Abstract
We are developing liposome-encapsulated hemoglobin (LEH) as an artificial oxygen carrier for resuscitation in indications, such as acute blood loss and surgery. Earlier attempts to formulate a viable LEH met with constraints of scale up and limited hemoglobin content. In this work, we report an LEH formulation containing novel anionic non-phospholipid (CHHDA) that enhances the encapsulation efficiency of hemoglobin inside the liposome bilayer. CHHDA was synthesized from inexpensive ingredients in high yields. The formulation was evaluated in vitro to investigate the cytotoxic effects on RAW 264.7 macrophages and HUVEC endothelial cells in culture by LDH, MTT and hexosaminidase assays. Under optimal conditions of manufacturing, the presence of 28 mole % of CHHDA enhanced the hemoglobin content to over 4 g/dL. The LEH containing CHHDA shows some cytotoxicity in HUVEC and RAW cells in vitro, especially by LDH assay. MTT assay was negative for cytotoxicity in both cells lines. By hexosaminidase assay, the proliferation of RAW cells, but not HUVEC cells, was inhibited. When CHHDA-LEH was incubated with isolated human platelets in vitro, no platelet activation was observed. The LEH formulation with novel anionic lipid and high hemoglobin content reported in this article is an improvement from the past preparations.
Keywords: Liposome, Hemoglobin, Resuscitation, Oxygen carrier, Hemorrhagic shock
1. INTRODUCTION
Safety and adequate availability of blood is still a major concern in transfusion medicine. The requirement of pre-transfusion processing, storage and cross-matching of blood are other factors that have given impetus to the search for safe, shelf-stable and efficacious oxygen carrying fluids. An oxygen carrying fluid mimicking red blood cells (RBCs), in efficacy and safety profile is the goal. An ideal oxygen carrier would be hemoglobin that is encapsulated and is supplemented with the oxido-reductive system of RBC. One approach to compartmentalize hemoglobin is to encapsulate hemoglobin in liposomes [1–6]. This encapsulated product has been variably termed hemoglobin vesicles (HbV), neo-red cells (NRC) or liposome-encapsulated hemoglobin (LEH) and contains highly concentrated (>36 g/dl) purified hemoglobin within the phospholipid membranes. Hemoglobin vesicles or liposome-encapsulated hemoglobin (LEH) mimics membrane enclosed cellular structure of red blood cells [2, 5, 7]. Compared to free modified hemoglobin preparations, LEH is characterized by spatial isolation of hemoglobin by an oxygen permeable lipid layer that eliminates the toxicity associated with free modified or unmodified hemoglobin. In addition, co-encapsulation of reductants, antioxidative enzymes and oxygen-affinity modifiers inside liposomes is readily possible to enhance resuscitative capacity of LEH.
A major impediment in the development of LEH has been the low encapsulation efficiency of hemoglobin inside the vesicles. To increase the encapsulation of proteins inside liposomes, anionic lipids, such as dimyristoyl- and dipalmitoyl- phosphatidyl glycerol (DMPG and DPPG) are usually incorporated in the lipid composition [8, 9]. However, anionic liposomes rapidly interact with the biological system subsequent to their opsonization with complement and other circulating proteins [10–12]. Such an interaction has at least two acute consequences- a rapid uptake by the reticuloendothelial system (RES), and toxic effects, such as pseudoallergy that is manifested as vasoconstriction, pulmonary hypertension, dyspnea, drop in circulating platelets and leukocytes, etc. [13, 14]. Since these reactions are mostly dependent on lipid dose, the problem is more challenging when huge quantities of liposomes need to be administered, such as in the use of LEH as a resuscitative fluid in acute blood loss. It is a challenge therefore, to encapsulate maximum amounts of hemoglobin in the least amount of lipid using anionic lipids and to keep the charge-associated toxicity in check. In this work we report synthesis of a simple anionic non-phospholipid to enhance hemoglobin encapsulation inside liposomes. We also show that this LEH preparation does not activate platelets in vitro. As a part of optimization of LEH formulation, in this article we present synthesis of novel anionic lipid and LEH formulation development as an oxygen carrier.
2. MATERIALS AND METHODS
2.1 Materials
The chemicals were obtained from Sigma-Aldrich (St. Louis, MO) and/or various suppliers through VWR Scientific (West Chester, PA) and were used without further purification. Tetradecenyl succinic anhydride was a kind gift from Vertellus Specialties Inc (Indianapolis, IN). For liposome preparations, the phospholipids were purchased from Lipoid (Ludwigshafen, Germany), Avanti Polar Lipids (Alabaster, AL) or NOF Corporation, (Tokyo, Japan). Cholesterol was obtained from Calbiochem (Gibbstown, NJ). Outdated red blood cell units were kind gift from Oklahoma Blood Institute (Oklahoma City, OK). For NMR and mass spectroscopy, analytical services in the Chemistry Department of the University of Oklahoma (Norman, OK) were used.
2.2 Synthesis of anionic nonphospholipids
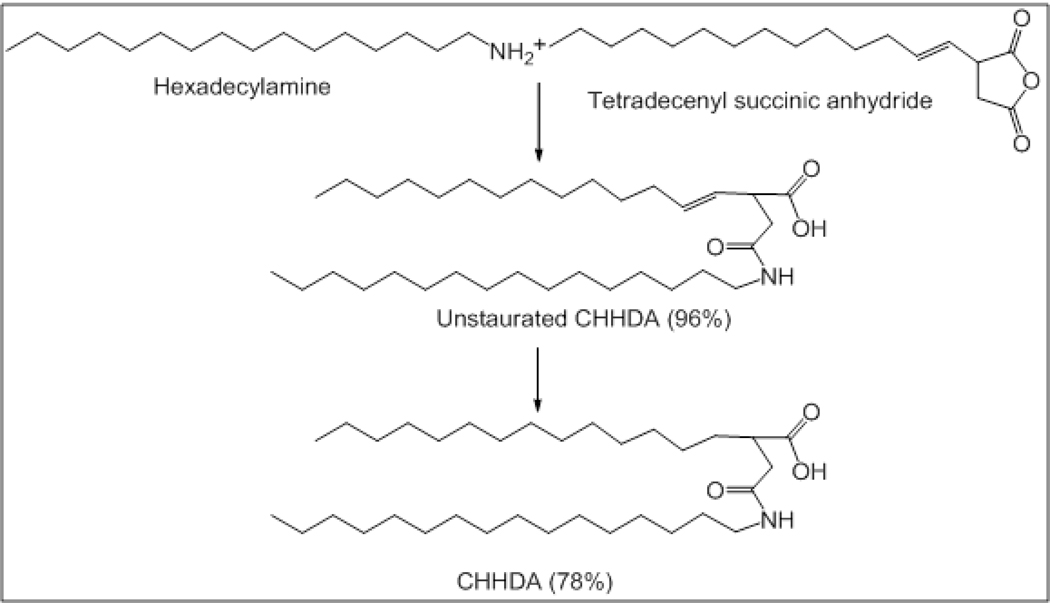
2-Carboxyheptadecanoyl heptadecylamide (CHHDA, Fig. 1)
Figure 1.
Synthesis scheme for DPTSA.
The scheme for the synthesis of CHHDA is shown in figure 1. Tetradecenylsuccinic anhydride (4.3 g, 17.8 mmole) and hexadecylamine (2.93 g, 10.4 mmol) were weighed in an oven-dried round bottom flask. Pyridine (4.5 ml) was added to the flask and the reaction was allowed to occur at 80° C for 3 hr. The reaction mixture was extracted into dichloromethane (DCM, 150 ml) and washed with 10% HCl. The organic phase was separated, dried with sodium sulfate and concentrated to obtain white solid of unsaturated CHHDA (wt. 6.88 g, yield 96%). The unsaturated CHHDA was subjected to catalytic reduction to obtain saturated CHHDA. Briefly, unsaturated CHHDA was dissolved in hexane at 60° C. Reduction was carried out by passing hydrogen gas in presence of catalyst palladium/charcoal (5%, 40 mg) at atmospheric pressure for 16 hrs. The reaction mixture was filtered on a Buchner funnel and hexane was evaporated under vacuum to obtain CHHDA as white solid (wt. 5.36 g, yield 78%). 1H NMR (300 MHz, CDCl3): δ 5.72 (br, 1H, NH, exchanged with D2O), 3.25 (q, 2H, CH2, J = 3.6), 2.80-2.35 (m, 4H, CH2), 1.60-1.40 (m, 4H, CH2), 1.35-1.15 (m, 50 H, CH2), 0.87 (t, 6H, CH3). 13C NMR (75 MHz, CDCl3): δ 176.04, 171.43, 41.62, 37.41, 31.56, 31.51, 29.34, 29.30, 29.16, 29.00, 22.34, 13.85. ESI HRMS calculated for C34H68NO3 (M++1) 538.42, found 538.40. CHHDA was also evaluated by differential scanning calorimetry (DSC) on a Q1000 (TA Instruments, New Castle, DE). DSC was performed, courtesy Dr. Brian P. Grady, in the School of Chemical, Biological and Materials Engineering of the University of Oklahoma (Norman, OK).
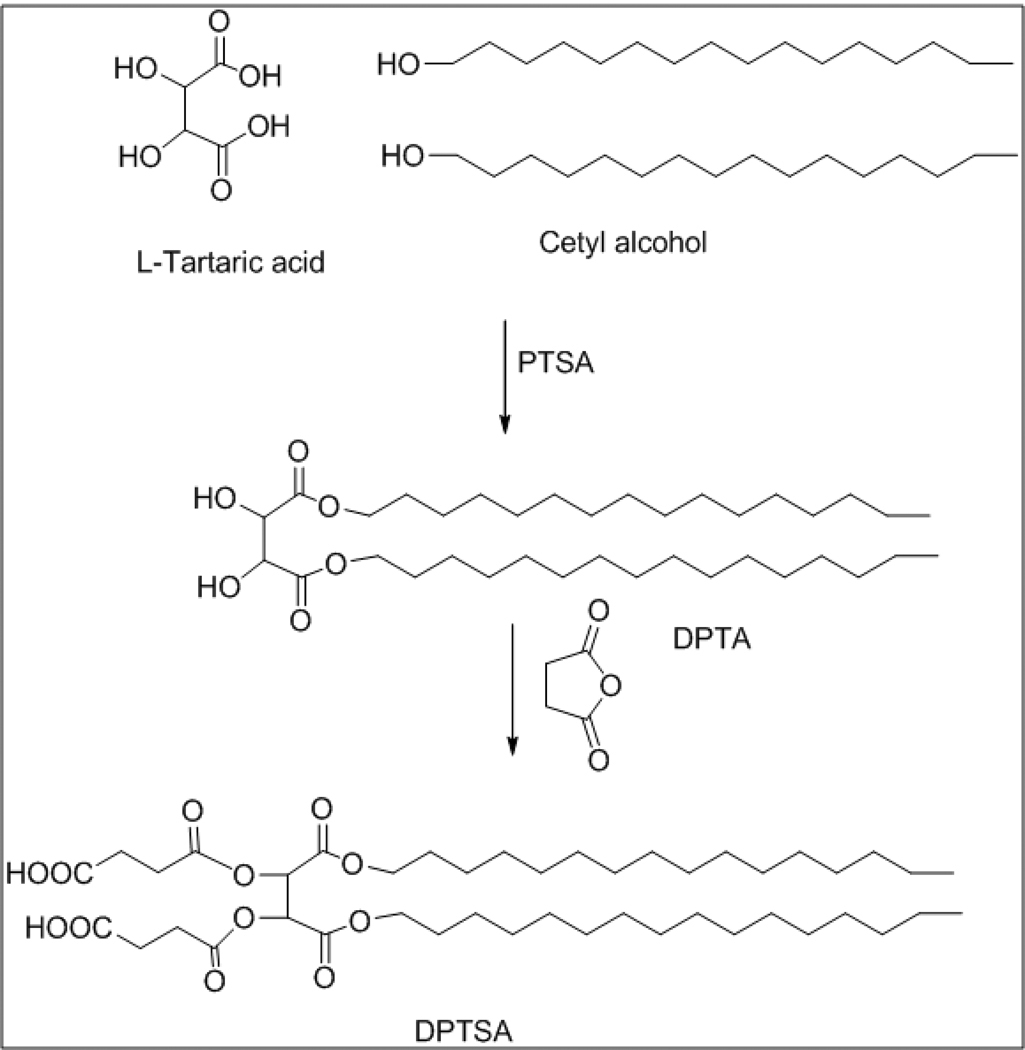
1,4-dipalmitoyl-tartarate-2,3-disuccinic acid (DPTSA, Fig 2)
Figure 2.
