SUMMARY
RASSF1A is a tumor suppressor gene that is epigenetically silenced in a wide variety of sporadic human malignancies. Expression of alternative RASSF1 isoforms cannot substitute for RASSF1A-promoted cell-cycle arrest and apoptosis. Apoptosis can be driven by either activating Bax or by activation of MST kinases. The Raf1 proto-oncogene binds to MST2, preventing its activation and proapoptotic signaling. Here we show that key steps in RASSF1A-induced apoptosis are the disruption of the inhibitory Raf1-MST2 complex by RASSF1A and the concomitant enhancement of MST2 interaction with its substrate, LATS1. Subsequently, RASSF1A-activated LATS1 phosphorylates and releases the transcriptional regulator YAP1, allowing YAP1 to translocate to the nucleus and associate with p73, resulting in transcription of the proapoptotic target gene puma. Our results describe an MST2-dependent effector pathway for RASSF1A proapoptotic signaling and indicate that silencing of RASSF1A in tumors removes a proapoptotic signal emanating from p73.
INTRODUCTION
Apoptosis is a critical process that safeguards against unlicensed growth of aberrant cells. Thus, signaling pathways that induce apoptosis are often silenced in tumors (Cox and Der, 2003; Evan et al., 1995). RASSF1 is a tumor suppressor gene on chromosome 3p21 that is inactivated in a wide variety of carcinomas (Agathanggelou et al., 2005). By alternative splicing and distinct promoter usage, the RASSF1 gene can express several isoforms, the predominant forms being 1A and 1C, which differ in their N termini. Whereas RASSF1C is ubiquitously expressed in normal and tumor cells, the expression of exon 1α, and consequently the RASSF1A isoform, is frequently lost in tumor cells via epigenetic promoter methylation. Introducing RASSF1A back into tumor cells can both restrict cell-cycle progression via inactivation of the APC/cyclosome (Song and Lim, 2004) or induce apoptosis. Recently, RASSF1A has been described to promote apoptosis by binding to the BH3 domain protein modulator of apoptosis protein-1 (MAP-1). This relieves an intramolecular inhibitory interaction in MAP-1 that enables it to bind to Bax and induce Bax to adopt its proapoptotic conformation (Baksh et al., 2005; Vos et al., 2006). RASSF1A also can activate apoptosis through the proapoptotic kinase MST1 (Oh et al., 2006). However, the signaling cascade downstream of MST that leads to apoptosis of mammalian cells has remained obscure.
In Drosophila melanogaster, the MST2 homolog Hippo can promote apoptosis by several mechanisms. Hippo can repress the transcriptional induction of the bantam microRNA, which counteracts the expression of the proapoptotic caspase activator Hid (Brennecke et al., 2003). Further, Hippo can signal through Warts to promote apoptosis by decreasing the levels of the caspase inhibitor dIAP (Edgar, 2006). A mammalian homolog of Warts, termed LATS1, is a candidate tumor suppressor (Hisaoka et al., 2002; St John et al., 1999). Warts and LATS1 are direct substrates of Hippo (Pantalacci et al., 2003; Xu et al., 1995) and MST2 (Chan et al., 2005), respectively. Warts also has been described to phosphorylate and inactivate Yorkie, a Drosophila homolog of the yes-associated protein (YAP). Because Yorkie is required for the transcription of the diap1 and cyclin E genes, its inactivation leads to growth arrest and apoptosis. Conversely, overexpression of Yorkie phenocopies loss-of-function mutations in the hippo/mst2 and warts genes (Huang et al., 2005). However, although the Hippo-Warts-Yorkie pathway regulates cell cycle and apoptosis in Drosophila, the functional conservation of this pathway in mammalian cells only starts to be elucidated. In mammalian cells, MST2 enhances the interaction between RASSF1A and WW45, the mammalian homolog of the Drosophila scaffold Salvador, and RASSF1A and WW45 promote MST2 activation and LATS1 phosphorylation in order to ensure proper mitotic progression and cytokinesis (Guo et al., 2007). These data show a role for mammalian MST2 that has not been described in the Drosophila model. However, a physiological activation signal for this pathway and its potential role in apoptosis regulation in mammalian cells are still lacking.
During the execution phase of apoptosis in mammalian cells, MST1 and MST2 can be activated by caspase cleavage. In the case of MST1, this leads to a constitutive phosphorylation of H2B, resulting in nuclear DNA fragmentation (Cheung et al., 2003). In addition, the activation of Jun N-terminal kinase (JNK) signaling also has been implicated in MST-promoted apoptosis (Ura et al., 2001). Recently, MST1 was reported to induce apoptosis in neuronal cells by phosphorylating and activating FOXO3 transcription factors in response to oxidative stress (Lehtinen et al., 2006). These findings indicate that in mammalian cells MST kinases may cause apoptosis via several pathways. The same paper showed that CST-1, the C. elegans MST1 homolog, delays aging and extends life span. Thus, although signaling modules may be conserved between lower and higher organisms, their functions may have changed and adapted to serve different requirements. We have previously described that the protective function of the Raf1 proto-oncogene against apoptosis involves the inhibition of MST2 activity by direct sequestration and inhibition of MST2 activation (O’Neill et al., 2004). Thus, MST2 may serve as a hub that functionally connects proliferative and proapoptotic pathways.
Regulation of apoptosis is an important mechanism for tumor suppression that is well described for the p53 protein. p53 senses DNA damage and initiates cellular responses that, dependent on the extent of the damage, lead to cell cycle arrest or apoptosis. The latter is caused by regulation of an apoptotic transcriptional program and by signaling to key proteins involved in the mitochondrial apoptotic cascade (Balint and Vousden, 2001). The precise contribution of p53 to proapoptotic responses has been blurred by the discovery of p53-related proteins p63 and p73, which can have distinct functions as well as overlapping responses, e.g., transcriptional induction of the proapoptotic gene puma (Yang et al., 2002).
Here we have investigated the individual steps in an apoptotic signaling pathway initiated by RASSF1A. This pathway was initially reconstructed by examining dynamic changes in the composition of RASSF1A/C and MST2 protein complexes with mass spectrometry-based proteomics. The rationale was to follow the transmission of a signal by tracing changes in protein interactions that change in response to specific stimuli. As a result, we describe a pathway emanating from the RASSF1A tumor suppressor that finally results in the p73-mediated transcription of puma, and show that RASSF1A-promoted apoptosis requires p73 and YAP1. The intermediate steps encompass the activation of MST2 by relieving Raf1 inhibition and the subsequent association of MST2 with RASSF1A and LATS1. This induces phosphorylation and release of YAP1, allowing its translocation to the nucleus and binding to p73. We further demonstrate that this pathway can be triggered by Fas death receptor signaling and that downregulation of individual pathway components prevents Fas-mediated cell death.
