Abstract
Background
Detection of HIV-1 in patients is limited by the sensitivity and selectivity of available tests. The nanotechnology-based bio-barcode-amplification method offers an innovative approach to detect specific HIV-1 antigens from diverse HIV-1 subtypes. We evaluated the efficacy of this protein-detection method in detecting HIV-1 in men enrolled in the Chicago component of the Multicenter AIDS Cohort Study (MACS).
Methods
The method relies on magnetic microparticles with antibodies that specifically bind the HIV-1 p24 Gag protein and nanoparticles that are encoded with DNA and antibodies that can sandwich the target protein captured by the microparticle-bound antibodies. The aggregate sandwich structures are magnetically separated from solution, and treated to remove the conjugated barcode DNA. The DNA barcodes (hundreds per target) were identified by a nanoparticle-based detection method that does not rely on PCR.
Results
Of 112 plasma samples from HIV-1-infected subjects, 111 were positive for HIV-1 p24 Gag protein (range: 0.11–71.5 ng/ml of plasma) by the bio-barcode-amplification method. HIV-1 p24 Gag protein was detected in only 23 out of 112 men by the conventional ELISA. A total of 34 uninfected subjects were negative by both tests. Thus, the specificity of the bio-barcode-amplification method was 100% and the sensitivity 99%. The bio-barcode-amplification method detected HIV-1 p24 Gag protein in plasma from all study subjects with less than 200 CD4+ T cells/μl of plasma (100%) and 19 out of 20 (95%) HIV-1-infected men who had less than 50 copies/ml of plasma of HIV-1 RNA. In a separate group of 60 diverse international isolates, representative of clades A, B, C and D and circulating recombinant forms CRF01_AE and CRF02_AG, the bio-barcode-amplification method identified the presence of virus correctly.
Conclusions
The bio-barcode-amplification method was superior to the conventional ELISA assay for the detection of HIV-1 p24 Gag protein in plasma with a breadth of coverage for diverse HIV-1 subtypes. Because the bio-barcode-amplification method does not require enzymatic amplification, this method could be translated into a robust point-of-care test.
Keywords: bio-barcode-amplification method, HIV-1, major core protein of HIV-1 (p24 Gag)
Accurate diagnostic tests for HIV-1 have a key role in clinical care and the control of HIV/AIDS [1–6]. Commonly used methods rely on the detection of antibodies to HIV-1 in plasma or sera [2,7–9]. Unfortunately, these techniques are of little value in the earliest stages of infection before the development of antibodies against HIV-1 (average infectious-window period of 25 days; 95% confidence interval: 9–41 days) [10–14] and also in neonates because maternal antibodies can persist in the newborn for up to 12 months or more [5,15–17]. Detection of the major core protein of HIV-1 (p24 Gag) in serum or plasma by an ELISA is specific but does not offer the requisite sensitivity [5,6,18–21]. Other methods for assessing the presence of virus include PCR-based detection of the viral genome, specific primers and probes in plasma and viral co-culture with normal donor lymphocytes [3,7,8,16]. Although accurate, these techniques are time-consuming and require specialized laboratories, elaborate equipment, special specimen-transport conditions to maintain viability and highly skilled personnel.
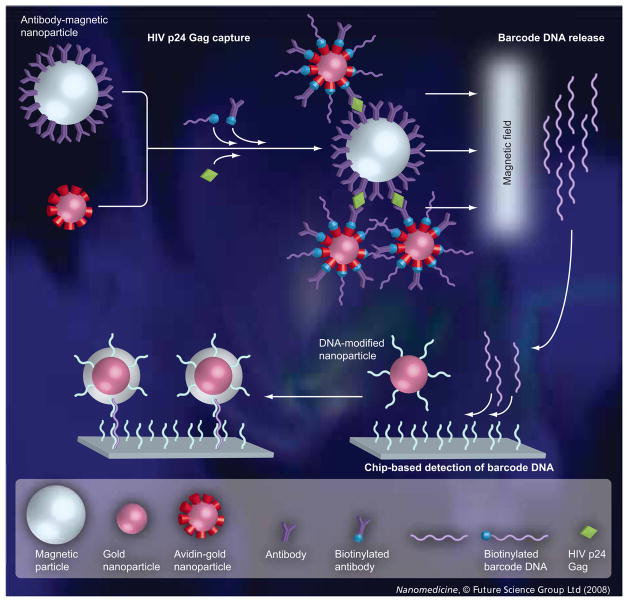
Technological advances in nanotechnology provide the opportunity to overcome many shortcomings of other diagnostic approaches [22–25]. The nanoparticle-based bio-barcode-amplification method offers an innovative approach to protein detection. There are several variations of this assay but most have two common components (Figure 1): magnetic microparticles (1-μm diameter tosyl-activated polyamine particles with magnetic iron oxide cores) functionalized with antibodies to the target protein and gold nanoparticles functionalized with both antibodies directed to a nonoverlapping region of the cognate protein and barcode DNA that are unique to the protein target of interest. In the presence of the target protein, the magnetic microparticles and the gold nanoparticles form aggregate sandwich structures that are separated magnetically from solution, followed by removal of the conjugated barcode DNA. The DNA barcodes can be identified quickly by nanoparticle-based detection methodologies [25,26].
Figure 1. Representation of the bio-barcode-amplification method and the chip-based scanometric detection used for HIV-1 p24 Gag-specific barcode DNA identification.
The sandwich capture of the HIV-1 p24 Gag target protein with magnetic microparticles functionalized with a mixture of sheep polyclonal antibodies against the HIV-1 p24 Gag protein and gold nanoparticles functionalized with both mouse monoclonal antibodies directed to a nonoverlapping region of HIV-1 p24 Gag and barcode oligonucleotides. The aggregate sandwich structures are separated magnetically from solution, followed by removal of the hybridized barcode DNA and chip-based detection of the dehybridized barcode DNA for HIV-1 p24 Gag protein identification. For real-time PCR-based measurements, the isolated barcode DNA, which has primers and probe-binding sites incorporated into the DNA sequence design, is added to the reaction mixture containing the appropriate primers and then the solution was thermally cycled.