Synthesis scheme for DPTGA.
Cetyl alcohol (71 gm, 0.29 mol) and p-toluene sulfonic acid (PTSA, 30.4 gm, 0.16 mol) were added to a solution of tartaric acid (20 gm, 0.13 mol) in toluene. The reaction mixture was refluxed for 16 h using dean stark apparatus. The toluene layer was given a water wash (100 ml), and the organic phase was separated, dried over sodium sulfate and concentrated to obtain white solid of DPTA. The solid was recrystallized from DCM and hexane to get 1,4-dipalmitoyl tartarate as white crystalline solid (75 gm, 86% yield). The melting point was determined to be 40–41 °C. 1H NMR (300 MHz, CDCl3): δ 4.52 (s, 2H), 4.25 (t, 4H, CH2), 1.75-1.60 (m, 4H, CH2), 1.40-1.10 (m, 52H, CH2), 0.87 (t, 6H, CH3). Disuccinate ester of DPTA was synthesized by adding succinic anhydride (0.91 gm, 9.1 mmol) to a solution of DPTA (2 gm, 3.0 mmol) in pyridine (5 ml) and heating at 80°C for 2 h. The resultant compound was extracted into chloroform (50 ml) and washed with 10% HCl followed by a water wash. The organic phase was dried over anhydrous sodium sulfate to obtain white solid of 1,4-dipalmitoyl-tartarate-2,3-disuccinic acid or DPTSA (2.45 gm, 94% yield, melting point 65°C). 1H NMR (300 MHz, CDCl3): δ 5.72 (s, 2H), 4.14 (t, 4H, CH2, J = 6.0), 2.80-2.65 (m, 4H, succinyl), 1.67-1.58 (m, 4H, CH2), 1.32-1.20 (m, 52H, CH2), 0.88 (t, 6H, CH3, J = 6.4). ESI HRMS calculated for C44H78NaO12 (M++Na) 821.54, found 821.40.
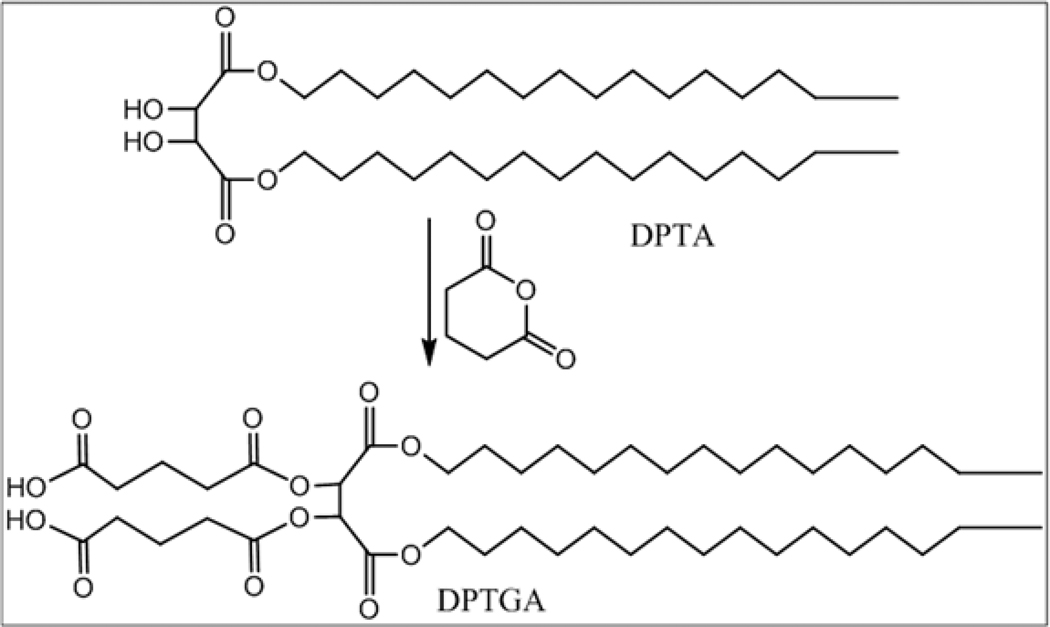
1,4-dipalmitoyl-tartarate-2,3-diglutaric acid (DPTGA, Fig. 3)
Figure 3.
Synthesis scheme for CHEMS.
Glutaric anhydride (2.61 gm, 22.7 mmol) and 1,4-dipalmitoyl tartarate (6 gm, 9.17 mmol) in pyridine (15 ml) were heated at 80°C for 2 h. The compound, 1,4-dipalmitoyl-tartarate-2,3-diglutaricacid (DPTGA) was extracted into chloroform (100 ml) and washed with 10% HCl (50 ml) followed by a wash with water (50 ml). The organic phase was separated, dried over Na2SO4 to obtain white solid (7.76 gm, 96% yield). 1H NMR (300 MHz, CDCl3): δ 5.69 (s, 2H), 4.20-4.05 (m, 4H), 2.60-2.40 (m, 8H), 2.05-1.95 (m, 4H), 1.65-1.55 (m, 4H), 1.40-1.10 (m, 52H), 0.87 (t, 6H, CH3).
1,4-disteroyl-tartarate-2,3-disuccinic acid (DSTSA)
DSTSA was synthesized following the scheme similar to that used for DPTSA, except distearoyl tartaric acid ester (DSTA) was used as the starting lipid. DSTA was obtained commercially from VWR Scientific. DSTSA was collected as white solid (93% yield, melting point 81°C). 1H NMR (300 MHz, CDCl3): δ 5.75 (s, 2H), 4.20-4.05 (m, 4H, CH2), 2.80-2.55 (m, 4H, succinyl), 1.70-1.55 (m, 4H, CH2), 1.45-1.15 (m, 60H, CH2), 0.88 (t, 6H, CH3).
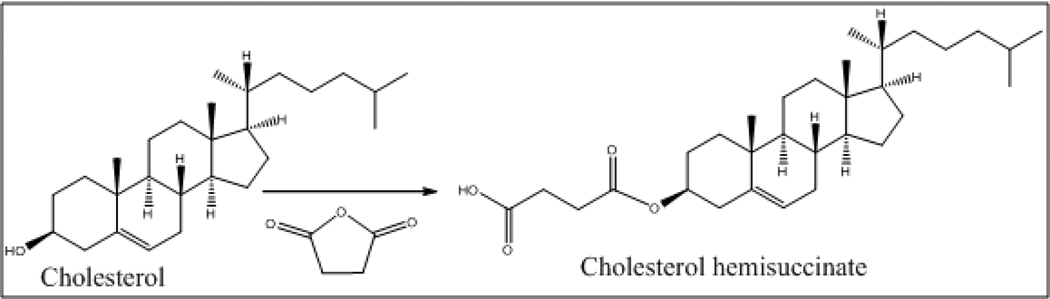
Cholesteryl hemisuccinate (CHEMS, Fig. 4)
Figure 4.
Differential scanning calorimetry of CHHDA.
Succinic anhydride (1.55 gm, 15.5 mmol) was added to a solution of cholesterol (5 gm, 12.9 mmol) in pyridine (10 ml). After heating at 80°C for 3 h, the reaction mixture was diluted with DCM and the organic phase was washed with 10% HCl (50 ml) followed by a water wash (50 ml). The organic phase was separated, dried over Na2SO4 to obtain cholesterol hemisuccinate as white solid. The solid was recrystallized from DCM and hexane (5.78 gm, 92% yield, and melting point 176–178°C). 1H NMR (300 MHz, CDCl3): δ 5.47 (d, vinylic-H, J = 5.0), 4.70-4.60 (m, 1H), 2.75-2.65 (m, 4H), 2.38-2.35 (d, 7H), 2.20-1.80 (m, 12H), 1.60-1.40 (m, 12H), 0.78-0.75 (m, 3H).
2.3 Isolation of stroma-free hemoglobin
Concentrated stroma-free hemoglobin was isolated from the outdated red blood cell (RBC) units using a previously described method [15]. Outdated RBC units were obtained from Sylvan Goldman Center, Oklahoma Blood Institute (Oklahoma City, OK). To enhance the stability of hemoglobin during the isolation process, the RBC suspension was purged with 0.22 µm-filtered carbon monoxide gas (CO) which converts hemoglobin into a relatively more stable carbonylhemoglobin (CO-Hb). The carbonylated RBCs (20 g/dl, 300 ml) were mixed with dichloromethane (DCM, 60 ml) and mixed for 10 min. The precipitate was allowed to settle, and the supernatant was again extracted with DCM. The extraction was repeated 3 times to remove all the lipid material soluble in organic phase. The residual DCM in the aqueous phase containing CO-Hb solution was removed under vacuum in an R-210 rotavapor (Buchi Corporation, New Castle, DE) at 40° C in dark. The resultant CO-Hb solution was further heated at 60°C for 1 hr to denature and precipitate any methemoglobin in the solution. The precipitate was removed by centrifugation at 8000 rpm for 20 min at 4° C in a Sorvall RC-5B refrigerated superspeed centrifuge. The supernatant was purified from fine remnants of particulate matter by sequentially passing it through tangential-flow FiberFlow capsules having 100 and 50 nm cut-off (Minntech, Minneapolis, MN). Finally, the purified hemoglobin solution was concentrated to about 38g/dl using a 30 kDa cut-off Prep/Scale-TFF filter cartridge (Millipore, Billerica, MA). The final preparation was characterized for oxygen affinity, CO-Hb content, MetHb concentration and endotoxin.
2.4 Preparation of liposome-encapsulated hemoglobin (LEH)
The LEH was prepared in several steps described as follows:
2.4.1 Preparation of pro-liposomes
Freeze-thaw (FT) method was used for the preparation of pro-liposomes. 1,2-disteroyl-sn-glycero-3-phosphatidylcholine (DSPC), Cholesterol (CHO), anionic lipid, 1,2-disteroyl-sn-glycero-3-phosphoethanolamine-N-[monomethoxy poly(ethylene glycol) (5000)] (DSPE-PEG5000) and vitamin E in 38.85:38.85:20:0.3:2 mol% were dissolved in a mixture of chloroform: methanol (2:1) and transferred to a round bottom flask. The solvent mixture was evaporated at 58°C on rotavapor to form a thin film. Any trace of organic solvent was removed by keeping the film under vacuum for 12 h. The phospholipid film was rehydrated with sodium hydroxide solution equimolar to the carboxyl groups present in the anionic lipid to maintain total lipid concentration to 12 mM. The pH of the lipid suspension was adjusted to 6.8–7.0. This suspension of large multilamellar vesicles was subjected to 8 FT cycles. An FT cycle consisted of snap-freezing the suspension in liquid nitrogen followed by immediate thawing in a 58°C water bath. DPPC-based pro-liposomes were prepared using DPPC, CHO, anionic lipid, DSPE-PEG5000 and vitamin E in 38.85:38.85:20:0.3:2 mol% and processed as above except that the thawing was carried out at 42°C. After the final FT cycle, the pro-liposome suspension was shell-frozen in a lyophilization flask and subjected to a 48 h lyophilization cycle in a Triad Lyophilizer (Labconco, Kansas City, Missouri). The dried pro-liposome material was stored at −20° C until further used.
