Abstract
Whether the lusitropic potential of short-term exercise in aged rats is linked to an augmentation in the growth hormone/insulin-like growth factor-1 (GH/IGF-1) axis and an alteration in the cardiac renin angiotensin system (RAS) is unknown. Old (28-month-old) male, Fischer 344×Brown Norway rats were randomized to 4 weeks of GH supplementation (300 μg subcutaneous, twice daily) or 4 weeks of treadmill running, or were used as sedentary controls. Six-month-old rats, sedentary or exercised, were used as young controls. Training improved exercise capacity in old animals. Exercise and GH attenuated age-related declines in myocardial relaxation despite an exercise-induced suppression of IGF-1. The regulatory protein, sarcoplasmic Ca2+ adenosine triphosphatase (SERCA2), increased with exercise but not GH. Among aged rats, the cardiac RAS was not altered by training or GH. Thus, the signaling pathway underlying the lusitropic benefit of short-term habitual exercise in the aged rat may be distinct from GH-mediated benefits and independent of the cardiac RAS.
Keywords: Aging, Diastolic function, Growth hormone, Insulin-like growth factor-1, Treadmill training
BOTH diastolic function and exercise tolerance decline in the course of normal aging (1,2). Diastolic dysfunction is characterized by incomplete relaxation of the ventricle and increased ventricular stiffness, impairing early and late filling, respectively (3). Although older patients with primary diastolic dysfunction may be asymptomatic at rest, this age-related cardiac phenotype often becomes apparent with exercise. Specifically, patients with diastolic dysfunction are not able to augment left ventricular (LV) filling appropriately with the tachycardia accompanying exercise, unless left atrial pressures are excessively increased (4). This type of exercise intolerance, in part, defines diastolic heart failure (DHF) (5), the most common form of heart failure (HF) in older patients. Exercise intolerance among HF patients with diastolic dysfunction is as severe as that among age-matched HF patients with severe systolic dysfunction (6), and this impairs quality of life.
Multiple lines of data from aging humans and animals suggest that aerobic exercise training may improve diastolic function and exercise tolerance (7-13). Although the mechanisms underlying this exercise-induced benefit are unclear, studies have shown that long-term exercise training in senescent rats increases sarcoplasmic reticular (SR) Ca2+ uptake (13,14) and attenuates age-associated changes in collagen deposition (15), two factors known to influence LV relaxation and stiffness, respectively (3). However, given the integral effects of exercise on cardiovascular structure and function (16-20), additional molecular mechanisms and signaling pathways through which exercise enhances diastolic function in the elderly population remain to be explored.
One age-related physiologic change that may be partially responsible for diastolic dysfunction and exercise intolerance is a decline in the pulsatility of growth hormone (GH) and its circulating local effector, insulin-like growth factor-1 (IGF-1). Although GH and IGF-1 are involved in heart development and in maintaining cardiac structure and performance (21), clinical and experimental evidence (21-27), including results from our laboratory (28), indicate that age-related reductions in the GH/IGF-1 axis are also associated with diastolic dysfunction, HF, and reduced exercise performance. Furthermore, we previously demonstrated that long-term GH replacement in aged rats increases circulating IGF-1 and attenuates diastolic dysfunction and LV remodeling, in part via a reduction in cardiac angiotensin II (Ang II) (28).
Because another stimulus of the GH/IGF-1 axis is physical exercise (29,30), the mechanisms that underlie the lusitropic potential of habitual exercise training and GH/IGF-1 may be interrelated, and this is the focus of our study. To date, no study has compared the influence of short-term exercise training on IGF-1 production and diastolic function in aged and mature adult rats. Brown Norway × Fisher 344 (BNF344) male rats were specifically chosen for the present study, because these animals display mild diastolic dysfunction and a reduction in GH and IGF-1 production as they age (28,31), without overt cardiovascular disease. These changes are similar to changes that occur in healthy persons >60 years of age (32,33). We also investigated the interactions between the GH/IGF-1 axis and exercise training with respect to the expression of sarcoplasmic Ca2+ adenosine triphosphatase (SERCA2). We hypothesized that brief exercise training (4 weeks) during old age would attenuate age-induced down-regulation of IGF-1, and that improved IGF-1 signaling would lead to an increase in the SR calcium regulatory protein, SERCA2, suggesting another mechanism by which exercise training limits cardiac aging. We also evaluated the role of cardiac angiotensin peptides with respect to aging, exercise, and GH, because activation of the renin angiotensin system (RAS) has been implicated in the pathogenesis of diastolic heart disease (34) and has been linked to GH/IGF-1 (28). To the best of our knowledge, this is the first study to investigate the effect of short-term exercise or GH replacement, late in life, on diastolic function of “healthy” aging rats.
Methods
Animals
Fifty 6- and 26-month-old male BNF344 rats were purchased from the National Institute on Aging colony at Harlan Industries (Indianapolis, IN) and were studied after 2 months, so that “young adult” rats were 8 months and “old” rats were 28 months of age. Rats were housed two per cage and were maintained on a 12-hour light/dark cycle, at constant temperature and humidity, in a facility approved by the Association for Assessment and Accreditation of Laboratory Animal Care. Rats had ad libitum access to standard rat chow (Nestle Purina, St. Louis, MO) and tap water. Body weights of all rats were recorded biweekly. All procedures were in compliance with the Guide for the Care and Use of Laboratory Animals, published by the National Institutes of Health and reviewed and approved by the Wake Forest University School of Medicine’s Animal Care and Use Committee before commencement of the study.
Treatment Protocol and Exercise Tolerance Measures
Rats were acclimated to a one-lane rodent treadmill (Scientific Instruments, Stoelting, Wood Dale, IL) by walking at a speed of 20 cm/s, 10 min/d, for 1 week. After acclimation, all rats underwent a pretreatment exercise tolerance test. The protocol for the exercise tolerance test consisted of walking at 20 cm/s for 3 minutes, followed by 2 cm/s increases in speed every 2 minutes until the rat reached exhaustion. Time to exhaustion (in seconds) was determined when the rat sat at the lower end of the treadmill, near a shock bar, for more than 5 seconds. Following the pretreatment treadmill stress test, young adult rats (10 per group) were randomized to either a sedentary (YSED) or exercise (YEX) group, whereas old rats were randomized into 1 of 3 groups (10 per group): sedentary (OSED), exercise (OEX), or GH replacement (OGH 300 μg porcine subcutaneously, twice daily for 4 weeks). This supplementation regimen was chosen based on our data indicating that it is sufficient to increase plasma IGF-1 levels in aged animals (28,31). Recombinant porcine GH was provided by Alpharma, Inc. (Victoria, Australia).
The 4-week exercise training program consisted of running on the treadmill at a speed of either 36 cm/s (adult) or 27 cm/s (old) for 25 min/d, 5 d/wk. All exercising rats were trained at their respective speeds within 2 weeks. The untrained rats, including YSED, OSED, and OGH groups, remained familiarized to the treadmill by walking at a speed of 20 cm/s for 10min/d, once or twice per week. The treadmill was set at 15° during walking, training, and testing. To prevent avoidance and insure exercise training, rats received a light electrical shock (6 mA) if they sat at the base of the treadmill. Within 1 week of training, the rates of exercise avoidance were minimal, and electrical shock was no longer needed. All rats underwent an exercise tolerance post-test after 4 weeks of training. During the periods between the exercise tolerance tests and echocardiograms, exercise routines were continued in moderation (15 min/d). Two of 10 OGH rats were killed prior to completion of the study, due to lower extremity paralysis. One OSED rat was eliminated from analyses, due to aberrantly high IGF-1 levels, which were >2 standard deviations from the group mean.
Echocardiographic Measures of Systolic and Diastolic Function
For the echocardiogram, rats were anesthetized with an isoflurane (2%)/oxygen mixture by nose cone during spontaneous ventilation. Anesthetized, spontaneously breathing animals were placed in a shallow left lateral decubitus position, with electrocardiographic adhesive electrodes applied to the paws. The left hemithorax was shaved and prepped with acoustic coupling gel to increase probe contact. Animals were secured to a warming table to maintain normothermia. Using a commercially available sector scanner equipped with a 12 MHz phased-array transducer (Envisor; Philips Medical Systems, Andover, MA), images were obtained at 100 mm/s sweep speed, and recorded on a digital storage optical disc for off-line analysis. LV M-mode images were obtained in the two-dimensional short-axis view, close to the papillary muscles. Diastolic posterior wall thickness and LV end-diastolic and end-systolic dimensions (LVDD, LVSD) were measured using the leading-edge method of the American Society of Echocardiography (35). The percentage of LV fractional shortening, an index of global systolic function, was calculated as ((LVDD – LVSD)/LVDD) × 100. Mitral inflow measurements of early filling velocities (Emax), early deceleration slope (Edecslope), and early deceleration time (Edectime) were obtained using pulsed Doppler, with the sample volume placed at the tips of mitral leaflets from an apical four-chamber orientation. Due to relatively high heart rates and fusion of the early and late Doppler profiles, only Emax was measured. Doppler tissue imaging to assess septal annular velocities (e’) of the mitral valve was also obtained from the four-chamber view. All measured and calculated systolic and diastolic indices are represented as the average of at least five consecutive cardiac cycles to minimize beat-to-beat variability.