RESULTS
RASSF1A Dissociates the Inhibitory Raf1-MST2 Complex
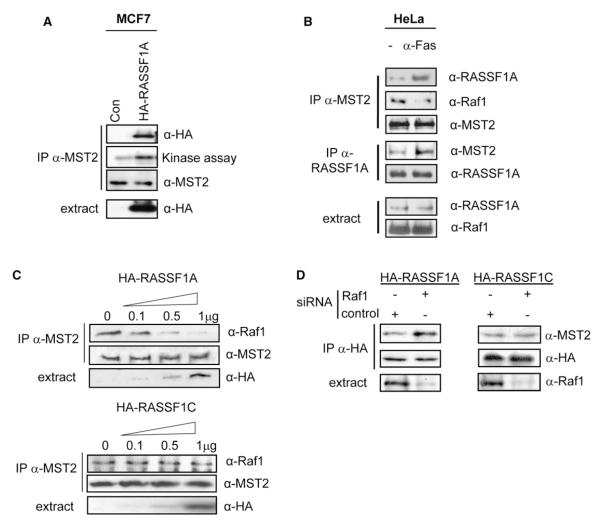
We used two cell lines, MCF7, which lacks endogenous RASSF1A expression, and HeLa, one of the few cell lines that retains endogenous RASSF1A expression. Transfection of RASSF1A into MCF7 cells induced RASSF1A binding to MST2 and activation of the MST2 kinase (Figure 1A). Endogenous MST2 and RASSF1A proteins could be coimmunoprecipitated in HeLa cells (Figure 1B and Figure S1 in the Supplemental Data available with this article online). This association was enhanced by stimulation of the Fas death receptor, interestingly at the expense of Raf1 binding to MST2 (Figure 1B). Graded overexpression of RASSF1 in MCF7 cells showed that RASSF1A, but not RASSF1C, could dissociate Raf1 from MST2 in a dose-dependent manner (Figure 1C). Conversely, downregulation of Raf1 expression by siRNA selectively promoted the binding of MST2 to RASSF1A but did not affect the MST2-RASSF1C complex (Figure 1D). These results suggested that RASSF1A can induce MST2 activation by dissociating it from Raf1. In vitro binding of Raf1 protein to a peptide array representing the full MST2 sequence showed that the Raf1 binding region is situated in the SARAH domain and overlaps with the RASSF1A binding domain (Figure S2), providing further evidence for mutually exclusive association to MST2. These results suggest that, in cells with intact RASSF1A expression, MST2-RASSF1A and MST2-Raf1 complexes exist in a dynamic equilibrium that is regulated by Fas.
Figure 1. RASSF1A Disrupts MST2 Interaction with Raf1.
(A) MCF7 cells were transfected with 1 μg HA-RASSF1A or empty vector. After 16 hr serum starvation (0.1% serum), cells were lysed. MST2 immunoprecipitates were subjected to an in-gel kinase assay and western blotted with the indicated antibodies.
(B) Endogenous MST2 and RASSF1A immunoprecipitates from serum-starved and anti-Fas CD95-treated HeLa cells were analyzed by western blotting with the indicated antibodies.
(C) MCF7 cells were transfected with increasing amounts of HA-RASSF1A or HA-RASSF1C. After serum starvation, MST2 immunoprecipitates were western blotted for coprecipitating Raf1.
(D) MCF7 cells were transfected with 50 ng/ml Raf1 or a nontargeting siRNA pool (control) and 1 μg of HA-RASSF1A or HA-RASSF1C. MST2 immunoprecipitates were examined for associated Raf1 by western blotting.
Raf1 Counteracts RASSF1A-Induced Apoptosis
RASSF1A triggered apoptosis of MCF7 cells in a dose-dependent manner, whereas RASSF1C was completely ineffective (Figure 2A). Depletion of MST2 by siRNA abrogated RASSF1A-induced apoptosis, whereas the downregulation of Raf1 expression enhanced it (Figure 2B). The protective role of Raf1 was independent of its ability to activate the MEK-ERK pathway, as pharmacological inhibition of MEK robustly inhibited ERK but failed to reproduce the enhancing effect of Raf1 downregulation on RASSF1A-induced apoptosis (Figure S3). Increasing the expression of RASSF1A sensitized MCF7 cells to Fas-promoted apoptosis (Figure 2C) in a dose-dependent manner (data not shown). Thus, although Fas can induce apoptosis on its own, the presence of RASSF1A enhances its proapoptotic capability, suggesting that Fas signaling can involve a RASSF1A-dependent arm. This arm is dependent on the RASSF1A-MST2 axis, as siRNA-mediated downregulation of MST2 had only a small effect on the basal apoptosis induced by Fas in the RASSF1A-deficient MCF7 cells but completely abolished the enhancement of Fas apoptosis by RASSF1A (Figure 2C).
Figure 2. MST2 Is Required for Fas- and RASSF1A-Induced Apoptosis.
(A) Serum-starved MCF7 cells were transfected with increasing amounts of HA-RASSF1A or HA-RASSF1C plasmids. After 20 hr of serum starvation, apoptosis was measured by assaying DNA fragmentation by FACS. Error bars show standard deviation. Aliquots of cell extracts were examined for the expression of transfected genes by using tubulin as loading control.
(B) MCF7 cells were transfected with 0.5 μg of HA-RASSF1A and 50 ng/ml of MST2 siRNAi (left panel) or 50 ng/ml of Raf1 siRNA (right panel) or control siRNA and analyzed as in Figure 1.
(C and D) MCF7 cells transfected with 0.5 μg of HA-RASSF1A and 50 ng/ml siRNAs for MST2, Raf1, or control were starved for 16 hr and treated with anti-Fas CD95 (50 nM) plus cyclohexamide (5 μg/ml) for 4 hr and assayed as above. Error bars show standard deviation.
(E) Raf-1 is required for protection from Fas-induced hepatitis in adult mice. PCR analysis of genomic DNA isolated from the liver of c-raf-1flox/flox and c-raf-1Δ/Δ;MxCre+ littermates after induction of MxCre in vivo, showing successful conversion of the f to the Δ allele (upper panel). This also resulted in the elimination of Raf1 protein as shown by immunoblot analysis of liver lysates. Fas expression was not affected by the Raf1 knockout, and an ERK2 immunoblot is shown as a loading control.
(F) c-raf-1Δ/Δ mice are sensitized to challenge by an anti-Fas Abs (Jo-2, 200 μg/kg). Survival was monitored for 48 hr. Animals that did not die within the first 8 hr recovered.
(G) Caspase 3 activation in the liver of c-raf-1Δ/Δ animals was determined by immunoblotting whole-cell lysates with antisera recognizing the cleaved, active forms of caspase-3. An ERK immunoblot is shown as a loading control. The livers were isolated either before or after (2 hr) i.v. injection with anti-Fas.
Raf1 ablation by siRNA also increased Fas-stimulated apoptosis. Fas-induced apoptosis was further enhanced when both RASSF1A was expressed and Raf-1 was depleted by siRNA (Figure 2D). These results show that RASSF1A can enhance Fas proapoptotic signaling and suggest that Raf1 is a rate-limiting step in Fas-mediated apoptosis. We previously observed that MST2 activity was constitutively elevated in Raf-1-/- fibroblasts (O’Neill et al., 2004). Therefore, we tested the sensitivity of conditional Raf-1-/- knockout mice toward Fas activation. Conditional ablation of c-raf-1 in the liver was performed by the induction of Cre expression by poly inosinic:cytidylic acid (poly I:C) treatment of Mx-Cre;c-raf-1flox/flox mice in vivo as previously described (Jesenberger et al., 2001). The successful knockout of Raf-1 was confirmed by genomic PCR and western blotting for Raf1 protein (Figure 2E). Injection of Fas-activating antibodies led to hepatitis and eventual death of Raf-1-/- knockout mice that were significantly increased in comparison to control animals (Figure 2F). The elimination of Raf1 in mice was accompanied by the activation of caspase 3 (Figure 2G), confirming that hepatitis is due to liver cell death by apoptosis. Thus, these data show that Raf1 counteracts Fas-mediated apoptosis in vivo.
