Abstract
The mechanisms that control fibroproliferation and matrix deposition in lung fibrosis remain unclear. We speculate that vitamin D deficiency may contribute to pulmonary fibrosis since vitamin D deficiency has been implicated in several diseases. First, we confirmed the presence of vitamin D receptors (VDR) in cultured NIH/3T3 and lung fibroblasts. Fibroblasts transfected with a vitamin D response element – reporter construct and exposed to the active vitamin D metabolite, 1,25(OH)2D3, showed increased promoter activity indicating VDR functionality in these cells. Testing the effects of 1,25(OH)2D3 on fibroblasts treated with transforming growth factor β1 (TGFβ1), considered a driver of many fibrotic disorders, we found that 1,25(OH)2D3 inhibited TGFβ1-induced fibroblast proliferation in a dose-dependent fashion. 1,25(OH)2D3 also inhibited TGFβ1 stimulation of α-smooth muscle actin expression and polymerization and prevented the upregulation of fibronectin and collagen in TGFβ1-treated fibroblasts. Finally, we examined how 1,25(OH)2D3 affects epithelial-mesenchymal transformation of lung epithelial cells upon exposure to TGFβ1. We showed that the TGFβ1-induced upregulation of mesenchymal cell markers and abnormal expression of epithelial cell markers were blunted by 1,25(OH)2D3. These observations suggest that under TGFβ1 stimulation, 1,25(OH)2D3 inhibits the profibrotic phenotype of lung fibroblasts and epithelial cells.
Keywords: Vitamin D, VDR, TGFβ, myofibroblast differentiation, epithelial mesenchymal transformation, fibrosis
(2) INTRODUCTION
Dysregulated synthesis and tissue accumulation of matrix molecules are the hallmarks of fibrotic diseases and are directly responsible for organ destruction and abnormal physiology. In the lung, these changes may be seen in two anatomically distinct diseases: idiopathic pulmonary fibrosis (IPF) and post-transplant obliterative bronchiolitis (OB). Both IPF and OB carry poor prognoses, with mortality rates upwards of fifty percent at 3 years [1–3], and to date, there are no safe and effective treatments capable of halting and/or reversing the fibrotic processes in these diseases.
While not enough is known about the mechanisms responsible for these events, some hypotheses implicate an aberrant wound healing process in response to some known or unspecified injurious stimulus. Characteristically, there is fibroblast activation with a cellular phenotype typified by a resistance to apoptosis, the overproduction of connective tissue matrices, and the appearance of a contractile apparatus [4]. What triggers the induction of these putative myofibroblasts likely involves TGFβ, a pro-fibrotic cytokine consistently found in fibrotic tissues [5]. Interestingly, the changes in fibroblasts promoted by TGFβ also seem to be mimicked by epithelial cells under similar conditions [6]. Thus, a greater understanding of host and environmental factors influencing fibroproliferation and matrix expression in the lung is needed in order to identify novel targets for the development of effective therapies.
Vitamin D3 is a steroid pre– pro– hormone that requires sequential enzymatic modification, 25– and 1α– hydroxylation, in the liver and kidney, respectively, to have maximal biological activity as the hormone, 1,25(OH)2D. 1,25(OH)2D acts in an endocrine fashion on target organs (i.e. parathyroid, bone, intestine and kidney) to regulate bone, calcium, and phosphate metabolism; and its effects are mediated through a specific protein, the Vitamin D Receptor (VDR).
VDR is a ligand-dependent transcription factor belonging to the super-family of nuclear hormone receptors. VDR binds to its ligand, 1,25(OH)2D, dimerizes with the retinoic acid receptor (RXR), and attaches to specific genomic sequences termed Vitamin D response elements (VDRE). Like others in the nuclear hormone receptor class, VDRs are modular in structure, with an N-terminal transcriptional activation domain and C-terminal ligand-binding domain. The mid-region DNA binding domain, which consists of two zinc fingers, specifically recognizes DR3-type DNA motifs in target promoters to modulate gene expression. In point of fact, the expression of over two-hundred genes are controlled via 1,25(OH)2D / VDR –dependent pathways, either directly or indirectly, including genes that regulate proliferation, differentiation, and apoptosis, amongst others [7].
There does appear to be a role for 1,25(OH)2D in the “non-calcemic” homeostasis of extra-skeletal organs as suggested in a recent clinical review on Vitamin D [7]. Evidence for this is provided by data demonstrating the expression of VDRs and 1α–hydroxylases in brain, prostate, breast, colon tissues, and circulating leukocytes. However, the exact role of vitamin D in such organs remains unelucidated and the biological consequences of vitamin D deficiency remain unclear. Of note, epidemiologic studies suggest a role for Vitamin D deficiency in the pathogenesis of numerous chronic illnesses [7]. For one, there is circumstantial evidence that living at higher latitudes (where decreased solar UVB radiation is a cause of Vitamin D deficiency) increases the risk of multiple sclerosis, Crohn’s disease, hypertension and cardiovascular disease, hematological and solid organ malignancy, and asthma. The strongest data come from the cancer literature linking Vitamin D deficiency with increased incidence of and mortality from colon, prostate, and breast cancer. Moreover, higher maternal intake of vitamin D during pregnancy was found in one study to be associated with a lower risk of recurrent wheeze in children at 3 years of age. Interestingly, a recent meta-analysis of 18 randomized clinical trials enrolling over 57,000 subjects suggested that intake of vitamin D supplements may decrease all-cause mortality [8].
Little is known about the role of vitamin D in lung or its impact on the function of lung cells. In particular, vitamin D status has not been systematically studied in patients with fibrotic lung disease. Unfortunately, such data are only found in studies of end-stage lung disease patients on a transplant waiting list containing a subgroup of pulmonary fibrotics [9, 10]. We speculate that vitamin D may influence lung fibrosis through direct effects on lung fibroblasts. Specifically, we hypothesize that vitamin D opposes intracellular pro-fibrogenic signals. To begin to address this hypothesis, we studied NIH3T3 fibroblastic cells and primary lung fibroblasts and found that they express functional vitamin D receptors. In these cells, vitamin D opposed the effects of transforming growth factor β1 (TGFβ1), a well-studied pro-fibrotic factor. Interestingly, vitamin D seems to have similar anti-TGFβ1 effects in lung epithelial cells thereby extending its protective functions to several pulmonary cells.
(3) METHODS
(4) General reagents
The following antibodies were used against E-cadherin (BD Transduction Laboratories, San Jose, CA), α-smooth muscle actin (αSMA) (Sigma, St. Louis, MO), VDR (Affinity BioReagents, Golden, CO), Pro-Collagen Type I (Novus Biologicals, Littleton, CO and Developmental Studies Hybridoma Bank, University of Iowa), fibronectin (Sigma), ZO-1 (Invitrogen, Carlsbad, CA), cytokeratin (DSHB, Iowa and Santa Cruz Biotechnology, Santa Cruz, CA), and PCNA (Cell Signal Technology, Danvers, MA). 1,25(OH)2D3 and rhTGFβ (10 ng/ml) were purchased from Alexis Biochemicals (San Diego, CA) and R& D Systems (Minneapolis, MN), respectively. The 1,25(OH)2D3 antagonist, ZK159222, was obtained from Bayer Schering Pharma AG (Berlin, Germany).
