Abstract
Objective
The role of chemokines and their transporters are poorly described in rheumatoid arthritis (RA). Evidence suggests that CXCL5 plays an important role as it is abundant in RA tissue and its neutralization moderates joint damage in animal models of arthritis. The chemokine transporter, Duffy Antigen Receptor for Chemokines (DARC), is also upregulated in early RA. Here we investigate the role of CXCL5 and DARC in regulating neutrophil recruitment using an in vitro model of the RA synovium.
Methods
To model the RA synovium, rheumatoid fibroblasts (RAF) were cocultured with endothelial cells (EC) for 24h. Gene expression in cocultured cells was investigated using TaqMan gene arrays. Roles of CXCL5 and DARC were determined by incorporating cocultures into a flow-based adhesion assay, where their function was demonstrated by blocking neutrophil recruitment with neutralizing reagents.
Results
EC-RAF coculture induced chemokine expression in both cell types. While CXC chemokines were modestly upregulated in EC, CXCL1, CXCL5 and CXCL8 expression were greatly increased in RAF. RAF also promoted the recruitment of flowing neutrophils to EC. Anti-CXCL5 antibody abolished neutrophil recruitment by neutralizing CXCL5 expressed on EC, or when used to immuno-deplete coculture conditioned medium. DARC was also induced on EC by coculture and an anti-Fy6 antibody or siRNA targeting of DARC expression effectively abolished neutrophil recruitment.
Conclusion
For the first time in a model of human disease, the function of DARC has been demonstrated as essential for editing the chemokine signals presented by EC and for promoting unwanted leukocyte recruitment.
The existence of a tissue specific address code for leukocyte recruitment during immune surveillance and acute inflammation is well established with adhesion receptors and chemokines being the primary coding elements (1, 2). However, the mechanisms of leukocyte recruitment into the rheumatoid (RA) joint are ill-defined. In chronic inflammation the address code presented on endothelial cells (EC) may reflect the abnormal inflammatory status of the tissue, with stromal cells producing an inappropriate profile of chemokines for presentation to circulating leukocytes. CXCL5 (ENA-78) is secreted by fibroblasts of the RA synovium and is elevated in synovial fluid and plasma of RA patients compared to other arthritic diseases (3). Furthermore, CXCL5 is reported to be as important as CXCL8 as a neutrophil chemoattractant in RA synovial fluid, with immuno-neutralization abrogating over 40% of chemotaxis (3). In adjuvant-induced arthritis models in rats, CXCL5 is elevated in the serum and joint homogenates with levels correlating with disease progression and clinical scores (4). Severity of disease was reduced by pre-treatment with antibodies against CXCL5 (4). Together these observations demonstrate an important role for CXCL5 in neutrophil recruitment in RA.
The Duffy Antigen Receptor for Chemokines (DARC) probably plays an important role in editing the leukocyte recruitment code on EC. DARC is a promiscuous receptor which binds some inflammatory chemokines with high affinity (5-7). However, DARC does not signal, rather it facilitates the transcytosis of chemokines from the stromal to the apical side of EC (8, 9) where glycosaminoglycans (GAGs) may present chemokines to leukocytes (7). DARC is expressed on the synovial vasculature in RA (10), and is increased in the synovium during early RA (11). These observations lead to the speculation that DARC might contribute to inflammation by presenting chemokines generated by stromal cells within diseased synovium (10, 11).
As both CXCL5 and DARC expression are increased in RA we speculate that presentation of CXCL5 is regulated by DARC. However, such a role for DARC has never been demonstrated in a human disease model. Here we used a coculture model of the RA synovium (12) to reconstruct the chronically inflamed RA microenvironment in vitro, by coculturing RA fibroblasts (RAF) or skin fibroblasts (SkF), as genetically matched control stromal cells, with human EC. After a period of conditioning, fibroblasts and EC were isolated and screened using microarray analysis. We show that recapitulating the RA environment upregulated message for a number of CXC-chemokines in EC and RAF, an effect absent in EC and SkF cocultures. Flow adhesion assays demonstrated that only CXCL5 was functional on EC cocultured with RAF and recruited flowing neutrophils. EC expression of DARC was induced by coculture with RAF and antibody blocking CXCL5 interactions with DARC or siRNA targeting DARC expression abolished neutrophil recruitment. Thus for the first time, we demonstrate a role for DARC in editing the leukocyte recruitment code in a chemokine specific manner and in a model of human inflammatory disease.
