Abstract
We have demonstrated that epithelial derived MMP-9 is upregulated during inflammatory bowel disease, mediates tissue damage during colitis and regulates goblet cell differentiation through proteoltic cleavage of Notch-1 in colon. Association of MMP-9 and Notch-1 with colon cancer has been well documented. In this study, we sought to address the role and mechanism by which MMP-9 mediates the colitis-associated colon cancer (CAC). Wild type (WT) and MMP-9 knock-out (MMP-9-/-) mice were used for in vivo studies and enterocyte cell line, Caco2-BBE, were used for in vitro studies. Azoxymethane and dextran sodium sulfate were used to induce CAC. MMP-9-/- mice showed increased susceptibility to CAC as evidenced by increased tumor multiplicity, size and mortality. CAC in MMP-9-/- is associated with increased proliferation and decreased apoptosis compared to CAC in WT mice. MMP-9-/- mice exhibited p21WAF1/Cip1 expression, but increased β-catenin expression compared to WT mice in CAC. In vitro studies of MMP-9 overexpression showed increased Notch-1 activation and reciprocal decrease in β-catenin. Notch and β-catenin/Wnt signaling are well documented as crucial molecular pathways deciding the gut epithelium differentiation and carcinogenesis. Despite being a mediator of pro-inflammatory response, MMP-9 plays a protective role and acts as a tumor suppressor in CAC by modulating Notch activation resulting in the activation of p21WAF1/Cip1 leading to suppression of β-catenin.
Introduction
Matrix metalloproteinases (MMPs) are zinc-dependent neutral endopeptidases which participate in degradation of extra cellular matrix proteins and are important for normal tissue remodeling but are also involved in several pathological processes, such as arthritis, atherosclerosis, myocardial infarction, colorectal cancer, tumor invasion and Inflammatory Bowel Disease (IBD) (1) (2) (3). MMP-9 is a 92 kD protein, one of the two MMPs known as gelatinases. MMP-9 is absent from most normal adult tissues including the intestinal epithelial cells. However, we and several others have shown MMP-9 is highly expressed during intestinal inflammation in different animal models and human IBD (4) (5). We have also shown that MMP-9-/- mice exposed to dextran sodium sulfate (DSS) or Salmonella typhimurium (ST) had dramatically reduced inflammation and mucosal injury and showed protection against acute colitis indicating that MMP-9 is a mediator of inflammation (4). Further, our studies showed that epithelial- but not immune cell- expressed MMP-9 mediates intestinal inflammation.
In addition to its role in inflammation, recent studies from our laboratory have demonstrated that MMP-9 plays a role in epithelial cell differentiation. We demonstrated that MMP-9-/- mice have increased secretory lineage as evidenced by increased MUC-2 expression and goblet cells and decreased absorptive epithelial cells as demonstrated by carbonic anhydrase. Being a protease, we reasoned that MMP-9 may play a role in cleaving Notch-1, a transcripton factor that plays a pivotal role in determining epithelial cell lineages and requires metalloproteinase for its cleavage (6). Indeed, MMP-9 cleaves Notch-1 and activation of Notch-1 is inhibited in MMP-9-/- mice (7). Recent studies have implicated Notch-1 signaling in the pathogenesis of colon cancer and given, the role of MMP-9 in Notch-1 activation and inflammation, in this study we addressed the role of MMP-9 in colitis-associated colon cancer (CAC).
IBD, which includes ulcerative colitis (UC) and Crohn’s disease (CD) is a chronic inflammatory disease of the intestine that affects 1 in 200 to 1 in 1000 individuals in the U.S. (8). UC and CD are characterized by relapses (acute flare) and remission which involve immune-mediated tissue injury followed by repair (9, 10). CAC is an important complication of UC or colonic Crohn’s disease that results in significant morbidity and mortality (11). Indeed, it causes 1/6 of all deaths in patients with ulcerative colitis and thus is a dreaded complication of the disease (12). The incidence of colon cancer is increased by 2% at 10 years, 8% at 20 years and 18% at 30 years in patients with ulcerative colitis and the risk of developing colon cancer correlates with the extent, duration and severity of disease (13). Although both sporadic colon cancer and CAC are malignancies of the colon, several features make CAC distinct from sporadic colon cancer (14). For example, unlike most sporadic colon cancers that arise from adenomatous polyps, colon cancer develops commonly in flat dysplastic tissue among the individuals pre-exposed to IBD. In addition, CAC is often multiple, anaplastic, broadly infiltrating, rapidly growing and occurs at a younger age. Genetic alterations also differ in CAC compared to sporadic colon cancer (15) (16). The pathogenesis of CAC is poorly understood and remarkably, the role of MMP-9 in CAC has not been explored. The role of MMP-9 in colon cancer is well recognized. MMP-9 has also been implicated to play a role in colon cancer progression and metastasis in animal models as well as in humans (17). It has been documented that MMP-9 regulates metastatic progression in colorectal cancer (18) and was found to be expressed by macrophages, neutrophils, and mast cells. Further, MMP-9 is found to generate angiostatin which is a crucial factor controlling the growth rate of certain tumors (19).
Though individual and independent roles of MMP-9 during inflammation and cancer in colon or different organs have been documented very well, the function of MMP-9 in mediating inflammation-associated colon cancer has not been studied. The aim of the present study is to understand the role of MMP-9 in an animal model of CAC.
Materials and Methods
Animal Models
See supplementary data.
Induction of AOM+DSS- induced cancer
Age (9-10 weeks-old) and sex matched C57B6 MMP-9-/- mice and their WT littermates were injected intraperitoneally with 7.6 mg/kg azoxymethane (AOM, Sigma, St. Louis, MO) on day 0. On day 7, one group (20) of both WT and MMP-9-/- mice, were exposed to 3% (wt/vol) dextran sodium sulfate (DSS, ICN Biomedicals, Aurora, OH, USA) by oral administration through their drinking water ad libitum for 7 days. On day 14, their water was changed to regular drinking water. On day 28, their drinking water was changed back to 3% DSS for a 2nd cycle of DSS exposure. After a week, these mice were returned to regular drinking water and were sacrificed on day 56. Their colons were opened longitudinally. We monitored body weight, stool consistency, and stool occult blood of all the mice during the DSS treatment and recovery phase. One group of mice were assessed for CAC mortality. To perform mortality studies, the same protocol was followed with the exception of one cycle of DSS (21) and animals were followed for 140 days or when the mice developed rectal prolapses and/or >20% body weight loss. For both the long term (140 days) and short (56 days) protocols, one group of mice were maintained with regular water and were sacrificed after 140 days and 56 days respectively. After macroscopic assessment for polyps, tissue sections were embedded in paraffin. Colon of the WT and MMP-9-/- mice were cut open and the number and size of the polyps were determined under the Zeiss microscope (Olympus, Center Valley, PA).
