Abstract
We investigated the effects of a γ-tocopherol–rich mixture of tocopherols (γ-TmT, containing 57% γ-T, 24% δ-T, and 13% α-T) on colon carcinogenesis in azoxymethane (AOM)/dextran sulfate sodium (DSS)–treated mice. In experiment 1, 6-week-old male CF-1 mice were given a dose of AOM (10 mg/kg body weight, i.p.), and 1 week later, 1.5% DSS in drinking water for 1 week. The mice were maintained on either a γ-TmT (0.3%)–enriched or a standard AIN93M diet, starting 1 week before the AOM injection, until the termination of experiment. In the AOM/DSS–treated mice, dietary γ-TmT treatment resulted in a significantly lower colon inflammation index (52% of the control) on day 7 and number of colon adenomas (9% of the control) on week 7. γ-TmT treatment also resulted in higher apoptotic index in adenomas, lower prostaglandin E2, leukotriene B4, and nitrotyrosine levels in the colon, and lower prostaglandin E2, leukotriene B4, and 8-isoprostane levels in the plasma on week 7. Some of the decreases were observed even on day 7. In experiment 2 with AOM/DSS–treated mice sacrificed on week 21, dietary 0.17% or 0.3% γ-TmT treatment, starting 1 week before the AOM injection, significantly inhibited adenocarcinoma and adenoma formation in the colon (to 17–33% of the control). Dietary 0.3% γ-TmT that was initiated after DSS treatment also exhibited a similar inhibitory activity. The present study showed that γ-TmT effectively inhibited colon carcinogenesis in AOM/DSS–treated mice, and the inhibition may be due to the apoptosis-inducing, anti-inflammatory, antioxidative, and reactive nitrogen species–trapping activities of tocopherols.
Tocopherols are a family of phenolic compounds, each containing a chromanol ring system and a 16-carbon phytyl tail. These lipophilic compounds are synthesized by plants to serve as free radical scavengers (i.e., chain-breaking antioxidants) and are an important group of dietary antioxidants for humans (1, 2). γ-Tocopherol (γ-T) and α-tocopherol (α-T) are the major dietary tocopherols present in vegetable oils, such as oils from soybean, corn, and cottonseeds and nuts (3, 4). α-T is trimethylated at the 5-, 7-, and 8-positions of the chroman ring, whereas γ-T is dimethylated at the 7- and 8-positions. The structures of these tocopherols are shown in Fig. 1A. Although γ-T is more abundant than α-T in the human diet, the latter is the major tocopherol found in human tissues. α-T has been traditionally considered “the” vitamin E because of its superior activity over other tocopherols in the classic fertility restoration assay (1). However, as pointed out by several reviews, γ-T has stronger antioxidative and anti-inflammatory activities than α-T and may be more effective in the prevention of cardiovascular diseases, neurodegenerative diseases, and cancers (2, 5, 6).
Fig. 1.
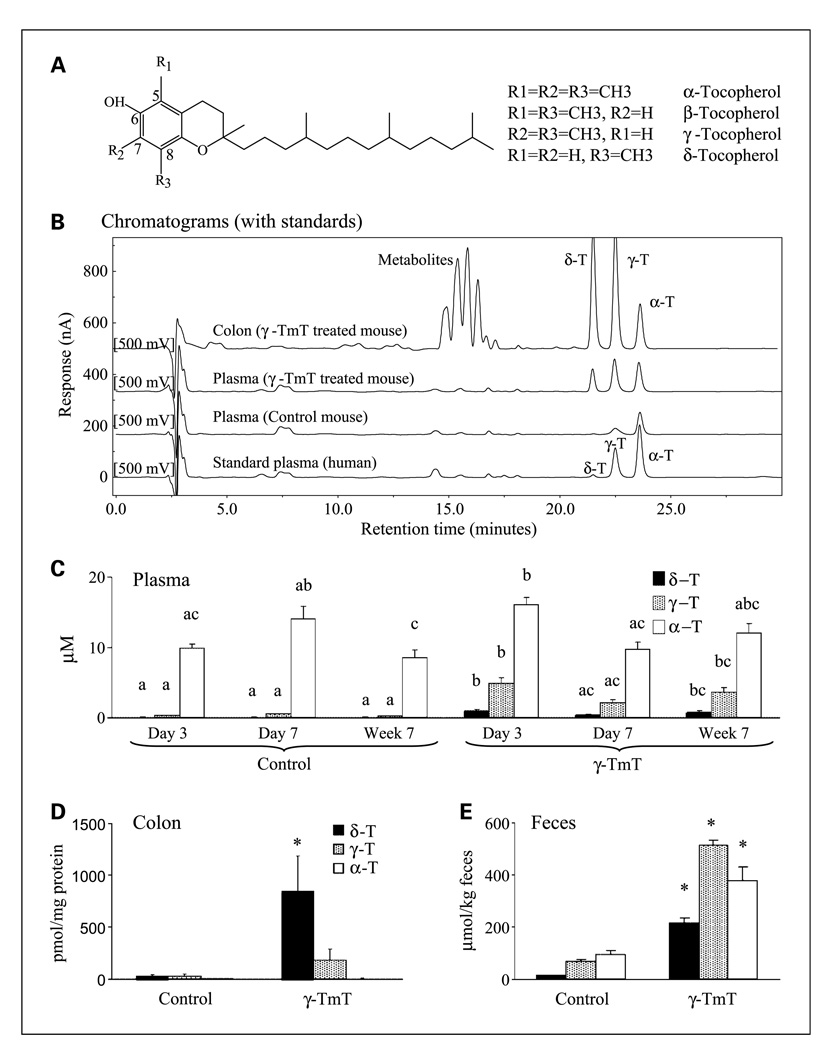
Structures of tocopherols (A) and effects of γ-TmT treatment (0.3% in the diet) on tocopherol levels in the plasma, colon tissues, and feces of the AOM/DSS–treated mice. Samples from experiment 1 were used. Levels of α-T, γ-T, and δ-T were determined according to Materials and Methods, and typical chromatograms of mouse samples and standard plasma (from human) at the 500 mV channel are shown (B). The detection limit of each tocopherol in our analysis was 0.02 ng. For a clear illustration, the baseline of each sample is set at an arbitrary level. Levels of α-T, γ-T, and δ-T in the plasma (C, n = 10/group) of AOM/DSS–treated mice sacrificed at day 3, day 7, and week 7, as well as in the colon homogenates (D, n = 9/group) and feces (E, n = 3/group) of AOM/DSS–treated mice on week 7, are shown as mean ± SE of each group. All three tocopherols were detected in all the samples, but the value of certain tocopherols were too low to be shown in the figures. Several unknown peaks between 14 and 18 min were observed in the colon samples from the γ-TmT–treated mice (B). Different superscripts (a, b, and c) indicate statistical difference among levels of each specific tocopherol (by one-way ANOVA; P < 0.05). *, statistical difference between the γ-TmT–treated and the control group in a specific tocopherol (P < 0.05 by the two-tailed t test).
In a study with healthy subjects, supplementation with a γ-T–rich mixture of tocopherols was more effective than with α-T at preventing platelet aggregation (7). In the Women's Health Study, supplementation with α-T did not show a protective effect against cardiovascular disease or cancer (8). In another trial, α-T supplementation produced unexpected adverse effects on the occurrence of second primary cancers and on cancer-free survival (9). It is known that excessive intake of α-T decreases blood levels of γ-T because of their competition for tocopherol transfer proteins; γ-T has a lower affinity for this protein and is excreted more extensively (5). One speculation is that the adverse effects of supplementation with large doses of α-T are caused by its lowering of the level of γ-T in the body.
Higher blood levels of γ-T have been correlated with lowered risk of prostate cancer in two nested case-control studies (CLUE I and CLUE II; ref. 10), but such an association has not been observed in the Physician's Healthy Study (11). In a nested-control study on Japanese Americans in Hawaii, higher serum γ-T levels have been nonsignificantly associated with lower prostate cancer risk and significantly associated with lower risk for cancers of the upper aerodigestive tract (12). Several studies have indicated that, compared with α-T, γ-T is far more effective at inhibiting cyclooxygenase (13) activity, pro-inflammatory eicosanoids formation, inflammatory damage, and sphingolipid synthesis (14–17), at trapping reactive nitrogen species (18) and at increasing peroxisome proliferator-activated receptor-γ expression (19, 20). γ-T has also been shown to be more effective than α-T at inhibiting the growth of colon, breast, prostate, and lung cancer cells in culture (17, 21–24). There is, however, insufficient information on the effect of tocopherols on carcinogenesis in animal models. Most studies have been conducted with α-T, the classic vitamin E, but the effects are either weak or inconsistent. For example, out of 10 studies on α-T and colon tumorigenesis, 8 have shown no effect (25–32), 1 has shown an inhibitory effect (33), and 1 has shown an enhancement effect (34). Out of five studies on α-T and mammary tumorigenesis, four studies have shown a protective effect (30, 35–37), but one study has shown no effect (38).
