Abstract
Protective humoral immune responses critically depend on the optimal differentiation of B cells into antibody secreting cells. Because of the important role of antibodies in fighting infections and in successful vaccination, it is imperative to identify mediators that control B cell differentiation. Activation of B cells through toll-like receptor 9 (TLR-9) by CpG-DNA induces plasma cell differentiation and antibody production. Herein, we examined the role of the PPARγ/RXRα pathway on human B cell differentiation. We demonstrated that activated B cells upregulate their expression of PPARγ. We also show that nanomolar levels of natural (15d-PGJ2) or synthetic (Rosiglitazone) PPARγ ligands enhanced B cell proliferation and significantly stimulated plasma cell differentiation and antibody production. Moreover, the addition of GW9662, a specific PPARγ antagonist, abolished these effects. RXR is the binding partner for PPARγ and is required to produce an active transcriptional complex. The simultaneous addition of nanomolar concentrations of the RXRα ligand (9-cis-RA) and PPARγ ligands to CpG-activated B cells resulted in additive effects on B cell proliferation, plasma cell differentiation and antibody production. Furthermore, PPARγ ligands alone or combined with 9-cis-RA enhanced CpG-induced expression of Cox-2 and the plasma cell transcription factor BLIMP-1. Induction of these important regulators of B cell differentiation provides a possible mechanism for the B cell enhancing effects of PPARγ ligands. These new findings indicate that low doses of PPARγ/RXRα ligands could be used as a new type of adjuvant to stimulate antibody production.
Keywords: PPARγ, B lymphocytes, antibody production, differentiation, retinoic acid
Introduction
The differentiation of B cells into immunoglobulin-secreting plasma cells is crucial for protective humoral immune responses to combat infection (1). The innate immune system recognizes microorganisms through pattern recognition receptors, such as toll-like receptors (TLRs). Activation of human B cells by unmethylated CpG DNA motifs, a TLR-9 ligand, induces B cell differentiation, as well as increased cytokine and antibody production (1). During humoral immune responses, naive B cells that become activated first proliferate and secrete immunoglobulin-M (IgM), followed by IgG. Some B cells become long-lived plasma cells that secrete copious amounts of antibody or further differentiate into memory B cells (2). Activation of B cells also results in the expression of key transcription factors, such as BLIMP-1, that lead to the expression of genes necessary for terminal B cell differentiation (3).
We recently published that peroxisome proliferator-activated receptor gamma (PPARγ) overexpression and knockdown influence BLIMP-1 expression in Burkitt’s lymphoma (4). PPARs belong to the nuclear hormone receptor superfamily of transcription factors (5), of which there are three isoforms: PPARα, PPARβ/δ and PPARγ. PPARγ and its ligands are involved in regulating proliferative, inflammatory and in some cases differentiating properties of immune and cancer cells (6, 7). We previously demonstrated that normal and malignant B lymphocytes express PPARγ and that exposure to micromolar levels of certain types of electrophilic PPARγ ligands inhibit B cell proliferation (8–10). PPARγ ligands are diverse and at high concentrations (µM) can have PPARγ-independent effects. Endogenous ligands include 15-deoxy-Δ12,14 prostaglandin J2 (15d-PGJ2), as well as fatty acid derivatives (i.e. oxidized low-density lipoproteins (OxLDL)). PGD2 and 15d-PGJ2 are derived from arachidonic acid by the catalytic activities of cyclooxygenase-2 (Cox-2) and prostaglandin D synthase (11–14). PGD2 spontaneously undergoes a series of dehydration reactions to form the PGJ family of prostaglandins, including 15d-PGJ2, and 15d-PGD2, which can also transactivate PPARγ (12, 15–17). PPARγ is also activated by synthetic ligands belonging to the thiazolidinediones (TZDs) class of anti-diabetic drugs, which includes Rosiglitazone. Following ligand binding, PPARγ forms a heterodimer with retinoid X receptors (RXRs) and subsequently binds to the peroxisome proliferator response element (PPRE) found in target gene promoters. RXR is an obligate partner of PPARγ It is required to induce transcription (18) and is activated by 9-cis-retinoic acid (9-cis-RA), a vitamin A metabolite (19).
It is unknown whether PPARγ/RXR regulates B cell differentiation. We hypothesized that during human B cell activation, PPARγ protein levels would increase and would stimulate differentiation and antibody production. We also proposed that PPARγ would interact with RXR to increase plasma cell formation and antibody production. Herein, we report our studies on PPARγ expression and how low doses of PPARγ ligands enhance B cell function.
Materials and Methods
Reagents and culture conditions
CpG oligodeoxynucleotides 2395 5′-TCGTCGTTTTCGGCGCGCGCCG-3′ were purchased from the Coley Pharmaceutical Group (Wellesley, MA) and used at a concentration of 1 µg/ml. A rabbit anti-human F(ab')2 anti-IgM Ab (Jackson ImmunoResearch Laboratories) was used at 2 µg/ml to crosslink the B cell receptor (BCR). Rosiglitazone and the irreversible PPARγ antagonist GW9662 were purchased from Cayman (Ann Harbor, MI) and 15d-PGJ2 was purchased from Biomol (Plymouth meeting, PA). 9-cis-retinoic acid was obtained from Sigma (St. Louis, MO). The anti-BLIMP-1 antibody was purchased from Novus Biologicals (Littleton, CO). The anti-PPARγ antibodies were purchased from Abcam (Cambridge, MA) and Santa Cruz (Santa Cruz, CA). Total actin (CP-01) antibody was from Oncogene (Cambridge, MA). The Cox-2 selective inhibitor SC-58125 was purchased from Cayman Chemical (Ann Arbor, MI)
B cell isolation
Normal B lymphocytes were isolated from a unit of whole blood from healthy donors with ethical permission from the Research Subjects Review Board at the University of Rochester. The isolation of normal B lymphocytes has been previously described (20). Briefly, buffy coats were obtained from whole blood and peripheral blood mononuclear cells (PBMCs) were obtained using Ficoll-Paque (Amersham Biosciences AB) gradient centrifugation. PBMCs were then incubated with anti-CD19 antibody-coated Dynabeads (Dynal Biotech, Oslo, Norway) and subjected to a magnetic field to separate B lymphocytes; negatively selected cells were washed out. B lymphocytes were then detached from the beads using an equal volume of CD19 Detachabeads (Dynal Biotech). B lymphocyte purity was >98% CD19 positive (as determined by flow cytometry, data not shown). Purified B cells were cultured in RPMI 1640 tissue culture medium (Life Technologies, Grand Island, NY) supplemented with 10% fetal bovine serum (FBS), 5 × 10−5 M β-mercaptoethanol (Eastman Kodak, Rochester, NY), 10 mM HEPES (US Biochemical Corp., Cleveland, OH), 2 mM L-glutamine (Life Technologies) and 50 µg/ml gentamicin (Life Technologies). All experiments were conducted with B cells from at least three different donors.
PPARγ gene reporter analysis
Transient transfections of normal B lymphocytes with a PPRE-luciferase reporter plasmid containing three copies of the ACO-PPRE (PPAR response element) from rat acyl CoA oxidase (a gift from Dr. B. Seed, Massachusetts General Hospital, Boston, MA) (21, 22) were conducted using the nucleofector protocol from Amaxa Biosystems (Cologne, Germany). Eighteen hours post-transfection cells were left untreated or were treated with 1 µg/ml CpG in the presence or absence of Rosiglitazone (0.5 µM) or 15d-PGJ2 (0.2 µM). These optimal doses were chosen based on pilot experiments. Twenty-four hours after treatments, luciferase activity was assayed using the Promega Luciferase Assay System (Madison, WI). Relative light units (RLU) were determined with a Lumicount Microplate Luminometer (Packard Instrument Co., Meriden, CT, USA). Relative light units (RLU) were normalized to transfection efficiency that was monitored by cotransfection of GFP expression vector. Transfection efficiency was approximately 40 % (data not shown).
Proliferation
For cell division, a CellTrace™ CFSE Cell Proliferation Kit (Invitrogen, Carlsbad, CA) was used according to the manufacturer’s protocol. Briefly, cells were labeled with 0.5 µM CFSE (carboxyfluorescein diacetate succinimidyl ester) for 15 minutes at 37°C, followed by two washes with 1x PBS, and then resuspended in RPMI culture media containing 10% FBS. Cells were then plated at a density of 1×105 cells/ well in a 96-round bottom plate. Five days later, cells were acquired using a BD Biosciences FACS Calibur flow cytometer and analyzed using FlowJo software (Tree Star, Inc. Ashland, OR).
Intracellular and surface labeling
B cells were incubated with mouse anti-human CD19-APC (BD Biosciences), anti-human CD38-PE (BD Biosciences) and/or anti-human CD27-APC (BD Biosciences) in cold PBS with sodium azide (0.02%) and BSA (0.3%) for 20 min at 20°C. COX-2 intracellular staining was performed as described previously (8). All samples were acquired on a BD Biosciences FACS Calibur flow cytometer and analyzed using FlowJo software (Tree Star, Inc. Ashland, OR).
For intracellular staining for PPARγ, untreated or activated B lymphocytes were surface stained with 20 µl of APC anti-human CD19 mAb (BD Biosciences) for 30 minutes in the dark at room temperature (RT). Cells were then fix and permeabilized with BD Cytofix/Cytoperm Fixation/ Permeabilization Kit following the manufacturer’s instructions. A FITC-Conjugated anti-human PPARγ antibody was used at a 1/100 dilution. An equal amount of IgG1 FITC mAb was used as an isotype control.
Antibody production
Purified human B lymphocytes (5 × 105 cells/ml) were cultured in 96-well round-bottom microtiter plates. Cells were treated for 5–6 days with activating agents in the presence and absence of PPARγ ligands and/or 9-cis-RA (100 nM). Pilot experiments were performed to optimize the doses of PPARγ and RXR ligands. For some experiments, cells were also treated with an optimal dose of GW9662 (500 nM). Supernatants were harvested and the concentrations of IgM and IgG were analyzed using human-specific ELISAs (Bethyl Laboratories).