Materials and Methods
EC and fibroblast cell culture
Methodology for the culture and coculture of RAF, SkF and EC has been described previously (12, 13). Briefly, RAF and SkF were explanted from synovium or skin obtained at total knee arthroplasty from consenting patients who fulfilled 1987 ARA criteria for RA and represented genetically matched controls isolated from chronically inflamed and non-inflamed tissue respectively (14, 15). Human umbilical vein EC were enzymatically isolated and cultured to confluence. EC-fibroblast cocultures were grown on opposing sides of porous culture plastic inserts (pore size; 0.4μm; BD Falcon) (12, 13). Cells were conditioned for 24h prior to parallel plate flow-based leukocyte adhesion assay, or being isolated for mRNA extraction or immunofluorimetry. Alternatively, conditioned medium from cocultures was used to stimulate EC monolayers for 24h before being incorporated in the flow assay
Flow-based adhesion assay
The parallel plate assay was performed at 37°C on an upright fluorescent microscope (Olympus BX61) at a wall shear stress of 0.1 Pa (12, 13). Human neutrophils were isolated by density gradient centrifugation (Histopaque 1077 and 1119, Sigma), suspended in PBS containing 0.1% BSA and fluorescently labeled with 1 μg/ml bisbenzimide. Neutrophils were perfused at 106 cells/ml for 3 min, followed by cell-free buffer to remove non-adherent cells. After a 2 min wash, video records were made of 10 fields along the centre of the insert. Video was digitized using Image-Pro Plus (USA) and adherent cells expressed as number /mm2/106 cells perfused.
TaqMan gene expression assays
EC and fibroblasts were isolated from either side of the culture membrane by trypsin digestion. mRNA was extracted using an RNeasy Mini Kit 50 (Qiagen, Crawley, UK). Duplicate analysis of the expression of 96 genes from cells isolated from two experiments were assessed using TaqMan® low density arrays (Applied Biosystems [ABI], CA, USA). Genes altered by a factor of 2-fold or more were analyzed in two further experiments by quantitative real time-PCR (qRT-PCR). Primers and FAM-labeled probe sets for CXCL1 (forward: CGAAGT CATAGCCACACTCAAGAA; reverse: GTTCAGCATCTTTTCGATGATTT TC; Probe: TGCCTCAATCCTGCATCCCCCAT) and CXCL8 (forward: AAACCAC CGGAA GGAACCA; reverse: TACCTTCACACAGAGCTGCAGAA; probe:TCCAA GCTGGC CGTGGCTCTCTT) were designed using Primer Express® software v2.0 (ABI). Probes for CXCL5, DARC and VIC-labeled beta-actin were Assay on Demand probes (ABI). In all samples, qRT-PCR cycle threshold (Ct) values were normalized to ß-actin and expressed relative to the calibrator samples of EC, SkF or RAF monocultures (RQ value).
Functional blocking antibodies and cytokine assays
In blocking experiments, purified anti-DARC (10 μg/ml; Fy6; New York Blood Centre) was included in culture supernatant during coculture. For flow cytometry, EC were labeled for 15 min with anti-Fy6 or an isotype matched (IgG1) control antibody. FITC conjugated goat anti-mouse IgG (DAKO Ltd, High Wycombe, UK) was the secondary antibody. Fluorescent intensity was assessed on a Coulter EPICS XL flow cytometer (Beckman Coulter, High Wycombe, UK). CXCL5 was blocked or depleted from the conditioned medium of EC and RAF cocultures using anti-CXCL5 (10 μg/ml; Clone 33160.111; R&D systems) conjugated Protein-G agarose beads (Upstate, Lake Placid, NY, USA). Anti-VCAM-1 (Clone 1.4C3; DAKO) conjugated Protein-G agarose beads were used as the isotype control.