2.4.2 Optimization of hemoglobin encapsulation
The effect of synthesized anionic lipids on the efficacy of hemoglobin encapsulation was examined. The lyophilized DPPC-based pro-liposomes containing 20 mol% of the anionic lipid were allowed to warm up to 25°C, and the powder was aseptically and gradually added to a highly concentrated, purified 5 ml Hb solution (38g/dl, <0.25 endotoxin units/ml) with constant stirring. The entire process was carried out under HEPA-filtered laminar flow hood; during entire processing, the temperature was maintained to 25°C. After 2 hr of incubation the suspension was ultracentrifuged at 35,000 rpm at 5° C for 30 min in an Optima L-100 XP ultracentrifuge (Beckman Coulter, Fullerton, CA). The supernatant was removed and the pellet was resuspended in phosphate-buffered saline (PBS, pH 7.4). The centrifugation cycle was repeated two times to completely get rid of free hemoglobin. Finally the pellet was resuspended in 1.0 ml of PBS (pH 7.4) and the amount of encapsulated hemoglobin was determined by monitoring the absorbance of LEH lysate in OBG at 540 nm [16]. In order to determine the effect of increasing mol% of anionic lipid on hemoglobin encapsulation, three batches of DPPC-based formulation were tested with 20, 28 and 40 mol% of CHHDA and encapsulated Hb in respective formulations was estimated [16].
2.4.3 Optimization of homogenization conditions
The homogenization process was optimized separately using pro-liposome batches of identical composition, except that no hemoglobin was introduced in these preparations. The particle size of LEH was reduced by high pressure homogenization using Emulsiflex-C3 (Avestin Inc., Ottawa, Ontario, Canada). Parameters, such as number of homogenization cycles and pressure were optimized to obtain the LEH of size 200–300 nm.
2.4.4 Preparation of LEH
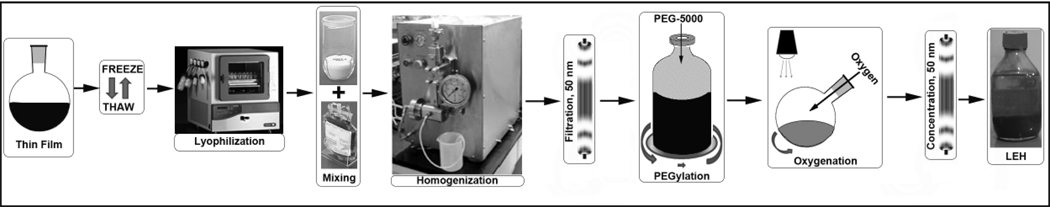
In order to validate the optimized lipid composition and production protocol, two small LEH batches (LEH 1 and LEH 2, 25 ml each) were manufactured. The composition chosen was DPPC, CHO, CHHDA, DSPE-PEG5000, vitamin E in 34.85:34.85:28:0.3:2 mol%. In batch LEH 2, the p50 of hemoglobin was adjusted by adding pyridoxal-5’-phosphate (PLP, 2.5 molar times of hemoglobin), to the carbonylhemoglobin solution and this solution was used for rehydration of lyophilized pro-liposomes. No PLP was used in LEH 1. Strict aseptic conditions were maintained throughout the preparation in a laminar flow hood. Final size reduction of hemoglobin containing pro-liposomes was carried out by high pressure homogenization in Emulsiflex-C3 at 20K psi for 4 cycles- each cycle separated by at least 30 minutes. The processing temperature was maintained at about 20°C by immersing the heat transfer coil in an ice-cold water-bath. Further processing of LEH was performed according to the method earlier published [17, 18]. Briefly, the free hemoglobin was separated from encapsulated hemoglobin by tangential-flow filtration through 50 nm hollow fiber filter using PBS (pH 7.4) as the diluting solvent. The purified LEH was post-inserted with PEG-lipid by mixing an aqueous solution of DSPE-PEG5000 with a dilute dispersion of LEH, such that the PEG-lipid concentration remains below its critical micelle concentration. To convert encapsulated carbonylhemoglobin into oxyhemoglobin, the PEGylated LEH was exposed to a bright visible light from a 500W halogen lamp under saturating oxygen atmosphere at 4–8° C. The conversion was monitored spectrophotometrically. The dilute oxygenated LEH was further subjected to tangential-flow filtration (50 nm filter, PBS wash-fluid) to eliminate remnants of free hemoglobin. Finally, the LEH was concentrated to the desired batch volume and stored at 4°C.
2.5 Characterization of LEH
To visually document the formation of liposomes using the anionic non-phospholipid, we performed electron microscopy at the University of Oklahoma at Norman (OK). The LEH preparations were characterized for hemoglobin content, methemoglobin, size, oxygen affinity and lipid concentration. Hb encapsulation was estimated by monitoring the absorbance of the OBG lysate of LEH at 540 nm [16]. The phospholipid concentration was determined by Stewart assay [19]. Methemoglobin content was also measured [20]. Oxygen affinity (p50) was measured in a Hemox-analyzer (TCS Scientific, New Hope, PA). Briefly, a 50 µl sample of LEH was dispersed in a 4 ml phosphate buffer (pH 7.4) containing albumin and an antifoaming agent. The mixture was incubated at 37°C for 5 minutes and aspirated into the cuvette for dual wavelength spectrophotometry. The sample was purged with N2 gas to PO2 < 2.0 mm Hg, before allowing air to attain PO2 >140 mm Hg. The results were analyzed by HAS software provided with the instrument. The particle size was determined by photon correlation spectroscopy using a Brookhaven particle size analyzer equipped with Mas Option software. Zeta potential of preparations was measured in a Zeta PLUS Zeta potential analyzer (Brookhaven Instruments Corp, Holtsville, NY). Zeta potential of LEH was estimated, both, before (non-PEGylated LEH) as well as after (PEGylated LEH) PEG post-insertion and the values were compared to zeta potential of empty liposomes containing 28% CHHDA or 20% DMPG. Preparations equivalent to about 40 µg of phospholipid in 1.5 ml of 0.22 µm filtered de-ionizd water were scanned at 25°C for 10 runs, each run consisting of 20 cycles. Zeta potential values were obtained as millivolt ± standard error of mean.
2.6 Cell culture and cytotoxicity studies
As a long-circulating oxygen carrier, LEH is expected to mostly interact with two cell types-endothelial cells in the vasculature and macrophages responsible for clearing particulate material. Human Umbilical Vein Endothelial Cells (HUVEC, ATCC, Manassas, VA) were grown in M-199 medium supplemented with 10% (v/v) plain FBS, endothelial cell grown supplement (ECGS) (20 µg /ml), heparin (90 mg/L), penicillin G (100 U/ml ) and streptomycin (100 µg/ml) (Sigma-Aldrich, St. Louis, MO). Cells were maintained at 37° C in 5% CO2 atmosphere. Cells were used after 3 passes for cytotoxicity study. RAW 264.7 cells (ATCC, Manassas, VA) were grown in Dulbecco's modified Eagle's medium supplemented with 10% FBS (ATCC) at 37° C in 5% CO2 atmosphere. Cells were grown in 96-well plates at a density of 104 cells/well. After 24 h of culture, the cells were treated with 100µl of either, 1, 2 or 5mg/ml of PEGylated or non-PEGylated LEH. Separately, cytotoxicity of empty liposomes carrying 10, 20 and 28mol% of CHHDA was also evaluated (5mg/ml, 100µl). Empty liposomes containing 0 mol% CHHDA and those containing DMPG at 20 mol% were taken as controls for comparison. The cytotoxicity was measured as the decrease in hexosaminidase activity in cells after 24 h as described by Landegren [21]. Para-nitrophenol-N-acetyl-beta-D-glucosaminide was used as the substrate for hexosaminidase enzyme.
The medium from the treated wells was used for lactate dehydrogenase (LDH) assay using Cytoscan-LDH cytotoxicity assay kit (G-Biosciences, St. Louis, MO). The cell treatment was identical to that described above. The culture plates were centrifuged for 20 min at 1500 rpm in a plate centrifuge. Supernatant, 50 µL, was collected from each well and transferred into a new plate. The supernatant from untreated cells and that from lysed cells using lysis buffer in the kit was used as negative and positive controls, respectively. Substrate, 50 µL, was added to each well and incubated at room temperature for 30 min in dark. The reaction was stopped by adding 50 µL of stop solution and the absorbance (ABS) was recorded at 490 nm. Percent cytotoxicity was calculated by using following formula-
To confirm the cytotoxicity, we also performed colorimetric MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] assay. Only LEH was tested with MTT assay at 5 mg/ml dose. After exposing the cells for either 6 or 24 hrs to LEH formulations at 37 °C, 10 µL of MTT reagent was added per well and incubation continued for further 2 h. At the end of incubation period, 100 µL detergent reagent provided with the kit (ATCC MTT cell proliferation assay kit, ATCC, VA) was added per well. The incubation was performed in dark for 2 h at room temperature and the absorbance was recorded at 570 nm. The results were presented as percent proliferation compared to untreated control.
2.7 Platelet activation by LEH in vitro
Fresh human whole blood (5 ml) was obtained from a normal healthy volunteer. The blood was anticoagulated by acid-citrate-dextrose solution (9 blood: 1 ACD, volume ratio). Platelet rich plasma (PRP) was separated from other cellular components by centrifugation at 150 g for 8 min. The aliquots of PRP (about 0.5 million platelets) were treated with 5 mg/ml of lipid as LEH. Two preparations of LEH were tested- one with PEG-coating and the other without PEG-coating. Adenosine diphosphate (ADP, 100 µM) was used as the positive control. After 5 and 30 min of incubation The samples were labeled with anti-human platelet antibodies for flowcytometry [22].
We used murine antihuman monoclonal fluorescein isothiocyanate (FITC)-conjugated CD61 to identify all platelets. Murine antihuman monoclonal phycoerythrin (PE)-conjugated CD62P was used to detect platelet activation. Isotype controls were run in parallel with all monoclonal antibodies: FITC-conjugated immunoglobulin G1 and PE-conjugated immunoglobulin G1. All antibodies and isotype controls were obtained from eBioscience (San Diego, CA). Briefly, about 10 µL of 1:10 diluted PRP was incubated in the dark at room temperature with a mixture of 20 µL of FITC-conjugated CD61 antibody and 20 µL of PE-conjugated CD62 antibody (each equal to about 0.25 µg and 0.125 µg antibodies, respectively). After 20 minutes, the platelets were thrice washed with PBS, followed by overnight fixing with 1 mL of ice-cold 1% paraformaldehyde in dark at 4°C. The samples were subjected to flow cytometric analysis in the Core Facility at the OUHSC on a FACS Calibur (BD and Company, Franklin Lakes, NJ) equipped with an argon ion laser and CellQuest software (BD and Company, Franklin Lakes, NJ). Forward light scatter and 2 fluorescent signals were determined for each cell, and at least 10000 platelet events were collected. The platelet population was logic gated by a forward scatter versus side scatter dot plot gate and by an FITC-conjugated CD61 versus side scatter dot plot gate. CD62P-positive activated platelets were identified by fluorescein intensity greater than that of the appropriate isotype control staining sample. The frequencies of CD62P-positive platelets were expressed as percentages of the total platelet population [22].
2.8 Data analysis
Data are presented as mean ± SD. The statistical differences were determined by the paired Student t test for P < 0.05.