Western blots
Whole cell extracts were collected using ELB buffer (50 mM HEPES (pH 7), 250 mM NaCl, 0.1% Nonidet P-40, 5 mM EDTA, 10 mM NaF, 0.1 mM Na3VO4, 50 µM ZnCl2, supplemented with 0.1 mM PMSF, 1 mM DTT, and a mixture of protease and phosphatase inhibitors) and total protein was quantified using bicinchoninic acid protein assay (BCA assay kit) (Pierce, Rockford, IL). Twenty-five micrograms of protein was electrophoresed on 8–16 % Precise™ protein gels (Pierce, Rockford, IL) and transferred to polyvinylidene fluoride (PVDF) membrane (Millipore, Billerica, MA). The membranes were analyzed for immunoreactivity with the indicated primary antibody, washed and then incubated with an appropriate horseradish peroxidase-conjugated secondary antibody. The membranes were visualized by chemiluminescence using an ECL kit (Pierce, Rockford, IL).
Statistical analysis
Statistical analysis was performed using GraphPad Prism (GraphPad Software, Inc, La Jolla, CA). For comparison between groups of three or more, an analysis of variance (ANOVA) with Newman-Keuls multiple comparison test was used to determine differences between treatments. A t-test was used to compare vehicle and PPARγ ligand. Results are expressed as the mean ± standard error of the mean (SEM). P values less that 0.05 were considered significant. All experiments were repeated at least 3 times.
Results
PPARγ expression is upregulated by B cell activation
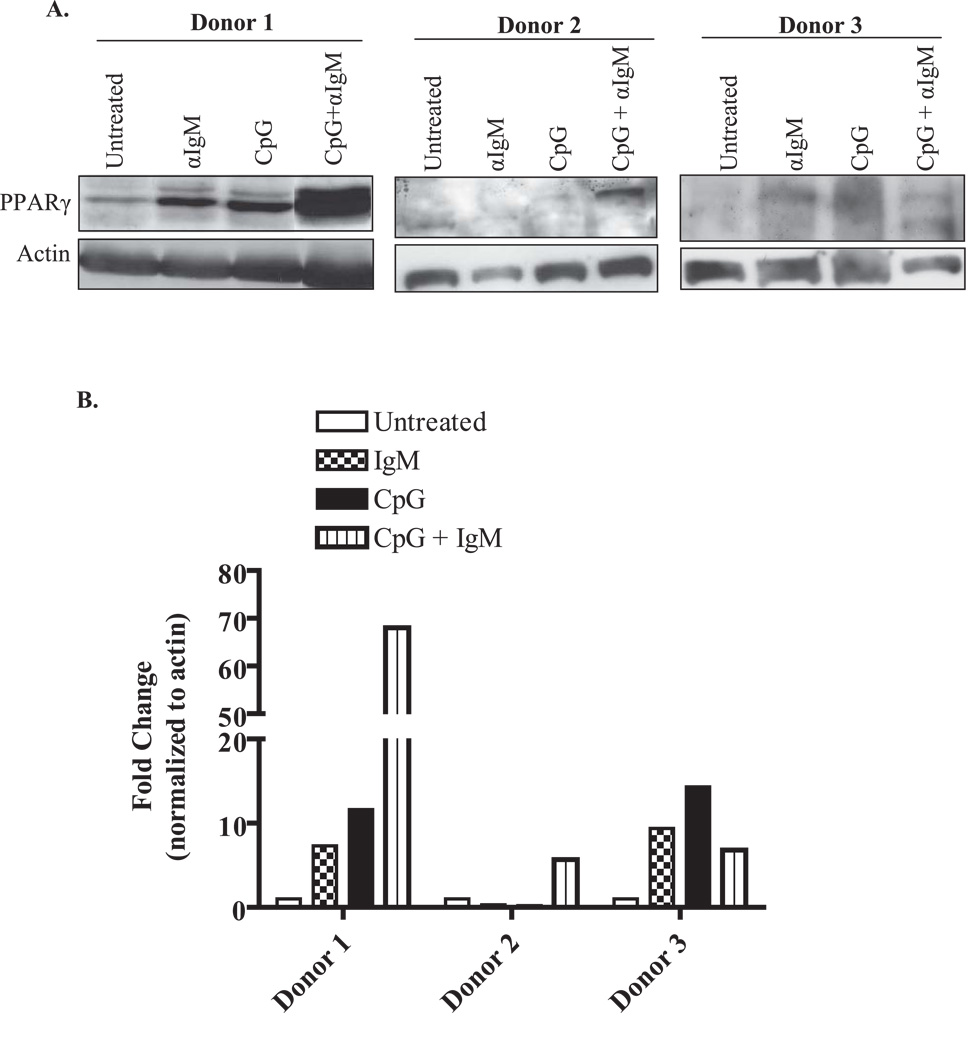
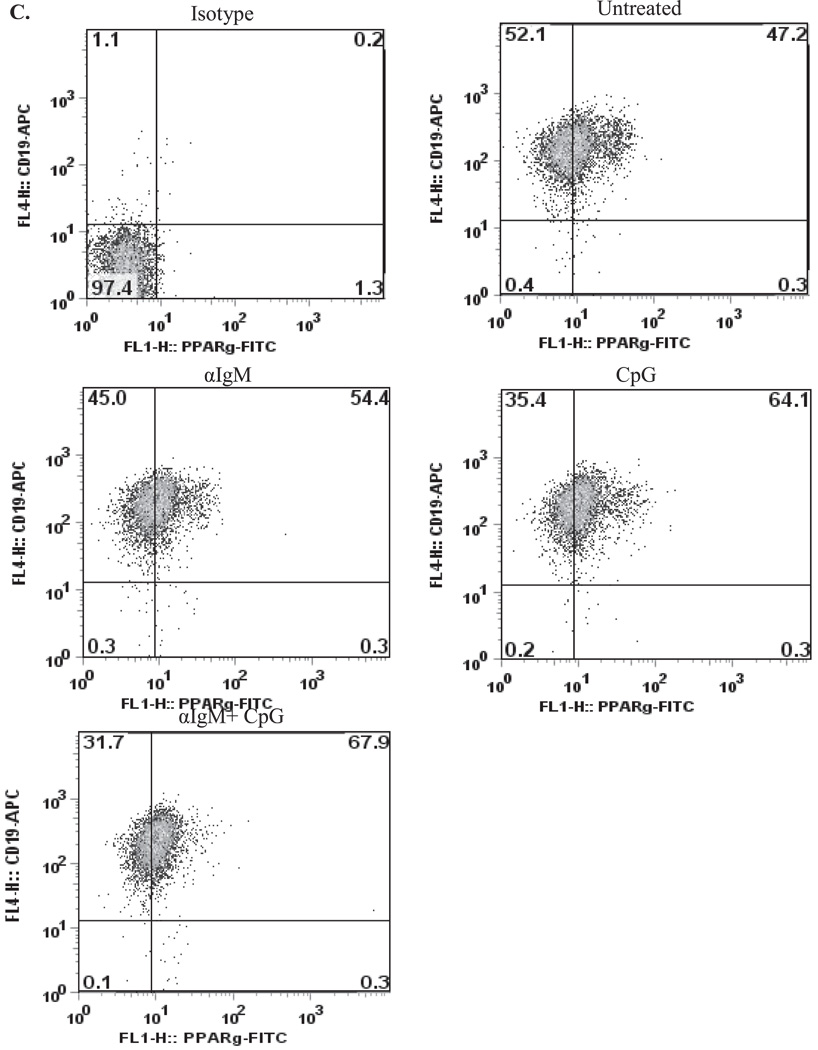
We previously showed that freshly isolated normal human B cells express low levels of PPARγ protein (8). However, it is unknown if PPARγ expression changes after provocation with stimulatory agents. Therefore, normal peripheral blood B cells were left untreated or activated with unmethylated CpG DNA (a TLR-9 ligand) (23), with or without anti-IgM, and PPARγ expression examined by Western blot. PPARγ is expressed in human B cells (≈ molecular weight is 54 kDa), with PPARγ levels in untreated B cells being variable (undetectable to low) between three individual donors (Figure 1A). However, PPARγ expression was increased in B cells that were activated with anti-IgM or CpG or a combination of anti-IgM plus CpG (Figure 1, Donors 1–3). Densitometric analysis demonstrates that the range of induction in PPARγ protein levels in activated B cells after 48 hours was between 10- and 70-fold (Figure 1B). We also performed intracellular staining for PPARγ in activated B cells. Here, treatment with anti-IgM, CpG or anti-IgM increased intracellular PPARγ levels compared to B cells that were untreated (Figure 1C). Collectively, these results indicate that PPARγ expression is increased by agents that trigger B cell activation and differentiation.
Figure 1. PPARγ expression is up-regulated by B cell activation.
A. Highly purified human B lymphocytes isolated from peripheral blood were left untreated or were treated for 48 hr with 2 µg/ml anti-IgM Ab, 1 µg/ml of CpG DNA alone, or a combination of CpG plus anti-IgM. Western blots from three individual donors shows immunoreactivity of PPARγ in B cells. PPARγ expression was detectable in untreated cells, with inter-individual variability in expression noted. Upon B cell stimulation, there was an increase in PPARγ expression in all three donors, with Donor 1 exhibiting the greatest increase in protein expression. Total actin expression was used as a protein-loading control. B. Densitometry of the Western blot for all three human B cell donors shows that PPARγ protein levels increase up to 9-fold for anti-IgM, 14.3-fold for CpG and 70-fold with CpG+anti-IgM compared to untreated B cells. C. Flow cytometric analysis of intracellular PPARγ expression confirmes that the level of PPARγ increases upon B cell activation (from ≈ 47% in untreated B cells to 68% in B cells activated with anti-IgM+CpG).
