Abstract
Microstructured and high surface energy titanium substrates increase osseointegration in vivo. In vitro, osteoblast differentiation is increased, but effects of the surface directly on multipotent mesenchymal stem cells (MSCs) and consequences for MSCs in the peri-implant environment are not known. We evaluated responses of human MSCs to substrate surface properties and examined the underlying mechanisms involved. MSCs exhibited osteoblast characteristics (alkaline phosphatase, RUNX2, and osteocalcin) when grown on microstructured Ti; this effect was more robust with increased hydrophilicity. Factors produced by osteoblasts grown on microstructured Ti were sufficient to induce co-cultured MSC differentiation to osteoblasts. Silencing studies showed that this was due to signaling via α2β1 integrins in osteoblasts on the substrate surface and paracrine action of secreted Dkk2. Thus, human MSCs are sensitive to substrate properties that induce osteoblastic differentiation; osteoblasts interact with these surface properties via α2β1 and secrete Dkk2, which acts on distal MSCs.
INTRODUCTION
The aim of most dental and orthopaedic implants is osseointegration of the implant with the surrounding bone as well as the maintenance and functional restoration of the existing bone. To accomplish this successfully, there are many factors to consider in the interaction between the implant material, most commonly pure titanium or titanium alloys in dental and orthopaedic applications, and the surrounding tissue in the patient. The first event after implant collocation is the attachment of proteins from the blood and serum to the implant surface. These protein-material interactions are regulated by the chemical composition, energy, and micro-nanotopography of the material’s surface [1-3].
The cells that colonize the surface of the implant do not have direct contact with the biomaterial but only with the proteins that attach to it [4, 5]. Most studies examining the osteogenic potential of implant surfaces in vitro have used cells that are either immature osteoblasts or osteoblast cell lines [6-8]. However, the first group of mesenchymal cells to colonize the implant surface must be able to migrate through the peri-implant clot, and are likely to be multipotent progenitor cells [9, 10]. The chemical composition of the adsorption layer on the surface can impact the lineage progression of these cells [11]. Surface nano and microstructure not only influence protein adsorption, but also the shape of cells that attach to the substrate [12, 13]. he mechanical stresses on the cytoskeleton can affect gene transcription [14], with resulting changes in cell response. Moreover, cells on the implant surface can affect cells distal to the implant via paracrine regulation. For this reason, how the cells sense the adsorbed layer and its underlying structure is important to the biological outcome.
In vivo studies support this. Topographical and chemical modifications such as sand blasting or sand blasting and acid etching [SLA] as well as modifications to increase surface energy (modified SLA [modSLA]) have greatly improved bone to implant contact, anchorage and removal torque [15-20]. In vitro studies using various osteoblast cell culture models show that SLA and modSLA surfaces cause osteoblasts to assume a more differentiated morphology than when they are grown on smooth Ti or tissue culture polystyrene (TCPS) surfaces [13]. Osteoblasts adhere more tightly to the rougher surfaces and they exhibit a more differentiated phenotype [13, 21, 22]. Recently, we showed that the α2β1 integrin is critical to this response. This integrin is recognized as the major receptor for collagen type I [23], the most abundant protein in bone. Targeted knockdown of either integrin subunit reduced the stimulatory effects of microstructure and hydrophilicity on maturation of MG63 osteoblasts [24-26].
In vitro studies show that osteoblasts cultured on microstructured Ti surfaces exhibit greater production of factors that regulate osteogenesis than osteoblasts cultured on smooth Ti substrates or TCPS, including prostaglandin E2 (PGE2), osteoprotegerin (OPG), transforming growth factor beta-1 (TGF-β1) [21] and BMP-2 [27]. These factors can act as autocrine and paracrine mediators of osteoblast activity [28]. Most recently, we showed that these cells also produce increased levels of factors that modulate the Wnt signaling pathway, including Dickkopf-1 (Dkk1) and Dkk2 [29]. Activation of Wnt is a critical step in the differentiation and maturation of osteoblasts and both canonical and alternate signaling pathways are involved [30]. We found that silencing of Dkk2, an inhibitor of the canonical Wnt pathway, in osteoblast-like cells abolished osteoblast maturation on microrough titanium surfaces [29]. In contrast, silencing of Dkk2, a more potent inhibitor of the same pathway, reduced differentiation of osteoblasts on smooth Ti and TCPS, but it did not block osteoblast maturation on the microtextured SLA and modSLA substrates.
These results indicate that the surface structure and chemistry of an implant material can modulate autocrine regulation of cells that are on the surface and suggest that they have the potential to act in a paracrine manner as well. It is not known, however, whether multipotent mesenchymal stem cells (MSCs) will exhibit surface dependent changes in the same manner as the various osteoblast models examined previously, or if they will differentiate into osteoblasts in a substrate-dependent manner. Moreover, it has not been shown in a definitive manner whether osteoblasts produce factors when grown on these substrates that act as paracrine regulators of osteogenic differentiation in MSC populations within the peri-implant environment but not on the implant surface. The present work evaluated the possible effects of surface roughness on MSC differentiation towards an osteogenic linage. In addition, we developed a co-culture model to investigate the possible role of the local microenvironment created by osteoblast-like cells cultured on rough surfaces on MSCs.
MATERIALS AND METHODS
Ti Disk Preparation
Ti disks were prepared from 1mm thick sheets of grade 2 unalloyed Ti and supplied to us by Institut Straumann AG (Basel, Switzerland). The disks were punched to 15mm in diameter to fit snuggly into the well of a 24W tissue culture plate. The methods used to produce pretreatment (PT, Ra=0.08μm), sandblasted acid etched (SLA, Ra=3.22μm), and modified SLA (modSLA, Ra=3.22μm) have been reported previously [13, 31]. Advancing contact angles were used to calculate the hydrophilicity of the surfaces as PT (95.8°), SLA (139.80°), and modSLA (~0°).
Direct Response to Ti Surfaces
Human MSCs were purchased from Lonza (Walkersville, MD) and grown in MSC Growth Medium (MSCGM, Lonza) at 37°C with 5% CO2 and 100% humidity. Cells were plated on TCPS, PT, SLA, or modSLA surfaces at a density of 5,000 cells/cm2 and grown to confluence on TCPS (about 7 days). At confluence, the media were changed and cells were incubated for 24 hours. After incubation, the conditioned media were collected and used to measure osteocalcin levels by radioimmunoassay (Biomedical Technologies Inc., Stoughton, MA) and OPG (modulates osteoclastogenesis by preventing RANKL from interacting with its receptor RANK), VEGF-A (promotes angiogenesis), and TGF-β1 (stimulates osteoblast matrix synthesis and differentiation and inhibits osteoclast activity) by ELISA (R&D Systems, Minneapolis, MN) following manufacturer’s instructions, as described previously [32, 33]. The cells on the surfaces were trypsinized twice, counted, and alkaline phosphatase specific activity and protein levels were measured.
