Abstract
Avian pulmonary capillaries differ from those of mammals in three important ways. The blood-gas barrier is much thinner, it is more uniform in thickness, and the capillaries are far more rigid when their transmural pressure is altered. The thinness of the barrier is surprising because it predisposes the capillaries to stress failure. A possible mechanism for these differences is that avian pulmonary capillaries, unlike mammalian, are supported from the outside by air capillaries, but the details of the support are poorly understood. To clarify this we studied the blood and air capillaries in chicken lung using transmission electron microscopy (EM) and two relatively new techniques that allow 3D visualization: electron tomography and serial block-face scanning EM. These studies show that the pulmonary capillaries are flanked by epithelial bridges composed of two extremely thin epithelial cells with large surface areas. The junctions of the bridges with the capillary walls show thickening of the epithelial cells and an accumulation of extracellular matrix. Collapse of the pulmonary capillaries when the pressure outside them is increased is apparently prevented by the guy wire-like action of the epithelial bridges. The enlarged junctions between the bridges and the walls could provide a mechanism that limits the hoop stress in the capillary walls when the pressure inside them is increased. The support of the pulmonary capillaries may also be explained by an interdependence mechanism whereby the capillaries are linked to a rigid assemblage of air capillaries. These EM studies show the supporting structures in greater detail than has previously been possible, particularly in 3D, and they allow a more complete analysis of the mechanical forces affecting avian pulmonary capillaries.
Keywords: epithelial bridges, epithelial plates, stress failure, electron tomography, serial block-face scanning electron microscopy
1. Introduction
It is remarkable that the two classes of vertebrates capable of sustained high oxygen consumptions, the mammals and birds, have radically different lungs. The mammalian lung uses reciprocating ventilation with large terminal air spaces (alveoli). By contrast, the avian lung has a flow-through system with small air capillaries. As a consequence, the environment of the pulmonary capillaries is very different between mammals and birds.
The present study was prompted by three important differences between avian and mammalian pulmonary capillaries. First, the blood-gas barrier is much thinner in birds than in mammals. For example, an analysis of data from 34 species of birds and 37 of mammals found that the mean harmonic thickness of the barrier was 0.19 µm and 0.47 µm respectively, that is it differed by a factor of 2.5 (Maina and West, 2005; West, 2009). The extreme thinness of the barrier is paradoxical because flying is very energetic, and the thinness of the barrier predisposes it to stress failure. It is known that mammals that have thicker pulmonary capillary walls can develop stress failure of the barrier during heavy exercise (West, 2009).
Second, the blood-gas barrier is much more uniform in thickness in the chicken than in mammals such as rabbit, dog, and horse (Watson et al., 2007). The reason is that mammals have a type I collagen cable that threads its way along the alveolar wall thickening one side of each capillary in the process. It has been suggested that this cable is necessary for the mechanical integrity of the long alveolar wall (Weibel, 1973), but it is not required for the avian pulmonary capillaries which are nested in a honeycomb-like arrangement of small, supporting air capillaries.
A third striking difference is that in birds, the capillaries are essentially rigid whereas in mammals they show a remarkable degree of recruitment and distention. The rigidity of avian pulmonary capillaries is highlighted by changing the pressure difference between the inside and outside of the capillary. For example, when the pressure outside the avian capillaries was increased to 35 cm H2O (26 mm Hg) higher than the pressure inside, the capillaries remained open whereas in mammalian lung they are squashed completely flat under these conditions (Watson et al., 2008).
It seems likely that these differences between avian and mammalian capillaries are related to the immediate environment of the small blood vessels. In mammals, the pulmonary capillaries are strung out along the long alveolar wall and are unsupported at right angles to wall. By contrast in birds, the pulmonary capillaries are intimately surrounded by air capillaries of approximately the same diameter, and the presumption is that the blood capillaries are therefore supported in some way. However the micromechanics of this support, if indeed it exists, are not understood.
The purpose of the present study was to obtain better information on the structures surrounding the pulmonary capillaries in birds in the hope that we can gain a better understanding of how the avian blood-gas barrier can be so much thinner and more uniform than in mammals, and why avian pulmonary capillaries are so rigid. Studies were made by transmission electron microscopy of chicken lung fixed by intratracheal instillation of glutaraldehyde. In addition, we exploited two relatively new techniques for obtaining three-dimensional information, namely electron tomography, and serial block-face scanning electron microscopy. Both techniques are well suited to studying the environment of avian pulmonary capillaries but we are not aware of any previous studies of this material using these methods.
2. Methods
2.1. Preparation of specimens
The animal protocols for these experiments were approved by the Animal Subject Committees of the University of California San Diego. White Leghorn chickens (Gallus gallus domesticus) were anesthetized with intravenous pentobarbital sodium (40 mg/kg) and a cannula was inserted into the trachea. The lungs were then fixed by intratracheal installation of 3% glutaraldehyde using a pressure of 25 cm H2O for 15 min.
Lung samples were taken from the paleopulmo portion of lung from each animal at about one-third distance from the most caudal aspect of the lung. A section of tissue 0.5 cm thick was excised from the entire width of each lung transverse to the cranial-caudal axis. This section was then cut into approximately 10 vertical slices and trimmed into blocks.
2.2. Transmission electron microscopy
Blocks were rinsed overnight in 0.1 M phosphate buffer (350 mOsm, pH 7.4) and postfixed for two hours in osmium tetroxide (1% osmium tetroxide in 0.125 sodium cacodylate buffer; 400 mOsm, pH 7.4). The samples were then passed through stepwise dehydration in increasing concentrations of ethanol (50–100 percent), rinsed with propylene oxide and embedded in Araldite. Blocks were then cut into ultra thin sections (50–70 nm) and contrast stained with saturated uranyl acetate and bismuth subnitrate. Sections were examined at an accelerating voltage of 60 kV using a Zeiss EM 10C transmission electron microscope. Micrographs of a carbon grating replica were taken for calibration.
