Abstract
Background
Ischemic mitral regurgitation (IMR) is caused by systolic traction on the mitral leaflets related to ventricular distortion. Little is known about how chronic tethering affects leaflet area, in part because it cannot be measured repeatedly in situ. Recently, a new method for 3D echo measurement of mitral leaflet area was developed and validated in vivo against sheep valves, later excised. Clinical studies (n=80) showed that mitral leaflet area increased by >30% in patients with inferior myocardial infarction (IMI) and dilated cardiomyopathy versus normal; greater adaptation independently predicted less MR. This study explored whether mitral valve area changes over time within the same heart with IMR.
Methods
Twelve sheep were studied at baseline and 3 months after IMI by 3D echo; 6 were untreated and 6 were treated initially with an epicardial patch to limit LV dilatation and MR.
Results
Untreated sheep developed LV dilatation at 3 months with global dysfunction (mean±SD, EF 24±10% vs. 44±10% with patching, p=0.05) and moderate MR (vena contracta 5.0±1.0 vs 0.8±1.0 mm, p<0.0001). In untreated sheep, total diastolic leaflet area increased from 13.1±1.3 to 18.1±2.5 cm2, p=0.0014. In patched sheep, leaflet area at 3 months was not significantly different from baseline sheep values (13.0±1.1 vs baseline, 12.1±1.8 cm2, p=0.31).
Conclusion
Mitral valve area, independent of systolic stretch, increases over time as the LV remodels after IMI. This increase, however, fails to compensate adequately for tethering to prevent MR. Understanding the mechanism of valve adaptation can potentially suggest new biological and surgical therapeutic targets.
Keywords: Mitral regurgitation, mitral valve leaflets, left ventricular remodeling
Functional mitral regurgitation (MR) is a frequent complication of ischemic heart disease as the left ventricle (LV) dilates and the papillary muscles (PMs) are displaced away from the annulus.1–6 Ischemic MR has consistently been associated with increased mortality, heart failure, and poor prognosis after myocardial infarction7–10.
Recently, patients with mitral leaflet tethering caused by dilated cardiomyopathy or inferior myocardial infarction (IMI) have been shown to have larger mitral leaflet areas than controls with normal hearts11 measured by 3D echocardiographic reconstruction of the open valvular surface. Patients with greater valve adaptation to the tethered valve geometry had less MR. Several potential mechanisms have been suggested for leaflet adaptation in patients with functional MR.12–18 Significant increases over 15 days in systolic leaflet length have also recently been described in sheep with pacing-induced cardiomyopathy even without an increase in the tethering distance from the PMs to the annulus that is seen in IMI.19 However, longitudinal changes in total leaflet area measured in diastole without added systolic leaflet stretch have not yet been studied in the inferior MI setting. Also, whether therapeutic procedures targeting LV remodeling can alter the long-term effect of valve tethering on leaflet remodeling is unknown.
Our aim was therefore to explore in an animal model whether mitral valve area changes over time within the same heart with inferior wall-motion abnormality and LV dilatation caused by an important IMI. Also, we intended to determine whether infarct patching, known to reduce LV remodeling and leaflet tethering, limits mitral leaflet changes.