This method offers several advantages over existing protein-detection methods [22,23,25]. Unlike the conventional ELISA assay, the target-binding portion of the bio-barcode assay is performed in solution (as opposed to on the flat surface of an ELISA plate). Accordingly, a large quantity of magnetic microparticles can be added to facilitate binding kinetics between the antibody and target protein. Homogeneous mixing makes this method faster and more efficient than ELISA (i.e., the equilibrium is pushed toward the captured-protein state by increasing the concentration of magnetic microparticles). Because the nanoparticle probe carries with it a large number of DNA barcodes per protein-binding event, there is substantial amplification and protein can be detected at attomolar concentration [23].
Here, we show that the bio-barcode-amplification method can detect HIV-1 p24 Gag protein in plasma sampled from HIV-1-infected men, regardless of the amount of HIV-1 RNA in their peripheral blood, with greater sensitivity than the conventional ELISA method, which found HIV-1 p24 Gag in plasma from only 23 out of 112 HIV-1-infected subjects. The bio-barcode-amplification method could also detect HIV-1 p24 Gag protein in samples including 60 diverse international isolates, demonstrating the clinical efficacy of the bio-barcode-amplification method for the detection of HIV-1 p24 Gag protein.
Methods
Study participants
The study subjects were men enrolled in the Chicago component of the Multicenter AIDS Cohort Study (MACS), a natural history study of men who have sex with men. Participants were followed at approximately 6-month-interval study visits, queried about risk behaviors, tested for antibodies to HIV-1 and had their CD4+ and CD8+ T-cell numbers counted. Blood specimens were collected in acid-citrate-dextrose. Plasma was collected after centrifugation to ensure cell-free specimens. The plasma samples were coded and replicate aliquots were stored at −80°C until use. Measurements were made with a single frozen plasma sample that was divided into replicate portions.
HIV-1-infected men (n = 112) were selected on the basis of differences in their CD4+ T-cell numbers and plasma levels of HIV-1. As some of the men began therapy, their levels of plasma viral RNA dropped below the threshold of detection. Study subjects were stratified by clinically relevant criteria, separating out those with less than 200 CD4+ T cells/μl (26 out of 112) or less than 50 copies of HIV-1 RNA/ml of plasma (20 out of 112). A total of 34 HIV-1-noninfected men were included as negative controls. The laboratory personnel were blind to study subject category.
Detection of HIV-1 p24 Gag protein in plasma by ELISA
Infected men had HIV-1 p24 Gag measured using the Alliance HIV-1 p24 Ag ELISA kit (Perkin-Elmer Life Sciences, Waltham, MA, USA) after immune-complex dissociation of the antibody–antigen complexes using low pH.
Detection of HIV-1 p24 RNA in plasma by PCR
HIV-1 RNA in plasma was measured by a quantitative real-time PCR assay (AMPLICOR HIV-1 MONITOR Test, Roche Molecular Diagnostic Systems, Pleasanton, CA, USA) that has a cutoff value of 50 copies/ml of plasma.
Antibodies against HIV-1 p24 Gag protein
Sheep polyclonal antibodies against the HIV-1 p24 Gag protein were raised against three different synthetic peptides (amino acid positions 283–297 [LDIRQGPKEPFRDYV], 173–188 [SALSEGATPQDLNTML] and 226–237 [GQMREPRGSDIA] relative to p24 Gag in the HIV-1 reference strain HXB2) and were then affinity purified against the immunogenic peptide (Cliniqa, Inc., San Marcos, CA, USA). The mouse monoclonal antibody against HIV-1 p24 Gag was raised against a conserved oligopeptide (positions 193–227 [GHQAAMQMLKETINEEAAEWDRLH-PVHAGPIAPG] and 253–267 [NPPIPVGEI-YKRWII]) (Cliniqa, Inc.). The peptides were selected to be highly conserved in HIV-1, corresponding as closely as possible to the HIV-1 p24 Gag consensus sequence of each of the most common clades and circulating recombinant forms and to a translation of a reconstruction of the HIV-1 M group ancestral sequence [27].
Preparation of functionalized magnetic microparticles
Tosyl-activated magnetic microparticles (MyOne™ Dynabeads®; Invitrogen, Inc., Carlsbad, CA, USA) were heavily functionalized with a mixture of sheep polyclonal antibodies against the HIV-1 p24 Gag protein (Cliniqa, Inc.). Briefly, 80 μl HIV-1 p24 polyclonal antibody (1 mg/ml), 20 μl magnetic microparticles, 84 μl 3 M (NH4) 2SO4 and 66 μl borate buffer were combined in a 0.2 ml PCR tube and incubated at 37°C at 1400 oscillations/min for 24 h. The microparticles were separated magnetically after incubation. To block any remaining active sites on the microparticles, 250 μl of blocking buffer, which consisted of phosphate-buffered saline (PBS) pH 7.4 with 0.5% bovine serum albumin (BSA) and 0.05% Tween20, was added and the microparticles were then incubated at 37°C at 1400 oscillations/min for 24 h. Following incubation, the microparticles were once again separated magnetically and were washed twice with 1 ml of magnetic-probe solution that contained PBS pH 7.4, 0.1% BSA and 0.05% Tween20. The magnetic microparticles were resuspended in 200 μl of magnetic-probe solution and were stored at 4°C until use. Functionalized microparticles prepared in this manner retained their activity for 4–6 months.
