands
While the data presented thus far indicate that TLR2 is capable of inducing type I IFN, these findings contradict a large body of work demonstrating that TLR2 does not induce type I IFN. One major difference between our work and previous studies is the use of viral as opposed to bacterial TLR2 ligands. To address whether the nature of the microbial ligands could account for differential induction of type I IFN, we compared induction of IFNβ and IFNα4 in response to vaccinia virus and TLR2 ligands. In contrast to vaccinia virus, the triacylated lipid Pam3SK4 (a TLR2/1 agonist25) did not induce IFNβ or IFNα4 in bone marrow cultures (Fig 3a).
Figure 3. Differential induction of type I IFN by TLR2 in response to viral and bacterial ligands.
a) IFNβ transcripts were measured in bone marrow cells treated for 12 hours with the bacterial TLR2 ligand Pam3SK4 (Pam3) or vaccinia virus (VV). b) Bone marrow or splenocytes from MOB mice were stimulated as indicated for 20 hours, and YFP production was measured by flow cytometry. The percentage of YFP-positive cells is indicated for each plot. All data are representative of at least 3 experiments.
As an alternative way to measure IFNβ induction, we used IFNβ reporter mice in which cDNA encoding yellow fluorescent protein has been knocked in downstream of the IFNβ locus (hereafter referred to as MOB mice)26. YFP was clearly detectable in bone marrow and splenocytes from these mice after stimulation with vaccinia virus. In contrast, there was no YFP signal when the same cells were stimulated with Pam3SK4 or Fsl-1 (a TLR2/6 agonist27) (Fig. 3b). This lack of type I IFN was not due to poor stimulation, though, as TNFα was induced by all TLR2 ligands (Sup. Fig. 2). Thus, a population of cells in the bone marrow and spleen is able to discriminate between viral and bacterial TLR2 stimuli and selectively induce type I IFN in response to virus.
Both bone marrow derived DCs and macrophages express TLR2 and respond to vaccinia virus. If the ability to discriminate between bacterial and viral ligands is a general property of TLR2 on all cells, then we would expect to observe TLR2-dependent type I IFN in response to viral ligands. However, type I IFN production by bone marrow derived DCs and HEK-293Ts was TLR2 independent and likely due to the activation of the cytosolic DNA sensor(s) (Sup. Fig. 3). Therefore we conclude that the ability to produce type I IFN in a TLR2-dependent manner is restricted to a specialized cell type present in spleen and bone marrow.
Identification of the cell type responsible for TLR2-dependent type I interferon production
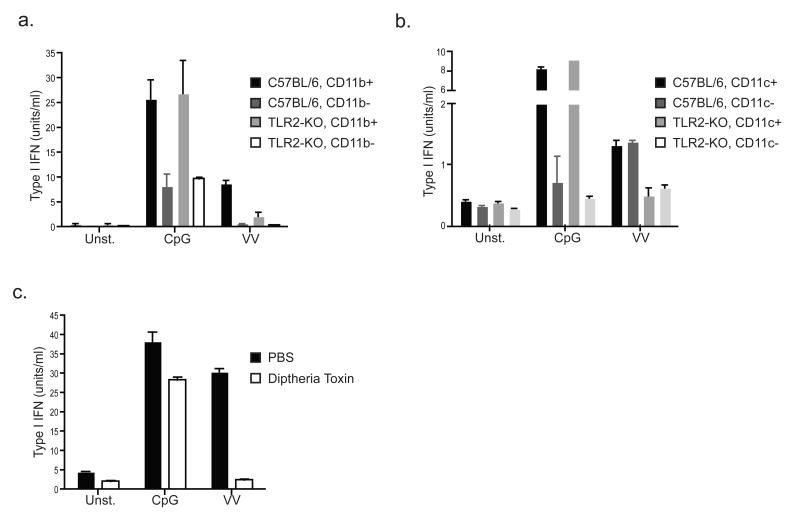
We next sought to identify the population of cells in the bone marrow and spleen responsible for TLR2-dependent type I IFN induction. As an initial approach we used magnetic bead cell sorting to separate bone marrow cells based on expression of the common myeloid marker CD11b or the common DC marker CD11c. Strikingly, CD11b positively sorted cells were enriched for TLR2-dependent type I IFN production in response to vaccinia virus, while the CD11b negative cells no longer responded (Fig. 4a). In contrast, sorting based on CD11c did not alter the response (Fig. 4b). We obtained similar results with cells from transgenic mice expressing the diptheria toxin receptor (DTR) driven by the CD11b promoter (CD11b-DTR mice)28. Splenocytes harvested from CD11b-DTR mice injected with diptheria toxin no longer responded to vaccinia virus (Fig. 4c). These results suggest that a CD11b+CD11c- population of cells is responsible for TLR2-dependent type I IFN production.
Figure 4. A population of CD11b+CD11c- cells is responsible for TLR2-dependent type I IFN production.
a) Bone marrow cells from C57BL/6 or TLR2-deficient mice were MACS sorted into CD11b-positive and CD11b-negative populations. The sorted cells were stimulated as indicated, and type I IFN was measured after 24 hours by bioassay. b) The same experiment was performed as described in (a), except that MACS sorting was based on CD11c. c) Splenocytes were harvested from CD11b-DTR transgenic mice 24 hours after injection with saline (PBS) or diptheria toxin. The resulting cells were stimulated as indicated and type I IFN production was measured after 24 hours by bioassay. All data are representative of at least 3 experiments.