Methods
Sheep model and design
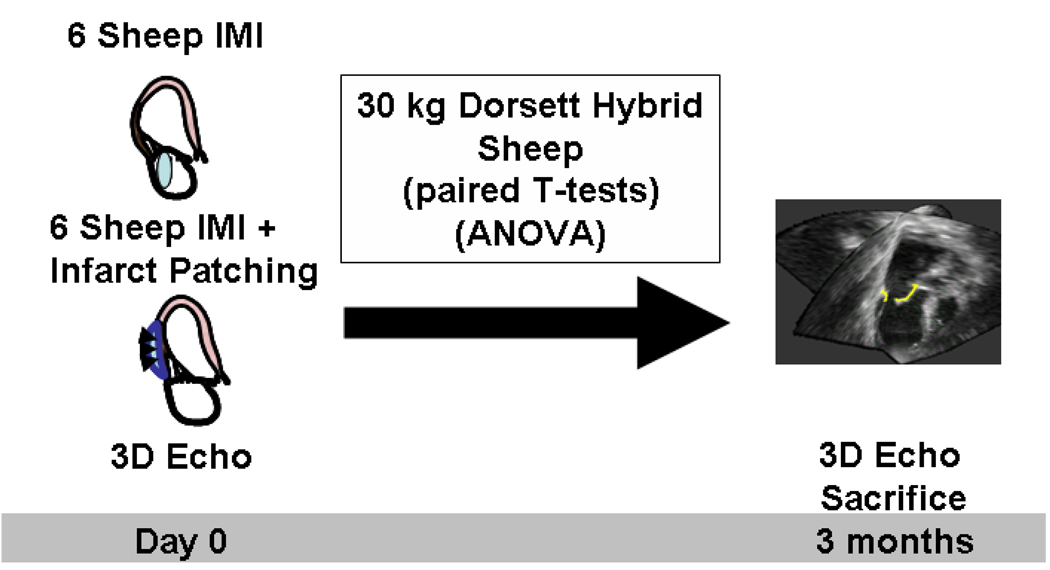
Twelve Dorsett hybrid sheep were studied (figure 1). As previously detailed by Llaneras et al,5, 20 anesthesia was induced with sodium thiopental (12.5 mg/kg IV), and the trachea intubated and ventilated with 2% isoflurane and oxygen. Animals received glycopyrrolate (0.4 mg IV) and vancomycin (0.5 gm IV) one hour before incision. Via left thoracotomy, the heart was exposed and chronic MR was produced by ligating the second and third circumflex obtuse marginal branches. Six animals were treated with inferior wall patching (see below) and 6 were closed without treatment. Before thoracotomy closure, all animals had baseline echocardiographic imaging. Animals were cared for over 12 weeks, following which a second thoracotomy was performed for repeat echocardiographic imaging. This study was reviewed and approved by our institutional Animal Care Committee.
Figure 1.
Study design. IMI, inferior myocardial infarction.
PM repositioning
The patch-balloon device was sewn onto the myocardium over the region of infarction using interrupted sutures as previously described21, 22. An elongated oval balloon parallel to the LV long axis was contained between the patch and the myocardium. The patch buttresses the balloon so that its inflation displaces the myocardium inward toward the anterior mitral annulus. Patch placement and degree of balloon inflation were guided in situ by echocardiography to reduce MR and achieve normal leaflet seating by injecting the minimum amount of fluid necessary (0–15 ml) intraoperatively (14±5 ml total).
Echocardiography
Basic views were obtained using a Philips iE33 scanner and a 5 MHz transducer. Images were analyzed offline using QLab 5.1 (Philips, Andover, MA). LV end-diastolic volume, end-systolic volume (LVESV) and ejection fraction (EF) were measured by biplane Simpson’s technique. The increase in LV volumes from baseline to 3 months was also reported and calculated as (3 month-baseline)/baseline. MR was quantified by the width of the proximal jet (vena contracta) in the apical long-axis view.23 Device application was adjusted to reduce MR based on visual assessment of the proximal jet width.
Leaflet area measurements
Images were obtained using an X3 matrix array transducer (Philips) to acquire 3D volumetric data sets of the mitral valve from 4 heart beats, while temporarily suspending mechanical ventilation. Leaflet areas were analyzed in mid-systole and end-diastole using custom software running on a personal computer (Omni4D: M. Handschumacher) as previously described11. Total leaflet area was measured at end-diastole, since in systole the area of each leaflet involved in coaptation cannot be uniformly visualized and since systole involves passive leaflet stretch. Therefore, total leaflet area was assessed at full end-diastolic leaflet opening, one frame before closure motion.
