Abstract
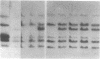
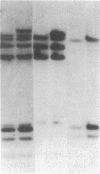
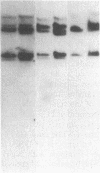
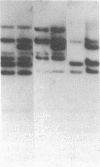
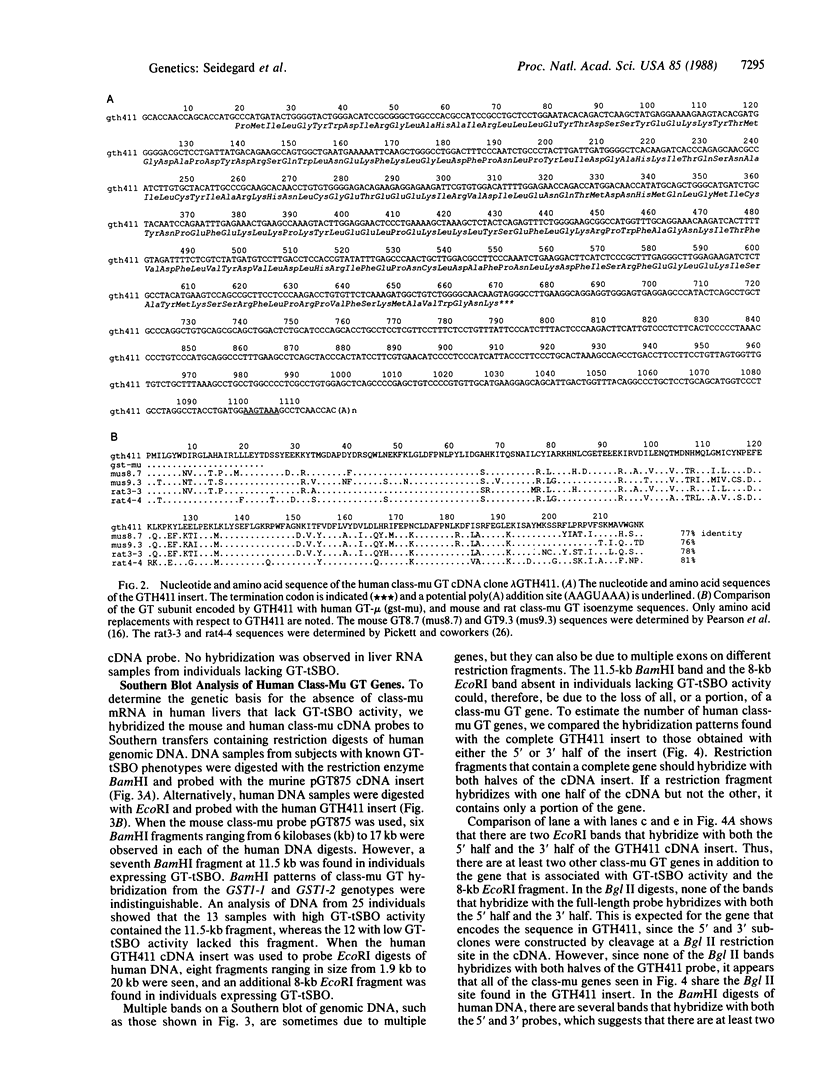
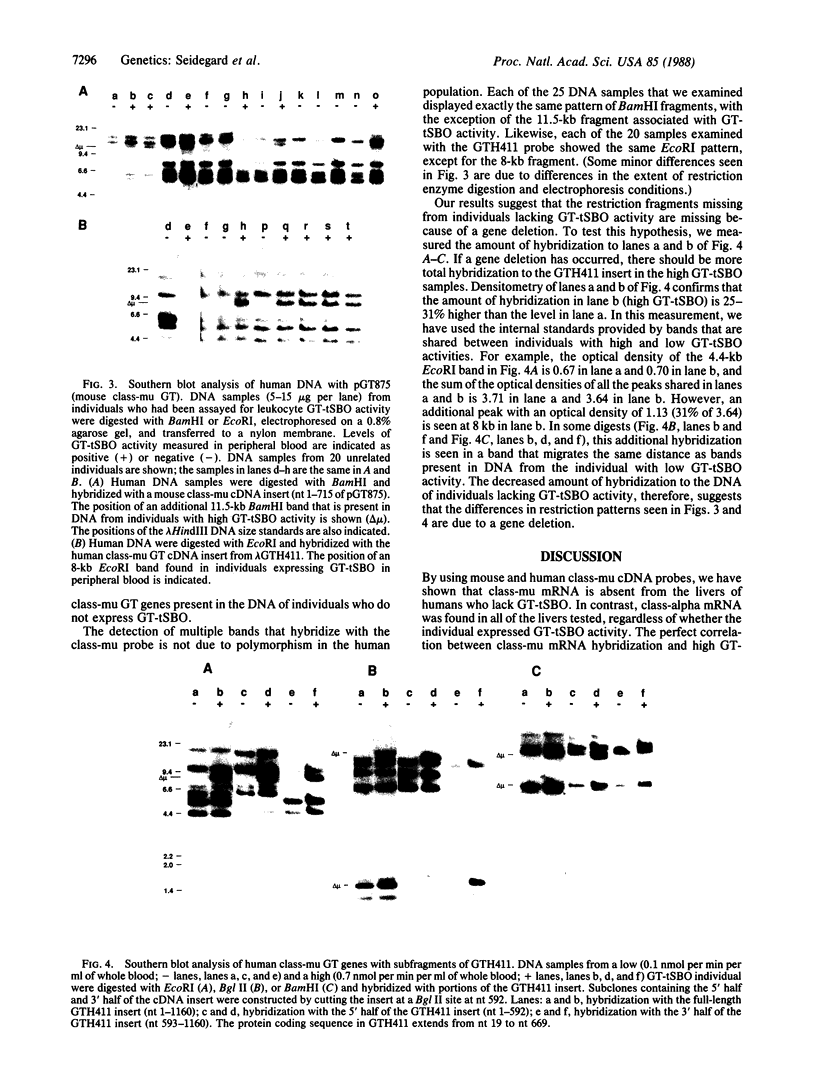
Glutathione transferase (GT; EC 2.5.1.18) mRNA levels were measured in human liver samples by using mouse and human cDNA clones that encode class-mu and class-alpha GT. Although all the RNA samples examined contained class-alpha GT mRNA, class-mu GT mRNA was found only in individuals whose peripheral leukocytes expressed GT activity on the substrate trans-stilbene oxide. The mouse class-mu cDNA clone was used to identify a human class-mu GT cDNA clone, lambda GTH411. The amino acid sequence of the GT encoded by lambda GTH411 is identical with the 23 residues determined for the human liver GT-mu isoenzyme and shares 76-81% identity with mouse and rat class-mu GT isoenzymes. The mouse and human class-mu GT cDNA inserts hybridize with multiple BamHI and EcoRI restriction fragments in the human genome. One of these hybridizing fragments is missing in the DNA of individuals who lack GT activity on trans-stilbene oxide. Hybridizations with nonoverlapping subfragments of lambda GTH411 suggest that there are at least three class-mu genes in the human genome. One of these genes appears to be deleted in individuals lacking GT activity on trans-stilbene oxide.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Board P. G. Biochemical genetics of glutathione-S-transferase in man. Am J Hum Genet. 1981 Jan;33(1):36–43. [PMC free article] [PubMed] [Google Scholar]
- Board P. G. Gene deletion and partial deficiency of the glutathione S-transferase (ligandin) system in man. FEBS Lett. 1981 Nov 30;135(1):12–14. doi: 10.1016/0014-5793(81)80933-7. [DOI] [PubMed] [Google Scholar]
- Board P. G., Webb G. C. Isolation of a cDNA clone and localization of human glutathione S-transferase 2 genes to chromosome band 6p12. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2377–2381. doi: 10.1073/pnas.84.8.2377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Church G. M., Gilbert W. Genomic sequencing. Proc Natl Acad Sci U S A. 1984 Apr;81(7):1991–1995. doi: 10.1073/pnas.81.7.