Normal B cell proliferation and antibody production is enhanced by PPARγ ligands
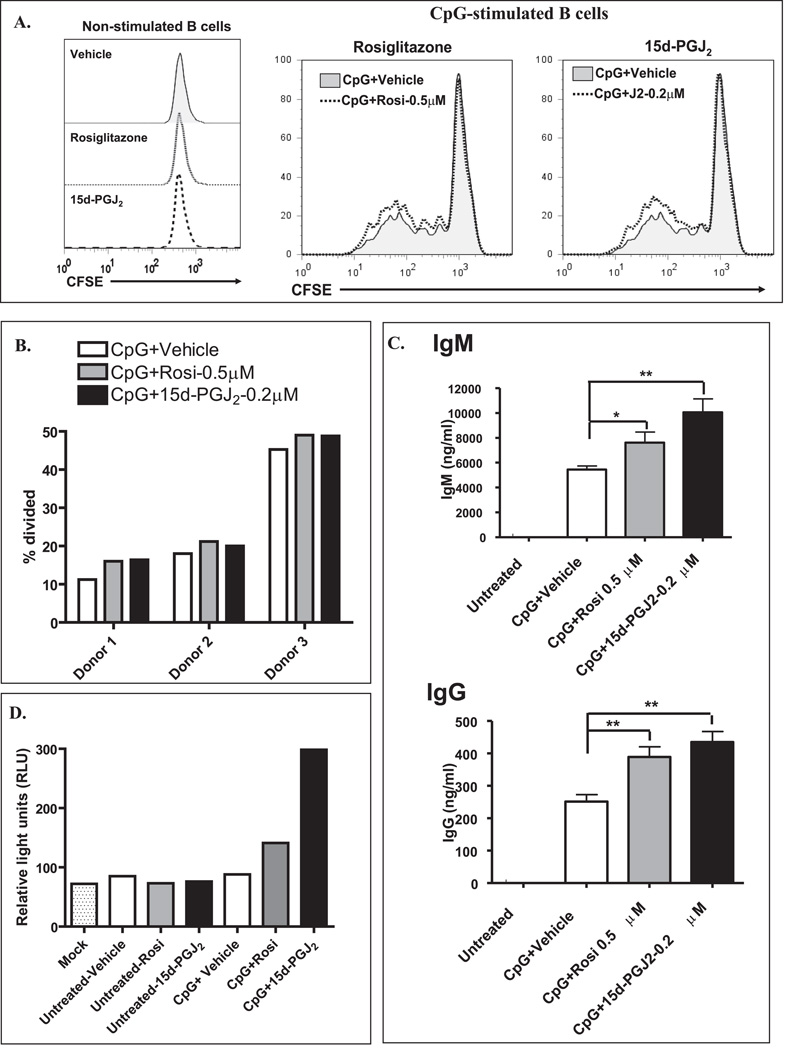
The natural PPARγ ligand 15d-PGJ2 is derived from its precursor, PGD2, by a series of dehydration steps (24). Physiological concentrations of 15d-PGJ2 are estimated to reach at least nanomolar concentrations (25). Additionally, therapeutic blood levels of the synthetic PPARγ ligand Rosiglitazone reach low micromolar levels (26). To examine the role of PPARγ in B cell function, we first examined the effects of physiologically relevant concentrations of Rosiglitazone and 15d-PGJ2 on B cell proliferation. B cells were labeled with the cell-division-tracking dye CFSE and activated for 5 days with CpG in the presence or absence of Rosiglitazone (0.5 µM) or 15d-PGJ2 (0.2 µM). These doses were chosen based on pilot experiments; these concentrations did not adversely affect cell viability (based on 7-AAD incorporation, cell size and 3H-thymidine incorporation; data not shown). Five days after activation, cells were analyzed by flow cytometry. Non-activated B cells treated with PPARγ ligands did not proliferate (Figure 2A, left panel). However, activated B cells incubated with either Rosiglitazone (0.5 µM) or 15d-PGJ2 (0.2 µM) increased cell division (Figure 2A, dotted histograms) compared to vehicle control (Figure 2A, shaded histograms). Figure 2B illustrates the percent of cell division for three different B cell donors. A similar trend was observed with all three donors (Figure 2B), where there was an increase in the percent of cell division (≈ 8 to 40%) in activated B cells treated with PPARγ ligands compared to vehicle control. These results indicate that low doses of PPARγ ligands enhance B cell proliferation.
Figure 2. Normal B cell proliferation and antibody production is enhanced by PPARγ ligands.
A. Purified human B cells (0.5×106 cells/ml) were labeled with CFSE and were left untreated (non-stimulated B cells) or were cultured with CpG (1 µg/ml) with or without Rosiglitazone (0.5 µM) or 15d-PGJ2 (0.2 µM) (CpG-stimulated B cells). Cell division was analyzed by flow cytometry at day 5. A total of 25,000 events were collected for each sample and the data were gated on the live cell population based on forward and side-scatter. The results are representative of three separate experiments. B. The percent cell division for three separate donors is shown. Note that a similar trend was observed with all three donors; PPARγ ligands increased the percentage of cell division from 8–40%. C. Purified B cells were stimulated with CpG (1 µg/ml) for 5 days in the presence and absence of 0.5 µM Rosiglitazone or 0.2 µM of 15d-PGJ2 and IgM and IgG levels were analyzed by ELISA. Vehicle (DMSO) was included as a negative control. Low doses of both PPARγ ligands significantly induced both IgM and IgG levels. D. Purified human B cells were transfected as described in Materials and Methods with a PPRE-Luciferase construct. Eighteen hours post-transfection, cells were treated with PPARγ ligands in the presence or absence of CpG (1 µg/ml). Twenty-four after treatments, cells were lysed and a luciferase assay was performed. CpG-activated B cells showed increased luciferase activity upon PPARγ ligand treatment.
Next, we evaluated whether activation of PPARγ influenced the differentiation of B cells into antibody-secreting plasma cells. Figure 2C shows that CpG significantly induced both IgM and IgG production. Moreover, both Rosiglitazone (0.5 µM) and 15d-PGJ2 (0.2 µM) further enhanced IgM and IgG production, by up to 2-fold, over CpG alone (Figure 2C). Thus, activation of PPARγ significantly increases antibody production.
To test whether the PPARγ ligand concentrations used here activated PPARγ, normal B cells were transfected with a PPARγ luciferase reporter construct. Eighteen hours post-transfection, cells were either treated with Rosiglitazone (0.5 µM) or with 15d-PGJ2 (0.2 µM) in the presence or absence of CpG. Non-activated B cells did not increase luciferase activity when treated with PPARγ ligands (Figure 2D). CpG-activated B cells with no exogenous PPARγ ligand also did not induce PPARγ activity. However, both Rosiglitazone and 15d-PGJ2 increased luciferase activity, indicating activation of PPARγ (Figure 2D). Therefore, activated B cells, which have higher PPARγ levels, can respond to PPARγ ligands, while non-activated B cells, with low PPARγ expression, were not able to activate PPARγ upon low dose PPARγ ligand exposure.
PPARγ ligands and 9-cis-RA enhance CpG-induced B cell proliferation
PPARγ forms a heterodimer with the 9-cis-retinoic acid receptor, RXRα (27). We hypothesized that 9-cis-RA, in conjunction with PPARγ activation, would enhance B cell proliferation. Normal B cells activated with CpG were treated with vehicle, Rosiglitazone (Rosi) or 15d-PGJ2 with or without 9-cis-RA (9-RA) and proliferation was measured at 5 days post-activation, using CFSE labeling (Figure 3). Five days post-CpG-activation, cells that were treated with PPARγ ligands alone or 9-cis-RA alone increased the percentage of dividing cells (Figure 3). Moreover, results from three separate donors indicate that there was a 2–3-fold increase in the percentage of cells dividing with 9-cis-RA plus Rosiglitazone or 15d-PGJ2 (Figure 3). These results show that PPARγ ligands and RXRα ligands enhance B cell proliferation.
Figure 3. PPARγ ligands enhance 9-cis-RA induced B cell proliferation.
Human B cells were stimulated with CpG (1 µg/ml) and treated with vehicle or with PPARγ ligands (0.5 µM Rosiglitazone or 0.2 µM of 15d-PGJ2), 9-cis-RA (100 nM) alone or a combination of a PPARγ ligand plus 9-cis-RA for 5 days. CFSE results were expressed graphically as mean percent division at 5 days. Results from three donor preparations are shown.