2.3. Electron tomography
Electron tomography was carried out on sections of 0.5 and 1.0 µm thickness. Images were acquired using a JEM4000EX IVEM (400 kV) microscope equipped with a NCMIR custom design 4K lens coupled CCD camera (Fan et al., 2000). The sections were successively tilted through 2 degree increments from −64 to +64 degrees. Part of an epithelial bridge was collected as a single tilt series and a central portion of the epithelial bridges was collected as a double tilt series. The backprojected volume images were reconstructed using both the Boulder Laboratory’s IMOD Tomography package suite and NCMIR’s Transform Based Reconstruction (TxBR) software. Visualizations of the backprojected volumes was completed using Visage Imaging’s Amira software suite. General information about electron tomography is available in Frank (1992).
2.4. Serial block-face scanning electron microscopy
Blocks of lung tissue were specially prepared for serial block-face scanning EM as follows. After primary aldehyde fixation the tissue was rinsed in 0.1 sodium cacodylate and post-fixed in 0.1% potassium ferrocyanide-reduced 2% osmium tetroxide in cacodylate buffer for 1 hour. Tissue was then rinsed in distilled water and treated with 0.1% aqueous thiocarbohydrazide for 20 minutes. After further rinsing in distilled water the tissue was again treated with 2% osmium tetroxide for 30 minutes, rinsed in distilled water , dehydrated in an ethanol series and infiltrated with Durcupan ACM resin.
The tissue blocks were mounted on an aluminum pin and trimmed to 1 mm × 0.5 mm in size. The specimen was placed in a scanning electron microscope (FEI Quanta FEG) equipped with a serial block-face sectioning unit (Gatan 3 View) and a backscatter electron image of the face obtained. An automatic microtome then removed a 60 nm thick slice from the face and another image was recorded. This procedure was then repeated 500 times to give a data set from which a complete three-dimensional reconstruction 30 µm thick could be derived. The separate images were processed using Amira (Visage Imaging) to create maximum intensity projections, slice by slice renderings and segmentations of epithelial bridges. A general description of serial block face scanning EM is given in Denk and Horstmann (2004).
3. Results
3.1. Ultrastructure of the epithelial bridges
The epithelial structures that join two adjacent capillaries and that form part of the wall of the air capillaries are known as epithelial bridges. These are very thin in cross-section but the epithelial cells that make up each bridge have a large area and are here called epithelial plates. It is convenient to describe the central portions of the bridges first and then the junctions of the bridges with the capillary walls.
3.1.1. Central portions of the epithelial bridges
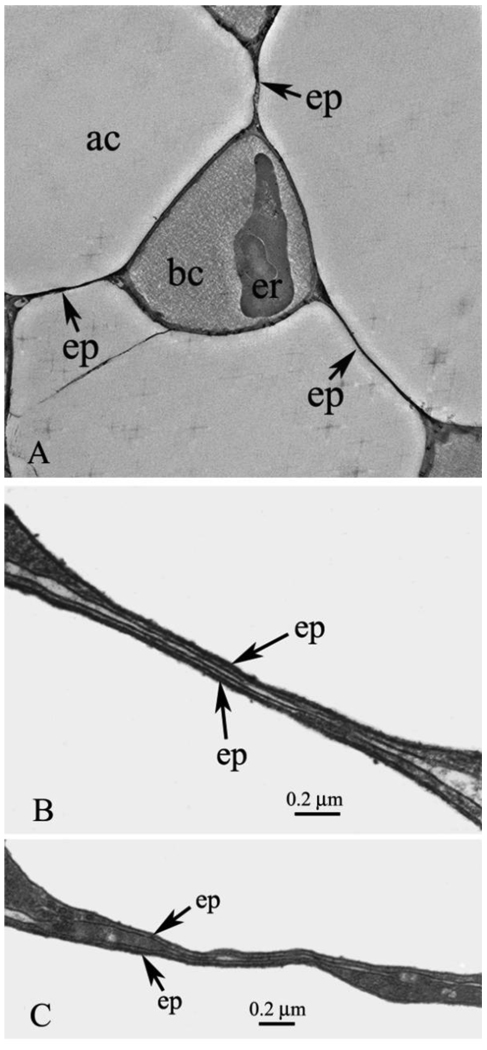
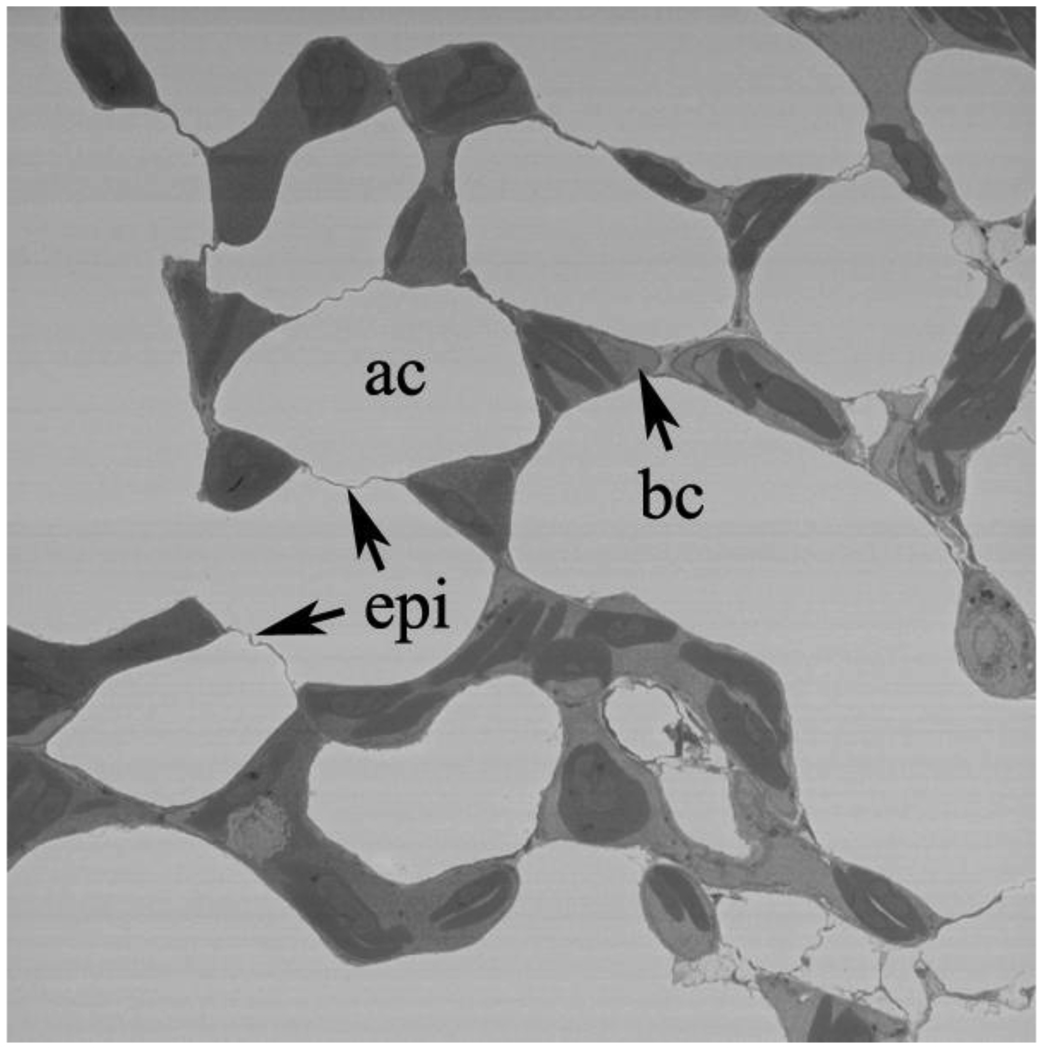
Figs. 1A, B, and C show central portions of epithelial bridges. Fig. 1A is a relatively low power EM but nicely shows three bridges and their attachments to a single capillary. This figure is a screen shot from Supplementary Material file SM1 which is an electron tomography study that shows more clearly how the bridges link to the capillary.
Fig. 1.