To quantify mRNA expression, cells were plated as described on TCPS, PT, SLA, and modSLA surfaces. At confluence, cells were incubated with fresh media for 12h and harvested using a TRIzol® (Invitrogen, Carlsbad, California) extraction method. mRNA was quantified using a NanoDrop spectrophotometer (Thermo Scientific, Waltham, Massachusetts). For the reverse transcription portion, 1ug of RNA was amplified (OmniScript RT, Qiagen, Valencia, California) using random oligiomers (Promega, Madison, Wisconsin). Starting quantities of mRNA were determined in an iQ5 Imaging System (BioRad, Hercules, California) using SybrGreen chemistry (BioRad). MSCs grown on TCPS were used to generate a standard curve for the reaction and values for each treatment extrapolated. Expression of mRNA was measured for RUNX2 (F: 5′-GTC TCA CTG CCT CTC ACT TG-3′, R: 5′-CAC ACA TCT CCT CCC TTC TG-3′), OCN (F: 5′- -3′, R: 5′- -3′), ITGα2 (F: 5′-ACT GTT CAA GGA GGA GAC-3′, R: 5′-GGT CAA AGG CTT GTT TAG G-3′), ITGβ1 (F: 5′-ATT ACT CAG ATC CAA CCA C-3′, R: 5′-TCC TCC TCA TTT CAT TCA TC-3′), DKK1 (F: 5′-CCA GAC CAT TGA CAA CTA CC-3′, R: 5′-CAG GCG AGA CAG ATT TGC-3′), DKK2 (F: 5′-TGA CTT GGG ATG GCA GAA TC-3′, R: 5′-CAG AAA TGA CGA GCA CAG C-3′). All genes are presented as normalized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH, F: 5′-GCT CTC CAG AAC ATC ATC C-3′, R: 5′-TGC TTC ACC ACC TTC TTG-3′).
Integrin Alpha-2 Silencing
Previous studies have shown that osteoblast differentiation on SLA and modSLA surfaces is mediated by α2β1 integrin signaling [24]. To determine if secretion of paracrine mediators of MSC differentiation also require signaling via this integrin, we took advantage of an MG63 cell line that was stably silenced for the α2 integrin subunit (ITGα2). Silenced cells exhibited 70% less of the ITGα2 subunit as assessed by Western blot and mRNA expression as described previously [24].
Dkk2 Silencing
Related studies in our lab indicate that Dkk2 is required for osteoblast differentiation on SLA and modSLA [29]. To determine if Dkk2 acts as a paracrine mediator of MSC differentiation, we took advantage of an MG63 cell line that was stably silenced for Dkk2. To generate this cell line, MG63 cells were transduced with either empty vector or one of four different shRNA lentiviral transduction clones. MG63 cells were plated at 20,000 cells/cm2 and cultured overnight as above. Mission® shRNA lentiviral transduction particles (Sigma-Aldrich, St. Louis, Missouri) were added to the cells at 7.5 multiplicity of infection and incubated for 18 hours. After incubation, transduced cells were selected with 0.25 ug/mL of puromycin (Sigma-Aldrich). Dkk2 expression and levels were assessed by RT-PCR and Western blot.
Co-culture Experiment
Successful osseointegration of implants depends not only on the MSCs that attach to the implant and undergo osteoblast differentiation due to the topography and chemistry of the biomaterial, but also on the osteoblasts that may migrate to the implant and affect the differentiation of cells distal to the implant surface itself. To study the paracrine effect that osteoblasts cultured on titanium surfaces have on nearby MSCs, we designed a co-culture system whereby factors produced by the osteoblasts would be allowed to exert their effect on MSCs. Using this system, we first looked at the effects of MG63 cells grown on titanium surfaces on MSCs. In subsequent studies, responses of MSCs co-cultured with wild type MG63 cells were compared to those of MG63 cells silenced for ITGα2 or Dkk2. MG63 cells were plated on cover glass (CG), PT, SLA, or modSLA at 10,000 cells/cm2 and grown to confluence on CG (about 7 days). At the same time, MSCs were plated at 5,000 cells/cm2 in 6 well plates and cultured in MSCGM. When the MG63 cells on the test surfaces were confluent, the disks were moved into cell culture inserts (BD Falcon, Franklin Lakes, New Jersey) above the MSCs in the 6 well plates. Cells were fed using DMEM, 10% FBS, and 1% penicillin-streptomycin for an additional 12 days in the co-culture system. After 12 days, the cell culture inserts with surfaces containing MG63 cells were removed and discarded and MSCs were fed with full media and incubated for additional 24 hours. After incubation, the conditioned media were collected and osteocalcin, OPG, VEGF-A, and TGF-β1 measured. MSCs were trypsinized, counted, and alkaline phosphatase specific activity and protein were measured in the cell lysate.
Statistical Analysis
Data presented are from one of two sets of experiments, with comparable results. Each data point is the mean ± SEM for six independent cultures, with the exception of the mRNA expression experiment, where n=4. Data were analyzed by analysis of variance and significant differences between groups determined using Bonferroni’s modification of the Student’s t-test. P<0.05 was considered to be significant.