In summary, these results suggest that RASSF1A is an effector arm of Fas-mediated apoptosis that is antagonized by Raf1 and mechanistically depends on RASSF1A displacing Raf1 from MST2 binding. These findings also validate our hypothesis that the signal flux through a pathway can be traced by following the dynamic changes of protein interactions, i.e., in this case MST2 switching from an inhibited complex with Raf1 to an apoptosis-inducing complex with RASSF1A.
RASSF1A Promotes MST2 Interaction with LATS1 and Proapoptotic Signaling
In order to identify downstream components of MST2 signaling, we immunoprecipitated MST2 and analyzed associated proteins by mass spectrometry, identifying LATS1 as an MST2 binding partner (data not shown). Interestingly, the formation of endogenous complexes between LATS1 and MST2 was stimulated by RASSF1A, while RASSF1C was without effect (Figure 3A), replicating the selectivity of induced MST2 interaction with RASSF1A versus RASSF1C (Figures 1C and 1D). Both MST2 and RASSF1A binding to LATS1 increased in LATS1 immunoprecipitates with the dose of RASSF1A expression (Figure 3B). LATS1 and RASSF1A also were found in MST2 immunoprecipitates (Figure S4), indicating that these proteins are able to form ternary complexes.
Figure 3. MST2-LATS1 Association Is Induced by RASSF1A and Required for Fas Proapoptotic Signaling.
(A) MCF7 cells were transfected with 0.5 μg of HA-RASSF1A and HA-RASSF1C constructs. Endogenous LATS1 immunoprecipitates from serum-starved cells were blotted with LATS1 and MST2 antibodies.
(B) MCF7 cells were transfected with 0.1, 0.5, and 1 μg of HA-RASSF1A. Endogenous LATS1 immunoprecipitates from serum-starved cells were blotted with LATS1, MST2, and HA antibodies.
(C) MCF7 cells were transfected with 0.5 μg HA-RASSF1A and HeLa cells with 50 ng/ml RASSF1A siRNA. After 16 hr serum starvation, cells were incubated with 50 nM CD95 antibody to Fas and cyclohexamide (5 μg/ml) for 4 hr where indicated and endogenous LATS1 immunoprecipitates were western blotted with LATS1 and MST2 antibodies.
(D) HeLa cells were transfected with 50 ng/ml RASSF1A, MST2, LATS1, or control siRNAs, serum starved, and treated with anti-Fas CD95 (50 nM) plus cyclohexamide (5 μg/ml) for 20 hr. Apoptosis was determined as in Figure 2. Error bars show standard deviation. Cell lysates were western blotted with the indicated antibodies to assess the efficiency of knockdown. Endogenous expression levels of tubulin were used as loading control.
As the RASSF1A-MST2 association was induced by Fas (Figure 1B), we tested whether this regulation also extended to the MST2-LATS1 interaction. In MCF7 cells, which lack endogenous RASSF1A expression, Fas stimulation enhanced the formation of endogenous MST2-LATS1 complexes only when RASSF1A was transfected (Figure 3C). In contrast, Fas stimulation readily augmented MST2-LATS1 association in HeLa cells, which express endogenous RASSF1A. This enhancement was completely prevented by siRNA-mediated downregulation of RASSF1A (Figure 3C). These data suggest that in response to Fas activation RASSF1A activates MST2 signaling by releasing MST2 from the inhibitory interaction with Raf1, inducing MST2 activation and binding to its substrate, LATS1.
This model predicts that Fas- and RASSF1A-induced apoptosis should be dependent on MST2 and LATS1. This indeed was the case, as the individual downregulation of endogenous RASSF1A, MST2, and LATS1 by siRNA in HeLa cells reduced Fas-triggered apoptosis (Figure 3D and Figure S5). Similarly, the downregulation of endogenous MST2 and LATS1 in MCF7 decreased RASSF1A-induced apoptosis (Figure S5). These results were confirmed by using mouse embryo fibroblasts from mice where RASSF1A or LATS1 was genetically deleted. RASSF1A-/- cells largely resisted Fas-mediated apoptosis (Figure S6A), and LATS1-/- cells resisted both Fas- and RASSF1A-induced apoptosis (Figure S6B). The reconstitution of LATS1-/- cells with myc-tagged LATS1 restored the susceptibility to Fas-induced apoptosis (Figure S6C), ruling out nonspecific adaptive effects that may have occurred during the establishment of the knockout cells. In summary, these results strongly suggest that these proteins are components of the same pathway.
LATS1 Associates with YAP1 and Is Regulated by RASSF1A
Genetic evidence from D. melanogaster suggests that the transcriptional coactivator Yorkie connects the LATS1 homolog Warts to the transcription of the nuclear target genes cyclin E and diap1 (Huang et al., 2005). Therefore, we examined whether LATS1 could associate with the mammalian Yorkie homolog YAP1. Endogenous LATS1-YAP1 complexes could be coimmunoprecipitated from HeLa and MCF7 cells. Despite very similar expression of LATS1 and YAP1 in the two cell types, less LATS1-YAP1 complex was recovered from HeLa cells (Figure 4A), suggesting that the expression of endogenous RASSF1A in HeLa cells may reduce the interaction. Indeed, exogenous expression of RASSF1A in MCF7 cells decreased the LATS1-YAP1 complex in a dose-dependent manner (Figure 4B). Again, RASSF1C had no effect (Figure 4B). RASSF1A inhibition of the LATS-YAP1 interaction was mediated by MST2, as downregulation of MST2 by siRNA abrogated the dissociation (Figure 4C), indicating that YAP1 is a downstream component of the RASSF1A pathway. Consistent with this conclusion, RASSF1A failed to induce apoptosis in MCF7 cells where YAP1 expression had been stably downregulated by expression of a shRNA (Figure 4D).
Figure 4. RASSF1A Regulates Apoptosis via LATS1 and YAP1.
(A) Endogenous LATS1 immunoprecipitates from MCF7 and HeLa cells were examined for associated endogenous YAP1 by western blotting.
(B) MCF7 cells were transfected with empty vector, 0.5 or 1 μg of HA-RASSF1A plasmid (top panels), or 1 μg of HA-RASSF1C (bottom panels). After 16 hr serum starvation, cell extracts were split in two aliquots, and endogenous YAP1 or LATS1 was immunoprecipitated and examined for association by immunoblotting with the indicated antibodies.
(C) MCF7 cells were transfected with 1 μg of HA-RASSF1A and 50 ng of MST2 or control siRNAs and examined for association of YAP1 and LATS1 as above.