A. Pulmonary capillary with three epithelial bridges linking it to surrounding blood capillaries. The expansions of the bridges at the junctions with the capillaries can be seen and also a nucleated red blood cell. ac, air capillary; bc, blood capillary; ep, epithelial bridge; er, erythrocyte. This is a screenshot from the video clip submitted as Supplementary Material SM1. B. High-power EM showing the detail of an epithelial bridge. The two extremely thin epithelial cells are clearly seen and both cell membranes of each can be identified. There is a small amount of matrix material between the cells. ep, epithelial cell. C. Another example of an epithelial bridge. Part of the bridge is very thin as in Fig. 1B but other parts show thickening of one of one of the epithelial cells. This may be because the plane of the section is not exactly at right angles to the plane of the cells. ep, epithelial cell.
Figs. 1B and C show the central portions of the bridges at high magnification. It can be seen that the bridges are made up of two separate epithelial cells with a small amount of matrix material between them. The total thickness of the central part of the bridges in these cross-sections is of the order of 50 nm. The four layers comprising the inner and outer cell membranes of both epithelial bridge cells are clearly seen. In Fig. 1B the bridge has a remarkably uniform thickness over most of its length whereas in Fig. 1C there is some variation in thickness. This may be because the micrograph of Fig. 1B happened to be at exactly right angles to the plane of the bridge, whereas in Fig. 1C this was not the case, and some thickening of the epithelial cell is seen because the plane of the section cuts slightly obliquely across the cell. In the thin central parts of the bridge the cytoplasm of the cells has a uniform appearance with no visible inclusions, but as the cells widen, approaching the junctions with the capillary wall, small circular structures in the cells are apparent. A notable feature of the bridges is that they do not include any intercellular junctions, and this is also the case in micrographs published by other investigators (Klika et al., 1997; Weidner et al., 1993).
3.1.2. Junctions of the epithelial bridges with the capillary walls
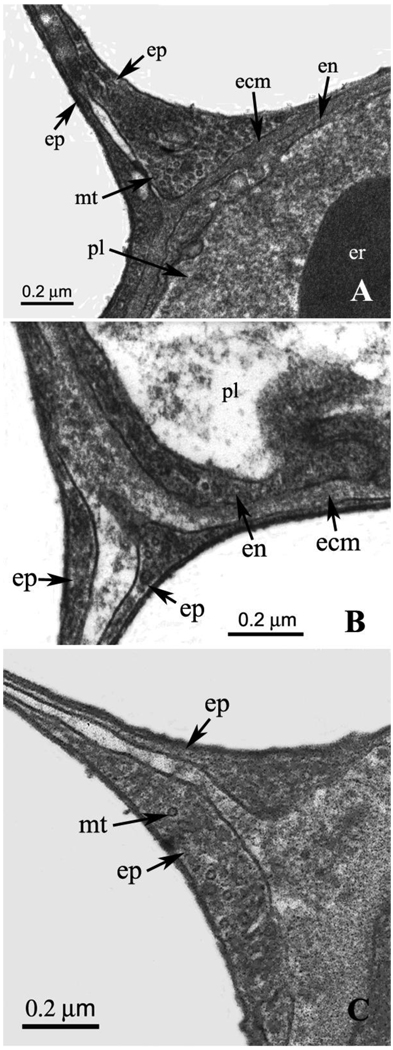
Examples of junctions are shown in Figs. 2A and B. It can be seen that the epithelial cells of the bridges are contiguous with the epithelium that forms the outer cellular layer of the pulmonary capillary. Fig. 2A shows that there is a marked widening of one of the epithelial bridge cells near the capillary wall. This is also seen in Fig. 2B to a lesser extent. Both figures show a communication between the extracellular matrix of the capillary wall and the space between the two epithelial cells of the bridge. However the material in the space between the cells is less electron dense as we move away from the junction. It is not clear how far the extracellular matrix of the capillary wall actually extends into the epithelial bridge or whether the matrix material in the center of the bridge has a different composition. This is potentially important because the extracellular matrix of the capillary wall is believed to contain type IV collagen which has a high ultimate tensile strength (West, 2009). Fig. 2B shows an enlargement of the extracellular matrix material at the junction between the bridge and the capillary wall. Both micrographs show very small circular structures of about 20 nm diameter within the epithelial cells close to the capillary wall. It is possible that these are microtubules.
