Abstract
This research provides estimates of the intergenerational persistence of Body Mass Index (BMI) between women and their children when both are at similar stages of the lifecycle. Using data from the National Longitudinal Survey of Youth 1979 (NLSY79) and the Young Adults of the NLSY79, associations between the weight status of women and their children are measured when both generations are between the ages of 16 and 24. In the entire sample, the measured intergenerational correlation of BMI is significantly different from zero and equal to 0.35. This result differs by gender with a BMI correlation between female children and their mothers of 0.38, compared to a significantly lower BMI correlation of 0.32 between mothers and their sons. Measures of this relationship across the distribution of BMI using quantile regression and quadrant dependence techniques indicate that the intergenerational persistence of BMI is strongest at higher levels of BMI. Strong dependence across generations is found when categorical outcomes of obesity and overweight are implemented. These results provide evidence of the strong persistence of weight problems across generations which may affect economic mobility within families.
Keywords: Intergenerational Mobility, Obesity, Quantile Regression
Both the genetic traits and household environments shared by parents and children influence the intergenerational transmission of health capital, which may influence economic success for both generations. The goal of this paper is to provide estimates of the intergenerational persistence of one form of health capital, weight status. The influence of obesity on income, wealth and other measures of socioeconomic status has been documented in recent research (e.g., Cawley (2004) and Zagorsky (2005)). Thus, the transmission of weight problems between generations may provide a mechanism to explain a portion of the relatively high degree of persistence in economic status found in Solon (1992) and Zimmerman (1992).1
Various dimensions of health status have been identified as potentially important mechanisms in the intergenerational transmission of economic status between generations.2 The measure of health capital used in this paper, Body Mass Index (BMI),3 has substantial variation across the population and combines the anthropometric measures of height and weight, whose intergenerational correlations have been previously studied.4 Of greatest interest for the current research is the persistence across generations of elevated BMI levels associated with obesity that are most likely to influence economic success.
Rapid expansion in rates of obesity in the United States and other developed countries has intensified the pursuit for explanations based on changes in food production technology and other aspects of the household environment such as video games and the internet. The intergenerational relationships estimated in this paper reflect social, cultural, genetic6 and environmental components, but attempts to identify the strength of BMI persistence across the distribution of BMI will receive the greatest attention.7 When parents make food consumption choices (or provide the feasible food consumption set) for children for meals eaten at home, this common environment generates a correlation between generations when both eat unhealthy (or healthy) food together. Since there exist substantial disparities in rates of obesity across various gradients of socioeconomic status and between ethnic groups,8 intergenerational BMI correlations are estimated separately for subgroups in certain specifications.
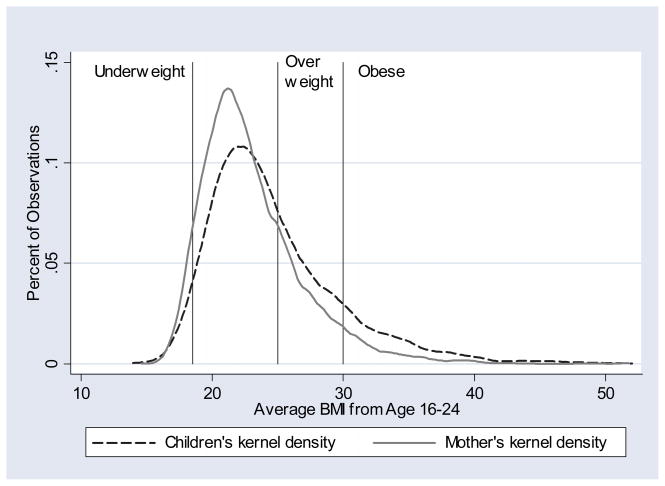
There exist numerous economic theories and empirical studies attempting to explain the enormous growth in rates of obesity in the United States, including changes in the relative price of unhealthy foods (caloric intake) and implicit costs of exercise (energy expenditure).9 While the goal of the present research is not to explain growth in rates of obesity, the data used in this paper span a period of rapid growth in obesity rates. Instead, emphasis is placed on understanding the degree of dependence in BMI and obesity between generations within families during this period of rapid weight growth. The women in the National Longitudinal Survey of Youth 1979 (NLSY79) were entering adulthood during a period of substantial growth in rates of adult obesity while their offspring in this sample were in late adolescence toward the end of the period of analysis in previous studies of obesity growth.10 The shift in the distribution of BMI between the NLSY79 mothers and their offspring detailed in Section 3 (Figure 1) reflects this substantial growth in rates of obesity.
Figure 1.
Distributions of Average BMI Ages 16–24
The longitudinal data provided in the NLSY79 allow for multiple observations of height and weight for both generations during late adolescence and early adulthood so that a portion of the potential bias resulting from transitory fluctuations in BMI is mitigated. The data also permit intergenerational associations to be measured when both generations are at similar points in the lifecycle so that the estimated relationships are not confounded by influences that affect both generations contemporaneously. The intergenerational persistence in BMI is first measured using traditional estimates of correlation coefficients that allow for comparison to previous studies of this topic (which typically use single observations of BMI or obesity status measured in the same year for both generations). Then, non-central measures of the association of BMI and obesity between generations are estimated that allow for the dependence of the relationship to vary across the distribution of weight outcomes.
Estimating the relationships of interest separately by the gender of the offspring, I find that the intergenerational correlation of BMI between mothers and daughters is significantly higher than between mothers and sons, with a correlation of approximately 0.38 (relative to 0.32 for sons to mothers). Using measures of this relationship estimated via quantile regression and quadrant dependence techniques, which allow for the magnitude of intergenerational BMI associations to vary across the distribution of the offspring’s BMI, the strength of this correlation appears to be largest at higher levels of BMI. This indicates that the intergenerational persistence of BMI is strongest for children born to mothers with the highest levels of BMI. Since the negative economic consequences of weight status occur at relatively higher levels of BMI, a higher rate of transmission of such weight problems among obese mothers could limit the economic mobility of their offspring. Estimates from probit models for obese and overweight outcomes add to this evidence of a strong dependence in potentially harmful (both economically and physiologically) weight outcomes across generations.
I. Previous Research on the Intergenerational Transmission of Obesity and its Economic Consequences
The influential role of obesity on measures of economic outcomes such as income, wealth and educational attainment has received increasing attention in recent studies. Research in this field has often found that the economic consequences of obesity vary by ethnicity and gender.11 A common result among these studies is that females are much more likely than males to suffer negative economic consequences due to elevated weight.
The causal influence of obesity on income is estimated by Cawley (2004) who uses an instrumental variable exploiting within-generation correlations of BMI in the NLSY79. Controlling for the potential endogeneity of weight status in determining income using siblings’ BMI as an instrument, he finds that white females in the NLSY79 who weigh two standard deviations above mean BMI earn 9 to 18% less than their average weight counterparts, while no significant differences were found for black or Hispanic females (or males of any ethnicity). Numerous other studies have found additional evidence of the negative consequence of obesity on wages for females, including Baum and Ford (2004), Conley and Glauber (2005) and Han, Norton and Stearns (2009). Brunello and D’Hombres (2007) find negative effects of obesity on wages for both males and females in Europe, especially in the southern part of Europe where obesity is relatively rare. Morris (2006) finds consistent evidence of a negative effect of obesity on occupational attainment (measured as the average wage rate associated with a given occupation) for women in England. Paraponaris et. al. (2005) find that obese individuals are less likely to be employed in France and experience longer unemployment spells. Other studies of the influence of obesity on wages find larger effects for women in several European countries (Atella et. al. (2008)), Denmark (Greve (2008)), and Finland (Johansson et. al. (2009)). A large and significant negative relationship between wealth and obesity is found for white females in Zagorsky (2005) who uses data from the NLSY79, and finds smaller effects for black females and white males. Crosnoe (2007) finds a significant reduction in the likelihood of college attendance for obese females with the strongest influence of weight problems on education attainment in schools with the lowest rates of obesity. Murasko (2009) identifies influential socioeconomic factors on the development of obesity during childhood while Cawley and Spiess (2008) find that obese children of both genders have lower verbal skill development at ages as young as 2 to 3 years old. Given the likely negative economic consequences of elevated BMI levels (and other measures of poor health), the persistence of such health problems within families may explain reduced prosperity in multiple generations. Obviously, the transmission of health outcomes between generations is a complicated process governed by a myriad of factors including genetics, culture, family values and consumption choices. One of the first discussions in the economics literature of such correlations in health status is provided by Ahlburg (1998). He reviews studies measuring intergenerational relationships in outcomes such as lifespan and certain diseases and reports estimates of intergenerational correlations for lifespan in the range of 0.15 to 0.3.