3. RESULTS
We synthesized several novel anionic non-phospholipids to enable enhanced encapsulation of hemoglobin inside the liposomes. Figure 1–4 show the synthetic scheme for various anionic non-phospholipids. Our observations in the development of an optimized and improved LEH formulation based on these anionic non-phospholipids are recorded in following paragraphs.
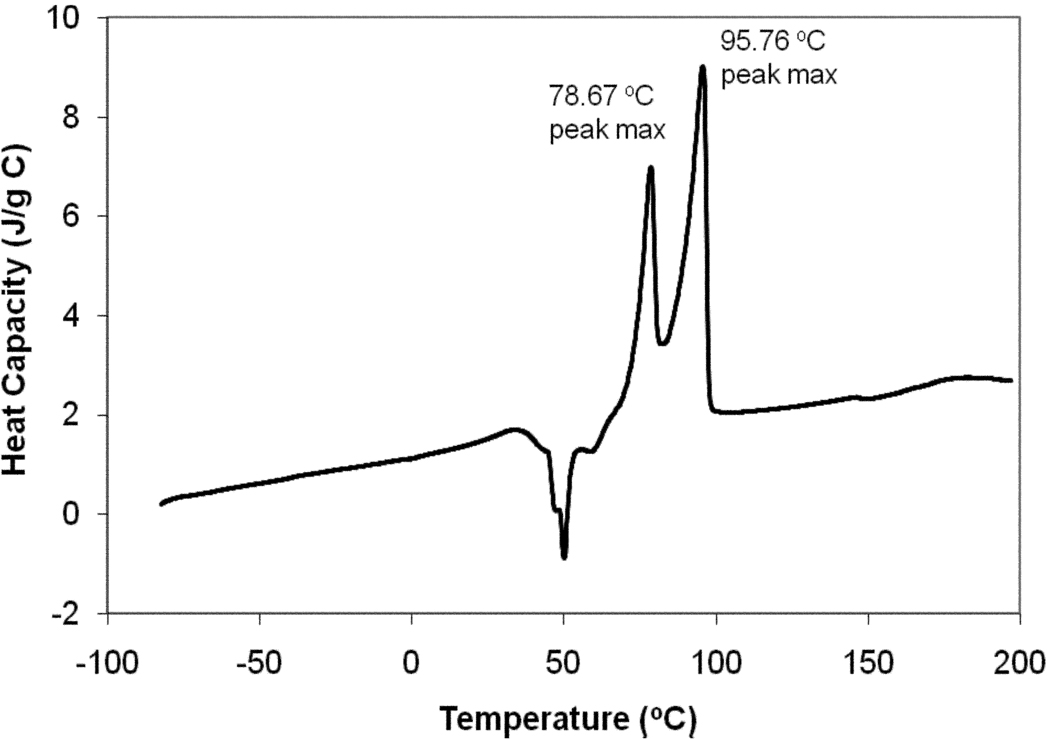
The reaction yields for CHHDA were routinely above 75%. CHHDA was characterized by proton NMR and high resolution mass spectrometry (HRMS). The diagnostic spectroscopic data described below confirmed the successful synthesis of CHHDA. ESI-HRMS showed molecular ion peak at 538.40 and 1H NMR showed the disappearance of vinylic protons at δ 5.80-5.20 ppm. The lipid peak intensity at δ 1.35-1.15 ppm was increased indicative of the hexadecylamine esterification. In DSC, CHHDA lipid showed endothermic events around 50°C, prior to the melting at around 80°C (Fig. 5). Noticeable exothermic events were observed around room temperature, and the lipid showed two melting events. The peak temperatures on the graph are printed by the instrument software. These may not be melting points, but are the temperatures corresponding to the maximum of the exothermic release of heat.
Figure 5.
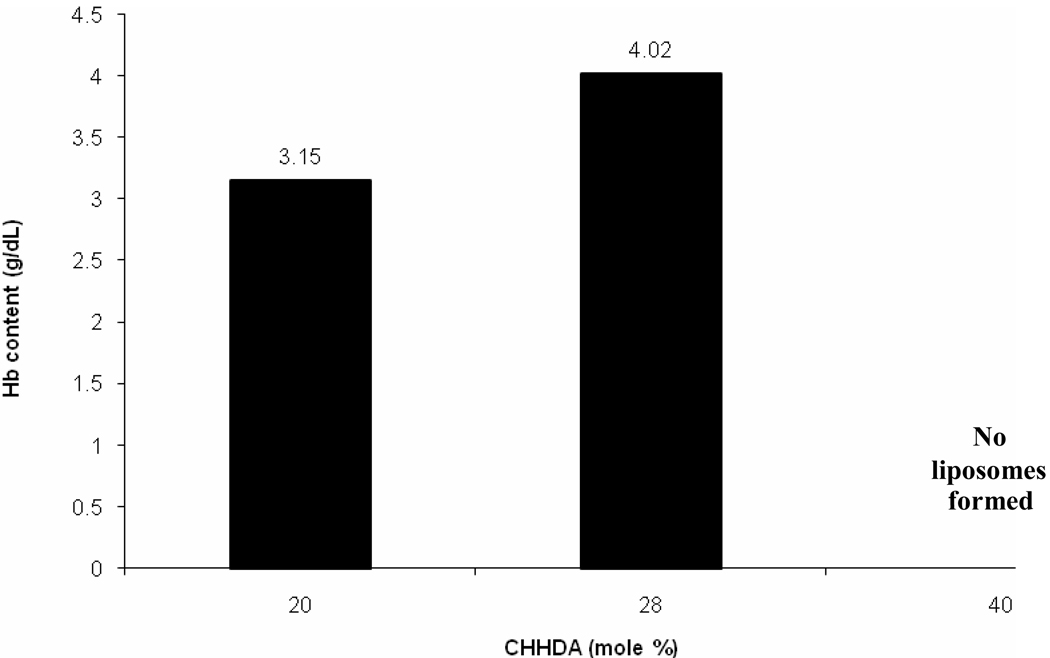
Effect of CHHDA mol % on Hb encapsulation inside liposomes.
DPTGA formation was confirmed by the appearance of glutaric protons at δ 4.20-4.05, lipid CH2 protons at 2.05-1.95 and 1.40-1.10 ppm as multiplets in 1H NMR. In case of DSTSA, the protons at the site of esterification shifted downfield to 5.69 from 4.52 ppm suggesting esterification. The stearoyl tartarate protons on –OH bearing carbons appeared as broad doublets at 4.50 ppm and shifted downfield to 5.75 as singlet after esterification of –OH groups. The succinyl protons appeared at 2.55-2.80 ppm as multiplets in 1H NMR spectrum. CHEMS showed melting point of 176–178°C where as cholesterol has a melting point of 148–150°C. The succinyl protons of CHEMS appeared at δ 2.75-2.60 in 1H NMR.
We used freeze-thaw method to prepare DSPC and DPPC based pro-liposomes, before encapsulation of hemoglobin. The particle size of DSPC-based pro-liposomes was reduced to 397.4±6 nm after 8 FT cycles (table 1). When sonication was used along with thawing, the pro-liposomes were reduced to an undesirably small size (210–215 nm). In case of DPPC-based composition, pro-liposomes of size 441.0±18 nm were produced after only 4 FT cycles. The effect of bath-sonication during thawing was similar to that seen with DSPC pro-liposomes, and the final size of DPPC pro-liposomes was about 213±2 nm.
Table 1.
Sizes of Pro-liposomes after Freeze-thaw
| Phospholipid | FT cycle | Sonication? | Size (nm) |
|---|---|---|---|
| DSPC | 4 | - | 639.4±13 |
| DSPC | 4 | Yes | 215.1±2 |
| DSPC | 8 | - | 397.4±6 |
| DSPC | 8 | Yes | 210.3±3 |
| DPPC | 4 | - | 441±18 |
| DPPC | 4 | Yes | 213.2±2 |
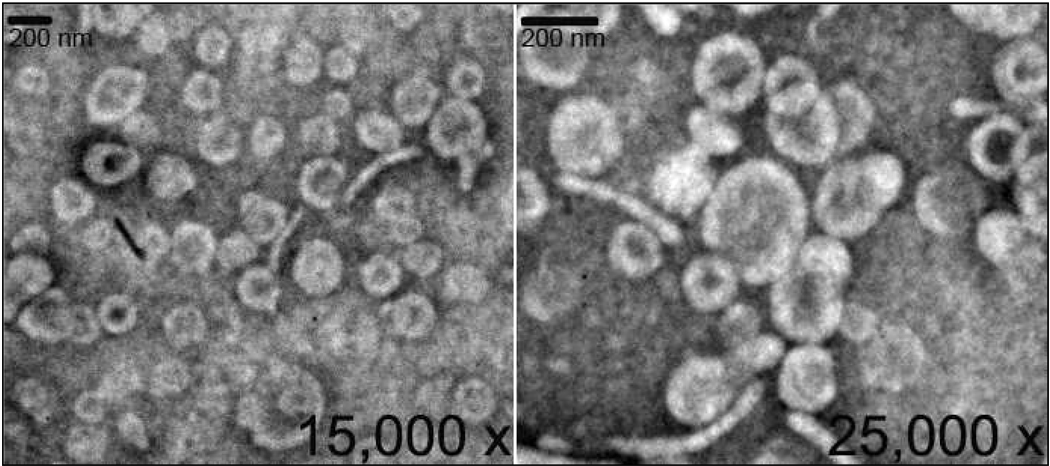
To investigate the effect of anionic lipids on hemoglobin encapsulation, DPPC based pro-liposome containing 20 mol% of one of the synthesized anionic lipids were incubated with carbonylhemoglobin solution. The hemoglobin content of CHHDA-containing liposomes was found to be the highest (3.15g/dl) whereas the hemoglobin content of DPTGA containing liposomes was the lowest (1.55 g/dl) (table 2). The hemoglobin-to-lipid ratio for CHHDA liposomes was 1.2. Methemoglobin formation in case of all the anionic lipids except CHEMS was less than 2%. For unknown and unexplored reasons, CHEMS tended to induce methemoglobin formation to the extent of 40% of total hemoglobin. The visible appearance of this preparation was dark brown whereas all other preparations were shiny deep red in color. Further, proliposomes containing increasing amounts of CHHDA were prepared to investigate the effect on Hb encapsulation. The Hb encapsulation was found to increase with increasing CHHDA mol% to a certain limit (Fig. 6). The highest encapsulation (4.02 g/dl) was observed with 28 mol% of CHHDA. At 40 mol%, CHHDA appeared to disrupt liposome structure as no LEH pellet was observed after ultracentrifugation. As maximum Hb ecapsulation was with 28mol% CHHDA, effect of 28 mol% CHHDA on the bilayer structure of liposomes was investigated by scanning electron microscopy. As shown in Figure 7, electron micrographs of LEH showed typical structure of liposomes. In summary, CHHDA up to 28% can be used as an anionic lipid component in LEH preparations.
Table 2.
Effect of various anionic lipids on hemoglobin encapsulation
| Phospholipid | Mole % | Hb content (g/dL) | Hb/lipid |
|---|---|---|---|
| DPTA | 20 | 2.6 | 1.26 |
| DPTGA | 20 | 1.55 | 1.2 |
| DSTSA | 20 | 2.09 | 1.7 |
| CDHHA | 20 | 3.15 | 1.2 |
| CHEMS | 20 | Hb oxidation | - |
Figure 6.
Scanning electron micrograph of LEH prepared with 28 mol% of CHHDA.
Figure 7.
Schematic illustration showing various stages of LEH manufacturing.