PPARγ ligands enhance the ability of 9-cis-RA to induce plasma cell differentiation
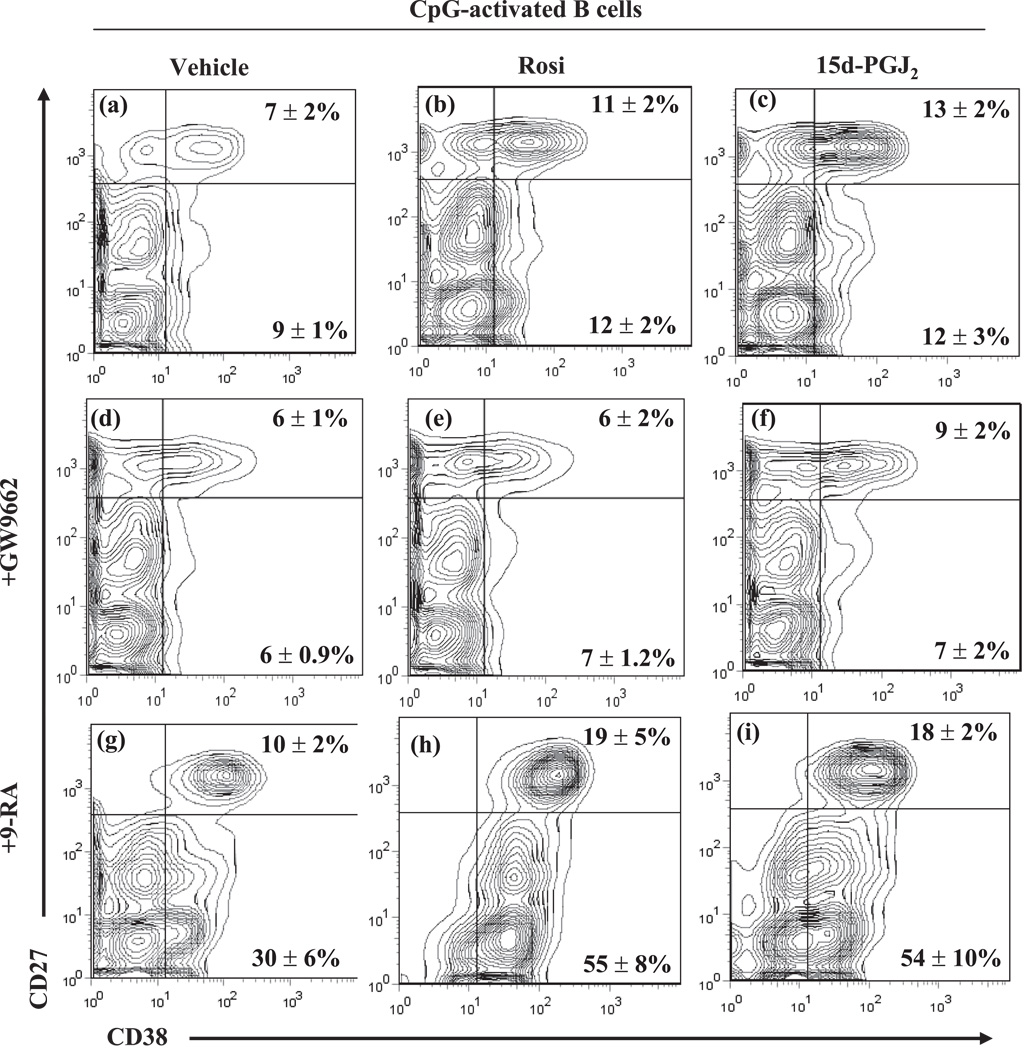
Peripheral-blood B lymphocytes include both naive and memory B cell populations. These two B cell subsets can be distinguished based on CD27 expression, which is a marker of memory B cells (28). Since CD38 upregulation is a marker of B cell differentiation (29, 30), we evaluated whether PPARγ ligands had an effect on CD38 surface expression in both naive (CD27−) and memory (CD27+) B cells. Non-stimulated B cells have no changes in differentiation markers upon PPARγ ligand treatment (data not shown). CpG treatment alone yielded 7.0 ± 1.7 % CD38highCD27high B cells, indicative of plasma cells (Figure 4a, see upper right quadrant). The percentage of CD38highCD27high cells increased to 10.7 ± .6 % with Rosiglitazone (Rosi, ~1.7 fold over vehicle) and to 12.5 ± 1.4 % with 15d-PGJ2 (~2 fold over vehicle) (Figure 4b and 4c). In contrast, PPARγ ligands had little effect on CD38 expression in naive (CD27−) B cells (Figure 4 a–c, see bottom right quadrant). This suggests that PPARγ ligands increase memory B cell differentiation to plasma cells.
Figure 4. PPARγ ligands enhance the ability of 9-cis-RA to induce plasma cell differentiation.
Peripheral blood B cells were treated with CpG (1 µg/ml) plus vehicle (a), Rosiglitazone at 0.5 µM (b), 15d-PGJ2 (0.2 µM) (c), GW9662 at (500 nM) alone (d) or in combination with Rosiglitazone (e) or 15d-PGJ2 (f). Some cells were treated with 9-cis-RA at a 100 nM (g–i) alone (g) or in combination with Rosiglitazone (h) or 15d-PGJ2 (i). The cells were harvested at 5 days and the frequency of cells with CD38highCD27high (Upper right quadrants) and CD38highCD27neg/low (Lower right quadrants) phenotype was determined. The values are representative of three separate experiments.
To assess whether the effects of the PPARγ ligands were PPARγ dependent, a widely used small molecule PPARγ irreversible antagonist, GW9662, was used. GW9662 covalently modifies the PPARγ ligand-binding site and acts as an irreversible antagonist (12, 31). The results indicate that PPARγ ligand-induced CD38 expression in memory B cells is attenuated with GW9662 (Figure 4d–f). Treatment with 9-cis-RA increased the percentage of CD38highCD27high to 10.3 ± 1.9 % (~1.7 fold vs. vehicle) and increased the percentage of CD38-expressing naive B cells (CD38highCD27low) from 9.1 ± 0.6% in CpG plus vehicle to 30.2 ± 5.5% in CpG plus 9-cis-RA (~3.5 fold vs. CpG plus vehicle) (Figure 4a and 4g, bottom right quadrant). Strikingly, the combination of PPARγ ligands plus 9-cis-RA further induced CD38 expression in both naive (bottom right quadrants) and memory (upper right quadrants) B cells by ~2 fold compared to 9-cis-RA alone (compare panel 4g with panels 4h and 4i). Thus, PPARγ ligands enhance B cell differentiation of CpG-stimulated memory B cells, but not naive B cells, in a PPARγ dependent manner. This suggests that activation of PPARγ/RXR heterodimers is a novel regulatory pathway for stimulating B cell differentiation.
PPARγ ligands act in concert with 9-cis-RA to enhance antibody production in CpG-stimulated B cells
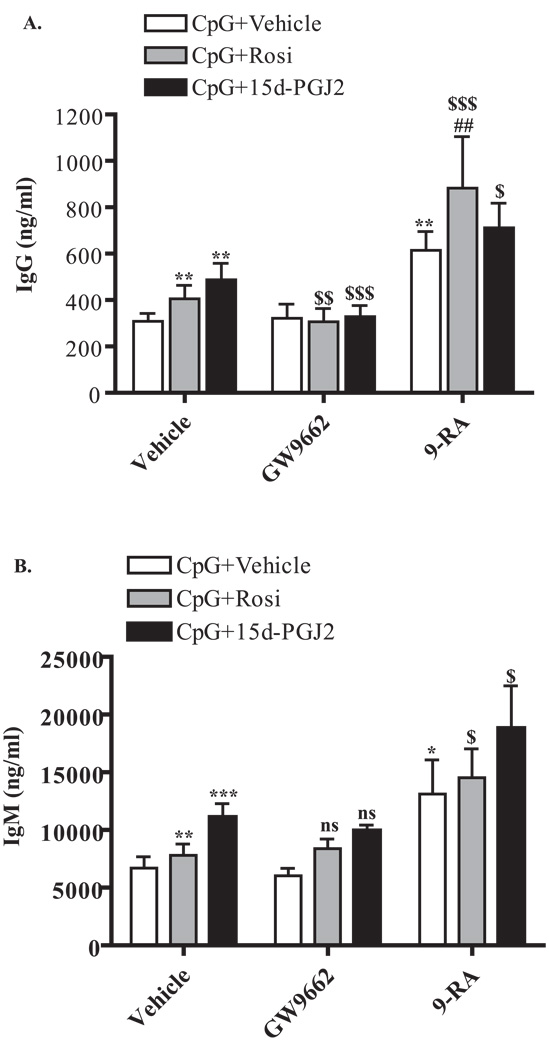
Since the effects of PPARγ ligands on B cell differentiation markers was PPARγ dependent (Figure 4), we next asked whether PPARγ ligand-induced antibody production was also PPARγ dependent. Again, PPARγ ligands significantly induced IgM and IgG production over vehicle control in CpG-activated cells (Figure 5A and 5B). Treatment with the PPARγ antagonist GW9662 abolished the effects of PPARγ ligands on IgG (Figure 5A), but not IgM, production (Figure 5B). This suggests that activation of PPARγ is necessary for PPARγ ligand-induced IgG, but not IgM, production.
Figure 5. PPARγ ligands and 9-cis-RA enhance antibody production.
Purified B cells were stimulated with CpG (1 µg/ml) for 6 days in the presence and absence of 0.5 µM Rosiglitazone or 0.2 µM 15d-PGJ2 and both IgG (A) and IgM (B) levels were analyzed by ELISA. Vehicle (DMSO) was added as a negative control (left bars). Some cells were also treated in the presence of the PPARγ antagonist GW9662 (500 nM, middle bars) or in the presence of 9-cis-RA (100 nM, right bars). PPARγ ligands significantly induced both IgM and IgG levels. GW9662 abrogated PPARγ ligand-induced IgG, but not IgM, levels. 9-cis-RA also induced both IgM and IgG levels, and when combined with PPARγ ligands, further enhanced IgM and IgG production. *p<0.05; **p< 0.01; ***p<0.001 vs. vehicle treated. $, p<0.05; $$, p<0.01, $$$, p<0.001 and ns (non significance) vs. respective PPARγ ligand alone. ##, p<0.01 vs. 9-cis-RA.
We also asked whether PPARγ ligands, in combination with 9-cis-RA, would further enhance antibody production. CpG-activated B cells were treated with 9-cis-RA (9-RA) alone or in combination with Rosiglitazone (Rosi) or 15d-PGJ2. Treatment with 9-cis-RA significantly induced both IgM and IgG production (Figure 5). Addition of Rosiglitazone with 9-cis-RA significantly enhanced IgG (Figure 5A), but not IgM (Figure 5B), production compared to 9-cis-RA alone. However, when combined treatment (9-RA plus Rosi) was compared to Rosiglitazone alone, both IgM and IgG were significantly induced. Addition of 15d-PGJ2, together with 9-cis-RA, also resulted in a significant increase in both IgM and IgG production compared to15d-PGJ2 alone (Figure 5A and 5B). These results indicate that combining PPARγ and RXR ligands further enhances antibody production.