Protein Extraction and Western Blot Analysis
See supplementary data.
Ki-67 Staining
See supplementary data.
TUNEL staining
See supplementary data.
Cell culture and transfection
See supplementary data.
Statistical Analysis
See supplementary data.
Results
MMP-9 is highly expressed in CAC
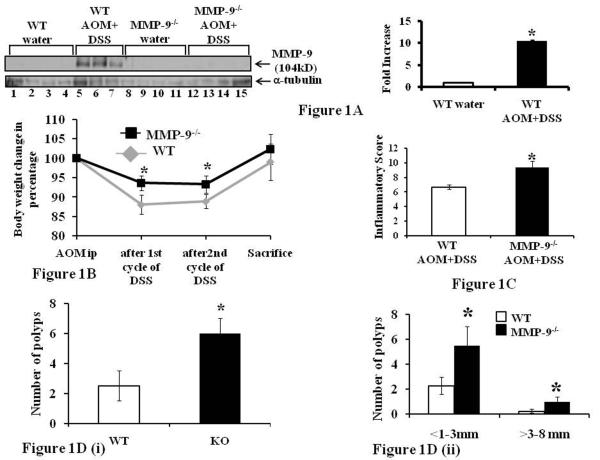
MMP-9-/- and WT mice were euthanized following short term protocol (56 days) and protein lysates were prepared from the colon mucosal stripping for western blot analysis as described in the Methods section. Western Blot analysis showed that MMP-9-/- mice induced with or without CAC lacked MMP-9 protein expression (Figure 1A, lanes 8-15). WT mice given water showed no MMP-9 protein expression (Figure 1A, lanes 1-4) but WT mice treated induced with CAC showed increased MMP-9 protein expression (Figure 1A, lanes 5-7). Densitometry analysis shown by the graphs revealed a 10.51±0.21 fold increase in MMP-9 protein expression in WT mice induced with CAC compared to WT mice treated with water (Mean ±S.E., 6 mice per group p<0.05) (Figure 1A).
Figure 1.
MMP-9 is highly expressed in CAC and MMP-9-/- mice show increased susceptibility to CAC. WT and MMP-9-/- mice (mice, n= 6 each group) were treated once with AOM and twice with 3% DSS to induce CAC. Mice were weighed once a week and were sacrificed after 56 days. 1A: western blot of proteins from the mucosal stripping of the colons of WT and MMP-9-/- mice with and without CAC probed with anti-MMP-9. Each lane shows protein (30μg/lane) from an individual mouse with or without CAC induction. Western blots were quantitated by scanning densitometry. Values are representative of three experiments, each bar represents mean ± S.E., *p<0.05. 1B: change in body weight of WT and MMP-9-/- mice (mice, n= 10 each group). 1C: inflammatory score, 1D(i): polyps count and 1D(ii): size of the polyp measured in diameter as described in the Methods section. Each bar represents mean ± S.E., *p< 0.05.
MMP-9-/- mice show increased susceptibility to CAC
MMP-9-/- and WT mice (mice, n= 10 each group) were treated with AOM/DSS to induce CAC and were sacrificed after 56 days. Mice were weighed once a week and the change in body weight shows no significant change in the body weight of MMP-9-/- mice after 1st and 2nd cycles of 3% DSS while there was a significant decrease in the body weight of WT mice after 1st and 2nd cycles of 3% DSS compared to initial body weight (Figure 1B). Both MMP-9-/- and WT mice showed no significant change in body weight at the end of 56 days as shown in Figure 1B. Mice were sacrificed after 56 days and the number of polyps were counted and measured in diameter with the help of Zeiss microscope. Colons were then processed for histology and inflammatory score was determined by H&E staining. Figure 1C shows that MMP-9-/- mice had significantly higher inflammatory score of 9.4±0.88 compared to WT mice (inflammatory score of 6.7±0.34) induced with CAC (4). Figure 1D (i) shows that there was significantly higher multiplicity of polyps among MMP-9-/- mice (6±1.65) compared to WT (2.5±0.75) mice induced with CAC. Figure 1D (ii) shows that MMP-9-/- mice had significantly more number of polyps in the range of <1-3mm diameter (5.5±1.5) as well as in the range of >3-8 (1.0±0.38) mm diameter compared to WT (2.25±0.5 in the range of <1-3 mm diameter, and 0.23±0.15 in the range of >3-8 mm diameter). Each bar represents mean ± S.E., p< 0.05.