Beadlyte® Human Multi-cytokine Beadmaster™ kit (Upstate) was used to assay CXCL1 and CXCL8 in coculture supernatants. CXCL5 levels were measured using sandwich ELISA (R&D Systems).
Transfection of EC with siRNA targeting DARC
EC were suspended by trypsin digestion and adjusted to 1 × 107 cells/ml in Nucleofector solution (Amaxa AG, Cologne, Germany). To 100 μl of cell suspension (106 cells) 100nM of either DARC siRNA (Invitrogen, Paisley, UK), or universal negative control siRNA (-VE; Invitrogen), or phosphate buffered saline (to control for the effects of Nucleofector Solution on EC viability and function) were added. Samples were transferred to Amaxa certified cuvettes and electroporated using Amaxa apparatus on program A-34. Following electroporation, 500 μl of pre-warmed EC culture medium was added to the cuvettes and cells were transferred into 6-well plates to be incubated at 37° C with 5% CO2 for 24 hours. Transfected EC from 2 donors were incorporated in to coculture with RAF from 2 different donors and neutrophil adhesion assessed by flow assay as described above.
Statistical analysis
Data are mean ± SEM. Statistical comparisons were carried out using paired or unpaired t-tests.
Results
An inflammatory environment is established in a coculture model of the RA synovium
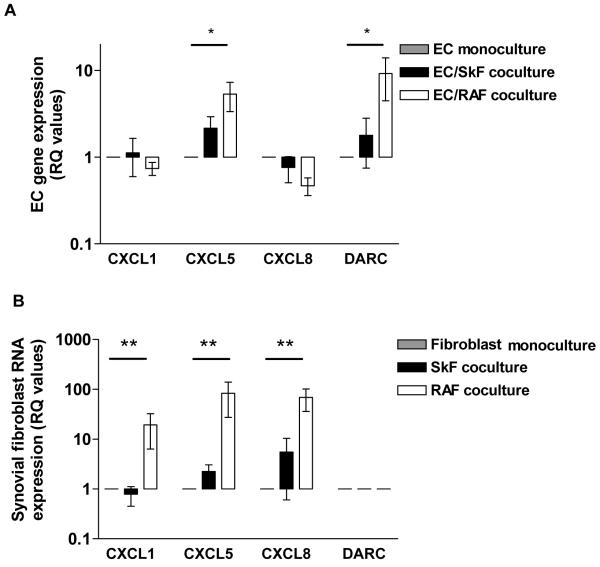
TaqMan® low density arrays were used to screen EC and fibroblasts isolated from mono- or co-culture (n=2). Table 1 provides a list of genes regulated in coculture by 2-fold or greater compared to monocultured cells. In cells from EC and SkF cocultures 20 genes were regulated (11 in EC and 9 in SkF, with 1 common to both). In the EC and RAF cocultures 14 genes were regulated (4 in EC and 11 in RAF, with 1 common to both cell types). Of these, we selected 4 genes (CXCL1, CXCL5, CXCL8 and DARC) being of greatest relevance to neutrophil recruitment, for further analysis in additional experiments. EC cocultured with RAF but not SkF showed an upregulation of CXCL5 and DARC message by 4- and 7-fold respectively compared to EC cultured alone (Figure 1a). Interestingly, in cocultured RAF, CXCL1, CXCL5 and CXCL8 were highly elevated compared to fibroblasts cultured alone (19-, 83- and 69-fold respectively; Figure 1b). DARC mRNA was not expressed in fibroblasts under monoculture or coculture conditions (Figure 1b). Thus, genomic analysis of RAF and EC indicated that the chemokine environment established upon coculture was more complex than previously reported (12).
Table I. TaqMan® low density array analysis of EC and SKF or RAF cocultures.