PPARγ ligands increase CpG-induced Cox-2 and BLIMP-1 expression
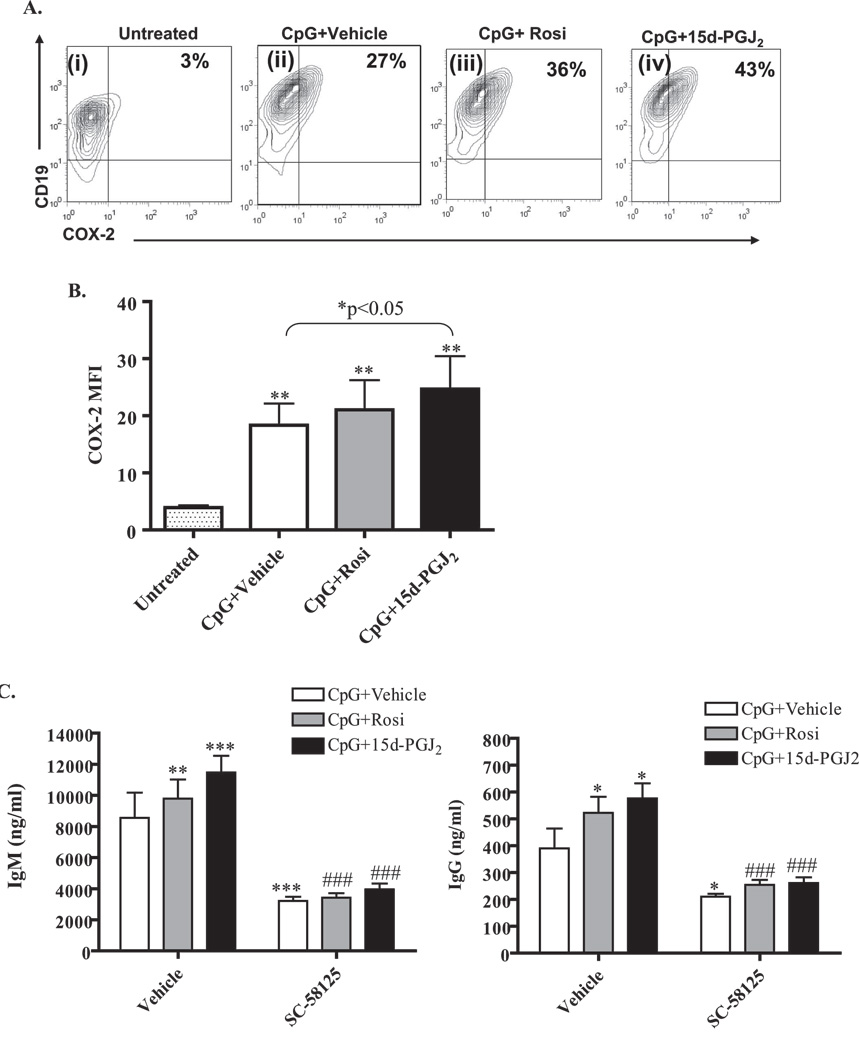
Our laboratory has demonstrated that CpG induces Cox-2 expression in B cells (32), which is important for B cell differentiation (20, 32). Therefore, we evaluated the levels of Cox-2 expression following PPARγ ligand treatment. Non-activated B cells treated with PPARγ ligands showed no increase in Cox-2 expression (data not shown). However, CpG activation increased the percentage of B cells, which express Cox-2, from 3% (untreated) to 27% (Figure 6A, compare panel ii with panel i). The percentage of Cox-2 positive B cells was further increased by Rosiglitazone (36%) and by 15d-PGJ2 (43%) (Figure 6A, panels iii and iv, upper right quadrants). Figure 6B shows the mean fluorescence intensity (MFI), indicative of the intensity of Cox-2 expression. Both CpG and PPARγ ligand treatment increased the levels of Cox-2 expression. These results further confirm that PPARγ stimulates Cox-2 expression in activated B cells. To determine whether the PPARγ-induced Cox-2 expression was responsible for increased antibody production, normal B cells were treated with PPARγ ligands in the presence or absence of the Cox-2 selective inhibitor SC-58125. We have previously shown that Cox-2 inhibitors reduce CpG-induced IgM and IgG production (32). Addition of SC-58125 to CpG-stimulated B cells significantly reduced IgM and IgG production (Figure 6C, open bars). Moreover, the increased antibody production upon PPARγ ligand treatment was also significantly reduced by the addition of SC-58125 (Figure 6C). These results suggest that PPARγ ligand-induced Cox-2 expression is at least partially responsible for the increase in antibody production.
Figure 6. PPARγ ligands increase CpG-induced COX-2 and BLIMP-1 expression.
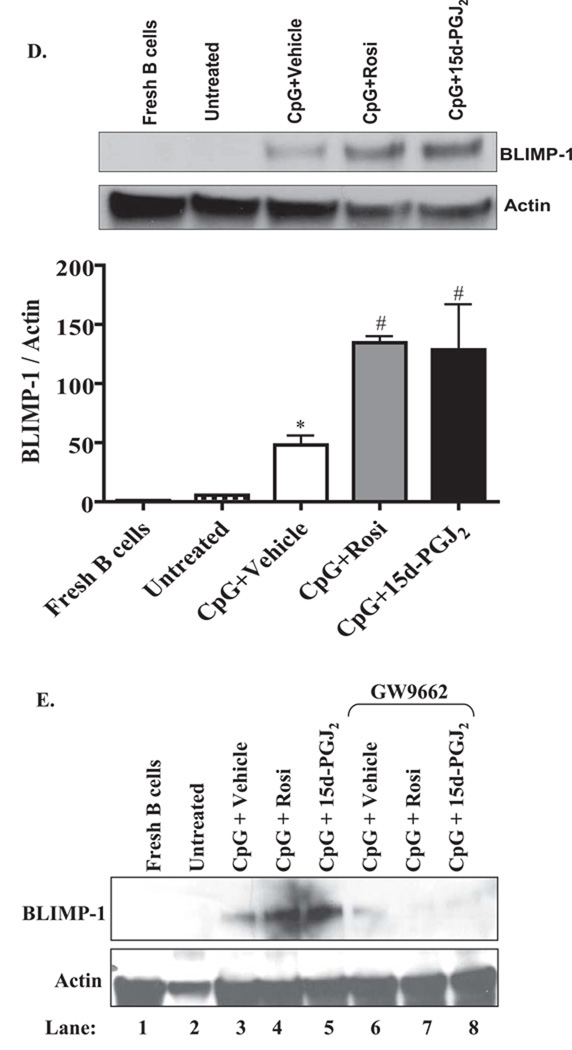
A. Purified B cells were either left untreated (panel i), or were treated with 1µg/ml of CpG and vehicle (panels ii and v), 0.5 µM of Rosiglitazone (panels iii and vi) or 0.2 µM of 15d-PGJ2 (panels iv and vii). Flow cytometry analysis of purified B cells shows that the percentage of CD19+ B cells expressing Cox-2 protein (upper right quadrants) was induced upon activation (27% on CpG+Vehicle vs. 3% on untreated). Cells treated with Rosiglitazone or 15d-PGJ2 further increased the percentage of Cox-2 positive cells (36% and 43%, respectively, compared to 27% of CpG+vehicle control). B. Results are expressed as Cox-2 mean fluorescence intensity (MFIs).**p<0.01 versus untreated; C. Purified B cells were stimulated with CpG (1 µg/ml) for 6 days in the presence and absence of 0.5 µM Rosiglitazone or 0.2 µM 15d-PGJ2 and both IgM and IgG levels were analyzed by ELISA. Vehicle (DMSO) was added as a negative control (left bars). Some cells were also treated in the presence of the Cox-2 selective inhibitor SC-58125 at a concentration of 10 µM (right bars). PPARγ ligands significantly induced both IgM and IgG levels. SC-58125 abrogated PPARγ ligand-induced IgG and IgM levels. *p<0.05, **, p< 0.01 and ***, p<0.001 vs. vehicle treated; ###p<0.001 vs. respective PPARγ ligand. D. Normal B cells were lysed immediately after isolation, were left untreated for 72 hr or were treated with CpG (1 µg/ml) alone or with PPARγ ligands for 72hrs. BLIMP-1 expression was analyzed by Western blot as indicated; representative western blot is shown. Total actin was used to normalize protein loading. BLIMP-1 levels were up-regulated upon CpG activation and PPARγ ligands further increased CpG-induced BLIMP-1 expression. Unstimulated B cells treated with PPARγ ligands had no effect on BLIMP-1 expression (data not shown). Graph: Densitometry of the Western blots show that the CpG-activated B cells increased BLIMP-1 protein levels. Treatment with either Rosiglitazone (Rosi) or 15d-PGJ2 significantly increased BLIMP-1 expression compared to CpG (*p<0.05). E. GW9662 attenuates BLIMP-1 protein expression. Expression of BLIMP-1 was assessed by western blot in B cells that were freshly isolated, untreated, or were activated by CpG in conjunction with Rosiglitazone (Rosi; 0.5 µM) or 15d-PGJ2 (0.2 µM); some cells were also exposed to the PPARγ antagonist GW9662 (500 nM). Treatment with GW9662 reduced BLIMP-1 expression in B cells that were treated with CpG+Vehicle, as well as those treated with Rosiglitazone or 15d-PGJ2.
Last, we evaluated BLIMP-1 expression, a transcription factor important in B cell differentiation (33). BLIMP-1 protein levels were significantly upregulated in response to CpG treatment in normal B cells compared to untreated or freshly isolated B cells (Figure 6D and 6E). When B cells were treated with a combination of CpG and PPARγ ligands, there was a further increase in BLIMP-1 expression. Densitometric analysis shows an induction of ~6-fold and ~9-fold with CpG plus Rosiglitazone and CpG plus 15d-PGJ2 treatment, respectively, over CpG-treated cells (Figure 6D).