RESULTS
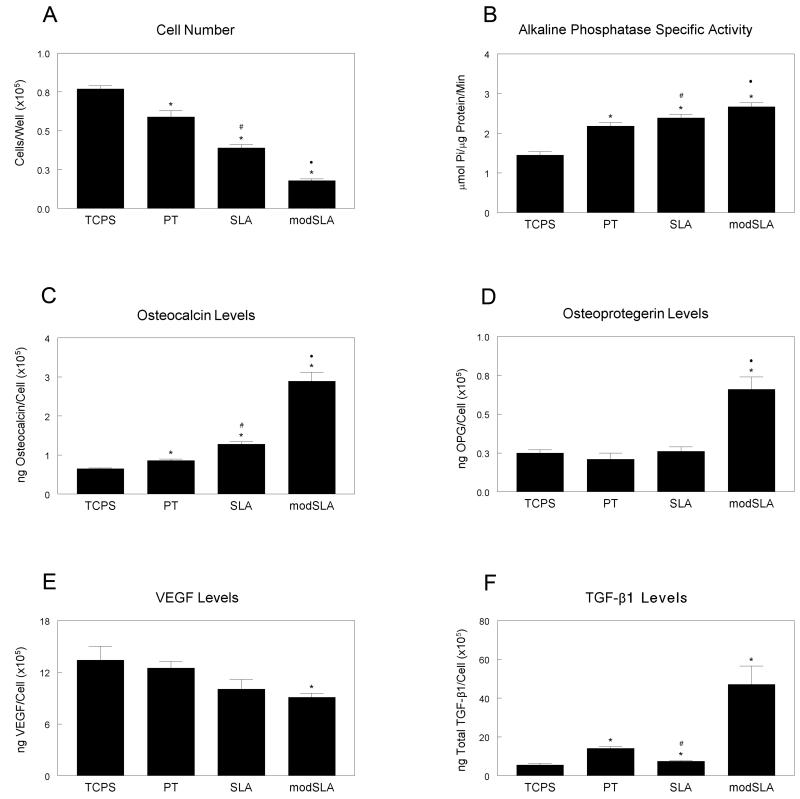
MSC differentiation to an osteoblast phenotype was sensitive to surface microstructure and hydrophilicity. Cell number, which decreases as cells move from a proliferative to a differentiated state, significantly decreased on all of the titanium substrates when compared to tissue culture polystyrene (TCPS) with greater decreases with increased roughness (SLA) and hydrophilicity (modSLA) (Fig. 1a). Alkaline phosphatase specific activity, an early marker of osteogenic differentiation, increased two-fold on titanium surfaces when compared to TCPS, with additional increases on SLA and modSLA surfaces (Fig. 1b). Osteocalcin, measured in the conditioned media and a later marker of osteoblastic differentiation, increased slightly in cultures grown on PT and SLA surfaces over basal levels but had a three-fold increase in cultures grown on modSLA surfaces (Fig. 1c). Secreted OPG increased only in MSCs grown on the hydrophilic modSLA surfaces (Fig. 1d). Levels of VEGF-A decreased slightly in the conditioned media of cells grown on titanium surfaces but were significantly decreased on the modSLA substrate (Fig. 1e). TGF-β1 increased on the smooth PT surface, but was significantly decreased on the rough SLA surface when compared to PT (Fig. 1f). TGF-β1 increased three-fold on modSLA when compared to the other substrates examined.
Figure 1.
Effect of titanium surface microstructure and hydrophilicity on human mesenchymal stem cell differentiation. MSCs were plated on TCPS, PT, SLA and modSLA surfaces and grown to confluence. At confluence, cell number (A), alkaline phosphatase specific activity (B), osteocalcin (C), osteoprotegerin (D), VEGF-A (E), and TGF-β1 (F) were measured. Data represented are mean ± SEM of six independent samples. *p<0.05, Ti vs. TCPS; #p<0.05, Ti vs. PT; ·p<0.05, surface vs. modSLA.
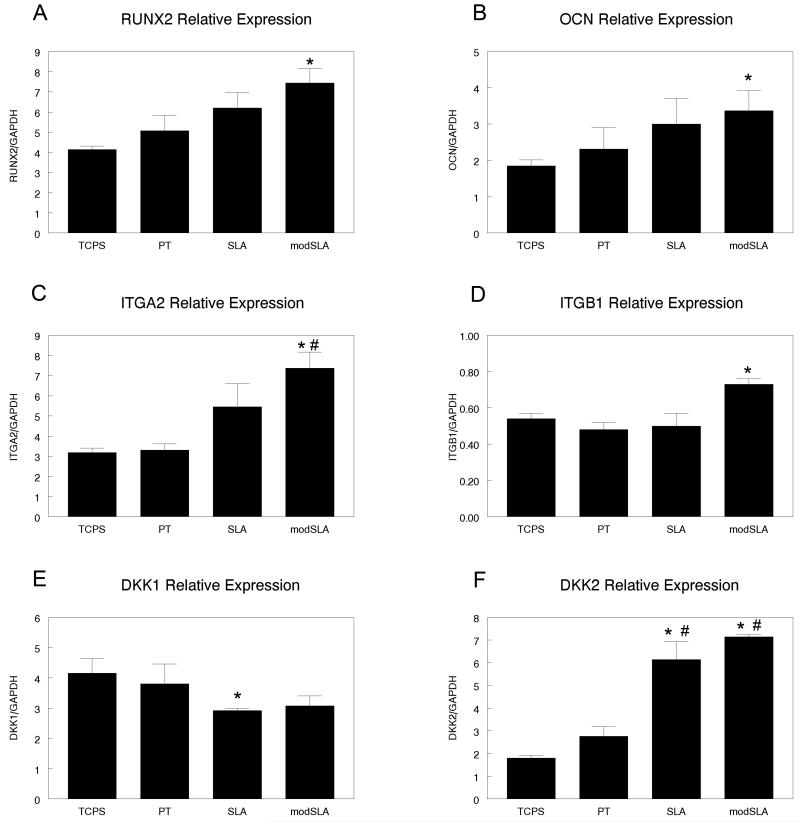
Osteoblastic gene expression in MSCs was also affected by the surface properties of the substrate. RUNX2 expression was increased on titanium surfaces and significantly increased on modSLA surfaces as compared to TCPS (Fig. 2a). Osteocalcin was also significantly upregulated on modSLA surfaces (Fig. 2b). MSCs grown on the rough SLA surface slightly increased expression of RUNX2 but expression on modSLA was significantly increased when compared to TCPS and PT surfaces (Fig. 2c). Integrin beta 1 expression increased 30% on modSLA in comparison to the other surfaces tested (Fig. 2d). DKK1 was slightly lower on modSLA surfaces and significantly down regulated on SLA than on the smooth TCPS and PT surfaces (Fig. 2e). DKK2 expression was 200% higher on SLA and modSLA surfaces than on TCPS or PT (Fig. 2f).
Figure 2.
Effect of titanium surface microstructure and hydrophilicity on human mesenchymal stem cell gene expression. MSCs were plated on TCPS, PT, SLA and modSLA surfaces and grown to confluence. At confluence, mRNA expression of (A) RUNX2, (B) Osteocalcin, (C) ITGα2, (D) ITGβ1, (E) DKK1, and (F) DKK2 was measured by Real-time PCR. Data represented are mean ± SEM of four independent samples. *p<0.05, Ti vs. TCPS; #p<0.05, Ti vs. PT; ·p<0.05, surface vs. modSLA.