Capture of HIV-1 p24 Gag in plasma by the bio-barcode-amplification method
The reaction was performed in 160 μl of sample with 1% Triton-X 100, 1% BSA, 0.2% Tween20, PBS (pH 7.4) and 0.5 μg of functionalized magnetic microparticles in 40 μl of 1% BSA, 1X PBS, 0.2% Tween20 and 2% sheep serum. HIV-1 p24 Gag antigen–antibody immune complexes in plasma were disrupted by immune-complex dissociation, as described previously [5]. After mixing 90 min (1400 cycles/min) at 37°C to enable HIV-1 p24-antigen binding, 20 μl of biotin-labeled monoclonal antibody against HIV-1 p24 Gag was added to a final concentration of 2.5 ng/μl and antibody binding continued for 45 min at 37°C. After magnetic separation, the magnetic microparticles were washed twice with 160 μl of 2 mM imidazole-buffered saline, 0.02% Tween20. This reaction was then adjusted to contain 0.4 nM streptavidin-coated 15 nm gold nanoparticles (Ted Pella, Inc., Redding, CA, USA) and then 1 nM 5′-biotin-labeled oligonucleotide (5′-AACGGTGCAATGAAGCCAAGTTAGAG CGAAGGTGCCATAACCACGAACTCT-GCTGCCACGAAAAAAAAAAAAAAAAAAA-3′; IDT Inc., Coralville, IA, USA) bound to the surface [28], 0.66 μg/μl tRNA (Sigma-Aldrich, Inc., St. Louis, MO, USA), 1% BSA, 1× PBS and 0.2% Tween20 in a total volume of 160 μl for 30 min. Barcode DNA in the aggregate-sandwich structure was eluted from the gold nanoparticle surface in 0.01 M EDTA, 0.02% Tween20, 98% formamide at 80°C for 5 min. Reactions were performed with the Evolution P3 microplate-processing platform (Perkin-Elmer, Waltham, MA, USA). Recombinant HIV-1 p24 Gag (Virology Quality Assurance Laboratory, NIAID, NIH, Bethesda, MD, USA) was used to create protein copy-number standards.
Quantification of barcode DNA eluted from the gold nanoparticle surface
After elution of the oligonucleotides from the gold nanoparticle surface, the eluted barcode DNA was quantified by both real-time PCR and chip-based scanometric methods. For barcode DNA identification by real-time PCR, one portion from each specimen was adjusted to contain primers RTP-BC-F (5′-CGGTGCAATGAAGCCAA-3′) and RTP-BC-R (5′-TTTTTCGTGGCAGCAGAGT-3′) and probe RTP-BC (FAM-5′-GAGCGAAGGTGCCATAACCACG-3′-TAMRA) (Qiagen Operon, Alameda, CA, USA). Amplification was performed as described previously [28]. Fluorescence data were recorded in real-time with an ABI Prism 7900 sequence-detection system (Applied Biosystems, Inc., Foster City, CA, USA). For barcode-DNA identification by a chip-based scanometric method, another portion was hybridized to chip-immobilized DNA complementary to the 5′ half of the barcode-DNA sequence to capture the barcode-DNA sequence and dT20 oligonucleotide-functionalized 15 nm gold nanoparticles were used to label the other 3′ half of the sequence in a sandwich-assay format [22,23]. Chips with hybridized nanoparticle probes were then subjected to silver amplification.
Light scatter from the developed spots were read with a Verigene-identification system (Nanosphere, Inc., Northbrook, IL, USA) and were analyzed with ImageJ software [101]. Signal intensity was normalized to the negative control.
Results
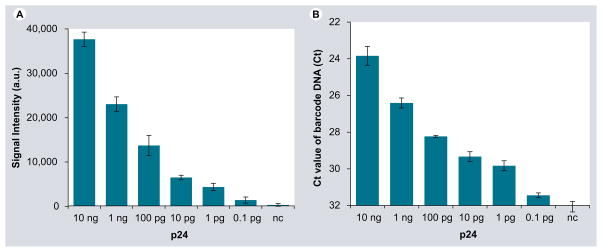
We first determined the sensitivity of the bio-barcode-amplification method by measuring tenfold serial dilutions of recombinant HIV-1 p24 Gag. Because the gold nanoparticles carry with them a large number of oligonucleotides per protein-binding event, there is substantial amplification and HIV-1 p24 Gag can be detected by chip-based scanometric and real-time PCR methods (Figure 2A & B, respectively). For both methods, the bio-barcode-amplification method exhibited a detection range between 0.1 pg/ml (4.2 fM) and 10 ng/ml (420 pM) HIV-1 p24 Gag in plasma and the response was linear (r2 = 0.9969; p < 0.0001).
Figure 2. Quantification of HIV-1 p24 Gag by the bio-barcode amplification method using chip-based scanometric and real-time PCR detection of HIV-1 p24 Gag-specific barcode DNA.
HIV-1 p24 Gag concentration was varied from 0.1 pg/ml to 10 ng/ml and a negative-control sample where no HIV-1 p24 Gag was added was also used. Plotted are the results of the (A) signal intensity (a.u.) versus the quantity of recombinant HIV-1 p24 Gag protein and (B) the Ct versus the quantity of recombinant HIV-1 p24 Gag protein. a.u.: Arbitrary units; Ct: Cycle threshold.
The threshold of sensitivity for HIV-1 p24 Gag was approximately 0.1 pg/ml of plasma (4.2 fM). Quantification was not reliable below this level. To obtain a clear differentiation from background signal and improve the accuracy of detection, we set a conservative cutoff between negative (within the normal HIV-1-noninfected range) and positive (above the normal HIV-1-noninfected range) at twice the standard deviation value (0.047). The selectivity of the barcode DNA was excellent, as demonstrated by the observation that there was little discernable signal when HIV-1 p24 Gag protein was absent (Figure 2A). The results of this protein-detection method were highly reproducible, regardless of the approach used to quantify the barcode DNA. Conventional enzyme immunoassays that are accepted and comparable clinically for detecting the same target have sensitivity limits of approximately 5 pg/ml (~0.2 pM), an order of magnitude less sensitive [9,29].
Because antigenic variation is a common strategy by which HIV-1 evades the immune system, the breadth of coverage by any given antibody is potentially limited [30]. At least one of the three different synthetic peptides (12-, 15- and 16-mers) used to create the mixture of sheep polyclonal antibodies against the HIV-1 p24 Gag protein bound to the surface of the gold nanoparticle were preserved perfectly in 1477 out of 1689 (87%) of HIV-1 M group sequences in the Gag alignment in the HIV database [102]. The 15- and 34-mer peptides used to create the mouse monoclonal antibody against the HIV-1 p24 Gag protein bound to the surface of the magnetic microparticle were less conserved; only 538 out of 1689 (32%) of M group sequences in the Gag alignment had a perfect match with one or the other peptide. Nevertheless, 1576 out of 1689 (93%) of M group sequences in the Gag alignment shared their amino acid sequence with at least one peptide with no more than one amino acid mismatch.