Biochemical Analyses
Ten days after the echocardiogram, rats were killed by rapid decapitation, blood was collected for IGF-1, and hearts were rapidly removed, weighed, and stored at −80°C for angiotensin peptide analyses. IGF-1 (Bachem, Torrance, CA) was radiolabeled using the lactoperoxidase and glucose oxidase method, and purified on a Sep-Pak silica cartridge (Waters, Milford, MA). Serum was extracted in acid–ethanol (36), and IGF-1 was measured by radioimmuno-assay (RIA) as previously described (37). The coefficient of variation for the RIA was 9%. Peptide values for Ang II, angiotensin 1-7 (Ang 1-7), and angiotensin I (Ang I) were verified in pooled samples by RIA. Relative levels of angiotensin converting enzyme-1 (ACE1), ACE2, and neprilysin were obtained from the peptide ratios Ang II/Ang I, Ang 1-7/Ang II, and Ang 1-7/Ang I, respectively.
Immunoblot Analysis
SERCA2 levels were determined by Western blot from isolated SR membranes (28), using rabbit polyclonal antibodies (Abcam, Cambridge, MA) and peroxidase-conjugated secondary anti-rabbit immunoglobulin G antibodies. Bands were identified by chemiluminescence. To normalize the variability of protein loading, the antibody to glyceraldehyde 3-phosphate dehydrogenase (GAPDH; Abcam) was probed on stripped blots (Recycling kit; Alpha Diagnostic International, Inc., San Antonio, TX). Each SERCA2 was normalized to its own GAPDH and expressed in arbitrary units.
Data Analysis
Data were analyzed using SPSS 13.0 (SPSS, Inc., Chicago, IL). Parametric assumptions were evaluated using histograms and descriptive statistics, and all variables satisfied the assumptions necessary for analysis of variance (ANOVA). For all conducted analyses, a 2 × 2 factorial ANOVA was conducted with Age (old vs young) and Exercise (sedentary vs exercised) as between-subjects factors. Because the GH treatment was applied only to old animals, its effect was examined in a separate one-way ANOVA, consisting of data only for old animals, and only post hoc comparisons between GH-treated and untreated animals were evaluated using Dunnett’s test. No attempts were made to control for multiple comparisons.
Because of the relative novelty of our hypotheses, a formal statistical power calculation was not conducted prior to conducting the study, for the intervention’s effect on our outcomes (e.g., short-term training or GH) was not known. Instead, the present study was primarily designed to examine the effect sizes (i.e., degree of difference) induced by the effects of age, exercise, and the interaction of age on exercise. For this reason, a standardized measure of effect size, Cohen’s d, is reported for all analyses (38). This measure reflects the degree of difference between two group means in terms of their standard deviation. Although rules-of-thumb interpretation of effect size measures can be problematic (38), d = 0.20 is generally considered to be a small difference (as the groups only differ by two tenths of their within-group standard deviations), d = 0.50 is considered a medium sized difference, and differences of d ≥ 0.80 are considered large. Where appropriate, the observed effect size is discussed in relation to its practical clinical significance.
Results
Animal Characteristics
The physical characteristics of the animals in all experimental groups are presented in Table 1. Body weight was significantly higher in old than in young animals. Exercise training did not induce significant changes in body weight in YEX rats, but was associated with a significant reduction in the weight of OEX rats. OGH rats exhibited a significant increase in body weight compared to the OEX group (F(2,24)=7.72, p=.003), probably as a result of the increased water retention associated with GH administration (39), combined with decreased body weight of the OEX rats. At the time of death, heart weights of old rats were greater than those of young rats. Among the old rats, OGH had greater heart weights than OEX or OSED (F(2,24) = 9.19, p=.001). However, the heart-to-body weight ratios of the OGH rats were not suggestive of cardiac hypertrophy when compared to ratios of OSED, OEX (F(2,24)=0.668), or young rats (Table 1).
Table 1.
Physical Characteristics of Experimental Groups
| Age |
Exercise |
Interaction |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Physical Variables | Sedentary | Exercised | GH | p Value | d | p Value | d | p Value | d* |
| Body weight, g | F(1,35) = 438 | 7.29 | F(1,35) = 9.73 | 1.06 | F(1,35) = 4.42 | 0.91 | |||
| Old | 591 ± 11 | 547 ± 9† | 630 ± 22 | ||||||
| Young | 401 ± 5 | 393 ± 6 | <.001 | .004 | .039 | ||||
| Body weight change, g | F(1,35) = 93 | 3.21 | F(1,35) = 10.23 | 1.09 | F(1,35) = 1.90 | 0.46 | |||
| Old | (−)37 ± 9 | (−)66 ± 8† | (−)10 ± 13 | ||||||
| Young | 27 ± 5 | 14 ± 3 | <.0001 | .003 | .176 | ||||
| Heart weight, g | F(1,35) = 175 | 4.42 | F(1,35) = 0.66 | 0.29 | F(1,35) = 2.33 | 0.51 | |||
| Old | 1.48 ± 0.4‡ | 1.41 ± .03† | 1.65 ± .05 | ||||||
| Young | 1.06 ± .02 | 1.08 ± .02 | <.001 | .423 | .136 | ||||
| LVW, g | F(1,35) = 299 | 6.00 | F(1,35) = 2.79 | 0.55 | F(1,35) = 3.79 | 0.67 | |||
| Old | 1.14 ± .02† | 1.08 ± .02† | 1.27 ± .03 | ||||||
| Young | 0.81 ± .01 | .82 ± .02 | <.001 | .104 | .006 | ||||
| LVW/Body weight, mg/g | F(1,35) = 10.58 | 1.09 | F(1,35) = 2.17 | 0.51 | F(1,35) = .053 | 0.00 | |||
| Old | 1.93 ± .04 | 1.97 ± .03 | 2.03 ± .03 | ||||||
| Young | 2.03 ± .02 | 2.08 ± .03 | .003 | .15 | .82 | ||||
| LVW/Tibia length, g/cm | F(1,35) = 27.27 | 1.77 | F(1,35) = .32 | 0.20 | F(1,35) = 0.56 | 0.20 | |||
| Old | .57 ± .007 | .57 ± .004 | .58 ± .003 | ||||||
| Young | .54 ± .004 | .55 ± .006 | <.001 | .573 | .56 | ||||
| IGF-1, pg/mL | F(1,35) = 4.68 | 0.74 | F(1,35) = 3.06 | 0.59 | F(1,35) = 0.07 | 0.00 | |||
| Old | 773 ± 51† | 709 ± 33† | 1093 ± 43 | ||||||
| Young | 877 ± 44 | 791 ± 42 | .038 | .089 | .80 | ||||
Notes:
Because there are four group means, there is no unique representation of d, so d = 2 × SDmeans = 2f.
p < .001 vs old GH.
p < .01 vs old GH.
GH = growth hormone treated; LVW = left ventricular weight; IGF-1 = insulin-like growth factor-1.
Serum levels of IGF-1 were significantly reduced in OSED and OEX rats compared to YSED and YEX rats (Table 1). Distinct from the age effect, there was a strong tendency for short-term training to reduce IGF-1 levels, as more than half a standard deviation difference in group means was observed. OGH rats displayed significantly higher levels of IGF-1 than both OSED and OEX rats (F(2,24) = 21.32, p < .0001), indicating that the supplementation regimen effectively restored systemic IGF-1 levels.
In Vivo Measurements
Exercise capacity
The effects of age, training, and GH on exercise capacity are shown in Figure 1. As expected, old rats had a significantly reduced exercise tolerance at baseline compared to young rats. Following 4 weeks of exercise training in both the young and old, treadmill tolerance times to exhaustion improved from respective baseline times and were significantly greater than those of their sedentary, age-matched counterparts. In fact, post-training exercise capacities achieved by the OEX rats were similar to those achieved by the YSED rats (Table 2). In contrast, OGH rats did not experience a notable improvement in pre- to postexercise tolerance times (Figure 1). Moreover, performance times in old rats after 4 weeks of GH were significantly lower than those of OEX rats and comparable to those of the OSED group.
