Abstract
Background
Cardiac resynchronization therapy (CRT) has been shown to reduce functional mitral regurgitation (MR). It has been proposed that the mechanism of MR reduction relates to geometric change or, alternatively, changes in left ventricular (LV) contractile function. Normal mitral valve (MV) function relies on a balance between tethering and closing forces on the MV leaflets. Functional MR results from a derangement of this force–balance relationship, and CRT may be an important modulator of MV function by its ability to enhance the force–balance relationship on the MV. We hypothesized that CRT improves the comprehensive force balance acting on the valve, including favorable changes in both geometry and LV contractile function.
Methods and Results
We examined the effect of CRT on 34 patients with functional MR before and after CRT (209±81 days). MR regurgitant volume, closing forces on MV (derived from Doppler transmitral pressure gradients), including dP/dt and a factor (closing pressure ratio) expressing how long the peak closing gradient is maintained over systole (closing pressure ratio=velocity time integral/MR peak velocity×mitral regurgitation time), and dyssynchrony by tissue Doppler were measured. End-diastolic volume, end-systolic volume, mitral valve annular area (MAA) and contraction (percent change in MAA from end-diastole to midsystole), leaflet closing area (leaflet area during valve closure), and tenting volume (volume under leaflets to annular plane) were measured by 3D echocardiography. After CRT, end-diastolic volume (253±111 versus 221±110 mL, P<0.001) and end-systolic volume (206±97 versus 167±91 mL, P<0.001) decreased and ejection fraction (19±6 versus 27±9%, P<0.001) increased. MR regurgitant volume decreased from 35±17 to 23±14 mL (P<0.001), MAA from 11.6±3.5 to 10.5±3.1 cm2 (P<0.001), leaflet closing area from 15.4±5 to 13.7±3.8 cm2 (P<0.001), and tenting volume from 5.7±2.6 to 4.6±2.2 mL (P<0.001). Peak velocity (and therefore transmitral closing pressure) was more sustained throughout systole, as reflected by the increase in the closing pressure ratio (0.77±0.1 versus 0.84±0.1 before CRT versus after CRT, P=0.01); dP/dt also improved after CRT. There was no change in dyssynchrony or MAA contraction.
Conclusions
Reduction in MR after CRT is associated with favorable changes in MV geometry and closing forces on the MV. It does so by favorably affecting the force balance acting on the MV in 2 ways: reducing tethering through reversal of LV remodeling and increasing the systolic duration of peak transmitral closing pressures.
Keywords: functional mitral regurgitation, 3D echocardiography, cardiac resynchronization therapy
Heart failure is a cause of significant morbidity and mortality, and its prevalence is increasing as the population ages.1 Cardiac resynchronization therapy (CRT) results in improvement in symptoms and survival in patients with advanced heart failure and widened QRS.2–4 Functional mitral regurgitation (FMR) occurs in approximately 50% of patients with left ventricular (LV) dysfunction and also negatively affects survival.5,6 CRT is associated with a reduction in MR, and this decrease in MR probably influences the favorable effects of CRT.7,8 Although reduction in MR has been described, its precise mechanism has been only incompletely defined. Normal mitral valve (MV) function results from a balance of both tethering and closing forces on the mitral valve. Tethering forces are transmitted via the chordae and keep the valve from prolapsing, whereas closing forces depend on the pressure generated by the ventricle to close the mitral valve. FMR results from a derangement in this force–balance relationship of tethering and closing forces.9,10 There are 2 potential mechanisms by which CRT may reduce MR. One relates to improved LV contraction with an increase in LV pressure generation after CRT, resulting in increased closing forces on the MV and improved leaflet coaptation. A second mechanism relates to reverse remodeling effects of CRT on the LV and MV geometry, resulting in improved spatial relationships of the MV to the ventricle and reduction in leaflet tethering, an important determinant of FMR.
Because CRT has the potential to modulate both contractile function and LV remodeling, elucidating the effects of CRT on MV geometry and function will provide important insights toward optimizing the therapeutic benefits of CRT and improve echocardiographic assessment of the efficacy of CRT. We hypothesized that CRT results in favorable effects on the force–balance relationship of MV function, improving both tethering and closing forces on the MV by its dual effect on LV contraction and remodeling. To look at these effects on the force–balance relationship, we used the strength of 3D echocardiography to quantify MV geometry and analyzed transmitral flow velocities to provide insights into the dynamics of MV closing forces, specifically including an integrated measure of LV force generation based on transmitral Doppler velocities.
Methods
Subjects
Consecutive patients with congestive heart failure with FMR (mild or greater) who received CRT for standard indications (New York Heart Association [NYHA] class ≥3 despite optimized medical therapy, ejection fraction [EF] ≤35%, and QRS duration ≥120 ms) were studied. Patients with acute heart failure decompensation (<3 months), echocardiographic evidence for organic mitral valve pathology, or aortic valve disease were excluded. Two- and three-dimensional baseline transthoracic echocardiography was performed before implant or within 24 hours after implant with biventricular pacing switched off. Follow-up studies were performed approximately 6 months after implant. Clinical status was assessed at baseline and at 6-month follow-up. The study was approved by an institutional review committee, and all patients gave informed consent.
Clinical Evaluation
Evaluation of clinical status included assessment of NYHA functional class, quality-of-life score (Minnesota Living With Heart Failure Questionnaire), and 6-minute walk test.
Echocardiography Protocol
Transthoracic echocardiography was performed at baseline (before CRT) and at 6 months (after CRT). All patients underwent 2D and 3D and color Doppler echocardiographic examination at rest in the lateral position. Tissue Doppler imaging (TDI) was used to assess intraventricular dyssynchrony. Three-dimensional echocardiography was used for the assessment of LV volumes, EF, and mitral valve geometry.