(5) Expression Plasmids
The expression plasmid ECFP VDR(119-C) [11], which lacks residues 1–118 that encode the DNA binding domain of the VDR, was kindly provided by Dr. A. Norman (University of California, Riverside, CA). pSPPx3-TK-Luc [12], which consists of the Vitamin D response element from the mouse osteopontin promoter upstream of a luciferase reporter, was a generous gift from Dr. D.W. Russell (University of Texas Southwestern Medical Center, San Antonio, TX). 3TP-Lux, purchased from Addgene (#11767, Cambridge, MA) is a luciferase, TGFβ-responsive reporter construct [13, 14]. The Renilla luciferase plasmid, pRL-CMV (Promega, Madison, WI), was included in all transfections for normalization.
(6) Cell culture
Primary lung fibroblasts were isolated from C57BL/6 mice as previously described [15] and used between passage two and ten. Primary murine lung and NIH/3T3 fibroblasts (ATCC, Manassas, VA) were cultured in Dulbecco’s modified Eagle medium (DMEM) with 4.5 g/L of glucose supplemented with 10% heat inactivated fetal bovine serum (FBS), 1% antibiotic-antimycotic solution (100 U/mL penicillin G sodium, 100 U/mL streptomycin, and 0.25 g/mL amphotericin B). The rat type II alveolar epithelial line (RLE-6TN, ATCC) was maintained in DMEM with 4.5 gm/l glucose, 10% FBS, 40 mM HEPES and 1% antibiotic-antimycotic solution. Where indicated, fibroblasts or RLE cells were serum-starved, pre-treated for 1 hour with the stated concentration of 1,25(OH)2D3 (or ethanol vehicle) and incubated with TGFβ (10 ng/ml) for the specified amount of time. Since the maximal concentration of 1,25(OH)2D3 achievable in lung tissue is not known, 1µM was chosen as the highest dose based on several in vitro studies of Vitamin D in fibroblasts and cancer cells [16].
(7) Cell transfection and luciferase assays
A calcium-phosphate transfection based method was used [17]. NIH/3T3s were plated in fibroblast media at a cell density of 1 × 105 cells per well in a 12-well dish the day prior to cell transfection. On the day of transfection, cells were incubated for one hour in fresh media, and DNA precipitate was made by adding 40 µg of plasmid (5 µg/µl) in 30 µl of 2.5M CaCl2 to 500 µl of bubbling 2× HEPES buffered saline (pH 7.05). After 20 min at room temperature, 45 µl of the precipitate was applied to each well. Cells were incubated with DNA overnight under standard growth conditions, washed twice with PBS the following day, and recultured with complete serum free media for 8 hours prior to treatment. After the appropriate time, treated cells were lysed with 5× Passive Lysis Buffer (Promega) and analyzed for Firefly and Renilla luciferase activity on a Thermo Luminoskan Ascent Luminometer (Waltham, MA) as described by Dyer et al [18].
(8) Reverse–Transcription and Polymerase Chain Reaction
Cells were cultured onto 6 well plates and incubated in complete serum free media for 24 hours prior to the addition of TGFβ1. Total RNA was isolated with a TRI reagent as previously described [19]. The reverse transcription reactions of the extracted RNA were performed by combining the following reagents: 0.625 µM dNTPs, 16 nmol random hexamer oligonucleotides (Roche Diagnostics, Indianapolis, IN), 5 µl First Strand Buffer (Invitrogen), 20 mM DTT, 200 units reverse transcriptase enzyme, 0.5 µl RNasin (Promega, Madison, WI), and 1 µg extracted RNA in a total volume of 25 µl. Murine primers for PCR reactions were based on GenBank published sequences and are as follows: αSMA (5’-ATG GAG TCA GCG GGC ATC-3’; 5’-AAC TGG AGG CGC TGA TCC-3’), VDR (5’-CAG CCA GCA CCT CCC TGC-3’; 5’-AGA AAC CCT TGC AGC CTT CA -3’), fibronectin (5’-GTT ATG ACG ATG GGA AGA-3’; 5’-ACT GGT TGT AGT TGT GGC-3’), type I collagen (5’-CTG CTG TTG GTG CTG CTG-3’; 5’-CAG GAG CAC CAG CAA TAC-3’), type III collagen (5’-TCT CAC CCT TCT TCA TCC CA-3’; 5’-GGC AGT CTA GTG GCT CCT CA -3’), PAI-1(5’- TCA TCA GAC AAT GGA AGG GC-3’; 5’- ACT GTG CCG CTC TCG TTT AC-3’), 18s (5’- TTG AAA ATC CGG GGG -3’; 5’- ACA TTG TTC CAA CAT GCC AG -3’).
Reactions containing 10× PCR buffer (Denville Scientific, South Plainfield, NJ), 1 unit Taq polymerase (Denville), 10µM each primer, and 800 ng cDNA in 7.5% glycerol, were performed using an optimized, primer-specific PCR thermocycling protocol. Amplicons were resolved on 1% agarose gels, stained with SYBR Green (Invitrogen, Carlsbad, CA), and visualized with a SafeImager transiluminator (Invitrogen).
(9) Western blotting
Fibroblasts were plated at near confluence, serum starved for 24 hours, and treated as indicated. Cells were washed with ice-cold PBS, incubated in 0.1 ml modified RIPA lysis buffer with protease inhibitors, and sonicated. Protein concentration was determined with the Bio-Rad protein assay reagent (Hercules, CA) according to the manufacturer’s directions. Equal aliquots of protein were fractionated by electrophoresis in 12% SDS-polyacrylamide gels and transferred onto nitrocellulose paper. The nitrocellulose paper was blocked with 10% milk in TBS-0.1% Tween-20 buffer and incubated overnight at 4°C with diluted primary antibody followed by a horseradish peroxidase-conjugated secondary antibody (Sigma). Immunoreactivity was visualized by chemiluminescence (Pierce, Rockford, IL). Membranes were stripped and reprobed for GAPDH (Abcam, Cambridge, MA).
(10) Immunofluorescence
Treated cells in chamber slides (Nalge Nunc International, Rochester, NY) were fixed in 4% paraformaldehyde for 15 minutes and permeablized with 0.5% Triton X-100 in Tris buffered saline (TBS). Slides were washed in TBS, blocked with 5% bovine serum albumin and 2% goat serum in 0.1% Triton X-100/TBS, and incubated with primary antibodies at 4 °C overnight. An Alexa Fluor® - labeled secondary antibody (Invitrogen) was applied for 1 hour to slides, which were then counterstained with DAPI (Sigma), briefly air-dried, and cover-slipped with ProLong® Gold mounting medium (Invitrogen). Slides were viewed under epifluorescence microscopy (Olympus BX41, Melville, MY) and images were captured using Magnafire 2.1 digital image acquisition software (Goleta, CA) using optimized exposure times for each fluor within each experimental condition. Brightness and contrast enhancement was applied equally to all images using a batch processing algorithm in Adobe Photoshop v7.0.
(11) Proliferation Assay
Cells were plated at a density of 2 × 103 cells per well for NIH/3T3s and 4 × 103 for primary lung fibroblast in a 96-well tissue culture dish. At the indicated time points, cells were rinsed with PBS, fixed with 100% ice-cold methanol for 5 minutes, washed with several changes of PBS, and incubated with 0.2% (w/v) crystal violet (Sigma) in 2.5% acetic acid for 30 minutes at room temperature. Culture plates were gently rinsed with tap water until clear, and allowed to dry. To extract the cell-bound dye, crystal violet stain was solubilized with 1% SDS for 1 hour at room temperature with shaking. The absorbance at 590 nm was determined on a AD 340C Beckman Coulter Absorbance Detector (Beckman Coulter, Fullerton CA).