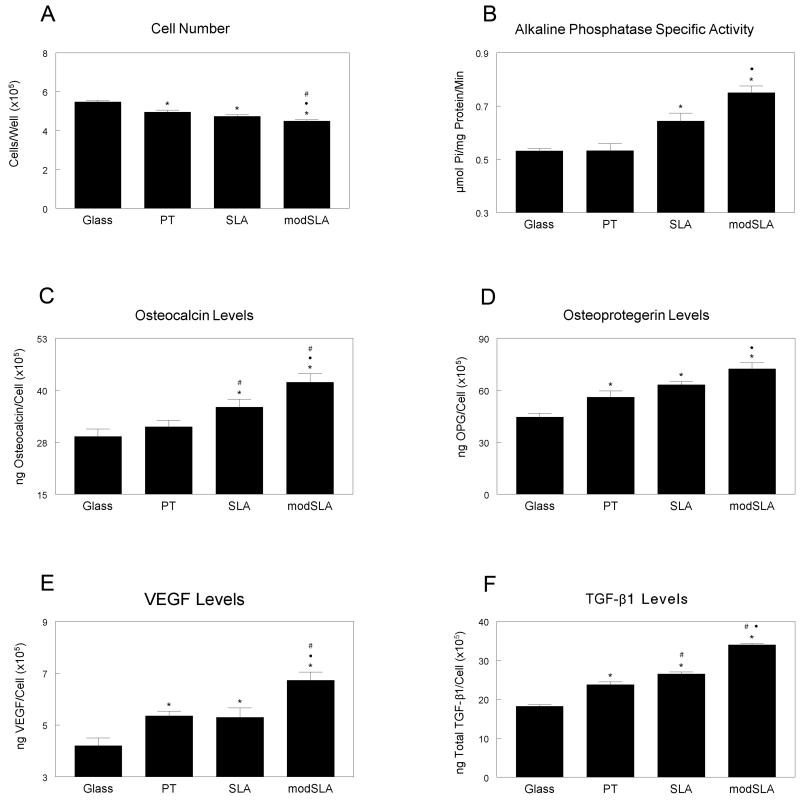
After 12 days in this culture system, MSC cell number significantly decreased in cultures exposed to osteoblasts on titanium surfaces with a further reduction when cells were co-cultured with MG63 on modSLA surfaces when compared to cover-glass (CG) control cultures (Fig. 3a). Alkaline phosphatase specific activity and osteocalcin levels in MSCs were not affected by MG63 cells grown on PT surfaces when compared to CG, but were significantly increased on microstructured SLA surfaces and hydrophilic modSLA surfaces (Fig. 3b, 3c). Osteoprotegerin levels increased when MSCs were exposed to titanium surfaces, with highest levels on modSLA surfaces (Fig. 3d). VEGF-A and TGF-β1 increased modestly on PT and SLA surfaces but was further increased on modSLA surfaces when compared to the other titanium substrates (Fig. 3e, 3f).
Figure 3.
Indirect effect of titanium surface microstructure and hydrophilicity on differentiation of human mesenchymal stem cells. MSCs were cultured together with MG63 cells grown on TCPS, PT, SLA, and modSLA surfaces. After 12 days of co-culture, MSCs were harvested and cell count (A), alkaline phosphatase specific activity (B), osteocalcin (C), osteoprotegerin (D), VEGF-A (E), and TGF-β1 (F) were measured. Data represented are mean ± SEM of six independent samples. *p<0.05, Ti vs. TCPS; #p<0.05, Ti vs. PT; ·p<0.05, surface vs. modSLA.
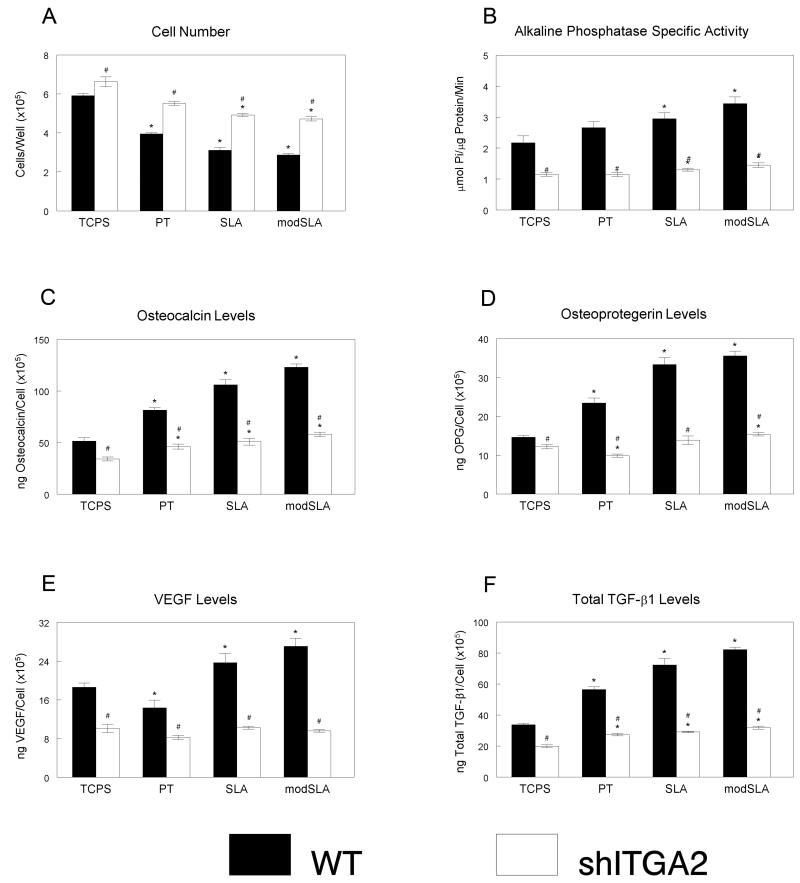
Signaling via α2β1 in osteoblasts grown on SLA and modSLA was required for MSC differentiation. While cell number decreased in MSCs cultured with ITGα2-silenced cells grown on SLA and modSLA surfaces when compared to CG, cell number was increased in MSCs exposed to growth factors from ITGα2-silenced cells (Fig. 4a) in comparison to the wild type MG63 cells. Alkaline phosphatase specific activity increased in MSCs exposed to MG63 cells grown on the rough SLA and modSLA surfaces over levels on CG and PT (Fig. 4b). When MSCs were grown with ITGα2-silenced cells, alkaline phosphatase specific activity was reduced on all cells when compared to the wild type, but activity was increased in cells exposed to the SLA and modSLA surfaces. Levels of osteocalcin in the conditioned media increased in cells exposed to the wild type MG63 (CG<PT<SLA<modSLA), but were significantly reduced in cells cultured with ITGα2-silenced cells with no difference between the titanium surfaces (Fig. 4c). Osteoprotegerin increased in cells exposed to MG63 cells on titanium surfaces, with significant increases on rough surfaces (SLA and modSLA, Fig. 4d). However, levels were decreased when cells were exposed to the silenced cells on surfaces, decreasing slightly on PT and increasing on modSLA when compared to CG. VEGF-A, which increased in the titanium surfaces with MG63 cells, was significantly decreased in the ITGα2-silenced cells with no significant difference between CG and the titanium surfaces (Fig. 4e). TGF-β1 in MSCs grown with ITGα2-silenced MG63 cells were significantly lower than those grown with the wild type MG63 cells, but the titanium substrates did increase TGF-β1 in the MSCs in both cell types (Fig. 4f).