Finally, we assessed if the increase in BLIMP-1 by Rosiglitazone and 15d-PGJ2 was PPARγ-dependent. In B cells treated with CpG and GW9662, there was a decrease in the expression of BLIMP-1 compared to CpG alone (Figure 6E, compare Lanes 3 and 6). The increase in BLIMP-1 by treatment of CpG-activated B cells with Rosiglitazone and 15d-PGJ2 was dramatically attenuated by GW9662 (Figure 6E, compare Lanes 4 and 5 with Lanes 7 and 8). Collectively, these results support our hypothesis that PPARγ ligands enhance B cell differentiation.
Discussion
The differentiation of B lymphocytes into antibody-producing plasma cells is necessary for protection against invading microorganisms and for successful vaccination. Augmenting antibody responses not only could improve normal humoral immune responses, but could also improve the outcome of patients with immune deficiencies or those who are immunosuppressed, elderly or very young. In this study, we present new evidence that PPARγ is a novel regulator of B cell differentiation and antibody production. We demonstrate that PPARγ levels increase in B cells upon TLR-9 activation and BCR cross-linking. Since these mitogenic stimuli induce B cell differentiation, our results suggest that PPARγ plays an important role in B cell function. Moreover, physiological (nM) doses of PPARγ ligands alone or in combination with RXRα ligands accelerated the differentiation of B cells into plasma cells and increased immunoglobulin synthesis. This supports the concept that, in normal B cells, PPARγ activation is an important pathway that can be exploited to boost humoral immune responses.
Certain PPARγ ligands are recognized as having anti-inflammatory properties and can be anti-proliferative agents in immune cells (34). In most studies, including our own (8–10, 35, 36), the effects of PPARγ ligands have been studied at high micromolar concentrations, at which PPARγ-independent effects can be observed (10), especially with electrophilic PPARγ ligands such as 15d-PGJ2. Herein, we demonstrated that nanomolar concentrations of both an endogenous PPARγ ligand (15d-PGJ2) and a synthetic ligand (Rosiglitazone) enhance B cell proliferation and immunoglobulin production. Many of the effects observed on B cell differentiation at nanomolar concentrations of 15d-PGJ2 and Rosiglitazone were reversible upon treatment with a highly specific PPARγ antagonist, GW9662 (31) (Figure 4, Figure 5 and Figure 6). These observations agree with findings on non-immune cells, such as epithelial cells, where nanomolar concentrations of PPARγ ligands increase cell proliferation in a PPARγ-dependent manner, whereas (high) micromolar concentrations inhibit proliferation in a PPARγ-independent manner (37–39). Additionally, the ability of a cell to respond to PPARγ ligands may be a direct reflection of the level of PPARγ protein expression. In the present study, we demonstrated that PPARγ levels increase upon B cell activation. These results mirror our previous studies on T cells, where PPARγ levels increased upon T cell activation (40). PPARγ expression also increases during the differentiation of monocytes to macrophages and PPARγ /RXR signaling induces macrophage differentiation (41, 42). This increase in PPARγ expression may help normal B cells respond to endogenous PPARγ ligands (e.g. 15d-PGJ2). Indeed, we did not observe any change in B cell function in non-activated B cells (i.e. those with low PPARγ expression) that were exposed to PPARγ ligands (data not shown). Taken together, these results indicate that physiologically relevant concentrations of PPARγ ligands induce differentiation of B lymphocytes through a PPARγ-dependent process.
The transcriptional actions of PPARγ depend on its dimerization partner RXR. Peripheral blood B lymphocytes express RXRα (43). Vitamin A is important for optimal humoral immune responses (44–49). Treatment of peripheral blood B cells with the vitamin A metabolite all-trans-retinoic acid (ATRA) induces CD38 expression and increases antibody production (45). Although ATRA does induce B cell differentiation, it only binds to the retinoid acid receptor (RAR). In contrast, 9-cis-RA, a vitamin A metabolite, is a ligand for both RAR and RXR (19, 50). RXR can heterodimerize with other receptors, including RAR (51). Thus, the ability of 9-cis-RA to robustly increase antibody production (Figure 5), compared to PPARγ ligands alone, may be a reflection of its ability to activate both RAR and PPARγ signaling pathways.
Moreover, certain studies have shown synergistic effects with RXR and PPARγ ligands on cell differentiation (52, 53). Herein, we observed additive effects on B cell differentiation when PPARγ ligands were used in combination with 9-cis-RA (Figure 3, Figure 4 and Figure 5). The combined effect observed with PPARγ ligands and 9-cis-RA suggests that activation of the PPARγ/RXR pathway enhances B cell differentiation. TLR signals such as CpG are sufficient to induce BLIMP-1 expression (54). We found that PPARγ ligands enhanced CpG-induced BLIMP-1 expression by 6 to 9 fold over CpG alone (Figures 6D and 6E). This increase in BLIMP-1 was PPARγ-dependent, as GW9662 reduced BLIMP-1 expression in CpG-activated B cells that were treated with Rosiglitazone or 15d-PGJ2 (Figure 6E). Thus, BLIMP-1 induction may be due to a direct transcriptional regulation by PPARγ on BLIMP-1.
The ability of the PPARγ ligands Rosiglitazone or 15d-PGJ2 to regulate antibody production is partially PPARγ-dependent. This was demonstrated by the fact that the PPARγ antagonist GW9662 significantly decreased PPARγ ligand-induced IgG (Figure 5A) but not IgM (Figure 5B). This suggests that PPARγ may not regulate the primary immune response, in which IgM is the first Ig class produced but rather, may regulate the ability of B cells to class-switch. In addition, these ligands also increased CpG-induced Cox-2 expression (Figures 6A and 6B). We have previously published that Cox-2 is increased after B cell activation and its activity is crucial for optimal antibody production (20, 32, 55). This increase in Cox-2 may permit more B cells to differentiate to antibody-secreting cells. Indeed, the addition of a Cox-2 selective inhibitor attenuated IgM and IgG induction by PPARγ ligands (Figure 6C). Despite the fact that antibody production by PPARγ ligands is only partially PPARγ-dependent, our data clearly demonstrate that Cox-2 activity is essential for the enhanced antibody production elicited by Rosiglitazone or 15d-PGJ2. Thus, activation of PPARγ, in concert with Cox-2, may be a novel mechanism for regulating B cell differentiation and class switching during an immune response.
The ability of a B cell to mature, differentiate and produce antibody is a complex process and often involves cells within the periphery, particularly T cells. We and others have shown that PPARγ profoundly affects T cell function (56–58). It is interesting to note that a reduction in PPARγ expression increases T cell proliferation and skews toward Th1 immune response (59), which includes increased IFN-γ and IL-12 production (59, 60). These cytokines can directly influence B cell function, including plasma cell formation (61), proliferation (62) and antibody production (63). The alteration in T cell function caused by reduced PPARγ expression may account for the results obtained by Setoguchi and colleagues (60). Here, utilizing B cells derived from PPARγ haploinsufficient (PPARγ+/−) mice, where PPARγ expression is reduced by 50% (64), they demonstrated that this reduction in PPARγ expression resulted in enhanced B cell proliferation and serum IgG and IgM levels (60). In their study, it seemed that the loss of PPARγ in B cells, rather than its activation (as described herein in Figure 2, Figure 5 and Figure 6) exerts control over B cell function, particularly antibody production. However, the contribution of reduced PPARγ expression in mouse T cells (and other antigen-presenting cells) could not be excluded. It is possible that, in the PPARγ+/− mice, T cell activation (caused by reduced PPARγ expression), and subsequent interaction with primed B cells, accounts for the heightened B cell proliferation and antibody production observed in the PPARγ+/− mice.
B cells are a critical component of both innate and adaptive immunity. Activation and subsequent differentiation of B cells in response to antigenic challenge is required for successful clearance of a pathogen. Our new findings show that activation of normal human B cells increases PPARγ protein levels, and that PPARγ activation increases cell differentiation. The concomitant use of PPARγ ligands plus 9-cis-RA greatly enhances B cell differentiation. Up-regulation of PPARγ, together with its activation by prostaglandins and RXRα ligands, represent a novel regulatory pathway for B cell differentiation. This new pathway could be exploited to enhance desirable antibody responses.
Acknowledgments
Grant support: This study was supported by DE011390, ES01247, a Hematology Training Grant NHLBI- T32HL007152 and the Training Program in Oral Sciences T32-DE007202. Carolyn J. Baglole was supported by a Parker B. Francis Fellowship.