Imaging Acquisition
Full-volume 3D data sets were obtained in the apical 4-chamber view with either a Sonos 7500 or IE33 using a ×3 transducer (Philips Medical Systems, Andover, Mass) with ECG gating and suspended respiration. Three-dimensional datasets were transferred to a computer for offline analysis with customized software.
Data sets for TDI were obtained by 2D Doppler echocardiography using a General Electric Vivid 7 cardiac ultrasound machine (General Electric, Milwaukee, Wis). Images were obtained with a 2- to 5-MHz transducer. Color tissue Doppler imaging from 3 consecutive cardiac cycles was obtained from apical views, and frame rate was optimized (>140 frames/s).
Data Analysis
The quantification of MR was performed using the proximal isovelocity surface area method.11,12 Instantaneous peak velocity and dP/dt, defined as the slope traced between 1 and 3 m/s on the MR jet recorded at a sweep speed of 100 mm/s, were measured.13
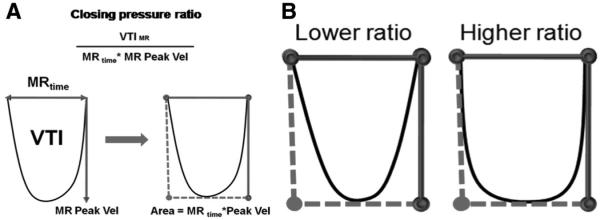
In addition to standard segmental measures of dyssynchrony (see below), LV and transmitral force generation as influenced by CRT was measured. These include dP/dt and the closing pressure ratio, which is an index measure that integrates LV force generation during systole based on transmitral Doppler velocities. This ratio is based on physiological principles of Doppler echocardiography examining and integrating the time course of closing pressure forces on mitral function as a measure of LV coordination14–16; it is calculated as velocity time integral divided by the MR time period and multiplied by MR peak velocity (velocity time integral/MR time×MR peak velocity) and is a measure of how sustained the maximum closing forces are on the MV during the MR time. The closing pressure ratio quantifies the impact of closing forces on the MV throughout systole and reflects an integrated measure of LV force generation. Because it is an integrative measure, it better reflects the overall closing forces on the MV rather than the instantaneous peak transmitral pressure. A higher ratio represents a transmitral gradient that rises faster and remains high for a longer period during systole (Figure 1).
Figure 1.
The ratio of velocity time integral (VTI) divided by the MR time period (MR time) and multiplied by MR peak velocity reflects the shape of the closing forces on the MV and is a measure of how sustained the maximum closing forces are on the MV during the MR time. A higher ratio represents a transmitral gradient that rises faster and remains high for a longer period during systole.
To validate the closing pressure ratio, we measured the ratio in an experimental model of ischemic mitral regurgitation17 with and without pacing. Pacing introduces ventricular dyssynchrony, and the closing pressure ratio should decrease with pacing. In this model, Dorsett sheep were placed under general anesthesia (12.5 mg/kg sodium thiopental IV) and ventilated at 15 mL/kg with a mixture of 2% isoflurane and oxygen. The hearts were exposed through a left thoracotomy and an epicardial pacing lead placed on the right ventricle to produce dyssynchrony. Epicardial scanning was performed to image the MV. The MR Doppler profile was obtained using continuous-wave Doppler with and without pacing. This protocol was reviewed and approved by our institutional animal care committee.
LV Volumes and MV Geometry
End-diastolic (EDV) and end-systolic (ESV) LV volumes were calculated by tracing the LV endocardial border using 6 imaging planes and applying a surface fitting algorithm to calculate volume.18 Mitral annular area (MAA) was measured at midsystole by tracing annular points. Mitral annular contraction was defined as percentage change in MAA from end-diastole (largest area) to midsystole (smallest area).19 The leaflet closing area is the surface area of the leaflets (midsystole using 6 planes, fitted to a surface fitting algorithm).20 Leaflet closing area increases in patients with LV dysfunction and indicates significant tethering. The tenting volume of the leaflets, which reflects the common end point of the force–balance relationship, incorporating both tethering and closing forces, was measured as the volume under the leaflets to the plane of mitral annulus at midsystole.
Dyssynchrony
Dyssynchrony was assessed by offline analysis of color tissue Doppler imaging. Indices of mechanical dyssynchrony calculated included (1) maximal time delay (MTD) defined as the difference between the maximal and minimal times to peak longitudinal systolic velocity among 12 segments, (2) standard deviation of the time to peak longitudinal systolic velocity for all 12 segments evaluated as Ts-SD, and (3) septal to lateral wall delay (SLD) defined as difference between time to peak systolic velocity of the basal inferoseptal and lateral walls. Left ventricular dyssynchrony was present when MTD ≥100 ms21,22 or Ts-SD ≥33 ms,23 or SLD ≥65 ms.24,25 Interpapillary muscle dyssynchrony was defined as the difference in time to peak longitudinal systolic velocity between the myocardial segments underlying each of the papillary muscles.26
Subgroup Analyses: MR Reduction and MR No-Reduction Subgroups and Nonresponder and Responder Subgroups
The patient population was subdivided into patients in whom MR RV was reduced ≥20% from baseline after CRT (MR reduction group) and patients whose MR RV was not reduced (increase in MR RV or a <20% reduction in MR RV: MR no-reduction group). In addition, patients were divided into echocardiographic responder (ESV reduced >15%) and nonresponder (ESV unchanged or increased) to CRT.21
Statistical Analysis
Continuous measurements are presented as mean±SD. Comparison between before and after CRT and were performed using a 2-tailed Student t test for paired continuous data. For paired ordinal variables, the Wilcoxon signed-rank test was performed. To assess the determinants of MR improvement after CRT, a multivariate stepwise linear regression analysis was performed with percent change in MR RV as the dependent variable. Pre-CRT variables included in the multivariate analysis were ESV, MAA, leaflet area, tenting volume, and closing pressure ratio. These variables were selected as the most likely mechanistic and functional variables to affect MR. For comparisons of subgroups (MR reduction versus MR no-reduction subgroups and responder and nonresponder groups), unpaired t tests were used to compare between baseline and after CRT.