Fig. 2.
A. High-power EM of a junction of an epithelial bridge with a capillary wall. Marked enlargement of one of the epithelial cells is well shown. This section of the cell clearly shows extremely small circular inclusions that have a diameter of approximately 20 nm. These may be microtubules. The space between the two epithelial cells making up the bridge is contiguous with extracellular matrix of the capillary wall. ecm, extracellular matrix; en, endothelial cell; ep, epithelial cell; er, erythrocyte; mt, microtubule; pl, plasma. B. Another example of a junction between them an epithelial bridge and a capillary wall. Again there is some enlargement of the epithelial cells near the junction. The small circular inclusions referred to in relation to Fig. 2A are just visible. Also the space between the two epithelial cells is contiguous with extracellular matrix of the capillary wall. ecm, extracellular matrix; en, endothelial cell; ep, epithelial cell; pl, plasma. C. Another example of a junction showing the continuation of the extracellular matrix of the capillary wall into the space between the two epithelial cells of the bridge. This section was cut diagonally which explains the thickness of the extracellular matrix layer. ep, epithelial cell; mt, microtubule.
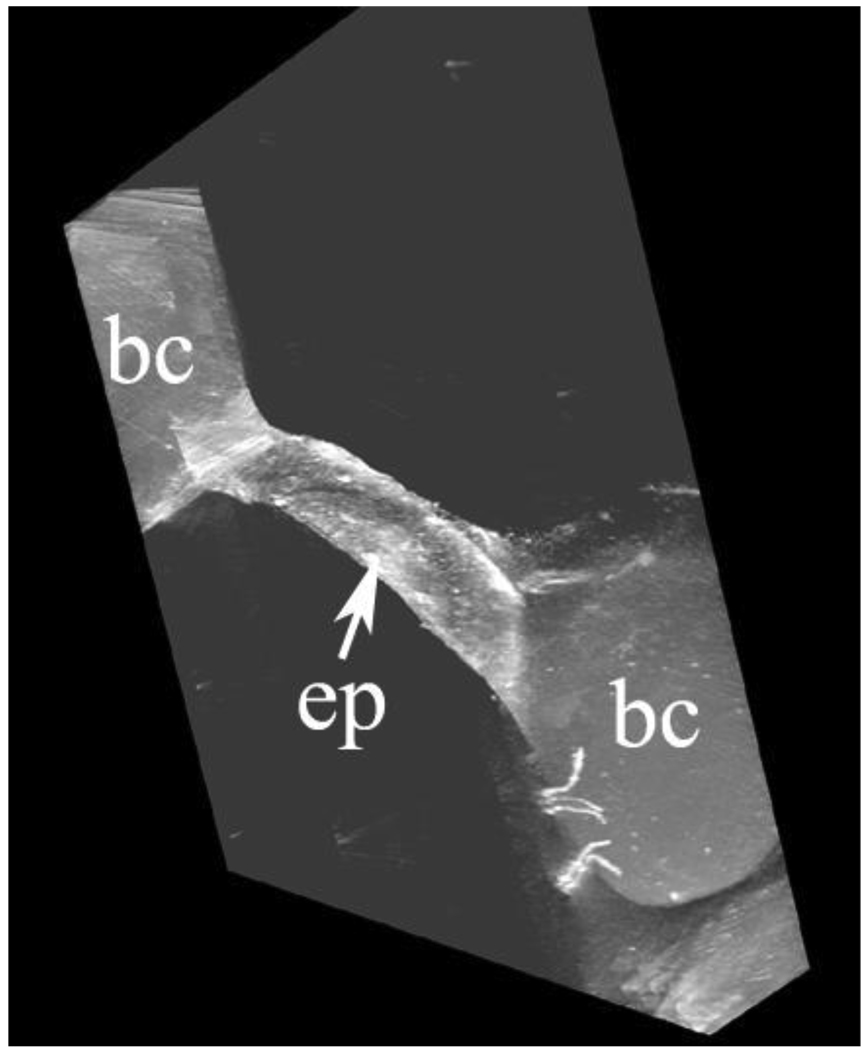
Fig. 3 shows part of an epithelial bridge from an electron tomography study. The figure is a screen shot from a file submitted as Supplementary Material SM2. The epithelial bridge is shown as a thin ribbon 1 µm wide. This is because the thickness of the tissue section is only 1 µm. However Fig. 3 and the SM2 clearly show the three-dimensional configuration of the junction between the bridge and the capillary. This emphasizes that the enlargements of the junctions shown in Figs. 2A and B extend along the capillary wall. It would be desirable to have thicker tissue sections so that a greater width of the epithelial bridge could be seen. However the thickness of the section is limited by the energy of the electrons. In this particular microscope the 300 KV electrons will not penetrate a thicker tissue slice satisfactorily especially when the slice is tilted so that the distance through which the electrons need to penetrate is increased.
Fig. 3.
Epithelial bridge connecting two pulmonary capillaries. The ribbon is 1 µm wide because this was the thickness of the tissue section but of course the bridge is much wider. The expansion of the epithelial cells at the junctions with the capillary walls is also shown. ep, epithelial bridge; bc, blood capillary. This is a screenshot from the video clip submitted as Supplementary Material SM2.