References
- 1.Chiron D, Bekeredjian-Ding I, Pellat-Deceunynck C, Bataille R, Jego G. Toll-like receptors: lessons to learn from normal and malignant human B cells. Blood. 2008;112:2205–2213. doi: 10.1182/blood-2008-02-140673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Klein U, Dalla-Favera R. Germinal centres: role in B-cell physiology and malignancy. Nat Rev Immunol. 2008;8:22–33. doi: 10.1038/nri2217. [DOI] [PubMed] [Google Scholar]
- 3.LeBien TW, Tedder TF. B lymphocytes: how they develop and function. Blood. 2008;112:1570–1580. doi: 10.1182/blood-2008-02-078071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Garcia-Bates TM, Peslak SA, Baglole CJ, Maggirwar SB, Bernstein SH, Phipps RP. Peroxisome proliferator-activated receptor gamma overexpression and knockdown: impact on human B cell lymphoma proliferation and survival. Cancer Immunol Immunother. 2008 doi: 10.1007/s00262-008-0625-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Braissant O, Foufelle F, Scotto C, Dauca M, Wahli W. Differential expression of peroxisome proliferator-activated receptors (PPARs): tissue distribution of PPAR-alpha, -beta, and -gamma in the adult rat. Endocrinology. 1996;137:354–366. doi: 10.1210/endo.137.1.8536636. [DOI] [PubMed] [Google Scholar]
- 6.Glass CK, Ogawa S. Combinatorial roles of nuclear receptors in inflammation and immunity. Nat Rev Immunol. 2006;6:44–55. doi: 10.1038/nri1748. [DOI] [PubMed] [Google Scholar]
- 7.Wang T, Xu J, Yu X, Yang R, Han ZC. Peroxisome proliferator-activated receptor gamma in malignant diseases. Crit Rev Oncol Hematol. 2006;58:1–14. doi: 10.1016/j.critrevonc.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 8.Padilla J, Leung E, Phipps RP. Human B lymphocytes and B lymphomas express PPAR-gamma and are killed by PPAR-gamma agonists. Clin Immunol. 2002;103:22–33. doi: 10.1006/clim.2001.5181. [DOI] [PubMed] [Google Scholar]
- 9.Ray DM, Akbiyik F, Bernstein SH, Phipps RP. CD40 engagement prevents peroxisome proliferator-activated receptor gamma agonist-induced apoptosis of B lymphocytes and B lymphoma cells by an NF-kappaB-dependent mechanism. J Immunol. 2005;174:4060–4069. doi: 10.4049/jimmunol.174.7.4060. [DOI] [PubMed] [Google Scholar]
- 10.Ray DM, Akbiyik F, Phipps RP. The peroxisome proliferator-activated receptor gamma (PPARgamma) ligands 15-deoxy-Delta12,14-prostaglandin J2 and ciglitazone induce human B lymphocyte and B cell lymphoma apoptosis by PPARgamma-independent mechanisms. J Immunol. 2006;177:5068–5076. doi: 10.4049/jimmunol.177.8.5068. [DOI] [PubMed] [Google Scholar]
- 11.Fitzpatrick FA, Wynalda MA. Albumin-catalyzed metabolism of prostaglandin D2. Identification of products formed in vitro. J Biol Chem. 1983;258:11713–11718. [PubMed] [Google Scholar]
- 12.Feldon SE, W O'Loughlin C, Ray DM, Landskroner-Eiger S, Seweryniak KE, Phipps RP. Activated human T lymphocytes express cyclooxygenase-2 and produce proadipogenic prostaglandins that drive human orbital fibroblast differentiation to adipocytes. Am J Pathol. 2006;169:1183–1193. doi: 10.2353/ajpath.2006.060434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Forman BM, Tontonoz P, Chen J, Brun RP, Spiegelman BM, Evans RM. 15-Deoxy-delta 12, 14-prostaglandin J2 is a ligand for the adipocyte determination factor PPAR gamma. Cell. 1995;83:803–812. doi: 10.1016/0092-8674(95)90193-0. [DOI] [PubMed] [Google Scholar]
- 14.Kliewer SA, Lenhard JM, Willson TM, Patel I, Morris DC, Lehmann JM. A prostaglandin J2 metabolite binds peroxisome proliferator-activated receptor gamma and promotes adipocyte differentiation. Cell. 1995;83:813–819. doi: 10.1016/0092-8674(95)90194-9. [DOI] [PubMed] [Google Scholar]
- 15.Soderstrom M, Wigren J, Surapureddi S, Glass CK, Hammarstrom S. Novel prostaglandin D(2)-derived activators of peroxisome proliferator-activated receptor-gamma are formed in macrophage cell cultures. Biochim Biophys Acta. 2003;1631:35–41. doi: 10.1016/s1388-1981(02)00322-0. [DOI] [PubMed] [Google Scholar]
- 16.Kim J, Yang P, Suraokar M, Sabichi AL, Llansa ND, Mendoza G, Subbarayan V, Logothetis CJ, Newman RA, Lippman SM, Menter DG. Suppression of prostate tumor cell growth by stromal cell prostaglandin D synthase-derived products. Cancer Res. 2005;65:6189–6198. doi: 10.1158/0008-5472.CAN-04-4439. [DOI] [PubMed] [Google Scholar]
- 17.Fukushima M. Biological activities and mechanisms of action of PGJ2 and related compounds: an update. Prostaglandins Leukot Essent Fatty Acids. 1992;47:1–12. doi: 10.1016/0952-3278(92)90178-l. [DOI] [PubMed] [Google Scholar]
- 18.Issemann I, Prince RA, Tugwood JD, Green S. The retinoid X receptor enhances the function of the peroxisome proliferator activated receptor. Biochimie. 1993;75:251–256. doi: 10.1016/0300-9084(93)90084-6. [DOI] [PubMed] [Google Scholar]
- 19.Mangelsdorf DJ, Borgmeyer U, Heyman RA, Zhou JY, Ong ES, Oro AE, Kakizuka A, Evans RM. Characterization of three RXR genes that mediate the action of 9-cis retinoic acid. Genes Dev. 1992;6:329–344. doi: 10.1101/gad.6.3.329. [DOI] [PubMed] [Google Scholar]
- 20.Ryan EP, Pollock SJ, Murant TI, Bernstein SH, Felgar RE, Phipps RP. Activated human B lymphocytes express cyclooxygenase-2 and cyclooxygenase inhibitors attenuate antibody production. J Immunol. 2005;174:2619–2626. doi: 10.4049/jimmunol.174.5.2619. [DOI] [PubMed] [Google Scholar]
- 21.Burgess HA, Daugherty LE, Thatcher TH, Lakatos HF, Ray DM, Redonnet M, Phipps RP, Sime PJ. PPARgamma agonists inhibit TGF-beta induced pulmonary myofibroblast differentiation and collagen production: implications for therapy of lung fibrosis. Am J Physiol Lung Cell Mol Physiol. 2005;288:L1146–L1153. doi: 10.1152/ajplung.00383.2004. [DOI] [PubMed] [Google Scholar]
- 22.Jiang C, Ting AT, Seed B. PPAR-gamma agonists inhibit production of monocyte inflammatory cytokines. Nature. 1998;391:82–86. doi: 10.1038/34184. [DOI] [PubMed] [Google Scholar]
- 23.Huggins J, Pellegrin T, Felgar RE, Wei C, Brown M, Zheng B, Milner EC, Bernstein SH, Sanz I, Zand MS. CpG DNA activation and plasma-cell differentiation of CD27- naive human B cells. Blood. 2007;109:1611–1619. doi: 10.1182/blood-2006-03-008441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shibata T, Kondo M, Osawa T, Shibata N, Kobayashi M, Uchida K. 15-deoxy-delta 12,14-prostaglandin J2. A prostaglandin D2 metabolite generated during inflammatory processes. J Biol Chem. 2002;277:10459–10466. doi: 10.1074/jbc.M110314200. [DOI] [PubMed] [Google Scholar]
- 25.Kobayashi Y, Ueki S, Mahemuti G, Chiba T, Oyamada H, Saito N, Kanda A, Kayaba H, Chihara J. Physiological levels of 15-deoxy-Delta12,14-prostaglandin J2 prime eotaxin-induced chemotaxis on human eosinophils through peroxisome proliferator-activated receptor-gamma ligation. J Immunol. 2005;175:5744–5750. doi: 10.4049/jimmunol.175.9.5744. [DOI] [PubMed] [Google Scholar]
- 26.Cox PJ, Ryan DA, Hollis FJ, Harris AM, Miller AK, Vousden M, Cowley H. Absorption, disposition, and metabolism of rosiglitazone, a potent thiazolidinedione insulin sensitizer, in humans. Drug Metab Dispos. 2000;28:772–780. [PubMed] [Google Scholar]
- 27.Berger J, Moller DE. The mechanisms of action of PPARs. Annu Rev Med. 2002;53:409–435. doi: 10.1146/annurev.med.53.082901.104018. [DOI] [PubMed] [Google Scholar]
- 28.Agematsu K, Hokibara S, Nagumo H, Komiyama A. CD27: a memory B-cell marker. Immunol Today. 2000;21:204–206. doi: 10.1016/s0167-5699(00)01605-4. [DOI] [PubMed] [Google Scholar]
- 29.Arpin C, Dechanet J, Van Kooten C, Merville P, Grouard G, Briere F, Banchereau J, Liu YJ. Generation of memory B cells and plasma cells in vitro. Science. 1995;268:720–722. doi: 10.1126/science.7537388. [DOI] [PubMed] [Google Scholar]
- 30.Campana D, Suzuki T, Todisco E, Kitanaka A. CD38 in hematopoiesis. Chem Immunol. 2000;75:169–188. doi: 10.1159/000058768. [DOI] [PubMed] [Google Scholar]
- 31.Leesnitzer LM, Parks DJ, Bledsoe RK, Cobb JE, Collins JL, Consler TG, Davis RG, Hull-Ryde EA, Lenhard JM, Patel L, Plunket KD, Shenk JL, Stimmel JB, Therapontos C, Willson TM, Blanchard SG. Functional consequences of cysteine modification in the ligand binding sites of peroxisome proliferator activated receptors by GW9662. Biochemistry. 2002;41:6640–6650. doi: 10.1021/bi0159581. [DOI] [PubMed] [Google Scholar]
- 32.Bernard MP, Phipps RP. CpG oligodeoxynucleotides induce cyclooxygenase-2 in human B lymphocytes: implications for adjuvant activity and antibody production. Clin Immunol. 2007;125:138–148. doi: 10.1016/j.clim.2007.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shaffer AL, Lin KI, Kuo TC, Yu X, Hurt EM, Rosenwald A, Giltnane JM, Yang L, Zhao H, Calame K, Staudt LM. Blimp-1 orchestrates plasma cell differentiation by extinguishing the mature B cell gene expression program. Immunity. 2002;17:51–62. doi: 10.1016/s1074-7613(02)00335-7. [DOI] [PubMed] [Google Scholar]