We determined the breadth of the bio-barcode-amplification assay by measuring the presence of HIV-1 p24 Gag in samples of 60 diverse viruses, including ten viruses from each of the six internationally most prevalent clades A, B, C and D and circulating recombinant forms CRF01_AE and CRF02_AG (obtained from the NIH AIDS Research & Reference Reagent Program [31]). At least one of the three synthetic peptides (12-, 15- and 16-mers) we used to create the mixture of sheep polyclonal antibodies against the HIV-1 p24 Gag protein were preserved perfectly in 56 out of 60 strains. Of the 15- and 34-mer peptides used to create the mouse monoclonal antibody against the HIV-1 p24 Gag protein, 20 out of 60 strains were preserved perfectly. All of the 60 international strains in the Gag alignment shared their amino acid sequence with at least one peptide with no more than one amino acid mismatch. HIV-1 p24 Gag was detected by the bio-barcode-amplification method for all 60 of these virus strains (data not shown). Accordingly, the minimal amino acid mismatches did not affect the results.
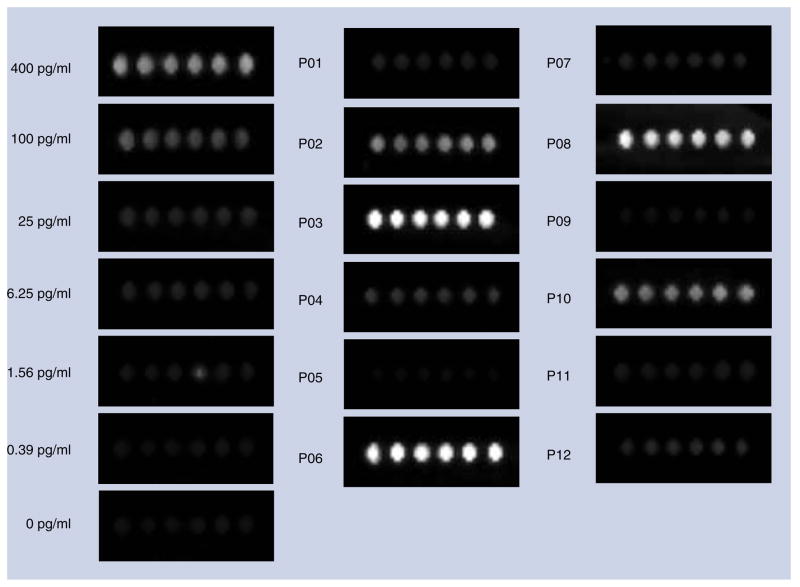
Next, we evaluated blinded samples of plasma obtained from HIV-1-infected men from the MACS study by ELISA and the bio-barcode-amplification method. The amount of HIV p24 antigen in plasma was measured in triplicate. We determined the quantity of HIV-1 p24 Gag protein in plasma by comparing the signal intensity of the unknown to a standard curve constructed by adding varying concentrations of recombinant HIV-1 p24 Gag to plasma obtained from a healthy donor (range: 0.39–400.00 pg/ml of plasma or 16.3 fM to 16.7 pM). The threshold of detection for the bio-barcode-amplification method exceeded the highest signal for the samples that were HIV-1 negative (both plasma samples and negative controls) by at least 2 logs. Figure 3 shows the chip-based scanometric detection of HIV-1 p24 Gag protein in plasma from 12 representative subjects (ten who were infected with HIV-1 and two who were not).
Figure 3. Chip-based scanometric detection of HIV-1 p24 Gag-specific barcode DNA.
HIV-1 p24 Gag concentration was varied from 0.39 to 400.00 pg/ml and a negative-control sample where no HIV-1 p24 Gag was added is shown. Also shown is detection of HIV-1 p24 Gag in plasma from ten HIV-1-infected (P01–P04, P06–P08 and P10–P12) and two HIV-1-noninfected (P05 and P09) men enrolled in the Chicago component of the Multicenter AIDS Cohort Study. The amount of HIV-1 p24 Gag protein in plasma was extrapolated from a standard curve of recombinant HIV-1 p24 Gag protein in plasma from a normal donor included in each run.
HIV-1 p24 Gag was detected in the plasma of 111 out of 112 men at all stages of HIV-1 infection by the bio-barcode-amplification method, and 23 out of 112 men by the conventional ELISA. Of the 34 men who were not infected with HIV-1, no HIV-1 p24 Gag was found by either method. The amount of HIV-1 p24 Gag protein measured by the bio-barcode-amplification method ranged from 0.04 to 753.00 pg/ml of plasma (corresponding to 1.67 fM to 31.4 pM) for the 112 HIV-1-infected men; one subject had an amount of HIV-1 p24 Gag protein (0.04 pg/ml of plasma that was below the cutoff value of 0.1 pg/ml of plasma) and two other subjects had measures near the cutoff value (0.11 and 0.12 pg/ml of plasma). The HIV-1 p24 Gag protein values obtained by the chip-based scanometric and real-time PCR methods were concordant (r2 = 0.9659; p < 0.0001; data not shown). Accordingly, the percentage of false-positive results for each protein-detection method was 0% and the specificity was 33% for conventional ELISA and 99% for the bio-barcode-amplification method.
We separated the 112 HIV-1-infected men into discrete groups based on their CD4+ T-cell number or the level of HIV-1 RNA in their plasma at the time the sample was taken. When grouped according to their CD4+ T-cell number, 26 men had less than 200 cells/μl and 86 men had more than 200 cells/μl. When grouped by plasma HIV-1 RNA levels, 20 men had less than 50 copies/ml and 92 men had more than 50 copies/ml. We then determined whether these clinical groups were associated with the amount of HIV-1 p24 Gag protein in plasma measured by the bio-barcode-amplification method.
For the men with less than 200 CD4+ T cells/μl, the HIV-1 p24 Gag protein median value was 14.68 pg/ml plasma (range: 0.11–753.30); for the 86 subjects with more than 200 CD4+ T cells/μl, the median value was 4.53 pg/ml plasma (range: 0.04–83.06). HIV-1 p24 Gag protein was not detected for one subject with more than 500 CD4+ T cells/μl. There was no relationship between the levels of HIV-1 p24 Gag antigen determined by the bio-barcode-amplification assay and the CD4+ T-cell number for those subjects with less than 200 CD4+ T cells/μl (r2 = 0.1258; p < 0.075) or more than 200 CD4+ T cells/μl (r2 = 0.0009; p < 0.79). HIV-1 p24 Gag antigen was detected by conventional ELISA in plasma samples from eight out of 26 (31%) of men with less than 200, 11 out of 47 (23%) men with more than 200 and less than 500 and four out of 39 (10%) men with more than 500 CD4+ T cells/μl.