Figure 4.
Indirect effect of ITGα2 silencing, titanium surface microstructure and hydrophilicity on differentiation of human mesenchymal stem cells. MSCs were cultured together with wild type MG63 and ITGα2-silenced cells grown on TCPS, PT, SLA, and modSLA surfaces. After 12 days of co-culture, MSCs were harvested and cell count (A), alkaline phosphatase specific activity (B), osteocalcin (C), osteoprotegerin (D), VEGF-A (E), and TGF-β1 (F) were measured. Data represented are mean ± SEM of six independent samples. *p<0.05, Ti vs. TCPS; #p<0.05, WT vs. silenced MG63 cells.
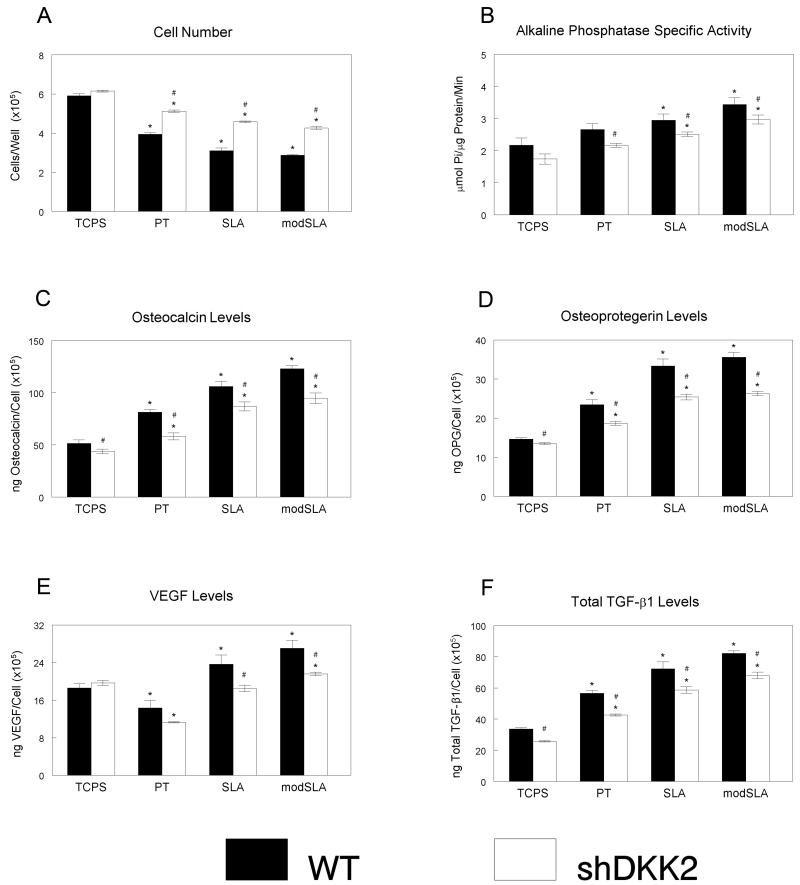
Osteoblast production of Dkk2 was important for MSC differentiation. When cultured with Dkk2-silenced cells, cell number decreased on titanium as compared to CG surfaces, but increased when compared to the wild type cells (Fig. 5a). Alkaline phosphatase specific activity was decreased in Dkk2-silenced cells when compared to wild type MG63, but both cell types did show increased activity on titanium and with increased surface roughness (Fig. 5b). Osteocalcin and osteoprotegerin levels increased with co-culture on titanium surfaces (Fig. 5c, 5d), independent of the cell type; however, the levels were reduced in MSCs exposed to Dkk2-silenced cells. VEGF-A levels were reduced in MSCs co-cultured with MG63 and Dkk-2 silenced MG63 cells on PT surfaces as compared to the glass control (Fig. 5e). The increase in VEGF-A in MSCs co-cultured with wild type cells on SLA was not seen in the Dkk2-silenced cells, but levels were increased for both cell types on SLA surfaces. TGF-β1 increased on titanium and with increased surface roughness in MSCs cultured with MG63 and Dkk2-silenced MG63, but again levels of this factor were decreased overall in the cells exposed to the silenced cell line (Fig. 5f).
Figure 5.
Indirect effect of Dkk2 silencing, titanium surface microstructure and hydrophilicity on differentiation of human mesenchymal stem cells. MSCs were cultured together with wild type MG63 and Dkk2-silenced cells grown on TCPS, PT, SLA, and modSLA surfaces. After 12 days of co-culture, MSCs were harvested and cell count (A), alkaline phosphatase specific activity (B), osteocalcin (C), osteoprotegerin (D), VEGF-A (E), and TGF-β1 (F) were measured. Data represented are mean ± SEM of six independent samples. *p<0.05, Ti vs. TCPS; #p<0.05, WT vs. silenced MG63 cells.
DISCUSSION
Several studies have shown that titanium surface properties such as microtopography, chemistry, and surface energy decrease osteoblast proliferation and modulate osteoblast maturation [25, 33-35]. These studies have focused on the behavior of committed osteoblasts on titanium surfaces. However, osteoprogenitor cells are more likely than osteoblasts to migrate to the implant surface from the surrounding tissue and their differentiation on the implant surface is a less studied, but potentially an important component of successful osseointegration. In the present study, we used a co-culture model to examine the effect of surface microstructure and surface energy on mesenchymal stem cell differentiation and the contribution of osteoblast-biomaterial interaction in the regulation of mesenchymal stem cell differentiation.