(12) Cytotoxicity Assay
NIH/3T3s were plated at a density of 2 × 104 cells per well in a 96-well tissue culture dish and treated as indicated. After 24 hours, 100 µL of cell culture media was analyzed for adenylate kinase activity using a bioluminescent cytotoxicity assay kit (Biovision, Mountain View, CA). The background luminescence of water (in the absence of cells) was used as a negative control. The positive control consisted of supernatants from cells treated with toxic doses of H2O2.
(13) Collagen Gel Contraction Assay
Serum-starved primary lung fibroblasts were treated 1,25(OH)2D3 and/or TGFβ as indicated for 72 hours, harvested with trypsin, and diluted in collagen type I (Sigma) to achieve a final collagen concentration of 0.65 mg/ml with 1.25 × 105 cells/ml. 250 µL of the collagen cell suspension were again treated 1,25(OH)2D3 and/or TGFβ, placed in a 35mm tissue culture dish in triplicates, allowed to polymerize for 2 hours, and covered in fresh culture media supplemented with the indicataed treatments. Diameters of the collagen gels were measured daily.
(14) Data analysis
Western blotting and RT-PCR experiments were performed in duplicates and repeated at least three times to ensure consistency. Reporter and proliferation data were also repeated thrice each with 3–4 replicates per experiment. All results are presented as mean ± S.E. GraphPad Prism v3.0 was used to analyze data by one-way ANOVA computation with Tukey’s multiple comparisons test. A ‘p’ value of 0.05 was considered significant.
(15) RESULTS
(16) Lung fibroblasts express functional Vitamin D receptors
To date, it remains unclear whether lung cells are capable of recognizing 1,25(OH)2D3 through the classic vitamin D receptor (VDR). To test this, we began by evaluating the expression of VDRs in lung tissue, and since fibroblasts are thought to be the predominant effector cell in organ fibrosis, we also assessed the expression of VDRs in this cell type. As depicted in Figure 1, VDR mRNA and protein expression were detected in the NIH/3T3 cell line (confirming previous reports [20]), primary lung fibroblasts, and whole lung homogenates, via RT-PCR (Figure 1A) and western blotting (Figure 1B), respectively. As expected, immunofluorescence staining (Figure 1C) in NIH/3T3 (left panel) and lung fibroblasts (right panel) revealed fibroblast nuclei as the main site of localization of vitamin D nuclear receptors.
FIG 1.
VDRs are expressed in whole lungs and lung fibroblasts. Extracts of NIH/3T3 cells and primary lung fibroblasts and homogenates of whole mouse lung were analyzed for Vitamin D receptor expression by (A) RT-PCR and (B) Western blotting. VDRs in NIH/3T3 and murine lung fibroblasts were detected using indirect immunofluorescence (E).
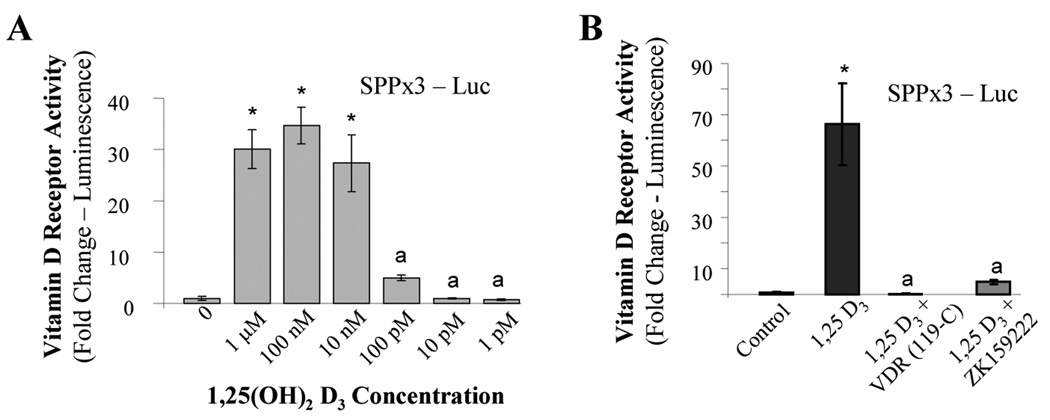
As a test of VDR functionality in fibroblasts, NIH/3T3 cells were transfected with a Vitamin D response element (VDRE) connected to a luciferase reporter gene and treated with 1,25(OH)2D3. We found that 1,25(OH)2D3 stimulated the expression of the gene with a maximal effect observed at 100 nM followed by a subsequent dose-dependent decline (Figure 2A). Next, fibroblasts were either pretreated with a specific antagonist of the VDR, ZK159222, or transfected with a truncated VDR capable of binding 1,25(OH)2D3 without affecting gene transcription and thereby competing with endogenous VDR. Both of these interventions were able block the VDRE promoter activity in fibroblasts stimulated by 1,25(OH)2D3 back to baseline indicating that the effects of 1,25(OH)2D3 on lung fibroblasts were indeed dependent on VDR (Figure 2B).
FIG 2.
VDRs are functional in fibroblasts. (A) NIH/3T3 fibroblasts were transfected with a luciferase reporter driven by three copies of a Vitamin D response element taken from the mouse osteopontin promoter (pSPPx3-Luc). Transfected fibroblasts were then treated with the indicated concentration of 1,25(OH)2D3 for 24 hours followed by testing for luciferase activity. (B) 3T3 fibroblasts were either co-transfected with pSPPx3-Luc and a mutated VDR (119-C) or treated with the VDR antagonist, ZK159222, after transfection with pSPPx3-Luc. Afterwards, cells were incubated overnight with 1µM 1,25(OH)2D3 and analyzed for luciferase expression. Data are expressed as the average normalized fold change over control ± SE. * = p<0.001. a = not statistically significant compared to control
(17) Vitamin D opposes the effects of TGFβ1 in lung fibroblasts
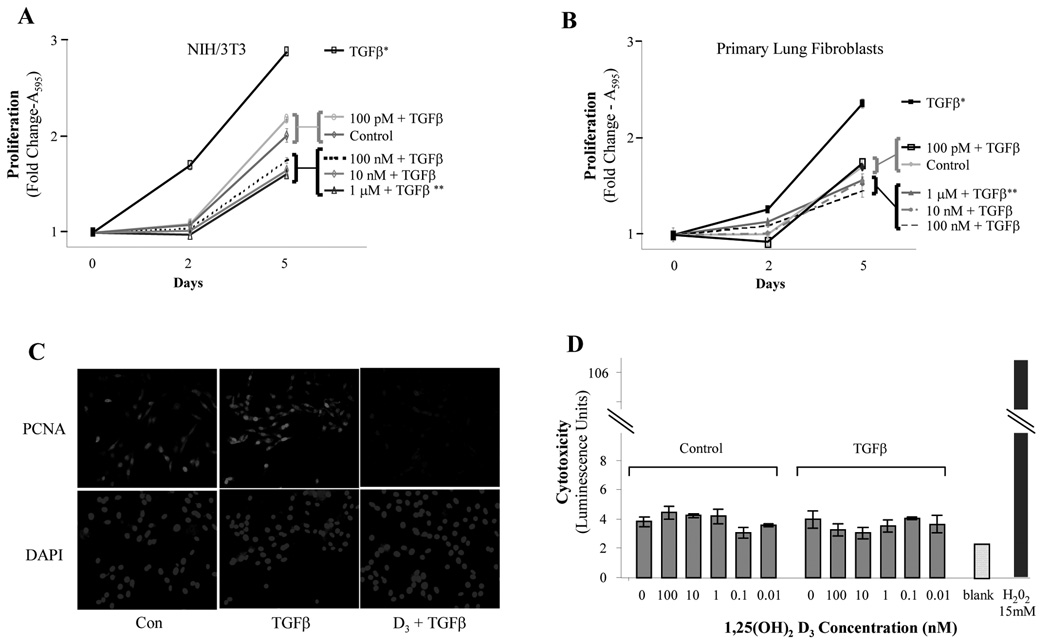
Having established the presence of functional VDRs in lung fibroblasts, we turned our attention to TGFβ. TGFβ1 is considered a master switch in fibrotic lung disorders and is known for its ability to stimulate cell proliferation and matrix expression in lung fibroblasts [5]. Therefore, we set out to test if active vitamin D influences these effects by treating fibroblasts with TGFβ1 in the presence or absence of 1,25(OH)2D3. As shown in Figure 3, A and B, TGFβ1 stimulated the proliferation of primary lung fibroblasts and NIH/3T3 fibroblasts, respectively. 1,25(OH)2D3 inhibited this effect. The highest concentrations of 1,25(OH)2D3 reduced the proliferation of TGFβ1-treated fibroblasts lower than that observed for unstimulated fibroblasts, and 100 pM 1,25(OH)2D3 was able to blunt TGFβ-mediated proliferation to near-baseline levels. Also, the expression of PCNA, proliferating cell nuclear antigen, by fluorescence immunocytochemistry was examined as a marker of actively replicating cells (Figure 3C). 1,25(OH)2D3 at 1 µM was able to block the upregulation of PCNA by TGFβ to levels even below that at baseline. Most notably, the inhibitory effect of 1,25(OH)2D3 was not associated with cytotoxicity as data were not meaningfully different than negative controls (Figure 3D).