Intraobserver and interobserver variabilities were assessed by intraclass correlation coefficients.27 All the statistical analyses were performed using SPSS 16.0 (SPSS, Inc, Chicago, Ill).
Results
Forty-seven patients with at least mild mitral regurgitation who underwent CRT were reviewed for inclusion. Twelve patients were excluded either for intrinsic mitral valve abnormalities (5 patients) or for suboptimal image quality for 3D analysis (7 patients). One patient died before the 6-month follow-up period. Thus, a total of 34 patients with a mean age of 66±12 years and 209±81 days of follow-up were included in the study. Baseline characteristic of these patients are summarized in Table 1. There were more men than women, although there were no differences in the underlying etiology (ischemic versus nonischemic) of cardiomyopathy.
Table 1. Baseline Characteristics of the Study Population.
| Age, y | 66±12 |
| Sex, n (%) | |
| Male | 23 (68) |
| Female | 11 (32) |
| Origin, n (%) | |
| Ischemic | 16 (47) |
| Nonischemic | 18 (53) |
| NYHA functional class | |
| III | 30 (88) |
| IV | 4 (12) |
| Quality-of-life score | 54±17 |
| 6-Minute walk test, m | 284±143 |
| Heart rate (BPM) | 70±11 |
| Mean blood pressure (mm Hg) | 85±12 |
BPM indicates beats per minute.
After CRT, patients had a clinical improvement in terms of NYHA functional class (P<0.0001), 6-minute walk test (284±143 to 408±100 meters; before versus after CRT, P<0.001), and quality-of-life score (54±17 to 21±15; P<0.001). There were no significant changes in heart rate (70±11 to 69±10 bpm; P=0.8) or mean blood pressure (85±12 to 86±12 mm Hg; P=0.9) between baseline and 6-month follow-up.
Table 2 shows the changes in echocardiographic measures before and after CRT. There was a significant reduction in MR regurgitant volume after CRT. LV volumes decreased and EF increased. There were favorable changes in MV geometry after CRT, with a decrease in MAA, leaflet closing area, and tenting volume (Figure 2). The closing pressure ratio increased after CRT, consistent with more sustained closing forces on MV during systole (Figure 3). In addition, in an experimental validation model, the closing pressure ratio was measured with and without pacing (pacing would introduce a more dyssynchronous contraction and hence decrease the ratio). In the paced heart, there was a significant decrease in closing pressure ratio compared with nonpaced hearts (0.85±0.03 to 0.72±0.03, nonpaced versus paced, P<0.001) (Figure 4), without significant change in heart rate. Mitral annular contraction and dyssynchrony by TDI did not change significantly despite improvement in LV function. After adjusting for closing pressure ratio (R2=0.17; B=−0.42; P=0.03), no other variable was significantly associated with percent decrease in MR.
Table 2. Echocardiographic Parameters at Baseline (Pre-CRT) and 6-Month Follow-Up (Post-CRT).
| Pre-CRT | Post-CRT | P | |
|---|---|---|---|
| MR (RV-ml) | 35±17 | 23±14 | <0.001 |
| EDV, mL | 253±111 | 221±100 | <0.001 |
| ESV, mL | 206±97 | 167±91 | <0.001 |
| EF, % | 19±6 | 27±9 | <0.001 |
| MAA, cm2 | 11.6±3.5 | 10.5±3.1 | <0.001 |
| MAA contr, % | 4±0.9 | 8±0.6 | 0.1 |
| TV, mL | 5.7±2.6 | 4.6±2.2 | <0.001 |
| Leaflet closing area, cm2 | 15.4±4 | 13.7±3.8 | <0.001 |
| Closing pressure ratio | 0.77±0.1 | 0.84±0.1 | 0.01 |
| dP/dt, mm Hg/s | 534±202 | 794±362 | <0.001 |
| MTD, ms | 102±34 | 108±36 | 0.2 |
| Ts-SD, ms | 36±13 | 40±14 | 0.07 |
| SLD, ms | 43±30 | 53±42 | 0.1 |
| PMs Dys, ms | 54±36 | 60±33 | 0.4 |
RV indicates regurgitant volume; TV, tenting volume; PMs Dys, interpappilary muscle dyssynchrony.
Figure 2.
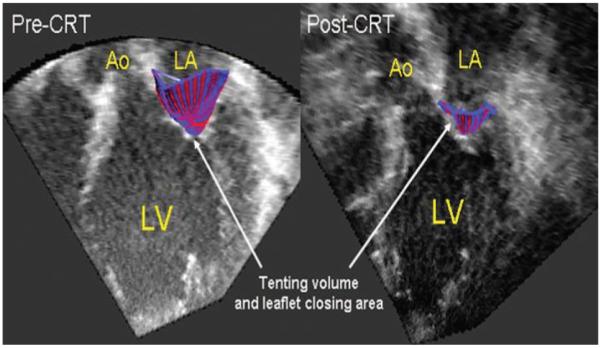
Three-dimensional reconstruction of the tenting volume and leaflet closing area in midsystole superimposed on 2D imaging slice from the 3D dataset. Both tenting volume and leaflet closing area decrease after CRT. Ao indicates aorta; LA, left atrium; LV, left ventricle.
Figure 3.