Figure 1.
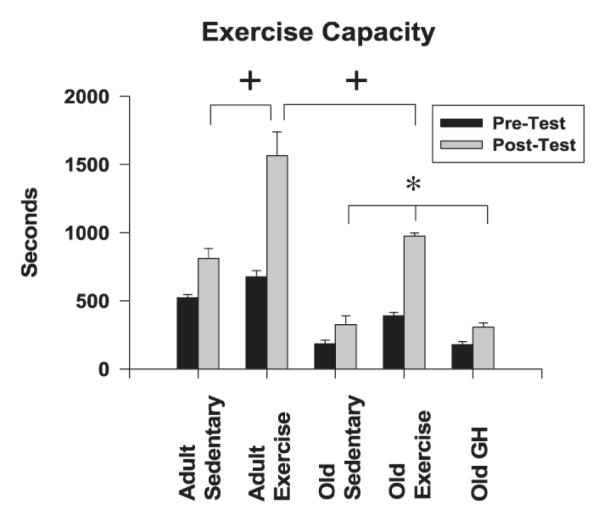
Exercise tolerance, or time to exhaustion, was increased by short-term training but not growth hormone (GH) supplementation in old rats. Data indicate exercise capacity times before and after treatment (training or GH) with respect to age. Values are means ± standard error. #p < .05 Young, pretest times vs Old, pretest times; + p < .05 vs young sedentary, old sedentary (OSED), old exercise, old GH-supplemented (OGH) post-test times; *p < .05 vs OSED and OGH post-test times.
Table 2.
In Vivo Measurements of Exercise Capacity and Cardiac Structure and Function
| Age |
Exercise |
Interaction |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| In Vivo Measures | Sedentary | Exercised | GH | p Value | d | p Value | d | p Value | d* |
| Exercise capacity, s | F(1,35) = 86 | 3.21 | F(1,35) = 147 | 4.13 | F(1,35) = 0.81 | 0.29 | |||
| Old | 325 ± 61 | 975 ± 22† | 306 ± 29 | ||||||
| Young | 810 ± 73 | 1564 ± 55 | <.0001 | <.0001 | .375 | ||||
| LVESD, cm | F(1,35) = 20 | 1.57 | F(1,35) = .15 | 0.00 | F( 1,35) = 0.01 | 0.00 | |||
| Old | 0.482 ± 0.33 | 0.491 ± .023 | 0.483 ± .020 | ||||||
| Young | 0.389 ± .007 | 0.396 ± .010 | <.0001 | .700 | .941 | ||||
| LVEDD, cm | F(1,35) = 26 | 1.77 | F(1,35) = .02 | 0.00 | F(1,35) = 0.62 | 0.29 | |||
| Old | 0.849 ± .026 | 0.831 ± .025 | 0.875 ± .033 | ||||||
| Young | 0.717 ± .018 | 0.732 ± .021 | <.0001 | .883 | .438 | ||||
| FS, % | F(1,35) = 4.2 | 0.70 | F(1,35) = .62 | 0.29 | F(1,35) = .91 | 0.35 | |||
| Old | 44 ± 2.3 | 41 ± 1.5 | 45 ± 1.3 | ||||||
| Young | 46 ± 1.3 | 46 ± 0.9 | .049 | .438 | .434 | ||||
| PWTed, cm | F(1,35) = 47 | 3.06 | F(1,35) = 13 | 1.28 | F(1,35) = .28 | 0.20 | |||
| Old | 0.204 ± .007 | 0.188 ± .004‡ | 0.215 ± .007 | ||||||
| Young | 0.171 ± .005 | 0.149 ± .004 | <.0001 | <.0001 | .602 | ||||
| IVRT, s | F(1,35) = 1.2 | 0.55 | F(1,35) = 2.0 | 0.55 | F(1,35) = .005 | 0 | |||
| Old | .020 ± .001 | .019 ± .001 | .021 ± .001 | ||||||
| Young | .019 ± .001 | .019 ± .001 | .288 | .169 | .945 | ||||
| Emax, cm/s | F(1,35) = .41 | 0.20 | F(1,35) = 1.4 | 0.41 | F(1.35) = .27 | 0.20 | |||
| Old | 95 ± 4 | 99 ± 4 | 102 ± 2 | ||||||
| Young | 92 ± 4 | 99 ± 4 | .530 | .247 | .606 | ||||
| Edec slope, cm/s2 | F(1,35) = 1.8 | 0.46 | F(1,35) = 1.2 | 0.41 | F(1,35) = 3.9 | 0.87 | |||
| Old | 27 ± 3 | 26 ± 2 | 24 ± 1 | ||||||
| Young | 21 ± 1 | 27 ± 2 | .188 | .278 | .057 | ||||
| Edec time, s | F(1,35) = 1.6 | 0.41 | F(1,35) = 2.6 | 0.55 | F(1,35) = 5.6 | 0.81 | |||
| Old | 0.040 ± .003 | .041 ± .002 | 0.46 ± .002 | ||||||
| Young | 0.047 ± .001 | .039 ± .001 | .221 | .117 | .024 | ||||
| e’, cm/s | F(1.35) = 4.4 | 0.74 | F(1,35) = .72 | 0.29 | F(1,35) = 2.3 | 0.55 | |||
| Old | 4.97 ± 0.34 | 6.16 ± .54§ | 6.53 ± .45§ | ||||||
| Young | 6.76 ± 0.62 | 6.43 ± 0.31 | .044 | .404 | .135 | ||||
| E/e’ | F(1,35) = 3.8 | 0.67 | F(1,35) = .08 | 0.00 | F(1,35) = 1.2 | 0.35 | |||
| Old | 19 ± 2 | 17 ± 1 | 16 ± 1 | ||||||
| Young | 15 ± 1 | 16 ± 1 | .061 | .778 | .275 | ||||
| Heart rate, bpm | F(1,35) = 3.7 | 0.70 | F(1,35) = 9.4 | 1.06 | F(1,35) = 1.90 | 0.46 | |||
| Old | 332 ± 14 | 318 ± 6 | 323 ± 3 | ||||||
| Young | 364 ± 10 | 323 ± 3 | .061 | .004 | .177 | ||||
Notes:
Because there are four group means, there is no unique representation of d, so d = 2 × SD mean = 2f.
p < .001 vs old GH and old sedentary.
p < .001 vs old GH.
p = .06 vs old sedentary.
GH = growth hormone treated; LVESD = left ventricular end systolic dimension; LVEDD = left ventricular end diastolic dimension; FS = fractional shortening; PWTed = posterior wall thickness at end diastole; IVRT = isovolumic relaxation time; Emax = peak velocity of early filling; Edec slope = deceleration slope of early filling; Edec time = deceleration time of early filling; e’ = mitral annular descent of early filling; E/e’ = early filling-to-mitral annular descent ratio.
Cardiac function
The effect of age, training, and GH on resting echocardiographic measures of myocardial structure and function are shown in Table 2. Old rats, regardless of treatment, had greater LV dimensions and wall thickness than their younger counterparts had. Although LV chamber dimensions were not affected by exercise, habitual training led to significant reductions in wall thickness, independent of age. Among the old animals, OGH rats had significantly thicker LV walls than did OEX rats (F(2,23) = 4.316, p = .026). Although systolic function was modestly lower in old than in young rats (p = .049), this age effect was not influenced by either exercise or GH.
Diastolic function during early LV filling was evaluated using both pulse wave and tissue Doppler. The time between aortic valve closure and mitral valve opening, isovolumic relaxation time, and Emax were not influenced by age or treatment (Table 2). However, habitual exercise led to a significant reduction in Edectime in the YEX rats, but not in OEX rats (Age × Exercise effect, F(1,35) = 5.6, p = .024). As expected, the tissue Doppler measure of myocardial relaxation, or mitral annular descent (e’), was significantly lower in old rats than in their younger counterparts. Among the old rats, short-term exercise and GH led to an increased e’ (p = .06) (Figure 2). Moreover, there was a strong tendency for filling pressures, as depicted by the E/e’ ratio, to be higher in old than in young rats (p = .061), as more than two thirds a standard deviation difference between group means was observed (Table 2). Among the old rats, neither exercise training nor GH affected E/e’ (F(2,24)=0.984). Heart rates under anesthesia were significantly reduced in exercised as compared to sedentary rats, regardless of age.
Figure 2.