FIG 3.
Effects of Vitamin D on fibroblast proliferation and viability. (A) Primary lung and (B) NIH/3T3 fibroblasts were treated with increasing doses of 1,25(OH)2D3 for 1 hour followed by exposure to TGFβ1 (10 ng/ml). Cell counts were determined colorimetrically at days 2 and 5. (C) 3T3 fibroblasts were stimulated with 1,25(OH)2D3 (1 µM) and/or TGFβ (10 ng/ml) and analyzed for PCNA expression by indirect immunofluorescence. (D) NIH/3T3s were incubated for 48 hours with the indicated concentrations of 1,25(OH)2D3 in the presence or absence of TGFβ1. Cytotoxicity was determined using a bioluminescent assay. Background luminescence, i.e. negative control, was measured using plain water. Complete cell lysis with 15 mM H2O2 was used as a positive control. Data shown are means ± SE. * = p<0.001 compared to control. ** = p <0.01 compared to control.
1,25(OH)2D3 also opposed the effects of TGFβ1 on myofibroblast transdifferentiation as demonstrated by its ability to inhibit TGFβ1-induced expression of α-smooth muscle actin (αSMA), a well known marker of myofibroblast transdifferentiation [21]. Immunofluorescence staining of NIH/3T3 fibroblasts also revealed that TGFβ1 stimulated the organization of αSMA into the tubular structure of the actin cytoskeleton, a process that was inhibited by 1,25(OH)2D3 (Figure 4A) as a function of concentration. RT-PCR analysis revealed that 1,25(OH)2D3 inhibited TGFβ1-induced αSMA mRNA expression in a dose-dependent manner in these cells (Figure 4B). Similarly, 1,25(OH)2D3–treated primary lung fibroblasts displayed diminished TGFβ-related expression of αSMA at the transcript and protein level (Figure 4, C and D).
FIG 4.
1,25(OH)2D3 inhibits myofibroblast transdifferentiation. NIH/3T3 fibroblasts were treated for 1 hour with the indicated amount of 1,25(OH)2D3, stimulated with TGFβ1 (10 ng/ml). (A) Immunofluorescence microscopy was used to assess the degree of αSMA (green) and f-actin (red) cytoskeletal organization after 72 hours. (B) αSMA expression by RT-PCR was detected after 24 hours. Primary lung fibroblasts were treated similarly. (C) RNA and (D) protein were extracted and analyzed for αSMA.
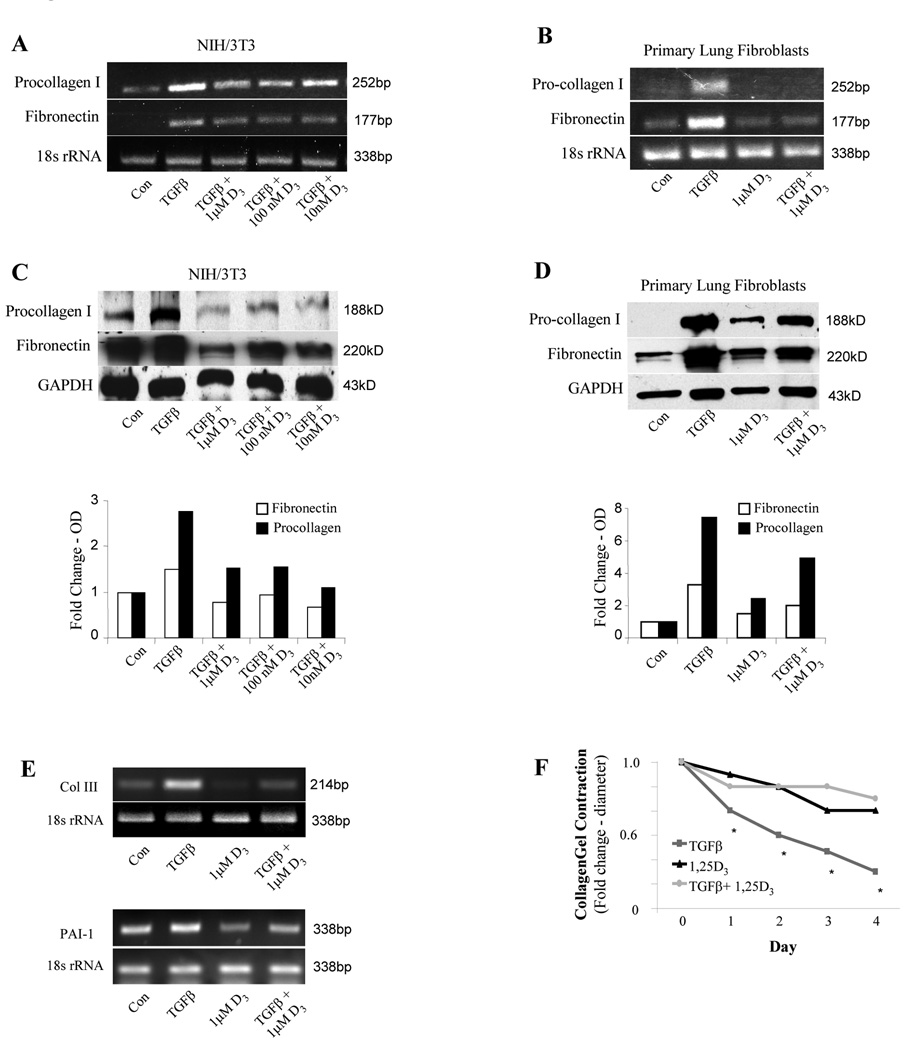
We next tested the effects of 1,25(OH)2D3 on the myofibroblast phenotype induced by TGFβ1. This phenotype is characterized by augmented matrix expression, and consistent with this, TGFβ1 is known to stimulate the expression of type I collagen and fibronectin in fibroblasts as depicted in Figure 5, A–D. In NIH/3T3 and primary lung fibroblasts, 1,25(OH)2D3 inhibited this effect in a dose-dependent manner by preventing induction of matrix mRNA (Figure 5, A and B) and protein (Figure 5, C and D) expression in response to TGFβ1. In similar fashion, 1,25(OH)2D3 blocked the upregulation of the TGFβ-dependent genes, type III collagen and PAI-1 (Figure 5E). Additionally, the myofibroblastic phenotype induced by TGFβ1 is also distinguished by increased contractility of collagen gels. Therefore, we tested the effect of 1,25(OH)2D3 on gel contractility induced by TGFβ1-treated fibroblasts. As shown in Figure 5F, TGFβ1 enhanced gel contractility, whereas 1,25(OH)2D3 eliminated the effect.