3.1.3. Epithelial plates
The appearance of the whole of the epithelial bridges in three dimensions was studied using serial block-face SEM. A screen shot from a file is shown in Fig. 4 and the complete video clip is in Supplementary Material SM3. For this preparation, the perimeter of a single epithelial bridge was traced by an operator using a video pen and assigning it a green color to distinguish it from the surrounding capillaries that were colored blue. The complete epithelial bridge comprised of two epithelial plates is clearly seen. The printed version of Figure 4 is in black and white whereas the online version and SM3 show the colors. In part of the video sequence we can see through the thin semi-transparent epithelial bridge to the capillaries behind. In another part of the sequence we look down through the center of an air capillary and see the epithelial bridge completely closing the gap between pulmonary capillaries at the far end. In another part of the sequence the blue pulmonary capillaries were removed by appropriate software allowing the bridge and its component plates to be clearly seen. The plates are seen in both plan view and from the side.
Fig. 4.
A. Plane view of an epithelial plate which is one of the two cells making up the bridge. Note that it is connected to the surrounding pulmonary capillaries around its complete perimeter. The epithelial bridge is so thin that it is possible to see structures on the other side of the plate in the video clip. ep, epithelial plate; bc, blood capillary. B. Side view of an epithelial bridge showing the two epithelial plates. For this view the capillaries were removed by the operator using appropriate software. epi pl, epithelial plate. Both Figs. 4A and 4B are from the video clip submitted as Supplementary Material SM3.
Figure 4 and SM3 emphasize that the epithelial bridge is part of the wall of the air capillary. They also show how the junction of the bridge with the capillary wall follows any curvature of the wall. This is important when we consider the role of the junction in influencing the mechanical properties of the pulmonary capillaries as discussed below.
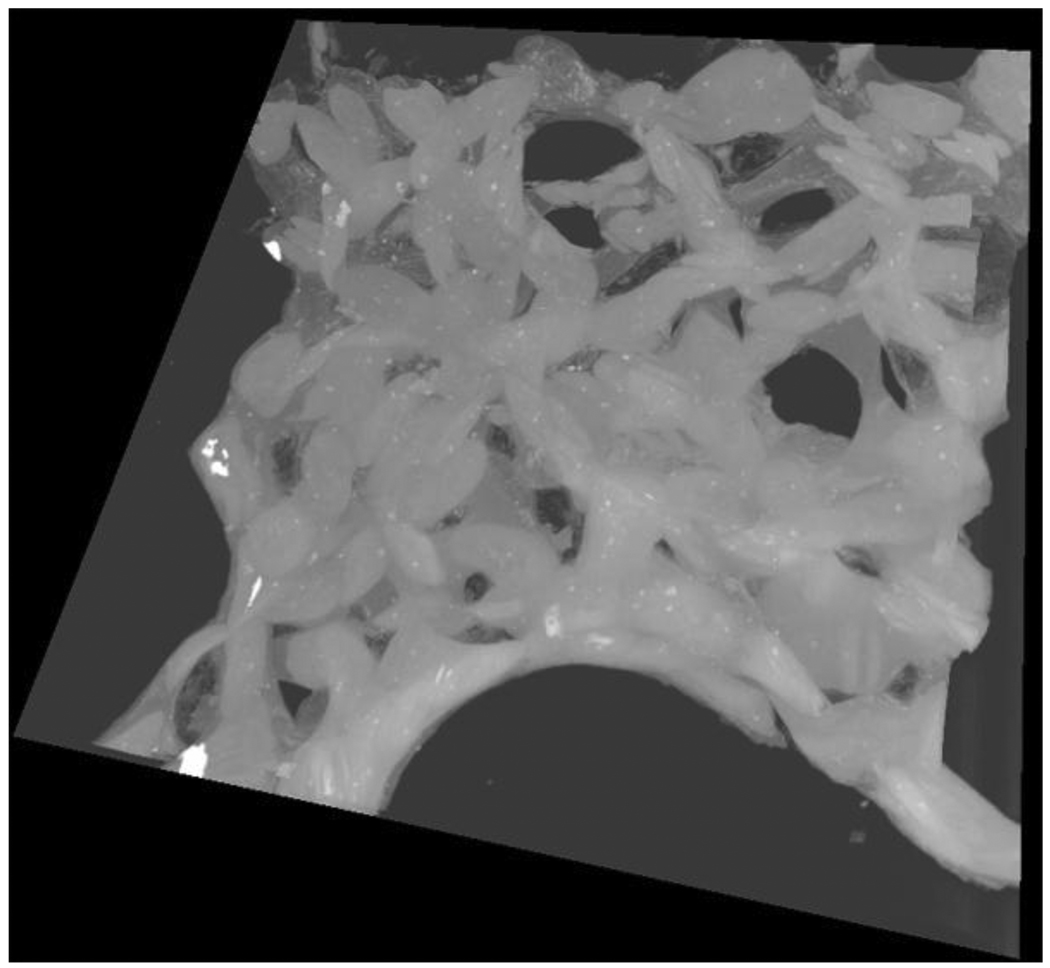
3.2. Three-dimensional relationships between the blood and air capillaries
The three-dimensional relationships between the blood capillaries and air capillaries are difficult to determine from conventional thin sections. However serial block-face SEM studies show the configuration clearly. A screen shot is shown in Fig. 5, and the file itself is in Supplementary Material SM4. It can be seen that the blood capillaries form a series of short interconnecting tubes of random orientation. The air capillaries are freely communicating spaces between the blood capillaries. No obvious constriction points in the air capillaries are seen. The whole arrangement emphasizes the very efficient apposition of air and blood in the gas-exchanging tissue.
Fig. 5.
Screen short from the video clip submitted as Supplementary Material SM4. This shows the network of pulmonary capillaries with the clear spaces formed by the air capillaries between them.