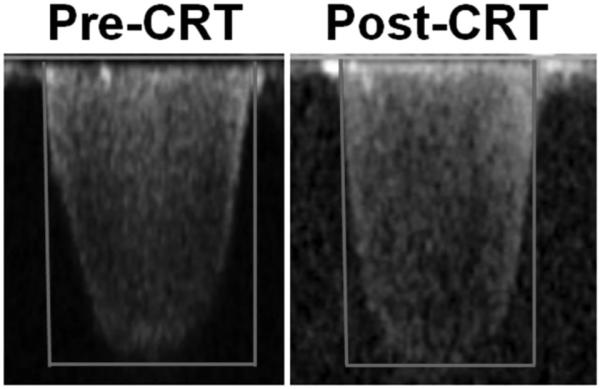
Continuous-wave Doppler regurgitant jet in a patient before CRT (right) and after CRT (left). Estimated closing pressure ratio increases from 0.682 to 0.894.
Figure 4.
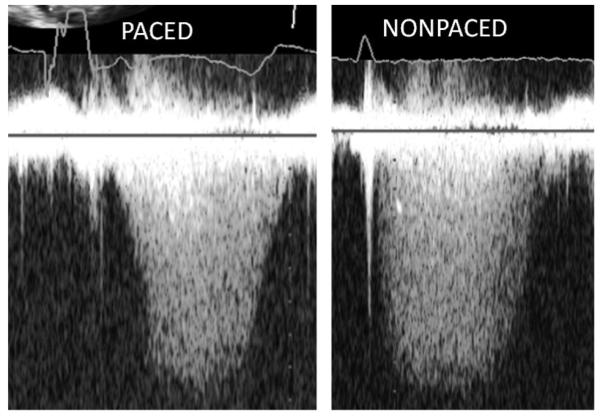
Continuous-wave Doppler regurgitant jet in paced (left) and nonpaced (right) patients. Estimated closing pressure ratio increases from 0.638 (paced) to 0.899 (nonpaced).
MR Reduction Versus MR No-Reduction Subgroups
Table 3 compares changes in echocardiographic parameters before and after CRT within each subgroup. Fifty-three percent of the patients had a >20% reduction in MR RV after CRT (MR reduction) and 47% had lesser reduction or increases in MR RV after CRT (MR no reduction). Both groups showed a reduction in LV volumes; however, the EF increased by 53% in the MR reduction group and only by 20% in the MR no-reduction group. Importantly, only in the MR reduction group were there associated beneficial MV geometry remodeling changes. Both groups demonstrated a significant increase in dP/dt. However, only the MR reduction group was associated with a significant increase in the closing pressure ratio compared with after CRT versus before CRT (Table 3).
Table 3. Comparison of Echocardiographic Parameters at Baseline and 6-Month Follow-Up After CRT in the MR Reduction Group and the MR No-Reduction Group.
| MR Reduction (n=18) |
MR No-Reduction (n=16) |
|||||
|---|---|---|---|---|---|---|
| Pre-CRT | Post-CRT | P | Pre-CRT | Post-CRT | P | |
| MR (RV-ml) | 43±13 | 18±10 | <0.001 | 26±17* | 29±15 | 0.008 |
| EDV, mL | 265±88 | 228±77 | 0.009 | 239±134 | 215±134 | 0.02 |
| ESV, mL | 216±78 | 167±81 | 0.001 | 195±118 | 168±113 | 0.004 |
| EF, % | 19±5 | 28±10 | <0.001 | 20±7 | 24±12 | 0.03 |
| MAA, cm2 | 12.4±3.2 | 11±3.4 | <0.001 | 10.8±3.9 | 10±2.8 | 0.1 |
| MAA contr, % | 3.9±0.7 | 7.8±0.5 | 0.07 | 4.2±1 | 8.8±0.7 | 0.3 |
| TV, mL | 6.4±2.5 | 4.8±2.4 | <0.001 | 5±2.8 | 4.6±2.1 | 0.1 |
| Leaflet closing area, cm2 | 16.9±3.8 | 14.5±4.1 | 0.008 | 14.2±4 | 13.1±3.6 | 0.2 |
| Closing pressure ratio | 0.77±0.04 | 0.85±0.1 | 0.02 | 0.78±0.1 | 0.81±0.1 | 0.2 |
| dP/dt, mm Hg/s | 548±227 | 867±447 | 0.02 | 519±181 | 750±264 | 0.01 |
RV indicates regurgitant volume; TV, tenting volume.
P compares pre-CRT versus post-CRT
P<0.05 (pre-CRT MR reduction versus pre-CRT MR no reduction).
Responder Versus No-Responder Subgroups
Table 4 compares changes in echocardiographic parameters before and after CRT within each subgroup. Among the 34 patients, there were 62% responders to CRT and 38% nonresponders. The responders had significant beneficial changes in MV geometry and improvement in closing forces after CRT compared with before CRT, whereas the nonresponder group did not have similar improvements in mitral valve geometry after CRT compared with before CRT (Table 4).
Table 4. Comparison of Echocardiographic Parameters at Baseline and 6-Month Follow-Up After CRT in Responders and Nonresponders to LV Reverse Remodeling.
| Responder (n=21) |
Nonresponder (n=13) |
|||||
|---|---|---|---|---|---|---|
| Pre-CRT | Post-CRT | P | Pre-CRT | Post-CRT | P | |
| MR (RV-ml) | 38±17 | 23±17 | <0.001 | 29±10 | 24±6 | 0.2 |
| EDV, mL | 247±84 | 195±66 | <0.001 | 262±148 | 265±130 | 0.6 |
| ESV, mL | 203±73 | 140±54 | <0.001 | 212±130 | 210±120 | 0.7 |
| EF, % | 19±6 | 29±10 | <0.001 | 20±6 | 22±9 | 0.2 |
| MAA, cm2 | 11.5±3 | 9.9±2.6 | <0.001 | 11.7±4.4 | 11.3±3.8 | 0.3 |
| MAA contr, % | 4±0.4 | 7±0.5 | 0.2 | 4±1 | 9±0.7 | 0.3 |
| TV, mL | 5.9±2.4 | 4.5±1.8 | <0.001 | 5.3±3.1 | 4.8±2.4 | 0.1 |
| Leaflet closing area, cm2 | 15.6±3.8 | 13±3.2 | <0.001 | 15.1±5.3 | 14.6±4.5 | 0.5 |
| Closing pressure ratio | 0.74±0.11 | 0.0.81±0.11 | 0.04 | 0.82±0.08 | 0.87±0.13 | 0.1 |
| dP/dt, mm Hg/s | 491±191 | 838±357 | 0.01 | 590±211 | 729±230 | 0.1 |
RV indicates regurgitant volume; TV, tenting volume.