FIG 5.
Matrix expression and collagen gel contraction are inhibited by 1,25(OH)2D3. NIH/3T3 (A and C) and primary lung fibroblasts (B and D) were exposed to 1,25(OH)2D3 at the specified concentrations and incubated with TGFβ (10 ng/ml) for 24 hours for RT-PCR experiments (A and B) and 72 hours for immunoblot studies (C and D). Densitometric analyses for the immunoblots are shown. Samples were analyzed for procollagen I and fibronectin expression. (E) RNA expression of type III collagen and PAI-1 were determined by RT-PCR in 3T3 fibroblasts exposed to 1,25(OH)2D3 (1 µM) and/or TGFβ (10 ng/ml). (F) 1,25(OH)2D3 / TGFβ - treated primary lung fibroblasts were encased in collagen gels containing the same treatments. Diameters of the gels were measured daily for 4 days. Data were obtained in triplicate and are expressed as mean fold change in diameter over control ± SE.
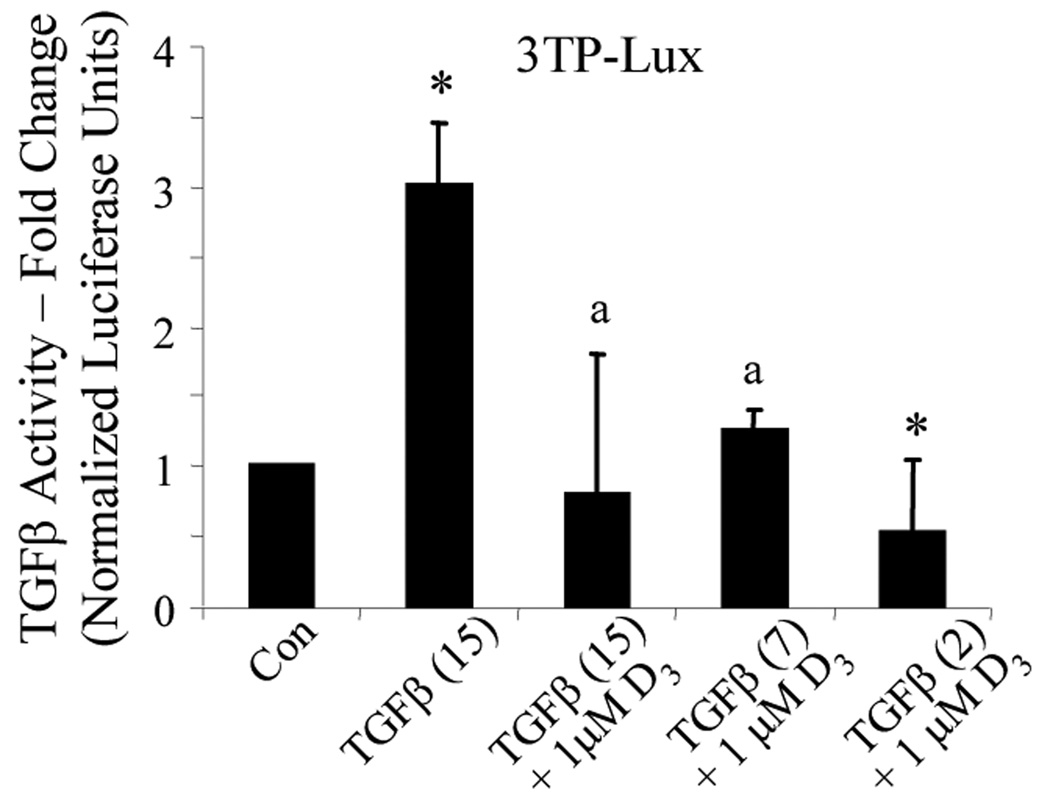
To begin studying the mechanisms by which active Vitamin D inhibits TGFβ effects in lung fibroblasts, TGFβ signal transduction was studied using the 3TP-Lux plasmid. TGFβ drives luciferase reporter expression by activating promoter elements of this construct, taken from the human collagenase and PAI-1 genes. For this purpose, NIH/3T3 cells were transiently transfected with 3TP-Lux, treated with 1,25(OH)2D3, and exposed to increasing concentrations of TGFβ. The effects of high (15 ng/ml) and medium (7 ng/ml) concentrations of TGFβ were completely abolished by 1,25(OH)2D3. Moreover, 1,25(OH)2D3 suppressed TGFβ activity below that of basal levels even under stimulation by low (2 ng/ml) amounts of TGFβ.