In a separate set of experiments, the homogenization process was optimized for pressure and number of passes. At 15K psi pressure, homogenization for 4 or 5 passes did not reduce the particle size below 400 nm (table 3). Increasing the pressure to 20K psi produced the liposomes in 200 nm range. As the desired size range was achieved at a combination of 4 passes at 20K psi, this set of conditions was selected for scale up of manufacturing further batches of LEH. We prepared two batches of LEH- one containing PLP and the other without PLP. LEH 1 and LEH 2 had particle size of 273.4±4.2 and 222.6±1.3 nm, respectively. LEH 1 and LEH 2 were characterized as described in table 4. Both the batches had a high hemoglobin content and hemoglobin-to-lipid ratio. Both preparations had less than 2% methemoglobin. The entire LEH processing has been schematized in Fig. 8. Separately we investigated the possibility of CHHDA having any effect on the oxygen affinity of hemoglobin (Table 5). It is clear that even at 28 mole %, CHHDA does not have any significant impact on p50 value on its own. On the other hand, the addition of PLP to hemoglobin prior to encapsulation altered the p50 of the final product from about 19 to 33 Torr (Table 4). The p50 value of LEH 1 without PLP was 18.94, whereas LEH 2 with PLP had p50 value of 33.14 Torr.
Table 3.
Optimization of homogenization process
| Sr. No | Number of Passes |
Pressure (psi) | Size (nm) |
|---|---|---|---|
| 1 | 4 | 15K | 405.1±30 |
| 2 | 5 | 15K | 435.3±16 |
| 3 | 5 | 20K | 199.1±2.8 |
| 4 | 4 | 20K | 222.6±1.3 |
| 5 | 3 | 22K | 263.7±4 |
Table 4.
Characteristics of LEH Batches
| Parameter | LEH 1 | LEH 2 |
|---|---|---|
| Size (nm) | 273.4±4.2 | 222.6±1.3 |
| Hb (g/dl) | 4.45 | 4.19 |
| Hb/Lipid ratio |
1.2 | 1.16 |
| p50 (Torr) | 18.94 | 33.14 |
| Met-Hb (%) | <2 | <2 |
Figure 8.
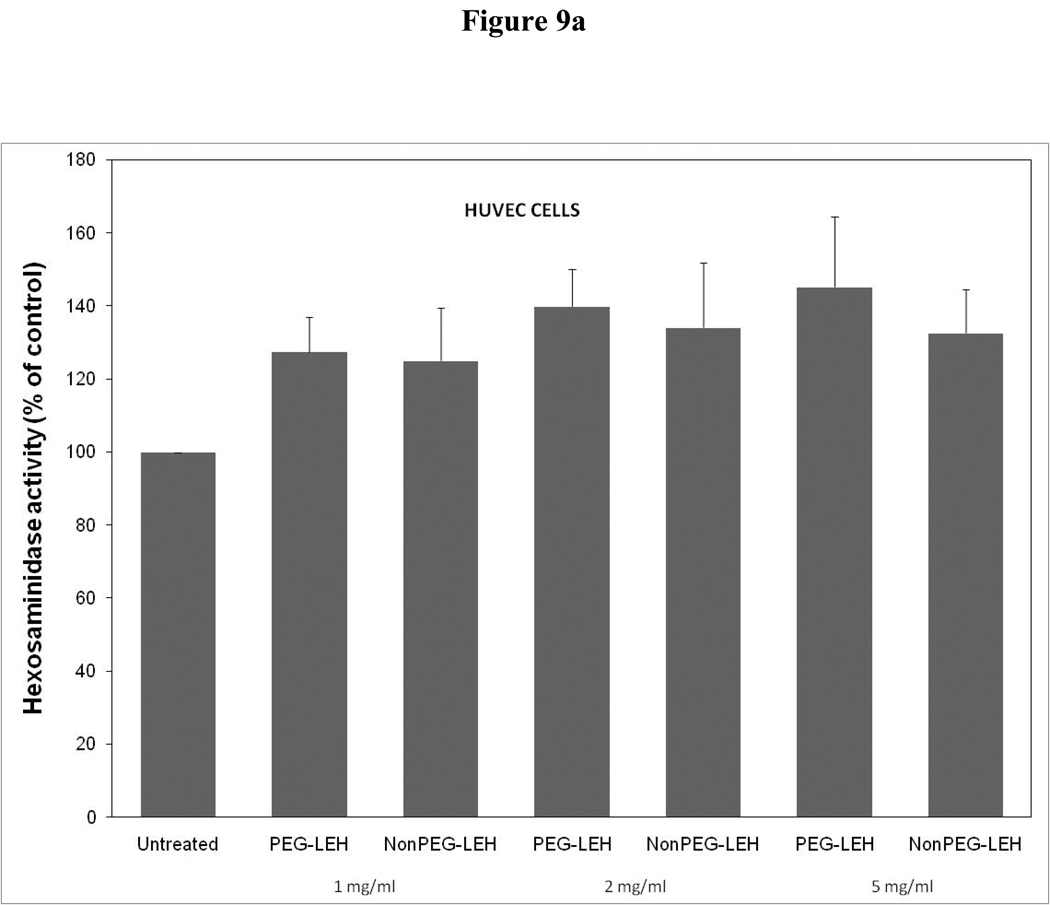
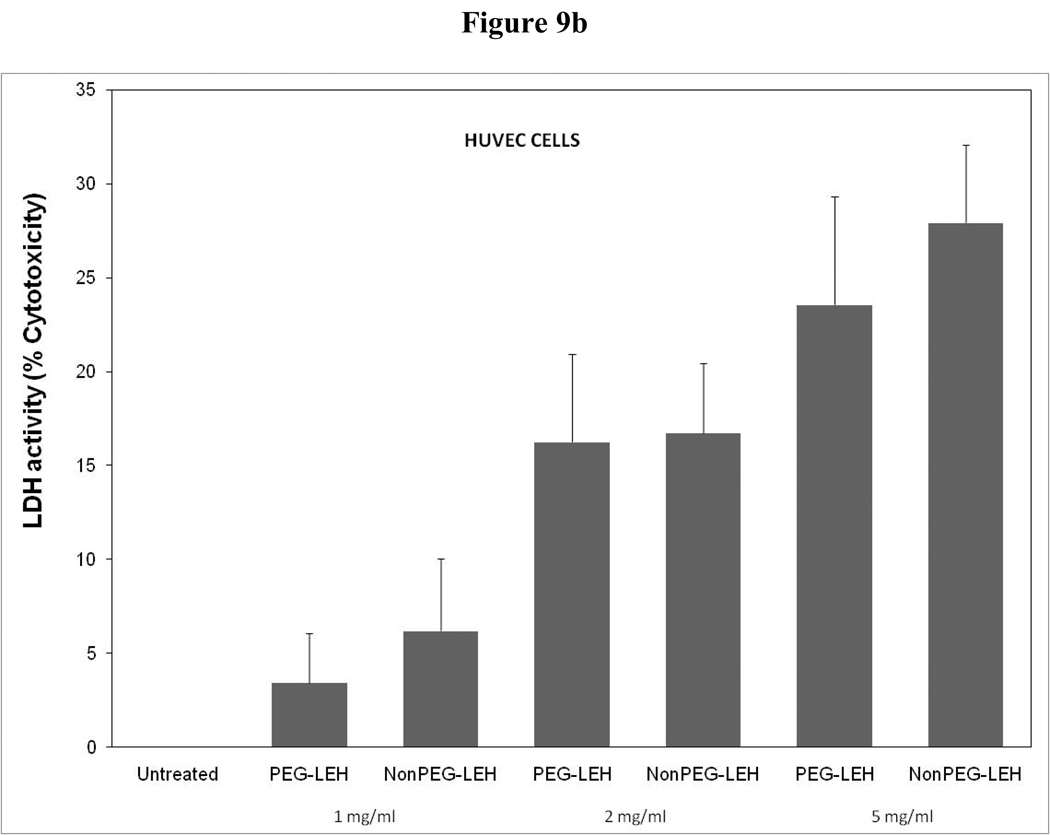
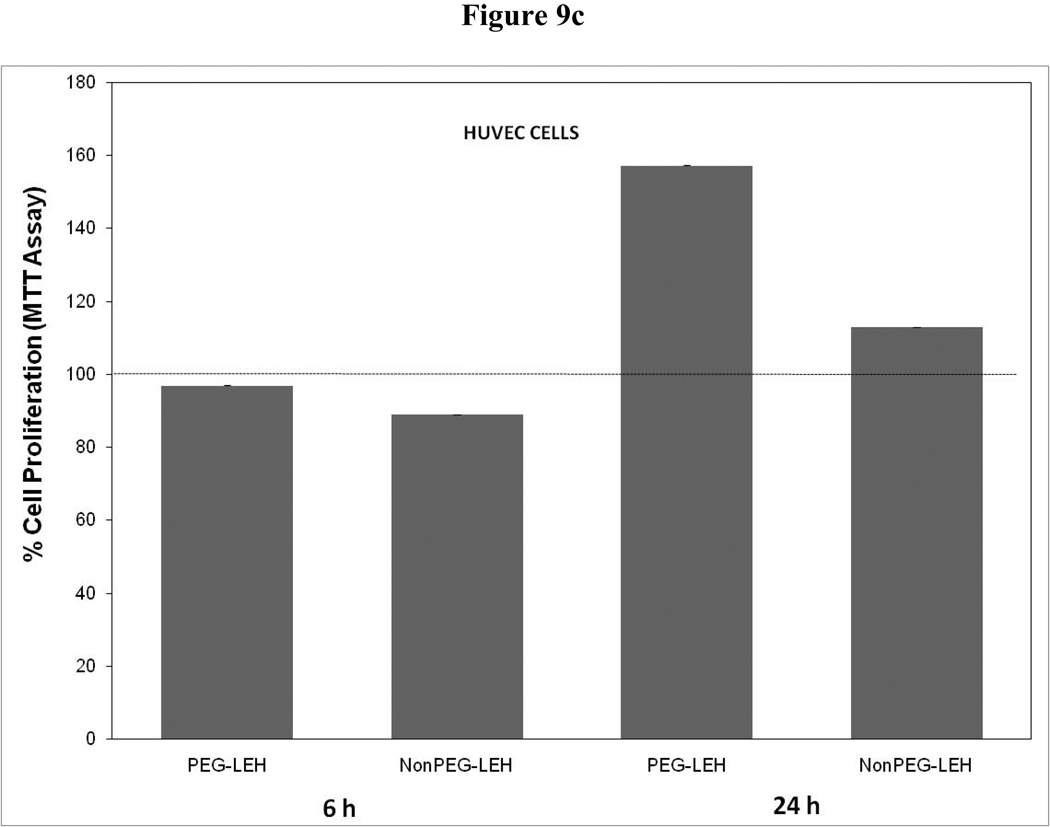
Cytotoxicity of LEH in HUVEC cells evaluated by (a) hexosaminidase assay, (b) lactate dehydrogenase assay, and (c) 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide, or MTT assay. The cells for MTT assay were treated only at 5 mg/ml lipid dose.
Table 5.
Effect of CDHHA on p50 of hemoglobin
| Sr. No. | TDSA-H (mol%) | p50 (Torr) |
|---|---|---|
| 1 | 0 | 9.94 |
| 2 | 20 | 10.18 |
| 3 | 28 | 10.85 |
Under the conditions of measurement, the zeta potentials of empty 20 mol% DMPG liposomes and empty 28% CHHDA liposomes were found to be −90.91±2.62 and −74.67±0.65 mV, respectively. Clearly, even at 28% concentration, the apparent negative potential of the CHHDA liposome particle was significantly lower than that of liposomes containing about 2/3rd the amount of DMPG. The liposome’s surface charge is governed by how the negatively-charged constituent is packed in the liposome structure, and how ionized the constituent becomes under measuring conditions. In comparison, the non-PEGylated LEH showed zeta potential value of −33.86±1.0. Presence of hemoglobin seemed to reduce negative potential of the liposome particles, possibly by interacting and neutralizing the negative charge of the liposome bilayer. PEGylation of LEH further reduced zeta potential to −27.98±2.4 mV. PEG coating of particles is known to decrease the charge effect under in vivo conditions.