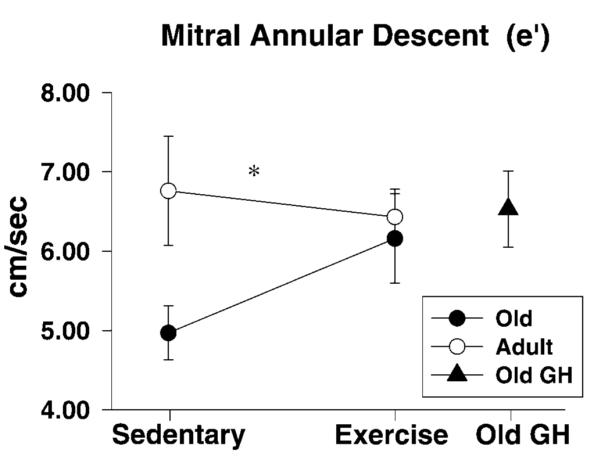
Tissue Doppler measure of diastolic function, mitral annular descent (e’), was lower in old as compared to young rats. Although short-term exercise did not affect e’ in the young, it enhanced myocardial tissue velocities in old trainers, as did growth hormone (GH) supplementation. Data indicate differences in septal e’ between sedentary and trained rats with respect to age. The effect of GH supplementation among old rats is also presented. Values are means ± standard error. *p < .05, effect of aging.
In Vitro Measurements
Immunoblots
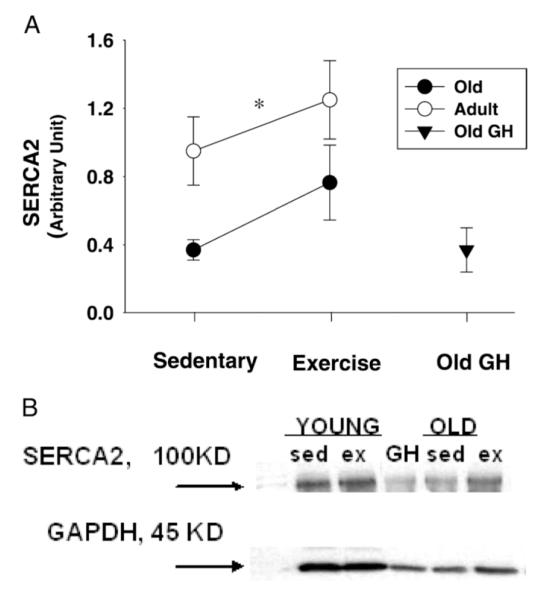
As expected, SERCA2 expression in heart tissue was significantly lower in old than in young rats (F(1,26) = 5.44, p = .028) (Figure 3A and B). Exercise training tended to increase SERCA2 in both age groups (F(1,26) = 2.34, p = .14) as it led to more than half a standard deviation (d = 0.59) difference in group means between trained and sedentary rats. Four weeks of GH administration failed to alter SERCA2 levels of OGH rats compared to those of OSED rats.
Figure 3.
A, Cardiac sarcoplasmic Ca2+ adenosine triphosphatase (SERCA2) levels were reduced in old rats. Even though brief exercise training enhanced SERCA2 expression among old trainers, protein levels of this important calcium regulatory protein were also improved in young trainers when compared to their sedentary counterparts. No changes in SERCA2 were observed as a result of growth hormone (GH) supplementation. Data indicate differences in SERCA2 levels between sedentary and trained rats with respect to age. The effect of GH supplementation among old rats is also presented. Values are means ± standard error. *p < .05, effect of aging. B, Immunoblot examples of SERCA2 and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) from each age and treatment group are also displayed. Sed = sedentary; ex = exercised.
Biochemistry
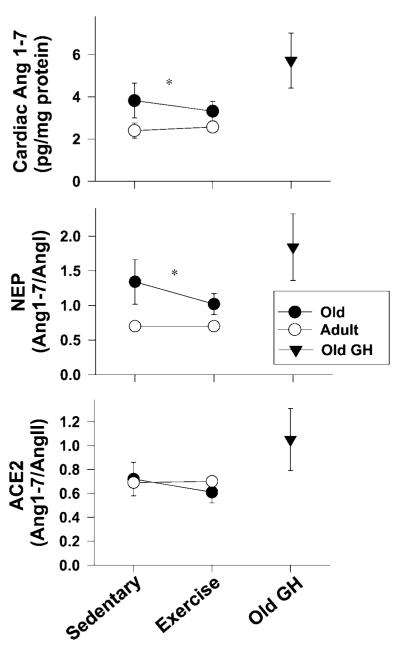
Results indicating the contribution of the cardiac RAS to diastolic dysfunction of aging and the potential effect of short-term exercise or GH supplementation on angiotensin peptide levels are shown in Table 3 and Figure 4. Cardiac Ang 1-7 was modestly elevated in the old rats, whereas its substrate, Ang II, was higher in young rats. The enzyme surrogates, ACE2 and neprilysin, also showed an age effect: The ratio of Ang 1-7/Ang II (or ACE2) (age: F(1,26)=7.84, p=.009) and Ang 1-7/Ang 1 (or neprilysin) (age: F(1,26) = 5.18, p = .03) was higher in old than in young rats. In addition, cardiac levels of the antiproliferative peptide, Ang 1-7, tended to be increased by GH treatment (p = .06). Exercise training appeared to have a different effect than GH on cardiac RAS components, increasing Ang I and Ang II levels without altering Ang 1-7 levels.
Table 3.
Angiotensin Peptides Related to Age, Exercise, and Growth Hormone (GH)
| Age |
Exercise |
Interaction |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Angiotensin Peptides | Sedentary | Exercised | GH | p Value | d | p Value | d | p Value | d* |
| Angiotensin I | F(1,28) = 2.37 | 0.59 | F(1,28) = 1.15 | 0.41 | F(1,28) = .03 | 0.00 | |||
| Old | 2.97 ± 0.16 | 3.25 ± 0.16 | 3.31 ± 0.28 | ||||||
| Young | 3.36 ± 0.34 | 3.57 ± 0.18 | .135 | .292 | .855 | ||||
| Angiotensin II | F(1,28) = 4.09 | 0.77 | F(1,28) = 2.95 | 0.67 | F(1,28) = .05 | 0.00 | |||
| Old | 5.18 ± 0.31 | 5.82 ± 0.25 | 5.72 ± 0.56 | ||||||
| Young | 5.96 ± 0.64 | 6.80 ± 0.47 | .053 | .097 | .823 | ||||
| Angiotensin 1-7 | F(1,28) = 3.76 | 0.74 | F(1,28) = .08 | 0.00 | F(1,28) = .56 | 0.20 | |||
| Old | 3.82 ± 0.82 | 3.32 ± 0.46 | 5.73 ± 1.31 | ||||||
| Young | 2.40 ± 0.36 | 2.57 ± 0.16 | .063 | .774 | .556 | ||||
Note:
Because there are four group means, there is no unique representation of d, so d = 2 × SDmeans = 2f.
Figure 4.
Effect of age and treatment on cardiac levels of the surrogate renin angiotensin system enzymes, angiotensin converting enzyme-2 (ACE2), and neprilysin (NEP), are shown. The observed age-related increase in angiotensin 1-7 (Ang 1-7) among old rats suggests that an endogenous protective or compensatory mechanism (e.g., antifibrotic or vasorelaxant) may be occurring in the “healthy” aging BNF344 rats, slowing the progression of diastolic dysfunction. The enzymatic pathway leading to Ang 1-7 in the aged rats preferentially involved neprilysin. *p < .05, effect of aging. GH = growth hormone.
Discussion
Understanding the influence of exercise training and GH supplementation on the senescent heart is important, considering the high prevalence of DHF in elderly people (40) and the frequent need for treatments that limit the progression of its precursor, diastolic dysfunction. Results of the present study demonstrated that short-term exercise training or GH supplementation initiated late-life attenuated diastolic dysfunction in old BNF344 rats. Despite comparable levels of circulating IGF-1, the cardioprotective action of exercise in the old rats was associated with improvements in the regulatory protein, SERCA2. In contrast, GH treatment of old rats restored IGF-1 levels to levels found in young rats and improved myocardial tissue velocities, but did not affect exercise capacity or SERCA2. Although we cannot rule out the possibility that an exercise-induced increase in myocardial uptake of IGF-1 (41,42) or a local autocrine or paracrine effect of IGF-1 (43) contributed to diastolic “health” in the OEX rats, the cardioprotective action of exercise training does not appear to be related to an augmented peripheral somatotropic axis. Thus, our results suggest that different mechanisms underlie the lusitropic benefit of short-term exercise versus GH supplementation.