Although the lack of CD11c expression suggested that pDCs were not responsible for the TLR2-dependent type I IFN production, we addressed this possibility directly using MOB mice. We compared the responding cells (YFP+) in bone marrow and spleen stimulated with vaccinia virus or CpG oligos. CpG oligos induce TLR9-dependent type I IFN production by pDCs, and the YFP+ cells in these cultures were B220+CD11c+, a surface phenotype consistent with that of pDCs (Fig. 5a). In contrast, the cells responding to vaccinia virus were B220- and had lower surface levels of CD11c (Fig. 5a). These distinct surface phenotypes clearly demonstrate that different cell types are responding to vaccinia virus and CpG oligos.
Figure 5. Ly6Chigh inflammatory monocytes produce IFNβ in response to vaccinia virus.
a) Bone marrow or splenocytes from MOB mice were stimulated with CpG or vaccinia virus (VV) for 20 hours, stained with antibodies against B220 or CD11c, and analyzed by flow cytometry. Comparisons between YFP-gated cells (orange line) versus total ungated cells (shaded) are shown. b) Cells were treated as in (a) but stained with antibodies against Ly6C, CD11b, or Ly6G. Comparisons between YFP-gated cells (orange line) versus total ungated cells (shaded) are shown. c) TLR2 is expressed on inflammatory monocytes. Bone marrow or splenocytes from C57BL/6 or TLR2-deficient mice were stained with antibodies specific for CD11b, Ly6C, and TLR2. d) TLR2 on inflammatory monocytes mediates type I IFN production in response to vaccinia virus. Bone marrow cells from MOB mice were cultured in the presence or absence of an anti-TLR2 blocking monoclonal antibody and stimulated as indicated. The percentage of YFP+ cells was determined by flow cytometry. e) Purified inflammatory monocytes are sufficient to produce type I IFN when stimulated with vaccinia virus. Bone marrow cells from C57BL/6 or TLR2-KO mice were sorted based on CD11b and Ly6C as shown. The plots shown have excluded B220+ and CD11c+ cells. Post-sort populations are shown and the percentage of inflammatory monocytes is indicated. The positive and negative populations were stimulated as indicated, and type I IFN production was measured after 24 hours by bioassay. All data are representative of at least 3 experiments.
To characterize more completely the CD11b+ cells producing IFNβ in response to vaccinia virus we stained bone marrow cells and splenocytes with a panel of antibodies against common hematopoetic surface markers. Based on the absence of certain lineage markers, we were able to exclude B cells, T cells, NK cells and neutrophils as the source of type I IFN (data not shown). The expression of CD11b suggested that the cells responding to vaccinia virus may represent a subset of monocytes. Monocytic subsets have been classified based on differential expression of the surface markers Ly6C and Ly6G29-31. Cells expressing YFP in response to vaccinia virus were Ly6ChighLy6G- (Fig. 5b), suggesting that they represent the subset of monocytes often referred to as “inflammatory” monocytes (IMs). While the expression of CD11c on IMs is inconsistent with published reports describing these cells, one possible explanation for this discrepancy is that activation of these cells leads to upregulation of CD11c29, 31. Taken together with the fact that sorting based on CD11c sorting did not alter the response to vaccinia (Fig. 4b), we conclude that the cells that make TLR2-dependent type I IFN are not initially CD11c+, but upregulate CD11c upon stimulation with virus. Staining with a TLR2-specific antibody confirmed that IMs in the spleen and bone marrow express TLR2 (Fig. 5c). Moreover, treatment of MOB bone marrow with TLR2 blocking antibodies prior to stimulation with vaccinia virus significantly reduced the number of YFP+ cells, demonstrating that the type I IFN production by these cells requires TLR2 (Fig. 5d).
To demonstrate formally that IMs are solely responsible for the TLR2-dependent type I IFN, we sorted these cells based on Ly6C, CD11b, CD11c, and B220 (Fig. 5e). Ly6ChighCD11b+CD11c-B220- cells sorted from the bone marrow produced type I IFN when stimulated with vaccinia virus in a TLR2-dependent manner. Moreover, the “negative” population (i.e., all other cells falling outside the Ly6ChighCD11b+CD11c-B220- gate) did not produce type I IFN in response to virus, indicating that IMs were solely responsible for the response (Fig. 5e). Importantly, these “negative” cells did produce type I IFN when stimulated with CpG oligos, while the IMs did not, demonstrating that our sorting parameters had effectively separated pDCs from IMs. Finally, quantitative PCR analysis of cDNA generated from the sorted IMs confirmed that they expressed elevated levels of CCR2 as previously reported for these cells30 (Sup. Fig. 4). Additionally, we observed slightly elevated levels of IRF7 transcripts in IMs, although not as high as the expression in pDCs (Sup. Fig 4).