P compares pre-CRT versus post-CRT.
Intraobserver and Interobserver Differences
The intraobserver variability as assessed by intraclass correlation coefficient (r) were 0.93 (95% CI, 0.75 to 0.98) for MAA, 0.94 (95% CI, 0.77 to 0.98) for leaflet closing area, and 0.93 (95% CI, 0.76 to 0.98) for tenting volume for interpapillary muscle dyssynchrony and MTD. The interobserver variability on these measurements were 0.90 (95% CI, 0.35 to 0.99), 0.91 (95% CI, 0.42 to 0.99), and 0.88 (95% CI, 0.41 to 0.98), respectively. The intravariability and intervariability values for the closing pressure ratio were 0.93 (95% CI, 0.53 to 0.99) and 0.90 (95% CI, 0.28 to 0.98), respectively. The intraobserver variability for dyssynchrony measurements were MTD, 0.83 (95% CI, −0.19 to 0.97); Ts-SD, 0.9 (95% CI, 0.3 to 0.98); and opposite wall delay (OPWD), 0.96 (95% CI, 0.61 to 0.99). The interobserver variability for these measurement were 0.86 (95% CI, 0.3 to 0.98), 0.93 (95% CI, 0.61 to 0.91), and 0.96 (95% CI, 0.69 to 0.99), respectively.
Discussion
The main finding of this study is that the reduction in FMR after CRT is associated with beneficial changes in MV geometry and improved LV closing pressures on the MV.
Force–Balance Relationship
Normal MV function relates to a balance between tethering and closing forces. The tethering forces are transmitted through the chordae to prevent mitral leaflets from prolapsing and are dependent on normal LV-MV spatial relationships.10,28,29 This spatial relationship depends on the position of the papillary muscle relative to the mitral valve leaflets, and LV geometry is a major determinant of this. Closing forces depend on the pressure generated by the ventricle to close the MV. FMR results from imbalance between tethering and closing forces. Apical displacement of the papillary muscles caused by a dilated or ischemically distorted LV wall increases tethering forces. Decreased LV contractility decreases closing forces.16 Both lead to incomplete mitral leaflet coaptation and mitral regurgitation. We demonstrated that CRT results in favorable effects on the force–balance relationship of MV function, improving both tethering and closing pressures on the MV by its dual effect on LV contraction and remodeling. These favorable effects result in a greater coaptation zone for closure. Our results suggest that CRT results in more sustained peak closing pressures on the MV during systole as demonstrated by a higher closing pressure ratio after CRT. A higher closing pressure ratio may result from either improved LV contractility or improved coordination of LV contraction or both. In our study, dP/dt also improved after CRT, suggesting that enhanced LV contractility plays a role in enhancing the closing pressures on the valve.
Studies have shown that FMR varies during the cardiac cycle and that it is determined by changes in the transmitral pressure gradient.30,31 A biphasic pattern has been described with early and late systolic peaks and a midsystole minimum.15 CRT may act to improve closing forces by improving the temporal pattern of closing forces on the MV throughout systole. Breithardt et al16 demonstrated that CRT acutely reduces the severity of FMR in patients with heart failure with left bundle-branch block. LV contractility improves; as a consequence, transmitral pressure gradient rises faster and to a higher maximal, as reflected by increases in dP/dt.
Beneficial remodeling changes in MV geometry and closing forces occurred despite the apparent lack of improvement in intraventricular or inter papillary muscle dyssynchrony after CRT in our study. Tissue velocity–derived dyssynchrony indices did not improve after CRT, even in patients who respond favorably to CRT. Neither patients who had an improvement in LV volume after CRT nor patients in whom the degree of MR improved exhibited a resolution of mechanical dyssynchrony after CRT. Other investigators have noted similar findings.32 The principal objective of this study was to examine the effects of CRT on the mitral valve and not to demonstrate improvements in dyssynchrony.
Assessment of cardiac force generation by transmitral pressure may provide an alternate window to examine the benefit of CRT in synchronizing LV contraction.
There are a number of measures to assess dyssynchrony that are inherently segmental in nature. However, none currently integrate the effect of LV contraction as a whole. The closing pressure ratio is a measure of global and coordinated LV contraction and may provide a unique index that is a complementary or independent measure of CRT benefit.
Comparison of the MR reduction and MR no-reduction groups provided confirmatory support for the importance of improving the force–balance relationship in reducing MR. Despite similar reductions in LV volumes and improvements in LVEF, patients in the MR reduction group had significant decreases in MV geometric measures after CRT compared with before CRT, whereas patients without MR reduction did not have favorable changes in MV geometry. Both groups demonstrated a significant increase dP/dt, but the MR reduction group also had a significant increase in the closing pressure.