Gene arrays were performed on EC, SkF or RAF samples after monoculture or coculture for 24h (n=2). The quantitative expression of target genes was determined relative to the housekeeping gene GAPDH and data presented as the fold-increase or decrease of cocultured samples compared to the calibrator samples (EC, SkF or RAF monocultures=1). Genes regulated by at least 2-fold were considered effected by coculture.
| Target Gene | EC with RAF | RAF with EC | EC with SkF | SkF with EC |
|---|---|---|---|---|
| α7 Integrin | 3.7 ± 1.1 | NR | 2.4 ± 0.8 | NR |
| α10 Integrin | NR | 2.3 ± 0.9 | NR | 13.6 ± 5.6 |
| β3 Integrin | NR | NR | NR | 2.8 ±1.1 |
| β7 Integrin | NR | NR | 2.9 ± 0.7 | NR |
| CCL2 | NR | 4.3 ± 2.1 | NR | 6.8 ±1.9 |
| CD44 | NR | NR | NR | 0.44 ± 0.1 |
| COX2 | NR | 6.8 ± 4.4 | NR | NR |
| CXCL1 | NR | 6.4 ±1.6 | 0.4 ± 0.1 | NR |
| CXCL5 | 3.8 ± 1.2 | 27.6 ± 13.8 | 2.1 ± 1.3 | 12.8 ±11.2 |
| CXCL8 | NR | 36.4 ± 8.7 | NR | 30.7 ± 19.0 |
| DARC | 4.8 ± 2.0 | NR | 2.3 ± 1.0 | NR |
| E-selectin | NR | 0.3 ± 0.1 | NR | NR |
| FLT-1 | NR | NR | 2.5 ± 0.5 | NR |
| 11βHSDI | NR | 4.3 ± 2.2 | NR | 6.3 ± 1.5 |
| ICAM-1 | NR | 3.1 ± 0.3 | NR | 12.5 ±6.2 |
| ICAM-2 | NR | 2.2 ± 0.6 | NR | NR |
| OPG | NR | 0.4 ± 0.1 | NR | NR |
| P-selectin | NR | 0.3 ± 0.1 | NR | NR |
| RANK | NR | NR | 3.8 ± 0.6 | NR |
| SREBF-1 | NR | NR | NR | 2.0 ± 0.3 |
| VCAM-1 | 0.4 ± 0.1 | NR | NR | NR |
| VEGF | NR | 2.1 ± 0.3 | 2.4 ± 0.4 | 2.4 ± 0.4 |
NR= not regulated.
Figure 1.
Effects of EC-RAF coculture on CXCL1, CXCL5, CXCL8 and DARC message in A) EC or B) SkF and RAF from the RA joint. Data are expressed as mean ± SEM of 4 experiments using different fibroblast explants and EC cultures. * = P<0.05; ** = P<0.01 for comparison of cocultured cells against monocultured calibrator samples.