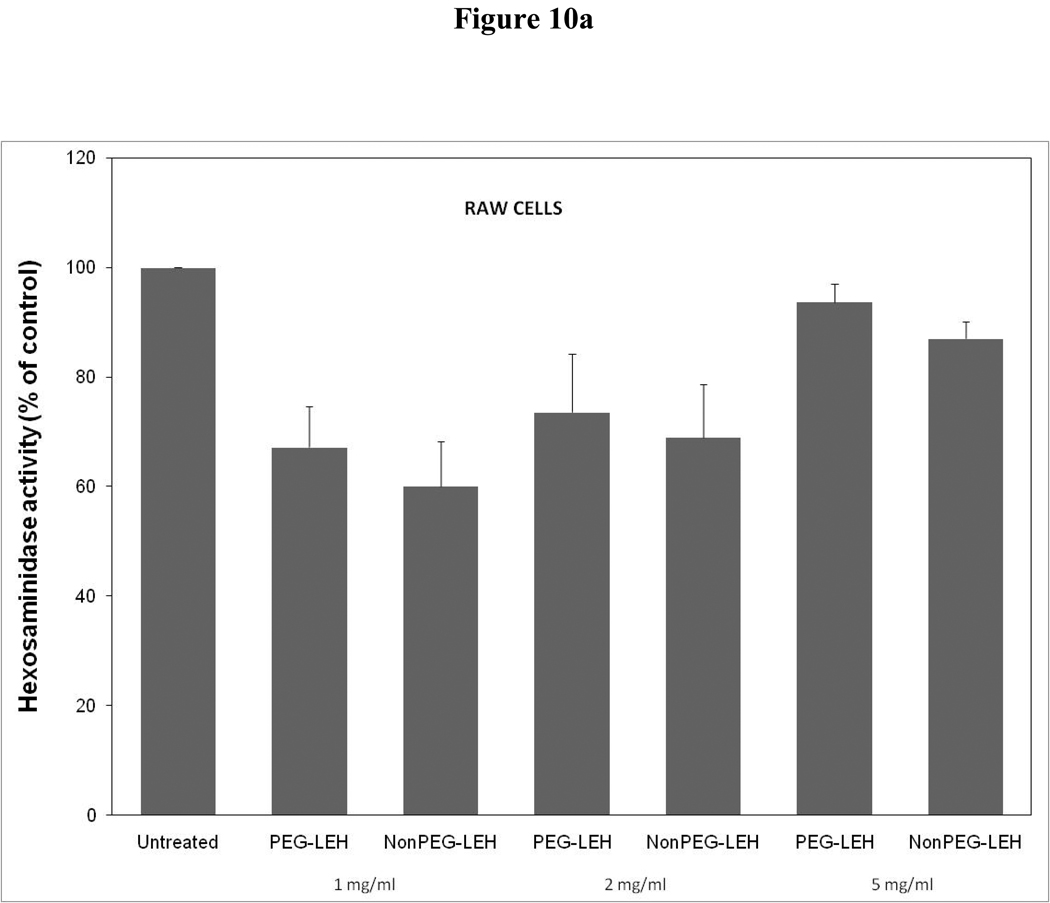
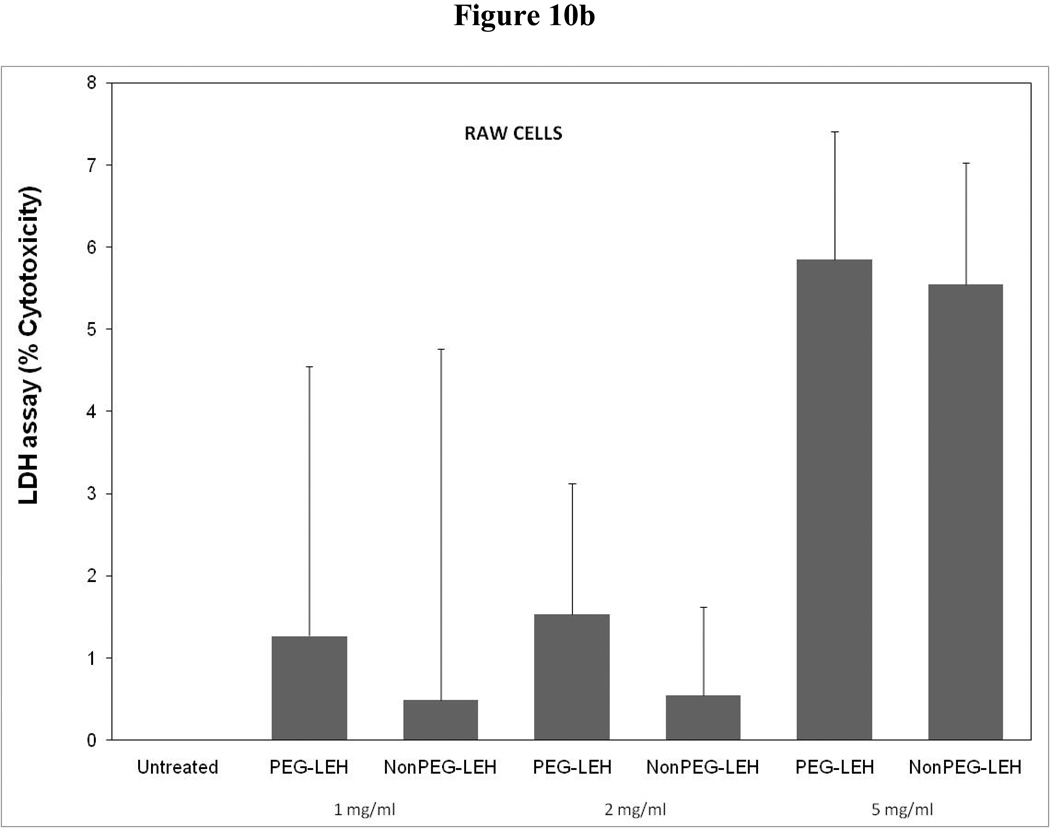
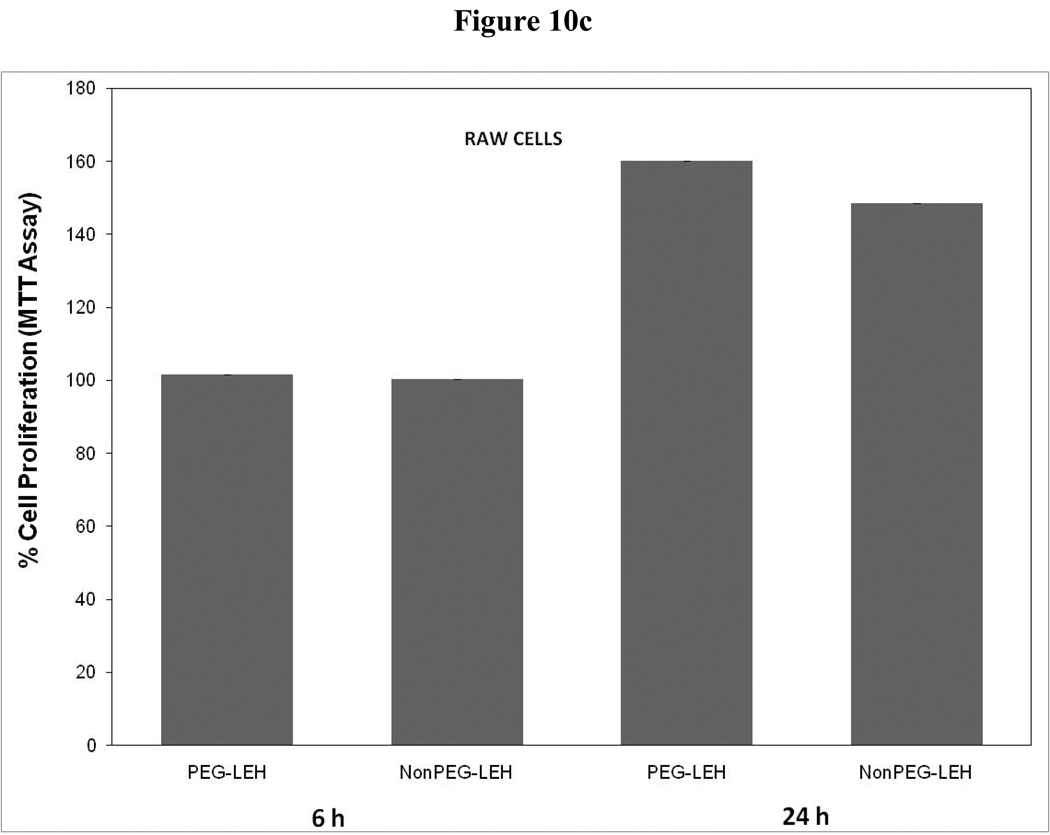
As a long-circulating oxygen carrier, LEH is expected to mostly interact with two cell types-endothelial cells in the vasculature and macrophages responsible for clearing particulate material. We studied the toxicity of our LEH formulation in HUVEC and RAW cell lines. Cells were grown in 96-well plates at a density of 104 cells/well. After 24h of culture, the cells were treated with LEH and empty liposomes carrying varying amounts of CHHDA; the cytotoxicity was measured as the decrease in hexosaminidase activity in cells after 24 h. As shown in Fig. 9a, the LEH showed minimal change in hexosaminidase activity in the HUVEC cells as compared to controls (100%) at any concentration that was tested. However, RAW cells (Fig. 10a) demonstrated significant toxicity. No significant difference was observed in cytotoxicity pro of PEGylated and non-PEGylated liposome at the tested concentration (p>0.05). All the empty liposome formulations tested appeared to be non-toxic to the cells (p>0.05). However, when LDH activity was measured in the medium of cultured cells, significant toxicity was observed at higher lipid concentrations (Fig. 9b and 10b). With MTT assay, the results did not show any measurable toxicity in any of the cell line at 6 h. Over 24 h exposures to LEH provided data suggestive of induction of cell growth which may be result of interference caused by various factors in the MTT assay [23–25].
Figure 9.
Cytotoxicity of LEH in RAW 264.7 cells evaluated by (a) hexosaminidase assay, (b) lactate dehydrogenase assay, and (c) 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide, or MTT assay. The cells for MTT assay were treated only at 5 mg/ml lipid dose.
Figure 10.