Patients who were responders to CRT, defined as an ESV reduction of >15%, had corresponding beneficial changes compared with pre-CRT levels, in MV geometry, closing pressure ratio, and dP/dt, whereas nonresponders did not have these beneficial changes after CRT versus before CRT. These changes in responders support the notion that the MV geometric and closing forces changes mirror the LV remodeling and function changes.33
Mechanistic Insights and Clinical Implications
Our findings demonstrated the importance of closing pressure ratio as a measure of success of CRT on MV function. An increase in closing pressure ratio after CRT reflects a more coordinated and integrated LV force generation across the MV. The closing pressure ratio mirrored favorable effects of CRT in terms of LV volume and MR reduction, and this ratio may be an important echocardiographic target to assess the efficacy of CRT.
Limitations
This study had a small sample size, and all variables considered for multivariate analyses could not be entered into the same model.
Matrix-array 3D probes have reduced frame rates and image resolution compared with 2D imaging. A reduction in frame rates may result in decreased image quality to assess complex mitral valve changes. However, image quality is typically adequate or better in patients with cardiomyopathy due to the proximity of the heart to the chest wall, especially in the apical windows.
Mitral regurgitation and LV ejection fraction can be affected by differences in loading conditions. However, the effects of different loading conditions should be minimized because these patients were stable outpatients on an optimal medical regimen. All studies were performed in the outpatient setting, not during a hospitalization for acute exacerbation, which may change loading conditions. In addition, there were no significant differences in heart rate and blood pressure among the patient visits.
We did not directly assess the acute effects of CRT on MV function. Prior studies have demonstrated that improved interpapillary muscle dyssynchrony plays an important role in beneficial acute effects after CRT.8,26,34 Our study did not examine acute effects, focusing on late remodeling effects of CRT.
Conclusion
CRT is associated with a reduction in MR. The mechanism relates to optimization of the force–balance relationship, with favorable changes in both closing and tethering forces on MV function. It does so by favorably affecting the force balance acting on the MV in 2 ways: reducing tethering through reversal of LV remodeling and increasing the systolic duration of high transmitral closing pressures.
Acknowledgments
Sources of Funding
This work was supported in part by the National Institutes of Health/National Institute for Biomedical Imaging and Bioengineering (grant R21 EB005294, to J.H.), an American Society of Echocardiography Career Development Award, and the Spanish Society of Cardiology (postresidency grant, to J.S.).
Footnotes
Disclosures
Dr Singh is a consultant and recipient of research grants and speaking fees for the following companies: St Jude Medical, Medtronic, Boston Scientific, Biotronix, Sorin, and Philips Medical Systems.
CLINICAL PERSPECTIVE
Cardiac resynchronization therapy (CRT) is associated with a reduction in functional mitral regurgitation (MR). However, the precise mechanism of MR reduction is incompletely defined. Normal mitral valve (MV) function results from a balance of both tethering and left ventricular (LV) closing forces on the MV. Tethering forces, transmitted via the chordae, keep the valve from prolapsing, whereas closing forces depend on the pressure generated by the ventricle to close the mitral valve. Functional MR results from a derangement in this force–balance relationship of tethering and closing forces. There are 2 potential mechanisms by which CRT may reduce MR. One mechanism relates to reverse remodeling effects of CRT on the LV and MV geometry, resulting in improved spatial relationships of the MV to the ventricle and reduction in leaflet tethering. A second mechanism relates to improved LV contraction with an increase in LV pressure generation after CRT and increased closing forces on the MV with improved leaflet coaptation. To better understand the mechanism of MR reduction after CRT, we examined LV and MV geometry using 3D echocardiography and the transmitral closing pressure pattern using Doppler echocardiography, at baseline and 6 months post-CRT. The latter is an integrated measure of LV force generation and reflects the coordinated impact of closing forces on the MV throughout systole. Our results show that MR reduction post-CRT is associated with both reduced tethering of the mitral valve through beneficial affects on LV remodeling and increased LV contraction forces on the mitral valve. It has been proposed that the mechanism of MR reduction relates to geometric change or, alternatively, changes in LV contractile function. Normal MV function relies on a balance between tethering and closing forces on the MV leaflets. Functional MR results from a derangement of this force–balance relationship, and CRT may be an important modulator of MV function by its ability to enhance the force–balance relationship on the MV. We hypothesized that CRT improves the comprehensive force balance acting on the valve, including favorable changes in both geometry and LV contractile function.