CXCL5 is selectively posted on cocultured EC and promotes neutrophil recruitment
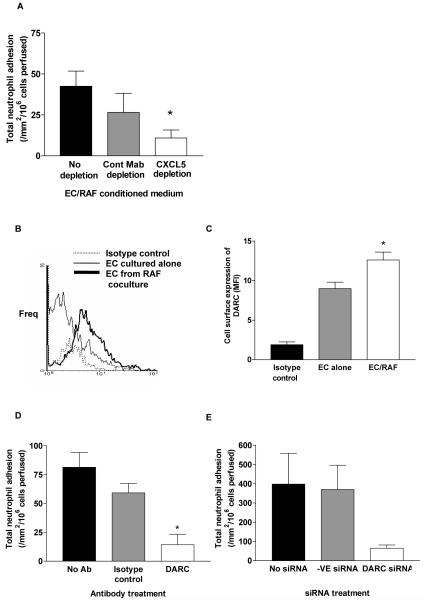
Coculture of EC with RAF but not with SkF activated EC allowing recruitment of neutrophils from flow (12). Neutrophil recruitment was β2 integrin and CXCR2 dependent (12). Importantly, a function neutralizing antibody against CXCL5 reduced neutrophil recruitment from 59 ± 8 /mm2/106 cells perfused to 10 ± 2 /mm2/106 cells perfused. Here, adhesion assays conducted using EC monocultures activated with conditioned medium from EC and RAF coculture for 24h, were also able to recruit neutrophils from flow (Figure 2A). When CXCL5 was depleted from the conditioned medium, EC monocultures could not support the recruitment of neutrophils (Figure 2A). Interestingly, cytokine analysis of coculture supernatants showed that CXCL8 was present at high concentrations in both monocultures (EC; 746 ±156 pg/ml) and cocultures (EC/SkF; pg/ml 892 ± 199 and EC-RAF; 437 ± 112 pg/ml respectively; p=N.S.), although, CXCL1 and CXCL5 were below the level of detection in the assays used. This strongly indicated that CXCL5 alone was posted on the EC surface despite the presence of substantial concentrations of at least one other CXC chemokine (i.e. CXCL8).
Figure 2.
A) The effect of incubating monolayers of EC with coculture conditioned medium before or after immuno-depletion using an anti-CXCL5 or control antibody; data are mean ± SEM of 4 experiments; * = P <0.05 for comparison of non depleted and antibody depleted conditioned media; B) A representative histogram of the expression of DARC protein on EC cultured alone or after coculture with RAF assessed by immunofluorimetry; C) Quantification of EC surface expression of DARC, data are mean ± SEM of 4 experiments; * = P <0.05 for comparison of EC monocultures and EC/RAF cocultures. D) The effect of inhibiting the binding of chemokines to DARC using an anti-FY6 monoclonal antibody; data are mean ± SEM of 4-12 experiments; * = P <0.05 for comparison of untreated and antibody treated cells. E) The effect of siRNA targeting of DARC in EC in coculture with RAF, data are mean ± SEM of 2 experiments.
DARC is essential for posting CXCL5 on cocultured EC
The specific nature of CXCL5 driven neutrophil recruitment indicated that a selective mechanism of chemokine transport and presentation might be operative. As mRNA for DARC was significantly upregulated in cocultured EC we postulated that this receptor might fulfill this role. DARC protein was found to be significantly upregulated on EC cocultured with RAF (Figure 2B and C) and an antibody (anti-Fy6) that specifically blocks the interaction of chemokines with DARC, abolished the adhesion of neutrophils to cocultured EC (Figure 2D). Furthermore, when DARC expression in EC cocultured with RAF was abrogated using siRNA, a similar reduction in the adhesion of neutrophils was seen (Figure 2E). Taken together, these results strongly imply that the activity of CXCL5 was dependent upon the function of DARC in cocultured EC.
Discussion
Here we modelled the RA synovium in vitro to identify mechanisms by which leukocytes are recruited to this environment. Transcriptional changes in EC and RAF were found after a period of residence in the recapitulated ‘synovium’. Importantly, we could utilise a functional assay (a leukocyte adhesion assay) to demonstrate that transcriptional changes, in particular of CXCL5 and DARC, were functionally relevant and were essential for the recruitment of flowing neutrophils.
The chemokines used to recruit neutrophils to the RA synovium are not well-defined. However, immunohistochemistry on diseased human tissue and inhibitor studies in animal models of arthritis indicate an important role for CXCL5 (3, 4). We have previously demonstrated a specific role for CXCL5 (rather than CXCL8 or CXCL1) in the recruitment of neutrophils to our model of the RA synovium using function blocking antibodies (12). Here we confirmed the importance of CXCL5 in neutrophil recruitment by demonstrating its transcriptional upregulation during coculture and loss of function in our adhesion assay upon immuno-depletion. The specificity of CXCL5 dependent neutrophil activation, even though CXCL8 is abundantly secreted into coculture supernatants, suggests that selective chemokine transport and presentation is occurring in this model of chronic inflammation.