Regular exercise improves cardiovascular performance in older people (44) and is considered an important therapeutic adjunct in the management of patients with DHF (45). Although the effect of exercise training on diastolic function has been variable in human studies (8,46-48), treadmill training of aged rats has been shown to enhance ventricular lusitropy and compliance (7,13,14). Our results similarly showed that 4 weeks of regular treadmill exercise improved myocardial relaxation, that is, increased the velocity of mitral annular descent, and exercise tolerance in old rats. The lack of diastolic improvement in the YEX rats may indicate that diastolic function was maximized prior to commencement of the training protocol. Likewise, tissue Doppler indices of diastolic function from high level, endurance-trained, young adult athletes were similar to those of sedentary, age-matched cohorts (49). Moreover, the inconsistent response of young rats to exercise training—enhancement of exercise tolerance with no change in mitral annular descent—implies that training has a greater impact on performance or tolerance than on resting diastolic function.
Many of the health-promoting effects of exercise result from the interaction of specific hormones and growth factors, such as insulin and IGF-1 (50). In humans and rodents, aging is associated with a reduction in GH pulsatility and hepatic GH resistance, resulting in a decline in serum IGF-1 (31,33), whereas physical activity is usually associated with constant or increased circulating levels of IGF-1 (29,30). The present study confirmed that IGF-1 decreases with age in rats; however, blood levels of this GH mediator remained unchanged or were slightly reduced by exercise training in rats, regardless of age. Discrepancies between results of human and animal studies might be at least partly due to differences in type, duration, and intensity of exercise. IGF-1 levels in humans were unaffected by chronic resistance training (51), unless the stimulus was of high intensity (52). Rodent responses also depend on the intensity of exercise. In one study, 8 weeks of treadmill training had no effect on circulating IGF-1, but increased hepatic IGF-1 (53), whereas 6 weeks of weighted swim training resulted in increased blood levels of IGF-1 (54). Interestingly, even when circulating levels remained unchanged, many of these exercise protocols resulted in increased local tissue production of IGF-1. For example, increased myocardial IGF-1 gene expression occurred after swim training (55), and skeletal muscle production of IGF-1 was stimulated by treadmill training (42). In addition, running may induce an increase in peripheral IGF-1 uptake in the target organ (43). Therefore, in the present study, we cannot rule out the possibility that exercise increased the myocardial uptake of blood IGF-1, resulting in lower circulating levels, and/or induced a local myocardial IGF-1 autocrine/paracrine response (56). Either or both of these effects could have contributed to enhanced diastolic function in the OEX rats. Furthermore, cardiac-specific overexpression of IGF-1 was recently shown to attenuate aging-associated cardiac diastolic dysfunction in aged transgenic mice (57).
The mechanism of exercise-induced attenuation of cardiac function in senescence is potentially multifactorial. Advantageous LV remodeling (58), enhanced calcium sequestration in sarcoplasmic reticulum (13,14), reduced aortic stiffness (18), and lowered heart rate have all been reported. Our finding of increased SERCA2 expression in OEX rats indicates that exercise may also facilitate intracellular Ca2+ resequestration. Tate and colleagues (13,14) similarly found enhancement of SERCA2 expression and function in hearts of exercise-trained old rats. The fact that exercise also increased SERCA2 in our young rats, but without a significant change in diastolic function, confirms that other factors besides intracellular calcium regulation were involved in the exercise-induced cardiac benefit.
One age-related physiological change associated with cardiac aging that may be altered by physical exercise is a decline in GH/IGF-1 activity. Low levels of IGF-1 are strongly related to increased risk of HF (59), particularly in elderly patients without previous myocardial infarction (25). Furthermore, reductions in global systolic and diastolic function in GH-deficient humans and experimental animals (21-24,60,61) have been partially ameliorated by GH replacement therapy (23,60). Beneficial effects of short-term (2–4 weeks) GH administration on cardiac performance and LV remodeling have also been reported in experimental models of HF, such as postmyocardial infarction in rats (62,63). Restoration of myocyte function has been associated with increases in SERCA2 in the left ventricle, suggesting GH-mediated synthesis of the proteins involved in intracellular calcium regulation (57). Moreover, we previously demonstrated that senescent rats with low GH/IGF-1 had reduced diastolic function, whereas 6 months of GH replacement preserved diastolic function and was associated with significant changes in serum IGF-1 and SERCA2 expression (28). In contrast, GH treatment in the present study did not alter the expression of SERCA2 in old rats, possibly due to the short duration of GH treatment (4 weeks). However, given the improved myocardial tissue velocities in the OGH rats compared to the OSED rats, a GH/IGF-1–mediated anti-inflammatory (64), antiapoptotic (65), or antifibrotic (66) effect on the myocardium could account for the attenuated diastolic dysfunction associated with aging. Although systemic blood pressures were not recorded, IGF-1–mediated nitric oxide release from vascular smooth muscle cells could have also had an effect on vascular tone, further improving lusitropic function in the OGH rats (67).
In addition to the effects described above, GH/IGF-1 could limit diastolic dysfunction of aging through interactions with the RAS. The RAS is an important regulator of cardiac growth and has been implicated in the pathogenesis of diastolic dysfunction (34). Increases in cardiac Ang II and aldosterone levels in aged rats have been associated with LV remodeling (68). Ang II promotes myocyte hypertrophy, increases myocardial collagen synthesis, and is mitogenic to neonatal cardiac fibroblasts (69-71). Previous work in our laboratory showed that reductions in Ang II were associated with limitations in cardiac collagen and diastolic dysfunction through long-term GH supplementation (28). Thus, aging, exercise, and/or GH/IGF-1 may affect diastolic dysfunction through the formation of Ang 1-7, the vasodilator, anti-proliferative arm of the RAS (61). Although the physiologic role of Ang 1-7 in the aged heart is not known, data suggest that ACE2, through the combined actions of metabolizing Ang II and increasing Ang 1-7, may serve to counterbalance the profibrotic effects of the ACE/Ang II pathway (72). Moreover, ACE inhibition and angiotensin receptor-1 antagonism reduced collagen deposition in the myocardium in spontaneously hypertensive rats and in the myocardium following myocardial infarction (73). Importantly, both of these treatments increase either circulating or tissue levels of Ang 1-7. Although we did not evaluate the short-term effects of GH or exercise on age-related cardiac fibrosis, results of the present study showed that aging did affect the RAS. Interestingly, levels of Ang 1-7 and the surrogate enzymes (ACE2 and neprilysin) that lead to the production of Ang 1-7 were higher in hearts of old than in hearts of young rats. Whether this increased preponderance of Ang 1-7 in the “healthy” aging BNF344 rats is a protective mechanism against more advanced stages of diastolic dysfunction or a counterregulatory mechanism, as noted in hypertensive rats (74), is currently under investigation. Interestingly, there was a strong tendency for short-term GH treatment to enhance Ang 1-7 production, as nearly three quarters of a standard deviation (d = 0.74) difference among old rats in Ang 1-7 was observed. Exercise training appeared to have a different effect than GH on the angiotensin peptides, tending to increase Ang II levels without altering Ang 1-7 levels. Future studies will address whether the cardiac components of the RAS including Ang II and the antiproliferative metabolite Ang 1-7, as well as their receptors, are differentially altered in aged rats following long-term GH replacement and/or exercise late in life.
The present study has several limitations. First, cardiac function was based on noninvasive evaluation of hemodynamics and myocardial performance. Therefore, we do not have direct measurements of filling pressures or of the decrease in LV isovolumetric pressure and its time constant, τ. However, our investigation used both transmitral and tissue Doppler echocardiography, which are the methods of choice for routine noninvasive evaluation of diastolic function in humans (75). Compared with conventional Doppler flow, tissue Doppler of the early diastolic velocity at the mitral annulus (e’) is less influenced by loading conditions (76,77), and has been closely related to invasive measurements of diastolic function in humans and animals (78). Second, as mentioned above, the possibility that exercise training enhanced myocardial uptake of blood IGF-1 or induced a local IGF-1 autocrine/paracrine response cannot be ruled out, because we did not measure cardiac IGF-1. Third, as we were primarily interested in comparing effects of treadmill exercise training and GH supplementation on diastolic function late in life, this preliminary study did not include the combination of GH supplementation and exercise training. Our future studies will investigate whether the combination of exercise training and GH supplementation at advanced age has an added, even synergistic, effect on diastolic function and exercise tolerance.
Clinical Perspective
Our results support the concept that a sedentary lifestyle is a risk factor for diastolic dysfunction, and they emphasize the importance of regular exercise, even late in life, to attenuate progression of the disease process. If, however, a patient is unable to exercise for a period of time (e.g., due to an orthopedic problem or illness), brief intervention with GH or a GH/IGF-1 analogue may be a reasonable alternative, even though the lusitropic benefits of GH repletion and aerobic exercise training appear to have different mechanisms. A risk/benefit assessment should be conducted prior to the initiation of GH treatment, given that GH supplementation in older adults has been associated with fluid retention, joint pain, carpal tunnel syndrome, impaired glucose tolerance (79), and possibly an increased incidence of cancer (80).