The transmission of obesity from parents to children is studied for families within a single health insurance organization in the state of Washington in Whitaker et. al. (1997). Using observations of BMI during childhood and adolescence combined with data on parental BMI levels, they find that children who are obese early in life and have at least one obese parent are three times more likely to become obese adults than non-obese children in households where neither parent is obese. While this effect dissipates slightly as children grow older, they find a very strong correlation between obesity in adolescence and becoming an obese adult with parental obesity raising the likelihood of obesity of offspring in early adulthood.
Classen and Hokayem (2005) measure the influence of maternal obesity on the likelihood of child and adolescent obesity with controls for several influential socioeconomic and demographic characteristics included. Using data from the NLSY79, they find that children of extremely obese mothers (with BMI greater than 40) are 50% more likely to be obese than their counterparts with mothers having BMI levels in the recommended range of 18.5 to 25.
Martin (2008) measures the intergenerational relationship of BMI using data from the National Longitudinal Survey of Adolescent Health (Add Health) which includes measures of BMI for adolescent children and reports of parental obesity status. She finds that children whose parents are both reported to be obese have BMI levels one standard deviation above the sample mean. The primary limitation of this study is that parental weight status is limited to a simple indicator for obesity and likely measured with substantial reporting error since the question asks one parental respondent to report whether the child’s mother and father are obese, but no measures of actual parental height and weight are collected. Furthermore, only the contemporaneous relationship of obesity status across generations can be measured, rather than correlations generated when both generations are at similar stages of life as in the current study.
Anderson, Butcher and Schanzenbach (2007) use data from repeated cross sections of the National Health and Nutrition Examination Survey (NHANES) to measure the contemporaneous correlation of BMI between women and their children in a given survey year. They find that the intergenerational BMI elasticity between women and their children has increased over time (using observations from 1971 to 2004), but does not vary significantly between families of different income levels. They also find similar intergenerational BMI elasticities for both fathers and mothers to their children. Overall, they find an intergenerational BMI elasticity between women and their children in the most recent NHANES of roughly 0.2 which they attribute to the interaction of common environments and genes between parents and children, with the role of common environments becoming increasingly influential over time.
Relative to previous studies, the current paper has several advantages. The longitudinal structure of the NLSY data allows for averaging over multiple observations of BMI to avoid potential measurement error resulting from observing height and weight at only a single point in time. Furthermore, observations of BMI for women in the NLSY79 and their children are available when both generations are at similar points in the lifecycle (late adolescence and early adulthood) instead of contemporaneous observations of BMI for both generations in a single year as in previous studies. The implementation of quantile regression and quadrant dependence estimates provide measures that allow the dependence of BMI between generations to vary across the entire distribution of weight outcomes, rather than relying solely on measures of central tendency such as correlation coefficients. A limitation of the NLSY data relative to the NHANES data is that height and weight is self-reported for a majority of observations in the NLSY.12 If the bias resulting from self-reported weight levels is constant across generations within families, then this would not introduce substantial bias in the coefficients of interest in this paper, but could be of concern in the case of large changes in BMI across generations.13
II. Description of Data
To estimate the degree of correlation in BMI between generations, I use data from the National Longitudinal Survey of Youth 1979 (NLSY79) and the Children and Young Adults of the NLSY79 (YA NLSY79). The NLSY79 began in 1979 with interviews of a nationally-representative sample of people born between 1957 and 1964 (so that all respondents were between ages 14 and 22 at the beginning of the survey). This sample was interviewed annually until 1986 and biennially since 1988. Height and weight data for women in the NLSY79 were first collected in the 1981 survey. Beginning in 1986, data on the children of the women in the NLSY79 sample were collected. In 1994, children aged 14 or older of women in the NLSY79 were surveyed in the YA NLSY79.
The structure of these surveys provides the highly desirable feature of being able to measure BMI at similar stages of life for mothers and their children. Previous studies of intergenerational mobility in economics have identified two critical shortcomings of data used to measure such relationships: potential measurement error from using only a single observation of the outcome of interest (assuming there exists temporal variance in the measure of mobility studied) and observing the outcome at different stages of the lifecycle (such as in cross sectional observations of earnings for two generations at a single point in time).14 For example, an observation of income for a father at age 50 might be paired with an observation on his son’s earnings in the same year at age 25. While measurements of the age of each generation are often incorporated to attempt to adjust for such lifecycle differences, it is evident that knowledge of the son’s earnings when he is age 50 would provide much more accurate estimates of the intergenerational correlation of income. Using the NLSY79 and YA NLSY79 data, I calculate the weight status of mothers and their children when both generations are between the ages of 16 and 24. This generates an estimate of the intergenerational persistence of BMI across generations at a crucial stage for determining the likelihood of developing weight problems later on in life.
A second critique of studies of intergenerational mobility focuses on whether the observed data are representative of the true level of the variable of interest. The transitory nature of weight and height during late adolescence could result in significant deviations from a more permanent measurement when data are only collected for a given year. Obviously, weight is a variable that is likely to fluctuate over time and multiple observations are desirable for generating an accurate measure of weight status. The longitudinal structure of the NLSY data allows for multiple observations of BMI for both generations to generate an estimate of its intergenerational dependence.15
Since the NLSY79 cohort contains individuals who were first interviewed at age 14 to 22 in 1979 and height and weight data are only available beginning with the 1981 survey, the age range for averaging BMI observations extends up to age 24. Although the period from age 16 to 24 is likely to feature significant changes in BMI (potentially as a result of individuals making food choices without supervision from parents for the first time), averaging BMI values over this range should accurately reflect weight status during this phase of development and reduce potential bias from only a single observation of BMI.16 For the determination of whether individuals are obese, overweight or underweight, the cutoffs for adults of BMI over 30 indicating obesity, BMI above 25 and below 30 as indicating overweight and BMI below 18.5 indicating underweight are used.17
Among the 12,686 people initially surveyed in the NLSY79, 6,283 were female. Of these women, approximately two-thirds have had children who have been included in at least one of the YA surveys. The selection criterion for inclusion in this analysis requires that the child have reached at least age 16 by the 2004 YA NLSY79 survey. This leaves 4,748 children born to 2,560 women in the NLSY79 sample for analysis. Given this criterion, the included sample contains an oversampling of younger mothers and the demographics displayed in Table 1 make apparent that the racial composition of the sample is not nationally representative. Thus, results for several of the specifications will be presented separately by racial and ethnic categories.
Table 1.
Summary Statistics for NLSY Sample
| NLSY79 Mothers | YA NLSY79 Children | |||
|---|---|---|---|---|
| (n = 2,560) | (n = 4,748) | |||
| Mean | Std. Dev. | Mean | Std. Dev. | |
| Avg. BMI, age 16–24 | 23.0 | 3.9 | 24.7 | 5.0 |
| Percent Overweight | 24.3% | 37.2% | ||
| BMI>25 | ||||
| Percent Obese | 5.7% | 13.1% | ||
| BMI>30 | ||||
| White | 42.0% | 39.5% | ||
| Black | 36.0% | 37.2% | ||
| Hispanic | 22.0% | 23.3% | ||
| Male | 50.6% | |||
Due to the structuring of the data for this analysis, there is no evident method to incorporate the available sample weights from the NLSY79 and YA NLSY79 surveys since height and weight measurements are collected for both generations in multiple survey years. Sample weights are provided for each NLSY survey year to generate a representative cross-section for a given survey year. However, since this study combines observations on mothers interviewed in the beginning of the NLSY79 panel (who were assigned sample weights for the first survey in 1979) and averages BMI over multiple survey years (each of which has unique sampling weights) with observations on their children who are also assigned sample weights in each survey year, this structure of data from multiple NLSY surveys does not lend itself to using the cross-section NLSY sample weights. The results reported in this paper are unweighted, but an appendix includes results generated using custom longitudinal sampling weights available for the YA NLSY data that provide representative weights for multiple survey years of the survey. The limitation of these custom weights for this application is that height and weight observations for the offspring are not all taken from the same set of survey years. Thus, the weighted results in the appendix use the custom sample weights for the YA NLSY survey from 1986 to 2004. Results (Tables A1 and A2) are not sensitive to the oversampling of black and Hispanic individuals in the unweighted data, but most results are presented separately by ethnicity given this potential concern.
Table A1.