Inflammatory monocytes induce TLR2-dependent type I IFN during viral infection in vivo
Our in vitro analyses of cells from bone marrow and spleen implicate IMs in the recognition of vaccinia virus and suggest that TLR2 activation in these cells leads to production of type I IFN. To address the relevance of these cells during vaccinia virus infection in vivo, we utilized the CD11b-DTR mice described earlier. Although the DTR transgene is driven by the CD11b promoter, previous analyses of these mice have demonstrated that a limited population of CD11b positive cells are efficiently deleted upon injection of diptheria toxin (DT)28. While monocytes and some tissue resident macrophages are removed, other CD11b positive cells (such as neutrophils and activated lymphocytes) remain largely unaffected. Indeed, we observed similar numbers of Ly6G+CD11b+ neutrophils in mice injected with DT or PBS, and the overall profile of CD11b-expressing cells in the spleen remained mostly unchanged (Fig. 6a). In contrast, IMs were deleted quite efficiently, providing a nice system with which to probe the functional relevance of these cells in vivo. Remarkably, when DTR-injected mice were subsequently challenged with vaccinia virus, serum levels of type I IFN were reduced to levels comparable to uninfected mice (Fig. 6b). Injection of DT into non-transgenic mice followed by vaccinia virus infection had no effect on type I IFN production, as expected (Sup. Fig. 5). To assess the relevance of these cells for viral clearance, we determined viral titers in CD11b-DTR mice depleted of IMs prior to infection. Mice lacking IMs displayed higher titers of vaccinia virus in the liver and ovaries (Fig. 6c and Sup. Fig. 6). Collectively, these data indicate that IMs are a key early source of type I IFN during viral infection and are necessary for early restriction of viral replication.
Figure 6. Inflammatory monocytes are required for early production of type I IFN and efficient viral clearance in vivo.
a) Deletion of inflammatory monocytes in CD11b-DTR mice. Splenocytes were harvested from CD11b-DTR transgenic mice 24 hours after intravenous injection of diptheria toxin or PBS followed by staining with antibodies against Ly6C, CD11b, or Ly6G. b) Inflammatory monocytes are required for production of type I IFN in response to vaccinia virus. CD11b-DTR transgenic mice where injected with diptheria toxin or PBS 24 hours before infection with 1×106 PFU of vaccinia virus. Serum was collected 24 hours post infection and type I IFN were quantified by bioassay. c) Depletion of inflammatory monocytes impairs viral clearance. CD11b-DTR transgenic mice were infected with 1×106 PFU of vaccinia virus 24 hours after intravenous injection of diptheria toxin or PBS. PFU were determined in the liver 48 hours after infection. The data presented are representative of at least 2 experiments.
Induction of type I IFN by TLR2 requires receptor internalization
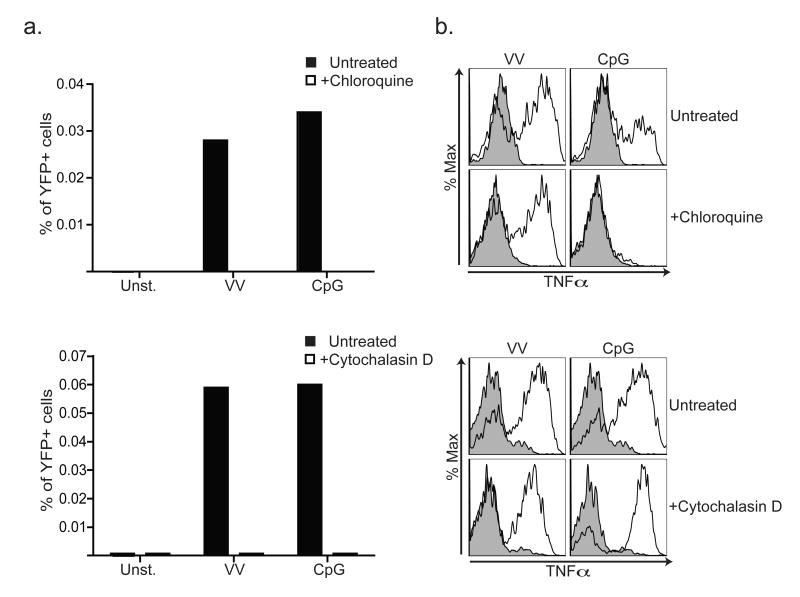
Finally, we sought to address the selective production of type I IFN in response to viruses by IMs. The fact that IMs produce TNFα in response to both viral and bacterial ligands indicates that the differential type I IFN response is not simply due to lack of recognition. Recent studies of TLR4 signal transduction have revealed that induction of type I IFN requires receptor internalization while signals leading to TNFα and IL-6 production can occur at the plasma membrane32. To determine whether similar cell biological regulation controls type I IFN production downstream of TLR2, we blocked endocytosis with the actin depolymerizing drug cytochalasin D or blocked endosomal maturation with chloroquine. Treatment of bone marrow cultures with either inhibitor prior to stimulation with vaccinia virus completely abrogated type I IFN production (Fig. 7a). In contrast, the production of TNFα was unaffected by either inhibitor, indicating that these agents do not prevent overall recognition of vaccinia virus or signaling by TLR2 (Fig. 7b). Instead, receptor internalization appears necessary only for TLR2-dependent production of type I IFN.
Figure 7. TLR2-dependent type I IFN production requires receptor internalization.
a) Bone marrow from MOB mice was incubated with 15μM chloroquine, 1μM cytochalasin D, or left untreated prior to stimulation with vaccinia virus (VV) or CpG. The percentages of YFP+ cells were measured 20h later by flow cytometry. b) Cells were treated as in (a) except that intracellular TNFα was measured in inflammatory monocytes cells by intracellular cytokine staining and flow cytometry. The data presented are representative of at least 2 experiments.