Acknowledgments
These studies were supported in part by grants from the Dennis W. Jahnigen Career Development Program (L.G.) and National Institutes of Health Grants KO8-AG026764-03 Paul Beeson award (L.G.) and AGR3718915 (D.A.K.).
References
- 1.Higginbotham MB, Morris KG, Williams RS, Coleman RE, Cobb FR. Physiologic basis for the age-related decline in aerobic work capacity. Am J Cardiol. 1986;57:1374–1379. doi: 10.1016/0002-9149(86)90221-3. [DOI] [PubMed] [Google Scholar]
- 2.Schulman SP, Lakatta EG, Fleg JL, Lakatta L, Becker LC, Gerstenblith G. Age-related decline in left ventricular filling at rest and exercise. Am J Physiol. 1992;263(6 Pt 2):H1932–H1938. doi: 10.1152/ajpheart.1992.263.6.H1932. [DOI] [PubMed] [Google Scholar]
- 3.Groban L. Diastolic dysfunction in the older heart. J Cardiothorac Vasc Anesth. 2005;19:228–236. doi: 10.1053/j.jvca.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 4.Little WC, Kitzman DW, Cheng CP. Diastolic dysfunction as a cause of exercise intolerance. Heart Fail Rev. 2000;5:301–306. doi: 10.1023/a:1026503028065. [DOI] [PubMed] [Google Scholar]
- 5.Kitzman DW, Higginbotham MB, Cobb FR, Sheikh KH, Sullivan MJ. Exercise intolerance in patients with heart failure and preserved left ventricular systolic function: failure of the Frank-Starling mechanism. J Am Coll Cardiol. 1991;17:1065–1072. doi: 10.1016/0735-1097(91)90832-t. [DOI] [PubMed] [Google Scholar]
- 6.Kitzman DW, Little WC, Brubaker PH, et al. Pathophysiological characterization of isolated diastolic heart failure in comparison to systolic heart failure. JAMA. 2002;288:2144–2150. doi: 10.1001/jama.288.17.2144. [DOI] [PubMed] [Google Scholar]
- 7.Brenner DA, Apstein CS, Saupe KW. Exercise training attenuates age-associated diastolic dysfunction in rats. Circulation. 2001;104:221–226. doi: 10.1161/01.cir.104.2.221. [DOI] [PubMed] [Google Scholar]
- 8.Forman DE, Manning WJ, Hauser R, Gervino EV, Evans WJ, Wei JY. Enhanced left ventricular diastolic filling associated with long-term endurance training. J Gerontol. 1992;47:M56–M58. doi: 10.1093/geronj/47.2.m56. [DOI] [PubMed] [Google Scholar]
- 9.Levy WC, Cerqueira MD, Abrass IB, Schwartz RS, Stratton JR. Endurance exercise training augments diastolic filling at rest and during exercise in healthy young and older men. Circulation. 1993;88:116–126. doi: 10.1161/01.cir.88.1.116. [DOI] [PubMed] [Google Scholar]
- 10.Li Y, Lincoln T, Mendelowitz D, Grossman W, Wei JY. Age-related differences in effect of exercise training on cardiac muscle function in rats. Am J Physiol. 1986;251(1 Pt 2):H12–H18. doi: 10.1152/ajpheart.1986.251.1.H12. [DOI] [PubMed] [Google Scholar]
- 11.Starnes JW, Beyer RE, Edington DW. Myocardial adaptations to endurance exercise in aged rats. Am J Physiol. 1983;245:H560–H566. doi: 10.1152/ajpheart.1983.245.4.H560. [DOI] [PubMed] [Google Scholar]
- 12.Takemoto KA, Bernstein L, Lopez JF, Marshak D, Rahimtoola SH, Chandraratna PA. Abnormalities of diastolic filling of the left ventricle associated with aging are less pronounced in exercise-trained individuals. Am Heart J. 1992;124:143–148. doi: 10.1016/0002-8703(92)90932-l. [DOI] [PubMed] [Google Scholar]
- 13.Tate CA, Taffet GE, Hudson EK, Blaylock SL, McBride RP, Michael LH. Enhanced calcium uptake of cardiac sarcoplasmic reticulum in exercise-trained old rats. Am J Physiol. 1990;258(2 Pt 2):H431–H435. doi: 10.1152/ajpheart.1990.258.2.H431. [DOI] [PubMed] [Google Scholar]
- 14.Tate CA, Helgason T, Hyek MF, et al. SERCA2a and mitochondrial cytochrome oxidase expression are increased in hearts of exercise-trained old rats. Am J Physiol. 1996;271(1 Pt 2):H68–H72. doi: 10.1152/ajpheart.1996.271.1.H68. [DOI] [PubMed] [Google Scholar]
- 15.Thomas DP, Zimmerman SD, Hansen TR, Martin DT, McCormick RJ. Collagen gene expression in rat left ventricle: interactive effect of age and exercise training. J Appl Physiol. 2000;89:1462–1468. doi: 10.1152/jappl.2000.89.4.1462. [DOI] [PubMed] [Google Scholar]
- 16.Blomqvist CG, Saltin B. Cardiovascular adaptations to physical training. Annu Rev Physiol. 1983;45:169–189. doi: 10.1146/annurev.ph.45.030183.001125. [DOI] [PubMed] [Google Scholar]
- 17.Bloor CM, Leon AS. Interaction of age and exercise on the heart and its blood supply. Lab Invest. 1970;22:160–165. [PubMed] [Google Scholar]
- 18.Gates PE, Tanaka H, Graves J, Seals DR. Left ventricular structure and diastolic function with human ageing. Relation to habitual exercise and arterial stiffness. Eur Heart J. 2003;24:2213–2220. doi: 10.1016/j.ehj.2003.09.026. [DOI] [PubMed] [Google Scholar]
- 19.Iemitsu M, Miyauchi T, Maeda S, et al. Exercise training improves cardiac function-related gene levels through thyroid hormone receptor signaling in aged rats. Am J Physiol Heart Circ Physiol. 2004;286:H1696–H1705. doi: 10.1152/ajpheart.00761.2003. [DOI] [PubMed] [Google Scholar]
- 20.Tanaka H, Dinenno FA, Monahan KD, Clevenger CM, DeSouza CA, Seals DR. Aging, habitual exercise, and dynamic arterial compliance. Circulation. 2000;102:1270–1275. doi: 10.1161/01.cir.102.11.1270. [DOI] [PubMed] [Google Scholar]
- 21.Khan AS, Sane DC, Wannenburg T, Sonntag WE. Growth hormone, insulin-like growth factor-1 and the aging cardiovascular system. Cardiovasc Res. 2002;54:25–35. doi: 10.1016/s0008-6363(01)00533-8. [DOI] [PubMed] [Google Scholar]
- 22.Colao A, Cuocolo A, Di Somma C, et al. Impaired cardiac performance in elderly patients with growth hormone deficiency. J Clin Endocrinol Metab. 1999;84:3950–3955. doi: 10.1210/jcem.84.11.6112. [DOI] [PubMed] [Google Scholar]
- 23.Colao A, di Somma C, Pivonello R, et al. The cardiovascular risk of adult GH deficiency (GHD) improved after GH replacement and worsened in untreated GHD: a 12-month prospective study. J Clin Endocrinol Metab. 2002;87:1088–1093. doi: 10.1210/jcem.87.3.8336. [DOI] [PubMed] [Google Scholar]
- 24.Colao A, Vitale G, Pivonello R, Ciccarelli A, Di Somma C, Lombardi G. The heart: an end-organ of GH action. Eur J Endocrinol. 2004;151(Suppl 1):S93–S101. doi: 10.1530/eje.0.151s093. [DOI] [PubMed] [Google Scholar]
- 25.Vasan RS, Sullivan LM, D’Agostino RB, et al. Serum insulin-like growth factor I and risk for heart failure in elderly individuals without a previous myocardial infarction: the Framingham Heart Study. Ann Intern Med. 2003;139:642–648. doi: 10.7326/0003-4819-139-8-200310210-00007. [DOI] [PubMed] [Google Scholar]
- 26.Wannenburg T, Khan AS, Sane DC, Willingham MC, Faucette T, Sonntag WE. Growth hormone reverses age-related cardiac myofilament dysfunction in rats. Am J Physiol Heart Circ Physiol. 2001;281:H915–H922. doi: 10.1152/ajpheart.2001.281.2.H915. [DOI] [PubMed] [Google Scholar]
- 27.Woodhouse LJ, Mukherjee A, Shalet SM, Ezzat S. The influence of growth hormone status on physical impairments, functional limitations, and health-related quality of life in adults. Endocr Rev. 2006;27:287–317. doi: 10.1210/er.2004-0022. [DOI] [PubMed] [Google Scholar]