Weighted Intergenerational BMI Elasticities and Correlations by Ethnicity and Gender, 1981 – 2004
| Relationship | Elasticity β1 | Std. Error | R2 | p-value of difference by gender | Correlation in BMI ρBMI |
|---|---|---|---|---|---|
| Full Sample | |||||
| Mother-Child | 0.414 | 0.022 | 0.117 | 0.342 | |
| Mother-Daughter | 0.479 | 0.034 | 0.137 | } 0.005 | 0.370 |
| Mother-Son | 0.356 | 0.028 | 0.100 | 0.316 | |
| Whites | |||||
| Mother-Child | 0.403 | 0.034 | 0.106 | 0.326 | |
| Mother-Daughter | 0.444 | 0.054 | 0.117 | } 0.259 | 0.343 |
| Mother-Son | 0.367 | 0.042 | 0.099 | 0.314 | |
| Blacks | |||||
| Mother-Child | 0.392 | 0.030 | 0.111 | 0.333 | |
| Mother-Daughter | 0.437 | 0.045 | 0.116 | } 0.064 | 0.340 |
| Mother-Son | 0.332 | 0.037 | 0.102 | 0.320 | |
| Hispanics | |||||
| Mother-Child | 0.388 | 0.042 | 0.105 | 0.324 | |
| Mother-Daughter | 0.461 | 0.068 | 0.143 | } 0.146 | 0.379 |
| Mother-Son | 0.324 | 0.058 | 0.077 | 0.277 | |
Note: Results use longitudinal custom sample weights from NLSY YA data for 1986–2004.
Table A2.
Weighted Distributions of Intergenerational Weight Status Transitions
| Full Sample | Child’s Weight Status | ||||||
|---|---|---|---|---|---|---|---|
| BMI | Category | <18.5 Underweight | 18.5–25 Recommended | 25–30 Overweight | >30 Obese | Mother’s Distribution | |
| Mother’s Weight Status | <18.5 | Underweight | 12.7% | 70.8% | 12.4% | 4.1% | 6.9% |
| 18.5–25 | Recommended | 4.7% | 66.4% | 20.4% | 8.6% | 70.8% | |
| 25–30 | Overweight | 2.3% | 43.9% | 32.0% | 21.9% | 17.5% | |
| >30 | Obese | 2.4% | 32.7% | 29.1% | 35.9% | 4.8% | |
| Child’s Distribution | 4.7% | 61.1% | 22.3% | 11.9% | |||
| Daughters | Daughter’s Weight Status | ||||||
| BMI | Category | <18.5 Underweight | 18.5–25 Recommended | 25–30 Overweight | >30 Obese | Mother’s Distribution | |
| Mother’s Weight Status | <18.5 | Underweight | 19.2% | 66.6% | 9.7% | 4.5% | 6.8% |
| 18.5–25 | Recommended | 7.3% | 66.4% | 17.6% | 8.7% | 70.1% | |
| 25–30 | Overweight | 2.7% | 43.2% | 28.8% | 25.3% | 18.0% | |
| >30 | Obese | 3.6% | 32.7% | 26.7% | 37.1% | 5.1% | |
| Daughter’s Distribution | 7.1% | 60.5% | 19.5% | 12.9% | |||
| Sons | Son’s Weight Status | ||||||
| BMI | Category | <18.5 Underweight | 18.5–25 Recommended | 25–30 Overweight | >30 Obese | Mother’s Distribution | |
| Mother’s Weight Status | <18.5 | Underweight | 6.6% | 74.7% | 14.9% | 3.8% | 7.0% |
| 18.5–25 | Recommended | 2.2% | 66.3% | 23.0% | 8.5% | 71.5% | |
| 25–30 | Overweight | 1.8% | 44.6% | 35.2% | 18.5% | 17.0% | |
| >30 | Obese | 4.5% | 32.7% | 31.7% | 34.5% | 4.5% | |
| Son’s Distribution | 2.4% | 61.7% | 24.9% | 11.0% | |||
Note: Weight Status for both generations are determined based on average BMI levels between ages 16 and 24. Data are weighted using longitudinal custom sample weights for NLSY YA from 1986 to 2004.
A limitation of the health data available in the NLSY79 is the lack of measures of actual body fatness (adiposity) which limits the analysis to using height and weight in the calculation of BMI which may be a poor proxy for adiposity. While some studies include measures of skinfold thickness and waist circumference, only self-reported height and weight are available in the NLSY data.18
III. Measures of Intergenerational BMI Correlations
This section provides estimates of intergenerational elasticities and correlations of BMI for the NLSY79 sample described in the previous section. In order to estimate the correlation of BMI between generations, this section specifies an empirical model of obesity transmission. While there are numerous studies of the genetic epidemiology of obesity and relationships between generations (e.g. Bouchard et. al. (2003) and Garn et. al. (1989)), there have been relatively few attempts to measure this correlation in a large data set with broad geographic diversity when both generations are at similar stages of physical development.
A. Intergenerational Elasticities and Correlations of BMI
Following a method for measuring intergenerational correlations developed in Goldberger (1989), the general form for the regressions estimated in this section is:
where BMIijg+1 is the natural logarithm of average BMI between ages 16 and 24 of child i in family j of generation g+1. Similarly, BMI jg is the natural logarithm of average BMI between ages 16 and 24 of the mother (belonging to generation g) in family j. The stochastic disturbance term eijg+1 for the regression has family and individual-specific components. The standard errors reported in this paper are adjusted (using the cluster command in Stata) to account for the presence of multiple children from a single mother in the data. With this specification, β1 represents the elasticity of a child’s BMI with respect to his or her mother’s BMI, with an elasticity of zero indicating no persistence in weight across generations. The intergenerational correlation of BMI is then given by
where σg and σg+1 are the standard deviations of BMIg and BMIg+1, respectively. In order to mitigate possible measurement error from temporal variation in BMI, I average all available, observations for mothers and their children when both are between ages 16 and 24.
A kernel density approximation of the distribution of average BMI for both generations between ages 16 and 24 indicates an evident shift toward higher BMI levels among children of the women in the NLSY79 (Figure 1).
Summary statistics of relevant variables in the NLSY sample (Table 1) show that average BMI increased by more than 7% between generations, while rates of overweight and obesity have both increased by more than 50% across generations in the NLSY and YA NLSY samples.
B. Estimates of the Intergenerational Correlation of BMI
Estimates of the intergenerational elasticities and correlations of BMI between mothers and their children for the entire sample and separately by gender and ethnicity indicate a strong degree of persistence across generations (Table 2a). For the full sample, the estimated intergenerational elasticity of BMI is significantly different from zero and equal to 0.42 which implies a correlation of 0.35.19 The elasticity of BMI between mothers and their daughters is nearly 0.5 which implies a correlation of BMI across generations of 0.38 for females. Males have a significantly lower correlation of BMI to their mothers of 0.32. The estimated correlations do not vary significantly between ethnic groups. Significant differences between genders in intergenerational BMI elasticity persist only for the black sub-sample. Results using the custom sample weights for the NLSY YA data indicate similar levels of correlations and significant differences by gender for the full sample (Table A1).
Table 2.