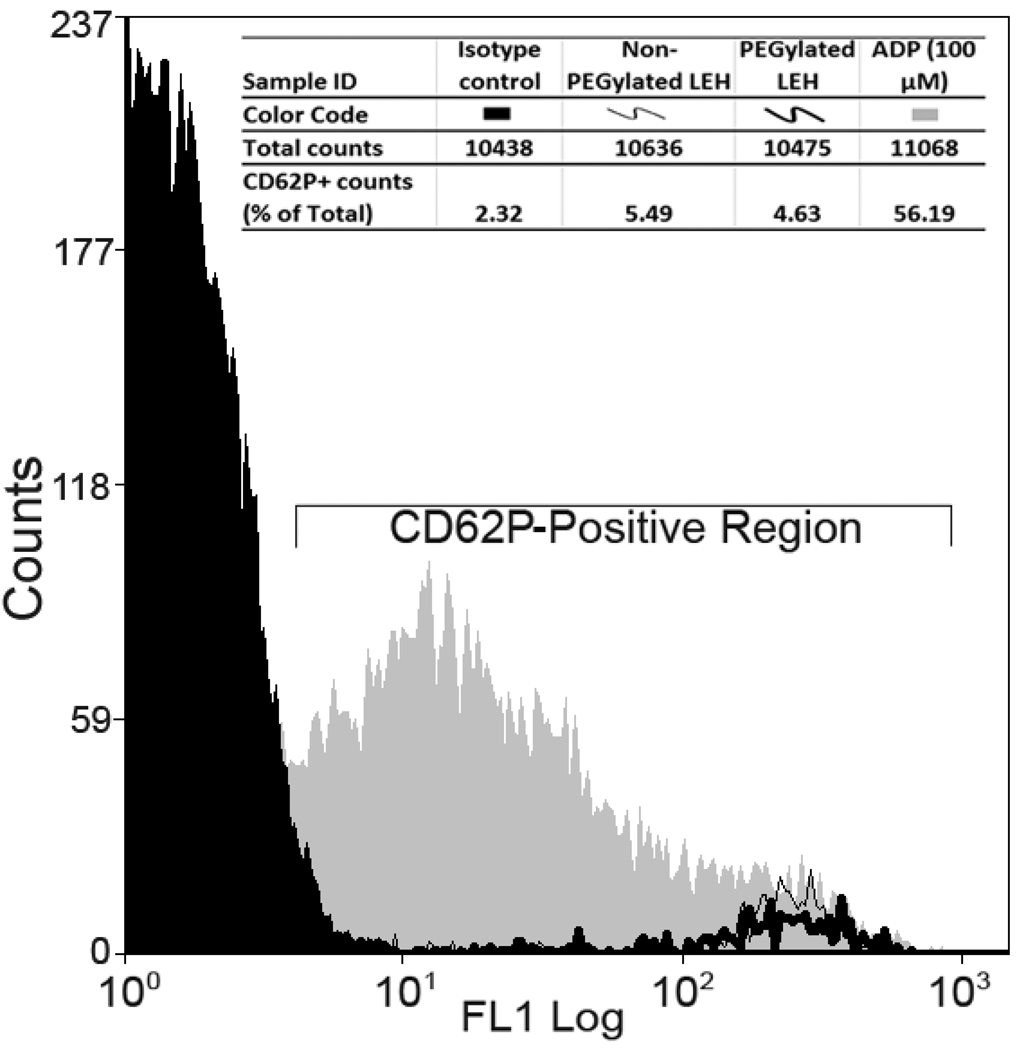
Effect of LEH on activation of platelets in vitro. ADP (100 µM) induces platelet activation characterized by increase in fluorescence intensity within CD62P-positive region. However, similar incubation with LEH demonstrated no such platelet activation.
Previous studies have shown that liposome preparations, such as LEH have a tendency to activate platelets. We performed a flow cytometry-based in vitro assay to investigate if the LEH treatment activates platelets and whether PEGylation has any impact on this phenomenon. CD61 is a marker of platelet glycoprotein IIIa, which is found on both normal (resting) and activated platelets. On the other hand, CD62P is found on the external membrane of activated platelets only. As shown in figure 11, LEH treatment did not demonstrate any platelet activation phenomenon, at least in the assay conditions employed in the study. PEGylation of LEH had no effect on the LEH-mediated response of the platelets. Treatment with ADP (100 µM) caused about 56% activation of platelets. The results were identical when the lipid-platelet interaction was allowed to occur for 30 min (data not shown).
Figure 11.
Effect of LEH on activation of platelets in vitro. ADP (100 µM) induces platelet activation characterized by increase in fluorescence intensity within CD62P-positive region. However, similar incubation with LEH demonstrated no such platelet activation.
4. DISCUSSION
LEH is primarily mostly composed of a combination of saturated high-carbon phospholipids, such as distearoyl phosphatidylcholine (DSPC, Tm 55°C) and dipalmitoyl phosphatidylcholine (DPPC, Tm 41°C) and cholesterol. Although, hemoglobin encapsulation in the liposomes has not been possible to match hemoglobin content of RBCs, it is desirable to encapsulate large amounts of hemoglobin within a minimum amount of lipid. Hemoglobin interacts with the phospholipid bilayer by both hydrophobic and ionic forces [26]. Ionic interaction seems stronger than the hydrophobic interaction and is dependent on pH and ionic strength of the medium [27]. As such, negatively charged lipids enhance encapsulation by interacting with oppositely charged domains of proteins. Using about 9 mol% of DPPG, in conjunction with optimal encapsulating conditions, Tsuchida and coworkers achieved the Hemoglobin-to-lipid ratio of 1.61 [5, 28]. Below its isoelectric point, Hb carries a positive charge and electrostatically interacts with negatively charge lipids resulting in increased encapsulation [5].
Apart from the beneficial interaction with hemoglobin, anionic lipids are known to undesirably enhance interaction of liposomes with complement and other opsonizing proteins in vivo [10, 12, 29]. Such interactions result in a rapid uptake of LEH by the RES, and toxic effects manifested as vasoconstriction, pulmonary hypertension, dyspnea, etc. Anionic phospholipids also enhance the rate of hemoglobin oxidation and displace heme relative to globin [30]. It is possible to partially reduce the toxicity of anionic phospholipids by PEG modification of LEH surface [18]. It is believed that a hydrophilic PEG coating on the liposome surface creates a steric barrier, enabling liposomes to circulate longer [31]. Recently, we have shown that PEGylation significantly reduces the thrombocytopenic effect of LEH administration in a rabbit model [13]. In view of the drawbacks of anionic phospholipids, an amino acid-based synthetic anionic lipid, 1,5-dipalmitoyl-l-glutamate-N-succinic acid, has been used in LEH [32]. It is believed that this amino acid-based lipid is better tolerated than anionic phospholipids.
The overall object of this work was to develop an LEH formulation with high Hb encapsulation based on ionic interaction with members of liposomal bilayer. In order to achieve this objective we synthesized several anionic lipids without phosphate groups. Four structurally different anionic lipids, as well as a cholesterol hemisuccinate (CHEMS), were synthesized for this purpose. All the anionic lipids were synthesized keeping in mind that they will be incorporated as a component of liposome membrane. All the lipids have features normally observed in phospholipid and considered essential for stable liposome formation, except that they do not have a phosphate group. These include a polar head group, at least a 16-member carbon double chain and melting points and molecular weights in the range of commonly used phospholipid. Negative charge was imparted by having either one (CHHDA,) or two (DTPA, DSTSA, DPGA and CHEMS) –COOH groups on the polar head.
The first step in LEH production was to make either DSPC or DPPC based pro-liposomes containing the novel anionic lipids. FT method effectively produced pro-liposomes in the size range of 397–441 nm for DSPC and DPPC based pro-liposomes, respectively. FT cycles reduce the lamellarity of liposomes thus converting multilamellar vesicles into unilamellar vesicles. Reduction in lamellarity is helpful in increasing encapsulation efficiency on lipid basis. During freezing process ice crystals are formed and disrupt the bilayers. Disrupted bilayers reassemble to form newer vesicles. Thus FT increases population of vesicles while decreasing the lamellarity [32, 33]. When FT process was coupled with sonication during thawing, the size reduction reached undesirably lower values. Hence the use of sonication was discontinued during further developmental stages. As expected, the number of cycles required to attain optimal pro-liposome size was dependent on the phospholipid used- DPPC (Tm=41°C) pro-liposomes took fewer cycles than DSPC (Tm=55°C) pro-liposomes. DPPC based formulation was considered more suitable for protein encapsulation due to milder processing conditions required. Hence this formulation was persisted with during further experiments.
The DPPC-based pro-liposomes were used to encapsulate hemoglobin. It was observed that liposomes containing CHHDA had the highest encapsulation efficiency. The experience with CHEMS was poor. Cholesterol is an essential component of most of the liposomes and is used sometimes as high as 50 mol %. An attempt was made to examine if some or all of the cholesterol can be replaced with CHEMS in LEH. It was believed that difference in the spatial orientation of CHEMS compared to anionic lipids in bilayers was responsible for the poor encapsulation. Somehow, CHEMS-containing LEH demonstrated high Met-Hb formation. The exact cause of superior performance of CHHDA is not known at this stage. CHHDA has only one –COOH group whereas other anionic lipids have two. This clearly indicates that the encapsulation is not a function of number of –COOH groups only. Orientation of lipids within the bilayer might affect packing of liposomes and rigidity. In this context it is worthwhile to note our observation that all preparations except those containing CHHDA revealed swelling-like phenomenon to varying degrees. Swelling did not increase with number of FT cycles and particle size was in the desired range of 400–450nm immediately after FT. However pro-liposomes (except CHHDA) were observed to undergo gel formation after 24 hr which was an indication of vesicle fusion. This might have some relation to the low encapsulation efficiency observed with these lipids. Since CHHDA resulted in maximum Hb encapsulation, further optimization was carried out using this anionic lipid. As the highest Hb encapsulation (4.02 g/dl, Hb/lipid ratio 1.2) was observed at 28 mol% of CHHDA, LEH 1 and LEH 2 were prepared with 28 mol% of CHHDA. At 40 mol% of CHHDA, liposomes did not form. High mol% of CHHDA might have resulted in a very high electrostatic repulsion and disruption of bilayers.
The oxygen affinity is measured in terms of the partial pressure of oxygen required to saturate 50% of hemoglobin or p50. p50 of hemoglobin is altered by several allosteric modifiers. Since anionic lipids interact with hemoglobin fairly intimately, it was interesting to study if CHHDA at 28 mol% concentration influences p50 of Hb. There was insignificant effect of CHHDA on the p50 of encapsulated hemoglobin. More importantly, interaction between CHHDA and Hb did not affect the ability of PLP to enhance p50. PLP is an established allosteric modifier and its use in LEH2 easily altered p50 to 33 Torr. Our previous experience suggested that LEH made up of the purified stroma-free hemoglobin (SFH) should have shown lower p50 value (<10 Torr) (Table 1 d). However, in case of LEH 1, the batch without PLP, the p50 value was about 19. The reasons for this discrepancy are not known, but are suggestive of some role played by the age of the outdated RBCs, state of their storage and the method of isolation. The previously reported purified SFH with high oxygen affinity was isolated from outdated RBCs kept frozen for several months; the isolation technique was based on hypotonic lysis of RBCs followed by sequential filtration through filters of decreasing pore size [17, 18]. In comparison, the SFH used in this work was from RBCs collected within 1 week of being outdated, kept at 4°C, and hemoglobin isolated by method based on the use of DCM [15].
Using high-pressure homogenization, we were able to reduce the particle size of LEH to a desired range. It has been reported earlier that the optimum size of PEGylated LEH for prolonged circulation after administration is 210–240 nm [18, 34].
As a long-circulating oxygen carrier, LEH is expected to mostly interact with two cell types-endothelial cells in the vasculature and macrophages responsible for clearing particulate material. We studied the toxicity of our LEH formulation in HUVEC and RAW cell lines. Our choice of hexosaminidase assay as the preferred method to test cytotoxicity was based on the apparent inability of MTT assay to provide accurate data in certain situations. For instance, it has been shown that in macrophage cell line RAW 264.7, macrophage activation causes interference in MTT assay [24]. The investigators also suggested that artifacts may also arise if the tested cell line is iNOS-expressing. Apparently, HUVEC cells express inducible iNOS [35]. Both these observations make MTT assay a questionable test in these two cells lines. Our own results from MTT assay provide contradictory findings that are suggestive of induction of cell proliferation rather than its inhibition. In yet another published study on MTT, it has been shown that presence of colored substances in the samples may result in significant inaccuracy [25]. Our own samples contain hemoglobin containing liposomes. Lastly, hemoglobin has been shown by MTT assay to induce colon cancer cell proliferation [23]. In light of these studies and our own experience, it appears that MTT assay may not be the appropriate assay in our test system. Based on hexosaminidase assay, it appears that HUVEC cells do not show any toxicity, but RAW cells show significant toxicity. On the other hand, LDH assay results indicate towards toxicity in both HUVEC as well as RAW cells. There are no other published reports on such long-term in vitro toxicity of LEH-like preparations, except the one by Centis and Vermette, who found 60–70% cell death after 24 h of treatment with the formulation consisting of DPPC:Chol: PA:DMPE-PEG (5:5:1:0.3%) [36]. Upon comparison, our formulation appears to be better tolerated by endothelial cells and macrophages.