(D) MCF7 cells stably expressing shRNA against YAP1 or control (Basu et al., 2003) were transfected with 0.25, 0.5, 1, and 1.5 μg of HA-RASSF1A. Apoptosis was determined by assessing caspase activation by zVAD-fmk-FITC after serum starvation for 16 hr and anti-Fas CD95 (50 nM) plus cyclohexamide (5 μg/ml) treatment. Error bars show standard deviation. Tubulin and exogenous HA-RASSF1A levels were determined by western blotting.
RASSF1A Promotes YAP1 and p73 Nuclear Translocation and Complex Formation
In unstimulated cells, YAP1 is mainly sequestered in the cytosol, preventing it to function as a transcription factor (Basu et al., 2003). RASSF1A is also cytosolic and mainly bound to microtubules in interphase cells (Dallol et al., 2004; Song and Lim, 2004), suggesting that RASSF1A may reduce YAP1 binding to LATS1 in order to release YAP1 for nuclear translocation. This seems to be the case. RASSF1A, but not RASSF1C, caused a translocation of YAP1 from the cytoplasm to the nucleus as shown by cell fractionation (Figure 5A). Similarly, downregulation of LATS1 by siRNA promoted YAP1 nuclear localization (Figure S7A), and YAP1 accumulated in the nucleus in LATS1-/- fibroblasts. Reconstitution with myc-tagged LATS1 reversed the nuclear localization of YAP1 in unstimulated cells and in addition made YAP1 nuclear localization dependent on RASSF1A stimulation (Figure S7B). These results confirm that LATS1 serves as a RASSF1A-regulated cytosolic anchor for YAP1.
Figure 5. RASSF1A Induces Subcellular Relocalization of YAP1 and p73, Phosphorylation of YAP1 by LATS1, and Formation of a YAP1-p73 Complex.
(A) MCF7 cells were transfected with control, 1 μg of HA-RASSF1A, or 1 μg of HA-RASSF1C plasmids. Nuclear and cytoplasmic fractions were examined for the presence of p73 and YAP1. Lamin A and HSP70 were monitored as markers for the purity of the nuclear and cytoplasmic fractions, respectively.
(B) MCF7 cells were transfected with control, 1 μg of HA-RASSF1A, or 1 μg of HA-RASSF1C plasmids. After 16 hr serum starvation, p73 immunoprecipitates were assessed for the copurification of YAP1 by western blotting.
(C) Endogenous LATS1 immunoprecipitates prepared from RASSF1A-transfected or control MCF7 cells were incubated with purified bacterial GST-YAP1 in the presence of 32P-γ-ATP, showing that RASSF1A stimulates YAP1 phosphorylation by LATS1.
(D) A kinase-dead myc-tagged LATS-1 mutant (myc-LATS1-KD) did not phosphorylate YAP1, showing that the phosphorylation is due to LATS1.
(E) Recombinant GST-YAP1 was phosphorylated by LATS1 in vitro. Association of phosphorylated (pYAP) and nonphosphorylated YAP with in vitro-translated HA-p73α was determined by coimmunoprecipitation and western blotting for GST in HA immunoprecipitates.
In addition, RASSF1A, but not RASSF1C, also caused a redistribution of the p73 protein from the cytosol to the nucleus (Figure 5A). Concomitantly, the interaction between endogenous YAP1 and p73 increased robustly (Figure 5B). This enhancement of p73-YAP1 association seems mediated by LATS1 phosphorylation of YAP1. LATS1-phosphorylated recombinant YAP1 in vitro, and its kinase activity toward YAP1, was greatly increased when LATS1 was immunoprecipitated from RASSF1A-transfected cells (Figure 5C). A kinase-negative LATS1 mutant failed to phosphorylate YAP1, demonstrating that phosphorylation is due to genuine LATS1 kinase activity and not to a contaminating kinase (Figure 5D). Interestingly, phosphorylated YAP1 bound stronger to p73 (Figure 5E), explaining why LATS1 stimulates the association between p73 and YAP1. These data suggest a dual role for LATS1. First, LATS1 is a cytosolic anchor for its substrate LATS1 in unstimulated cells. Second, in response to RASSF1A, it phosphorylates YAP1, promoting YAP1 binding to p73 and nuclear translocation.
RASSF1A Promotes p73-YAP1-Mediated Transcription of the Proapoptotic Gene puma and p53-Independent Apoptosis
YAP1 can stabilize p73 by preventing nuclear export and subsequent degradation (Basu et al., 2003; Dobbelstein et al., 2005; Melino et al., 2004). It also functions as a coactivator of p73-dependent gene transcription that directs p73 to specifically transcribe proapoptotic genes such as bax, p53AIP1(Strano et al., 2005), and puma (Melino et al., 2004). Therefore, we investigated whether the RASSF1A-induced p73-YAP1 complex could mediate the transcription of proapoptotic genes. Although Bax levels did not change in response to RASSF1A, puma mRNA was strongly induced (Figure S8A). Likewise, RASSF1A, but not RASSF1C, could enhance the expression of PUMA protein (Figure 6A), while BAX protein levels were unaffected (Figure 6B). The induction of PUMA expression correlated with enhanced occupancy of the puma gene promoter by both p73 and YAP1 as shown by chromatin immunoprecipitation (Figure S8B), suggesting that the p73-YAP1 complex directly enhances transcription of the puma gene.
Figure 6. A YAP1-p73 Complex Induces PUMA Expression and Mediates RASSF1A-Induced Apoptosis.
(A) MCF7 cells were transfected with control, 1 μg of HA-RASSF1A, or HA-RASSF1C plasmids and serum starved for 16 hr. Extracts were immunoblotted for endogenous PUMA levels, and tubulin was used as loading control.
(B) MCF7 cells were transfected with 1 μg of HA-RASSF1A and 70 ng/ml siRNA against YAP1 or control in the indicated combinations. Cell extracts were immunoblotted for endogenous protein levels of PUMA, Bax, YAP1, and tubulin (as loading control). Transfected HA-RASSF1A was detected with anti-HA antibody.
(C) MCF7 cells were transfected as in (B) and examined for apoptosis by using the zVAD-fmk-FITC method. Endogenous YAP1, tubulin, and exogenous HA-RASSF1A levels were determined by western blotting.
(D) MCF7 cells were transfected with 1 μg of HA-RASSF1A and 50 ng/ml p73 siRNA in the combinations indicated. Apoptosis was determined as above. Endogenous p73, tubulin, and exogenous HA-RASSF1A levels were determined by western blotting.
(E) Saos2 cells with doxycycline-inducible HA-p73α were transfected with Flag-RASSF1A or empty vector. Apoptosis was determined by FACS analysis of zVAD-fmk-FITC in the presence or absence of HA-p73α induction (doxycycline for 24 hr).
In (C)–(E), error bars indicate standard deviation.