| Table 2a. Intergenerational BMI Elasticities and Correlations by Ethnicity and Gender, 1981 – 2004 | ||||||
|---|---|---|---|---|---|---|
| Relationship | Sample Size | Elasticity β1 | Std. Error | R2 | p-value of difference by gender | Correlation in BMI ρBMI |
| Full Sample | ||||||
| Mother-Child | 4,748 | 0.420 | 0.019 | 0.122 | 0.350 | |
| Mother-Daughter | 2,348 | 0.486 | 0.030 | 0.144 | } 0.001 | 0.379 |
| Mother-Son | 2,400 | 0.356 | 0.025 | 0.102 | 0.319 | |
| Whites | ||||||
| Mother-Child | 1,875 | 0.401 | 0.033 | 0.106 | 0.326 | |
| Mother-Daughter | 923 | 0.433 | 0.052 | 0.114 | } 0.346 | 0.338 |
| Mother-Son | 952 | 0.370 | 0.042 | 0.101 | 0.318 | |
| Blacks | ||||||
| Mother-Child | 1,769 | 0.403 | 0.030 | 0.120 | 0.346 | |
| Mother-Daughter | 909 | 0.455 | 0.045 | 0.129 | } 0.035 | 0.359 |
| Mother-Son | 860 | 0.332 | 0.038 | 0.103 | 0.321 | |
| Hispanics | ||||||
| Mother-Child | 1,104 | 0.406 | 0.041 | 0.110 | 0.332 | |
| Mother-Daughter | 516 | 0.465 | 0.064 | 0.138 | } 0.208 | 0.371 |
| Mother-Son | 588 | 0.358 | 0.055 | 0.090 | 0.300 | |
| Table 2b. Intergenerational BMI Elasticities and Correlations by SES measures | |||||||
|---|---|---|---|---|---|---|---|
| Sample Size | Elasticity β1 | Std. Error | R2 | p-value of F-test for all coeff. equal | p-value of F-test for higest-lowest diff. | Correlation in BMI ρBMI | |
| Income Level (Child aged 2–18) | |||||||
| <25th percentile | 1,399 | 0.36 | 0.032 | 0.130 | 0.183 | 0.105 | 0.314 |
| 25–50th percentile | 1,280 | 0.38 | 0.033 | 0.324 | |||
| 50–75th percentile | 1,140 | 0.46 | 0.045 | 0.363 | |||
| >75th percentile | 810 | 0.47 | 0.061 | 0.359 | |||
| Mother’s Education Level | |||||||
| Less than high school | 713 | 0.35 | 0.044 | 0.126 | 0.123 | 0.042** | 0.320 |
| High School | 2,371 | 0.44 | 0.025 | 0.368 | |||
| Some college | 1,136 | 0.39 | 0.048 | 0.299 | |||
| College graduate | 409 | 0.53 | 0.079 | 0.404 | |||
| Poverty Level (while Child age 2–18) | |||||||
| 75–100% of time in poverty | 883 | 0.37 | 0.039 | 0.129 | 0.071* | 0.034** | 0.334 |
| 25–75% of time in poverty | 1,045 | 0.39 | 0.039 | 0.319 | |||
| 1–25% of time in poverty | 647 | 0.35 | 0.051 | 0.287 | |||
| Never in poverty vs. Ever in Poverty | 2,054 | 0.47 | 0.033 | 0.374 | |||
| 2,575 | 0.38 | 0.025 | 0.021** | 0.021** | 0.326 | ||
Notes: Income quartiles are not uniformly distributed due to quartiles being calculated for entire YA NLSY79 sample.
indicates statistically significant difference at 10% level,
indicates statistically significant difference at 5% level
Estimates of the persistence of BMI across generations within families of different SES measures indicate higher levels of intergenerational correlations among higher SES families (Table 2b). Using income measured as the average family income per household member for the offspring during the period of life from age 2 to 18 (available in the NLSY79 interviews with the mothers), children are organized into quartiles based on their family’s relative income within the NLSY79 sample.20 Intergenerational BMI correlations range from 0.31 in the lowest income quartile to 0.36 in the highest, but there are not statistically significant differences in elasticities between income quartiles.
Another measure of SES disparities considers the duration of time during childhood that the offspring’s family was in poverty. The poverty variable is calculated as the percentage of time the child’s family was classified as being in poverty (reported for mothers in the NLSY surveys) when the child was age 2 to 18.21 The correlation of BMI across generations is significantly higher for children who grew up in families who were never in poverty relative to children whose families were classified as having income below the Federal Poverty Line at least once (1 – 25% of the time in poverty). Comparing families that were ever classified as being in poverty during the offspring’s upbringing relative to families that were never in poverty reveals a significantly lower intergenerational BMI correlation for families who were ever impoverished, but the magnitude of the correlation increases as the duration of poverty rises.
Results for differences in BMI correlations by maternal education indicate a higher persistence of BMI at higher education levels, with the correlation significantly larger in households in which the mother has completed college relative to households in which the mother did not complete high school. However, the largest disparity in magnitudes of intergenerational correlations is between the two highest levels of education (college graduates relative to those who attended some college). The relationship between intergenerational BMI correlations and these measures of SES are not monotonic in any of the three cases, but there exists some evidence of stronger intergenerational BMI persistence in higher SES families. This is in contrast to the results in Anderson, Butcher and Schanzenbach (2007) who do not find significant differences between SES groups in BMI correlations between women and their children in the NHANES data.
IV. Non-Central Measures of the Intergenerational Persistence of BMI
The previous section provides estimates of intergenerational elasticities (and associated correlations) of BMI using least-squares regressions. Such estimates allow for inference for changes in BMI levels between generations around the population mean of the maternal BMI distribution. However, since relatively high BMI levels substantially above the mean (indicative of obesity) are most likely to influence economic outcomes, results in this section measure the strength of intergenerational BMI transmission across the entire distribution of observed BMI levels. The consequences of weight status for economic outcomes are most relevant for BMI levels above 30 indicating obesity, and thus, understanding the intergenerational transmission of such undesirable health outcomes is of greatest interest.
A. Quantile Regression Estimates of BMI Correlations
Quantile regressions estimate parameters by minimizing sums of absolute deviations (rather than squared residuals as in classical linear regression) for a specified division of the dependent variable into quantiles.22 Such estimates provide measures of the influence of a covariate (maternal BMI) at multiple quantiles of the distribution of the dependent variable (in this case, the BMI of a woman’s offspring).23 The particular quantiles used in quantile regressions can be chosen based on areas in the distribution of greatest interest, number of available observations or a variety of other criterion. This flexibility allows for potentially nonlinear relationships to be more readily recognized than in an ordinary least squares regression. For the application here, we are interested in whether the association to maternal BMI is constant across the distribution of the offspring’s BMI.24
Parameters in a quantile regression model of intergenerational BMI transmission are estimated for the θth quantile (0 < θ < 1) by solving for the elasticity β1θ by defining Diffi = BMIijg+1−(βo+β1BMI jg) for n observations and solving
with the indicator function 1Diff>0 defined to equal one for individuals having positive values of Diffi, and zero otherwise.
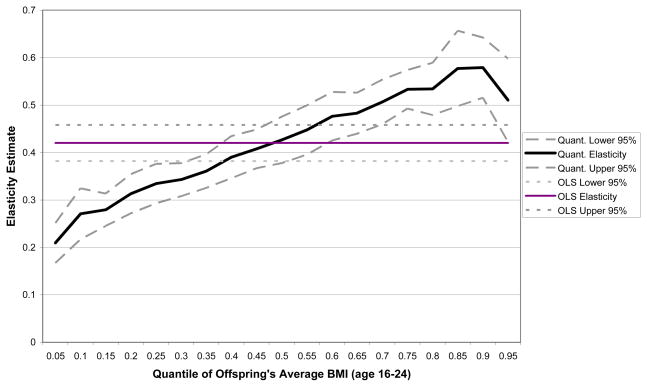
This produces a range of estimates for the intergenerational elasticity of BMI between women and their children across the distribution of BMIg+1 as θ varies. Figure 2 displays estimates of the elasticity of BMI between generations for quantile regressions allowing θ to vary from .05 to .95 in uniform increments of .05 with the quantile cutoffs based on the distribution of the offspring’s BMI in the YA NLSY79 data.26 The estimated elasticities around the median of the distribution are similar to those estimated using ordinary least squares (from the elasticity column in Table 2a). The strength of the intergenerational relationship declines at quantiles below the median and increases significantly at higher quantiles in the BMI distribution. These results show a clear trend of an increased elasticity of BMI transmission at higher levels of the offspring’s BMI. Table 3 provides results for selected quantile levels. The estimated elasticities around the 90th percentile (BMI levels just above the cutoff for obesity) vary from 0.53 for sons to 0.62 for daughters, with the elasticity estimate for the full sample more than twice as large at the 90th percentile as at the 10th percentile (where BMI levels are just above the cutoff indicating underweight). Thus, the estimated intergenerational dependence in BMI is strongest among offspring at the upper end of the distribution of BMI.26 Such a result indicates that the degree of similarity in weight outcomes between generations is highest among those children who are obese or overweight in late adolescence.
Figure 2.
Results for Quantile Estimates of Intergenerational BMI Elasticity Relative to OLS Elasticity
Table 3. Quantile Regression Results for Intergenerational Elasticity of BMI.