Recently, we showed that dietary treatment with a γ-T–rich mixture of tocopherols (γ-TmT; 0.1% in diet) significantly inhibited azoxymethane (AOM)–induced colon aberrant crypt foci formation (39) and N-methyl-N-nitrosourea–induced mammary tumorigenesis in rats (40). γ-TmT is a commercially available by-product in the refining of edible vegetable oil. Dietary supplementation with this tocopherol mixture may be beneficial, especially in a population that has inadequate dietary intake of tocopherols. It is, therefore, important to have a more thorough understanding of the cancer-preventive activities of this mixture of tocopherols.
In the present work, we investigated the effects of this γ-TmT preparation on colon carcinogenesis in mice that received treatment with AOM and dextran sulfate sodium (DSS). DSS is known to produce colonic inflammation (41) and promote colon carcinogenesis (42). This mouse model enabled us to also investigate the effects of the treatment on colonic inflammation and related mechanisms.
Materials and Methods
Animal experiments
All animal experiments will be done under protocol no. 02-027 approved by the Institutional Animal Care and Use Committee at Rutgers University. In experiment 1, male CF-1 mice at 6 wk of age (Charles River Laboratories) were given a dose of AOM (10 mg/kg body weight, i.p.) or the vehicle (sterile saline). One week later, they were given 1.5% DSS (molecular weight of 36,000–50,000, ICN Biochemicals, Inc.) in drinking water for 1 wk. The mice were maintained on either a γ-TmT–enriched AIN93M diet (0.3% in the diet) or AIN93M diet, starting 1 wk before the AOM injection (from 5 wk of age), until the experiment was terminated at 3 d, 7 d, or 7 wk after the DSS treatment (referred to as day 3, day 7, and week 7, respectively). The γ-TmT–enriched diet was formulated by adding 0.3% of γ-TmT to the semipurified AIN93M diet (Research Diets, Inc.). The γ-TmT used was a mixture containing 57% γ-T, 24% δ-T, 13% α-T, and ~0.5% β-T (Cognis Corporation). Body weights, food and fluid consumption, and general health status were monitored weekly. For terminating the experiment, mice were sacrificed by CO2 asphyxiation. Blood was taken by cardiac puncture and centrifuged for 15 min at 3,000 × g. Plasma was then collected and frozen at −80°C. At necropsy, colons were removed, washed with ice-cold saline, cut open longitudinally from anus to cecum, and flattened on filter paper. Proximal colons (~2cm segment), which did not contain any visible tumors, were cut and frozen at −80°C for biochemical analyses. The rest of the colon was then fixed in 10% buffered formalin for 24 h for histopathologic analyses. Spleens and livers were removed, washed with ice-cold saline, blotted, and weighed.
In experiment 2, to compare the effects of different γ-TmT treatments on the development of adenocarcinomas, male CF-1 mice at 6 wk of age were given AOM (5 mg/kg body weight, i.p.) twice at 4 d interval or the vehicle (sterile saline). One week later, they were given 1.5% DSS in drinking water for 1 wk. The mice were maintained on 0.17% γ-TmT, 0.3% γ-TmT, or the standard AIN93M diet, starting 1 wk before the first AOM injection (from 5 wk of age), until the experiment was terminated at 21 wk after the DSS treatment. In another group, 0.3% γ-TmT treatment was initiated after the 1 wk DSS treatment. Body weights, food and fluid consumption, and general health status were monitored weekly. At the end of experiment, mice were sacrificed by CO2 asphyxiation. Blood and tissues were harvested and stored as described above.
Histopathological and immumohistopathological analyses
The formalin-fixed colon tissues from AOM/DSS–treated mice (sacrificed at week 7) were stained with 0.2% methylene blue solution for 3 to 5 min to visualize small colon polyps. The stained polyps and tumors were then dissected with surrounding normal colon tissues, paraffin-embedded, and sectioned serially at 4-µm thickness. All of the individual tumors and polyps were evaluated histopathologically in two H&E–stained sections (sections 1 and 10) per tumor. Histopathologically confirmed tumors were then classified into three categories—colon adenomas with mild, moderate, and severe dysplasia. Colonic neoplasms and dysplasia were diagnosed according to the typical criteria described previously (43, 44). The visible colon tumors from the AOM/DSS–treated mice sacrificed at week 21 were also similarly dissected and evaluated histopathologically.
In two H&E–stained sections of the colon from AOM/DSS– or DSS-treated mice, sacrificed at 3 and 7 days after the DSS treatment, the inflammation index was determined. The inflammation index was the sum of scores of four individual inflammatory parameters: inflammation severity, ulceration, inflammation area involved, and hyperplasia and dysplasia (45, 46). The inflammation severity was scored as 0 (normal colonic mucosa), 1 (mild inflammation: either focal or wildly separated multifocal inflammation limited to the basal one third of the mucosa with lost crypts), 2(moderate inflammation: either multifocal or locally extensive inflammation and/or fibrosis up to two third of the crypts), or 3 (severe inflammation: mucosal ulcers with monocytes and polymorphonuclear leukocytes infiltrated into the mucosa, submucosa, muscularis propria and/or subserosa). The ulceration was scored as 0 (absent) or 1 (present). Ulceration was defined as an area of mucosa where the epithelial lining was missing. The inflammation area, which accounts for 0%, 1%, to 25%, 26–50%, 51–75%, or 76–100% of the surface area examined, was scored as 0, 1, 2, 3, or 4, respectively. Hyperplasia and dysplasia were scored as 0 (normal), 1 (mild hyperplasia: epithelial cells lined normally, but crypts 2 to 4 times thicker than normal crypts), 2(low-grade dysplasia: 2to 4 times thicker epithelium, hyperchromatic cells, fewer goblet cells, and scattered crypts developing an arborizing pattern), or 3 (high-grade dysplasia: >4 times thicker epithelium, hyperchromasia, few or no goblet cells, highly mitotic cells in the crypts with arborizing pattern, and crypts extended to muscularis mucosa or submucosa).
For immunohistochemistry, tissue sections were deparaffinized in xylene and rehydrated in distilled water, and the endogenous peroxidase was quenched in 0.3% hydrogen peroxide in methanol for 30 min. Subsequently, sections were subjected to antigen retrieval by heating the slides in sodium citrate buffer (0.01 mol/L, pH 6.0) in a pressure cooker for 3 min after reaching full pressure. Sections were then blocked for 1 h at room temperature in PBS containing 3% normal horse or goat serum. The sections were then immunostained with anti–cleaved caspase-3 (1: 200, Cell Signaling) antibodies overnight at room temperature. The antibodies were diluted in 10% goat or horse serum. The sections were rinsed in PBS and incubated with a biotinylated secondary antibody and subsequently incubated in Vectorstain Elite ABC reagent for 30 min, and 3,3′-diaminobenzidine (Vector Laboratories) was used as the chromogen. Sections were then counterstained for 2 to 3 min with hematoxylin (Sigma) and mounted with Permount. Apoptotic cells were identified by staining with antibodies against cleaved caspase-3. Quantification of the number of total cells and cleaved caspase-3–positive cells in adenomas was done by using the Image-Pro Plus system as described previously (47). Apoptotic indexes were then expressed as the percentage of positive cells in total adenoma cells counted.
Analysis of tocopherols by high-performance liquid chromatography
The procedure for the determination of tocopherol levels in plasma was described previously (48). The methods was further improved and used for the determination of tissue and fecal levels of tocopherols and their metabolites. In brief, plasma sample (10 µL) was mixed with 140 µL deionized water and 150 µL ethanol, and fat-soluble materials were extracted with 1 mL hexane twice. For colon and fecal samples, 10 mg of sample were mixed with 140 µL deionized water and 150 µL ethanol. After homogenization, the supernatant was extracted with 1 mL hexane twice. The material in the dried hexane extract was redissolved in 100 µL ethanol and then injected onto high-performance liquid chromatography. For high-performance liquid chromatography analysis, a Supelcosil C18 reversed-phase column (150 × 4.6 mm; 5 µm particle size) was used. For the analysis of δ-, γ-, and α-tocopherols, an isocratic mobile phase of 82% ethanol in water containing 20 mmol/L ammonium acetate (pH 4.4) was used at a flow rate of 1.2 mL/min. The eluant was monitored with an ESA 5600A Coulochem electrode array system (CEAS, ESA, Inc.) with potentials set at 200, 300, 500, and 700 mV. We have previously established a “standard plasma,” with its tocopherol concentrations determined with standard curves constructed from standards of pure α-, γ-, and δ-tocopherols from the Center for Disease Control and Prevention (Atlanta, GA; ref. 48). The tocopherol concentrations in mouse plasma and tissues were determined by comparison with the peak heights of the standard plasma. Because the δ-T level in the standard plasma was low, γ-T in the standard was used to as a surrogate for δ-T. We have established that the electrochemical responses of γ-T and δ-T are same.