Discussion
Here we report the identification of a novel antiviral signaling pathway in which TLR2 activation leads to production of type I IFN. Prior to this work, TLR2-dependent type I IFN production had not been reported, and, indeed, in most cell types TLR2 does not induce this antiviral response. We demonstrate that inflammatory monocytes are uniquely capable of responding to viral TLR2 ligands by producing type I IFN, and our work suggests that these cells represent another specialized antiviral cell type with functional and conceptual parallels to pDCs. In addition, these data solidify the interpretation that viral recognition by TLR2 is a host strategy, as opposed to manipulation by viruses, and argue that certain viral proteins are sufficiently constrained to serve as targets for innate immune receptors. Overall, this work has important implications for our understanding of how the innate immune system recognizes viruses.
Until recently, the specific and differing roles played by monocytic subpopulations during immune responses have not been well appreciated. In the last few years, though, several studies have identified IMs as a largely bone marrow resident cell type that is rapidly recruited to sites of infection in a CCR2-dependent manner29-31. These cells have been named “inflammatory monocytes” to distinguish them from Ly6C- monocytes, which are thought to play a more important role in maintaining tissue homeostasis29. IMs can differentiate into a number of different DC subsets at sites of inflammation, including TNF- and inducible nitric oxide synthase (iNOS)-producing DCs (TipDCs) as well as inflammatory DCs29-31. These cells have been implicated in bacterial, parasitic, and viral immunity31. An additional role for Ly6Chigh monocytes has been observed in a mouse model of induced lupus. In this model, Ly6Chigh monocytes accumulate in the peritoneal cavity of mice after injection of 2,6,10,14-tetramethylpentadecane33. Surprisingly, the Ly6Chigh monocytes express type I IFN in this model. While the activation signal for these cells has not been defined in this context, the observation that IMs can produce type I IFN during disease supports our contention that they may function as specialized IFN producing cells. The ability of IMs cells to secrete pro-inflammatory cytokines and type I IFN suggests that these cells may play a key role early during viral infection. In addition, differentiation of IMs into DCs upon activation by viruses may further enhance their contribution to the antiviral immune response through induction of adaptive responses.
A surprising aspect of TLR2 function on IMs is the ability to distinguish between viral and bacterial ligands; type I IFN is only induced in response to viral ligands while TNFα production occurs in response to both classes of stimuli. This differential response may be partially explained by the observation that activation of the signal transduction pathway leading to type I IFN production requires receptor internalization, as both chloroquine and cytochalasin D disrupt IFN but not TNF production. This dichotomy is reminiscent of TLR4 signaling, in which Trif activation occurs at endosomal membranes while MyD88 activation occurs at the plasma membrane32. Our data suggest that TLR2 signaling is also regulated through localization, yet, in contrast to TLR4, all TLR2 signaling is MyD88-dependent. In this sense, differential signaling by TLR2 may be more conceptually similar to TLR9 signaling in that different ligands produce distinct responses through the same signaling adaptor (MyD88). For example, in pDCs class B CpG oligos lead to the production of cytokines such as TNFα and IL-12 but not to the production of type I IFN, while class C CpG oligos lead to the production of TNFα, IL-12 and type I IFN5, 34. IMs have a similar ability to use one TLR and produce two unique responses. How such specificity can be generated downstream of a particular TLR is not understood in pDCs or in our system. It is possible that viruses are more efficiently internalized than bacterial ligands or specifically traffic to a specialized compartment from which type I IFN signaling can be initiated. Still, such a mechanism cannot explain why cDCs or macrophages are unable to make type I IFN in a TLR2-dependent manner when stimulated with virus. Thus, it seems possible that a specialized co-receptor might be required to generate the specificity that we have discovered.
The fact that innate immune recognition of vaccinia virus is so heavily TLR2-dependent is somewhat unexpected based on the additional innate receptors capable of viral recognition. For example, TLR9 clearly plays a role in innate recognition of many DNA viruses, yet our data (Fig. 2a) as well as the work of others18 suggest that TLR9 does not play a major role in the recognition of vaccinia virus. The ability of some viruses to evade certain innate receptors has undoubtedly required the host to evolve additional strategies of viral recognition. Cytosolic DNA sensors play a major role in detection of viral nucleic acid in the host cytosol35-37. In DCs and macrophages stimulated with vaccinia virus, we observe a TLR-independent type I IFN response, which is presumably due to activation of cytosolic DNA sensors (Sup. Fig. 3). In IMs as well as during in vivo infection, though, induction of type I IFN is largely TLR2-dependent. This dependence suggests that vaccinia virus can evade innate receptors, such as TLR9 and cytosolic DNA sensors, yet remains detectable by TLR2.
While nucleic acid recognition appears to be the dominant strategy employed by the innate immune system to detect viral infection, there is accumulating evidence that the host can recognize certain viruses independently of nucleic acid. The work presented here supports this view and provides evidence for a novel host signaling pathway linking viral recognition to induction of type I IFN. Our data and the work of others suggest that the innate immune system must have evolved specificity for certain viral proteins that are unable to mutate and escape recognition. Such a scenario is not unprecedented. Bacterial flagellin is recognized by several innate receptors (TLR538, Ipaf 39, 40, and Naip541), yet most bacteria appear unable to mutate flagellin to avoid recognition. Importantly, mutations that abrogate flagellin recognition result in nonmotile bacteria41, 42. Thus, it would appear that certain pathogen encoded proteins are sufficiently constrained that they can serve as targets for innate immune receptors.