(18) Vitamin D opposes the effects of TGFβ1 in epithelial cells
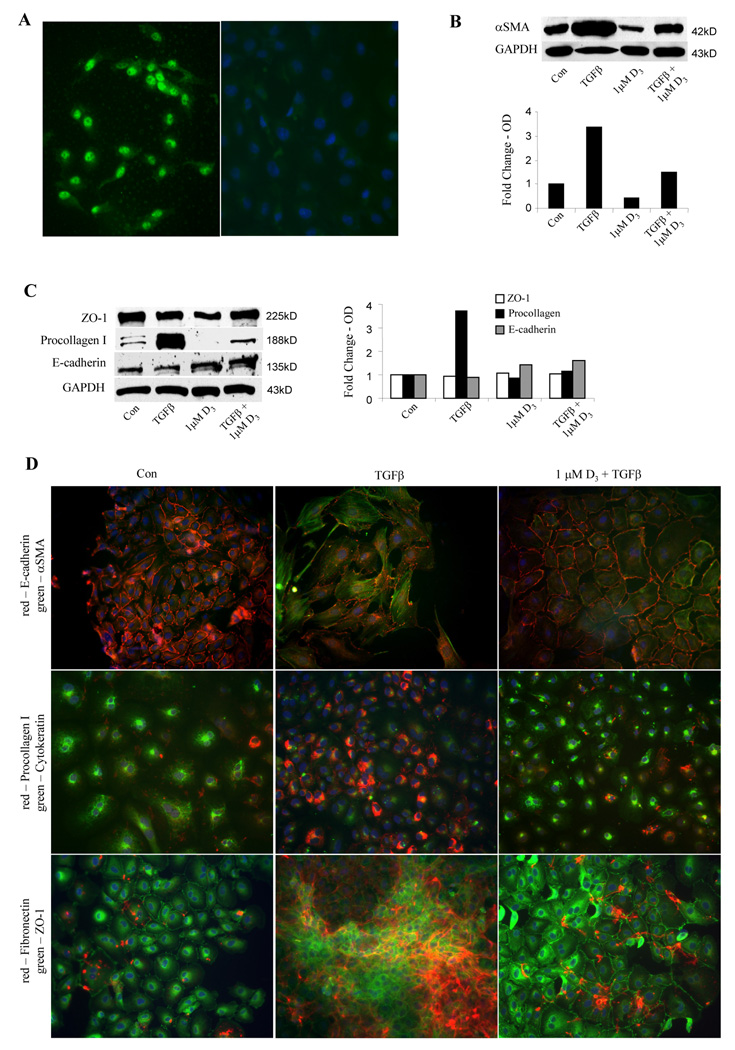
The above data suggest that 1,25(OH)2D3 opposes the pro-fibrogenic effects of TGFβ1 in lung fibroblasts. In addition to its effects on fibroblasts, TGFβ1 has also been implicated in the transdifferentiation of epithelial cells into myofibroblasts, a processed termed epithelial-mesenchymal transformation. This concept has received much attention lately due to its potential role in lung fibrosis [6]. To study this, we began by evaluating the expression of VDRs in a cultured rat lung epithelial cell line by immunofluorescence and as expected, found it localized in their nuclei (Figure 6A; left image). EMT is characterized by an increase in the expression of mesenchymal markers and a loss of epithelial cell markers, which, in this epithelial cell line, may take up to 10 days of incubation with TGFβ1 [6]. We examined the early events (at 3 days) driven by TGFβ in lung epithelial cells and demonstrated that TGFβ1 greatly stimulated the expression of mesenchymal markers, αSMA and procollagen I, whereas 1,25(OH)2D3 opposed this effect (Figure 6, B and C). Under our experimental conditions, the total expression of the epithelial markers, E-cadherin, cytokeratin, and ZO-1, in the presence or absence of 1,25(OH)2D3 or TGFβ, was marginally affected when detected within 72 hours by Western blot in whole cell lysates (Figure 6C). Immunofluorescence microscopic analysis was also used as a complementary approach to discern any phenotypic changes of EMT associated with 1,25(OH)2D3 and TGFβ. We demonstrated that quiescent epithelial cells negligibly express αSMA, collagen, and fibronectin (Figure 6D, left panels), yet upon stimulation by TGFβ, cells were noted to organize αSMA into stress fibers, express collagen, and secrete fibronectin (middle panels). These changes mediated by TGFβ were, however, mitigated by 1,25(OH)2D3 (right panels). TGFβ did, interestingly, induce derangements as quickly as 3 days in the pattern of cellular localization of epithelial markers. Specifically, when compared to control, TGFβ treatment resulted in the disruption of cell membrane E-cadherin contiguity, the failure of cytokeratin filamentous organization, and the shifting of crisp, perinuclear and membrane ZO-1 staining to a diffuse, cytoplasmic staining. These changes suggested dysregulation of protein expression early on in lung epithelial cells after stimulation by TGFβ1, even before a decrement in expression. More importantly, prior exposure of epithelial cells to 1,25(OH)2D3 was able to abrogate these processes.
FIG 6.
1,25(OH)2D3 interrupts TGFβ signaling. (A) NIH/3T3 fibroblasts were transiently transfected with a luciferase reporter under the control of a TGFβ-sensitive promoter (3TP-Lux). Transfected fibroblasts were then treated with the 1,25(OH)2D3 (1 µM) for 1 hour, exposed to TGFβ at the indicated concentration (ng/ml) for 24 hours, followed by quantification of luciferase activity. Data are expressed as the average normalized fold change over control ± SE. * = p<0.001 compared to control. a = not statistically significant compared to control
(19) DISCUSSION
The role of vitamin D in lung remains largely unexplored. Specifically, it is unclear whether lung cells recognize vitamin D and whether vitamin D levels affect lung cell functions. We hypothesized that vitamin D influences pro-fibrogenic processes by targeting lung fibroblasts and tested this hypothesis in NIH/3T3 cells and primary murine lung fibroblasts. We demonstrated VDR expression in lung tissue homogenates and in lung fibroblasts. Furthermore, we found that the VDR localizes to the nucleus, consistent with its role as a nuclear receptor, and that it is functional since NIH/3T3 fibroblasts transfected with a VDRE fused to a luciferase reporter showed increased expression of the transfected gene when exposed to 1,25(OH)2D3. Having demonstrated the expression of functional VDRs in lung fibroblasts, we proceeded to test the effect of 1,25(OH)2D3 in cells exposed to TGFβ1, a well known pro-fibrotic growth factor. As expected, TGFβ1 stimulated the proliferation of lung fibroblasts and their myofibroblastic transdifferentiation as highlighted by increased expression and organization of αSMA, increased secretion of matrix proteins, and increased contractility of collagen gels. All of these effects were inhibited in a dose-dependent fashion by 1,25(OH)2D3. Of interest, a similar effect was noted in epithelial cells since 1,25(OH)2D3 inhibited their TGFβ1-induced EMT.
These observations are important for the following reasons. First, they confirm that lung fibroblasts and epithelial cells are indeed capable of recognizing 1,25(OH)2D3 through functional VDRs. VDRs have long been described in the fetal lung, as the immature lung is a known target organ for 1,25(OH)2D3. Owing to its pro-differentiation role, it is not surprising then that Vitamin D promotes lung development and alveolarization in the prenatal and neonatal lung. However, in order to regulate lung maturation properly, its effects on the developing lung sit in stark contrast to our findings in mature lung cells. For instance, fetal lung fibroblasts proliferate more in the presence of 1,25(OH)2D3 than in its absence [22, 23]. Additionally, 1,25(OH)2D3 may actually contribute to the EMT in the lung that is a necessary, programmed process for organogenesis [24].
In the mature lung, data are only emerging as to the cellular targets for Vitamin D. VDRs were recently detected in the epithelia of normal and malignant human bronchial tissue [25], while VDR responsive genes were found to be upregulated in lung epithelial cells treated with vitamin D metabolites [26] Similarly, airway smooth muscle cells express functional VDRs, and under stimulation by 1,25(OH)2D3 demonstrate differential gene expression [27].
Secondly, another important observation relates to the fact that 1,25(OH)2D3 was capable of inhibiting the TGFβ-mediated tissue remodeling responses in cultured lung fibroblasts. Though quite limited, much of the work on the role of vitamin D in TGFβ-related fibrosis has been studied, not unexpectedly, in cells or organs known to be vitamin D targets. For instance, similar to our data, in vitro myofibroblastic transformation of kidney interstitial fibroblasts by TGFβ, as measured by αSMA and collagen expression, was also blocked with 1,25(OH)2D3 [28], while cultured normal murine dermal fibroblasts are less efficient in contracting collagen gels [29]. More recently, 1,25(OH)2D3 was shown in mesenchymal multipotent cells to prevent their differentiation into a pro-fibrotic cellular phenotype in addition to induce the expression of anti-fibrotic soluble factors [30].
Correspondingly, treatment of animals with 1,25(OH)2D3 one of its analogues appears to confer protection against fibrosis in various animal models. Activation of Vitamin D pathways have been observed to decrease matrix expression and/or affect TGFβ signaling in experimental renal fibrosis [31, 32], UV-induced skin fibrosis [33], and viral myocarditis [34]. As a curiosity, mice lacking VDRs begin to display evidence of cardiac remodeling, even in the absence of concomitant injury [35].