Enzyme immunoassay
Procedures for prostaglandin E2 (PGE2) and leukotriene B4 (LTB4) enzyme immunoassay were the same as previously described (49). In brief, frozen colon tissues were placed into ice-cold T-PER tissue protein extraction buffer (Pierce Biotechnology) containing protease inhibitor cocktails (Sigma), indomethacin (a cyclooxygenase inhibitor, Cayman Chemical), and nordihydroguaiaretic acid (a lipoxygenase inhibitor, Sigma) and then homogenized using Polytron (Brinkmann Instruments Co.). After centrifugation for 10 min at 12,000 × g, the supernatants were retained as colon homogenates. Levels of nitrotyrosine were determined in the colon homogenate using an EIA kit from Cell Sciences, Inc., following the manufacturer's protocol. For the determination of PGE2, LTB4, and 8-isoprostane levels, the colon homogenates or plasma samples were mixed with ethyl acetate, voltexed for 30 min, and then centrifuged at 10,000 × g for 20 min (Sorvall RT 6000B). The organic layer was collected and dried using a Speed Vacuum Evaporator (VWR International, Inc.). The dried samples were then reconstituted in EIA buffer (Cayman Chemical), and levels of PGE2, LTB4, and 8-isoprostane were determined using EIA kits (Cayman Chemical).
Statistical analyses
One-way ANOVA combined with Tukey's post hoc test was used for comparisons among multiple groups. The two-tailed Student's t test was used for simple comparisons between two groups. The one-tailed Student's t test was used as a back-up test if the two-tailed t test showed a borderline significance. The Fisher's exact test was used for comparison of tumor incidence data. The Q-spread method was used for identifying outliers (50). Data point greater than the third quartile + 3(Q-spread) (Q-spread is defined as the difference between the third and first quartile) was identified as an extreme outlier.
Results
Effects of dietary γ-TmT treatment on general health and tocopherol levels in the plasma and tissues of AOM/DSS–treated mice (experiment 1)
The body weights of AOM/DSS–treated mice on γ-TmT–enriched AIN93M diet were similar to those of the control group (on AIN93M diet) throughout the experiment. The γ-TmT–treated mice looked healthy throughout the experiment and no signs of toxicity were observed. The liver weights of the γ-TmT–treated mice on week 7 did not differ from those in the control mice. Plasma alanine aminotransferase levels were not affected by γ-TmT treatment.
Some typical chromatograms of tocopherol analysis are shown in Fig. 1B. The effect of γ-TmT treatment (experiment 1) on plasma levels of tocopherols was examined at three time points (Fig. 1C). The major tocopherol found in the plasma of the control mice was α-T (~12 µmol/L). The γ-TmT treatment did not significantly change the plasma α-T levels but significantly increased the plasma levels of γ-T (4- to 23-fold) and δ-T (13- to 37-fold). The effect of treatment with γ-TmT on colonic and fecal levels of tocopherols was examined at week 7. In the colon homogenates of the control mouse (Fig. 1D), δ-T and γ-T levels (both at ~28 µmol/kg) were much higher than α-T levels (~2 µmol/kg) and the γ-TmT treatment significantly increased levels of δ-T (30 fold) and γ-T (6 fold) but did not affect the α-T levels. The feces had high levels of α-T, γ-T, and δ-T (93, 69, and 13 µmol/kg, respectively; Fig. 1E), and these levels were significantly increased by the γ-TmT treatment (4-, 7-, and 16-fold over the control, respectively). Several unknown peaks between 14 and 18 min were observed in the high-performance liquid chromatography analysis of the colon samples from the γ-TmT–treated mice (Fig. 1B), but not in those of the control mice. Several peaks between 22 and 45 min were also observed in the analysis of the fecal samples from γ-TmT–treated mice, but not in those of the control mice (data not shown).
Effect of γ-TmT on colon adenoma formation and apopotosis
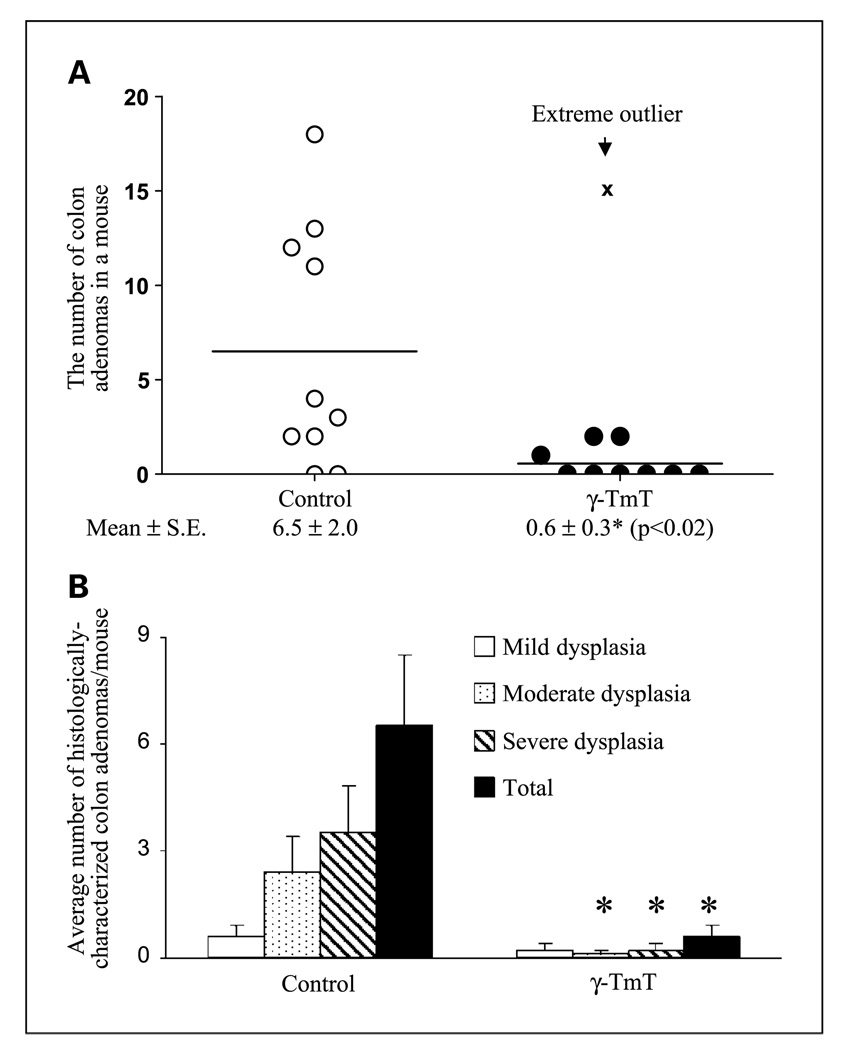
In experiment 1, the AOM/DSS–treated mice on the AIN93M diet had 6.5 ± 2.0 tumors per mouse in the colon, and dietary treatment with γ-TmT resulted in a significantly lower number of colon tumors (0.6 ± 0.3; P < 0.05; Fig. 2).
Fig. 2.
Effects of γ-TmT treatment (0.3% in the diet) on colon adenoma formation in AOM/DSS–treated CF-1 mice. Formalin-fixed visible tumors were dissected, embedded, and sectioned for histopathological analysis. Two H&E-stained sections per tumor were analyzed. All of the individual colon tumors were histologically characterized as tubular adenomas. Scatter plot for the number of colon adenomas per mouse in AOM/DSS–treated mice on week 7 (A, n = 10/group). One mouse in the γ-TmT–treated mice had 15 adenomas in the colon (x), and the number was identified as an extreme outlier (by Q-spread analysis); it was therefore excluded for subsequent statistical analysis. A two-tailed t test yields P = 0.09 and a one-tailed t test yields P = 0.04 when all animals were included. The numbers of colon adenomas with mild, moderate, and severe dysplasia per mouse are also shown as mean ± SE in B. *, the value is different from that in the control group (P ≤ 0.05 by the two-tailed t test).