MSCs had reduced cell numbers on Ti substrates compared to TCPS and numbers were further reduced on the microstructured SLA and modSLA substrates. Substrates with high energy decreased MSC number even more. Although this decrease in cell number may result from reduced initial attachment and increased cell death, the fact that the cells exhibited markers associated with a mature secretory osteoblast phenotype suggests that they have committed to an osteoblastic lineage on the microstructured substrates, which is correlated with reduced proliferation [36]. Similar results were found in previous studies using human osteoblasts, rat osteoblasts, and osteosarcoma cells, showing that a combination of rough surface and high energy increased osteoblast maturation [13, 37-39]. In addition to increased alkaline phosphatase and osteocalcin, MSCs also increased production of important local factors that create a more suitable osteogenic environment, including OPG, VEGF-A and TGF-β1. These factors are also increased in primary osteoblasts and osteoblast-like osteosarcoma cells in a similar manner, TCPS<PT<SLA<modSLA. These data suggest that surface properties like microstructure or surface energy have the potential to modulate osteoblast differentiation via autocrine and paracrine regulation.
Most of the studies assessing osteogenic differentiation of MSCs have focused on the synergistic effect of BMPs and substrates as titanium, hydroxyapatite, or the osteoblast differentiation capacity of the MSCs treated with osteogenic media in diverse substrates [40-43]. It is important to note that MSC differentiation towards an osteoblastic linage in this study is the result of the surface properties and the microenvironment that the cells produced in vitro and is not the result of osteogenic factors added to the media or the treatment with an osteogenic media as has been shown in other work [44, 45]. Others have reported that modulating bulk material properties of a surface such as stiffness can direct stem cell differentiation and that stem cells grown on these surfaces can undergo neurogenic, myogenic, or osteogenic differentiation [46], supporting our observation that modifying surface properties such as roughness and hydrophilicity also directs stem cell differentiation.
Once MSCs or progenitor cells fully differentiate to an osteoblastic linage, the factors that they produce in response to the micron and submicron surface topography influence the MSCs and progenitor cells in the surrounding tissue, which will induce new bone formation from a distal area. This process is important for the success of the osseointegration process. As an alternative to the traditional technique of treating cell monolayers with collected conditioned media, we adapted a co-culture method in which factors produced by osteoblast-like cells on the biomaterial directly affect MSCs in real time. Thus, MSCs were exposed to factors produced by osteoblasts at early and late states of differentiation on the Ti substrates. This is by its design a cross-talk assay; factors produced by the MSCs also had potential to act on the osteoblasts during the co-culture period, similar to the cross-talk that occurs in vivo. The results of these experiments show that factors present in the conditioned media reduced MSC proliferation in a substrate-dependent manner with the greatest reduction found in co-cultures where osteoblasts were grown on modSLA (modSLA>SLA>PT>CG). Conversely, the greatest production of osteocalcin, OPG, and TGF-β1 was seen in MSCs grown in co-culture with osteoblasts on SLA and modSLA. These results suggest that the local factors released by MG63 cells grown on titanium surfaces affect MSC differentiation, but only when the osteoblasts are grown in SLA and modSLA is there a significant induction of an osteoblastic phenotype. Moreover, these results suggest that the cell/substrate interaction also modulates angiogenesis, since levels of VEGF-A were increased in the media produced by the osteoblasts grown on the microstructured and high energy substrates.
Our experiments using cell lines silenced for the α2 integrin subunit demonstrated that the osteoblasts on the material substrates released factors that mediated osteoblastic differentiation in the distal MSC cultures. Knockdown of the α2 integrin to approximately 30% expression reduced the osteogenic differentiation of the MSCs in the co-culture, supporting our previous findings showing that osteoblast response to microstructured Ti is mediated by α2β1 [24]. Knockdown of Dkk2 also significantly reduced osteogenic differentiation of the MSCS. Dkk2 is a soluble factor released by osteoblasts that acts on the cells via non-canonical Wnt signaling pathways to promote the differentiation of osteoblasts. Levels of secreted Dkk2 are increased in cultures of osteoblasts grown on SLA and modSLA and knockdown of this protein reduces osteoblast differentiation of MG63 cells grown on microstructured Ti [29]. The results of the present study indicate that the secreted Dkk2 not only acts in an autocrine manner on the osteoblasts on the Ti substrates but also on distal MSCs to promote their differentiation in the osteoblast lineage.
CONCLUSIONS
We have shown that surface microstructure and surface energy are able to direct mesenchymal stem cells toward an osteoblast lineage, through measurements of proteins and mRNA commonly used as markers of osteoblasts. We have also shown, using the co-culture model, that differentiated osteoblasts on implant surfaces create a sufficient environment for osteogenic differentiation of the surrounding MSCs. Finally, we showed the contributions of ITGα2 and DKK2 in the differentiation of the surrounding tissue.
ACKNOWLEDGEMENTS
This study was supported by a grant from the ITI Foundation, NIH AR052102, and the PROFIP of the Universidad Nacional Autonoma de Mexico. Ti disks were provided by Institut Straumann AG (Basel, Switzerland) as a gift. Dr. Marco Wieland was the Vice President for Research at Institut Straumann AG at the time the study was performed; he participated in the study as a contributing scientist.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Protivinsky J, Appleford M, Strnad J, Helebrant A, Ong JL. Effect of chemically modified titanium surfaces on protein adsorption and osteoblast precursor cell behavior. Int J Oral Maxillofac Implants. 2007;22(4):542–550. [PubMed] [Google Scholar]