The RASSF1A-induced increase of PUMA protein levels could be prevented by siRNA-mediated downregulation of YAP1 (Figure 6B), and RASSF1A could not induce PUMA in MCF7 cells with a stable shRNA-mediated knockdown of YAP1 (data not shown). YAP1 downregulation substantially inhibited the induction of apoptosis in response to RASSF1A (Figure 6C). Similarly, the downregulation of p73 also reduced RASSF1A-triggered apoptosis (Figure 6D), suggesting that RASSF1A uses the p73-YAP1 protein complex to mediate transcription of puma and induce apoptosis. Although LATS1 downregulation promoted the nuclear accumulation of YAP1 (Figure S7), the elimination of LATS1 was not sufficient to enhance PUMA expression (Figures S9A and S9B). PUMA expression was dependent on the expression of both LATS1 and RASSF1A (Figure S9A) and in the case of LATS1-/- cells required reconstitution with LATS1 as well as RASSF1A stimulation (Figure S9C). These findings support the conclusion that LATS1 has a dual function. Besides retaining YAP1 in the cytosol, it also is needed to enable the formation of a YAP1 and p73 complex by phosphorylating YAP1 (Figures 5C–5E). This phosphorylation is stimulated by RASSF1A, which activates LATS1 kinase activity (Figure 5C) and enables the formation of a functional p73-YAP1 transcriptional complex competent to enhance puma gene expression (Figure S8).
Apoptosis regulation is commonly more tightly linked with p53 than with p73, and although p73 can induce apoptosis, it is not entirely clear under which conditions (Dobbelstein et al., 2005). In addition, p73 can induce apoptosis by p53-dependent as well as p53-independent mechanisms (Ramadan et al., 2005). To address a potential role of p53 in RASSF1A-induced apoptosis, we used Saos2 cells, which are deficient in p73 and p53 and have been engineered to express a doxycycline-inducible p73 transgene. These cells were refractory to RASSF1A-mediated apoptosis but became responsive when p73 expression was induced by doxycycline (Figure 6E). This mode of RASSF1A-induced apoptosis could be inhibited by siRNA downregulation of MST2, LATS1, and YAP1 (Figure S10), confirming the dependency of RASSF1A-mediated apoptosis on MST2, LATS1, YAP1, and p73 also in this cell system. Further, as Saos2 cells are devoid of p53, these results also demonstrate that p73 does not require p53 to induce apoptosis in response to RASSF1A.
DISCUSSION
Examining the mechanism underlying the promotion of apoptosis by RASSF1A, we show that in mammalian cells RASSF1A can engage p73-directed transcription of the proapoptotic puma gene. Signaling through this pathway is tractable through regulated changes in protein interactions. Quantitative proteomics methods that can capture dynamic changes in protein expression or interactions (Gingras et al., 2007; Ong and Mann, 2005) now permit the systematic mapping of signaling pathways and eventually also the exploration of the network structures of signaling pathways. Our results suggest the following model (Figure 7). RASSF1A activates MST2 kinase by releasing it from the inhibitory complex with Raf1 and allowing it to bind to its substrate LATS1. This in turn stimulates the phosphorylation and subsequent release of YAP1 from LATS1, followed by translocation of YAP1 into the nucleus, where phosphorylated YAP1 binds to p73 and stimulates transcription of the proapoptotic puma gene. The activation of this proapoptotic signaling pathway is specific for RASSF1A, while RASSF1C is completely inert. This selectivity further suggests that this pathway may be important in tumors where RASSF1A expression is lost, while RASSF1C expression is usually retained (Agathanggelou et al., 2005). Silencing of RASSF1A removes the ability to promote proapoptotic signaling through the MST2 route. This pathway also may contribute to Fas death receptor signaling, which is part of antitumor surveillance mechanisms that restrict tumor cell survival.
Figure 7. Model for RASSF1A Activation of puma Expression.
Fas activation releases MST2 from the inhibitory complex with Raf-1 and induces binding to RASSF1A, leading to MST2 activation. RASSF1A further promotes the interaction of MST2 with its substrate, LATS1. This in turn induces the phosphorylation of YAP1 by LATS1 and dissociation. YAP1 phosphorylation increases the affinity for p73, permitting YAP1 to form a nuclear complex with p73, which induces the transcription of the proapoptotic puma gene.
The Fas pathway is emerging as a central target for Raf1. Raf1-/- knockout mice are hypersensitive to Fas-mediated apoptosis (Figure 2E), a defect that can be largely rescued by crossing Raf1-/- mice to mice heterozygous for functional Fas or Fas ligand, thereby reducing Fas or Fas ligand gene dosis (Piazzolla et al., 2005). Raf1 can interfere with Fas signaling at several levels. By inhibiting ROK-α kinase, Raf1 can regulate Fas surface expression and clustering, restraining Fas signaling at physiological levels during development (Piazzolla et al., 2005). Further, Raf1 can interfere with Fas signaling by preventing MST2 activation (O’Neill et al., 2004) and binding of MST2 to RASSF1A (Figure 1). Interestingly, Fas can both activate MST2 (independent of caspase activation) and regulate the LATS1-YAP1 interaction. However, we could not conclusively determine regulation of YAP1-p73 association in response to Fas (data not shown). The reason is unclear but may indicate that the endogenous RASSF1A expression in HeLa cells is too low to promote phosphorylation or that additional signals, which are circumvented by RASSF1A overexpression, are needed to regulate the YAP1-p73 complex. Such signals may include Akt, which has been shown to phosphorylate YAP1 at serine 127, thereby creating a docking site for 14-3-3, which sequesters YAP1 in the cytosol (Basu et al., 2003). Our results suggest that LATS1 also can sequester YAP1 in the cytosol. Downregulation of LATS1 expression leads to nuclear translocation of YAP1. The induction of puma further requires phosphorylation of YAP1 by LATS1, which facilitates the formation of a YAP1-p73 complex that binds to the puma gene promoter and induces puma transcription. Thus, LATS1 has a dual function as an anchoring protein that retains YAP1 in the cytosol and as a kinase that phosphorylates YAP1 to facilitate its association with p73.
p73 can trigger apoptosis by inducing Bax translocation to mitochondria in a PUMA-dependent manner (Melino et al., 2004). RASSF1A can induce apoptosis by promoting MAP-1 binding to Bax, which forces Bax into its proapoptotic conformation and insertion into the mitochondrial membrane (Baksh et al., 2005). We also observed conformational activation and translocation of Bax to the mitochondria in response to RASSF1A (Figure S11). Thus, Bax may be a target for two converging RASSF1A activated pathways: one via p73-PUMA and another via MAP-1. The exact contributions of these pathways to apoptosis are likely to be dependent on a fine balance between respective protein expression levels and hence may vary between different cellular conditions and cell types. These findings also highlight the central position of RASSF1A as regulator of apoptosis and hence may explain why its expression is so frequently lost in cancer.
The central module of this pathway, MST2/Hippo-LATS1/Warts-YAP1/Yorkie, is conserved in D. melanogaster controlling apoptosis and cell-cycle progression (Edgar, 2006; O’Neill et al., 2005). However, there are important differences. In Drosophila, upstream input into the Hippo pathway is coming through Merlin and Expanded (Hamaratoglu et al., 2006), two cytoskeletal proteins that regulate cell shape and adhesion (Edgar, 2006). In mammalian cells, proapoptotic input comes through RASSF1A. D. melanogaster only has one RASSF homolog, termed dRASSF, which is most closely related to mammalian RASSF2 and distantly to RASSF1A. In contrast to mammalian cells, where RASSF1A stimulates MST kinases (Oh et al., 2006) or targets them to substrates (Praskova et al., 2004), dRASSF impairs Hippo activity (Polesello et al., 2006). Further, it is also unlikely that Raf controls the Hippo pathway, because the single D. melanogaster Raf gene is homologous to mammalian B-Raf, which cannot interact with MST2 (O’Neill et al., 2004). Downstream, Yorkie seems to be strictly antiapoptotic in D. melanogaster, whereas its mammalian homolog, YAP1, has a more diversified function.