Duffy Antigen Receptor for Chemokines (DARC) selectively binds most inflammatory but not homeostatic chemokines (5). The chemokine binding properties of DARC have been elucidated using radio-isotype competitive binding assays or the anti-Fya, Fy3 or Fy6 antibodies (16-18). Anti-Fy6 is a monoclonal antibody generated against Duffy antigen receptor found on human red blood cells and can effectively block the interaction of CXCL1, CXCL8, and CCL5 to DARC (17). Structural analysis of the receptor/ligand interaction between DARC and its chemokine ligands has revealed that the location of the FY6 epitope of DARC is closely involved in the chemokine binding properties of this receptor (16). Here, we demonstrate that anti-Fy6 antibody blockade can also inhibit neutrophil recruitment from flow onto EC/RAF cocultures probably through its ability to displace CXCL5 binding to DARC. The functional effects of DARC in our coculture system were confirmed using siRNA targeting EC DARC expression. Taken together these results indicate that the regulation of DARC expression on EC by disease-transformed fibroblasts is an important component in the recruitment of neutrophils to the inflamed RA synovium.
Although increased expression of DARC can be visualised by immuno-histochemistry in inflamed and/or diseased human tissue, a definitive role for DARC in any human disease has yet to be demonstrated. In vitro experiments, where immortalised EC have been transfected with the human DARC gene, indicate that DARC expression increases the efficiency of leukocyte recruitment, probably by transporting chemokines for presentation to circulating leukocytes (8, 9). The use of more relevant study systems is hampered by the loss of DARC on cultured EC and an inability to induce its expression with inflammatory cytokines (9). This makes the current coculture model of the RA synovium of particular importance. Firstly, it provides a disease specific leukocyte recruitment assay in which the contributions of DARC and chemokines can be dissected. Secondly, it provides a model in which the agent(s) that regulate the expression of DARC in EC can be investigated. To date we have described a role for IL-6 in the cross-talk between RAF and EC (12). However, neutralising this cytokine, or inhibiting its secretion by the addition of hydrocortisone to cocultures, does effect DARC expression, at least at the level of mRNA production (12 and unpublished observations). Thus, an unknown agent(s) is generated during RAF/EC coculture which induces DARC expression and is essential for neutrophil recruitment to our model synovium. Through further characterisation of this model we may be able to identify new therapeutic targets which moderate chronic inflammation in RA.
References
- 1.Springer TA. Traffic signals on endothelium for lymphocyte recirculation and leukocyte emigration. Ann. Rev. Physiol. 1995;57:827–72. doi: 10.1146/annurev.ph.57.030195.004143. [DOI] [PubMed] [Google Scholar]
- 2.Imhof BA, Dunon D. Leukocyte migration and adhesion. Adv. Immunol. 1995;58:345–416. doi: 10.1016/s0065-2776(08)60623-9. [DOI] [PubMed] [Google Scholar]
- 3.Koch AE, Kunkel SL, Harlow LA, Mazarakis DD, Haines GK, Burdick MD, Pope RM, Walz A, Strieter RM. Epithelial neutrophil activating peptide-78: a novel chemotactic cytokine for neutrophils in arthritis. J Clin. Invest. 1994;94:1012–8. doi: 10.1172/JCI117414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Halloran MM, Woods JM, Strieter RM, Szekanecz Z, Volin MV, Hosaka S, et al. The Role of an Epithelial Neutrophil-Activating Peptide-78-Like Protein in Rat Adjuvant-Induced Arthritis. J Immunol. 1999;162:7492–500. [PubMed] [Google Scholar]