Another informative sequence is obtained when the viewer is enabled to move through the complete volume slice by slice. A screen shot of this is shown in Fig. 6, and the video clip is in Supplementary Material SM5. As indicated earlier, this study was done by taking a series of separate SEM images while the tissue block was shaved by the automatic microtome which removed 60 nm thick slices sequentially for 500 slices. This means that we can gradually move through a 30 µm thickness of the block. At any particular point in time, we can see the blood capillaries with their red cells, the epithelial bridges, and the air capillaries. If we concentrate on a single capillary as we gradually move through the block, its configuration changes and we eventually reach an epithelial bridge that connects it to the next capillary. In some instance there is no epithelial bridge at the end of the capillary but simply the space of an air capillary. The appearances of this sequence are essentially predictable from the image shown in Fig. 5, but in the video clip the transition from blood capillary to epithelial cell is emphasized. These experiments were not designed to measure the size of the blood and air capillaries. However, in a previous study on similar material we found that the average diameter of the blood capillaries in a thin microscopic section varied from 3.9 to 5.4 µm when the capillary transmural pressure was altered over a range of 60 cm water (Watson et al., 2008). For ducks, Woodward and Maina (2005 and 2008) reported the diameter of blood capillaries to be about 3 µm, and for air capillaries the mean diameter was 15 µm.
Fig. 6.
Screenshot from the video clip submitted as Supplementary Material SM5. The blood and air capillaries can be seen with the epithelial bridges. ac, air capillary; bc, blood capillary; ep, epithelial bridge.
4. Discussion
Previous investigators have described the structures outside the pulmonary capillaries in avian lung notably Maina (2005), Klika et al. (1997), Scheuermann et al. (1997), and Weidner et al. (1993). However the present study is the first to clarify the three dimensional arrangement of the junctions of the epithelial bridges with the pulmonary capillaries and as such it allows a better analysis of the forces acting on the capillaries than has previously been available. In a recent review (West, 2009), possible modes of support of the blood capillaries were discussed but the present article is the first to provide the ultrastructural basis of the physiology.
4.1. Epithelial bridges
Klika et al. (1997) and Scheuermann et al. (1997) obtained electron micrographs of lung tissue from pigeon, barn owl, domestic chicken, and quail and their results are similar to those reported here although the resolution in their published micrographs is not as high. Another difference is that in that study, many of the epithelial bridges show a much larger space between the two epithelial plates than is the case here. A likely explanation of this is that the lungs developed some degree of interstitial pulmonary edema before the tissue was fixed. In support of this, the study of Weidner et al. (1993) showed a progressive increase in the width of the space between the epithelial bridges of chickens following graded intravenous infusion of avian Ringer’s solution to produce increasing degrees of interstitial edema. In none of the micrographs reproduced in either of these studies was there any example of an epithelial cell junction in the epithelial bridge itself, and this is consistent with our findings.
4.2. Epithelial plates
The epithelial plates are the two very thin cells that have an extensive area and that make up the bridges as shown in Figs. 4A and B, and Supplementary Material SM2. These plates are single epithelial cells with no cellular junctions and are remarkable for their extraordinary thinness but large extent. We have not been able to find similar illustrations of these plates although there have been several published three-dimensional studies of the gas-exchanging tissue of avian lung. For example, Woodward and Maina (2005; 2008) performed three-dimensional reconstructions of Muscovy duck lung using serial sections of light micrographs. In addition there have been scanning electron micrograph studies of blood capillaries that give a three-dimensional perspective (Brackenbury and Akester, 1978; Maina, 1982), and also an image of a cast of air capillaries in chicken lung (Maina, 1982). However none of these studies clearly show the anatomy of the epithelial plates. An interesting feature of Supplementary Material SM2 is that the plates are so thin that they are transparent to some extent and other tissue can be seen behind them. Perhaps we should not be too surprised that these epithelial plates can be extremely thin and have such a large area because this is the typical appearance of the type I alveolar epithelial cells that cover most of the surface of the alveoli in mammalian lungs.
4.3. Junctions of the epithelial bridges with the capillary walls
As discussed below, these junctions may have a critical role in reducing the hoop stresses that otherwise might damage the capillary wall when the capillary transmural pressure is raised. As Fig. 1A, Fig. 2A, and B, and Supplementary Material SM1 and SM2 show, these junctions are characterized by expansions of the epithelial cells as they join the capillary wall. In addition there is some evidence that the extracellular matrix of the capillary wall that contains the stress-bearing type IV collagen is thickened at the junction and perhaps may extend somewhat into the gap between the two epithelial cells at the beginning of the bridge. This is best seen in Fig. 2B. Another feature of the epithelial cells at the junction is the large number of cytoplasmic inclusions that appear as extremely small circular structures that are possibly microtubules. The significance of these is not known.
4.4. Mode of support of the pulmonary capillaries
As noted earlier, there are three major differences between avian and mammalian pulmonary capillaries. These are 1) Avian capillaries have very much thinner walls than in the case in mammals, and furthermore the thickness of the extracellular matrix which is believed to be responsible for the mechanical strength of the capillaries is very much less in birds. For example, Watson et al. (2007) reported that the average thickness of the extracellular matrix in pulmonary capillaries of chicken was 0.045 ± 0.02 µm as compared with 0.319 ± 0.51 µm in dog. This is a difference of over sevenfold. 2) The blood-gas barrier is much more uniform in thickness in birds compared with mammals (Watson et al., 2007). 3) Avian pulmonary capillaries are much more rigid than those of mammals in that the change in average diameter when the capillary transmural pressure is altered is very much less. A feasible mechanism for these differences is that avian pulmonary capillaries receive some support from the outside. This is in contrast to the situation in mammals where the capillaries are unsupported at right angles to the alveolar wall.
There seem to be two possible ways in which the pulmonary capillaries can receive mechanical support from the outside.
The epithelial bridges themselves could support the capillaries by a guy wire (extension) or buttressing (compression) action.
The junctions between the epithelial bridges and the capillary walls could themselves share some of the hoop stress that otherwise might damage the capillary wall.