3D tracing
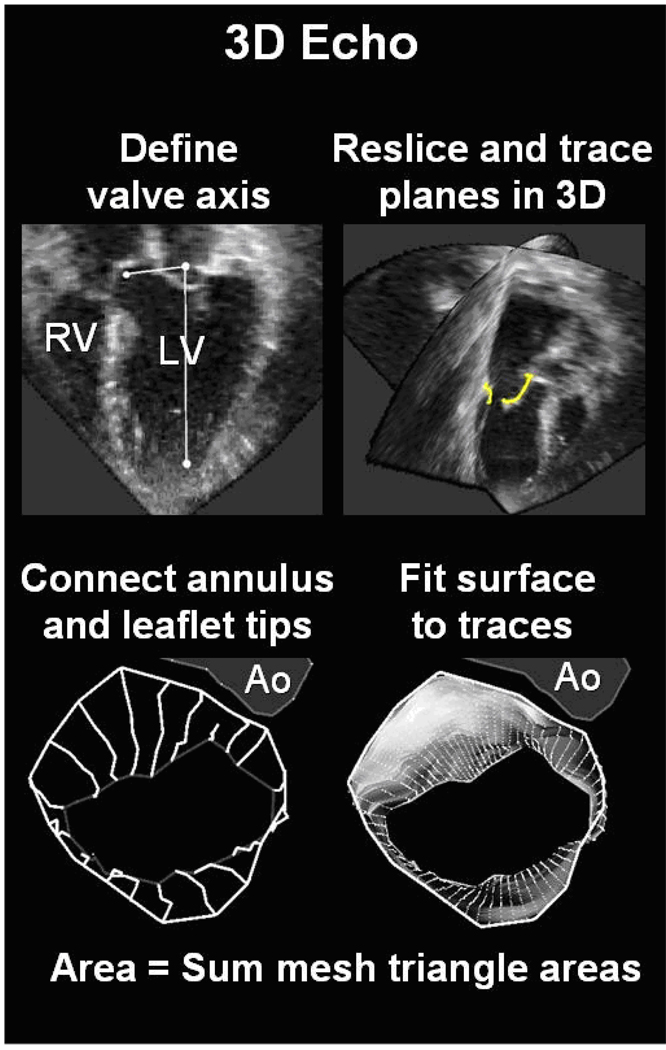
The technique of 3D leaflet tracing and measurement of total leaflet area and closure area has been recently described and validated in a sheep model using the explanted leaflet area as reference. Intra- and interobserver variability of this method were minimal.11 In brief, after standardization of the axis of reference, tracings were performed by automatically obtaining a set of 9 equiangular intersecting image planes (0–180 degrees) from the 3D data set, with the 0-degree view passing through the center of the aortic valve (Figure 2). The annular points, leaflets, and open leaflet tips were manually traced, providing two leaflet traces per plane for a total of 18 traces.
Figure 2.
Method of mitral leaflet area measurement (left) showing leaflet reconstruction in a normal beating sheep heart.
An automated computer algorithm connected the individual annular points to form a closed 3D annular loop and then computed an open tube-like 3D polygonal surface conforming to the leaflet traces. Leaflet area was calculated by summing the elements of this 3D polygonal surface.
In mid-systole, the closed leaflet surface was similarly computed to provide the closure area as the minimal area of the leaflets necessary to occlude the orifice based on their three-dimensional shape, which is dictated by leaflet tethering.24 The closure area was measured as a continuous surface area separating the LA and LV cavities; this measurement excludes the juxtaposed leaflet surface portions that meet in systole but do not separate the two cavities. The regurgitant orifice itself is not directly visualized, and therefore not excluded from this area; the closure area is therefore the area necessary for the leaflets to completely close the orifice between the two cavities. Mitral annular area was calculated as the projection of the annular trace onto its average or least-squares plane.24 Tethering was also confirmed by measuring mid-systolic tenting volume between leaflets and annular least-squares plane.
Statistical analysis
Chronic effects of IMI and the effect of the patch-device were tested by repeated measures analysis of variance after verification that all underlying statistical assumptions for such ANOVA (normal distribution of samples and homogeneous variances) were satisfied. Measures were taken at baseline, acutely after infarction and at 3 months follow up. For MR severity, values measured acutely after infarction and at 3 months follow up were compared (no MR or variance at baseline). For LV volumes, EF, and leaflet areas, values measured at baseline and at 3 months follow up were compared. A 2-tailed p-value of 0.05 was considered significant. For multiple comparisons, significance was examined by Student-Newman-Keuls tests. Values are reported as mean±SD.