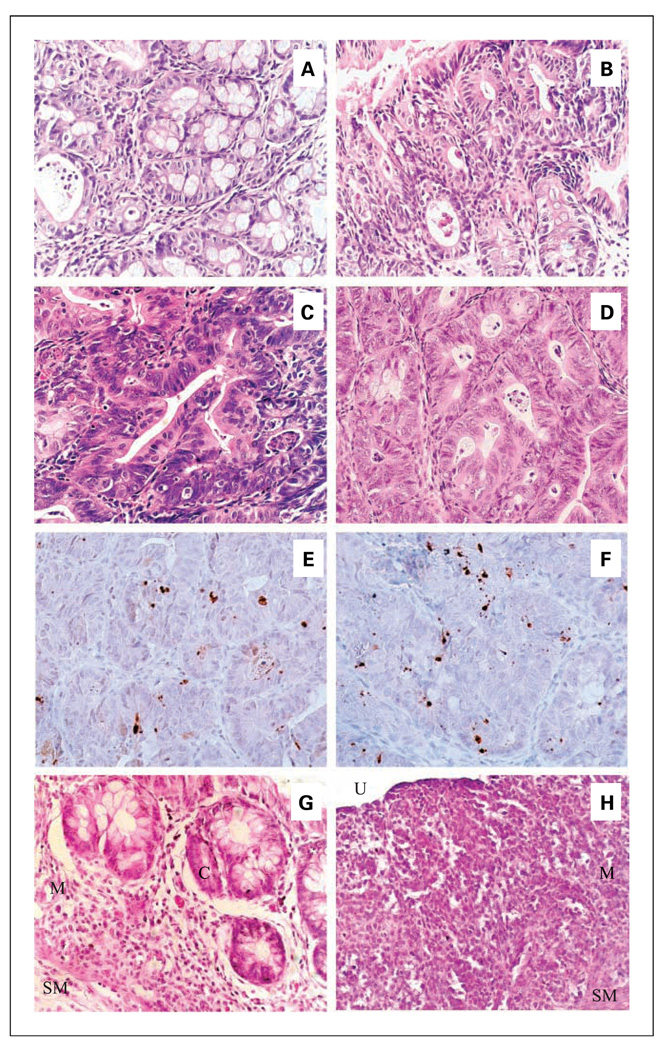
All of the macroscopic tumors (>1 mm in diameter) were found in the middle and distal regions of colons. All of the tumors were histopathologically characterized as adenomas, mostly tubular adenomas. We subsequently classified the adenomas into three different categories: mild, moderate, and severe dysplastic adenomas (Fig. 2B and 3A–C). The majority of colon adenomas in the control mice contained moderate and severe dysplasia (accounting for 34% and 54%, respectively). The γ-TmT–treated group had fewer colon adenomas than the control group, and those with mild, moderate, and severe dysplasia were lower by 63%, 95%, and 94%, respectively. Most of the methylene blue–stained small polyps (<1 mm in diameter) were identified as a gut-associated lymphoid tissue.
Fig. 3.
Histological characterization of colon adenomas and inflammation in AOM/DSS–treated mice. Tubular adenomas were classified into those with mild (A), moderate (B), and severe (C) dysplasia in the colons of AOM/DSS–treated mice on week 7 (in experiment 1). Well-differentiated adenocarcinomas (D) were found in the colons of AOM/DSS–treated mice on week 21 (in experiment 2). Magnification, ×400. Compared with adenomas from the control group (E), adenomas from the γ-TmT–treated group (F) had an increased number of cleaved caspase-3–positive cells on week 7. Cleaved caspase-3–positive cells displayed in the nucleus of adenoma cells, but rarely in that of normal cells. Magnification, ×400. Effects of γ-TmT on the colonic inflammation in the AOM/DSS–treated mice on day 7 are shown in G and H. G, mild inflammation in the colon of γ-TmT–treated mice showing mononuclear and polymorphonuclear leukocytes infiltration into the basal one third of the mucosa (M). H, severe inflammation in the colon of control AOM/DSS–treated mice showing mononuclear and polymorphonuclear leukocytes infiltration into both mucosa and submucosa (SM), loss of crypts (C, which are seen in panel G), and ulceration (U).
To determine the effect of γ-TmTon apoptosis, adenomas from γ-TmT–treated group and the control group were analyzed for apoptosis by immunohistochemistry with anti–cleaved caspase-3 antibody (Fig. 3E, F). Cleaved caspase-3–positive cells displaying nuclear staining were observed in adenomas but rarely in the normal mucosa. Apoptotic index was expressed as the percentage of cleaved caspase-3 positively stained cells among total cells in adenomas observed in each tissue section. The γ-TmT–treated group had a significantly higher apoptotic index (6.3% ± 1.7; 6 adenomas analyzed) than the control group (3.6% ± 0.4; 20 adenomas analyzed; P = 0.03). γ-TmT treatment did not cause any appreciable increase of cleaved caspase-3–positive cells in normal mucosa.
Effect of γ-TmT on colonic inflammation
In AOM/DSS–treated mice, the inflammation seemed to progress from day 3 to day 7. The γ-TmT treatment resulted in a lower inflammation index in the colon in mice sacrificed on day 7 (52% of the control; P < 0.05; Table 1; Fig. 3G, H), but not those sacrificed on day 3. Among individual inflammatory parameters, the decrease in the grade for hyperplasia and dysplasia was most prominent (P < 0.05). In the mice that received DSS only, the inflammation index on day 7, however, was significantly lower than that on day 3, apparently due to recovery from the DSS-induced damage. In the mice that only received DSS, γ-TmT treatment did not decrease the inflammation index on days 3 and 7.
Table 1.
Effects of dietary treatment with γ-TmT on colonic inflammation in AOM/DSS–treated or DSS-treated CF-1 mice
| Group (n) | Severity | Ulceration | Area involved | Hyperplasia and dysplasia | Inflammation index |
|---|---|---|---|---|---|
| 3 d after DSS treatment (day 3) | |||||
| AOM/DSS–control (10) | 1.7 ± 0.3 | 1.4 ± 0.6 | 0.8 ± 0.3 | 0.3 ± 0.2 | 4.2 ± 1.3 |
| AOM/DSS–γ-TmT (10) | 2.3 ± 0.3 | 2.8 ± 0.9 | 1.3 ± 0.4 | 0.4 ± 0.3 | 6.6 ± 1.3 |
| DSS-control (4) | 1.8 ± 0.5 | 2.8 ± 0.6 | 1.0 ± 0 | 0.3 ± 0.3 | 5.8 ± 1.1 |
| DSS-γ-TmT (5) | 2.0 ± 0.5 | 2.0 ± 0.6 | 1.8 ± 0.8 | 0.8 ± 0.4 | 6.5 ± 2.0 |
| 7 d after DSS treatment (day 7) | |||||
| AOM/DSS–control (10) | 1.9 ± 0.4 | 1.5 ± 0.4 | 1.1 ± 0.4 | 1.6 ± 0.3* | 6.1 ± 1.3 |
| AOM/DSS–γ-TmT (10) | 1.4 ± 0.3 | 0.7 ± 0.3 | 0.6 ± 0.2 | 0.5 ± 0.3† | 3.2 ± 1.0‡ |
| DSS-control (3) | 1.3 ± 0.9 | 0.3 ± 0.3§ | 0.3 ± 0.3§ | 0 ± 0‖ | 2.0 ± 1.5§ |
| DSS-γ-TmT (5) | 2.3 ± 0.7 | 2.8 ± 1.4 | 1.0 ± 0.4 | 1.3 ± 0.4‡ | 7.3 ± 2.6 |
NOTE: The samples were from experiment 1. All the analyzable field of colons were sectioned and H&E stained, and two sections per colon were analyzed. The inflammation index was the sum of the individual scores for disease severity, ulceration, area of inflammatory involvement, and grade of hyperplasia and dysplasia. Values are mean ± SE per mouse.
The value is different from that of AOM/DSS–treated group on day 3 (P < 0.01 by the two-tailed t test).
The value is different from that of the control group on day 7 (P < 0.05 by the two-tailed t test).
The value is different from that of the control group on day 7 (P < 0.05 by the one-tailed t test).
The value is different from that of the DSS-treated group on day 3 (P = 0.09 by two-tailed t test; P < 0.05 by the one-tailed t test).
The value is different from that of AOM/DSS–treated group on day 7 (P < 0.05 by the two-tailed t test).