In addition, the LEH preparations containing CHHDA were not found to activate platelets in vitro. Studies in animal models receiving LEH or liposomes noted a formulation-dependent transient decrease in circulating platelets immediately following an intravenous infusion [37]. Such an undesired reaction remains an obstacle to the successful application of LEH as a universal resuscitation fluid. The observation that in vitro platelet aggregation is not affected by incubation with LEH indicates an essential role of a systemic element in inducing platelet reaction [38]. It has been since demonstrated that complement system plays an integral role in LEH-induced thrombocytopenia [39], causing activation of classical complement pathway after interaction between the phospholipid bilayer and C reactive protein. A recent article has also shown that anionic charge on the surface of the liposome plays a key role in activation of both classical and alternative complement pathway [40]. Liposomes containing negatively-charged phospholipids induce a very rapid decline in circulating platelets that is more severe, and that takes a longer time to recover to normal levels as compared to the liposomes consisting of neutral phospholipids. Liposomes containing phosphatidylglycerol form micro-aggregates with platelets in vitro, and it has been suggested that the sequestration of these micro-aggregates by the reticuloendothelial system causes the decline in circulating platelets [41].
The utility of LEH is most obvious when there is reduction in the oxygen carrying capacity secondary to a severe blood loss. Uncontrolled hemorrhage is also characterized by progressive depletion of circulating thrombocytes resulting in an inability to initiate effective hemostasis. In these circumstances, a further thrombocytopenic reaction to resuscitative fluid would exacerbate an already compromised hemostasis condition. Therefore, the LEH preparation should be formulated to eliminate the platelet reaction seen with liposome administration. In our recent publication, we reported the effect of charge and PEGylation on platelet activation by LEH in a rabbit model [13]. The tested LEH preparation contained dimyristoylphosphatidylglycerol (DMPG) as the anionic lipid.
5. Conclusions
In this report we presented a formulation of LEH containing novel anionic non-phospholipid to enhance hemoglobin encapsulation. The LEH formulation showed minimal toxicity to endothelial cells and macrophages in culture, and did not demonstrate any platelet activating tendency. With enhanced hemoglobin inside the liposomes and optimal particle size, this LEH formulation is being investigated in our laboratory as a resuscitative fluid in hemorrhagic shock model.
Acknowledgements
The authors acknowledge the help of Dr. Brian Grady (University of Oklahoma at Norman, OK) for his help in DSC. Oklahoma Blood Institute’s willingness to share outdated RBC units in this research is appreciated. The work was funded by a grant from National Institutes of Health/National Institute of Biomedical Imaging and Bioengineering (5R21EB005187-03).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Awasthi V. Curr Drug Deliv. 2005;2:133–142. doi: 10.2174/1567201053586029. [DOI] [PubMed] [Google Scholar]
- 2.Phillips WT, Klipper RW, Awasthi VD, Rudolph AS, Cliff R, Kwasiborski V, Goins BA. J Pharmacol Exp Ther. 1999;288:665–670. [PubMed] [Google Scholar]
- 3.Takaori M, Fukui A. Artif Cells Blood Substit Immobil Biotechnol. 1996;24:643–653. doi: 10.3109/10731199609118889. [DOI] [PubMed] [Google Scholar]
- 4.Usuba A, Motoki R, Sakaguchi K, Suzuki K, Kamitani T. Artif Cells Blood Substit Immobil Biotechnol. 1994;22:503–516. doi: 10.3109/10731199409117878. [DOI] [PubMed] [Google Scholar]
- 5.Sakai H, Hamada K, Takeoka S, Nishide H, Tsuchida E. Biotechnol Prog. 1996;12:119–125. doi: 10.1021/bp950068w. [DOI] [PubMed] [Google Scholar]
- 6.Farmer MC, Johnson SA, Beissinger RL, Gossage JL, Lynn AB, Carter KA. Adv Exp Med Biol. 1988;238:161–170. doi: 10.1007/978-1-4684-7908-9_13. [DOI] [PubMed] [Google Scholar]
- 7.Rudolph AS, editor. Encapsulation of hemoglobin in liposomes. Boston: Birkhauser; 1995. [Google Scholar]
- 8.Drummond DC, Meyer O, Hong K, Kirpotin DB, Papahadjoulos D. Pharmacol. Rev. 1999;51:691–743. [PubMed] [Google Scholar]
- 9.Walde P, Ichikawa S. Biomol. Eng. 2001;18:143–177. doi: 10.1016/s1389-0344(01)00088-0. [DOI] [PubMed] [Google Scholar]
- 10.Miller CR, Bondurant B, McLean SD, McGovern KA, O'Brien DF. Biochemistry. 1998;37:12875–12883. doi: 10.1021/bi980096y. [DOI] [PubMed] [Google Scholar]
- 11.Semple SC, Chonn A, Cullis PR. Adv. Drug Delivery Rev. 1998;32:3–17. doi: 10.1016/s0169-409x(97)00128-2. [DOI] [PubMed] [Google Scholar]
- 12.Szebeni J. Crit Rev Ther Drug Carrier Syst. 1998;15:57–88. [PubMed] [Google Scholar]
- 13.Awasthi VD, Goins B, Phillips WT. Am J Pharmacol Toxicol. 2007;2:98–105. [Google Scholar]
- 14.Szebeni J, Baranyi L, Savay S, Bodo M, Morse DS, Basta M, Stahl GL, Bunger R, Alving CR. Am J Physiol Heart Circ Physiol. 2000;279:H1319–H1328. doi: 10.1152/ajpheart.2000.279.3.H1319. [DOI] [PubMed] [Google Scholar]
- 15.Sakai H, Takeoka S, Yokohama H, Seino Y, Nishide H, Tsuchida E. Protein Expr Purif. 1993;4:563–569. doi: 10.1006/prep.1993.1074. [DOI] [PubMed] [Google Scholar]
- 16.Tomita S EY, Santa M, Yoshida H, Yasumitsu Y. J Nara Med Assoc. 1968;19:1–6. [Google Scholar]
- 17.Awasthi VD, Garcia D, Klipper R, Phillips WT, Goins BA. International journal of pharmaceutics. 2004;283:53–62. doi: 10.1016/j.ijpharm.2004.06.015. [DOI] [PubMed] [Google Scholar]
- 18.Awasthi VD, Garcia D, Klipper R, Goins BA, Phillips WT. J Pharmacol Exp Ther. 2004;309:241–248. doi: 10.1124/jpet.103.060228. [DOI] [PubMed] [Google Scholar]
- 19.Stewart JCM. Anal. Biochem. 1980;104:10–14. doi: 10.1016/0003-2697(80)90269-9. [DOI] [PubMed] [Google Scholar]
- 20.Matsuoka T. Biol Pharm Bull. 1997;20:1208–1211. doi: 10.1248/bpb.20.1208. [DOI] [PubMed] [Google Scholar]
- 21.Landegren U. J Immunol Methods. 1984;67:379–388. doi: 10.1016/0022-1759(84)90477-0. [DOI] [PubMed] [Google Scholar]
- 22.Shigeta K, Taniguchi N, Omoto K, Madoiwa S, Sakata Y, Mori M, Hatake K, Itoh K. J Ultrasound Med. 2003;22:365–373. doi: 10.7863/jum.2003.22.4.365. [DOI] [PubMed] [Google Scholar]
- 23.Lee RA, Kim HA, Kang BY, Kim KH. World J Gastroenterol. 2006;12:5644–5650. doi: 10.3748/wjg.v12.i35.5644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pozzolini M, Scarfi S, Benatti U, Giovine M. Analytical biochemistry. 2003;313:338–341. doi: 10.1016/s0003-2697(02)00631-0. [DOI] [PubMed] [Google Scholar]
- 25.Wang X, Ge J, Wang K, Qian J, Zou Y. Assay and drug development technologies. 2006;4:203–207. doi: 10.1089/adt.2006.4.203. [DOI] [PubMed] [Google Scholar]
- 26.Szebeni J, Hauser H, Eskelson CD, Watson RR, Winterhalter KH. Biochemistry. 1988;27:6425–6434. doi: 10.1021/bi00417a034. [DOI] [PubMed] [Google Scholar]
- 27.Pitcher WH, 3rd, Keller SL, Huestis WH. Biochim Biophys Acta. 2002;1564:107–113. doi: 10.1016/s0005-2736(02)00405-4. [DOI] [PubMed] [Google Scholar]
- 28.Takeoka S, Ohgushi T, Terase K, Ohmori T, Tsuchida E. Langmuir. 1996;12:1755–1759. [Google Scholar]
- 29.Cullis PR, Chonn A, Semple SC. Adv Drug Deliv Rev. 1998;32:3–17. doi: 10.1016/s0169-409x(97)00128-2. [DOI] [PubMed] [Google Scholar]
- 30.Szebeni J, Hauser H, Eskelson CD, Winterhalter KH. Biomater Artif Cells Artif Organs. 1988;16:301–312. doi: 10.3109/10731198809132579. [DOI] [PubMed] [Google Scholar]
- 31.Torchilin VP, Papisov MI. J. Liposome Res. 1994;4:725–739. [Google Scholar]
- 32.Sou K, Naito Y, Endo T, Takeoka S, Tsuchida E. Biotechnol Prog. 2003;19:1547–1552. doi: 10.1021/bp0201004. [DOI] [PubMed] [Google Scholar]
- 33.Sriwongsitanont MUS. Colloid Polym Sci. 2004;282:753–760. [Google Scholar]
- 34.Awasthi VD, Garcia D, Goins BA, Phillips WT. International journal of pharmaceutics. 2003;253:121–132. doi: 10.1016/s0378-5173(02)00703-2. [DOI] [PubMed] [Google Scholar]
- 35.Persson K, Sauma L, Safholm A, Xu L, Li W, Yuan XM. Apmis. 2009;117:1–9. doi: 10.1111/j.1600-0463.2008.00001.x. [DOI] [PubMed] [Google Scholar]
- 36.Centis V, Vermette P. Colloids and surfaces. 2008;65:239–246. doi: 10.1016/j.colsurfb.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 37.Rabinovici R, Rudolph AS, Vernick J, Feuerstein G. Critical care medicine. 1994;22:480–485. [PubMed] [Google Scholar]
- 38.Phillips WT, Klipper R, Fresne D, Rudolph AS, Javors M, Goins B. Exp Hematol. 1997;25:1347–1356. [PubMed] [Google Scholar]
- 39.Goins B, Phillips WT, Klipper R, Rudolph AS. The Journal of surgical research. 1997;68:99–105. doi: 10.1006/jsre.1997.5014. [DOI] [PubMed] [Google Scholar]
- 40.Moghimi SM, Hamad I, Andresen TL, Jorgensen K, Szebeni J. Faseb J. 2006;20:2591–2593. doi: 10.1096/fj.06-6186fje. [DOI] [PubMed] [Google Scholar]
- 41.Loughrey HC, Bally MB, Reinish LW, Cullis PR. Thrombosis and haemostasis. 1990;64:172–176. [PubMed] [Google Scholar]