YAP1 has been found amplified in breast cancer (Overholtzer et al., 2006) and liver cancer where it can accelerate tumorigenesis (Zender et al., 2006). However, YAP1 also can divert p73 to selectively activate the transcription of proapoptotic genes (Melino et al., 2004; Strano et al., 2005). In order to further investigate the role of YAP1 in human tumors, we mined gene array expression datasets and tested for correlations between YAP1 expression and clinical outcome. In three out of four breast cancer studies with documented clinical endpoints, we found no significant correlation between YAP1 expression and survival. One study, which included the largest number of patients (n = 251, with 136 presenting changes in YAP1 expression), showed a significant positive correlation of YAP1 expression with survival (Figure S12). Although these data do not support a role of YAP1 as oncogene in human cancer, the situation may be more complicated. In the liver cancer model, YAP1 was coamplified with cIAP1, an inhibitor of apoptosis (Zender et al., 2006). This finding is intriguing in several aspects. It indicates that the transforming potential of YAP1 may be reliant on the coexpression of antiapoptotic genes. In Drosophila, YAP1 enhances the expression of dIAP (inhibitor of apoptosis) and cyclin E (Harvey et al., 2003; Udan et al., 2003; Wu et al., 2003). Thus, a selection for the concomitant amplification of YAP1 and cIAP1 in cancer is surprising, as increasing YAP1 gene dosage should also increase IAP levels, if the mammalian MST2 pathway was a simple functional replica of the Drosophila Hippo pathway. However, this is not the case. Despite repeated attempts, we could not obtain convincing evidence that MST2 regulates the expression of IAPs and cyclin E in mammalian cells (data not shown). These data suggest that the biological outcome of YAP1 signaling may be highly context dependent. Although clearly YAP1 can engage proapoptotic signaling pathways, it also may have oncogenic potential in situations where its proapoptotic signaling is thwarted. Such apparently mutually exclusive functions in promoting cell transformation and apoptosis alike were first discovered as a property of Myc (Evan et al., 1992). YAP1 seems a similar case. What causes this functional dichotomy is currently unknown but may be dictated by the interaction partners. An obvious and attractive speculation is that one of these crucial interaction partners is p73. As D. melanogaster has no p73 homolog (Sutcliffe and Brehm, 2004), Yorkie may be unable to engage proapoptic signaling. These data suggest that mammalian cells use the core of an ancient signaling pathway but have reengineered it to serve more complex requirements of mammalian cell signaling by linking it to new inputs and outputs.
EXPERIMENTAL PROCEDURES
Reagents and Cells
HEK293T, MCF7, and HeLa cells were grown in DMEM plus 10% fetal calf serum (GIBCO-BRL). Transient transfection used Lipofectamine 2000 (Invitrogen) according to the manufacturer’s instructions. HA-RASSF1A and HA-RASSF1C have been described (Ortiz-Vega et al., 2002). MCF7 cells stably expressing shRNA against YAP1 were a kind gift from Subham Basu (Basu et al., 2003). Antibodies are described in the Supplemental Data.
siRNA and shRNAs
Typically, between 50 and 100 ng/ml siRNA duplexes were transfected with Lipofectamine 2000. In order to avoid unspecific effects, several different siRNAs and shRNAs have been used for the knockdown of each protein. For detailed information, see the Supplemental Data.
Immunoprecipitation and Immunoblotting
Cells were lysed in 20 mM HEPES (pH 7.5), 150 mM NaCl, 1% NP-40, 2 mM NaF, 10 mM β-glycerophosphate, 2 mM Na4P2O4, and protease and phosphatase inhibitors. Immunoprecipitates were washed three times with lysis buffer containing 0.5% NP-40, separated by SDS-PAGE, and analyzed by western blotting. For YAP1-p73 coimmunoprecipitations, cells were lysed in 50 mM Tris (pH 8), 100 mM NaCl, 10% glycerol, 1% Triton X-100, 1 mM MgCl2, 2 mM PMSF, and protease and phosphatase inhibitors. These immunoprecipitates were washed three times with NET-buffer (50 mM Tris [pH 7.5], 150 mM NaCl, 1 mM EDTA, 0.25% gelatine, and 0.1% Nonidet P-40) and immunoblotted as described (Strano et al., 2001). Nucleo-cytoplasmic fractionation was performed with NE-PER nuclear cytoplasmic extraction reagents (Pierce).
Kinase Assays and LATS1-p73 Association
In-gel kinase assays were performed as previously described (O’Neill et al., 2004). LATS1 kinase assay was performed by incubating endogenous LATS1 immunoprecipitates with bacterial-purified GST-YAP (Basu et al., 2003) in a kinase assay buffer consisting of 25 mM Tris-HCl (pH 7.5), 5 mM β-glycerophosphate, 2 mM dithiothreitol (DTT), 0.1 mM Na3VO4, 10 mM MgCl2, 50 μM ATP, and 32P-ATP. Reactions were incubated for 90 min at 30°C prior to either SDS-PAGE electrophoresis and detection on a BioRad PhosphorImager or incubated with in vitro-transcribed and -translated (Promega) HA-p73a for a further 30 min in the same buffer.
Apoptosis Assays
Samples for apoptosis assays were divided in two aliquots that were analyzed by FACS for (1) DNA fragmentation using propidium iodide staining for determination of the SubG1 fraction as previously described (O’Neill et al., 2004) and (2) caspase activity by using the fluorescent cleavable substrate of caspases zVAD-fmk-FITC (Promega) according to the manufacturer’s instructions. Results shown indicate fold increase in SubG1 population or increase in cleavage of zVAD-fmk-FITC. All error bars show standard deviation.
Supplementary Material
ACKNOWLEDGMENTS
We thank Farida Latif and Ashraf Dallol for RASSF1 expression vectors, Gerd Pfeifer for RASSF1A-/- MEFs, Tian Xu for LATS1-/- MEFs, Subham Basu for YAP1 reagents, Karen Vousden, Bob Ludwig, and Karim Bensaad for advice and p73 and PUMA reagents, and the Sir Henry Wellcome Functional Genomics Facility for mass spectrometry. This work was supported by Cancer Research UK, the European Union FP6 grants “Interaction Proteome” (LSHG-CT-2003-505520 to W.K.) and “Growthstop” (LSHC-CT-2006-037731 to W.K. and M.B.), and the Wellcome Trust.
Footnotes
Supplemental Data Supplemental Data include Supplemental Experimental Procedures, Supplemental References, and twelve figures and can be found with this article online at http://www.molecule.org/cgi/content/full/27/6/962/DC1/.