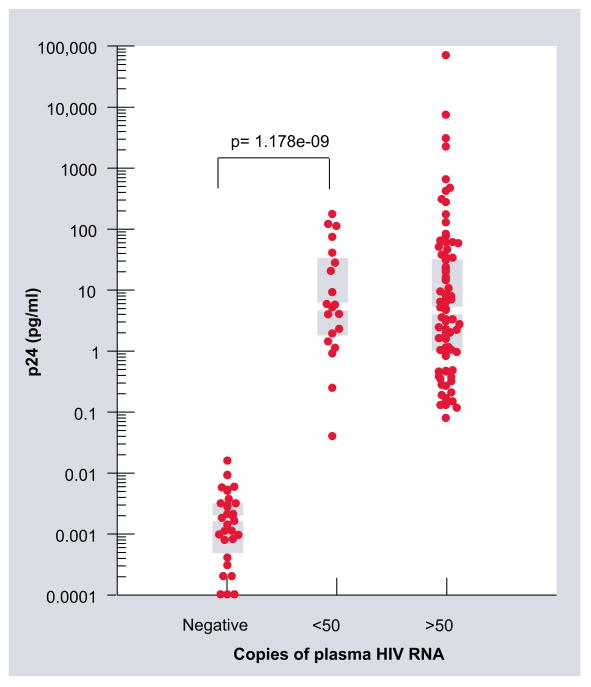
The signal from eluted barcode DNA and the level of HIV-1 RNA in plasma is shown in Figure 4. For the subjects with more than 50 copies of HIV-1 RNA/ml plasma (n = 92), the HIV-1 p24 Gag protein median value was 5.12 pg/ml (range: 0.11–71500.00). Subjects with less than 50 copies of HIV-1 RNA/ml of plasma (n = 20) had a HIV-1 p24 Gag protein median value of 4.40 pg/ml (range: 0.25–40.75). The HIV-1 p24 Gag protein median log value was 0.001 (cutoff value of 0.1 pg/ml; range: 1.0 × 10−5–0.005) for the noninfected healthy donors (n = 34). None of the 20 HIV-1-infected men with fewer than 50 copies of HIV-1 RNA/ml plasma and only 23 out of the 92 HIV-1-infected men with more than 50 copies of HIV-1 RNA/ml plasma had HIV-1 p24 Gag protein detected by conventional ELISA.
Figure 4. A plot of the HIV-1 p24 Gag concentration in plasma measured by the bio-barcode-amplification method with chip-based scanometric detection (signal intensity) against the level of HIV-1 RNA in plasma measured by a real-time PCR assay.
The ordinate is the log-transformed concentration of HIV-1 p24 Gag in plasma. The abscissa is the amount of HIV-1 RNA in plasma from subjects not infected with HIV-1 (n = 34) and from subjects infected with HIV-1 that have more than 50 copies/ml (n = 92) and less than 50 copies/ml (n = 20) of plasma RNA. The gray bars indicate the median and interquartile range. The Wilcoxon rank sum test p-values show the comparison between the HIV-1-noninfected and HIV-1-infected subjects with less than 50 copies of RNA/ml of plasma. The signal intensity for all plasma samples from subjects infected with HIV-1 scored higher than the highest signal intensity for the samples from the negative controls.
For one subject with fewer than 50 copies/ml of plasma HIV-1 RNA, the amount of HIV-1 p24 Gag protein measured by the bio-barcode-amplification method was at the cutoff level for one sample and less than the cutoff value of 0.1 pg/ml of plasma (0.04 pg/ml) for another. Nevertheless, the signal intensity for the lowest sample was 2.6-fold greater than the signal intensity for the highest negative-control value. Accordingly, the difference between the distributions of the negative controls and the HIV-1-infected subjects with fewer than 50 copies of HIV-1 RNA/ml of plasma was profound (Figure 4) and it is possible that, with further refinement of the cutoff values based on larger samples, the baseline cutoff could be improved.
We found a highly significant difference between the signal intensity for the eluted bar-code DNA in plasma obtained from the HIV-1-noninfected men (n = 34) and the HIV-1-infected men with more than 50 copies of viral RNA/ml of plasma (n = 92; Wilcoxon rank statistic p < 2 × 10−16) and less than 50 copies of HIV-1 RNA/ml (n = 20; p = 1.18 × 10−9). Among the 112 HIV-1-infected men, 17 out of 20 (85%) with less than 50 copies of HIV-1 RNA/ml plasma and 29 out of 92 (31%) with more than 50 copies of HIV-1 RNA/ml plasma were treated with viral protease inhibitors. Levels of HIV-1 p24 Gag antigen determined by the bio-barcode-amplification assay and HIV-1 RNA levels measured by real-time PCR methods in plasma for those subjects with more than 50 copies of HIV-1 RNA/ml of plasma were correlated (n = 92; r2 = 0.8882; p < 0.0001). Nevertheless, there was no association between the two assays when subjects had less than 50 copies of HIV-1 RNA/ml of plasma (n = 20; r2 = 0.01871; p < 0.56) and there was no difference between the two assays when subjects had less than 50 copies of HIV-1 RNA/ml of plasma (p = 0.72, Wilcoxon rank sum) (Figure 4). Thus, like the quantitative real-time PCR assay, the bio-barcode-amplification method is highly sensitive in terms of detection, although, when low levels of virus are present, it was not quantitative.
Discussion
The bio-barcode-amplification method offers an innovative approach to detect target proteins that overcomes many shortcomings of other diagnostics. It has high sensitivity and selectivity compared with many PCR-based approaches but without the need for enzymatic amplification. The bio-barcode-amplification method is exquisitely specific because antibodies bound to the gold nanoparticles and the magnetic microparticles are directed against two or more distinct epitopes.