4.5. Possible role of the epithelial bridges themselves
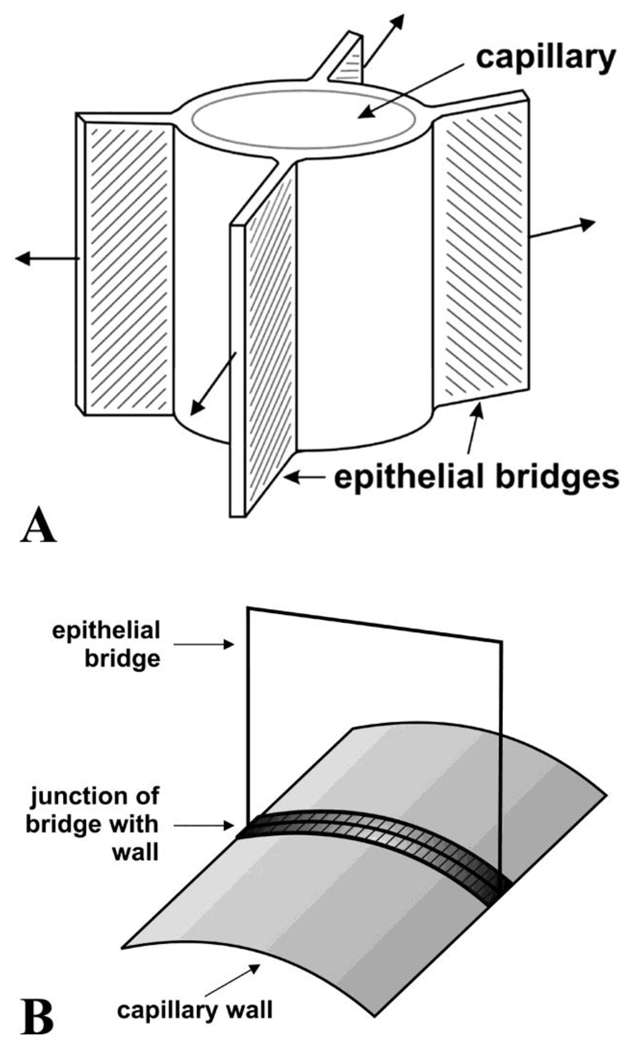
A striking feature of the behavior of pulmonary capillaries in chickens is that their diameter is little reduced when the pressure outside them exceeds the pressure inside (Watson et al., 2008). For example when the pressure in the air capillaries was raised from 0 to 35 cm H2O above the pressure inside the blood capillaries, the change in diameter was less than 20%. Contrast this with the behavior of mammalian pulmonary capillaries which completely collapse under these conditions, a situation known as Zone 1 (West et al., 1964). It seems reasonable to ascribe this behavior to the guy wire-like action of the epithelial bridges as suggested by Fig. 1A although the connections of the bridges to the capillaries are by means of plates rather than wires. This support requires that the epithelial plates that make up the bridges be strong in extension. A diagram is shown in Fig. 7A with arrows depicting the tensile forces that could hold the pulmonary capillary open.
Fig. 7.
A. Diagram to show how the epithelial bridges could exert tension on the blood capillaries and prevent their collapse. The direction of the tension is indicated by the arrows. The bridges might also resist enlargement of the capillaries thus reducing the hoop stress in the wall. Modified from West (2009). B. Diagram showing how the junctions between the epithelial cells and the capillary wall could reduce the wall hoop stress just as the iron hoops do the same around a barrel of beer. In this diagram, the junction of the epithelial cell with the capillary wall runs around the circumference of a capillary. In Figure 7A, the junction is shown running along the direction of the axis of the capillary. These drawing show the two extremes of directions. In practice, the junctions presumably have a random orientation.
However when we consider forces tending to increase the hoop stress in the pulmonary capillary wall, the situation is less clear. It is well known that capillary stress failure occurs in mammalian lungs under both physiological and pathological conditions in spite of the much thicker wall of the capillaries in mammals compared with birds (West, 2009). The fact that avian pulmonary capillaries can maintain their mechanical integrity with an extremely thin wall, and in particular with far less of the stress-bearing extracellular matrix, is difficult to explain. This would presumably require the epithelial bridges to be strong in compression, but they are so thin that this seems unlikely, and they would be expected to buckle. The situation might be different if there were appreciable surface tension forces in the air capillaries that resulted in a rigid assemblage. But in the absence of these forces it is not clear how the epithelial bridges could prevent large hoop stresses in the capillary wall that would result in failure.
4.6. Possible role of the junctions between the epithelial bridges and the capillary walls
We have seen that there is an expansion of tissue at the junctions between the bridges and the walls with an obvious increase in the thickening of the epithelial cell itself, and also possibly the stress-bearing extracellular matrix (Figs. 2A and B, and the Supplementary Material SM2). These junctions track along the capillary walls, and if they were sufficiently numerous and appropriately oriented, they could share some of the hoop stress which otherwise would cause stress failure in the walls. An analogy here is the iron hoops around a barrel of beer although the cellular junctions are part of the barrel rather than being outside it. A diagram is shown in Fig. 7B. Note that in this diagram the junction runs around the circular perimeter of the capillary rather than along the axis as shown in Figure 7A. In practice the junctions have random orientations as implied by Figure 5 and SM4.
4.7. Possible surface tension effects in the air capillaries
The effects of surface tension in the air capillaries could have a major influence on the support of the blood capillaries. Because the diameter of the air capillaries is approximately 10 to 20 µm, the average radius of curvature of say, 5 to 10 µm could result in large pressures tending to close the capillary. For example, if we consider an air capillary as a thin-walled cylindrical tube with a radius of curvature of 10 µm and a thin aqueous lining layer, the pressure tending to close the capillary can be calculated from the Laplace relationship. This states that the pressure is given by the surface tension divided by the radius of curvature. For water at 37° C, the surface tension is 70 mN.m−1 (70 dyn.cm−1), and for a radius of curvature of 10 µm this means that the pressure is about 7 kPa (53 mm Hg). For an air capillary of 5 µm radius of curvature, the pressure would be double this. Another way of looking at this is that a tube by itself would require a pressure inside it of 7 kPa to prevent it from collapsing. An assemblage of such tubes could be very rigid as is the case with the avian lung.