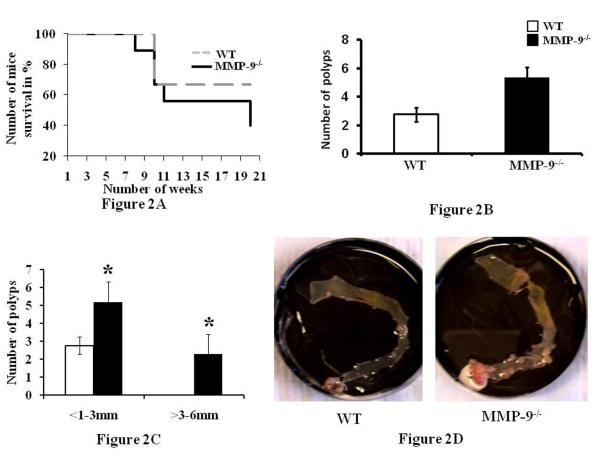
In another set of experiments, we followed mice for an additional 90 days to assess cancer development and mortality. MMP-9-/- and WT mice (mice, n= 10 each group) were induced with CAC as described in Methods section. Mice were weighed once a week and their mortality is represented by Figure 2A which shows the survival curve of MMP-9-/- and WT mice. 10% of MMP-9-/- mice given the CAC induction developed rectal prolapse, reflecting the severity of inflammation and significant weight loss starting at 7 weeks. There was 70% mortality in this group at 20 weeks vs 30% mortality for WT mice induced with CAC. This data indicates higher mortality among MMP-9-/- compared to WTs. After sacrifice, the colon was cut open, stained with methylene blue and the number and size of the polyps were assessed (Figure 2B and 2C). Figure 2B shows that MMP-9-/- mice had significantly increased multiplicity of tumors (5.4±2.04) compared to WT mice (2.8±0.48) and Figure 2C indicates that MMP-9-/- mice had significantly more number of polyps in the range of <1-3mm diameter (3.9±1.9) as well as in the range of >3-8 mm diameter (1.5±1.1) respectively compared to WT (2.8±0.48 in the range of <1-3 mm diameter, and no polyps in the range of >3-8 mm diameter). Each bar represents mean ± S.E., p< 0.05. Figure 2D shows the representative gross anatomy of the colon of the MMP-9-/- mice and WT mice reflecting the number of polyps among them.
Figure 2.
MMP-9-/- mice show increased susceptibility to CAC and mortality. Mortality studies were done as described in the Methods section. 2A: shows the mortality among WT and MMP-9-/- mice induced with CAC and followed for a total of 140 days or were sacrificed if they developed rectal prolapse and/or ≥20% body weight loss. 2B: polyps count and 2C: size of the polyp measured in diameter as described in the Methods section. Each bar represents mean ± S.E., *p< 0.05. 2D: representative gross anatomy of the colon of the MMP-9-/- mice and WT mice reflecting the number of polyps among them.
CAC in MMP-9-/- mice is associated with increased proliferation and decreased apoptosis compared to WT mice
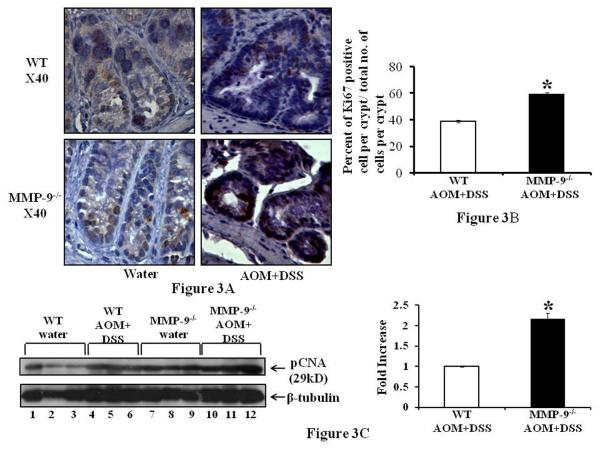
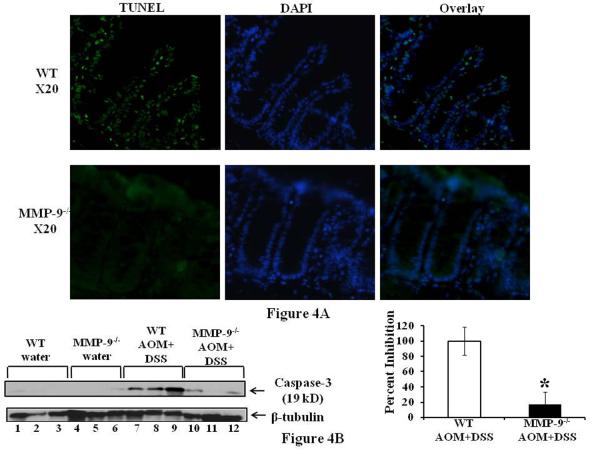
Alteration in cell proliferation is a significant factor in the multistep process of tumorigenesis and Ki67 is a well established marker for the assessment of cell proliferation (22). Figure 3A left panel shows the Ki67 staining as depicted by brown nuclei staining among the crypts of colonic epithelium of MMP-9-/- and WT mice without the induction of CAC indicating no significant difference among them while Figure 3A(i) right panel suggests an increase in the Ki67 staining among the crypts of MMP-9-/- compared to WT mice having CAC. Figure 3B shows the graphs representing the number of nuclei positive for Ki67 staining per crypt and indicates that MMP-9-/- mice had more proliferation (39.4±9.8) compared to WT mice (18.3±6.9) as indicated by the percentage of the number of Ki67 positive nuclei/ crypt. Figure 3C shows the western blot of pCNA (a marker for proliferation) and supports the immunohistochemistry data of Ki67 staining by showing increased protein expression of pCNA among MMP-9-/- mice compared to WT mice, both induced with CAC (lanes 10-12 and lanes 4-6 respectively). Both MMP-9-/- and WT mice showed some constitutive pCNA expression without the induction of CAC, which was higher among MMP-9-/- mice (Figure 3C lanes 7-9 and lanes 1-3 respectively) compared to WT mice. The adjacent graph shows the quantification of western blots by scanning densitometry and indicates a 2.2±0.14 fold increase in pCNA expression among MMP-9-/- mice compared to WT mice induced with AOM and two cycles of DSS. Apoptosis is the intrinsic property of tumor cells which is modulated by the expression of several oncogenes (23). Figure 4A left panel shows the TUNEL staining as depicted by fluorescent green nuclei staining among the crypts of colonic epithelium of MMP-9-/- and WT mice treated with AOM and two cycles of DSS, middle panel shows the fluorescent blue DAPI nuclear staining and the right panel shows the merged image of TUNEL and DAPI, indicating no significant level of apoptosis among MMP-9-/- mice while crypts of WT mice showed some apoptotic cells. Figure 4B showing the western blot of caspase-3 (another marker for apoptosis required in the cleavage of critical cellular substrates), supports the immunohistochemistry data of TUNEL staining by showing a decreased protein expression of caspase-3 among MMP-9-/- (lanes 10-12) mice compared to WT mice (lanes 7-9), both induced with CAC. The adjacent graph shows the quantification of western blots by scanning densitometry and indicates an inhibition of 82.96±16.47 percent in caspase-3 protein expression among MMP-9-/- mice compared to WT mice induced with CAC.