Following this reasoning, it is possible that the innate immune system has evolved to recognize viral proteins that are under similar functional constraints as flagellin. The viral fusion apparatus is a particularly attractive target in this regard. All viruses possess a fusion apparatus that is absolutely essential for propagation, making it an ideal target of the innate immune system. While fusion proteins from unrelated viruses share no homology at the amino acid level, structural studies have demonstrated that, in some cases, these proteins share surprising structural homology43. It is possible that TLR2 has evolved to recognize a conserved structural feature associated with certain viral fusion proteins. In support of this hypothesis, HCMV glycoprotein B, which is required for viral fusion, has been reported to activate TLR210. Additionally, a number of reports suggest that TLR4 can recognize viral structural proteins44-48. Identification of ligands from other viruses recognized by TLR2 and TLR4 will be necessary to address whether a common structural feature is targeted.
Methods
Mice and Viruses
C57BL/6, FVB/N, FVB/N-CD11b-DTR and IRF1-/- mice were purchased from Jackson Laboratories. UNC93b1 mutant mice were purchased from the Mutant Mouse Regional Resource Centers. Tlr2-/-, Tlr9-/-, Myd88-/-, and Myd88×Trif-/- mice (kindly provided by S. Akira) were backcrossed at least 7 generations onto the C57BL/6 background. IRF3-/- and IFNαβR-/- mice were provided by D. Portnoy (UC Berkeley). IRF7-/- and IRF3×IRF7-/- were provided by K. Fitzgerald (U. Mass). MOB mice have been previously described26. All mice were housed within the animal facilities at the University of California, Berkeley according to IACUC guidelines.
Vaccinia virus (Western Reserve strain) was a gift from D. Raulet (UC Berkeley). MCMV (Smith strain) was a gift from L. Coscoy (UC Berkeley). VV was propagated and plaqued on BHK21 cells. MCMV was propagated and plaqued on NIH 3T3 cells.
Flow cytometry and FACS
Antibodies were from eBioscience or BD Biosciences: anti-CD11b-APC-Cy7 and -PE-Cy5 (clone M1/70), anti-CD11c-FITC and -PE-Cy7 (clone N418), anti-B220-PE-Cy5 and -FITC (clone RA3-6B2), anti-Ly6C-PerCP-Cy5.5 (clone HK1.4), anti-Ly6G-PE and -FITC (clone 1A8), anti-TNFα-PE and-PacificBlue (clone MP6-XT22), and anti-TLR2-PE (clone T2.5). Before staining for surface markers, cells were incubated with an anti-CD16/CD32 antibody (clone 2.4G2, UCSF Monoclonal Antibody Core). Intracellular cytokine staining was performed with Fixation & Permeabilization Kit (eBioscience) according to manufacturer's instructions. Brefeldin was added 30 min after stimulation and cell were harvested after an additional 5 hours. Data were collected on a LSR II (Becton Dickinson) or FC-500 (Beckman Coulter). The data were analyzed with FloJo software (Tree Star).
Cell sorting was performed on a BD INFLUX. Isolation of IMs is described above. Sorted pDCs (Sup. Fig 4) were purified from bone marrow by sorting B220+Cd11c+ cells.
Cell lines and tissue culture
HEK293, NIH3T3, and BHK21 cells were obtained from ATCC, and HEK293-TLR2 cells have been previously described13. ISRE-L929 cells were obtained from D. Portnoy (UC Berkeley)49 and have been previously described. Briefly, these cells have been stably transfected with an interferon-sensitive responsive element upstream of the luciferase gene.
Cell lines were cultured in DMEM supplemented with 10% FCS, L-glutamine, penicillin/streptomycin, sodium pyruvate and HEPES (Invitrogen). Primary cells were cultured in RPMI 1640 supplemented with 10% FCS, L-glutamine, penicillin/streptomycin, sodium pyruvate and HEPES.
Bone-marrow-derived conventional DCs and macrophages were differentiated in RPMI supplemented with GM-CSF or M-CSF containing supernatant, respectively, as previously described50. After 5 days, cells were replated in RPMI media, and stimulated as indicated.
Luciferase Assays
ISRE-L929 cells were used to measure the amount of type I IFN present in supernatants or sera. In parallel, ISRE-L929 cells were treated with serial dilutions of recombinant IFNβ (R&D) to generate a standard curve in each experiment. In some cases supernatants or sera were diluted to achieve signal within the linear range of the standard curve.
All cells were lysed using Passive Lysis Buffer (Promega) and luciferase was measured on a LMaxII-384 luminometer (Molecular Devices).
Bone marrow and spleen cell harvesting/stimulation
Spleens were dispersed into single cell suspensions by passage through nylon mesh (BD Falcon). Bones were washed with ethanol and RPMI media, and bone marrow cells were released by gentle grinding with a mortar and pestle. Cells were passed through a filter to remove debris, red blood cells were removed by hypotonic lysis, and the remaining cells were washed three times with RPMI media, counted, and plated.
All viral stimulations were performed at an MOI of 0.2 using UV inactivated particles, unless otherwise noted. CpG oligonucleotide (TCCATGACGTTCCTGACGTT with phosphorothioate linkages from IDT or Invitrogen) was used at 1μM. CpG A oligonucleotide (G*GTGCATCGATGCAG*G*G*G*G*G with phosphorothioate linkages indicated with an asterisk were from IDT) was used at 1μM. Cultures were stimulated with FSL-1, Pam3SK4 and R848 (Invivogen) at 0.1 μg/ml, 1 μg/ml, and 1 μg/ml respectively. TLR2 blocking antibody (clone T2.5 - eBioscience) was added to cells 30 minutes before stimulation at 50 μg/ml.