Dependent variable is natural log of child’s average BMI from age 16–24 and elasticity reported is coefficient on natural log of mother’s average BMI from 16–24, n = 4,748 observations
| Quantile | ||||||
|---|---|---|---|---|---|---|
| Full Sample | 10 | 25 | 50 | 75 | 90 | OLS |
| Elasticity | 0.27 | 0.33 | 0.43 | 0.53 | 0.58 | 0.42 |
| Std. Error | (0.036) | (0.027) | (0.024) | (0.026) | (0.038) | (0.019) |
| Females | 10 | 25 | 50 | 75 | 90 | OLS |
| Elasticity | 0.32 | 0.41 | 0.50 | 0.62 | 0.62 | 0.49 |
| Std. Error | (0.045) | (0.044) | (0.036) | (0.046) | (0.060) | (0.030) |
| Males | 10 | 25 | 50 | 75 | 90 | OLS |
| Elasticity | 0.20 | 0.27 | 0.38 | 0.43 | 0.53 | 0.36 |
| Std. Error | (0.050) | (0.027) | (0.022) | (0.031) | (0.061) | (0.025) |
| BMI Value at Quantile Cutoff | ||||||
| 10 | 25 | 50 | 75 | 90 | Mean BMI | |
| Full Sample | 19.6 | 21.2 | 23.6 | 26.9 | 31.3 | 24.7 |
| Females | 19.2 | 20.9 | 23.3 | 27.3 | 32.0 | 24.7 |
| Males | 19.9 | 21.5 | 23.7 | 26.6 | 30.6 | 24.7 |
Note: Standard errors for quantile regressions are generated via bootstrap replications.
B. Quadrant Dependence in Intergenerational BMI Transmission
While correlation coefficients, such as those presented in Section 3, provide a parsimonious measure of the direction and degree of dependence between random variables, it is possible that the strength of this relationship may vary substantially across BMI distributions as was found in the quantile regression results presented above. Summaries of associations based on measures of central tendency may conceal (possibly non-linear) conditional relationships in ranges of the distributions of most interest. This section describes and provides results for techniques that allow for measuring the degree of dependence between random variables at multiple points across the distributions of BMI in both generations. These measures, known as quadrant dependence, were developed in Lehmann (1966) and are useful for describing the comovement of two (or more) random variables. Allowing the strength of the association of BMI across generations to vary in a more flexible fashion will provide insights on areas of the distributions of interest where persistence in weight is strongest.
Formally, a pair of random variables, X and Y, with a distribution F(X,Y) are said to exhibit positive quadrant dependence (PQD) if
with strict PQD holding if the inequality is strict for at least one (x,y). Negative quadrant dependence (NQD) is defined analogously with the inequality reversed. The family of distribution functions for which PQD and NQD hold are defined as F1 and G1, respectively. Karlin (1983) further develops these measures by providing a technique allowing for comparisons of the degree of quadrant dependence between pairs of random variables. In order to implement measures of quadrant dependence empirically, indicator functions are employed to approximate the space of increasing functions defined by F1. Following the notation of Karlin (1983), we define
where b varies within the support of x and y to generate a series of cutoffs. Correlations between the indicator functions φb (x) and ψb (y) are then calculated for selected values of b in the support of x and y to measure the dependence between x and y across different segments of their joint distribution. In the results presented here, b takes on the values of the 20th, 40th, 60th and 80th percentiles of the distributions of BMI for each generation. The measured correlations are then arranged into a 4 × 4 “association array” whose entries are the correlations between φi (x) and ψj (y) for i, j∈{20,40,60,80}. With rij = r(φi (x), ψj (y)) representing the correlation between φi (x) and ψj (y), arrays of the dependence between random variables are organized from r20,20 in the bottom left corner to r80,80 in the upper right corner with i increasing from the bottom to the top of the array and j increasing from left to right in the array.
Variables are said to exhibit strong PQD if all of the entries in the array are strictly positive. For comparison of the strength of dependence in two populations A and B, Karlin argues that if all of the entries in an association array for population A are strictly greater than the entries in the array for population B then outcomes are more associated in population A than B.
Comparisons of the degree and direction of quadrant dependence in BMI between generations by gender and ethnic groups indicate strong positive quadrant dependence in all cases (Tables 4 and 5). The magnitude of dependence generally increases toward the upper right in each table indicating stronger dependence at higher BMI levels. Comparing the degree of PQD for daughters and sons in Table 4, BMI is more associated between women and their daughters than women and their sons.27 No evident differences exist when comparing the quadrant dependence of BMI between ethnic groups in Table 5.
Table 4.
Measures of Quadrant Dependence in BMI Transmission
| Full Sample | ||||
|---|---|---|---|---|
| (Corr = 0.35) | ||||
| (n = 4,748) | ||||
| Mother’s BMI Percentile | Child’s BMI Percentile | |||
| 20 | 40 | 60 | 80 | |
| 80 | 0.135 | 0.205 | 0.232 | 0.231 |
| 60 | 0.160 | 0.226 | 0.235 | 0.222 |
| 40 | 0.166 | 0.214 | 0.215 | 0.190 |
| 20 | 0.160 | 0.194 | 0.178 | 0.124 |
| Daughters | ||||
| (Corr = 0.38) | ||||
| (n = 2,348) | ||||
| Mother’s BMI Percentile | Daughter’s BMI Percentile | |||
| 20 | 40 | 60 | 80 | |
| 80 | 0.171 | 0.241 | 0.260 | 0.238 |
| 60 | 0.213 | 0.260 | 0.261 | 0.232 |
| 40 | 0.216 | 0.234 | 0.236 | 0.222 |
| 20 | 0.201 | 0.201 | 0.192 | 0.153 |
| Sons | ||||
| (Corr = 0.32) | ||||
| (n = 2,400) | ||||
| Mother’s BMI Percentile | Son’s BMI Percentile | |||
| 20 | 40 | 60 | 80 | |
| 80 | 0.097 | 0.171 | 0.205 | 0.222 |
| 60 | 0.105 | 0.193 | 0.209 | 0.211 |
| 40 | 0.116 | 0.197 | 0.195 | 0.155 |
| 20 | 0.124 | 0.193 | 0.165 | 0.094 |
Table 5.
Quadrant Dependence Measures of BMI Transmission by Race
| Whites | ||||
|---|---|---|---|---|
| (Corr = 0.33) | ||||
| (n = 1,875) | ||||
| Mother’s BMI Percentile | Child’s BMI Percentile | |||
| 20 | 40 | 60 | 80 | |
| 80 | 0.128 | 0.211 | 0.226 | 0.227 |
| 60 | 0.167 | 0.237 | 0.234 | 0.228 |
| 40 | 0.174 | 0.214 | 0.217 | 0.178 |
| 20 | 0.156 | 0.182 | 0.161 | 0.102 |
| Blacks | ||||
| (Corr = 0.35) | ||||
| (n = 1,769) | ||||
| Mother’s BMI Percentile | Child’s BMI Percentile | |||
| 20 | 40 | 60 | 80 | |
| 80 | 0.132 | 0.193 | 0.217 | 0.231 |
| 60 | 0.129 | 0.191 | 0.207 | 0.215 |
| 40 | 0.121 | 0.176 | 0.164 | 0.189 |
| 20 | 0.112 | 0.169 | 0.164 | 0.148 |
| Hispanics | ||||
| (Corr = 0.33) | ||||
| (n = 1,104) | ||||
| Mother’s BMI Percentile | Child’s BMI Percentile | |||
| 20 | 40 | 60 | 80 | |
| 80 | 0.115 | 0.171 | 0.221 | 0.202 |
| 60 | 0.146 | 0.202 | 0.225 | 0.193 |
| 40 | 0.162 | 0.207 | 0.231 | 0.176 |
| 20 | 0.196 | 0.213 | 0.189 | 0.097 |
V. Intergenerational Obesity and Overweight Transmission
This section provides results for the empirical probabilities of transitions between weight status categories across generations. While the previous analysis provided correlations and measures of the dependence of BMI levels across generations, this section considers the distribution of categorical weight outcomes for young adults conditional on their mother’s weight status when she was at the same stage of life. Individuals with average BMI levels above 30 are classified as obese and those with BMI between 25 and 30 are categorized as overweight.
Distributions of offspring weight status conditional on maternal weight status indicate a high degree of persistence in weight problems across generations (Table 6). These results indicate that 70% of children born to women who were obese in early adulthood became obese or overweight themselves by early adulthood which is more than double the rate for children born to women who were in the recommended range of BMI at this stage of life. However, in accord with the expansion in rates of obesity and overweight during the 25 years covered by these data, more than 30% of children born to mothers who were of recommended BMI in early adulthood were obese or overweight when they were at a similar stage of development. Results using the custom NLSY sample weights indicate similar patterns in the conditional distributions of weight outcomes across generations with slightly lower rates of weight problems when the oversampling of black and Hispanic mothers is accounted for (Table A2).
Table 6.