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dao D. D., Partridge C. A., Kurosky A., Awasthi Y. C. Human glutathione S-transferases. Characterization of the anionic forms from lung and placenta. Biochem J. 1984 Jul 1;221(1):33–41. doi: 10.1042/bj2210033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding G. J., Ding V. D., Rodkey J. A., Bennett C. D., Lu A. Y., Pickett C. B. Rat liver glutathione S-transferases. DNA sequence analysis of a Yb2 cDNA clone and regulation of the Yb1 and Yb2 mRNAs by phenobarbital. J Biol Chem. 1986 Jun 15;261(17):7952–7957. [PubMed] [Google Scholar]
- Jakoby W. B., Ketterer B., Mannervik B. Glutathione transferases: nomenclature. Biochem Pharmacol. 1984 Aug 15;33(16):2539–2540. doi: 10.1016/0006-2952(84)90621-x. [DOI] [PubMed] [Google Scholar]
- Laisney V., Nguyen Van Cong, Gross M. S., Frezal J. Human genes for glutathione S-transferases. Hum Genet. 1984;68(3):221–227. doi: 10.1007/BF00418392. [DOI] [PubMed] [Google Scholar]
- Mannervik B., Alin P., Guthenberg C., Jensson H., Tahir M. K., Warholm M., Jörnvall H. Identification of three classes of cytosolic glutathione transferase common to several mammalian species: correlation between structural data and enzymatic properties. Proc Natl Acad Sci U S A. 1985 Nov;82(21):7202–7206. doi: 10.1073/pnas.82.21.7202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannervik B. The isoenzymes of glutathione transferase. Adv Enzymol Relat Areas Mol Biol. 1985;57:357–417. doi: 10.1002/9780470123034.ch5. [DOI] [PubMed] [Google Scholar]
- Pearson W. R., Mukai T., Morrow J. F. Repeated DNA sequences near the 5'-end of the silk fibroin gene. J Biol Chem. 1981 Apr 25;256(8):4033–4041. [PubMed] [Google Scholar]
- Pearson W. R., Windle J. J., Morrow J. F., Benson A. M., Talalay P. Increased synthesis of glutathione S-transferases in response to anticarcinogenic antioxidants. Cloning and measurement of messenger RNA. J Biol Chem. 1983 Feb 10;258(3):2052–2062. [PubMed] [Google Scholar]
- Rhoads D. M., Zarlengo R. P., Tu C. P. The basic glutathione S-transferases from human livers are products of separate genes. Biochem Biophys Res Commun. 1987 May 29;145(1):474–481. doi: 10.1016/0006-291x(87)91345-3. [DOI] [PubMed] [Google Scholar]
- Seidegård J., DePierre J. W., Pero R. W. Hereditary interindividual differences in the glutathione transferase activity towards trans-stilbene oxide in resting human mononuclear leukocytes are due to a particular isozyme(s). Carcinogenesis. 1985 Aug;6(8):1211–1216. doi: 10.1093/carcin/6.8.1211. [DOI] [PubMed] [Google Scholar]