- 2.Hao L, Lawrence J. Wettability modification and the subsequent manipulation of protein adsorption on a Ti6Al4V alloy by means of CO2 laser surface treatment. J Mater Sci Mater Med. 2007;18(5):807–817. doi: 10.1007/s10856-006-0002-4. [DOI] [PubMed] [Google Scholar]
- 3.Cai K, Bossert J, Jandt KD. Does the nanometre scale topography of titanium influence protein adsorption and cell proliferation? Colloids Surf B Biointerfaces. 2006;49(2):136–144. doi: 10.1016/j.colsurfb.2006.02.016. [DOI] [PubMed] [Google Scholar]
- 4.Raghavendra S, Wood MC, Taylor TD. Early wound healing around endosseous implants: a review of the literature. Int J Oral Maxillofac Implants. 2005;20(3):425–431. [PubMed] [Google Scholar]
- 5.Davies JE. Understanding peri-implant endosseous healing. J Dent Educ. 2003;67(8):932–949. [PubMed] [Google Scholar]
- 6.Dalby MJ, McCloy D, Robertson M, Agheli H, Sutherland D, Affrossman S, et al. Osteoprogenitor response to semi-ordered and random nanotopographies. Biomaterials. 2006;27(15):2980–2987. doi: 10.1016/j.biomaterials.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 7.Dalby MJ, McCloy D, Robertson M, Wilkinson CD, Oreffo RO. Osteoprogenitor response to defined topographies with nanoscale depths. Biomaterials. 2006;27(8):1306–1315. doi: 10.1016/j.biomaterials.2005.08.028. [DOI] [PubMed] [Google Scholar]
- 8.Dalby MJ, Gadegaard N, Curtis AS, Oreffo RO. Nanotopographical control of human osteoprogenitor differentiation. Curr Stem Cell Res Ther. 2007;2(2):129–138. doi: 10.2174/157488807780599220. [DOI] [PubMed] [Google Scholar]
- 9.Caplan AI. Why are MSCs therapeutic? New data: new insight. J Pathol. 2009;217(2):318–324. doi: 10.1002/path.2469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Caplan AI, Dennis JE. Mesenchymal stem cells as trophic mediators. J Cell Biochem. 2006;98(5):1076–1084. [Google Scholar]
- 11.Le Guillou-Buffello D, Bareille R, Gindre M, Sewing A, Laugier P, Amedee J. Additive effect of RGD coating to functionalized titanium surfaces on human osteoprogenitor cell adhesion and spreading. Tissue Eng Part A. 2008;14(8):1445–1455. doi: 10.1089/ten.tea.2007.0292. [DOI] [PubMed] [Google Scholar]
- 12.Yang Y, Tian J, Deng L, Ong JL. Morphological behavior of osteoblast-like cells on surface-modified titanium in vitro. Biomaterials. 2002;23(5):1383–1389. doi: 10.1016/s0142-9612(01)00259-9. [DOI] [PubMed] [Google Scholar]
- 13.Zhao G, Raines AL, Wieland M, Schwartz Z, Boyan BD. Requirement for both micron- and submicron scale structure for synergistic responses of osteoblasts to substrate surface energy and topography. Biomaterials. 2007;28(18):2821–2829. doi: 10.1016/j.biomaterials.2007.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Watson PA. Function follows form: generation of intracellular signals by cell deformation. FASEB J. 1991;5(7):2013–2019. doi: 10.1096/fasebj.5.7.1707019. [DOI] [PubMed] [Google Scholar]
- 15.Cochran DL, Nummikoski PV, Higginbottom FL, Hermann JS, Makins SR, Buser D. Evaluation of an endosseous titanium implant with a sandblasted and acid-etched surface in the canine mandible: radiographic results. Clin Oral Implants Res. 1996;7(3):240–252. doi: 10.1034/j.1600-0501.1996.070306.x. [DOI] [PubMed] [Google Scholar]
- 16.Cochran DL, Schenk RK, Lussi A, Higginbottom FL, Buser D. Bone response to unloaded and loaded titanium implants with a sandblasted and acid-etched surface: a histometric study in the canine mandible. J Biomed Mater Res. 1998;40(1):1–11. doi: 10.1002/(sici)1097-4636(199804)40:1<1::aid-jbm1>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 17.Cochran DL, Buser D, ten Bruggenkate CM, Weingart D, Taylor TM, Bernard JP, et al. The use of reduced healing times on ITI implants with a sandblasted and acid-etched (SLA) surface: early results from clinical trials on ITI SLA implants. Clin Oral Implants Res. 2002;13(2):144–153. doi: 10.1034/j.1600-0501.2002.130204.x. [DOI] [PubMed] [Google Scholar]
- 18.Bornstein MM, Harnisch H, Lussi A, Buser D. Clinical performance of wide-body implants with a sandblasted and acid-etched (SLA) surface: results of a 3-year follow-up study in a referral clinic. Int J Oral Maxillofac Implants. 2007;22(4):631–638. [PubMed] [Google Scholar]
- 19.Schwartz Z, Raz P, Zhao G, Barak Y, Tauber M, Yao H, et al. Effect of micrometer-scale roughness of the surface of Ti6Al4V pedicle screws in vitro and in vivo. J Bone Joint Surg Am. 2008;90(11):2485–2498. doi: 10.2106/JBJS.G.00499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bornstein MM, Hart CN, Halbritter SA, Morton D, Buser D. Early Loading of Nonsubmerged Titanium Implants with a Chemically Modified Sand-Blasted and Acid-Etched Surface: 6-Month Results of a Prospective Case Series Study in the Posterior Mandible Focusing on Peri-Implant Crestal Bone Changes and Implant Stability Quotient (ISQ) Values. Clin Implant Dent Relat Res. 2009 doi: 10.1111/j.1708-8208.2009.00148.x. [DOI] [PubMed] [Google Scholar]
- 21.Boyan BD, Batzer R, Kieswetter K, Liu Y, Cochran DL, Szmuckler-Moncler S, et al. Titanium surface roughness alters responsiveness of MG63 osteoblast-like cells to 1 alpha,25-(OH)2D3. J Biomed Mater Res. 1998;39(1):77–85. doi: 10.1002/(sici)1097-4636(199801)39:1<77::aid-jbm10>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 22.Lai HC, Zhuang LF, Liu X, Wieland M, Zhang ZY. The influence of surface energy on early adherent events of osteoblast on titanium substrates. J Biomed Mater Res A. 2009 doi: 10.1002/jbm.a.32542. [DOI] [PubMed] [Google Scholar]
- 23.White DJ, Puranen S, Johnson MS, Heino J. The collagen receptor subfamily of the integrins. Int J Biochem Cell Biol. 2004;36(8):1405–1410. doi: 10.1016/j.biocel.2003.08.016. [DOI] [PubMed] [Google Scholar]