Distributions of Intergenerational Weight Status Transitions
| Full Sample | Child’s Weight Status | ||||||
|---|---|---|---|---|---|---|---|
| BMI | Category | <18.5 Underweight | 18.5–25 Recommended | 25–30 Overweight | >30 Obese | Mother’s Distribution | |
| Mother’s Weight Status | <18.5 | Underweight | 11.6% | 71.9% | 12.6% | 3.9% | 6.0% |
| 18.5–25 | Recommended | 4.4% | 64.2% | 22.1% | 9.4% | 69.6% | |
| 25–30 | Overweight | 1.9% | 42.9% | 33.3% | 21.9% | 18.7% | |
| >30 | Obese | 2.2% | 28.4% | 29.9% | 39.5% | 5.7% | |
| Child’s Distribution | 4.2% | 58.6% | 24.1% | 13.1% | N=4,748 | ||
| Daughters | Daughter’s Weight Status | ||||||
| BMI | Category | <18.5 Underweight | 18.5–25 Recommended | 25–30 Overweight | >30 Obese | Mother’s Distribution | |
| Mother’s Weight Status | <18.5 | Underweight | 17.1% | 67.9% | 11.4% | 3.6% | 6.0% |
| 18.5–25 | Recommended | 6.4% | 63.8% | 19.5% | 10.3% | 68.4% | |
| 25–30 | Overweight | 2.2% | 41.5% | 31.4% | 25.0% | 19.4% | |
| >30 | Obese | 2.8% | 28.3% | 25.5% | 43.5% | 6.2% | |
| Daughter’s Distribution | 6.0% | 57.5% | 21.7% | 14.8% | N=2,348 | ||
| Sons | Son’s Weight Status | ||||||
| BMI | Category | <18.5 Underweight | 18.5–25 Recommended | 25–30 Overweight | >30 Obese | Mother’s Distribution | |
| Mother’s Weight Status | <18.5 | Underweight | 6.2% | 75.9% | 13.8% | 4.1% | 6.0% |
| 18.5–25 | Recommended | 2.4% | 64.6% | 24.5% | 8.5% | 70.7% | |
| 25–30 | Overweight | 1.6% | 44.4% | 35.4% | 18.5% | 18.0% | |
| >30 | Obese | 1.6% | 28.6% | 34.9% | 34.9% | 5.3% | |
| Son’s Distribution | 2.5% | 59.8% | 26.3% | 11.5% | N=2,400 | ||
Note: Weight Status for both generations are determined based on average BMI levels between ages 16 and 24. Data are from NLSY79 and Young Adult offspring of women in NLSY79.
Estimates of probit models of offspring obesity (in which the dependent variable equals 1 if the BMI of the offspring is greater than 30) and obese or overweight (where the dependent variable equals one if the offspring’s BMI is greater than 25) show large marginal effects of maternal weight status on the weight problems of offspring (Tables 7 and 8). Note that the dependent variable indicating an overweight or obese offspring in Table 8 includes all offspring with BMI above 25, while the mother’s overweight variable used as a regressor includes only those with BMI values between 25 and 30. The calculated marginal effects measure changes in the probability of being obese or overweight for children whose mothers had BMI levels outside of the recommended range. Results are provided for the entire sample, separately by gender and by ethnic categories for each gender given the oversample of certain demographic groups in this sample.
Table 7. Marginal Effects of Maternal Weight Status for Probit Estimates of Offspring’s Obesity (BMI > 30).
Change in likelihood of offspring’s obesity relative to mother in recommended weight range (18.5 < BMI < 25)
| Full Sample | All Females | All Males | |
|---|---|---|---|
| Maternal Weight Status, age 16–24 | |||
| Obese | 31.7% | 34.9% | 27.7% |
| BMI>30 | (12.31)** | (9.16)** | (7.79)** |
| Overweight | 12.9% | 15.2% | 10.3% |
| 25<BMI<30 | (8.61)** | (6.86)** | (5.54)** |
| Underweight | −7.0% | −8.7% | −5.4% |
| BMI<18.5 | (3.28)** | (2.71)** | (1.90) |
| Observations | 4,748 | 2,348 | 2,400 |
| Percent Obese (Fraction of Dependent Variable = 1) | 13.1% | 14.8% | 11.5% |
| Females | |||
| White | Black | Hispanic | |
| Maternal Weight Status, age 16–24 | |||
| Obese | 16.9% | 37.8% | 32.4% |
| BMI>30 | (2.78)** | (6.91)** | (3.76)** |
| Overweight | 21.7% | 12.5% | 7.2% |
| 25<BMI<30 | (6.08)** | (3.29)** | (1.85) |
| Underweight | −3.0% | −17.1% | −6.2% |
| BMI<18.5 | (0.86) | (2.13)* | (1.00) |
| Observations | 923 | 909 | 516 |
| Percent Obese (Fraction of Dependent Variable = 1) | 9.8% | 21.8% | 11.4% |
| Males | |||
| White | Black | Hispanic | |
| Maternal Weight Status, age 16–24 | |||
| Obese | 26.9% | 28.6% | 30.3% |
| BMI>30 | (3.87)** | (5.51)** | (4.33)** |
| Overweight | 10.2% | 13.2% | 6.8% |
| 25<BMI<30 | (3.33)** | (4.34)** | (1.85) |
| Underweight | −7.6% | 0.6% | −7.2% |
| BMI<18.5 | (2.01)* | (0.11) | (0.97) |
| Observations | 952 | 860 | 588 |
| Percent Obese (Fraction of Dependent Variable = 1) | 10.9% | 10.9% | 13.1% |
Robust z-statistics corrected for multiple children from same mother in parentheses.
significant at 5%;
significant at 1%
Table 8. Marginal Effects of Maternal Weight Status for Probit Estimates of Likelihood of Obese/Overweight Child (BMI > 25).
Change in likelihood of overweight or obese offspring relative to mother in recommended weight range (18.5 < BMI < 25)
| Full Sample | All Females | All Males | |
|---|---|---|---|
| Maternal Weight Status, age 16–24 | |||
| Obese | 37.7% | 38.9% | 36.6% |
| BMI>30 | (11.56)** | (8.80)** | (7.25)** |
| Overweight | 23.7% | 26.6% | 20.8% |
| 25<BMI<30 | (11.60)** | (9.04)** | (7.47)** |
| Underweight | −16.8% | −17.0% | −16.5% |
| BMI<18.5 | (4.92)** | (3.53)** | (3.45)** |
| Observations | 4,748 | 2,348 | 2,400 |
| Percent Obese or Overweight (BMI > 25) (Fraction of Dependent Variable = 1) | 37.2% | 36.6% | 37.8% |
| Females | |||
| White | Black | Hispanic | |
| Maternal Weight Status, age 16–24 | |||
| Obese | 33.9% | 35.6% | 32.5% |
| BMI>30 | (3.96)** | (6.23)** | (3.19)** |
| Overweight | 28.3% | 24.0% | 19.3% |
| 25<BMI<30 | (5.69)** | (5.35)** | (3.26)** |
| Underweight | −9.2% | −16.1% | −27.6% |
| BMI<18.5 | (1.42) | (1.96)* | (2.73)** |
| Observations | 923 | 909 | 516 |
| Percent Obese or Overweight (BMI > 25) (Fraction of Dependent Variable = 1) | 26.0% | 47.7% | 35.9% |
| Males | |||
| White | Black | Hispanic | |
| Maternal Weight Status, age 16–24 | |||
| Obese | 28.5% | 38.0% | 38.9% |
| BMI>30 | (2.92)** | (5.62)** | (3.23)** |
| Overweight | 24.3% | 19.1% | 17.4% |
| 25<BMI<30 | (4.96)** | (4.20)** | (3.47)** |
| Underweight | −13.1% | −14.3% | −26.5% |
| BMI<18.5 | (2.03)* | (1.64) | (2.30)* |
| Observations | 952 | 860 | 588 |
| Percent Obese or Overweight (BMI > 25) (Fraction of Dependent Variable = 1) | 33.4% | 40.0% | 41.8% |
Robust z-statistics corrected for multiple children from same mother in parentheses.
significant at 5%;
significant at 1%
Not surprisingly, having a mother who was obese between ages 16 and 24 significantly increases the likelihood that her child will also be overweight or obese at a similar stage of life in all specifications (Tables 7 and 8). The magnitude of the increase in the likelihood of obesity for a child born to a mother who was obese in early adulthood (relative to one with a recommended BMI level) ranges from 17% for white females to 38% for black females (Table 7). The estimated influence of having an obese mother is similar across the three demographic categories for sons. Having a mother who was overweight (with a BMI between 25 and 30) significantly increases the likelihood of becoming obese for both white and black females and males, but does not have a statistically significant effect for the likelihood of obesity among Hispanics.