Effects of γ-TmT on plasma and colonic PGE2 and LTB4 levels
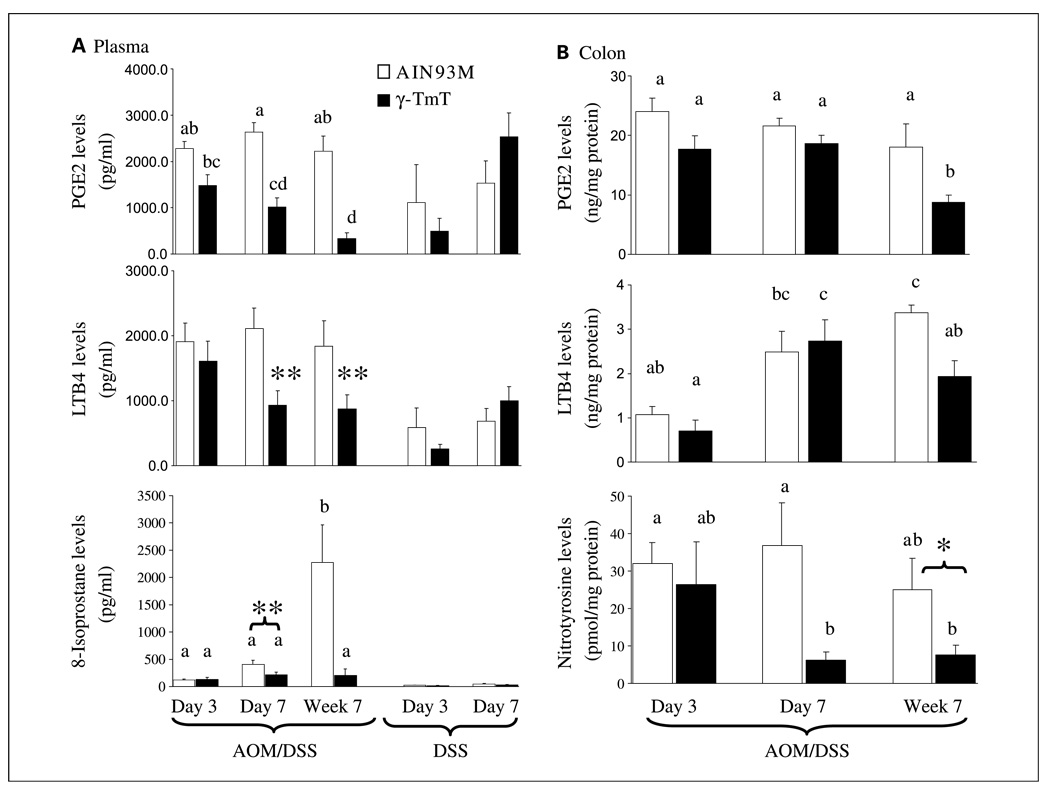
The plasma levels of PGE2 and LTB4 in AOM/DSS–treated mice on days 3 and 7 were 2- to 3-fold higher than in mice treated with DSS only (P ≤ 0.05, Fig. 4A) and 26- to 31-fold higher than in 9- to 10-week old male mice that did not receive any treatment (with PGE2: 87.7 ± 11.3 pg/mL; LTB4: 68.9 ± 12.4 pg/mL; n = 3; P < 0.006, results not shown). In AOM/DSS–treated mice, γ-TmT treatment resulted in significantly lower levels of both PGE2 and LTB4 in the plasma (15–48% of the control; P < 0.05) on day 7 and week 7. γ-TmT treatment, however, did not significantly affect the plasma levels of PGE2 and LTB4 in mice that received DSS only. In nontumorous colon homogenates of the AOM/DSS–treated mice on week 7, PGE2 and LTB4 levels were also lower in the γ-TmT–treated group than those in the control group (57% and 48% of the control, respectively; P < 0.05), but the effect was not seen at earlier time points (Fig. 4B). Tumor samples were processed for histologic analyses and were not suitable for biochemical analyses.
Fig. 4.
Effects of γ-TmT treatment on plasma or colonic levels of PGE2, LTB4, 8-isoprostane, and nitrotyrosine. A, PGE2, LTB4, and 8-isoprostane levels were determined in the plasma of AOM/DSS–treated mice (on day 3, day 7, and week 7) and DSS-treated mice (on days 3 and 7). B, PGE2, LTB4, and nitrotyrosine levels were determined in the colon (homogenates) of AOM/DSS–treated mice. Columns, mean of group (n = 9–10 per AOM/DSS–treated group; n = 4–5 per DSS-treated group); bars, SE. Different superscripts (a, b, c, d) indicate statistical difference among levels of AOM/DSS–treated mice (by one-way ANOVA; P < 0.05). Statistical difference between levels of the γ-TmT–treated and the respective control group: **, P < 0.05 by the two-tailed t test; *, P < 0.05 by the one-tailed t test (P = 0.07 by two-tailed t test).
Effects of γ-TmT on plasma 8-isoprostane and colonic nitrotyrosine levels
The plasma levels of 8-isoprostane in AOM/DSS–treated mice on days 3 and 7 were higher than those in the corresponding DSS-treated mice (P < 0.01; Fig. 4A) and much higher than those in mice that did not receive any treatment (with value of 6.9 ± 0.8 pg/mL; n = 3; P < 0.03). The plasma 8-isoprostane levels of the AOM/DSS–treated mice on week 7 were much higher than those at earlier time points, and γ-TmT treatment resulted in significantly lower levels (9% of the control; P < 0.05). The γ-TmT treatment also resulted in lower levels in the AOM/DSS–treated mice on day 7 (53% of the control; P < 0.05). The 8-isoprostane lowering effect by γ-TmT was not statistically significant in mice that received DSS only. Nitrotyrosine levels were determined in nontumorous colon homogenates of the AOM/DSS–treated mice. The levels were much lower in the γ-TmT–treated group than those in the control group (P < 0.05) on day 7 and week 7 (Fig. 4B). Under our experimental condition, nitrotyrosine levels in the plasma were not detectable.
Effects of γ-TmT on colon adenocarcinoma formation and other parameters in AOM/DSS–treated mice (experiment 2)
To investigate the effect of γ-TmT on colon adenocarcinoma formation, we conducted a longer experiment, in which male CF-1 mice were treated with the 0.3% γ-TmT, 0.17% γ-TmT, or AIN93M diet until the experiment was terminated at 21 weeks after DSS treatment (experiment 2). In this experiment, we modified the AOM dosing scheme; the mice were given AOM (5 mg/kg body weight, i.p.) twice at 4 days interval. The control mice (AOM/DSS–treated mice on AIN93M diet) on week 21 had 2.4 ± 0.8 tumors per mouse in the colon, and 75% of the tumors were histopathologically identified as well-differentiated adenocarcinomas (1.8 ± 0.7 per mouse; Table 2). Dietary treatment with 0.3% and 0.17% γ-TmT, starting 1 week before the first AOM injection, resulted in significantly lower numbers of colon tumors (adenocarcinomas lowered by 83% and 67%, respectively). Dietary 0.3% γ-TmT treatment that was initiated after the 1 week DSS treatment also exhibited similar inhibitory activity (adenocarcinomas lowered by 78%).
Table 2.
Effects of dietary γ-TmT treatments on colon adenocarcinoma and adenoma formation in AOM/DSS–treated mice
| Group | Visible tumors | No. histologically characterized tumors/mouse | |||
|---|---|---|---|---|---|
| % Mice | No./mouse | Adenocarcinomas | Adenomas | Total | |
| Control | 41.2 (7/17) | 2.4 ± 0.8 | 1.8 ± 0.7 | 0.6 ± 0.3 | 2.4 ± 0.8 |
| 0.17% γ-TmT | 15.8 (3/19) | 0.6 ± 0.5* | 0.6 ± 0.5 | 0.1 ± 0.1† | 0.6 ± 0.5* |
| 0.3% γ-TmT | 12.5 (2/16) | 0.3 ± 0.2† | 0.3 ± 0.2† | 0.1 ± 0.1† | 0.3 ± 0.2† |
| Control → 0.3% γ-TmT | 15.8 (3/19) | 0.5 ± 0.3† | 0.4 ± 0.3* | 0.1 ± 0.1† | 0.5 ± 0.3† |
NOTE: Results of experiment 2. The mice were maintained on the 0.17% γ-TmT, 0.3% γ-TmT, or AIN93M diet, starting 1 wk before the AOM injection (from 5 wk of age), until the experiment was terminated at 21 wk after the DSS treatment. The control → 0.3% γ-TmT group received 0.3% γ-TmT diet after the DSS treatment. Formalin-fixed visible tumors were dissected, embedded, sectioned, and H&E-stained for histopathologic analysis. All of the visible tumors scored were histologically confirmed as adenomas or adenocarcinomas. Values are mean ± SE per mouse.
P < 0.05 by one-tailed t test (P = 0.07–0.09 by two-tailed t test).
P < 0.05 by the two-tailed t test.
The plasma levels of PGE2, LTB4, and 8-isoprostane in AOM/DSS–treated mice on week 21 were 2,150.8 ± 29.1, 2,021.0 ± 564.2, and 1,107.4 ± 97.3 pg/mL, respectively, and all three γ-TmT treatment groups resulted in significantly lower levels of PGE2(t o <1% of the control), LTB4 (to 19–24% of the control), and 8-isoprostane (to 1.1–2.3% of the control) in the plasma (P < 0.001; data not shown in Table 2). In nontumorous colon homogenates of the AOM/DSS–treated mice, PGE2, LTB4, and nitrotyrosine levels were also lower in the γ-TmT–treated groups than those in the control group (38–89% of the control; P < 0.05).