Results
MR severity
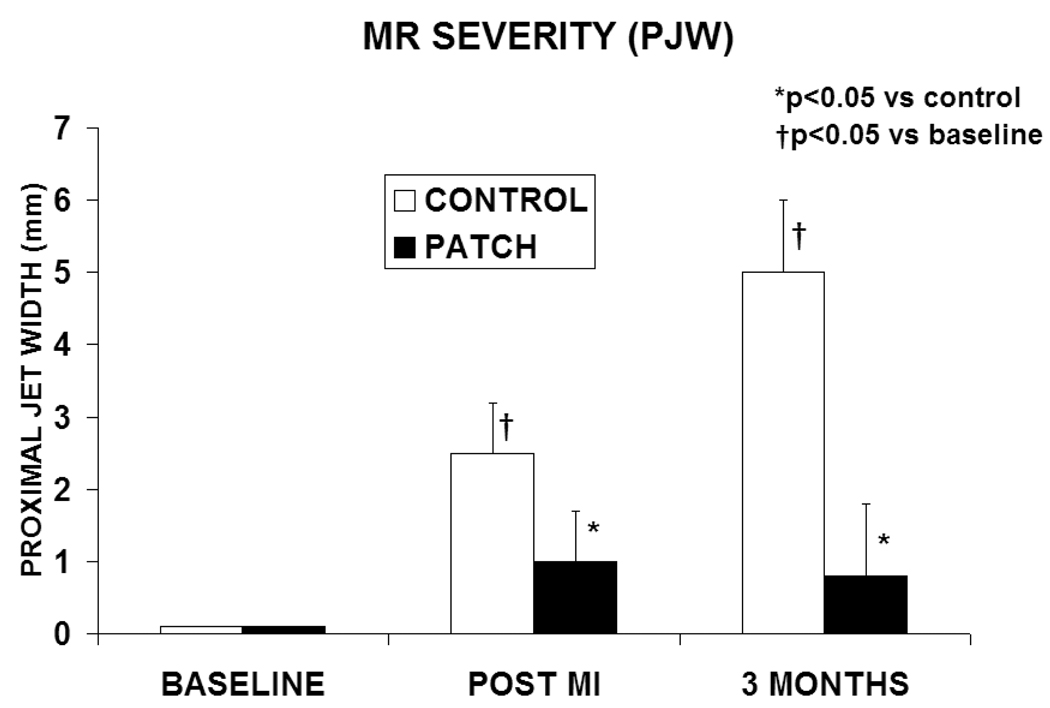
All animals in the control group developed moderate MR immediately after IMI (PJW=2.5±0.7 mm, mean±SD, figure 3), and severe MR at 3 months follow-up (PJW=5.0±1.0 mm, p= 0.0015 vs immediately post IMI). In the patch group, PJW immediately after IMI was 1.0±0.7 mm. At 3 months, MR did not increase in the patch group (PJW= 0.8±1.0 mm, p=0.4 vs immediately after IMI). MR severity was significantly higher in the control group than in the patch group at 3 months(p=0.00016).
Figure 3.
MR severity at baseline, immediately after IMI, and at 3 months follow-up (mean±SD). PJW, proximal jet width.
Hemodynamic data
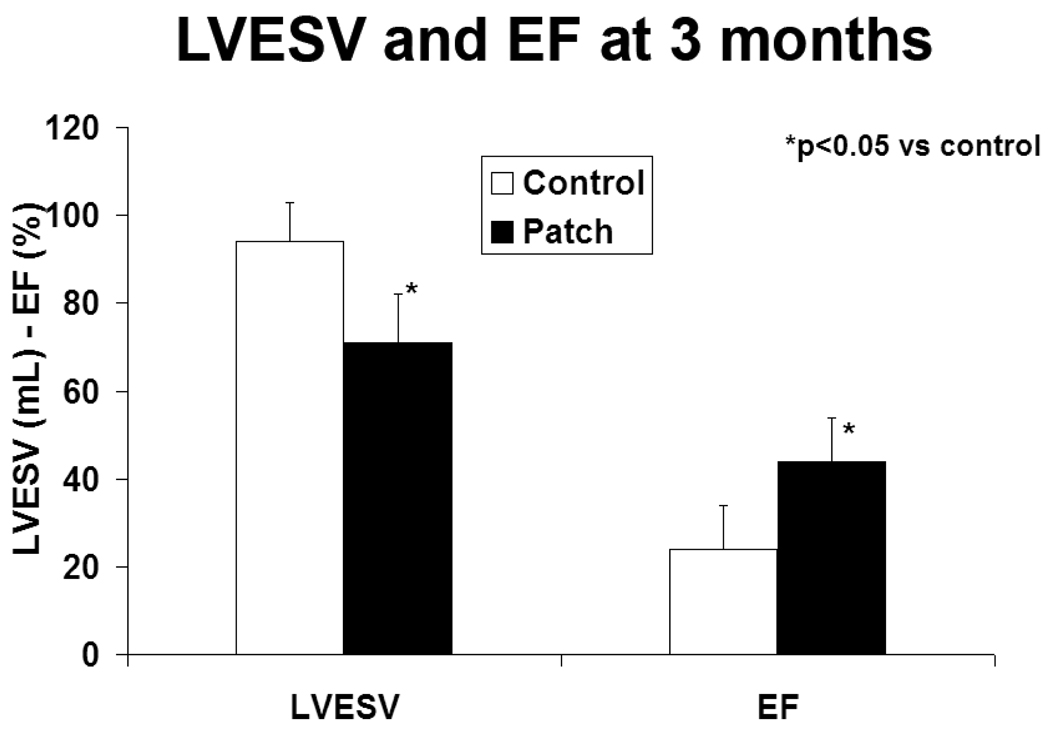
Results are summarized in figure 4. LVESV was increased at 3 months compared to baseline in both the control group (94±15 vs 53±14mL, p=0.01) and the patch group (71±9 vs 48±10 mL, p=0.05), although the increase in volume ([3 month-baseline]/baseline) was less in the patch group (47±9 vs 77±12 %, p=0.01). LVESV was therefore significantly increased at three months in the control group compared with the patch group (p=0.03). EF was decreased at 3 months in the control group (24±10 vs baseline 44±10 %, p=0.02) but remained stable in the patch group (44±10 vs baseline 43±11 %, p=0.80).