Quantitative real-time RT-PCR
For IFNβ/IFnα4 RT-PCR, at indicated times post-stimulation, cells were harvested and resuspended in TRIzol. For CCR2/IRF7 RT-PCR, cells were sorted on a BD INFLUX and were resuspended in TRIzol.
For all samples, the Trizol Plus RNA Purification System (Invitrogen) was used to isolate RNA. Quantative PCR was performed on an Applied Biosystems 7300 (Applied Biosystems) using SYBR green-based quantification. Gene-specific transcript levels were normalized to the amount of RPS17 mRNA. The following primers were used: RPS17: 5′-cgccattatccccagcaag-3′, 5′-tgtcgggatccacctcaatg-3′; IFNβ: 5′-ataagcagctccagctccaa-3′, 5′-ctgtctgctggtggagttca-3′; IFNα4: 5′-gcaatgacctccatcagcagc-3′, 5′-cactccttctcctcactcagtcttg-3′; IRF7: 5′-ctacaccatctacctgggttttgg-3′, 5′- agacaagcacaagccgagactg-3′; CCR2: 5′- tccttgggaatgagtaactgtgt-3′, 5′- tggagagataccttcggaactt-3′.
Magnetic Cell Sorting
CD11b+ and CD11c+ bone marrow cells were positively sorted by magnetic cell sorting. Cells were stained with anti-CD11b or anti-CD11c biotinylated antibodies and then incubated with anti-biotin microbeads (Miltenyi Biotec). Cells were sorted on an AutoMACS cell sorter (Miltenyi Biotec). The positive and negative (flow-through) populations were collected. The positive populations were enriched for the target cells but also contained CD11b- or CD11c- cells. s
CD11b-DTR mice
CD11b-DTR mice were injected intravenously with 500ng diptheria toxin (Sigma). For in vitro experiments, splenocytes were harvested 24h later and stimulated with vaccinia virus (0.2 MOI) or CpG oligos (1μM). Type I IFN was measured in supernatants after 24h by bioassay.
For in vivo experiments, mice were infected with live vaccinia virus (1×106 pfu) 24h after diptheria toxin injection. 24 hours post-infection serum was collected and type I IFN was measured by bioassay. 48 hours post-infection organs where collected and PFU counts where performed.
Inhibition of receptor internalization
To measure IFN production, bone marrow from MOB mice was treated with chloroquine (15μM) or cytochalasin D (1μg/ml) for 30 minutes before the addition of ligands. 20h post stimulation, cells where harvested and analyzed.
To measure TNF production, bone marrow from B6 mice was pretreated with chloroquine (15μM) or cytochalasin D (1μM) for 14h before the addition of ligands. 30 minutes after the addition of ligands, Brefeldin was added. Cells were harvested after an additional 5 hours. In all, the cells spent the same amount of time in drug to control for potential toxicity.
Supplementary Material
Acknowledgments
We acknowledge early contributions to this work by M. Tokuyama, M. Fontana, and K. Camfield. We thank M. Mouchess for technical assistance, N. An from the viral core facility of the Berkeley Center for Host Pathogen Studies for help with production of vaccinia virus, D. Stetson for providing key reagents, members of the Barton and Vance labs for helpful discussions and advice, R. Vance and L. Coscoy for comments on the manuscript, and H. Nolla for assistance with flow cytometry. This work was supported by University of California start-up funds, the Hellman Family Faculty Fund, the NIH (AI072429 to G.M.B.), and a NIH NRSA Trainee appointment (GM007232 to R.B.).
Footnotes
Author contributions: R.B. and G.M.B designed the experiments. R.B. and L.L. performed the experiments. R.M.L. provided reagents. R.B. and G.M.B. wrote the manuscript.