- 28.Groban L, Pailes NA, Bennett CD, et al. Growth hormone replacement attenuates diastolic dysfunction and cardiac angiotensin II expression in senescent rats. J Gerontol A Biol Sci Med Sci. 2006;61:28–35. doi: 10.1093/gerona/61.1.28. [DOI] [PubMed] [Google Scholar]
- 29.Hurel SJ, Koppiker N, Newkirk J, et al. Relationship of physical exercise and ageing to growth hormone production. Clin Endocrinol (Oxf) 1999;51:687–691. doi: 10.1046/j.1365-2265.1999.00852.x. [DOI] [PubMed] [Google Scholar]
- 30.Poehlman ET, Copeland KC. Influence of physical activity on insulin-like growth factor-I in healthy younger and older men. J Clin Endocrinol Metab. 1990;71:1468–1473. doi: 10.1210/jcem-71-6-1468. [DOI] [PubMed] [Google Scholar]
- 31.Sonntag WE, Steger RW, Forman LJ, Meites J. Decreased pulsatile release of growth hormone in old male rats. Endocrinology. 1980;107:1875–1879. doi: 10.1210/endo-107-6-1875. [DOI] [PubMed] [Google Scholar]
- 32.Corpas E, Harman SM, Blackman MR. Human growth hormone and human aging. Endocr Rev. 1993;14:20–39. doi: 10.1210/edrv-14-1-20. [DOI] [PubMed] [Google Scholar]
- 33.Rudman D, Feller AG, Nagraj HS, et al. Effects of human growth hormone in men over 60 years old. N Engl J Med. 1990;323:1–6. doi: 10.1056/NEJM199007053230101. [DOI] [PubMed] [Google Scholar]
- 34.McMurray J. Renin angiotensin blockade in heart failure with preserved ejection fraction: the signal gets stronger. Eur Heart J. 2006;27:2257–2259. doi: 10.1093/eurheartj/ehl249. [DOI] [PubMed] [Google Scholar]
- 35.Sahn DJ, DeMaria A, Kisslo J, Weyman A. Recommendations regarding quantitation in M-mode echocardiography: results of a survey of echocardiographic measurements. Circulation. 1978;58:1072–1083. doi: 10.1161/01.cir.58.6.1072. [DOI] [PubMed] [Google Scholar]
- 36.Daughaday WH, Parker KA, Borowsky S, Trivedi B, Kapadia M. Measurement of somatomedin-related peptides in fetal, neonatal, and maternal rat serum by insulin-like growth factor (IGF) I RIA, IGF-II radioreceptor assay (RRA), and multiplication-stimulating activity RRA after acid-ethanol extraction. Endocrinology. 1982;110:575–581. doi: 10.1210/endo-110-2-575. [DOI] [PubMed] [Google Scholar]
- 37.Sonntag WE, Lenham JE, Ingram RL. Effects of aging and dietary restriction on tissue protein synthesis: relationship to plasma insulin-like growth factor-1. J Gerontol. 1992;47:B159–B163. doi: 10.1093/geronj/47.5.b159. [DOI] [PubMed] [Google Scholar]
- 38.Cohn J. Statistical Power Analysis for Behavioral Sciences. 4th Ed Lawrence Erlbaum Associates; Mahwah, NJ: 1998. [Google Scholar]
- 39.Dimke H, Flyvbjerg A, Frische S. Acute and chronic effects of growth hormone on renal regulation of electrolyte and water homeostasis. Growth Horm IGF Res. 2007;17:353–368. doi: 10.1016/j.ghir.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 40.Kitzman DW, Gardin JM, Gottdiener JS, et al. Importance of heart failure with preserved systolic function in patients > or = 65 years of age. Am J Cardiol. 2001;87:413–419. doi: 10.1016/s0002-9149(00)01393-x. [DOI] [PubMed] [Google Scholar]
- 41.Carro E, Nuñez A, Busiguina S, Torres-Aleman I. Circulating insulin-like growth factor I mediates effects of exercise on the brain. J Neurosci. 2000;20:2926–2933. doi: 10.1523/JNEUROSCI.20-08-02926.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Eliakim A, Moromisato M, Moromisato D, Brasel JA, Roberts C, Jr, Cooper DM. Increase in muscle IGF-I protein but not IGF-I mRNA after 5 days of endurance training in young rats. Am J Physiol. 1997;273(4 Pt 2):R1557–R1561. doi: 10.1152/ajpregu.1997.273.4.R1557. [DOI] [PubMed] [Google Scholar]
- 43.Gillespie CM, Merkel AL, Martin AA. Effects of insulin-like growth factor-I and LR3IGF-I on regional blood flow in normal rats. J Endocrinol. 1997;155:351–358. doi: 10.1677/joe.0.1550351. [DOI] [PubMed] [Google Scholar]
- 44.Deley G, Kervio G, Van Hoecke J, Verges B, Grassi B, Casillas JM. Effects of a one-year exercise training program in adults over 70 years old: a study with a control group. Aging Clin Exp Res. 2007;19:310–315. doi: 10.1007/BF03324707. [DOI] [PubMed] [Google Scholar]
- 45.Smart N, Haluska B, Jeffriess L, Marwick TH. Exercise training in systolic and diastolic dysfunction: effects on cardiac function, functional capacity, and quality of life. Am Heart J. 2007;153:530–536. doi: 10.1016/j.ahj.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 46.Baldi JC, McFarlane K, Oxenham HC, Whalley GA, Walsh HJ, Doughty RN. Left ventricular diastolic filling and systolic function of young and older trained and untrained men. J Appl Physiol. 2003;95:2570–2575. doi: 10.1152/japplphysiol.00441.2003. [DOI] [PubMed] [Google Scholar]
- 47.Fleg JL, Shapiro EP, O’Connor F, Taube J, Goldberg AP, Lakatta EG. Left ventricular diastolic filling performance in older male athletes. JAMA. 1995;273:1371–1375. [PubMed] [Google Scholar]
- 48.Nottin S, Nguyen LD, Terbah M, Obert P. Long-term endurance training does not prevent the age-related decrease in left ventricular relaxation properties. Acta Physiol Scand. 2004;181:209–215. doi: 10.1111/j.1365-201X.2004.01284.x. [DOI] [PubMed] [Google Scholar]
- 49.Schmidt-Trucksäss A, Schmid A, Häussler C, Huber G, Huonker M, Keul J. Left ventricular wall motion during diastolic filling in endurance-trained athletes. Med Sci Sports Exerc. 2001;33:189–195. doi: 10.1097/00005768-200102000-00003. [DOI] [PubMed] [Google Scholar]
- 50.Manetta J, Brun JF, Maimoun L, Callis A, Préfaut C, Mercier J. Effect of training on the GH/IGF-I axis during exercise in middle-aged men: relationship to glucose homeostasis. Am J Physiol Endocrinol Metab. 2002;283:E929–E936. doi: 10.1152/ajpendo.00539.2001. [DOI] [PubMed] [Google Scholar]
- 51.Bermon S, Ferrari P, Bernard P, Altare S, Dolisi C. Responses of total and free insulin-like growth factor-I and insulin-like growth factor binding protein-3 after resistance exercise and training in elderly subjects. Acta Physiol Scand. 1999;165:51–56. doi: 10.1046/j.1365-201x.1999.00471.x. [DOI] [PubMed] [Google Scholar]
- 52.Kraemer WJ, Aguilera BA, Terada M, et al. Responses of IGF-I to endogenous increases in growth hormone after heavy-resistance exercise. J Appl Physiol. 1995;79:1310–1315. doi: 10.1152/jappl.1995.79.4.1310. [DOI] [PubMed] [Google Scholar]
- 53.Heo YR, King CW. Influence of exercise-training on insulin-like growth factor I and II in rats. Nutr Res. 2001;21:1191–1199. [Google Scholar]
- 54.Gomes RJ, de Mello MA, Caetano FH, et al. Effects of swimming training on bone mass and the GH/IGF-1 axis in diabetic rats. Growth Horm IGF Res. 2006;16:326–331. doi: 10.1016/j.ghir.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 55.Scheinowitz M, Kessler-Icekson G, Freimann S, et al. Short- and long-term swimming exercise training increases myocardial insulin-like growth factor-I gene expression. Growth Horm IGF Res. 2003;13:19–25. doi: 10.1016/s1096-6374(02)00137-5. [DOI] [PubMed] [Google Scholar]