In this proof-of-concept study, the bio-barcode-amplification method detected HIV-1 p24 Gag protein in plasma with comparable specificity (100%) but greater sensitivity (99% vs 20.5%) than the conventional HIV-1 p24 Gag antigen ELISA. The bio-barcode-amplification method detected HIV-1 p24 Gag protein reproducibly in all study subjects with advanced HIV-1 infection (<200 CD4+ T cells/μl of plasma). Furthermore, this protein-detection method could resolve the difference between the presence and absence of the virus in HIV-1-infected men with undetectable levels of plasma HIV-1 RNA by a standard real-time PCR assay. Lastly, the bio-barcode-amplification method detected 60 out of 60 diverse and representative samples of 60 M (for main) group viruses, the group of viruses that encompasses all of the major clades and their recombinant forms and that encompasses the diversity of the global AIDS pandemic.
Conventional ELISAs detect as little as 5–20 pg of HIV-1 p24 Gag antigen. Immune-complex dissociation to disrupt HIV-1 antigen-antibody complexes has resulted in more frequent detection of HIV-1 p24 Gag antigen late in disease [29]. Signal amplification using biotinylated tyramide [32], PCR [33] and microparticle probes with reporter barcode DNA [34] has extended the lower limit of detection of HIV-1 p24 Gag protein; however, because of the heterogeneous nature of the target-capture procedure and high background, the results using these techniques are mixed [23]. The bio-barcode-amplification method has high sensitivity based on co-loaded probes, with a lower limit of detection of 0.1 pg/ml of plasma HIV-1 p24 Gag; further refinement of this cutoff value is possible.
A simple model of stoichiometry suggests that 0.1 pg of p24 Gag protein corresponds to approximately 1000 virus particles or approximately 2000 copies of HIV-1 RNA [35]. The model assumes that all the HIV-1 p24 Gag protein in peripheral blood is in intact virions and a mature virus core contains 1000–1500 p24 Gag proteins [9,35]. The accuracy of the ratio of p24 Gag protein to viral genomic RNA is limited by the inaccuracy in the empirical determination of the amount of HIV-1 p24 Gag sufficient for mature-virus particle assembly and is confounded by the presence of HIV-1 p24 Gag in defective or immature virus particles and residual circulating immune complexes, degraded virus particles entangled in the follicular dendritic-cell network in lymphoid tissue and HIV-1 p24 Gag released from productively infected cells or leaked from cells killed by viral or immune-mediated cytotoxicity.
HIV-1 replication continues to sustain long-lived pools of infected cells, despite constraints on virus production by combinations of drugs that inhibit viral reverse transcriptase and protease and block new rounds of de novo infection [36]. Occasional expansion of an HIV-1-infected cell by antigenic stimulation may provide an opportunity for the virus to replicate for a short number of generations. HIV-1 protease inhibitors prevent cleavage of the Gag and Gag–Pol protein precursors in infected cells and thereby affect virus-particle assembly and RNA packaging [37,38]. Thus, assembly and release of virus particles occur in the presence of protease inhibitors, although the particles produced are immature and not infectious. More than twice the HIV-1 p24 Gag proteins (~5000 p24 Gag proteins) form the immature virus core [35]. A possible explanation for the lack of an association between the levels of HIV-1 RNA and HIV-1 p24 Gag in plasma is that HIV-1-infected subjects treated with a protease inhibitor have measurable amounts of HIV-1 p24 Gag despite indeterminate amounts of HIV-1 RNA in their plasma.
Because of the extreme genetic variability of HIV-1, diagnosis of infection by DNA probes and immunoassays is often difficult. The HIV-1 p24 Gag protein is one of the most well conserved proteins among HIV-1 variants. Most technologies capable of identifying HIV-1 in blood were developed for B-subtype viruses; this is a problem for much of the developing world, where C and A subtypes and circulating recombinant forms predominate [27]. The bio-barcode-amplification method performed consistently well with diverse HIV-1 subtypes because we selected peptides with the greatest level of conservation across global HIV-1 Gag protein sequences to serve as epitopes for the selected antibodies, enabling their cross-reactivity.
Technological improvements should make this protein-detection method useful in resource-limited settings in which technically demanding PCR-based assays may not be practical. Such improved diagnostic tests will probably affect measurements of disease incidence, the feasibility of disease elimination and problems associated with the appropriateness, expense and toxicity of therapy. Accordingly, this protein-detection method offers a significant opportunity to develop a simple, accurate diagnostic test that could remove an obstacle to progress against HIV/AIDS.
Executive summary
Accurate diagnostic tests for HIV-1 have a key role in clinical care and the control of HIV/AIDS.
Detection of the major core protein of HIV-1 (p24 Gag) in serum or plasma by an ELISA is specific, but does not offer the requisite sensitivity.
The nanoparticle-based bio-barcode-amplification method offers an innovative approach to protein detection.
The bio-barcode-amplification method exhibited a detection range between 0.1 pg/ml (4.2 fM) and 10 ng/ml (420 pM) HIV-1 p24 Gag, and the response was linear (r2 = 0.9969; p < 0.0001).
There was a highly significant difference between the signal intensity for the eluted barcode DNA in plasma obtained from the HIV-1-uninfected men (n = 34) and the HIV-1-infected men with more than 50 copies of HIV-1 RNA/ml of plasma (n = 92; Wilcoxon rank statistic p < 2 × 10−16) and less than 50 copies of viral RNA/ml (n = 20; p = 1.18 × 10−9).
In a separate group of 60 diverse international isolates, representative of clades A, B, C and D and circulating recombinant forms CRF01_AE and CRF02_AG, the bio-barcode-amplification method identified the presence of virus correctly.
This protein detection method offers a significant opportunity to develop an accurate diagnostic test that could remove an obstacle to progress against HIV/AIDS.
Acknowledgments
We thank J Moore, M Malim, J Storhoff and A Aldovini for helpful discussions. E-Y Kim, JP Phair, CA Mirkin and SM Wolinsky designed the study. JP Phair performed the specimen coding. E-Y Kim, J Stanton and K Krebs performed the bio-barcode amplification method. S Wu performed the specimen processing and HIV-1 RNA and CD4+ T-cell quantification. D Bogdan performed the real-time PCR and p24 ELISA. B Korber helped with peptide design and analysis. E-Y Kim, CA Mirkin, B Korber and SM Wolinsky contributed to the writing of the report. All the authors reviewed and contributed to the report.