Figure 3.
CAC in MMP-9-/- is associated with increased proliferation and decreased apoptosis. WT and MMP-9-/- mice (mice, n= 6 each group) with induced CAC and sacrificed after 56 days. Colon of the mice was processed for immunohistochemistry using anti-Ki67 antibody as described in the Methods section. 3A: representative sections of MMP-9-/- and WT mice with induced CAC. Proliferating cells are indicated by brown nuclei of the crypts with a counter staining of hematoxylin. 3B: graphs representing the number of nuclei positive for Ki67 staining per crypt. Each bar represents mean ± S.E., *p<0.05. 3C: shows the western blot of protein from the mucosal stripping of the colons of WT and MMP-9-/- with and without induction of CAC probed with anti-pCNA. Each lane shows protein (30μg/lane) from an individual mouse. Western blots were quantified by scanning densitometry and densities of MMP-9-/- mice relative to WT mice both induced with CAC are graphed adjacently.
Figure 4.
A: TUNEL and DAPI staining and apoptotic cells in the right panel. 4B: represents the western blot of whole tissue lysates of the colons of WT and MMP-9-/- mice with and without CAC induction probed with anti-caspase-3. Each lane shows protein (30μg/lane) from an individual mouse. Adjacent graph shows the quantification of Western blots by scanning densitometry and plotting the densities of MMP-9-/- mice relative to WT mice both induced with CAC. Values are representative of three experiments, mean ± SE; *p<0.05.
CAC in MMP-9-/- mice showed altered levels of p21WAF1/Cip1, β-catenin and NF-κB
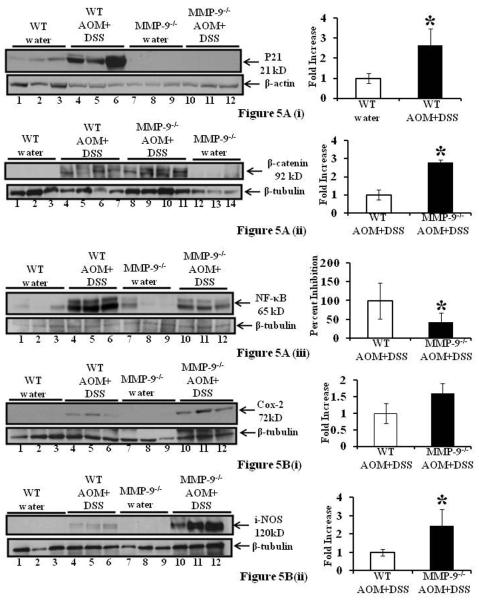
We have previously shown that MMP-9 activates Notch-1 (7). In this study we examined the expression levels of p21WAF1/Cip1, a ‘canonical’ Notch target, in keratinocytes that has been shown to suppress Wnt signaling and function as a negative regulator of stem cell potential and tumorigenesis (24), consequently suppressing protein levels of β-catenin. CAC was induced as described in Methods section. Colon of the mice was harvested at 56 days and mucosal strippings were processed for western blots. Western Blot analysis of p21WAF1/Cip1 showed that MMP-9-/- mice with or without the induction of CAC had almost negligible protein expression (Figure 5A(i), lanes 7-12) compared to WT mice with or without the induction of CAC (Figure 5A(i), lanes 1-6). CAC induction among the WT mice caused an increase in protein expression of p21WAF1/Cip1 compared to WT mice without CAC induction (Figure 5A(i), lanes 4-6 and 1-3 respectively). Densitometric analysis shown by the graphs revealed a 2.26±0.87 fold increase in p21WAF1/Cip1 protein expression among WT mice induced with CAC (Mean ±S.E., 6 mice per group p<0.05) [Figure 5A(i)]. Figures 5A(ii) shows that MMP-9-/- mice induced with CAC had increased expression of β-catenin (lanes 8-11 and lanes 4-7 respectively) compared to WT mice. The adjacent graph shows the quantification of western blots by scanning densitometry and indicates a 2.8±0.14 fold increase in β-catenin expression among MMP-9-/- mice compared to WT mice induced with CAC. Figure 5A(iii) shows that that MMP-9-/- mice compared to WT mice (lanes 10-12 and lanes 4-6 respectively) induced with CAC had decreased expression of NF-κB which participates in the control of intestinal homeostasis (25). Both MMP-9-/- and WT mice without the induction of CAC showed some constitutive expression of NF-κB (Figure 5A(iii) lanes 7-9 and lanes 1-3 respectively). The adjacent graph shows the quantification of western blots by scanning densitometry and indicates an inhibition of 57.78±24.92 percent in NF-κB expression among MMP-9-/- mice compared to WT mice induced with CAC. Values are representative of three individual experiments, mean ± SE; *p<0.05. Together these data demonstrate that CAC in MMP-9-/- is associated with decreased expression of NF-κB and p21WAF1/Cip1 and increased expression of β-catenin while CAC in WT type have increased MMP-9, NICD, p21 WAF1/Cip1 and a reciprocal decrease in Wnt signaling
Figure 5.
CAC in MMP-9-/- mice showed altered protein expressions of p21WAF1/Cip1, β-catenin, NF-κB, COX-2 and i-NOS. WT and MMP-9-/- mice (mice, n= 6 each group) induced with CAC were sacrificed after 56 days. Each lane shows protein (30μg/lane) from an individual mouse with or without induction of CAC. 5A(i-iii): western blots of protein from the mucosal stripping of the colons of WT and MMP-9-/- mice with and without CAC induction probed with anti-p21WAF1/Cip1, anti- β-catenin and anti- NF-κB respectively. Adjacent graphs show the quantification by scanning densitometry. 5B(i-ii): western blots of protein from the mucosal stripping of the colons of WT and MMP-9-/- mice with and without induction of CAC probed with anti-COX-2 and anti-i-NOS respectively. Western blots were quantitated by scanning densitometry and graphed adjacently. Values are representative of three experiments, each bar represents mean ± S.E., *p<0.05.