- 56.Ren J, Samson WK, Sowers JR. Insulin-like growth factor I as a cardiac hormone: physiological and pathophysiological implications in heart disease. J Mol Cell Cardiol. 1999;31:2049–2061. doi: 10.1006/jmcc.1999.1036. [DOI] [PubMed] [Google Scholar]
- 57.Li Q, Wu S, Li SY, et al. Cardiac-specific overexpression of insulin-like growth factor 1 attenuates aging-associated cardiac diastolic contractile dysfunction and protein damage. Am J Physiol Heart Circ Physiol. 2007;292:H1398–H1403. doi: 10.1152/ajpheart.01036.2006. [DOI] [PubMed] [Google Scholar]
- 58.Giannuzzi P, Temporelli PL, Corrà U, Tavazzi L, ELVD-CHF Study Group Antiremodeling effect of long-term exercise training in patients with stable chronic heart failure: results of the Exercise in Left Ventricular Dysfunction and Chronic Heart Failure (ELVD-CHF) trial. Circulation. 2003;108:554–559. doi: 10.1161/01.CIR.0000081780.38477.FA. [DOI] [PubMed] [Google Scholar]
- 59.Anker SD, Volterrani M, Pflaum CD, et al. Acquired growth hormone resistance in patients with chronic heart failure: implications for therapy with growth hormone. J Am Coll Cardiol. 2001;38:443–452. doi: 10.1016/s0735-1097(01)01385-7. [DOI] [PubMed] [Google Scholar]
- 60.Longobardi S, Cittadini A, Strömer H, et al. Echocardiographic assessment of cardiac morphology and function in mutant dwarf rats. Growth Horm IGF Res. 2000;10:242–247. doi: 10.1054/ghir.2000.0160. [DOI] [PubMed] [Google Scholar]
- 61.Trask AJ, Ferrario CM. Angiotensin-(1-7): pharmacology and new perspectives in cardiovascular treatments. Cardiovasc Drug Rev. 2007;25:162–174. doi: 10.1111/j.1527-3466.2007.00012.x. [DOI] [PubMed] [Google Scholar]
- 62.Cittadini A, Grossman JD, Napoli R, et al. Growth hormone attenuates early left ventricular remodeling and improves cardiac function in rats with large myocardial infarction. J Am Coll Cardiol. 1997;29:1109–1116. doi: 10.1016/s0735-1097(97)00010-7. [DOI] [PubMed] [Google Scholar]
- 63.Isgaard J, Kujacic V, Jennische E, et al. Growth hormone improves cardiac function in rats with experimental myocardial infarction. Eur J Clin Invest. 1997;27:517–525. doi: 10.1046/j.1365-2362.1997.1430692.x. [DOI] [PubMed] [Google Scholar]
- 64.Adamopoulos S, Parissis JT, Paraskevaidis I, et al. Effects of growth hormone on circulating cytokine network, and left ventricular contractile performance and geometry in patients with idiopathic dilated cardiomyopathy. Eur Heart J. 2003;24:2186–2196. doi: 10.1016/s0195-668x(03)00480-9. [DOI] [PubMed] [Google Scholar]
- 65.Parissis JT, Adamopoulos S, Karatzas D, Paraskevaidis J, Livanis E, Kremastinos D. Growth hormone-induced reduction of soluble apoptosis mediators is associated with reverse cardiac remodelling and improvement of exercise capacity in patients with idiopathic dilated cardiomyopathy. Eur J Cardiovasc Prev Rehabil. 2005;12:164–168. doi: 10.1097/01.hjr.0000159320.70090.3d. [DOI] [PubMed] [Google Scholar]
- 66.Brüel A, Oxlund H. The effect of growth hormone on rat myocardial collagen. Growth Horm IGF Res. 1999;9:123–130. doi: 10.1054/ghir.1999.0097. [DOI] [PubMed] [Google Scholar]
- 67.Cao N, Lau S, Nguyen TT, White PJ. Characterization of the acute cardiovascular effects of intravenously administered insulin-like growth factor-I in conscious Sprague-Dawley rats. Clin Exp Pharmacol Physiol. 2006;33:1190–1195. doi: 10.1111/j.1440-1681.2006.04510.x. [DOI] [PubMed] [Google Scholar]
- 68.Lacolley P, Safar ME, Lucet B, Ledudal K, Labat C, Benetos A. Prevention of aortic and cardiac fibrosis by spironolactone in old normotensive rats. J Am Coll Cardiol. 2001;37:662–667. doi: 10.1016/s0735-1097(00)01129-3. [DOI] [PubMed] [Google Scholar]
- 69.Sadoshima J, Izumo S. Molecular characterization of angiotensin II-induced hypertrophy of cardiac myocytes and hyperplasia of cardiac fibroblasts. Critical role of the AT1 receptor subtype. Circ Res. 1993;73:413–423. doi: 10.1161/01.res.73.3.413. [DOI] [PubMed] [Google Scholar]
- 70.Schorb W, Booz GW, Dostal DE, Conrad KM, Chang KC, Baker KM. Angiotensin II is mitogenic in neonatal rat cardiac fibroblasts. Circ Res. 1993;72:1245–1254. doi: 10.1161/01.res.72.6.1245. [DOI] [PubMed] [Google Scholar]
- 71.Sun Y, Zhang J, Lu L, Bedigian MP, Robinson AD, Weber KT. Tissue angiotensin II in the regulation of inflammatory and fibrogenic components of repair in the rat heart. J Lab Clin Med. 2004;143:41–51. doi: 10.1016/j.lab.2003.07.004. [DOI] [PubMed] [Google Scholar]
- 72.Crackower MA, Sarao R, Oudit GY, et al. Angiotensin-converting enzyme 2 is an essential regulator of heart function. Nature. 2002;417:822–828. doi: 10.1038/nature00786. [DOI] [PubMed] [Google Scholar]
- 73.Lijnen PJ, Petrov VV. Role of intracardiac renin-angiotensin-aldosterone system in extracellular matrix remodeling. Methods Find Exp Clin Pharmacol. 2003;25:541–564. doi: 10.1358/mf.2003.25.7.778094. [DOI] [PubMed] [Google Scholar]
- 74.Trask AJ, Averill DB, Ganten D, Chappell MC, Ferrario CM. Primary role of angiotensin-converting enzyme-2 in cardiac production of angiotensin-(1-7) in transgenic Ren-2 hypertensive rats. Am J Physiol Heart Circ Physiol. 2007;292:H3019–H3024. doi: 10.1152/ajpheart.01198.2006. [DOI] [PubMed] [Google Scholar]
- 75.Nishimura RA, Tajik AJ. Evaluation of diastolic filling of left ventricle in health and disease: Doppler echocardiography is the clinician’s Rosetta Stone. J Am Coll Cardiol. 1997;30:8–18. doi: 10.1016/s0735-1097(97)00144-7. [DOI] [PubMed] [Google Scholar]
- 76.Garcia MJ, Thomas JD, Klein AL. New Doppler echocardiographic applications for the study of diastolic function. J Am Coll Cardiol. 1998;32:865–875. doi: 10.1016/s0735-1097(98)00345-3. [DOI] [PubMed] [Google Scholar]
- 77.Ommen SR, Nishimura RA, Appleton CP, et al. Clinical utility of Doppler echocardiography and tissue Doppler imaging in the estimation of left ventricular filling pressures: a comparative simultaneous Doppler-catheterization study. Circulation. 2000;102:1788–1794. doi: 10.1161/01.cir.102.15.1788. [DOI] [PubMed] [Google Scholar]
- 78.Kasner M, Westermann D, Steendijk P, et al. Utility of Doppler echocardiography and tissue Doppler imaging in the estimation of diastolic function in heart failure with normal ejection fraction: a comparative Doppler-conductance catheterization study. Circulation. 2007;116:637–647. doi: 10.1161/CIRCULATIONAHA.106.661983. [DOI] [PubMed] [Google Scholar]
- 79.Liu H, Bravata DM, Olkin I, et al. Systematic review: the safety and efficacy of growth hormone in the healthy elderly. Ann Intern Med. 2007;146:104–115. doi: 10.7326/0003-4819-146-2-200701160-00005. [DOI] [PubMed] [Google Scholar]
- 80.Tripkovic I, Tripkovic A, Strnad M, Capkun V, Zekan L. Role of insulin-like growth factor-1 in colon cancerogenesis: a case-control study. Arch Med Res. 2007;38:519–525. doi: 10.1016/j.arcmed.2007.01.012. [DOI] [PubMed] [Google Scholar]