References
- 1.McFate S. Epidemiology of congestive heart failure. Am J Cardiol. 1985;55:3–8. doi: 10.1016/0002-9149(85)90789-1. [DOI] [PubMed] [Google Scholar]
- 2.Abraham WT, Fisher WG, Smith AL, Delurgio DB, Leon AR, Loh E, Kocovic DZ, Packer M, Clavell AL, Hayes DL, Ellestad M, Trupp RJ, Underwood J, Pickering F, Truex C, McAtee P, Messenger J. Cardiac resynchronization in chronic heart failure. N Engl J Med. 2002;346:1845–1853. doi: 10.1056/NEJMoa013168. [DOI] [PubMed] [Google Scholar]
- 3.Cleland JG, Daubert JC, Erdmann E, Freemantle N, Gras D, Kappenberger L, Tavazzi L. The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med. 2005;352:1539–1549. doi: 10.1056/NEJMoa050496. [DOI] [PubMed] [Google Scholar]
- 4.Abraham WT, Hayes DL. Cardiac resynchronization therapy for heart failure. Circulation. 2003;108:2596–2603. doi: 10.1161/01.CIR.0000096580.26969.9A. [DOI] [PubMed] [Google Scholar]
- 5.Trichon BH, Felker GM, Shaw LK, Cabell CH, O’Connor CM. Relation of frequency and severity of mitral regurgitation to survival among patients with left ventricular systolic dysfunction and heart failure. Am J Cardiol. 2003;91:538–543. doi: 10.1016/s0002-9149(02)03301-5. [DOI] [PubMed] [Google Scholar]
- 6.Grigioni F, Enriquez-Sarano M, Zehr KJ, Bailey KR, Tajik AJ. Ischemic mitral regurgitation: long-term outcome and prognostic implications with quantitative Doppler assessment. Circulation. 2001;103:1759–1764. doi: 10.1161/01.cir.103.13.1759. [DOI] [PubMed] [Google Scholar]
- 7.St John Sutton MG, Plappert T, Abraham WT, Smith AL, DeLurgio DB, Leon AR, Loh E, Kocovic DZ, Fisher WG, Ellestad M, Messenger J, Kruger K, Hilpisch KE, Hill MR. Effect of cardiac resynchronization therapy on left ventricular size and function in chronic heart failure. Circulation. 2003;107:1985–1990. doi: 10.1161/01.CIR.0000065226.24159.E9. [DOI] [PubMed] [Google Scholar]
- 8.Kanzaki H, Bazaz R, Schwartzman D, Dohi K, Sade LE, Gorcsan J., III A mechanism for immediate reduction in mitral regurgitation after cardiac resynchronization therapy: insights from mechanical activation strain mapping. J Am Coll Cardiol. 2004;44:1619–1625. doi: 10.1016/j.jacc.2004.07.036. [DOI] [PubMed] [Google Scholar]
- 9.Levine RA, Schwammenthal E. Ischemic mitral regurgitation on the threshold of a solution: from paradoxes to unifying concepts. Circulation. 2005;112:745–758. doi: 10.1161/CIRCULATIONAHA.104.486720. [DOI] [PubMed] [Google Scholar]
- 10.Otsuji Y, Handschumacher MD, Schwammenthal E, Jiang L, Song JK, Guerrero JL, Vlahakes GJ, Levine RA. Insights from three-dimensional echocardiography into the mechanism of functional mitral regurgitation: direct in vivo demonstration of altered leaflet tethering geometry. Circulation. 1997;96:1999–2008. doi: 10.1161/01.cir.96.6.1999. [DOI] [PubMed] [Google Scholar]
- 11.Recusani F, Bargiggia GS, Yoganathan AP, Raisaro A, Valdes-Cruz LM, Sung HW, Bertucci C, Gallati M, Moises VA, Simpson IA. A new method for quantification of regurgitant flow rate using color Doppler flow imaging of the flow convergence region proximal to a discrete orifice: an in vitro study. Circulation. 1991;83:594–604. doi: 10.1161/01.cir.83.2.594. [DOI] [PubMed] [Google Scholar]
- 12.Vandervoort PM, Rivera JM, Mele D, Palacios IF, Dinsmore RE, Weyman AE, Levine RA, Thomas JD. Application of color Doppler flow mapping to calculate effective regurgitant orifice area: an in vitro study and initial clinical observations. Circulation. 1993;88:1150–1156. doi: 10.1161/01.cir.88.3.1150. [DOI] [PubMed] [Google Scholar]
- 13.Bargiggia GS, Bertucci C, Recusani F, Raisaro A, de Servi S, Valdes-Cruz LM, Sahn DJ, Tronconi L. A new method for estimating left ventricular dP/dt by continuous wave Doppler-echocardiography: validation studies at cardiac catheterization. Circulation. 1989;80:1287–1292. doi: 10.1161/01.cir.80.5.1287. [DOI] [PubMed] [Google Scholar]
- 14.Schwammenthal E, Chen C, Benning F, Block M, Breithardt G, Levine RA. Dynamics of mitral regurgitant flow and orifice area: physiologic application of the proximal flow convergence method: clinical data and experimental testing. Circulation. 1994;90:307–322. doi: 10.1161/01.cir.90.1.307. [DOI] [PubMed] [Google Scholar]
- 15.Hung J, Otsuji Y, Handschumacher MD, Schwammenthal E, Levine RA. Mechanism of dynamic regurgitant orifice area variation in functional mitral regurgitation: physiologic insights from the proximal flow convergence technique. J Am Coll Cardiol. 1999;33:538–545. doi: 10.1016/s0735-1097(98)00570-1. [DOI] [PubMed] [Google Scholar]
- 16.Breithardt OA, Sinha AM, Schwammenthal E, Bidaoui N, Markus KU, Franke A, Stellbrink C. Acute effects of cardiac resynchronization therapy on functional mitral regurgitation in advanced systolic heart failure. J Am Coll Cardiol. 2003;41:765–770. doi: 10.1016/s0735-1097(02)02937-6. [DOI] [PubMed] [Google Scholar]
- 17.Llaneras MR, Nance ML, Streicher JT, Lima JA, Savino JS, Bogen DK, Deac RF, Ratcliffe MB, Edmunds LH., Jr. Large animal model of ischemic mitral regurgitation. Ann Thorac Surg. 1994;57:432–439. doi: 10.1016/0003-4975(94)91012-x. [DOI] [PubMed] [Google Scholar]