REFERENCES
- Agathanggelou A, Cooper WN, Latif F. Role of the Ras-association domain family 1 tumor suppressor gene in human cancers. Cancer Res. 2005;65:3497–3508. doi: 10.1158/0008-5472.CAN-04-4088. [DOI] [PubMed] [Google Scholar]
- Baksh S, Tommasi S, Fenton S, Yu VC, Martins LM, Pfeifer GP, Latif F, Downward J, Neel BG. The tumor suppressor RASSF1A and MAP-1 link death receptor signaling to Bax conformational change and cell death. Mol. Cell. 2005;18:637–650. doi: 10.1016/j.molcel.2005.05.010. [DOI] [PubMed] [Google Scholar]
- Balint EE, Vousden KH. Activation and activities of the p53 tumour suppressor protein. Br. J. Cancer. 2001;85:1813–1823. doi: 10.1054/bjoc.2001.2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu S, Totty NF, Irwin MS, Sudol M, Downward J. Akt phosphorylates the Yes-associated protein, YAP, to induce interaction with 14–3-3 and attenuation of p73-mediated apoptosis. Mol. Cell. 2003;11:11–23. doi: 10.1016/s1097-2765(02)00776-1. [DOI] [PubMed] [Google Scholar]
- Brennecke J, Hipfner DR, Stark A, Russell RB, Cohen SM. bantam encodes a developmentally regulated microRNA that controls cell proliferation and regulates the proapoptotic gene hid in Drosophila. Cell. 2003;113:25–36. doi: 10.1016/s0092-8674(03)00231-9. [DOI] [PubMed] [Google Scholar]
- Chan EH, Nousiainen M, Chalamalasetty RB, Schafer A, Nigg EA, Sillje HH. The Ste20-like kinase Mst2 activates the human large tumor suppressor kinase Lats1. Oncogene. 2005;24:2076–2086. doi: 10.1038/sj.onc.1208445. [DOI] [PubMed] [Google Scholar]
- Cheung WL, Ajiro K, Samejima K, Kloc M, Cheung P, Mizzen CA, Beeser A, Etkin LD, Chernoff J, Earnshaw WC, Allis CD. Apoptotic phosphorylation of histone H2B is mediated by mammalian sterile twenty kinase. Cell. 2003;113:507–517. doi: 10.1016/s0092-8674(03)00355-6. [DOI] [PubMed] [Google Scholar]
- Cox AD, Der CJ. The dark side of Ras: regulation of apoptosis. Oncogene. 2003;22:8999–9006. doi: 10.1038/sj.onc.1207111. [DOI] [PubMed] [Google Scholar]
- Dallol A, Agathanggelou A, Fenton SL, Ahmed-Choudhury J, Hesson L, Vos MD, Clark GJ, Downward J, Maher ER, Latif F. RASSF1A interacts with microtubule-associated proteins and modulates microtubule dynamics. Cancer Res. 2004;64:4112–4116. doi: 10.1158/0008-5472.CAN-04-0267. [DOI] [PubMed] [Google Scholar]
- Dobbelstein M, Strano S, Roth J, Blandino G. p73-induced apoptosis: a question of compartments and cooperation. Biochem. Biophys. Res. Commun. 2005;331:688–693. doi: 10.1016/j.bbrc.2005.03.155. [DOI] [PubMed] [Google Scholar]
- Edgar BA. From cell structure to transcription: Hippo forges a new path. Cell. 2006;124:267–273. doi: 10.1016/j.cell.2006.01.005. [DOI] [PubMed] [Google Scholar]
- Evan GI, Wyllie AH, Gilbert CS, Littlewood TD, Land H, Brooks M, Waters CM, Penn LZ, Hancock DC. Induction of apoptosis in fibroblasts by c-myc protein. Cell. 1992;69:119–128. doi: 10.1016/0092-8674(92)90123-t. [DOI] [PubMed] [Google Scholar]
- Evan GI, Brown L, Whyte M, Harrington E. Apoptosis and the cell cycle. Curr. Opin. Cell Biol. 1995;7:825–834. doi: 10.1016/0955-0674(95)80066-2. [DOI] [PubMed] [Google Scholar]
- Gingras AC, Gstaiger M, Raught B, Aebersold R. Analysis of protein complexes using mass spectrometry. Nat. Rev. Mol. Cell Biol. 2007;8:645–654. doi: 10.1038/nrm2208. [DOI] [PubMed] [Google Scholar]
- Guo C, Tommasi S, Liu L, Yee JK, Dammann R, Pfeifer GP. RASSF1A is part of a complex similar to the Drosophila Hippo/Salvador/Lats tumor-suppressor network. Curr. Biol. 2007;17:700–705. doi: 10.1016/j.cub.2007.02.055. [DOI] [PubMed] [Google Scholar]
- Hamaratoglu F, Willecke M, Kango-Singh M, Nolo R, Hyun E, Tao C, Jafar-Nejad H, Halder G. The tumour-suppressor genes NF2/Merlin and Expanded act through Hippo signalling to regulate cell proliferation and apoptosis. Nat. Cell Biol. 2006;8:27–36. doi: 10.1038/ncb1339. [DOI] [PubMed] [Google Scholar]
- Harvey KF, Pfleger CM, Hariharan IK. The Drosophila Mst ortholog, hippo, restricts growth and cell proliferation and promotes apoptosis. Cell. 2003;114:457–467. doi: 10.1016/s0092-8674(03)00557-9. [DOI] [PubMed] [Google Scholar]
- Hisaoka M, Tanaka A, Hashimoto H. Molecular alterations of h-warts/LATS1 tumor suppressor in human soft tissue sarcoma. Lab. Invest. 2002;82:1427–1435. doi: 10.1097/01.lab.0000032381.68634.ca. [DOI] [PubMed] [Google Scholar]
- Huang J, Wu S, Barrera J, Matthews K, Pan D. The Hippo signaling pathway coordinately regulates cell proliferation and apoptosis by inactivating Yorkie, the Drosophila Homolog of YAP. Cell. 2005;122:421–434. doi: 10.1016/j.cell.2005.06.007. [DOI] [PubMed] [Google Scholar]
- Jesenberger V, Procyk KJ, Ruth J, Schreiber M, Theussl HC, Wagner EF, Baccarini M. Protective role of Raf-1 in Salmonella-induced macrophage apoptosis. J. Exp. Med. 2001;193:353–364. doi: 10.1084/jem.193.3.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehtinen MK, Yuan Z, Boag PR, Yang Y, Villen J, Becker EB, DiBacco S, de la Iglesia N, Gygi S, Blackwell TK, Bonni A. A conserved MST-FOXO signaling pathway mediates oxidative-stress responses and extends life span. Cell. 2006;125:987–1001. doi: 10.1016/j.cell.2006.03.046. [DOI] [PubMed] [Google Scholar]
- Melino G, Bernassola F, Ranalli M, Yee K, Zong WX, Corazzari M, Knight RA, Green DR, Thompson C, Vousden KH. p73 Induces apoptosis via PUMA transactivation and Bax mitochondrial translocation. J. Biol. Chem. 2004;279:8076–8083. doi: 10.1074/jbc.M307469200. [DOI] [PubMed] [Google Scholar]