Figure 4.
LV end-systolic volume and ejection fraction at 3 months follow-up (mean±SD). LVESV, left ventricular end systolic volume, EF, ejection fraction.
Mitral valve areas
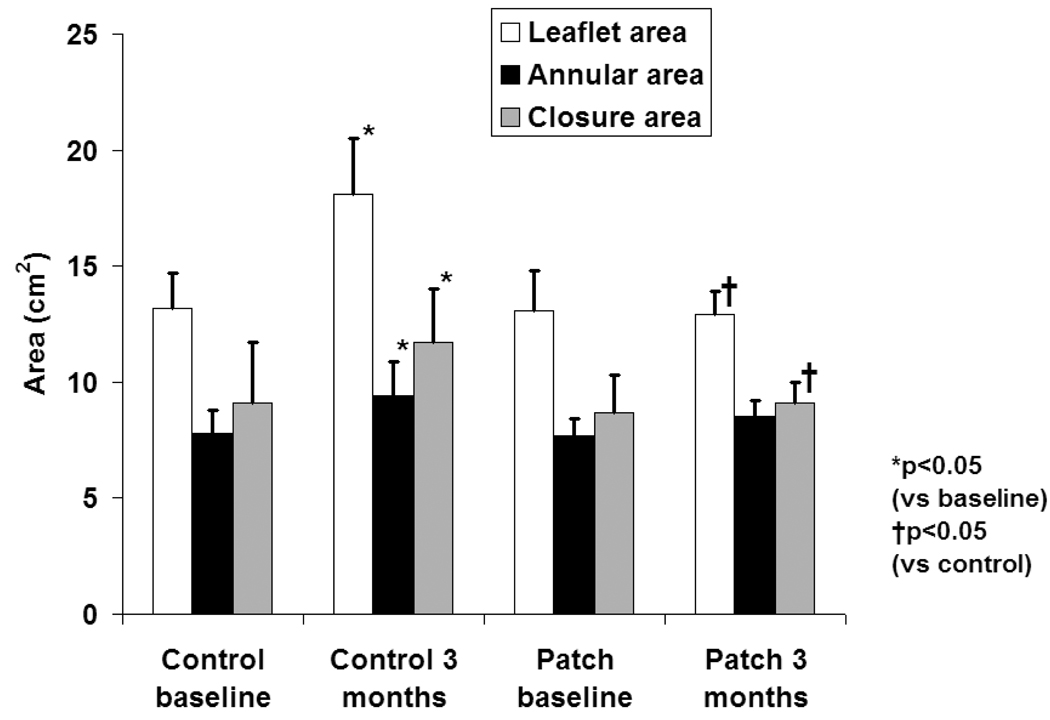
Total leaflet area increased at 3 months compared to baseline in the control group (18.1±2.5 vs 13.1±1.3 cm2, p=0.0001, figure 5). No significant increase in leaflet area was observed in the patch group (13.1±1.2 vs 12.1±1.9 cm2, p=0.31). Mitral annular area increased at 3 months in the control group (9.4±1.5 vs 7.8±1.0 cm2, p=0.02) but not significantly in the patch group (8.5±0.7 vs7.7±0.8 cm2, p=0.1), consistent with posterior cinching of the annulus and MR reduction by the patch.
Figure 5.
Total leaflet area, annular area and closure area at baseline and at 3 months follow-up (mean±SD).