A third observation that deserves highlighting relates to our findings in lung epithelial cells. Several recent studies have pointed to epithelial cell dysfunction as a potential early event in tissue fibrosis. Furthermore, epithelial cells are now considered a source of myofibroblasts through EMT, a process by which epithelial cells transform into smooth muscle/fibroblast-like cells capable of enhancing fibroproliferative responses driven by TGFβ1. We found that lung epithelial cells express VDR and that 1,25(OH)2D3 greatly upregulated the expression of mesenchymal markers in these cells while inhibiting the expression of epithelial cell markers, suggesting inhibition of TGFβ1-induced EMT. In fibrogenic EMT, nothing has been previously reported vis á vis Vitamin D in the lung. Tan et al. were able to inhibit EMT in a murine model of renal fibrosis with a 1,25(OH)2D3 analogue via an inhibition of TGFβ signals [31]. They reported that activation of Vitamin D signals also prevented TGFβ-mediated EMT in cultured renal tubular epithelial cells. Furthermore, it has been suggested that Vitamin D may induce the differentiation of carcinoma cells thereby potentially affecting oncogenic EMT by stimulating the expression of E-cadherin and blocking upregulation of mesenchymal genes [36, 37].
The relevance of the in vitro findings reported here to the situation in vivo is uncertain and further studies are required to confirm them in humans. However, our work in lung fibroblasts and epithelia suggests a pathway, previously unexplored in cells of this organ, capable of influencing TGFβ1-related pro-fibrotic responses. If confirmed, this would unveil a potential new target for fibrotic lung disorders.
FIG 7.
TGFβ1 induction of epithelial-mesenchymal transformation is antagonized by 1,25(OH)2D3. Indirect immunofluorescence for VDRs (A, left panel) was applied to cultured lung epithelial cells. An IgG control is also shown (A, right panel). Lung epithelial cells were pre-treated with 1,25(OH)2D3 (1µM) for 1 hour and incubated with TGFβ1 (10 ng./ml) for 72 hours. Western blotting of protein extracts was performed for (B) αSMA, (C) ZO-1, Pro-collagen I, and E-cadherin. Densitometric analyses of the immunoblots are shown. Double label immunofluorescence (D) was performed in untreated (left panels), TGFβ-treated (middle panels) and 1,25(OH)2D3/TGFβ- treated (right panels) lung epithelial cells. Epithelial/mesenchymal pairs that were studied included E-cadherin and αSMA (top panels), cytokeratin and procollagen I (middle panels), and ZO-1 and fibronectin (bottom panels).
ACKNOWLEGEMENTS
This work was supported by NIH grants, 1K08HL077533 (A.M.R.) and 5K08HL080293 (C.W.), a research award from the Roche Organ Transplant Research Foundation (A.M.R.), and the American Heart Association.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Valentine VG, Robbins RC, Berry GJ, Patel HR, Reichenspurner H, Reitz BA, et al. Actuarial survival of heart-lung and bilateral sequential lung transplant recipients with obliterative bronchiolitis. J Heart Lung Transplant. 1996;15(4):371–383. [PubMed] [Google Scholar]
- 2.American Thoracic Society. Idiopathic pulmonary fibrosis: diagnosis and treatment. International consensus statement. American Thoracic Society (ATS), and the European Respiratory Society (ERS) Am J Respir Crit Care Med. 2000;161(2 Pt 1):646–664. doi: 10.1164/ajrccm.161.2.ats3-00. [DOI] [PubMed] [Google Scholar]
- 3.Selman M, King TE, Pardo A. Idiopathic pulmonary fibrosis: prevailing and evolving hypotheses about its pathogenesis and implications for therapy. Ann Intern Med. 2001;134(2):136–151. doi: 10.7326/0003-4819-134-2-200101160-00015. [DOI] [PubMed] [Google Scholar]
- 4.Zhang K, Rekhter MD, Gordon D, Phan SH. Myofibroblasts and their role in lung collagen gene expression during pulmonary fibrosis. A combined immunohistochemical and in situ hybridization study. American Journal of Pathology. 1994;145(1):114–125. [PMC free article] [PubMed] [Google Scholar]
- 5.Blobe GC, Schiemann WP, Lodish HF. Role of transforming growth factor beta in human disease. N Engl J Med. 2000;342(18):1350–1358. doi: 10.1056/NEJM200005043421807. [DOI] [PubMed] [Google Scholar]
- 6.Willis BC, Liebler JM, Luby-Phelps K, Nicholson AG, Crandall ED, du Bois RM, et al. Induction of epithelial-mesenchymal transition in alveolar epithelial cells by transforming growth factor-beta1: potential role in idiopathic pulmonary fibrosis. Am J Pathol. 2005;166(5):1321–1332. doi: 10.1016/s0002-9440(10)62351-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Holick MF. Vitamin D Deficiency. N Engl J Med. 2007;357(3):266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 8.Autier P, Gandini S. Vitamin D Supplementation and Total Mortality: A Meta-analysis of Randomized Controlled Trials. Arch Intern Med. 2007;167(16):1730–1737. doi: 10.1001/archinte.167.16.1730. [DOI] [PubMed] [Google Scholar]
- 9.Shane E, Silverberg SJ, Donovan D, Papadopoulos A, Staron RB, Addesso V, et al. Osteoporosis in lung transplantation candidates with end-stage pulmonary disease. The American Journal of Medicine. 1996;101(3):262–269. doi: 10.1016/S0002-9343(96)00155-6. [DOI] [PubMed] [Google Scholar]
- 10.Aris RM, Neuringer IP, Weiner MA, Egan TM, Ontjes D. Severe osteoporosis before and after lung transplantation. Chest. 1996;109(5):1176–1183. doi: 10.1378/chest.109.5.1176. [DOI] [PubMed] [Google Scholar]
- 11.Vertino AM, Bula CM, Chen JR, Almeida M, Han L, Bellido T, et al. Nongenotropic, anti-apoptotic signaling of 1alpha,25(OH)2-vitamin D3 and analogs through the ligand binding domain of the vitamin D receptor in osteoblasts and osteocytes Mediation by Src, phosphatidylinositol 3-, and JNK kinases. J Biol Chem. 2005;280(14):14130–14137. doi: 10.1074/jbc.M410720200. [DOI] [PubMed] [Google Scholar]
- 12.Cheng JB, Motola DL, Mangelsdorf DJ, Russell DW. De-orphanization of Cytochrome P450 2R1: a microsomal vitamin D 25-hydroxylase. J. Biol. Chem. 2003;278(39):38084–38093. doi: 10.1074/jbc.M307028200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wrana JL, Attisano L, Cárcamo J, Zentella A, Doody J, Laiho M, et al. TGF[beta] signals through a heteromeric protein kinase receptor complex. Cell. 1992;71(6):1003–1014. doi: 10.1016/0092-8674(92)90395-s. [DOI] [PubMed] [Google Scholar]
- 14.Lagna G, Hata A, Hemmati-Brivanlou A, Massagué J. Partnership between DPC4 and SMAD proteins in TGF-beta signalling pathways. Nature. 1996;383:832–836. doi: 10.1038/383832a0. [DOI] [PubMed] [Google Scholar]