- Seidegård J., Guthenberg C., Pero R. W., Mannervik B. The trans-stilbene oxide-active glutathione transferase in human mononuclear leucocytes is identical with the hepatic glutathione transferase mu. Biochem J. 1987 Sep 15;246(3):783–785. doi: 10.1042/bj2460783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidegård J., Pero R. W., Miller D. G., Beattie E. J. A glutathione transferase in human leukocytes as a marker for the susceptibility to lung cancer. Carcinogenesis. 1986 May;7(5):751–753. doi: 10.1093/carcin/7.5.751. [DOI] [PubMed] [Google Scholar]
- Seidegård J., Pero R. W. The hereditary transmission of high glutathione transferase activity towards trans-stilbene oxide in human mononuclear leukocytes. Hum Genet. 1985;69(1):66–68. doi: 10.1007/BF00295531. [DOI] [PubMed] [Google Scholar]
- Strange R. C., Faulder C. G., Davis B. A., Hume R., Brown J. A., Cotton W., Hopkinson D. A. The human glutathione S-transferases: studies on the tissue distribution and genetic variation of the GST1, GST2 and GST3 isozymes. Ann Hum Genet. 1984 Jan;48(Pt 1):11–20. doi: 10.1111/j.1469-1809.1984.tb00829.x. [DOI] [PubMed] [Google Scholar]
- Suguoka Y., Kano T., Okuda A., Sakai M., Kitagawa T., Muramatsu M. Cloning and the nucleotide sequence of rat glutathione S-transferase P cDNA. Nucleic Acids Res. 1985 Sep 11;13(17):6049–6057. doi: 10.1093/nar/13.17.6049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki T., Coggan M., Shaw D. C., Board P. G. Electrophoretic and immunological analysis of human glutathione S-transferase isozymes. Ann Hum Genet. 1987 May;51(Pt 2):95–106. doi: 10.1111/j.1469-1809.1987.tb01051.x. [DOI] [PubMed] [Google Scholar]
- Tu C. P., Qian B. Human liver glutathione S-transferases: complete primary sequence of an Ha subunit cDNA. Biochem Biophys Res Commun. 1986 Nov 26;141(1):229–237. doi: 10.1016/s0006-291x(86)80358-8. [DOI] [PubMed] [Google Scholar]
- Vander Jagt D. L., Hunsaker L. A., Garcia K. B., Royer R. E. Isolation and characterization of the multiple glutathione S-transferases from human liver. Evidence for unique heme-binding sites. J Biol Chem. 1985 Sep 25;260(21):11603–11610. [PubMed] [Google Scholar]
- Whitlock J. P., Jr The regulation of cytochrome P-450 gene expression. Annu Rev Pharmacol Toxicol. 1986;26:333–369. doi: 10.1146/annurev.pa.26.040186.002001. [DOI] [PubMed] [Google Scholar]