References
- 1.Medzhitov R. Recognition of microorganisms and activation of the immune response. Nature. 2007;449:819–826. doi: 10.1038/nature06246. [DOI] [PubMed] [Google Scholar]
- 2.Kawai T, Akira S. Innate immune recognition of viral infection. Nature immunology. 2006;7:131–137. doi: 10.1038/ni1303. [DOI] [PubMed] [Google Scholar]
- 3.Stetson DB, Medzhitov R. Type I interferons in host defense. Immunity. 2006;25:373–381. doi: 10.1016/j.immuni.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 4.van den Broek MF, Muller U, Huang S, Zinkernagel RM, Aguet M. Immune defence in mice lacking type I and/or type II interferon receptors. Immunological reviews. 1995;148:5–18. doi: 10.1111/j.1600-065x.1995.tb00090.x. [DOI] [PubMed] [Google Scholar]
- 5.Gilliet M, Cao W, Liu YJ. Plasmacytoid dendritic cells: sensing nucleic acids in viral infection and autoimmune diseases. Nature reviews. 2008;8:594–606. doi: 10.1038/nri2358. [DOI] [PubMed] [Google Scholar]
- 6.Kadowaki N, et al. Subsets of human dendritic cell precursors express different toll-like receptors and respond to different microbial antigens. The Journal of experimental medicine. 2001;194:863–869. doi: 10.1084/jem.194.6.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yamamoto M, et al. Role of adaptor TRIF in the MyD88-independent toll-like receptor signaling pathway. Science (New York, NY. 2003;301:640–643. doi: 10.1126/science.1087262. [DOI] [PubMed] [Google Scholar]
- 8.Auerbuch V, Brockstedt DG, Meyer-Morse N, O'Riordan M, Portnoy DA. Mice lacking the type I interferon receptor are resistant to Listeria monocytogenes. The Journal of experimental medicine. 2004;200:527–533. doi: 10.1084/jem.20040976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.O'Connell RM, et al. Type I interferon production enhances susceptibility to Listeria monocytogenes infection. The Journal of experimental medicine. 2004;200:437–445. doi: 10.1084/jem.20040712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boehme KW, Guerrero M, Compton T. Human cytomegalovirus envelope glycoproteins B and H are necessary for TLR2 activation in permissive cells. J Immunol. 2006;177:7094–7102. doi: 10.4049/jimmunol.177.10.7094. [DOI] [PubMed] [Google Scholar]
- 11.Compton T, et al. Human cytomegalovirus activates inflammatory cytokine responses via CD14 and Toll-like receptor 2. Journal of virology. 2003;77:4588–4596. doi: 10.1128/JVI.77.8.4588-4596.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Szomolanyi-Tsuda E, Liang X, Welsh RM, Kurt-Jones EA, Finberg RW. Role for TLR2 in NK cell-mediated control of murine cytomegalovirus in vivo. Journal of virology. 2006;80:4286–4291. doi: 10.1128/JVI.80.9.4286-4291.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sato A, Linehan MM, Iwasaki A. Dual recognition of herpes simplex viruses by TLR2 and TLR9 in dendritic cells. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:17343–17348. doi: 10.1073/pnas.0605102103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kurt-Jones EA, et al. Herpes simplex virus 1 interaction with Toll-like receptor 2 contributes to lethal encephalitis. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:1315–1320. doi: 10.1073/pnas.0308057100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chang S, Dolganiuc A, Szabo G. Toll-like receptors 1 and 6 are involved in TLR2-mediated macrophage activation by hepatitis C virus core and NS3 proteins. Journal of leukocyte biology. 2007;82:479–487. doi: 10.1189/jlb.0207128. [DOI] [PubMed] [Google Scholar]
- 16.Zhou S, et al. MyD88 is critical for the development of innate and adaptive immunity during acute lymphocytic choriomeningitis virus infection. European journal of immunology. 2005;35:822–830. doi: 10.1002/eji.200425730. [DOI] [PubMed] [Google Scholar]
- 17.Bieback K, et al. Hemagglutinin protein of wild-type measles virus activates toll-like receptor 2 signaling. Journal of virology. 2002;76:8729–8736. doi: 10.1128/JVI.76.17.8729-8736.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhu J, Martinez J, Huang X, Yang Y. Innate immunity against vaccinia virus is mediated by TLR2 and requires TLR-independent production of IFN-beta. Blood. 2007;109:619–625. doi: 10.1182/blood-2006-06-027136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Toshchakov V, et al. TLR4, but not TLR2, mediates IFN-beta-induced STAT1alpha/beta-dependent gene expression in macrophages. Nature immunology. 2002;3:392–398. doi: 10.1038/ni774. [DOI] [PubMed] [Google Scholar]
- 20.Kawai T, et al. Lipopolysaccharide stimulates the MyD88-independent pathway and results in activation of IFN-regulatory factor 3 and the expression of a subset of lipopolysaccharide-inducible genes. J Immunol. 2001;167:5887–5894. doi: 10.4049/jimmunol.167.10.5887. [DOI] [PubMed] [Google Scholar]
- 21.Doyle S, et al. IRF3 mediates a TLR3/TLR4-specific antiviral gene program. Immunity. 2002;17:251–263. doi: 10.1016/s1074-7613(02)00390-4. [DOI] [PubMed] [Google Scholar]
- 22.Krug A, et al. TLR9-dependent recognition of MCMV by IPC and DC generates coordinated cytokine responses that activate antiviral NK cell function. Immunity. 2004;21:107–119. doi: 10.1016/j.immuni.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 23.Tabeta K, et al. The Unc93b1 mutation 3d disrupts exogenous antigen presentation and signaling via Toll-like receptors 3, 7 and 9. Nature immunology. 2006;7:156–164. doi: 10.1038/ni1297. [DOI] [PubMed] [Google Scholar]
- 24.Asselin-Paturel C, et al. Mouse type I IFN-producing cells are immature APCs with plasmacytoid morphology. Nature immunology. 2001;2:1144–1150. doi: 10.1038/ni736. [DOI] [PubMed] [Google Scholar]