CAC in MMP-9-/- mice showed altered protein expressions of COX-2 and i-NOS
Cox-2 and i-NOS are two proteins well associated with CAC (26) (27). MMP-9-/- and WT mice (mice, n= 6 each group) were treated with one dose of AOM and two cycles of 3% DSS to induce CAC and were sacrificed after 56 days. Western blots of protein from the mucosal stripping of the colons were performed as described in Methods section. Figure 5B(i) shows that MMP-9-/- mice (lanes 10-12) compared to WT (4-6) mice, both induced with CAC, had increased expression of COX-2, while both MMP-9-/- and WT mice without CAC induction showed no expression of COX-2 (Figure 5B(i), lanes 7-9 and lanes 1-3 respectively). The adjacent graph [Figure 5B(i)] shows the quantification of western blots by scanning densitometry and indicates a 1.6±1.13 fold increase in COX-2 expression among MMP-9-/- mice compared to WT mice induced with CAC. Figures 5B(ii) shows that MMP-9-/- mice (lanes 10-12) compared to WT mice (lanes 4-6), both induced with CAC, had increased expression of i-NOS, while both MMP-9-/- and WT mice treated with water showed no expression of i-NOS (Figure 5B(ii), lanes 7-9 and lanes 1-3 respectively). The adjacent graph [Figure 5B(ii)] shows the quantification of western blots by scanning densitometry and indicates a 2.5±0.91 fold increase in i-NOS expression among MMP-9-/- mice compared to WT mice with the treatment of AOM and two cycles of DSS. Values are representative of three individual experiments, mean ± SE; *p<0.05.
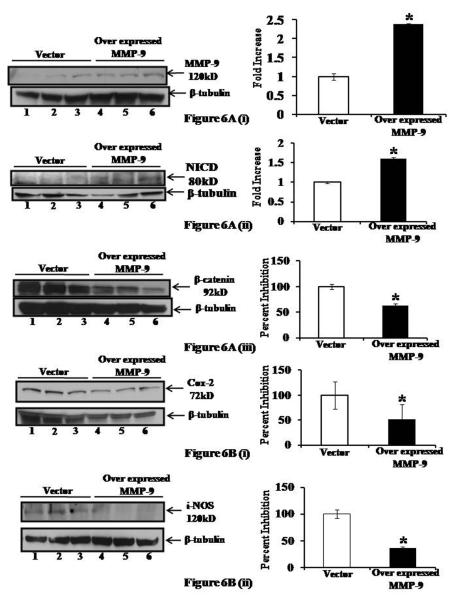
MMP-9 overexpression results in altered protein expressions of NICD, β-catenin, COX-2 and i-NOS
Our next goal was to determine the effect of MMP-9 overexpression on transcription factors and molecules associated with differentiation and proliferation of colonic epithelium. We used the Caco2BBE cell line stably transfected with a pEGFP plasmid, with or without the MMP-9 gene as described in the Methods section as our in vitro model to study the effect of MMP-9 on the expression of NICD, β-catenin, COX-2 and i-NOS. Figure 6A(i) represents the efficiency of transfection confirmed by western blot of MMP-9. Equal amounts of protein were separated by SDS-PAGE and probed with MMP-9 antibody. β-tubulin served as the loading control for proteins. The lane marked MMP-9 shows a band at 120 kDa (Figure 6A(i) lanes 4-6) consistent with the expression of GFP-MMP-9. MMP-9 is undetectable in the vector-transfected cells (Figure 6A(i) lanes 1-3), similar to normal colonic epithelia. Western blot using anti-NICD, anti-β-catenin, anti-COX-2 and anti-iNOS- specific antibodies (Figure 6A(ii-iii), 6B(i-ii), lanes 4-6 respectively) showed a significant increase in NICD, and a decrease in β-catenin, COX-2 and i-NOS in MMP-9 overexpressing cells compared to vector-transfected cells (Figure 6A(ii-iii), 6B(i-ii), lanes 1-3 respectively). Densitometric analysis revealed a 1.61±.02 fold increase in NICD protein expression and an inhibition of 37.17±3.93, 48.98±30.30 and 64.11±3.11 percent in protein expression of β-catenin, COX-2 and i-NOS, in MMP-9 overexpressing cells compared to vector (Mean ±S.E., replicates of three, p<0.05). Together, our in vitro data using the stably transfected Caco2BBE cell line, demonstrate that MMP-9 down-regulates β-catenin, COX-2 and i-NOS while activates NICD expression which are all key factors in regulating differentiation and proliferation of the gut epithelium.
Figure 6.
MMP-9 overexpression results in altered levels of NICD, β-catenin, COX-2 and i-NOS. Caco2-BBE cells stably transfected with a pEGFP plasmid, with or without the MMP-9 gene were plated on 6 well filters at confluency and cell lysates were collected and immunoblotted for 6A(i): MMP-9, 6A(ii): NICD, 6A(iii): β-catenin, 6B(i): COX-2 and 6B(ii): i-NOS. β-tubulin was used as the loading control. Western blots were quantitated by scanning densitometry and graphed adjacently. Each lane shows protein (30μg/lane). Values are representative of three experiments, mean ± SE; *p<0.05.
Discussion
Chronic inflammation is linked to cancer development in a number of organs (15). CAC advances through a series of genetic alterations that endow cells with growth advantages over normal cells. As advantaged cells proliferate, they progressively acquire even more DNA damage that further favors their proliferation (15), however, molecules released by inflammatory cells may either suppress or enhance tumor progression. MMPs have the ability to cleave various bioactive substrates and thereby can either promote or suppress tumor progression, resulting in a delicate balance of pro- and anti-tumor activity determining the fate of the tumors (19). During inflammation progressing to cancer, MMPs might be involved at different steps, starting from the growth of the primary tumor to the support of the tumor growth in the metastatic site. Some studies have shown the chronic inflammation associated with some cancers can further stimulate cancer progression due to the release of MMPs from the inflammatory cells (28) (29). There are only a few elaborate studies on the role of MMPs in colitis associated tumorigenesis (30), though some studies have shown that inhibition of gelatinases results in regression of tumor growth (31) (19). The gelatinases, especially MMP-9, can also stimulate tumor growth (19). Recently, Sinnamon et al have shown that in APC-Min mice genetically deficient in MMP-9 expression had fewer tumors than littermate controls and suggested the alteration of proliferation by MMP-9 among APC-Min adenoma cells (32). Burg-Roderfeld et al (33), has shown that MMP-9 hemopexin domain has as inhibitory effect on migration and adhesion of colorectal carcinoma cells. Another study by Pozzi et al (34) showed that reduction of plasma levels of MMP-9, in either normal or integrin alpha1-null mice, leads to decreased synthesis of angiostatin and consequent increased tumor growth and vascularization and was also supported by the study of Bjorklund and Koivunen (19). In melanoma, increased expression of MMP-9 is found initially, but at the later stage it is reversed (19) (35). It has been reported that in breast and colon cancer, MMP-9 expression has been correlated with both increased and decreased survival and formation of distant metastasis (19) (36).