The effects of dietary γ-TmT treatments on plasma, liver, and colon levels of tocopherols were also examined. As expected, the major tocopherol found in the control mice was α-T. Both the 0.17% and 0.3% γ-TmT treatments significantly increased plasma levels of γ-T (5-fold of the control), δ-T (17- to 21-fold), and even α-T (~2-fold), but no clear dose-dependent increases were found. Hepatic levels of γ-T and δ-T, but not α-T, were significantly increased by the γ-TmT treatments. The γ-TmT treatments increased γ-T (24- to 29-fold) and δ-T (52- to 66-fold) in the colon; both tocopherols were in the concentration range of 10 to 13 µmol/kg in the γ-TmT–treated mice.
Discussion
In the present study, we showed that dietary γ-TmT (0.3% and 0.17% in AIN93M diet) significantly inhibits colon inflammation and carcinogenesis in AOM/DSS–treated mice. We also found that the 0.3% γ-TmT treatment that began after the DSS treatment resulted in a similar inhibition in colon carcinogenesis (Table 2), suggesting that γ-TmT inhibits colon carcinogenesis at the postinitiation stage. To our knowledge, this is the first report on the inhibitory activity of a mixed tocopherol preparation on colon inflammation and carcinogenesis. The inhibitory activity of γ-TmT against colon carcinogenesis found in the present study was as strong as the inhibitory activities of other chemopreventive agents, such as a cyclooxygenase-2inhibitor (nimesulide), peroxisome proliferator-activated receptor ligands (triglitazone and bezafibrate), and a statin (pivastatin), in the AOM/DSS–treated mouse model (51, 52). This study is also the first study that systemically analyze blood and tissue levels of tocopherols in a carcinogenesis model. None of the γ-TmT treatments affected body weights, liver weights, or plasma alanine aminotransferase levels.
The 0.3% γ-TmT diet that we used contained ~74-, 44-, 8-, and 45-fold higher levels of γ-T, δ-T, α-T, and β-T, respectively, than those in the AIN93M diet (~2 4 mg γ-T, 15 mg δ-T, 82 mg α-T, and 1 mg β-T per kg diet). The dietary treatments with γ-TmT significantly increased γ-T and δ-T levels in the plasma, colon, and liver. The γ-TmT treatments did not significantly affect the α-T levels in the tissues, although prolonged γ-TmT treatments (in experiment 2) increased the plasma α-T levels (~2-fold of the control level). Dietary tocopherols are absorbed from the intestine as chylomicrons and transported to the liver via the lymphatic system. It has been suggested that the α-T transfer protein in the liver selectively transfers α-T to very low-density lipoproteins; α-T, therefore, preferentially enters into the circulation (53). This explains our results that α-T levels were higher than δ- and γ-T levels in the plasma of mice that received the control diet or even the γ-TmT (Fig. 1C). γ-T and δ-T were not effectively absorbed and their levels were high in the colon of the γ-TmT–treated mice (Fig. 1D). Several metabolites were observed in the colon tissues (Fig. 1B) and feces; those could be products with degraded phytyl chain or nitrated γ-T or δ-T. All the metabolites need to be further identified.
The inhibitory activity of α-T on colon tumorigenesis is either weak or inconsistent in previous studies (25–32). γ-T has been shown to have stronger antioxidative and anti-inflammatory activities than α-T (2, 5, 6). It has also been shown to be more effective than α-T at inhibiting the growth of colon, breast, prostate, and lung cancer cells (17, 21–24). γ-T is, therefore, likely the major tocopherol of the mixture that conferred the inhibitory activity against colon carcinogenesis. It is also possible that the interaction of γ-T with other tocopherols produced the strong inhibitory activity of the mixture. Because of the high concentration of γ-T and δ-T observed in the colon, it is interesting to suggest that γ-T, δ-T, and the interaction between these tocopherols play important roles in the inhibitory effect against colon carcinogenesis. Jiang et al. (15) showed that there was a possible synergy between γ-T and δ-T in inhibiting the growth of prostate cancer cells. We have observed in our preliminary cell culture study using HT29 and HCT116 colon cancer cell lines that the treatment with γ-T or δ-T (at the concentration of 25–75 µmol/L δ-T or γ-T for 48–72h) inhibited the growth of cell, whereas α-T was rather inactive.3
In this study, we characterized the colonic inflammation process in the AOM/DSS model, and these results suggest that the pretreatment of AOM prevents the resolution of DSS-induced inflammation and further promotes hyperplasia and dysplasia (Table 1). Pro-inflammatory eicosanoids, PGE2 and LTB4, are implicated in both inflammation and carcinogenesis. We found that γ-TmT treatments resulted in significantly decreased levels of PGE2 and LTB4 in the plasma and the nontumorous colon tissue of the AOM/DSS–treated mice (Fig. 4). The levels of key enzymes that were involved in the production of PGE2 and LTB4 (i.e., cytoplasmic phospholipase 2, cyclooxygenase-2, and 5-lipoxygenase) in the nontumorous colon homogenates, however, were not significantly affected by the γ-TmT treatment (data not shown). The biochemical analysis of nontumorous colon tissues should provide information on the preventive action of γ-TmT. The decreases in the PGE2 levels may be due to the inhibition of cyclooxygenase-2 activity by γ-T, as shown by Jiang et al. in lipopolysaccharide-stimulated macrophages and interleukin 1β–activated epithelial cells (15). Alternatively, the decreased levels of PGE2 and LTB4 are a consequence of the decreased inflammation or carcinogenesis. We found that the plasma levels of PGE2 and LTB4 in the AOM/DSS–treated mice were higher than in the mice treated with DSS only and much higher than in mice that did not receive any treatment (Fig. 4A), suggesting that DSS treatment elevates the levels of pro-inflammatory eicosanoids and the pretreatment with AOM further elevates the levels.
Tocopherols, as antioxidants, can scavenge reactive oxygen and nitrogen species. Isoprostanes are a family of eicosanoids that are produced by the random oxidation of phospholipids. 8-Isoprostane (8-isoPGF2α) is implicated as a causative mediator of pulmonary oxygen toxicity (54), and its level is elevated in heavy smokers (55). The plasma 8-isoprostane levels of the AOM/DSS–treated mice were higher than in the corresponding DSS-treated mice and markedly higher than in the mice that did not receive any treatment. The plasma 8-isoprostane levels of the AOM/DSS–treated mice on week 7 were much higher than those at earlier time points (Fig. 4A). The γ-TmT treatment resulted in significantly decreased 8-isoprostane levels on day 7, week 7, and week 21. These results suggest that oxidative stress is increased during tumorigenesis and it is inhibited by γ-TmT. Nitrotyrosine is a biomarker of NO-mediated protein modification and is commonly used to detect NO-mediated cellular damage. Colonic nitrotyrosine levels were markedly decreased in the γ-TmT–treated mice on day 7 and week 7 (Fig. 4B) and moderately decreased in the γ-TmT–treated mice on week 21, suggesting that the γ-TmT treatment inhibited nitrosative stress. The inhibition of protein nitration may be partially due to the NOxtrapping capability of γ-T, as previously shown in a rat model of zymosan-induced peritonitis (18) and in other experimental systems (56–59).
In summary, we showed that the dietary γ-TmT treatment (0.3% and 0.17% in AIN93M diet) significantly inhibited colon carcinogenesis in AOM/DSS–treated mice. We also systematically characterized the inflammation process in this model and showed the anti-inflammatory activity of γ-TmT. The inhibition may be due to the apoptosis-inducing, anti-inflammatory, antioxidative, and reactive nitrogen species–trapping activities of tocopherols, especially of γ-T and δ-T, which are present in rather higher concentration in the colon. γ-TmT is a by-product in the refining of edible vegetable oil with different tocopherols at ratios similar to the human diet. It is readily available, nontoxic, and may have a great potential for future use in cancer prevention. The present results provide the preclinical data for translation to clinical trials for the prevention of colon cancer.
Acknowledgments
We thank Anna Lu and Afsheen Khan for their help in animal studies.
Grant support: Jewels of Charity award to the Cancer Institute of New Jersey and Facility Cores supported by ES05022 and CA72720.