- 34.Straus DS, Glass CK. Anti-inflammatory actions of PPAR ligands: new insights on cellular and molecular mechanisms. Trends Immunol. 2007;28:551–558. doi: 10.1016/j.it.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 35.Padilla J, Kaur K, Cao HJ, Smith TJ, Phipps RP. Peroxisome proliferator activator receptor-gamma agonists and 15-deoxy-Delta(12,14)(12,14)-PGJ(2) induce apoptosis in normal and malignant B-lineage cells. J Immunol. 2000;165:6941–6948. doi: 10.4049/jimmunol.165.12.6941. [DOI] [PubMed] [Google Scholar]
- 36.Ray DM, Bernstein SH, Phipps RP. Human multiple myeloma cells express peroxisome proliferator-activated receptor gamma and undergo apoptosis upon exposure to PPARgamma ligands. Clin Immunol. 2004;113:203–213. doi: 10.1016/j.clim.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 37.Emi M, Maeyama K. The biphasic effects of cyclopentenone prostaglandins, prostaglandin J(2) and 15-deoxy-Delta(12,14)-prostaglandin J(2) on proliferation and apoptosis in rat basophilic leukemia (RBL-2H3) cells. Biochem Pharmacol. 2004;67:1259–1267. doi: 10.1016/j.bcp.2003.10.037. [DOI] [PubMed] [Google Scholar]
- 38.Fukunaga Y, Itoh H, Doi K, Tanaka T, Yamashita J, Chun TH, Inoue M, Masatsugu K, Sawada N, Saito T, Hosoda K, Kook H, Ueda M, Nakao K. Thiazolidinediones, peroxisome proliferator-activated receptor gamma agonists, regulate endothelial cell growth and secretion of vasoactive peptides. Atherosclerosis. 2001;158:113–119. doi: 10.1016/s0021-9150(01)00430-0. [DOI] [PubMed] [Google Scholar]
- 39.Berry EB, Keelan JA, Helliwell RJ, Gilmour RS, Mitchell MD. Nanomolar and micromolar effects of 15-deoxy-delta 12,14-prostaglandin J2 on amnion-derived WISH epithelial cells: differential roles of peroxisome proliferator-activated receptors gamma and delta and nuclear factor kappa B. Mol Pharmacol. 2005;68:169–178. doi: 10.1124/mol.104.009449. [DOI] [PubMed] [Google Scholar]
- 40.Harris SG, Phipps RP. Prostaglandin D(2), its metabolite 15-d-PGJ(2), and peroxisome proliferator activated receptor-gamma agonists induce apoptosis in transformed, but not normal, human T lineage cells. Immunology. 2002;105:23–34. doi: 10.1046/j.0019-2805.2001.01340.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bouhlel MA, Derudas B, Rigamonti E, Dievart R, Brozek J, Haulon S, Zawadzki C, Jude B, Torpier G, Marx N, Staels B, Chinetti-Gbaguidi G. PPARgamma activation primes human monocytes into alternative M2 macrophages with anti-inflammatory properties. Cell Metab. 2007;6:137–143. doi: 10.1016/j.cmet.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 42.Tontonoz P, Nagy L, Alvarez JG, Thomazy VA, Evans RM. PPARgamma promotes monocyte/macrophage differentiation and uptake of oxidized LDL. Cell. 1998;93:241–252. doi: 10.1016/s0092-8674(00)81575-5. [DOI] [PubMed] [Google Scholar]
- 43.Buck J, Myc A, Garbe A, Cathomas G. Differences in the action and metabolism between retinol and retinoic acid in B lymphocytes. J Cell Biol. 1991;115:851–859. doi: 10.1083/jcb.115.3.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sherr E, Adelman DC, Saxon A, Gilly M, Wall R, Sidell N. Retinoic acid induces the differentiation of B cell hybridomas from patients with common variable immunodeficiency. J Exp Med. 1988;168:55–71. doi: 10.1084/jem.168.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Morikawa K, Nonaka M. All-trans-retinoic acid accelerates the differentiation of human B lymphocytes maturing into plasma cells. Int Immunopharmacol. 2005;5:1830–1838. doi: 10.1016/j.intimp.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 46.Ballow M, Xiang S, Wang W, Brodsky L. The effects of retinoic acid on immunoglobulin synthesis: role of interleukin 6. J Clin Immunol. 1996;16:171–179. doi: 10.1007/BF01540916. [DOI] [PubMed] [Google Scholar]
- 47.Aukrust P, Muller F, Ueland T, Svardal AM, Berge RK, Froland SS. Decreased vitamin A levels in common variable immunodeficiency: vitamin A supplementation in vivo enhances immunoglobulin production and downregulates inflammatory responses. Eur J Clin Invest. 2000;30:252–259. doi: 10.1046/j.1365-2362.2000.00619.x. [DOI] [PubMed] [Google Scholar]
- 48.Blomhoff HK, Smeland EB, Erikstein B, Rasmussen AM, Skrede B, Skjonsberg C, Blomhoff R. Vitamin A is a key regulator for cell growth, cytokine production, and differentiation in normal B cells. J Biol Chem. 1992;267:23988–23992. [PubMed] [Google Scholar]
- 49.Ertesvag A, Aasheim HC, Naderi S, Blomhoff HK. Vitamin A potentiates CpG-mediated memory B-cell proliferation and differentiation: involvement of early activation of p38MAPK. Blood. 2007;109:3865–3872. doi: 10.1182/blood-2006-09-046748. [DOI] [PubMed] [Google Scholar]
- 50.Bastien J, Rochette-Egly C. Nuclear retinoid receptors and the transcription of retinoid-target genes. Gene. 2004;328:1–16. doi: 10.1016/j.gene.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 51.Wolf G. Is 9-cis-retinoic acid the endogenous ligand for the retinoic acid-X receptor? Nutr Rev. 2006;64:532–538. doi: 10.1111/j.1753-4887.2006.tb00186.x. [DOI] [PubMed] [Google Scholar]
- 52.Kliewer SA, Umesono K, Noonan DJ, Heyman RA, Evans RM. Convergence of 9-cis retinoic acid and peroxisome proliferator signalling pathways through heterodimer formation of their receptors. Nature. 1992;358:771–774. doi: 10.1038/358771a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shimizu M, Moriwaki H. Synergistic Effects of PPARgamma Ligands and Retinoids in Cancer Treatment. PPAR Res. 2008;2008:181047. doi: 10.1155/2008/181047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Calame K. Activation-dependent induction of Blimp-1. Curr Opin Immunol. 2008;20:259–264. doi: 10.1016/j.coi.2008.04.010. [DOI] [PubMed] [Google Scholar]
- 55.Mongini PK. COX-2 expression in B lymphocytes: links to vaccines, inflammation and malignancy. Clin Immunol. 2007;125:117–119. doi: 10.1016/j.clim.2007.07.013. [DOI] [PubMed] [Google Scholar]
- 56.Harris SG, Phipps RP. The nuclear receptor PPAR gamma is expressed by mouse T lymphocytes and PPAR gamma agonists induce apoptosis. Eur J Immunol. 2001;31:1098–1105. doi: 10.1002/1521-4141(200104)31:4<1098::aid-immu1098>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 57.Harris SG, Phipps RP. Peroxisome proliferator-activated receptor gamma (PPAR-gamma) activation in naive mouse T cells induces cell death. Ann N Y Acad Sci. 2000;905:297–300. doi: 10.1111/j.1749-6632.2000.tb06565.x. [DOI] [PubMed] [Google Scholar]
- 58.Thompson PW, Bayliffe AI, Warren AP, Lamb JR. Interleukin-10 is upregulated by nanomolar rosiglitazone treatment of mature dendritic cells and human CD4+ T cells. Cytokine. 2007;39:184–191. doi: 10.1016/j.cyto.2007.07.191. [DOI] [PubMed] [Google Scholar]
- 59.Natarajan C, Muthian G, Barak Y, Evans RM, Bright JJ. Peroxisome proliferator-activated receptor-gamma-deficient heterozygous mice develop an exacerbated neural antigen-induced Th1 response and experimental allergic encephalomyelitis. J Immunol. 2003;171:5743–5750. doi: 10.4049/jimmunol.171.11.5743. [DOI] [PubMed] [Google Scholar]
- 60.Setoguchi K, Misaki Y, Terauchi Y, Yamauchi T, Kawahata K, Kadowaki T, Yamamoto K. Peroxisome proliferator-activated receptor-gamma haploinsufficiency enhances B cell proliferative responses and exacerbates experimentally induced arthritis. J Clin Invest. 2001;108:1667–1675. doi: 10.1172/JCI13202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vogel LA, Showe LC, Lester TL, McNutt RM, Van Cleave VH, Metzger DW. Direct binding of IL-12 to human and murine B lymphocytes. Int Immunol. 1996;8:1955–1962. doi: 10.1093/intimm/8.12.1955. [DOI] [PubMed] [Google Scholar]
- 62.Dubois B, Massacrier C, Vanbervliet B, Fayette J, Briere F, Banchereau J, Caux C. Critical role of IL-12 in dendritic cell-induced differentiation of naive B lymphocytes. J Immunol. 1998;161:2223–2231. [PubMed] [Google Scholar]
- 63.Estes DM, Tuo W, Brown WC, Goin J. Effects of type I/type II interferons and transforming growth factor-beta on B-cell differentiation and proliferation. Definition of costimulation and cytokine requirements for immunoglobulin synthesis and expression. Immunology. 1998;95:604–611. doi: 10.1046/j.1365-2567.1998.00645.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kubota N, Terauchi Y, Miki H, Tamemoto H, Yamauchi T, Komeda K, Satoh S, Nakano R, Ishii C, Sugiyama T, Eto K, Tsubamoto Y, Okuno A, Murakami K, Sekihara H, Hasegawa G, Naito M, Toyoshima Y, Tanaka S, Shiota K, Kitamura T, Fujita T, Ezaki O, Aizawa S, Kadowaki T, et al. PPAR gamma mediates high-fat diet-induced adipocyte hypertrophy and insulin resistance. Mol Cell. 1999;4:597–609. doi: 10.1016/s1097-2765(00)80210-5. [DOI] [PubMed] [Google Scholar]