Closure area was increased in the control group at 3 months (11.7±2.3 vs 9.1±2.6 cm2, p=0.03), suggesting a tethered geometry. Closure area did not increase in the patch group (9.1±0.9 vs 8.7±1.6 cm2, p=0.73), consistent with stabilization of the LV-mitral valvular complex by the device. Tethering was confirmed by increased tenting volume in the control group at 3 months (1.83±1.14 cm3 versus 0.41±0.20 cm3 at baseline, p=0.039, without significant change in patched animals.
Discussion
Ischemic MR has been described as a disease in which distorted ventricular geometry and impaired function lead to increased systolic tethering and incomplete leaflet closure.1–6 Recently, however, we have begun to ask whether the leaflets themselves can adapt to chronic leaflet stretch and undergo changes that could improve coaptation. A clinical study has shown that MV area is increased by more than 30% in patients with IMI and dilated cardiomyopathy compared to patients with normal hearts at a single evaluation timepoint.11 In that work, a new echocardiographic method of in vivo leaflet area measurement had been first validated in sheep by correlating the echo-derived measure and the area measured by planimetry after explantation. To date, these changes in area had not been shown in longitudinal studies.
In the present study, we demonstrate that the mitral valve undergoes adaptive changes that translate in an increase in leaflet area over time within the same heart. In these animals, leaflet area increased by 38.2% three months after IMI. A recently validated 3D echocardiography technique for the reconstruction of the leaflets visualized in vivo allows such repeated measurements. In contrast, no significant change in leaflet area was observed in sheep in whom the papillary muscles were realigned by external patch placement, with associated limitation of both LV and annular remodeling, which appear to create the geometric stimuli for leaflet enlargement. This adaptation of the leaflets, however, may not be sufficient to overcome the effects of tethering and restore normal coaptation.
These results are consistent with the study of Timek, Miller et al. who described a pacing-induced cardiomyopathy model in sheep.19 In this model leaflet length was measured by radioopaque markers placed along the leaflet midline. Systolic leaflet length increased over 15 days as the LV dilated (15.2% for the anterior leaflet and 16.7% for the posterior leaflet), roughly corresponding to a 30% increase in total leaflet area. The current study adds the setting of a typical IMI, a common clinical scenario for ischemic MR, and the measurement of total leaflet area in diastole during which increased passive stretch is not a factor. These findings are also consistent with the pathological literature indicating changes in leaflet composition and collagen content, which may lead to changes in leaflet area but also decrease leaflet flexibility and ability to bend and coapt effectively.13–16, 18 Potential mechanisms for leaflet adaptation will require further study and include the generation of transforming growth factor (TGF)-β and related signals by mechanically stretched valve interstitial cells and myocytes in ischemic hearts.12–18 These molecules could induce endothelial-to-mesenchymal cell transdifferentation, with activation and proliferation of interstitial cells and increased matrix production. Studying these mechanisms can help us understand why leaflet adaptation may be greater in some patients and how it might be therapeutically augmented.
In summary, leaflet area increases substantially if LV remodeling is not limited by external constraint. These results suggest the need to further improve our understanding of mitral leaflet adaptation, and whether it can be targeted in our therapeutic approach to functional MR.
Footnotes
Disclaimer: The manuscript and its contents are confidential, intended for journal review purposes only, and not to be further disclosed.
Author Disclosures
Miguel Chaput: No disclosures
Mark D. Handschumacher: No disclosures
Luis J. Guerrero: No disclosures
Godtfred Holmvang: No disclosures
Jacob P. Dal-Bianco: No disclosures
Suzanne Sullivan: No disclosures
Gus J. Vlahakes: No disclosures
Judy Hung: No disclosures
Robert A. Levine: No disclosures
The authors had full access to the data and take full responsibility for its integrity. All authors have read and agree to the manuscript as written. There are no conflicts of interest to declare.