Footnotes
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Traber MG. Vitamin E. Baltimore (MD): Lippincott Williams and Wilkins; 2006. [Google Scholar]
- 2.Hensley K, Benaksas EJ, Bolli R, et al. New perspectives on vitamin E: γ-tocopherol and carboxyelthylhydroxychroman metabolites in biology and medicine. Free Radic Biol Med. 2004;36:1–15. doi: 10.1016/j.freeradbiomed.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 3.Eitenmiller T, Lee J. Vitamin E: food chemistry, composition, and analysis. New York: Marcel Dekker, Inc.; 2004. [Google Scholar]
- 4.U.S. Department of Commerce and U.S. Bureau of the Census. Fat and oils: production, consumption, and stocks 2004. 2005 [Google Scholar]
- 5.Dietrich M, Traber MG, Jacques PF, Cross CE, Hu Y, Block G. Does γ-tocopherol play a role in the primary prevention of heart disease and cancer? A review. J Am Coll Nutr. 2006;25:292–299. doi: 10.1080/07315724.2006.10719538. [DOI] [PubMed] [Google Scholar]
- 6.Reiter E, Jiang Q, Christen S. Anti-inflammatory properties of a- and g-tocopherol. Mol Aspects Med. 2007;5–6:668–691. doi: 10.1016/j.mam.2007.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu M, Wallmon A, Olsson-Mortlock C, Wallin R, Saldeen T. Mixed tocopherols inhibit platelet aggregation in humans: potential mechanisms. Am J Clin Nutr. 2003;77:700–706. doi: 10.1093/ajcn/77.3.700. [DOI] [PubMed] [Google Scholar]
- 8.Lee IM, Cook NR, Gaziano JM, et al. Vitamin E in the primary prevention of cardiovascular disease and cancer: the Women's Health Study: a randomized controlled trial. Jama. 2005;294:56–65. doi: 10.1001/jama.294.1.56. [DOI] [PubMed] [Google Scholar]
- 9.Bairati I, Meyer F, Gelinas M, et al. A randomized trial of antioxidant vitamins to prevent second primary cancers in head and neck cancer patients. J Natl Cancer Inst. 2005;97:481–488. doi: 10.1093/jnci/dji095. [DOI] [PubMed] [Google Scholar]
- 10.Huang HY, Alberg AJ, Norkus EP, Hoffman SC, Comstock GW, Helzlsouer KJ. Prospective study of antioxidant micronutrients in the blood and the risk of developing prostate cancer. Am J Epidemiol. 2003;157:335–344. doi: 10.1093/aje/kwf210. [DOI] [PubMed] [Google Scholar]
- 11.Gann PH, Ma J, Giovannucci E, et al. Lower prostate cancer risk in men with elevated plasma lycopene levels: results of a prospective analysis. Cancer Res. 1999;59:1225–1230. [PubMed] [Google Scholar]
- 12.Nomura AM, Stemmermann GN, Lee J, Craft NE. Serum micronutrients and prostate cancer in Japanese Americans in Hawaii. Cancer Epidemiol Biomarkers Prev. 1997;6:487–491. [PubMed] [Google Scholar]
- 13.Yong LC, Brown CC, Schatzkin A, et al. Intake of vitamins E, C, and A and risk of lung cancer. The NHANES I epidemiologic follow-up study. First National Health and Nutrition Examination Survey. Am J Epidemiol. 1997;146:231–243. doi: 10.1093/oxfordjournals.aje.a009258. [DOI] [PubMed] [Google Scholar]
- 14.O'Leary KA, de Pascual-Tereasa S, Needs PW, Bao YP, O'Brien NM, Williamson G. Effect of flavonoids and vitamin E on cyclooxygenase-2 (COX-2) transcription. Mutat Res. 2004;551:245–254. doi: 10.1016/j.mrfmmm.2004.01.015. [DOI] [PubMed] [Google Scholar]
- 15.Jiang Q, Elson-Schwab I, Courtemanche C, Ames BN. γ-Tocopherol and its major metabolite, in contrast to α-tocopherol, inhibit cyclooxygenase activity in macrophages and epithelial cells. Proc Natl Acad Sci U S A. 2000;97:11494–11499. doi: 10.1073/pnas.200357097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jiang Q, Ames BN. γ-Tocopherol, but not α-tocopherol, decreases proinflammatory eicosanoids and inflammation damage in rats. FASEB J. 2003;17:816–822. doi: 10.1096/fj.02-0877com. [DOI] [PubMed] [Google Scholar]
- 17.Jiang Q, Wong J, Fyrst H, Saba JD, Ames BN. γ-Tocopherol or combinations of vitamin E forms induce cell death in human prostate cancer cells by interrupting sphingolipid synthesis. Proc Natl Acad Sci U S A. 2004;101:17825–17830. doi: 10.1073/pnas.0408340102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jiang Q, Lykkesfeldt J, Shigenaga MK, Shigeno ET, Christen S, Ames BN. γ-Tocopherol supplementation inhibits protein nitration and ascorbate oxidation in rats with inflammation. Free Radic Biol Med. 2002;33:1534–1542. doi: 10.1016/s0891-5849(02)01091-2. [DOI] [PubMed] [Google Scholar]
- 19.De Pascale MC, Bassi AM, Patrone V, Villacorta L, Azzi A, Zingg JM. Increased expression of transglutaminase-1 and PPARγ after vitamin E treatment in human keratinocytes. Arch Biochem Biophys. 2006;447:97–106. doi: 10.1016/j.abb.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 20.Campbell SE, Stone WL, Whaley SG, Qui M, Krishnan K. Gamma (γ) tocopherol upregulates peroxisome proliferator activated receptor (PPAR) gamma (γ) expression in SW 480 human colon cancer cell lines. BMC Cancer. 2003;3:25. doi: 10.1186/1471-2407-3-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Campbell SE, Stone WL, Lee S, et al. Comparative effects of RRR-α- and RRR-γ-tocopherol on proliferation and apoptosis in human colon cancer cell lines. BMC Cancer. 2006;6:13. doi: 10.1186/1471-2407-6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yu W. Evaluation of the anticancer actions of natural vitamin E forms RRR-α-tocopherol and γ-tocopherol; Abstract for poster #68 at the ACS International Research Conference on Food, Nutrition and Cancer; July 13–14, 2006; Washington, DC. 2006. [Google Scholar]
- 23.Gysin R, Azzi A, Visarius T. γ-Tocopherol inhibits human cancer cell cycle progression and cell proliferation by down-regulation of cyclins. FASEB J. 2002;16:1952–1954. doi: 10.1096/fj.02-0362fje. [DOI] [PubMed] [Google Scholar]
- 24.Jiang Q, Wong J, Ames BN. γ-Tocopherol induces apoptosis in androgen-responsive LNCaP prostate cancer cells via caspase-dependent and independent mechanisms. Ann N Y Acad Sci. 2004;1031:399–400. doi: 10.1196/annals.1331.056. [DOI] [PubMed] [Google Scholar]
- 25.Reddy BS, Tanaka T. Interactions of selenium deficiency, vitamin E, polyunsaturated fat, and saturated fat on azoxymethane-induced colon carcinogenesis in male F344 rats. J Natl Cancer Inst. 1986;76:1157–1162. [PubMed] [Google Scholar]
- 26.Chester JF, Gaissert HA, Ross JS, Malt RA, Weitzman SA. Augmentation of 1,2-dimethylhydrazine-induced colon cancer by experimental colitis in mice: role of dietary vitamin E. J Natl Cancer Inst. 1986;76:939–942. [PubMed] [Google Scholar]
- 27.Temple NJ, el-Khatib SM. Cabbage and vitamin E: their effect on colon tumor formation in mice. Cancer Lett. 1987;35:71–77. doi: 10.1016/0304-3835(87)90058-9. [DOI] [PubMed] [Google Scholar]
- 28.Yao K, Latta M, Bird RP. Modulation of colonic aberrant crypt foci and proliferative indexes in colon and prostate glands of rats by vitamin E. Nutr Cancer. 1996;26:99–109. doi: 10.1080/01635589609514467. [DOI] [PubMed] [Google Scholar]
- 29.Maziere S, Meflah K, Tavan E, Champ M, Narbonne JF, Cassand P. Effect of resistant starch and/or fat-soluble vitamins A and E on the initiation stage of aberrant crypts in rat colon. Nutr Cancer. 1998;31:168–177. doi: 10.1080/01635589809514699. [DOI] [PubMed] [Google Scholar]