Expanding the classification of weight problems to include both obese and overweight (BMI greater than 25) offspring, the estimated probit results indicate that children of obese mothers are 38% more likely to be overweight or obese (Table 8). Conversely, mothers with BMI levels below 18.5 (classified as underweight) are significantly less likely to have obese or overweight children than women with BMIs in the recommended range between 18.5 and 25. Being born to an obese or overweight mother significantly increases the likelihood of becoming obese or overweight by early adulthood for all six demographic groups considered, with the magnitude of the effects similar across all three racial and ethnic categories for each gender. The measured reductions in the likelihood of developing weight problems among the offspring of a mother who was underweight are large and statistically significant among Hispanic males and females.
VI. Conclusions and Directions for Further Research
This paper provides several measures of the intergenerational persistence of weight problems that have been argued to causally influence economic success. If elevated parental BMI levels reduce familial resources (for instance, via a reduction in wages for obese workers or an increased likelihood of disability), then economic success for the subsequent generation may be limited by an increased likelihood of weight problems due both to genetic predisposition as well as to resource constraints that bias caloric consumption towards less healthy foods. This process of intergenerational health capital transmission may then serve to explain a portion of the relatively low levels of economic mobility found in studies over the last two decades (beginning with Solon (1992) and Zimmerman (1992)).
The estimated degree of correlation in BMI across generations in the NLSY data used in this study is roughly 0.35 and in line with previous epidemiological studies of the heritability of obesity. A significantly higher correlation of BMI between mothers and their daughters (0.38) than with their sons (0.32) was found. While there exists the possibility that bias in the self-reports of weight in the NLSY data leads to an overstated correlation, the intergenerational dependence is large across the entire range of the BMI distribution. The transmission of obesity appears to be most persistent at the highest levels of obesity. Quantile regressions indicate that toward the upper end of the distribution of BMI, the elasticity of offspring BMI with respect to mother’s BMI is above 0.5. Measures of quadrant dependence provide additional evidence that the strength of the relationship in BMI across generations is increasing at higher points in the distribution of BMI.
The data employed in these measurements also reflect the dramatic increase in obesity in the United States population during the period studied. Roughly 25% of the mothers in the NLSY79 were overweight or obese between ages 16 to 24, while 37% of their offspring were classified as overweight or obese by the same age range and rates of obesity have more than doubled between these two generations. Additionally, 70% of the offspring born to women who were obese were themselves at least overweight by early adulthood. Children born to women who were obese in early adulthood are more than 30% more likely to themselves be obese, relative to children born to women with a recommended BMI level.
The research presented in this paper provides estimates of the dependence of weight outcomes across two generations when both generations are at similar stages of the lifecycle. Given previous estimates of a significant causal effect of obesity on income and wages for females as well as correlations between obesity and accumulation of wealth and education, the substantial intergenerational persistence in BMI, especially at its highest levels, may provide a pathway to explain some portion of reduced levels of economic mobility. Future studies using the NLSY and other longitudinal data will be able to measure intergenerational relationships in economic mobility measured by income, wealth and education controlling for the strong persistence in weight outcomes found in this paper.
Footnotes
Bowles & Gintis (2002) review the broad topic of intergenerational transmission of economic status and Grawe and Mulligan (2002) provide a theory of economic interpretations of such correlations.
See Currie (2009) for a review of the pathways through which health may affect intergenerational mobility.
BMI is calculated as (weight in kilograms)/(height in meters2). Adults with a BMI of 30 and above are typically classified as obese, while those with BMI levels greater than or equal to 25 are classified as overweight.
See Galton’s 1889 Natural Inheritance for the genesis of this literature.
One pathway may be via rates of time preference that affect obesity (as in Komlos, Smith and Bogin (2007)) when such preferences are transmitted across generations.
For a review of genetic studies of obesity, see Maes et. al. (1997) and Bouchard et. al. (2003).
Odgen (2008) demonstrates significant differences in rates of adolescent obesity by ethnic groups. Baum and Ruhm (2008) provide evidence of disparities in levels and growth rates of obesity across SES levels.
See, for example, Cutler et. al. (2003); Chou et. al. (2004); Lakdawalla and Philipson (2009) and Komlos et. al. (2009).
Burkhauser, Cawley and Schmeiser (2009) provide evidence that growth in rates of obesity are sensitive to the measure of obesity used. Measures of skinfold thickness indicate that substantial growth in rates of obesity in United States occurred 10 to 20 years earlier than indicated by more commonly used BMI measures.
See Averett and Korenman (1999) for a comparison of the influence of obesity on economic outcomes for blacks and whites. Case and Menedez (2009) consider explanations of higher rates of obesity among females in low-income countries.
Cawley (2004) finds small effects on estimated coefficients of interest when correcting for self-reporting bias in the NLSY data, but Cawley and Burkhauser (2008) find significant underreporting of weight among those with elevated BMI levels in the NHANES data. Average BMI levels calculated from self-reported height and weight are 0.34 to 0.98 points lower than BMI values calculated from measured height and weight for demographic groups other than African American and Mexican American males where no significant differences are found.
This would result in the measured correlation overstating the true correlation (since an obese son of a woman with a recommended BMI level would be more likely to understate his weight while the mother is less likely to have substantial measurement error in a self-report if she is of recommended weight).
Problems arising from measurement of income at different points in the lifecycle for measuring intergenerational correlations are discussed in Haider and Solon (2006).
The average number of observations of BMI per young adult in the NLSY is 2.5. Approximately one-quarter of the YA sample includes only a single observation of BMI between ages 16 and 24, while more than 20 percent have four or more observations of BMI in this age range.
BMI values for women who were pregnant at the time of the survey are set to missing, but the women may still be included in the sample if they were ever interviewed between ages 16 and 24 while not pregnant.
The 2000 CDC Growth Charts for BMI have 85th percentile cutoffs of 24.2 and 24.7 and 95th percentile cutoffs of 27.6 and 28.9 for the youngest 16 year old males and females, respectively. These percentiles are generally used to classify adolescents as “at risk of overweight” and overweight, but often referred to as overweight (above 85th percentile) and obese (above 95th percentile). The 85th and 95th percentile cutoffs exceed 25 and 30 respectively for females by the end of their 17th year and for males early in their 19th year.
See Cawley and Burkhauser (2008) for a discussion of the limitations of BMI as a measure of actual body fatness.
The correlation is lower than the elasticity in this case since the standard deviation of the maternal BMI is less than that for their children’s BMI.
The oversampling of relatively younger mothers is evident with the overrepresentation of families in the lowest income quartile among the NLSY79 sample.
The NLSY provides indicator variables for whether the household income for each family is below the Federal Poverty Income Guideline, controlling for household size, for each survey year.
An example of quantile regression is a median regression which takes the median of the distribution of the dependent variable as its single quantile of interest.
Stifel and Averett (2009) use quantile regressions to study the influence of a variety of correlates on childhood obesity across the distribution of BMI.
Note that quantiles in this procedure are determined by the empirical distribution of BMI observed in the NLSY data, rather than from an external reference such as the CDC BMI growth charts.
As indicated in Table 3, the 75th percentile of child’s BMI in the YA NLSY surveys is above the threshold for overweight (BMI greater than 25), while the 90th percentile is above the cutoff for obesity classification (BMI greater than 30).