- 24.Olivares-Navarrete R, Raz P, Zhao G, Chen J, Wieland M, Cochran DL, et al. Integrin alpha2beta1 plays a critical role in osteoblast response to micron-scale surface structure and surface energy of titanium substrates. Proc Natl Acad Sci U S A. 2008;105(41):15767–15772. doi: 10.1073/pnas.0805420105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schwartz Z, Bell BF, Wang L, Zhao G, Olivares-Navarrete R, Boyan BD. Beta-1 integrins mediate substrate dependent effects of 1alpha,25(OH)2D3 on osteoblasts. J Steroid Biochem Mol Biol. 2007;103(35):606–609. doi: 10.1016/j.jsbmb.2006.12.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang L, Zhao G, Olivares-Navarrete R, Bell BF, Wieland M, Cochran DL, et al. Integrin beta1 silencing in osteoblasts alters substrate-dependent responses to 1,25-dihydroxy vitamin D3. Biomaterials. 2006;27(20):3716–3725. doi: 10.1016/j.biomaterials.2006.02.022. [DOI] [PubMed] [Google Scholar]
- 27.Ong JL, Bess EG, Bessho K. Osteoblast progenitor cell responses to characterized titanium surfaces in the presence of bone morphogenetic protein-atelopeptide type I collagen in vitro. J Oral Implantol. 1999;25(2):95–100. doi: 10.1563/1548-1336(1999)025<0095:OPCRTC>2.3.CO;2. [DOI] [PubMed] [Google Scholar]
- 28.Schwartz Z, Olivares-Navarrete R, Wieland M, Cochran DL, Boyan BD. Mechanisms regulating increased production of osteoprotegerin by osteoblasts cultured on microstructured titanium surfaces. Biomaterials. 2009;30(20):3390–3396. doi: 10.1016/j.biomaterials.2009.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Olivares-Navarrete R, Hyzy S, Weiland M, Boyan B, Schwartz Z. The Roles of Wnt Signaling Modulators Dickkopf-1 (Dkk1) and Dickkopf-2 (Dkk2) and Cell Maturation State in Osteogenesis on Microstructured Titanium Surfaces. Biomaterials. 2009 doi: 10.1016/j.biomaterials.2009.11.071. doi:10.1016/j.biomaterials.2009.11.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Milat F, Ng KW. Is Wnt signalling the final common pathway leading to bone formation? Mol Cell Endocrinol. 2009;310(12):52–62. doi: 10.1016/j.mce.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 31.Rupp F, Scheideler L, Olshanska N, de Wild M, Wieland M, Geis-Gerstorfer J. Enhancing surface free energy and hydrophilicity through chemical modification of microstructured titanium implant surfaces. J Biomed Mater Res A. 2006;76(2):323–334. doi: 10.1002/jbm.a.30518. [DOI] [PubMed] [Google Scholar]
- 32.Lossdorfer S, Schwartz Z, Wang L, Lohmann CH, Turner JD, Wieland M, et al. Microrough implant surface topographies increase osteogenesis by reducing osteoclast formation and activity. J Biomed Mater Res A. 2004;70(3):361–369. doi: 10.1002/jbm.a.30025. [DOI] [PubMed] [Google Scholar]
- 33.Kieswetter K, Schwartz Z, Hummert TW, Cochran DL, Simpson J, Dean DD, et al. Surface roughness modulates the local production of growth factors and cytokines by osteoblast-like MG-63 cells. J Biomed Mater Res. 1996;32(1):55–63. doi: 10.1002/(SICI)1097-4636(199609)32:1<55::AID-JBM7>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 34.Boyan BD, Bonewald LF, Paschalis EP, Lohmann CH, Rosser J, Cochran DL, et al. Osteoblast-mediated mineral deposition in culture is dependent on surface microtopography. Calcif Tissue Int. 2002;71(6):519–529. doi: 10.1007/s00223-001-1114-y. [DOI] [PubMed] [Google Scholar]
- 35.Schwartz Z, Lohmann CH, Oefinger J, Bonewald LF, Dean DD, Boyan BD. Implant surface characteristics modulate differentiation behavior of cells in the osteoblastic lineage. Adv Dent Res. 1999;13:38–48. doi: 10.1177/08959374990130011301. [DOI] [PubMed] [Google Scholar]
- 36.Stein GS, Lian JB, Owen TA. Relationship of cell growth to the regulation of tissue-specific gene expression during osteoblast differentiation. FASEB J. 1990;4(13):3111–3123. doi: 10.1096/fasebj.4.13.2210157. [DOI] [PubMed] [Google Scholar]
- 37.Zhao G, Schwartz Z, Wieland M, Rupp F, Geis-Gerstorfer J, Cochran DL, et al. High surface energy enhances cell response to titanium substrate microstructure. J Biomed Mater Res A. 2005;74(1):49–58. doi: 10.1002/jbm.a.30320. [DOI] [PubMed] [Google Scholar]
- 38.Zhao G, Zinger O, Schwartz Z, Wieland M, Landolt D, Boyan BD. Osteoblast-like cells are sensitive to submicron-scale surface structure. Clin Oral Implants Res. 2006;17(3):258–264. doi: 10.1111/j.1600-0501.2005.01195.x. [DOI] [PubMed] [Google Scholar]
- 39.Zinger O, Zhao G, Schwartz Z, Simpson J, Wieland M, Landolt D, et al. Differential regulation of osteoblasts by substrate microstructural features. Biomaterials. 2005;26(14):1837–1847. doi: 10.1016/j.biomaterials.2004.06.035. [DOI] [PubMed] [Google Scholar]
- 40.Lin L, Chow KL, Leng Y. Study of hydroxyapatite osteoinductivity with an osteogenic differentiation of mesenchymal stem cells. J Biomed Mater Res A. 2009;89(2):326–335. doi: 10.1002/jbm.a.31994. [DOI] [PubMed] [Google Scholar]
- 41.Muller P, Bulnheim U, Diener A, Luthen F, Teller M, Klinkenberg ED, et al. Calcium phosphate surfaces promote osteogenic differentiation of mesenchymal stem cells. J Cell Mol Med. 2008;12(1):281–291. doi: 10.1111/j.1582-4934.2007.00103.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Na K, Kim SW, Sun BK, Woo DG, Yang HN, Chung HM, et al. Osteogenic differentiation of rabbit mesenchymal stem cells in thermo-reversible hydrogel constructs containing hydroxyapatite and bone morphogenic protein-2 (BMP-2) Biomaterials. 2007;28(16):2631–2637. doi: 10.1016/j.biomaterials.2007.02.008. [DOI] [PubMed] [Google Scholar]
- 43.Tsiridis E, Bhalla A, Ali Z, Gurav N, Heliotis M, Deb S, et al. Enhancing the osteoinductive properties of hydroxyapatite by the addition of human mesenchymal stem cells, and recombinant human osteogenic protein-1 (BMP-7) in vitro. Injury. 2006;37(Suppl 3):S25–32. doi: 10.1016/j.injury.2006.08.021. [DOI] [PubMed] [Google Scholar]
- 44.Maegawa N, Kawamura K, Hirose M, Yajima H, Takakura Y, Ohgushi H. Enhancement of osteoblastic differentiation of mesenchymal stromal cells cultured by selective combination of bone morphogenetic protein-2 (BMP-2) and fibroblast growth factor-2 (FGF-2) J Tissue Eng Regen Med. 2007;1(4):306–313. doi: 10.1002/term.41. [DOI] [PubMed] [Google Scholar]
- 45.Kang Q, Song WX, Luo Q, Tang N, Luo J, Luo X, et al. A comprehensive analysis of the dual roles of BMPs in regulating adipogenic and osteogenic differentiation of mesenchymal progenitor cells. Stem Cells Dev. 2009;18(4):545–559. doi: 10.1089/scd.2008.0130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126(4):677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]