References
- 1.Godley RW, Wann LS, Rogers EW, Feigenbaum H, Weyman AE. Incomplete mitral leaflet closure in patients with papillary muscle dysfunction. Circulation. 1981;63(3):565–571. doi: 10.1161/01.cir.63.3.565. [DOI] [PubMed] [Google Scholar]
- 2.Sabbah HN, Kono T, Rosman H, Jafri S, Stein PD, Goldstein S. Left ventricular shape: A factor in the etiology of functional mitral regurgitation in heart failure. American Heart Journal. 1992;123(4 Part 1):961–966. doi: 10.1016/0002-8703(92)90703-x. [DOI] [PubMed] [Google Scholar]
- 3.Otsuji Y, Handschumacher MD, Schwammenthal E, Jiang L, Song J-K, Guerrero JL, Vlahakes GJ, Levine RA. Insights From Three-Dimensional Echocardiography Into the Mechanism of Functional Mitral Regurgitation : Direct In Vivo Demonstration of Altered Leaflet Tethering Geometry. Circulation. 1997;96(6):1999–2008. doi: 10.1161/01.cir.96.6.1999. [DOI] [PubMed] [Google Scholar]
- 4.Komeda M, Glasson JR, Bolger AF, Daughters GTI, Ingels NB, Jr, Miller DC. Papillary muscle-left ventricular wall "complex". Journal of Thoracic and Cardiovascular Surgery. 1997;113(2):292–301. doi: 10.1016/s0022-5223(97)70326-x. [DOI] [PubMed] [Google Scholar]
- 5.Llaneras MR, Nance ML, Streicher JT, Linden PL, Downing SW, Lima JA, Deac R, Edmunds LH., Jr Pathogenesis of ischemic mitral insufficiency. J Thorac Cardiovasc Surg. 1993;105(3):439–442. [PubMed] [Google Scholar]
- 6.Yiu SF, Enriquez-Sarano M, Tribouilloy C, Seward JB, Tajik AJ. Determinants of the Degree of Functional Mitral Regurgitation in Patients With Systolic Left Ventricular Dysfunction : A Quantitative Clinical Study. Circulation. 2000;102(12):1400–1406. doi: 10.1161/01.cir.102.12.1400. [DOI] [PubMed] [Google Scholar]
- 7.Grigioni F, Enriquez-Sarano M, Zehr KJ, Bailey KR, Tajik AJ. Ischemic Mitral Regurgitation : Long-Term Outcome and Prognostic Implications With Quantitative Doppler Assessment. Circulation. 2001;103(13):1759–1764. doi: 10.1161/01.cir.103.13.1759. [DOI] [PubMed] [Google Scholar]
- 8.Lamas GA, Mitchell GF, Flaker GC, Smith SC, Jr, Gersh BJ, Basta L, Moye L, Braunwald E, Pfeffer MA. Clinical Significance of Mitral Regurgitation After Acute Myocardial Infarction. Circulation. 1997;96(3):827–833. doi: 10.1161/01.cir.96.3.827. [DOI] [PubMed] [Google Scholar]
- 9.Barzilai B, Gessler C, Perez JE, Schaab C, Jaffe AS. Significance of Doppler-detected mitral regurgitation in acute myocardial infarction. The American Journal of Cardiology. 1988;61(4):220–223. doi: 10.1016/0002-9149(88)90919-8. [DOI] [PubMed] [Google Scholar]
- 10.Feinberg MS, Schwammenthal E, Shlizerman L, Porter A, Hod H, Freimark D, Matezky S, Boyko V, Mandelzweig L, Vered Z. Prognostic significance of mild mitral regurgitation by color Doppler echocardiography in acute myocardial infarction. The American Journal of Cardiology. 2000;86(9):903–907. doi: 10.1016/s0002-9149(00)01119-x. [DOI] [PubMed] [Google Scholar]
- 11.Chaput M, Handschumacher MD, Tournoux F, Hua L, Guerrero JL, Vlahakes GJ, Levine RA. Mitral Leaflet Adaptation to Ventricular Remodeling: Occurrence and Adequacy in Patients With Functional Mitral Regurgitation. Circulation. 2008;118(8):845–852. doi: 10.1161/CIRCULATIONAHA.107.749440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thubrikar MJ, Labrosse MR, Zehr KJ, Robicsek F, Gong GG, Fowler BL. Aortic root dilatation may alter the dimensions of the valve leaflets. European Journal of Cardio-Thoracic Surgery. 2005;28(6):850–855. doi: 10.1016/j.ejcts.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 13.Willems IEHM, Smits JF, Daemen MJ. Structural alterations in heart valves during left ventricular pressure overload in the rat. Laboratory Investigation. 1994;71:127–133. [PubMed] [Google Scholar]