Each entry in the association array for daughters is larger than the corresponding entry in the association array for sons.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Bibliography
- Ahlburg D. Intergenerational Transmission of Health. The American Economic Review Papers and Proceedings. 1998;88(2):265–270. [Google Scholar]
- Anderson P, Butcher K, Schanzenbach D. Childhood Disadvantage and Obesity: Is Nature Trumping Nurture? NBER Working Paper No. 13479 2007 [Google Scholar]
- Atella V, Pace N, Vuri D. Are employers discriminating with respect to weight?: European Evidence using Quantile Regression. Economics & Human Biology. 2008;6(3):305–329. doi: 10.1016/j.ehb.2008.06.007. [DOI] [PubMed] [Google Scholar]
- Averett S, Korenman S. Black-white differences in social and economic consequences of obesity. International Journal of Obesity. 1999;23:166–173. doi: 10.1038/sj.ijo.0800805. [DOI] [PubMed] [Google Scholar]
- Baum CL, Ford WF. The Wage Effects of Obesity: A Longitudinal Study. Health Economics. 2004;13(9):885–899. doi: 10.1002/hec.881. [DOI] [PubMed] [Google Scholar]
- Baum C, Ruhm CJ. Age, Socioeconomic Status and Obesity Growth. Journal of Health Economics. 2009;28(3):635–648. doi: 10.1016/j.jhealeco.2009.01.004. [DOI] [PubMed] [Google Scholar]
- Bowles S, Gintis H. The Inheritance of Inequality. Journal of Economic Perspectives. 2002;16(3):3–30. [Google Scholar]
- Bouchard C, Perusse L, Rice T, Rao DC. Genetics of Human Obesity. In: Bray GA, Bouchard C, editors. Handbook of Obesity. 2. Marcel Dekker; New York: 2003. pp. 157–200. [Google Scholar]
- Burkhauser RV, Cawley J, Schmeiser MD. The Timing of the Rise in U.S. Obesity Varies With Measure of Fatness. Economics & Human Biology. 2009;7(3):307–318. doi: 10.1016/j.ehb.2009.07.006. [DOI] [PubMed] [Google Scholar]
- Brunello G, D’Hombres B. Does body weight affect wages? Evidence from Europe. Economics and Human Biology. 2007;5(1):1–19. doi: 10.1016/j.ehb.2006.11.002. [DOI] [PubMed] [Google Scholar]
- Case A, Menedez A. Sex differences in obesity rates in poor countries: Evidence from South Africa. Economics and Human Biology. 2009;7(3):271–282. doi: 10.1016/j.ehb.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cawley J. The Impact of Obesity on Wages. Journal of Human Resources. 2004;39(2):451–474. [Google Scholar]
- Cawley J, Burkhauser R. Beyond BMI: The Value of More Accurate Measures of Fatness and Obesity in Social Science Research. Journal of Health Economics. 2008;27(2):519–529. doi: 10.1016/j.jhealeco.2007.05.005. [DOI] [PubMed] [Google Scholar]
- Cawley J, Spiess K. Obesity and skill attainment in early childhood. Economics & Human Biology. 2008;6(3):388–397. doi: 10.1016/j.ehb.2008.06.003. [DOI] [PubMed] [Google Scholar]
- Chou SY, Grossman M, Saffer H. An economic analysis of adult obesity: results from the Behavioral Risk Factor Surveillance. Journal of Health Economics. 2004;23:565–587. doi: 10.1016/j.jhealeco.2003.10.003. [DOI] [PubMed] [Google Scholar]
- Classen T, Hokayem C. Childhood Influences on Youth Obesity. Economics and Human Biology. 2005;3(2):165–187. doi: 10.1016/j.ehb.2005.05.008. [DOI] [PubMed] [Google Scholar]
- Conley D, Glauber R. Gender, Body Mass and Economic Status. National Bureau of Economic Working Paper No. 11343 2005 [Google Scholar]
- Crosnoe R. Gender, Obesity, and Education. Sociology of Education. 2007;80(3):241–260. [Google Scholar]
- Currie J. Healthy, Wealthy, and Wise: Socioeconomic Status, Poor Health in Childhood, and Human Capital Development. Journal of Economic Literature. 2009;47(1):87–122. [Google Scholar]
- Cutler D, Glaeser E, Shapiro J. Why Have Americans Become More Obese? Journal of Economic Perspectives. 2003;17:93–118. [Google Scholar]
- Garn SM, Sullivan TV, Hawthorne VM. Fatness and Obesity of the Parents of Obese Individuals. American Journal of Clinical Nutrition. 1989;50(4):740–745. doi: 10.1093/ajcn/50.6.1308. [DOI] [PubMed] [Google Scholar]
- Goldberger AS. Economic and Mechanical Models of Intergenerational Transmission. The American Economic Review. 1989;79(3):504–513. [Google Scholar]
- Grawe N, Mulligan C. Economic Interpretations of Intergenerational Correlations. Journal of Economic Perspectives. 2002;16(3):45–58. [Google Scholar]
- Greve J. Obesity and labor market outcomes in Denmark. Economics & Human Biology. 2008;6(3):350–362. doi: 10.1016/j.ehb.2008.09.001. [DOI] [PubMed] [Google Scholar]
- Haider S, Solon G. Lifecycle Variation in the Association between Current and Lifetime Earnings. American Economic Review. 2006;96(4):1308–20. [Google Scholar]
- Han E, Norton EC, Stearns SC. Weight and Wages: Fat Versus Lean Paychecks. Health Economics. 2009;18(5):535–548. doi: 10.1002/hec.1386. [DOI] [PubMed] [Google Scholar]
- Johansson E, Böckerman P, Kiiskinen U, Heliövaara M. Obesity and labour market success in Finland: The difference between having a high BMI and being fat. Economics & Human Biology. 2009;7(1):36–45. doi: 10.1016/j.ehb.2009.01.008. [DOI] [PubMed] [Google Scholar]
- Karlin S. Association arrays in assessing forms dependencies between bivariate random variables. Proceedings of the National Academy of Sciences USA. 1983;80(2):647–651. doi: 10.1073/pnas.80.2.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenker R, Hollack KF. Quantile Regression. Journal of Economic Perspectives. 2001;15(4):143–156. [Google Scholar]
- Komlos J, Smith PK, Bogin B. Obesity and the Rate of Time preference: Is there a Connection? Journal of Biosocial Science. 2004;36(2):209–219. doi: 10.1017/s0021932003006205. [DOI] [PubMed] [Google Scholar]
- Komlos J, Breitfelder A, Sunder M. The transition to post-industrial BMI values among US children. American Journal of Human Biology. 2009;21(2):151–160. doi: 10.1002/ajhb.20806. [DOI] [PubMed] [Google Scholar]
- Lakdawalla D, Philipson T. The Growth of Obesity and Technological Change. Economics and Human Biology. 2009;7(3):283–293. doi: 10.1016/j.ehb.2009.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann E. Some Concepts of Dependence. Annals of Mathematical Statistics. 1966;37:1137–1153. [Google Scholar]
- Maes HHM, Neale MC, Eaves LJ. Genetic and Environmental Factors in Relative Body Weight and Human Adiposity. Behavior Genetics. 1997;27(4):325–351. doi: 10.1023/a:1025635913927. [DOI] [PubMed] [Google Scholar]
- Martin M. The Intergenerational Correlation in Weight: How Genetic Resemblance Reveals the Social Role of Families. American Journal of Sociology. 2008;114(Supplement):S67–S105. doi: 10.1086/592203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris S. Body Mass Index and Occupational Attainment. Journal of Health Economics. 2006;25(2):347–364. doi: 10.1016/j.jhealeco.2005.09.005. [DOI] [PubMed] [Google Scholar]
- Murasko JE. Socioeconomic status, height, and obesity in children. Economics & Human Biology. 2009;7(3):376–386. doi: 10.1016/j.ehb.2009.04.004. [DOI] [PubMed] [Google Scholar]
- Odgen CL, Carroll MD, Flegal KM. High Body Mass Index for Age Among US Children and Adolescents, 2003–2006. New England Journal of Medicine. 2008;299(20):2401–2405. doi: 10.1001/jama.299.20.2401. [DOI] [PubMed] [Google Scholar]
- Paraponaris A, Bérengère S, Ventelou B. Obesity, weight status and employability: Empirical evidence from a French national survey. Economics & Human Biology. 2005;3(2):241–258. doi: 10.1016/j.ehb.2005.06.001. [DOI] [PubMed] [Google Scholar]
- Solon G. Intergenerational Income Mobility in the United States. The American Economic Review. 1992;82(3):393–408. [Google Scholar]
- Stifel DC, Averett SL. Childhood overweight in the United States: A quantile regression approach. Economics and Human Biology. 2009;7(3):387–397. doi: 10.1016/j.ehb.2009.05.005. [DOI] [PubMed] [Google Scholar]
- Whitaker R, Wright J, Pepe M, Seidel K, Dietz W. Predicting Obesity in Young Adulthood from Childhood and Parental Obesity. The New England Journal of Medicine. 1997;337(13):869–873. doi: 10.1056/NEJM199709253371301. [DOI] [PubMed] [Google Scholar]
- Zagorsky J. Health and wealth: The late-20th century obesity epidemic in the U.S. Economics and Human Biology. 2005;3(2):296–313. doi: 10.1016/j.ehb.2005.05.003. [DOI] [PubMed] [Google Scholar]
- Zimmerman G. Regression Toward Mediocrity in Economic Stature. The American Economic Review. 1992;82(3):409–429. [Google Scholar]