- 25.Jin MS. Crystal structure of the TLR1-TLR2 heterodimer induced by binding of a tri-acylated lipopeptide. Cell. 2007;130:1071–1082. doi: 10.1016/j.cell.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 26.Scheu S, Dresing P, Locksley RM. Visualization of IFNbeta production by plasmacytoid versus conventional dendritic cells under specific stimulation conditions in vivo. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:20416–20421. doi: 10.1073/pnas.0808537105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Okusawa T, et al. Relationship between structures and biological activities of mycoplasmal diacylated lipopeptides and their recognition by toll-like receptors 2 and 6. Infection and immunity. 2004;72:1657–1665. doi: 10.1128/IAI.72.3.1657-1665.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cailhier JF, et al. Conditional macrophage ablation demonstrates that resident macrophages initiate acute peritoneal inflammation. J Immunol. 2005;174:2336–2342. doi: 10.4049/jimmunol.174.4.2336. [DOI] [PubMed] [Google Scholar]
- 29.Auffray C, Sieweke MH, Geissmann F. Blood Monocytes: Development, Heterogeneity, and Relationship with Dendritic Cells. Annual review of immunology. 2009;27:669–692. doi: 10.1146/annurev.immunol.021908.132557. [DOI] [PubMed] [Google Scholar]
- 30.Geissmann F, Jung S, Littman DR. Blood Monocytes Consist of Two Principal Subsets with Distinct Migratory Properties. Immunity. 2003;19:71–82. doi: 10.1016/s1074-7613(03)00174-2. [DOI] [PubMed] [Google Scholar]
- 31.Serbina NV, Jia T, Hohl TM, Pamer EG. Monocyte-Mediated Defense Against Microbial Pathogens. Annual review of immunology. 2008;26:421–452. doi: 10.1146/annurev.immunol.26.021607.090326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kagan J, et al. TRAM couples endocytosis of Toll-like receptor 4 to the induction of interferon-beta. Nature immunology. 2008;9:361–368. doi: 10.1038/ni1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee PY, et al. A novel type I IFN-producing cell subset in murine lupus. J Immunol. 2008;180:5101–5108. doi: 10.4049/jimmunol.180.7.5101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Krieg AM. Therapeutic potential of Toll-like receptor 9 activation. Nat Rev Drug Discov. 2006;5:471–484. doi: 10.1038/nrd2059. [DOI] [PubMed] [Google Scholar]
- 35.Ishii KJ, et al. A Toll-like receptor-independent antiviral response induced by double-stranded B-form DNA. Nature immunology. 2006;7:40–48. doi: 10.1038/ni1282. [DOI] [PubMed] [Google Scholar]
- 36.Stetson DB, Medzhitov R. Recognition of cytosolic DNA activates an IRF3-dependent innate immune response. Immunity. 2006;24:93–103. doi: 10.1016/j.immuni.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 37.Takaoka A, et al. DAI (DLM-1/ZBP1) is a cytosolic DNA sensor and an activator of innate immune response. Nature. 2007;448:501–505. doi: 10.1038/nature06013. [DOI] [PubMed] [Google Scholar]
- 38.Hayashi F, et al. The innate immune response to bacterial flagellin is mediated by Toll-like receptor 5. Nature. 2001;410:1099–1103. doi: 10.1038/35074106. [DOI] [PubMed] [Google Scholar]
- 39.Franchi L, et al. Cytosolic flagellin requires Ipaf for activation of caspase-1 and interleukin 1beta in salmonella-infected macrophages. Nature immunology. 2006;7:576–582. doi: 10.1038/ni1346. [DOI] [PubMed] [Google Scholar]
- 40.Miao EA, et al. Cytoplasmic flagellin activates caspase-1 and secretion of interleukin 1beta via Ipaf. Nature immunology. 2006;7:569–575. doi: 10.1038/ni1344. [DOI] [PubMed] [Google Scholar]
- 41.Lightfield KL, et al. Critical function for Naip5 in inflammasome activation by a conserved carboxy-terminal domain of flagellin. Nature immunology. 2008;9:1171–1178. doi: 10.1038/ni.1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Smith KD, et al. Toll-like receptor 5 recognizes a conserved site on flagellin required for protofilament formation and bacterial motility. Nature immunology. 2003;4:1247–1253. doi: 10.1038/ni1011. [DOI] [PubMed] [Google Scholar]
- 43.Steven AC, Spear PG. Biochemistry. Viral glycoproteins and an evolutionary conundrum. Science (New York, NY. 2006;313:177–178. doi: 10.1126/science.1129761. [DOI] [PubMed] [Google Scholar]
- 44.Kurt-Jones EA, et al. Pattern recognition receptors TLR4 and CD14 mediate response to respiratory syncytial virus. Nature immunology. 2000;1:398–401. doi: 10.1038/80833. [DOI] [PubMed] [Google Scholar]
- 45.Jude BA, et al. Subversion of the innate immune system by a retrovirus. Nature immunology. 2003;4:573–578. doi: 10.1038/ni926. [DOI] [PubMed] [Google Scholar]
- 46.Triantafilou K, Triantafilou M. Coxsackievirus B4-induced cytokine production in pancreatic cells is mediated through toll-like receptor 4. Journal of virology. 2004;78:11313–11320. doi: 10.1128/JVI.78.20.11313-11320.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Georgel P, et al. Vesicular stomatitis virus glycoprotein G activates a specific antiviral Toll-like receptor 4-dependent pathway. Virology. 2007;362:304–313. doi: 10.1016/j.virol.2006.12.032. [DOI] [PubMed] [Google Scholar]
- 48.Jiang Z, et al. CD14 is required for MyD88-independent LPS signaling. Nature immunology. 2005;6:565–570. doi: 10.1038/ni1207. [DOI] [PubMed] [Google Scholar]
- 49.Crimmins GT, et al. Listeria monocytogenes multidrug resistance transporters activate a cytosolic surveillance pathway of innate immunity. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:10191–10196. doi: 10.1073/pnas.0804170105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ewald SE, et al. The ectodomain of Toll-like receptor 9 is cleaved to generate a functional receptor. Nature. 2008;456:658–662. doi: 10.1038/nature07405. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.