- 14.Quick DW, Kunzleman KS, Kneebone JM, Cochran RP. Collagen synthesis is upregulated in mitral valves subjected to altered stress. ASAIO J. 1997;43:181–186. [PubMed] [Google Scholar]
- 15.Kunzelman KS, Quick DW, Cochran RP. Altered collagen concentration in mitral valve leaflets: Biochemical and finite element analysis. Annals of Thoracic Surgery. 1998;66:s198–s205. doi: 10.1016/s0003-4975(98)01106-0. [DOI] [PubMed] [Google Scholar]
- 16.Sugi Y, Yamamura H, Okagawa H, Markwald RR. Bone morphogenetic protein-2 can mediate myocardial regulation of atrioventricular cushion mesenchymal cell formation in mice. Developmental Biology. 2004;269(2):505–518. doi: 10.1016/j.ydbio.2004.01.045. [DOI] [PubMed] [Google Scholar]
- 17.Ng CM, Cheng A, Myers LA, Martinez-Murillo F, Jie C, Bedja D, Gabrielson KL, Hausladen JMW, Mecham RP, Judge DP, Dietz HC. TGF-{beta}-dependent pathogenesis of mitral valve prolapse in a mouse model of Marfan syndrome. J. Clin. Invest. 2004;114(11):1586–1592. doi: 10.1172/JCI22715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grande-Allen KJ, Griffin BP, Ratliff NB, Cosgrove DM, III, Vesely I. Glycosaminoglycan profiles of myxomatous mitral leaflets and chordae parallel the severity of mechanical alterations. Journal of the American College of Cardiology. 2003;42(2):271–277. doi: 10.1016/s0735-1097(03)00626-0. [DOI] [PubMed] [Google Scholar]
- 19.Timek TA, Lai DT, Dagum P, Liang D, Daughters GT, Ingels NB, Jr, Miller DC. Mitral Leaflet Remodeling in Dilated Cardiomyopathy. Circulation. 2006;114(1suppl):I-518–I-523. doi: 10.1161/CIRCULATIONAHA.105.000554. [DOI] [PubMed] [Google Scholar]
- 20.Llaneras MR, Nance ML, Streicher JT, Lima JA, Savino JS, Bogen DK, Deac RF, Ratcliffe MB, Edmunds LH., Jr Large animal model of ischemic mitral regurgitation. Ann Thorac Surg. 1994;57(2):432–439. doi: 10.1016/0003-4975(94)91012-x. [DOI] [PubMed] [Google Scholar]
- 21.Hung J, Chaput M, Guerrero JL, Handschumacher MD, Papakostas L, Sullivan S, Solis J, Levine RA. Persistent Reduction of Ischemic Mitral Regurgitation by Papillary Muscle Repositioning: Structural Stabilization of the Papillary Muscle Ventricular Wall Complex. Circulation. 2007;116(11suppl):I-259–I-263. doi: 10.1161/CIRCULATIONAHA.106.679951. [DOI] [PubMed] [Google Scholar]
- 22.Hung J, Guerrero JL, Handschumacher MD, Supple G, Sullivan S, Levine RA. Reverse Ventricular Remodeling Reduces Ischemic Mitral Regurgitation: Echo-Guided Device Application in the Beating Heart. Circulation. 2002;106(20):2594–2600. doi: 10.1161/01.cir.0000038363.83133.6d. [DOI] [PubMed] [Google Scholar]
- 23.Zoghbi WA, Enriquez-Sarano M, Foster E, Grayburn PA, Kraft CD, Levine RA, Nihoyannopoulos P, Otto CM, Quinones MA, Rakowski H. Recommendations for evaluation of the severity of native valvular regurgitation with two-dimensional and doppler echocardiography. Journal of the American Society of Echocardiography. 2003;16(7):777–802. doi: 10.1016/S0894-7317(03)00335-3. [DOI] [PubMed] [Google Scholar]
- 24.Levine RA, Handschumacher MD, Sanfilippo AJ, Hagege AA, Harrigan P, Marshall JE, Weyman AE. Three-dimensional echocardiographic reconstruction of the mitral valve, with implications for the diagnosis of mitral valve prolapse. Circulation. 1989;80(3):589–598. doi: 10.1161/01.cir.80.3.589. [DOI] [PubMed] [Google Scholar]
- 25.Glower DD, Spratt JA, Snow ND, Kabas JS, Davis JW, Olsen CO, Tyson GS, Sabiston DC, Jr, Rankin JS. Linearity of the Frank-Starling relationship in the intact heart: the concept of preload recruitable stroke work. Circulation. 1985;71(5):994–1009. doi: 10.1161/01.cir.71.5.994. [DOI] [PubMed] [Google Scholar]