- 30.Hagiwara A, Boonyaphiphat P, Tanaka H, et al. Organ-dependent modifying effects of caffeine, and two naturally occurring antioxidants α-tocopherol and n-tritriacontane-16,18-dione, on 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP)-induced mammary and colonic carcinogenesis in female F344 rats. Jpn J Cancer Res. 1999;90:399–405. doi: 10.1111/j.1349-7006.1999.tb00761.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chung H, Wu D, Han SN, et al. Vitamin E supplementation does not alter azoxymethane-induced colonic aberrant crypt foci formation in young or old mice. J Nutr. 2003;133:528–532. doi: 10.1093/jn/133.2.528. [DOI] [PubMed] [Google Scholar]
- 32.Exon JH, South EH, Taruscio TG, Clifton GD, Fariss MW. Chemopreventive effect of dietary d-α-tocopheryl succinate supplementation on precancer colon aberrant crypt formation and vitamin E analogue levels in young and old rats. Nutr Cancer. 2004;49:72–80. doi: 10.1207/s15327914nc4901_10. [DOI] [PubMed] [Google Scholar]
- 33.Cook MG, McNamara P. Effect of dietary vitamin E on dimethylhydrazine-induced colonic tumors in mice. Cancer Res. 1980;40:1329–1331. [PubMed] [Google Scholar]
- 34.Toth B, Patil K. Enhancing effect of vitamin E on murine intestinal tumorigenesis by 1,2-dimethylhydrazine dihydrochloride. J Natl Cancer Inst. 1983;70:1107–1111. [PubMed] [Google Scholar]
- 35.Hirose M, Nishikawa A, Shibutani M, Imai T, Shirai T. Chemoprevention of heterocyclic amine-induced mammary carcinogenesis in rats. Environ Mol Mutagen. 2002;39:271–278. doi: 10.1002/em.10066. [DOI] [PubMed] [Google Scholar]
- 36.Dias MF, Sousa E, Cabrita S, Patricio J, Oliveira CF. Chemoprevention of DMBA-induced mammary tumors in rats by a combined regimen of α-tocopherol, selenium, and ascorbic acid. Breast J. 2000;6:14–19. doi: 10.1046/j.1524-4741.2000.98071.x. [DOI] [PubMed] [Google Scholar]
- 37.Hirose M, Takahashi S, Ogawa K, Futakuchi M, Shirai T. Phenolics: blocking agents for heterocyclic amine-induced carcinogenesis. Food Chem Toxicol. 1999;37:985–992. doi: 10.1016/s0278-6915(99)00092-7. [DOI] [PubMed] [Google Scholar]
- 38.Gould MN, Haag JD, Kennan WS, Tanner MA, Elson CE. A comparison of tocopherol and tocotrienol for the chemoprevention of chemically induced rat mammary tumors. Am J Clin Nutr. 1991;53:1068S–1070S. doi: 10.1093/ajcn/53.4.1068S. [DOI] [PubMed] [Google Scholar]
- 39.Newmark HL, Huang MT, Reddy BS. Mixed tocopherols inhibit azoxymethane-induced aberrant crypt foci in rats. Nutr Cancer. 2006;56:82–85. doi: 10.1207/s15327914nc5601_11. [DOI] [PubMed] [Google Scholar]
- 40.Suh N, Paul S, Lee HJ, et al. Mixed tocopherols inhibit N-methyl-N-nitrosourea-induced mammary tumor growth in rats. Nutr Cancer. 2007;59:76–81. doi: 10.1080/01635580701419022. [DOI] [PubMed] [Google Scholar]
- 41.Seril DN, Liao J, Ho KL, Warsi A, Yang CS, Yang GY. Dietary iron supplementation enhances DSS-induced colitis and associated colorectal carcinoma development in mice. Dig Dis Sci. 2002;47:1266–1278. doi: 10.1023/a:1015362228659. [DOI] [PubMed] [Google Scholar]
- 42.Tanaka T, Kohno H, Suzuki R, Yamada Y, Sugie S, Mori H. A novel inflammation-related mouse colon carcinogenesis model induced by azoxymethane and dextran sodium sulfate. Cancer Sci. 2003;94:965–973. doi: 10.1111/j.1349-7006.2003.tb01386.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ward JM. Morphogenesis of chemically induced neoplasms of the colon and small intestine in rats. Lab Invest. 1974;30:505–513. [PubMed] [Google Scholar]
- 44.Pascal RR. Dysplasia and early carcinoma in inflammatory bowel disease and colorectal adenomas. Hum Pathol. 1994;25:1160–1171. doi: 10.1016/0046-8177(94)90032-9. [DOI] [PubMed] [Google Scholar]
- 45.Seril DN, Liao J, Ho KL, Yang CS, Yang GY. Inhibition of chronic ulcerative colitis-associated colorectal adenocarcinoma development in a murine model by N-acetylcysteine. Carcinogenesis. 2002;23:993–1001. doi: 10.1093/carcin/23.6.993. [DOI] [PubMed] [Google Scholar]
- 46.Suzuki R, Kohno H, Sugie S, Tanaka T. Sequential observations on the occurrence of preneoplastic and neoplastic lesions in mouse colon treated with azoxymethane and dextran sodium sulfate. Cancer Sci. 2004;95:721–727. doi: 10.1111/j.1349-7006.2004.tb03252.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hao X, Sun Y, Yang CS, et al. Inhibition of intestinal tumorigenesis in Apc(min/+) mice by green tea polyphenols (polyphenon E) and individual catechins. Nutr Cancer. 2007;59:62–69. doi: 10.1080/01635580701365050. [DOI] [PubMed] [Google Scholar]
- 48.Miller KW, Yang CS. An isocratic high-performance liquid chromatography method for the simultaneous analysis of plasma retinol, α-tocopherol, and various carotenoids. Anal Biochem. 1985;145:21–26. doi: 10.1016/0003-2697(85)90321-5. [DOI] [PubMed] [Google Scholar]
- 49.Ju J, Liu Y, Hong J, Huang MT, Conney AH, Yang CS. Effects of green tea and high-fat diet on arachidonic acid metabolism and aberrant crypt foci formation in an azoxymethane-induced colon carcinogenesis mouse model. Nutr Cancer. 2003;46:172–178. doi: 10.1207/S15327914NC4602_10. [DOI] [PubMed] [Google Scholar]
- 50.Kitchens LJ. Exploring statistics, a modern introduction to data analysis and inference. Books/Cole Publishing Company; pp. 122–130. [Google Scholar]
- 51.Suzuki R, Kohno H, Sugie S, Nakagama H, Tanaka T. Strain differences in the susceptibility to azoxymethane and dextran sodium sulfate-induced colon carcinogenesis in mice. Carcinogenesis. 2006;27:162–169. doi: 10.1093/carcin/bgi205. [DOI] [PubMed] [Google Scholar]
- 52.Kohno H, Suzuki R, Sugie S, Tanaka T. Suppression of colitis-related mouse colon carcinogenesis by a COX-2 inhibitor and PPAR ligands. BMC Cancer. 2005;5:46. doi: 10.1186/1471-2407-5-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Traber MG, Kayden HJ. Preferential incorporation of α-tocopherol vs γ-tocopherol in human lipoproteins. Am J Clin Nutr. 1989;49:517–526. doi: 10.1093/ajcn/49.3.517. [DOI] [PubMed] [Google Scholar]
- 54.Vacchiano CA, Tempel GE. Role of nonenzymatically generated prostanoid, 8-iso-PGF2 α, in pulmonary oxygen toxicity. J Appl Physiol. 1994;77:2912–2917. doi: 10.1152/jappl.1994.77.6.2912. [DOI] [PubMed] [Google Scholar]
- 55.Morrow JD, Frei B, Longmire AW, et al. Increase in circulating products of lipid peroxidation (F2-isoprostanes) in smokers. Smoking as a cause of oxidative damage. N Engl J Med. 1995;332:1198–1203. doi: 10.1056/NEJM199505043321804. [DOI] [PubMed] [Google Scholar]
- 56.Cooney RV, Franke AA, Harwood PJ, Hatch-Pigott V, Custer LJ, Mordan LJ. γ-Tocopherol detoxification of nitrogen dioxide: superiority to α-tocopherol. Proc Natl Acad Sci U S A. 1993;90:1771–1775. doi: 10.1073/pnas.90.5.1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cooney RV, Harwood PJ, Franke AA. Products of γ-tocopherol reaction with NO2 and their formation in rat insulinoma (RINm5F) cells. Free Radic Biol Med. 1995;19:259–269. doi: 10.1016/0891-5849(95)00019-t. [DOI] [PubMed] [Google Scholar]
- 58.Christen S, Woodall AA, Shigenaga MK, South-well-Keely PT, Duncan MW, Ames BN. γ-Tocopherol traps mutagenic electrophiles such as NO(X) and complements α-tocopherol: physiological implications. Proc Natl Acad Sci U S A. 1997;94:3217–3222. doi: 10.1073/pnas.94.7.3217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hoglen NC, Waller SC, Sipes IG, Liebler DC. Reactions of peroxynitrite with γ-tocopherol. Chem Res Toxicol. 1997;10:401–407. doi: 10.1021/tx960200h. [DOI] [PubMed] [Google Scholar]