- 5.Gardner L, Patterson AM, Ashton BA, Stone MA, Middleton J. The human Duffy antigen binds selected inflammatory but not homeostatic chemokines. Biochem. Biophys. Res. Commun. 2004;321:306–12. doi: 10.1016/j.bbrc.2004.06.146. [DOI] [PubMed] [Google Scholar]
- 6.Kashiwazaki M, Tanaka T, Kanda H, Ebisuno Y, Izawa D, Fukuma N, et al. A high endothelial venule-expressing promiscuous chemokine receptor DARC can bind inflammatory, but not lymphoid, chemokines and is dispensable for lymphocyte homing under physiological conditions. Int. Immunol. 2003;15:1219–27. doi: 10.1093/intimm/dxg121. [DOI] [PubMed] [Google Scholar]
- 7.Middleton J, Patterson AM, Gardner L, Schmutz C, Ashton BA. Leukocyte extravasation: chemokine transport and presentation by the endothelium. Blood. 2002;100:3853–60. doi: 10.1182/blood.V100.12.3853. [DOI] [PubMed] [Google Scholar]
- 8.Lee JS, Frevert CW, Wurfel MM, Peiper SC, Wong VA, Ballman KK, et al. Duffy Antigen Facilitates Movement of Chemokine Across the Endothelium In Vitro and Promotes Neutrophil Transmigration In Vitro and In Vivo. J. Immunol. 2003;170:5244–51. doi: 10.4049/jimmunol.170.10.5244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rot A. Contribution of Duffy antigen to chemokine function. Cytokine Growth Factor Rev. 2005;16:687–694. doi: 10.1016/j.cytogfr.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 10.Patterson AM, Siddall H, Chamberlain G, Gardner L, Middleton J. Expression of the duffy antigen/receptor for chemokines (DARC) by the inflamed synovial endothelium. J. Pathol. 2002;197:108–16. doi: 10.1002/path.1100. [DOI] [PubMed] [Google Scholar]
- 11.Gardner L, Wilson C, Patterson AM, Bresnihan B, Fitzgerald O, Stone MA, et al. Temporal expression pattern of Duffy antigen in rheumatoid arthritis: up-regulation in early disease. Arthritis Rheum. 2006;54:2022–6. doi: 10.1002/art.21909. [DOI] [PubMed] [Google Scholar]
- 12.Lally F, Smith E, Filer A, Stone MA, Shaw JS, Nash GB, et al. A novel mechanism of neutrophil recruitment in a coculture model of the rheumatoid synovium. Arthritis Rheum. 2005;52:3460–9. doi: 10.1002/art.21394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rainger GE, Stone P, Moreland C, Nash GB. A novel system for investigating the ability of stromal cells to regulate adhesion of flowing leukocytes to endothelial cells. J. Immunol Meth. 2001;255:73–82. doi: 10.1016/s0022-1759(01)00427-6. [DOI] [PubMed] [Google Scholar]
- 14.Arnett FC, Edworthy SM, Bloch DA, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–24. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 15.Parsonage G, Falciani F, Burman A, Filer A, Ross E, Bofill M, et al. Global gene expression profiles in fibroblasts from synovial, skin and lymphoid tissue reveals distinct cytokine and chemokine expression patterns. Thromb. Haemost. 2003;90:688–97. doi: 10.1160/TH03-04-0208. [DOI] [PubMed] [Google Scholar]
- 16.Tournamille C, Van Kim CL, Gane P, et al. Close Association of the First and Fourth Extracellular Domains of the Duffy Antigen/Receptor for Chemokines by a Disulfide Bond Is Required for Ligand Binding. J Biol Chem. 1997;272:16274–80. doi: 10.1074/jbc.272.26.16274. [DOI] [PubMed] [Google Scholar]
- 17.Nichols ME, Rubinstein P, Barnwell J, Rodriguez de Cordoba S, Rosenfield RE. A new human Duffy blood group specificity defined by a murine monoclonal antibody. Immunogenetics and association with susceptibility to Plasmodium vivax. J Exp Med. 1987;166:776–785. doi: 10.1084/jem.166.3.776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Horuk R, Chitnis CE, Darbonne WC, et al. A receptor for the malarial parasite Plasmodium vivax: the erythrocyte chemokine receptor. Science. 1993;261:1182–1184. doi: 10.1126/science.7689250. [DOI] [PubMed] [Google Scholar]