Footnotes
Financial & competing interests disclosure
CA Mirkin and SM Wolinsky are affiliated with Nanosphere, Inc. A Doris Duke Charitable Foundation Innovation in Clinical Research Award, grants and contracts from the National Institutes of Health and the National Science Foundation supported this work. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Bibliography
- 1.Mylonakis E, Paliou M, Lally M, et al. Laboratory testing for infection with the human immunodeficiency virus: established and novel approaches. Am J Med. 2000;109:568–576. doi: 10.1016/s0002-9343(00)00583-0. [DOI] [PubMed] [Google Scholar]
- 2.Burgisser P, Vernazza P, Flepp M, et al. Performance of five different assays for the quantification of viral load in persons infected with various subtypes of HIV-1 Swiss HIV Cohort Study. J Acquir Immune Defic Syndr. 2000;23:138–144. doi: 10.1097/00126334-200002010-00005. [DOI] [PubMed] [Google Scholar]
- 3.de Baar MP, Timmermans EC, Bakker M, et al. One-tube real-time isothermal amplification assay to identify and distinguish human immunodeficiency virus type 1 subtypes A, B, and C and circulating recombinant forms AE and AG. J Clin Microbiol. 2001;39:1895–1902. doi: 10.1128/JCM.39.5.1895-1902.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lewin SR, Vesanen M, Kostrikis L, et al. Use of real-time PCR and molecular beacons to detect virus replication in human immunodeficiency virus type 1-infected individuals on prolonged effective antiretroviral therapy. J Virol. 1999;73:6099–6103. doi: 10.1128/jvi.73.7.6099-6103.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miles SA, Balden E, Magpantay L, et al. Rapid serologic testing with immune-complex-dissociated HIV p24 antigen for early detection of HIV infection in neonates Southern California Pediatric AIDS Consortium. N Engl J Med. 1993;328:297–302. doi: 10.1056/NEJM199302043280501. [DOI] [PubMed] [Google Scholar]
- 6.Pascual A, Cachafeiro A, Funk ML, et al. Comparison of an assay using signal amplification of the heat-dissociated p24 antigen with the Roche Monitor human immunodeficiency virus RNA assay. J Clin Microbiol. 2002;40:2472–2475. doi: 10.1128/JCM.40.7.2472-2475.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rouet F, Ekouevi DK, Chaix ML, et al. Transfer and evaluation of an automated, low-cost real-time reverse transcription-PCR test for diagnosis and monitoring of human immunodeficiency virus type 1 infection in a West African resource-limited setting. J Clin Microbiol. 2005;43:2709–2717. doi: 10.1128/JCM.43.6.2709-2717.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sivapalasingam S, Essajee S, Nyambi PN, et al. Human immunodeficiency virus (HIV) reverse transcriptase activity correlates with HIV RNA load: implications for resource-limited settings. J Clin Microbiol. 2005;43:3793–3796. doi: 10.1128/JCM.43.8.3793-3796.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jennings C, Fiscus SA, Crowe SM, et al. Comparison of two human immunodeficiency virus (HIV) RNA surrogate assays to the standard HIV RNA assay. J Clin Microbiol. 2005;43:5950–5956. doi: 10.1128/JCM.43.12.5950-5956.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pantaleo G, Fauci AS. Immunopathogenesis of HIV infection. Ann Rev Microbiol. 1996;50:825–854. doi: 10.1146/annurev.micro.50.1.825. [DOI] [PubMed] [Google Scholar]
- 11.Lackritz EM, Satten GA, Aberle-Grasse J, et al. Estimated risk of transmission of the human immunodeficiency virus by screened blood in the United States. N Engl J Med. 1995;333:1721–1725. doi: 10.1056/NEJM199512283332601. [DOI] [PubMed] [Google Scholar]
- 12.Bianco C, et al. U.S Public Health Service guidelines for testing and counseling blood and plasma donors for human immunodeficiency virus type 1 antigen. MMWR Morb Mortal Wkly Rep. 1996;45:1–9. [PubMed] [Google Scholar]
- 13.Prado JG, Shintani A, Bofill M, et al. Lack of longitudinal intrapatient correlation between p24 antigenemia and levels of human immunodeficiency virus (HIV) type 1 RNA in patients with chronic HIV infection during structured treatment interruptions. J Clin Microbiol. 2004;42:1620–1625. doi: 10.1128/JCM.42.4.1620-1625.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sterling TR, Hoover DR, Astemborski J, et al. Heat-denatured human immunodeficiency virus type 1 protein 24 antigen: prognostic value in adults with early-stage disease. J Infect Dis. 2002;186:1181–1185. doi: 10.1086/343807. [DOI] [PubMed] [Google Scholar]
- 15.Chadwick EG, Yogev R, Kwok S, et al. Enzymatic amplification of the human immunodeficiency virus in peripheral blood mononuclear cells from pediatric patients. J Infect Dis. 1989;160:954–959. doi: 10.1093/infdis/160.6.954. [DOI] [PubMed] [Google Scholar]
- 16.Cunningham CK, Charbonneau TT, Song K, et al. Comparison of human immunodeficiency virus 1 DNA polymerase chain reaction and qualitative and quantitative RNA polymerase chain reaction in human immunodeficiency virus 1-exposed infants. Pediatr Infect Dis J. 1999;18:30–35. doi: 10.1097/00006454-199901000-00009. [DOI] [PubMed] [Google Scholar]
- 17.Nadal D, Böni J, Kind C, et al. Prospective evaluation of amplification-boosted ELISA for heat-denatured p24 antigen for diagnosis and monitoring of pediatric human immunodeficiency virus type 1 infection. J Infect Dis. 1999;180:1089–1095. doi: 10.1086/315012. [DOI] [PubMed] [Google Scholar]