- 18.Handschumacher MD, Lethor JP, Siu SC, Mele D, Rivera JM, Picard MH, Weyman AE, Levine RA. A new integrated system for three-dimensional echocardiographic reconstruction: development and validation for ventricular volume with application in human subjects. JAm Coll Cardiol. 1993;21:743–753. doi: 10.1016/0735-1097(93)90108-d. [DOI] [PubMed] [Google Scholar]
- 19.Ormiston JA, Shah PM, Tei C, Wong M. Size and motion of the mitral valve annulus in man, I: a two-dimensional echocardiographic method and findings in normal subjects. Circulation. 1981;64:113–120. doi: 10.1161/01.cir.64.1.113. [DOI] [PubMed] [Google Scholar]
- 20.Chaput M, Handschumacher MD, Tournoux F, Hua L, Guerrero JL, Vlahakes GJ, Levine RA. Mitral leaflet adaptation to ventricular remodeling: occurrence and adequacy in patients with functional mitral regurgitation. Circulation. 2008;118:845–852. doi: 10.1161/CIRCULATIONAHA.107.749440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yu CM, Fung WH, Lin H, Zhang Q, Sanderson JE, Lau CP. Predictors of left ventricular reverse remodeling after cardiac resynchronization therapy for heart failure secondary to idiopathic dilated or ischemic cardiomyopathy. Am J Cardiol. 2003;91:684–688. doi: 10.1016/s0002-9149(02)03404-5. [DOI] [PubMed] [Google Scholar]
- 22.Yu CM, Fung JW, Zhang Q, Chan CK, Chan YS, Lin H, Kum LC, Kong SL, Zhang Y, Sanderson JE. Tissue Doppler imaging is superior to strain rate imaging and postsystolic shortening on the prediction of reverse remodeling in both ischemic and nonischemic heart failure after cardiac resynchronization therapy. Circulation. 2004;110:66–73. doi: 10.1161/01.CIR.0000133276.45198.A5. [DOI] [PubMed] [Google Scholar]
- 23.Yu CM, Chau E, Sanderson JE, Fan K, Tang MO, Fung WH, Lin H, Kong SL, Lam YM, Hill MR, Lau CP. Tissue Doppler echocardiographic evidence of reverse remodeling and improved synchronicity by simultaneously delaying regional contraction after biventricular pacing therapy in heart failure. Circulation. 2002;105:438–445. doi: 10.1161/hc0402.102623. [DOI] [PubMed] [Google Scholar]
- 24.Gorcsan J, III, Kanzaki H, Bazaz R, Dohi K, Schwartzman D. Usefulness of echocardiographic tissue synchronization imaging to predict acute response to cardiac resynchronization therapy. Am J Cardiol. 2004;93:1178–1181. doi: 10.1016/j.amjcard.2004.01.054. [DOI] [PubMed] [Google Scholar]
- 25.Bax JJ, Bleeker GB, Marwick TH, Molhoek SG, Boersma E, Steendijk P, van der Wall EE, Schalij MJ. Left ventricular dyssynchrony predicts response and prognosis after cardiac resynchronization therapy. J Am Coll Cardiol. 2004;44:1834–1840. doi: 10.1016/j.jacc.2004.08.016. [DOI] [PubMed] [Google Scholar]
- 26.Ypenburg C, Lancellotti P, Tops LF, Bleeker GB, Holman ER, Pierard LA, Schalij MJ, Bax JJ. Acute effects of initiation and withdrawal of cardiac resynchronization therapy on papillary muscle dyssynchrony and mitral regurgitation. J Am Coll Cardiol. 2007;50:2071–2077. doi: 10.1016/j.jacc.2007.08.019. [DOI] [PubMed] [Google Scholar]
- 27.Shrout PEFJ. Intraclass correlations: uses in assessing rater reliability. Psychol Bull. 1979;2:420–428. doi: 10.1037//0033-2909.86.2.420. [DOI] [PubMed] [Google Scholar]
- 28.Hung J, Papakostas L, Tahta SA, Hardy BG, Bollen BA, Duran CM, Levine RA. Mechanism of recurrent ischemic mitral regurgitation after annuloplasty: continued LV remodeling as a moving target. Circulation. 2004;110:II-85–II-90. doi: 10.1161/01.CIR.0000138192.65015.45. [DOI] [PubMed] [Google Scholar]
- 29.Hung J, Guerrero JL, Handschumacher MD, Supple G, Sullivan S, Levine RA. Reverse ventricular remodeling reduces ischemic mitral regurgitation: echo-guided device application in the beating heart. Circulation. 2002;106:2594–2600. doi: 10.1161/01.cir.0000038363.83133.6d. [DOI] [PubMed] [Google Scholar]
- 30.Yoran C, Yellin EL, Becker RM, Gabbay S, Frater RW, Sonnenblick EH. Dynamic aspects of acute mitral regurgitation: effects of ventricular volume, pressure and contractility on the effective regurgitant orifice area. Circulation. 1979;60:170–176. doi: 10.1161/01.cir.60.1.170. [DOI] [PubMed] [Google Scholar]
- 31.Yellin EL, Yoran C, Sonnenblick EH, Gabbay S, Frater RW. Dynamic changes in the canine mitral regurgitant orifice area during ventricular ejection. Circ Res. 1979;45:677–683. doi: 10.1161/01.res.45.5.677. [DOI] [PubMed] [Google Scholar]
- 32.Chung ES, Leon AR, Tavazzi L, Sun JP, Nihoyannopoulos P, Merlino J, Abraham WT, Ghio S, Leclercq C, Bax JJ, Yu CM, Gorcsan J, III, St John Sutton M, De Sutter J, Murillo J. Results of the Predictors of Response to CRT (PROSPECT) trial. Circulation. 2008;117:2608–2616. doi: 10.1161/CIRCULATIONAHA.107.743120. [DOI] [PubMed] [Google Scholar]
- 33.Vidal B, Sitges M, Marigliano A, Diaz-Infante E, Azqueta M, Tamborero D, Macias A, Roig E, Brugada J, Pare C, Mont L. Relation of response to cardiac resynchronization therapy to left ventricular reverse remodeling. Am J Cardiol. 2006;97:876–881. doi: 10.1016/j.amjcard.2005.09.137. [DOI] [PubMed] [Google Scholar]
- 34.Ypenburg C, Lancellotti P, Tops LF, Boersma E, Bleeker GB, Holman ER, Thomas JD, Schalij MJ, Pierard LA, Bax JJ. Mechanism of improvement in mitral regurgitation after cardiac resynchronization therapy. Eur Heart J. 2008;29:757–765. doi: 10.1093/eurheartj/ehn063. [DOI] [PubMed] [Google Scholar]