In the present study we observed that MMP-9-/- mice showed increased susceptibility to inflammation-associated colon cancer/ polyps as indicated by their number, size of polyps and the mortality rate, though we have shown previously (4) that MMP-9-/- mice are protected from inflammation. Interestingly, MMP-9-/- mice induced with inflammation-associated colon cancer/ polyps exhibited higher proliferation and lower apoptosis compared to WT mice inflammation. This implies the underlying role of MMP-9 in regulating proliferation and apoptosis during the carcinogenic process. This fact was further supported by the altered protein expressions of different inflammatory and transcription factors, which are well known to be involved in regulating the cell proliferation and differentiation or apoptosis.
Based on our data that MMP-9 functions as the protease that cleaves and activates Notch-1 (7), we explored the role of MMP-9-medited Notch-1 activation in CAC. In the present study, we observed the activation of p21WAF1/Cip1 expression due to activation of Notch-1 signaling in CAC in WT mice, which was clearly inhibited in CAC in MMP-9-/- mice. The in vitro results using our overexpressing MMP-9 cell line also corroborated with in vivo data supporting the hypothesis that despite being a mediator of pro-inflammatory response, MMP-9 plays a protective role in CAC by modulating carcinogenesis through its effect on Notch signaling.
Intestinal epithelium is a very dynamic tissue involving key cellular processes like proliferation and differentiation. It has been known that there are many genes which are involved in the normal development of intestine and also play a critical role in the process of human carcinogenesis (37) (38) (39) (40). Continuous renewal of the intestinal epithelium requires coordinated regulation between different signaling pathways and molecules to maintain the balance between proliferation and differentiation of epithelial stem cells and immature progenitor cells. One of the important signaling systems regulating the intestinal epithelium homestasis is the Wnt signaling pathway (41) which involves dephosphorylation and stabilization of β-catenin and its nuclear translocation, and activation of target genes by the complex consisting of β-catenin and the TCF family of transcription factors (42). Another important pathway is the highly conserved Notch signaling pathway, which controls selective cell fate decisions and subsequent differentiation in the intestine (43) (44) with oncogenic and growth-promoting roles. It can also function as a tumor suppressor (44) (38). It has been reported that Notch-1 deficiency results in increased β-catenin-mediated signaling in hyperproliferative skin and primary tumor lesions, indicating that Notch might suppress Wnt signaling (44) (45). This is mediated by Notch by indirect activation of p21 which subsequently binds to the Wnt4 promoter and down-regulates its expression (44) (24). Further, overactivation of the Wnt pathway due to mutations in β-catenin causes many of the epithelial cells to enter into a proliferative state and display a failure of the differentiation programs in these cells (43) (46). Additionally, Wnt signaling probably integrates with other stem cell niche derived signals such as BMP, Hedge-hog, and Notch (47) (43). Irrespective of whether these signals have a stromal or an epithelial origin, they reflect a constant cross talk among them which is very important for the maintenance of normal epithelium (48) (47). A few studies have shown that repeated exposure to DSS causes the appearance of dysplasia and/ or cancer in mice due to repeated mucosal erosion and regeneration of the colonic epithelium causing the increase in the susceptibility of mucosal epithelia for dysplasia and/ or cancer development (49). Although underlying mechanisms are still unknown, increased proliferative activity preceded dysplasia which results in the increase of proliferating cells to apoptotic cells. In the present study, our results have shown increased proliferation among the MMP-9-/- mice induced with inflammation-associated colon cancer as well as increased β-catenin expression. Similar to keratinocytes but in contrast to colonic epithelial cells, our data show that MMP-9-mediated Notch signaling is associated with inhibition of Wnt signaling and mediates cell survival.
In conclusion, we demonstrate that MMP-9 acts as a tumor suppressor in CAC, likely through its effect on the Notch signaling pathway. Absence of MMP-9 is associated with defective Notch-1 activation, suppressed p21WAF1/Cip1 expression and reciprocal activation of Wnt signaling and increased proliferation.
Supplementary Material
Acknowledgements
This work was supported by the National Institute of Health of Diabetes and Digestive and Kidney Diseases (R24-DK-064399 center grant, RO1-DK064711 & RO1-DK076825 to S.V.S.; RO1-DK-071594 to D.M.; RO1-DK061417 to A.T.G.); and a research fellowship grant from the Crohn’s Colitis Foundation of America to P.G. We appreciate the contribution of Dr. Tracy S. Obertone in proof reading the manuscript.