- 18.Respess RA, Cachafeiro A, Withum D, et al. Evaluation of an ultrasensitive p24 antigen assay as a potential alternative to human immunodeficiency virus type 1 RNA viral load assay in resource-limited settings. J Clin Microbiol. 2005;43:506–508. doi: 10.1128/JCM.43.1.506-508.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ribas SG, Ondoa P, Schüpbach J, et al. Performance of a quantitative human immunodeficiency virus type 1 p24 antigen assay on various HIV-1 subtypes for the follow-up of human immunodeficiency type 1 seropositive individuals. J Virol Methods. 2003;113:29–34. doi: 10.1016/s0166-0934(03)00219-2. [DOI] [PubMed] [Google Scholar]
- 20.Schupbach J, Böni J, Flepp M, et al. Antiretroviral treatment monitoring with an improved HIV-1 p24 antigen test: an inexpensive alternative to tests for viral RNA. J Med Virol. 2001;65:225–232. doi: 10.1002/jmv.2024. [DOI] [PubMed] [Google Scholar]
- 21.Schupbach J, Günthard H, Joos B, et al. HIV-1 p24 may persist during long-term highly active antiretroviral therapy, increases little during short treatment breaks, and its rebound after treatment stop correlates with CD4+ T cell loss. J Acquir Immune Defic Syndr. 2005;40:250–256. doi: 10.1097/01.qai.0000181281.75670.56. [DOI] [PubMed] [Google Scholar]
- 22.Bao YP, Wei TF, Lefebvre PA, et al. Detection of protein analytes via nanoparticle-based bio bar code technology. Anal Chem. 2006;78:2055–2059. doi: 10.1021/ac051798d. [DOI] [PubMed] [Google Scholar]
- 23.Nam JM, Thaxton CS, Mirkin CA. Nanoparticle-based bio-bar codes for the ultrasensitive detection of proteins. Science. 2003;301:1884–1886. doi: 10.1126/science.1088755. [DOI] [PubMed] [Google Scholar]
- 24.Shaikh KA, Ryu KS, Goluch ED, et al. A modular microfluidic architecture for integrated biochemical analysis. Proc Natl Acad Sci USA. 2005;102:9745–9750. doi: 10.1073/pnas.0504082102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tang S, Zhao J, Storhoff JJ, et al. Nanoparticle-based biobarcode amplification assay (BCA) for sensitive and early detection of human immunodeficiency type 1 capsid (p24) antigen. J Acquir Immune Defic Syndr. 2007;46:231. doi: 10.1097/QAI.0b013e31814a554b. [DOI] [PubMed] [Google Scholar]
- 26.Taton TA, Mirkin CA, Letsinger RL. Scanometric DNA array detection with nanoparticle probes. Science. 2000;289:1757–1760. doi: 10.1126/science.289.5485.1757. [DOI] [PubMed] [Google Scholar]
- 27.Gaschen B, Taylor J, Yusim K, et al. Diversity considerations in HIV-1 vaccine selection. Science. 2002;296:2354–2360. doi: 10.1126/science.1070441. [DOI] [PubMed] [Google Scholar]
- 28.Kim EY, Stanton J, Vega RA, et al. A real-time PCR-based method for determining the surface coverage of thiol-capped oligonucleotides bound onto gold nanoparticles. Nucleic Acids Res. 2006;34:e54. doi: 10.1093/nar/gkl147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ledergerber B, Flepp M, Böni J, et al. Human immunodeficiency virus type 1 p24 concentration measured by boosted ELISA of heat-denatured plasma correlates with decline in CD4 cells, progression to AIDS, and survival: comparison with viral RNA measurement. J Infect Dis. 2000;181:1280–1288. doi: 10.1086/315366. [DOI] [PubMed] [Google Scholar]
- 30.Ferns RB, Tedder RS, Weiss RA. Characterization of monoclonal antibodies against the human immunodeficiency virus (HIV) gag products and their use in monitoring HIV isolate variation. J Gen Virol. 1987;68:1543–1551. doi: 10.1099/0022-1317-68-6-1543. [DOI] [PubMed] [Google Scholar]
- 31.Brown BK, Darden JM, Tovanabutra S, et al. Biologic and genetic characterization of a panel of 60 human immunodeficiency virus type 1 isolates, representing clades A, B, C, D, CRF01_AE, and CRF02_AG, for the development and assessment of candidate vaccines. J Virol. 2005;79:6089–6101. doi: 10.1128/JVI.79.10.6089-6101.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bobrow MN, Harris TD, Shaughnessy KJ, Litt GJ. Catalyzed reporter deposition, a novel method of signal amplification Application to immunoassays. J Immunol Methods. 1989;125:279–285. doi: 10.1016/0022-1759(89)90104-x. [DOI] [PubMed] [Google Scholar]
- 33.Sano T, Smith CL, Cantor TR. Immuno-PCR: very sensitive antigen detection by means of specific antibody-DNA conjugates. Science. 1992;258:120–122. doi: 10.1126/science.1439758. [DOI] [PubMed] [Google Scholar]
- 34.Tang S, Zhao J, Storhoff JJ, et al. Nanoparticle-based biobarcode amplification assay for sensitive and early detection of human immunodeficiency type 1 capsid antigen. J Acquir Immune Defic Syndr. 2007;46:231–237. doi: 10.1097/QAI.0b013e31814a554b. [DOI] [PubMed] [Google Scholar]
- 35.Briggs JA, Simon MN, Gross I, et al. The stoichiometry of Gag protein in HIV-1. Nat Struct Mol Biol. 2004;11:672–675. doi: 10.1038/nsmb785. [DOI] [PubMed] [Google Scholar]
- 36.Furtado MR, Callaway DS, Phair JP, et al. Persistence of HIV-1 transcription in peripheral-blood mononuclear cells in patients receiving potent antiretroviral therapy. N Engl J Med. 1999;340:1614–1622. doi: 10.1056/NEJM199905273402102. [DOI] [PubMed] [Google Scholar]
- 37.Kaplan AH, Zack JA, Knigge M, et al. Partial inhibition of the human immunodeficiency virus type-1 protease results in aberrant virus assembly and the formation of noninfectious particles. J Virol. 1993;67:4050–4055. doi: 10.1128/jvi.67.7.4050-4055.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang SW, Aldovini A. RNA incorporation is critical for retroviral particle integrity after cell membrane assembly of Gag complexes. J Virol. 2002;76:11853–11865. doi: 10.1128/JVI.76.23.11853-11865.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Websites
- 101.Imaging Processing and Analysis in Java. http://rsb.info.nih.gov/ij/
- 102.Los Alamos National Laboratory HIV Database. www.hiv.lanl.gov/content/hiv-db/ALIGN_CURRENT/ALIGN-INDEX.html.