- O’Neill E, Rushworth L, Baccarini M, Kolch W. Role of the kinase MST2 in suppression of apoptosis by the proto-oncogene product Raf-1. Science. 2004;306:2267–2270. doi: 10.1126/science.1103233. [DOI] [PubMed] [Google Scholar]
- O’Neill EE, Matallanas D, Kolch W. Mammalian sterile 20-like kinases in tumor suppression: an emerging pathway. Cancer Res. 2005;65:5485–5487. doi: 10.1158/0008-5472.CAN-05-1453. [DOI] [PubMed] [Google Scholar]
- Oh HJ, Lee KK, Song SJ, Jin MS, Song MS, Lee JH, Im CR, Lee JO, Yonehara S, Lim DS. Role of the tumor suppressor RASSF1A in Mst1-mediated apoptosis. Cancer Res. 2006;66:2562–2569. doi: 10.1158/0008-5472.CAN-05-2951. [DOI] [PubMed] [Google Scholar]
- Ong SE, Mann M. Mass spectrometry-based proteomics turns quantitative. Nat. Chem. Biol. 2005;1:252–262. doi: 10.1038/nchembio736. [DOI] [PubMed] [Google Scholar]
- Ortiz-Vega S, Khokhlatchev A, Nedwidek M, Zhang XF, Dammann R, Pfeifer GP, Avruch J. The putative tumor suppressor RASSF1A homodimerizes and heterodimerizes with the Ras-GTP binding protein Nore1. Oncogene. 2002;21:1381–1390. doi: 10.1038/sj.onc.1205192. [DOI] [PubMed] [Google Scholar]
- Overholtzer M, Zhang J, Smolen GA, Muir B, Li W, Sgroi DC, Deng CX, Brugge JS, Haber DA. Transforming properties of YAP, a candidate oncogene on the chromosome 11q22 amplicon. Proc. Natl. Acad. Sci. USA. 2006;103:12405–12410. doi: 10.1073/pnas.0605579103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantalacci S, Tapon N, Leopold P. The Salvador partner Hippo promotes apoptosis and cell-cycle exit in Drosophila. Nat. Cell Biol. 2003;5:921–927. doi: 10.1038/ncb1051. [DOI] [PubMed] [Google Scholar]
- Piazzolla D, Meissl K, Kucerova L, Rubiolo C, Baccarini M. Raf-1 sets the threshold of Fas sensitivity by modulating Rok-alpha signaling. J. Cell Biol. 2005;171:1013–1022. doi: 10.1083/jcb.200504137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polesello C, Huelsmann S, Brown NH, Tapon N. The Drosophila RASSF homolog antagonizes the hippo pathway. Curr. Biol. 2006;16:2459–2465. doi: 10.1016/j.cub.2006.10.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Praskova M, Khoklatchev A, Ortiz-Vega S, Avruch J. Regulation of the MST1 kinase by autophosphorylation, by the growth inhibitory proteins, RASSF1 and NORE1, and by Ras. Biochem. J. 2004;381:453–462. doi: 10.1042/BJ20040025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramadan S, Terrinoni A, Catani MV, Sayan AE, Knight RA, Mueller M, Krammer PH, Melino G, Candi E. p73 induces apoptosis by different mechanisms. Biochem. Biophys. Res. Commun. 2005;331:713–717. doi: 10.1016/j.bbrc.2005.03.156. [DOI] [PubMed] [Google Scholar]
- Song MS, Lim DS. Control of APC-Cdc20 by the tumor suppressor RASSF1A. Cell Cycle. 2004;3:574–576. [PubMed] [Google Scholar]
- St John MA, Tao W, Fei X, Fukumoto R, Carcangiu ML, Brownstein DG, Parlow AF, McGrath J, Xu T. Mice deficient of Lats1 develop soft-tissue sarcomas, ovarian tumours and pituitary dysfunction. Nat. Genet. 1999;21:182–186. doi: 10.1038/5965. [DOI] [PubMed] [Google Scholar]
- Strano S, Munarriz E, Rossi M, Castagnoli L, Shaul Y, Sacchi A, Oren M, Sudol M, Cesareni G, Blandino G. Physical interaction with Yes-associated protein enhances p73 transcriptional activity. J. Biol. Chem. 2001;276:15164–15173. doi: 10.1074/jbc.M010484200. [DOI] [PubMed] [Google Scholar]
- Strano S, Monti O, Pediconi N, Baccarini A, Fontemaggi G, Lapi E, Mantovani F, Damalas A, Citro G, Sacchi A, et al. The transcriptional coactivator Yes-associated protein drives p73 gene-target specificity in response to DNA Damage. Mol. Cell. 2005;18:447–459. doi: 10.1016/j.molcel.2005.04.008. [DOI] [PubMed] [Google Scholar]
- Sutcliffe JE, Brehm A. Of flies and men; p53, a tumour suppressor. FEBS Lett. 2004;567:86–91. doi: 10.1016/j.febslet.2004.03.122. [DOI] [PubMed] [Google Scholar]
- Udan RS, Kango-Singh M, Nolo R, Tao C, Halder G. Hippo promotes proliferation arrest and apoptosis in the Salvador/Warts pathway. Nat. Cell Biol. 2003;5:914–920. doi: 10.1038/ncb1050. [DOI] [PubMed] [Google Scholar]
- Ura S, Masuyama N, Graves JD, Gotoh Y. MST1-JNK promotes apoptosis via caspase-dependent and independent pathways. Genes Cells. 2001;6:519–530. doi: 10.1046/j.1365-2443.2001.00439.x. [DOI] [PubMed] [Google Scholar]
- Vos MD, Dallol A, Eckfeld K, Allen NP, Donninger H, Hesson LB, Calvisi D, Latif F, Clark GJ. The RASSF1A tumor suppressor activates Bax via MOAP-1. J. Biol. Chem. 2006;281:4557–4563. doi: 10.1074/jbc.M512128200. [DOI] [PubMed] [Google Scholar]
- Wu S, Huang J, Dong J, Pan D. hippo encodes a Ste-20 family protein kinase that restricts cell proliferation and promotes apoptosis in conjunction with salvador and warts. Cell. 2003;114:445–456. doi: 10.1016/s0092-8674(03)00549-x. [DOI] [PubMed] [Google Scholar]
- Xu T, Wang W, Zhang S, Stewart RA, Yu W. Identifying tumor suppressors in genetic mosaics: the Drosophila lats gene encodes a putative protein kinase. Development. 1995;121:1053–1063. doi: 10.1242/dev.121.4.1053. [DOI] [PubMed] [Google Scholar]
- Yang A, Kaghad M, Caput D, McKeon F. On the shoulders of giants: p63, p73 and the rise of p53. Trends Genet. 2002;18:90–95. doi: 10.1016/s0168-9525(02)02595-7. [DOI] [PubMed] [Google Scholar]
- Zender L, Spector MS, Xue W, Flemming P, Cordon-Cardo C, Silke J, Fan ST, Luk JM, Wigler M, Hannon GJ, et al. Identification and validation of oncogenes in liver cancer using an integrative oncogenomic approach. Cell. 2006;125:1253–1267. doi: 10.1016/j.cell.2006.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.