- 15.Ramirez AM, Takagawa S, Sekosan M, Jaffe HA, Varga J, Roman J. Smad3 deficiency ameliorates experimental obliterative bronchiolitis in a heterotopic tracheal transplantation model. Am J Pathol. 2004;165(4):1223–1232. doi: 10.1016/S0002-9440(10)63382-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barsony J, Marx SJ. Receptor-mediated rapid action of 1 alpha,25-dihydroxycholecalciferol: increase of intracellular cGMP in human skin fibroblasts. Proc Natl Acad Sci U S A. 1988;85(4):1223–1226. doi: 10.1073/pnas.85.4.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kingston RE, Chen CA, Rose JK. Calcium phosphate transfection. In: Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, et al., editors. Curr Protoc Mol Biol. Somerset: John Wiley and Sons, Inc.; 2007. Unit 9.1. [Google Scholar]
- 18.Dyer BW, Ferrer FA, Klinedinst DK, Rodriguez R. A Noncommercial Dual Luciferase Enzyme Assay System for Reporter Gene Analysis. Analytical Biochemistry. 2000;282(1):158–161. doi: 10.1006/abio.2000.4605. [DOI] [PubMed] [Google Scholar]
- 19.Jarmuz T, Roser S, Rivera H, Gal A, Roman J. Transforming growth factor-beta1, myofibroblasts, and tissue remodeling in the pathogenesis of tracheal injury: potential role of gastroesophageal reflux. Ann Otol Rhinol Laryngol. 2004;113(6):488–497. doi: 10.1177/000348940411300614. [DOI] [PubMed] [Google Scholar]
- 20.Krishnan AV, Feldman D. Cyclic adenosine 3',5'-monophosphate up-regulates 1,25-dihydroxyvitamin D3 receptor gene expression and enhances hormone action. Mol Endocrinol. 1992;6(2):198–206. doi: 10.1210/mend.6.2.1314957. [DOI] [PubMed] [Google Scholar]
- 21.Ramirez AM, Shen Z, Ritzenthaler JD, Roman J. Myofibroblast transdifferentiation in obliterative bronchiolitis: tgf-beta signaling through smad3-dependent and -independent pathways. Am J Transplant. 2006;6(9):2080–2088. doi: 10.1111/j.1600-6143.2006.01430.x. [DOI] [PubMed] [Google Scholar]
- 22.Liebeskind A, Srinivasan S, Kaetzel D, Bruce M. Retinoic acid stimulates immature lung fibroblast growth via a PDGF-mediated autocrine mechanism. Am J Physiol Lung Cell Mol Physiol. 2000;279(1):L81–L90. doi: 10.1152/ajplung.2000.279.1.L81. [DOI] [PubMed] [Google Scholar]
- 23.Ormerod AK, Xing Z, Pedigo NG, Mishra A, Kaetzel DM. The calcitriol analogue EB1089 impairs alveolarization and induces localized regions of increased fibroblast density in neonatal rat lung. Exp Lung Res. 2008;34(4):155–182. doi: 10.1080/01902140801929325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nguyen TM, Guillozo H, Marin L, Tordet C, Koite S, Garabedian M. Evidence for a vitamin D paracrine system regulating maturation of developing rat lung epithelium. Am J Physiol Lung Cell Mol Physiol. 1996;271(3):L392–L399. doi: 10.1152/ajplung.1996.271.3.L392. [DOI] [PubMed] [Google Scholar]
- 25.Menezes RJ, Cheney RT, Husain A, Tretiakova M, Loewen G, Johnson CS, et al. Vitamin D Receptor Expression in Normal, Premalignant, and Malignant Human Lung Tissue. Cancer Epidemiol Biomarkers Prev. 2008;17(5):1104–1110. doi: 10.1158/1055-9965.EPI-07-2713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hansdottir S, Monick MM, Hinde SL, Lovan N, Look DC, Hunninghake GW. Respiratory Epithelial Cells Convert Inactive Vitamin D to Its Active Form: Potential Effects on Host Defense. J Immunol. 2008;181(10):7090–7099. doi: 10.4049/jimmunol.181.10.7090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bosse Y, Maghni K, Hudson TJ. 1{alpha},25-Dihydroxy-vitamin D3 stimulation of bronchial smooth muscle cells induces autocrine, contractility, and remodeling processes. Physiol. Genomics. 2007;29(2):161–168. doi: 10.1152/physiolgenomics.00134.2006. [DOI] [PubMed] [Google Scholar]
- 28.Li Y, Spataro BC, Yang J, Dai C, Liu Y. 1,25-dihydroxyvitamin D3 inhibits renal interstitial myofibroblast activation by inducing hepatocyte growth factor expression. Kidney Int. 2005;68(4):1500–1510. doi: 10.1111/j.1523-1755.2005.00562.x. [DOI] [PubMed] [Google Scholar]
- 29.Greiling D, Thieroff-Ekerdt R. 1alpha,25-dihydroxyvitamin D3 rapidly inhibits fibroblast-induced collagen gel contraction. J Invest Dermatol. 1996;106(6):1236–1241. doi: 10.1111/1523-1747.ep12348928. [DOI] [PubMed] [Google Scholar]
- 30.Artaza JN, Norris KC. Vitamin D reduces the expression of collagen and key profibrotic factors by inducing an antifibrotic phenotype in mesenchymal multipotent cells. J Endocrinol. 2009;200(2):207–221. doi: 10.1677/JOE-08-0241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tan X, Li Y, Liu Y. Paricalcitol Attenuates Renal Interstitial Fibrosis in Obstructive Nephropathy. J Am Soc Nephrol. 2006;17(12):3382–3393. doi: 10.1681/ASN.2006050520. [DOI] [PubMed] [Google Scholar]
- 32.Hullett DA, Laeseke PF, Malin G, Nessel R, Sollinger HW, Becker BN. Prevention of chronic allograft nephropathy with vitamin D. Transpl Int. 2005;18(10):1175–1186. doi: 10.1111/j.1432-2277.2005.00187.x. [DOI] [PubMed] [Google Scholar]
- 33.Koshiishi I, Mitani H, Sumita T, Imanari T. 1,25-Dihydroxyvitamin D3 Prevents the Conversion of Adipose Tissue into Fibrous Tissue in Skin Exposed to Chronic UV Irradiation. Toxicology and Applied Pharmacology. 2001;173(2):99–104. doi: 10.1006/taap.2001.9178. [DOI] [PubMed] [Google Scholar]
- 34.Szalay G, Sauter M, Haberland M, Zuegel U, Steinmeyer A, Kandolf R, et al. Osteopontin. A Fibrosis-Related Marker Molecule in Cardiac Remodeling of Enterovirus Myocarditis in the Susceptible Host. Circ Res. 2009 doi: 10.1161/CIRCRESAHA.109.193805. [epub 10.1161/circresaha.1109.193805] [DOI] [PubMed] [Google Scholar]
- 35.Rahman A, Hershey S, Ahmed S, Nibbelink K, Simpson RU. Heart extracellular matrix gene expression profile in the vitamin D receptor knockout mice. The Journal of Steroid Biochemistry and Molecular Biology. 2007;103(3–5):416–419. doi: 10.1016/j.jsbmb.2006.12.081. [DOI] [PubMed] [Google Scholar]
- 36.Palmer HG, Gonzalez-Sancho JM, Espada J, Berciano MT, Puig I, Baulida J, et al. Vitamin D3 promotes the differentiation of colon carcinoma cells by the induction of E-cadherin and the inhibition of {beta}-catenin signaling. J. Cell Biol. 2001;154(2):369–388. doi: 10.1083/jcb.200102028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pendas-Franco N, Gonzalez-Sancho JM, Suarez Y, Aguilera O, Steinmeyer A, Gamallo C, et al. Vitamin D regulates the phenotype of human breast cancer cells. Differentiation. 2007;75(3):193–207. doi: 10.1111/j.1432-0436.2006.00131.x. [DOI] [PubMed] [Google Scholar]