References
- 1.Egeblad M, Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer. 2002;2:161–74. doi: 10.1038/nrc745. [DOI] [PubMed] [Google Scholar]
- 2.Okamoto R, Watanabe M. Cellular and molecular mechanisms of the epithelial repair in IBD. Dig Dis Sci. 2005;50(Suppl 1):S34–8. doi: 10.1007/s10620-005-2804-5. [DOI] [PubMed] [Google Scholar]
- 3.Overall CM. Dilating the degradome: matrix metalloproteinase 2 (MMP-2) cuts to the heart of the matter. Biochem J. 2004;383:e5–7. doi: 10.1042/BJ20041433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Castaneda FE, Walia B, Vijay-Kumar M, et al. Targeted deletion of metalloproteinase 9 attenuates experimental colitis in mice: central role of epithelial-derived MMP. Gastroenterology. 2005;129:1991–2008. doi: 10.1053/j.gastro.2005.09.017. [DOI] [PubMed] [Google Scholar]
- 5.Baugh MD, Perry MJ, Hollander AP, et al. Matrix metalloproteinase levels are elevated in inflammatory bowel disease. Gastroenterology. 1999;117:814–22. doi: 10.1016/s0016-5085(99)70339-2. [DOI] [PubMed] [Google Scholar]
- 6.Brou C, Logeat F, Gupta N, et al. A novel proteolytic cleavage involved in Notch signaling: the role of the disintegrin-metalloprotease TACE. Mol Cell. 2000;5:207–16. doi: 10.1016/s1097-2765(00)80417-7. [DOI] [PubMed] [Google Scholar]
- 7.Garg P, Ravi A, Patel NR, et al. Matrix metalloproteinase-9 regulates MUC-2 expression through its effect on goblet cell differentiation. Gastroenterology. 2007;132:1877–89. doi: 10.1053/j.gastro.2007.02.048. [DOI] [PubMed] [Google Scholar]
- 8.Loftus EV., Jr. Clinical epidemiology of inflammatory bowel disease: Incidence, prevalence, and environmental influences. Gastroenterology. 2004;126:1504–17. doi: 10.1053/j.gastro.2004.01.063. [DOI] [PubMed] [Google Scholar]
- 9.Bouma G, Strober W. The immunological and genetic basis of inflammatory bowel disease. Nat Rev Immunol. 2003;3:521–33. doi: 10.1038/nri1132. [DOI] [PubMed] [Google Scholar]
- 10.Fiocchi C. Inflammatory bowel disease: etiology and pathogenesis. Gastroenterology. 1998;115:182–205. doi: 10.1016/s0016-5085(98)70381-6. [DOI] [PubMed] [Google Scholar]
- 11.Langholz E, Munkholm P, Davidsen M, Binder V. Colorectal cancer risk and mortality in patients with ulcerative colitis. Gastroenterology. 1992;103:1444–51. doi: 10.1016/0016-5085(92)91163-x. [DOI] [PubMed] [Google Scholar]
- 12.Prior P, Gyde S, Cooke WT, Waterhouse JA, Allan RN. Mortality in Crohn’s disease. Gastroenterology. 1981;80:307–12. [PubMed] [Google Scholar]
- 13.Eaden JA, Abrams KR, Mayberry JF. The risk of colorectal cancer in ulcerative colitis: a meta-analysis. Gut. 2001;48:526–35. doi: 10.1136/gut.48.4.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Choi PM, Zelig MP. Similarity of colorectal cancer in Crohn’s disease and ulcerative colitis: implications for carcinogenesis and prevention. Gut. 1994;35:950–4. doi: 10.1136/gut.35.7.950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feagins LA, Souza RF, Spechler SJ. Carcinogenesis in IBD: potential targets for the prevention of colorectal cancer. Nat Rev Gastroenterol Hepatol. 2009;6:297–305. doi: 10.1038/nrgastro.2009.44. [DOI] [PubMed] [Google Scholar]
- 16.Fukata M, Abreu MT. Role of Toll-like receptors in gastrointestinal malignancies. Oncogene. 2008;27:234–43. doi: 10.1038/sj.onc.1210908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mook OR, Frederiks WM, Van Noorden CJ. The role of gelatinases in colorectal cancer progression and metastasis. Biochim Biophys Acta. 2004;1705:69–89. doi: 10.1016/j.bbcan.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 18.Bernhard EJ, Gruber SB, Muschel RJ. Direct evidence linking expression of matrix metalloproteinase 9 (92-kDa gelatinase/collagenase) to the metastatic phenotype in transformed rat embryo cells. Proc Natl Acad Sci U S A. 1994;91:4293–7. doi: 10.1073/pnas.91.10.4293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bjorklund M, Koivunen E. Gelatinase-mediated migration and invasion of cancer cells. Biochim Biophys Acta. 2005;1755:37–69. doi: 10.1016/j.bbcan.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 20.Tanaka T, Kohno H, Suzuki R, Yamada Y, Sugie S, Mori H. A novel inflammation-related mouse colon carcinogenesis model induced by azoxymethane and dextran sodium sulfate. Cancer Sci. 2003;94:965–73. doi: 10.1111/j.1349-7006.2003.tb01386.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Okayasu I, Ohkusa T, Kajiura K, Kanno J, Sakamoto S. Promotion of colorectal neoplasia in experimental murine ulcerative colitis. Gut. 1996;39:87–92. doi: 10.1136/gut.39.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saleh HA, Jackson H, Khatib G, Banerjee M. Correlation of bcl-2 oncoprotein immunohistochemical expression with proliferation index and histopathologic parameters in colorectal neoplasia. Pathol Oncol Res. 1999;5:273–9. doi: 10.1053/paor.1999.0231. [DOI] [PubMed] [Google Scholar]
- 23.Hickman JA. Apoptosis induced by anticancer drugs. Cancer Metastasis Rev. 1992;11:121–39. doi: 10.1007/BF00048059. [DOI] [PubMed] [Google Scholar]
- 24.Devgan V, Mammucari C, Millar SE, Brisken C, Dotto GP. p21WAF1/Cip1 is a negative transcriptional regulator of Wnt4 expression downstream of Notch1 activation. Genes Dev. 2005;19:1485–95. doi: 10.1101/gad.341405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Inan MS, Tolmacheva V, Wang QS, Rosenberg DW, Giardina C. Transcription factor NF-kappaB participates in regulation of epithelial cell turnover in the colon. Am J Physiol Gastrointest Liver Physiol. 2000;279:G1282–91. doi: 10.1152/ajpgi.2000.279.6.G1282. [DOI] [PubMed] [Google Scholar]
- 26.Eberhart CE, Coffey RJ, Radhika A, Giardiello FM, Ferrenbach S, DuBois RN. Up-regulation of cyclooxygenase 2 gene expression in human colorectal adenomas and adenocarcinomas. Gastroenterology. 1994;107:1183–8. doi: 10.1016/0016-5085(94)90246-1. [DOI] [PubMed] [Google Scholar]
- 27.Ambs S, Merriam WG, Bennett WP, et al. Frequent nitric oxide synthase-2 expression in human colon adenomas: implication for tumor angiogenesis and colon cancer progression. Cancer Res. 1998;58:334–41. [PubMed] [Google Scholar]
- 28.Coussens LM, Raymond WW, Bergers G, et al. Inflammatory mast cells up-regulate angiogenesis during squamous epithelial carcinogenesis. Genes Dev. 1999;13:1382–97. doi: 10.1101/gad.13.11.1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Coussens LM, Tinkle CL, Hanahan D, Werb Z. MMP-9 supplied by bone marrow-derived cells contributes to skin carcinogenesis. Cell. 2000;103:481–90. doi: 10.1016/s0092-8674(00)00139-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Newell KJ, Matrisian LM, Driman DK. Matrilysin (matrix metalloproteinase-7) expression in ulcerative colitis-related tumorigenesis. Mol Carcinog. 2002;34:59–63. doi: 10.1002/mc.10049. [DOI] [PubMed] [Google Scholar]
- 31.Lubbe WJ, Zhou ZY, Fu W, et al. Tumor epithelial cell matrix metalloproteinase 9 is a target for antimetastatic therapy in colorectal cancer. Clin Cancer Res. 2006;12:1876–82. doi: 10.1158/1078-0432.CCR-05-2686. [DOI] [PubMed] [Google Scholar]
- 32.Sinnamon MJ, Carter KJ, Fingleton B, Matrisian LM. Matrix metalloproteinase-9 contributes to intestinal tumourigenesis in the adenomatous polyposis coli multiple intestinal neoplasia mouse. Int J Exp Pathol. 2008;89:466–75. doi: 10.1111/j.1365-2613.2008.00621.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Burg-Roderfeld M, Roderfeld M, Wagner S, Henkel C, Grotzinger J, Roeb E. MMP-9-hemopexin domain hampers adhesion and migration of colorectal cancer cells. Int J Oncol. 2007;30:985–92. doi: 10.3892/ijo.30.4.985. [DOI] [PubMed] [Google Scholar]
- 34.Pozzi A, LeVine WF, Gardner HA. Low plasma levels of matrix metalloproteinase 9 permit increased tumor angiogenesis. Oncogene. 2002;21:272–81. doi: 10.1038/sj.onc.1205045. [DOI] [PubMed] [Google Scholar]
- 35.van den Oord JJ, Paemen L, Opdenakker G, de Wolf-Peeters C. Expression of gelatinase B and the extracellular matrix metalloproteinase inducer EMMPRIN in benign and malignant pigment cell lesions of the skin. Am J Pathol. 1997;151:665–70. [PMC free article] [PubMed] [Google Scholar]
- 36.Pacheco MM, Mourao M, Mantovani EB, Nishimoto IN, Brentani MM. Expression of gelatinases A and B, stromelysin-3 and matrilysin genes in breast carcinomas: clinico-pathological correlations. Clin Exp Metastasis. 1998;16:577–85. doi: 10.1023/a:1006580415796. [DOI] [PubMed] [Google Scholar]
- 37.Guo RJ, Suh ER, Lynch JP. The role of Cdx proteins in intestinal development and cancer. Cancer Biol Ther. 2004;3:593–601. doi: 10.4161/cbt.3.7.913. [DOI] [PubMed] [Google Scholar]
- 38.Radtke F, Raj K. The role of Notch in tumorigenesis: oncogene or tumour suppressor? Nat Rev Cancer. 2003;3:756–67. doi: 10.1038/nrc1186. [DOI] [PubMed] [Google Scholar]
- 39.di Magliano M Pasca, Hebrok M. Hedgehog signalling in cancer formation and maintenance. Nat Rev Cancer. 2003;3:903–11. doi: 10.1038/nrc1229. [DOI] [PubMed] [Google Scholar]
- 40.Giles RH, van Es JH, Clevers H. Caught up in a Wnt storm: Wnt signaling in cancer. Biochim Biophys Acta. 2003;1653:1–24. doi: 10.1016/s0304-419x(03)00005-2. [DOI] [PubMed] [Google Scholar]
- 41.Fodde R, Brabletz T. Wnt/beta-catenin signaling in cancer stemness and malignant behavior. Curr Opin Cell Biol. 2007;19:150–8. doi: 10.1016/j.ceb.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 42.Morin PJ, Sparks AB, Korinek V, et al. Activation of beta-catenin-Tcf signaling in colon cancer by mutations in beta-catenin or APC. Science. 1997;275:1787–90. doi: 10.1126/science.275.5307.1787. [DOI] [PubMed] [Google Scholar]
- 43.Nakamura T, Tsuchiya K, Watanabe M. Crosstalk between Wnt and Notch signaling in intestinal epithelial cell fate decision. J Gastroenterol. 2007;42:705–10. doi: 10.1007/s00535-007-2087-z. [DOI] [PubMed] [Google Scholar]
- 44.Koch U, Radtke F. Notch and cancer: a double-edged sword. Cell Mol Life Sci. 2007;64:2746–62. doi: 10.1007/s00018-007-7164-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nicolas M, Wolfer A, Raj K, et al. Notch1 functions as a tumor suppressor in mouse skin. Nat Genet. 2003;33:416–21. doi: 10.1038/ng1099. [DOI] [PubMed] [Google Scholar]
- 46.Su LK, Kinzler KW, Vogelstein B, et al. Multiple intestinal neoplasia caused by a mutation in the murine homolog of the APC gene. Science. 1992;256:668–70. doi: 10.1126/science.1350108. [DOI] [PubMed] [Google Scholar]
- 47.Sancho E, Batlle E, Clevers H. Signaling pathways in intestinal development and cancer. Annu Rev Cell Dev Biol. 2004;20:695–723. doi: 10.1146/annurev.cellbio.20.010403.092805. [DOI] [PubMed] [Google Scholar]
- 48.Brabletz S, Schmalhofer O, Brabletz T. Gastrointestinal stem cells in development and cancer. J Pathol. 2009;217:307–17. doi: 10.1002/path.2475. [DOI] [PubMed] [Google Scholar]
- 49.Inoue T, Murano M, Kuramoto T, et al. Increased proliferation of middle to distal colonic cells during colorectal carcinogenesis in experimental murine ulcerative colitis. Oncol Rep. 2007;18:1457–62. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.