Unfortunately we are almost entirely ignorant about the role of surface tension in the avian lung. It is not known whether the air capillaries are lined with a liquid or are dry. We do know that avian lungs contain surfactant as do mammalian lungs (Pattle, 1978). It is also known that avian lung has a peculiar trilaminar substance (TLS) that is not found in other lungs, and the electron microscopic appearance of this suggests that it may have surfactant properties (Klika et al., 1997; Pattle, 1978). Pattle (1978) has suggested that the TLS might be concerned with keeping the air capillaries free of liquid. Bernhard et al. (2004) have reviewed differences in surfactant between birds and mammals.
4.8. Interdependence as a means of support of the pulmonary capillaries
Mead et al. (1970) introduced the term “interdependence” to refer to the stabilizing effect exerted on a structure embedded in an elastic continuum by virtue of the many connections between the embedded structure and the surrounding material. The original description considered a structure such as an airway or blood vessel, or perhaps a region of collapsed alveoli within an expanded lung. The analysis showed that the forces acting on the embedded structure could be very high. For example if the embedded region tended to collapse below its equilibrium volume, substantial forces would come into play to prevent this. In the same way, if the embedded region tended to expand beyond its normal volume, this would be opposed by the forces in the elastic continuum around it. This type of analysis might be very relevant to how an avian blood capillary shows very small changes in its caliber in spite of large changes in capillary transmural pressure. However whereas in the analysis of structures within the mammalian lung the pressure-volume properties of the lung are known, this information is not available for the parabronchial tissue in birds. Note also that the interdependence explanation does not concern itself with the geometry of the attachments as was discussed above, but simply the overall effects of a surrounding elastic continuum on an embedded structure that has a tendency to increase or decrease its size.
In summary, our studies clarify how the pulmonary capillaries of chicken lung can differ so much from those of mammals. Avian pulmonary capillaries have thinner and more uniform blood-gas barriers, and they show much less change in diameter when the capillary transmural pressure is altered. Compression of the capillaries from the outside is apparently resisted by the guy wire-like arrangement of the epithelial bridges as shown for example in Fig. 1A. A similar mechanism could support the capillaries when the pressure inside them is increased if the epithelial bridges resist compression. This would be most effective if there was appreciable surface tension in the air capillaries that promoted their rigidity. Another mechanism that could support the capillary walls when the pressure inside them was increased is the cord-like junctions between the epithelial bridges and the capillary wall. These junctions run along the wall and could share some of the hoop stress which otherwise would tend to damage the unprotected wall. This could explain why the blood-gas barrier, and in particular the extracellular matrix layer in it, can afford to be so thin. As noted earlier, the uniform thickness of the blood-gas barrier is explained by the absence of type I collagen cable that is necessary in an alveolar lung but not here where external support of the pulmonary capillaries is available. Another potential explanation for the support of the pulmonary capillaries is the interdependence mechanism that exerts a stabilizing action on an embedded structure in an elastic continuum. The net result of having a very thin blood-gas barrier of uniform thickness is advantageous for pulmonary gas exchange.
Supplementary Material
SM1. Electron tomograph showing the pulmonary capillary with its three bridges that make up Fig. 1A. By rotating the capillary in three dimensions, the junctions between the bridges and the capillary wall are more clearly seen.
SM2. Electron tomograph showing a ribbon of epithelial bridge running between two capillaries Rotation of the image shows the nature of the junctions between the bridge and the capillary walls clearly.
SM3. Serial block-face SEM study showing an epithelial plate surrounded by blood capillaries. The plate has been colored green while the capillaries are blue. This was done by tracing around the periphery of the plate with a video pen. In part of the clip it is possible to see structures through the plate because it is so thin. In a later part of the clip the capillaries have removed by the operator and the plate is well seen both in plane view and side view.
SM4. Serial block-face SEM study showing the blood and air capillaries in a block of tissue 30 µm thick. The configuration of the two sets of capillaries can be seen as the section is rotated.
SM5. Serial block-face SEM study in which we appear to move through a 30 µm thick tissue block in a series of small steps. As we do this and concentrate on a single capillary, its thickness changes and eventually we see the connecting epithelial bridge. For some of the capillaries there is no bridge on the far side but simply the empty air capillary.
Acknowledgments
The work was supported by NIH grant R01 HL 60968.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bernhard W, Haslam PL, Floros J. From birds to humans: new concepts on airways relative to alveolar surfactant. Am. J. Respir. Cell Mol. Biol. 2004;30:6–11. doi: 10.1165/rcmb.2003-0158TR. [DOI] [PubMed] [Google Scholar]
- Brackenbury J, Akester AR. A model of the capillary zone of the avian tertiary bronchus. In: Piiper J, editor. Respiratory Function in Birds Adult and Embryonic. Berlin: Springer-Verlag; 1978. [Google Scholar]
- Denk W, Horstmann H. Serial block-face scanning electron microscopy to reconstruct three-dimensional tissue nanostructure. PLOS Biol. 2004;2:1900–1909. doi: 10.1371/journal.pbio.0020329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan GY, Peltier S, Lamont S, Dunkelberger DG, Burke BE, Ellisman MH. Multiport-readout frame-transfer 5 megapixel CCD imaging system for TEM applications. Ultramicroscopy. 2000;84:75–84. doi: 10.1016/s0304-3991(00)00021-8. [DOI] [PubMed] [Google Scholar]
- Frank J, editor. Electron Tomography: Three-Dimensional Imaging with the Transmission Electron Microscope. New York: Plenum Press; 1992. [Google Scholar]
- Klika E, Scheuermann DW, De Groodt-Lasseel MHA, Bazantova I, Switka A. Anchoring and support system of pulmonary gas-exchange tissue in four bird species. Acta Anat. 1997;159:30–41. doi: 10.1159/000147962. [DOI] [PubMed] [Google Scholar]
- Maina JN. A scanning electron microscopic study of the air and blood capillaries of the lung of the domestic fowl (Gallus domesticus) Experientia. 1982;35:614–616. doi: 10.1007/BF02327080. [DOI] [PubMed] [Google Scholar]
- Maina JN. The Lung Air-Sac System of Birds. Berlin: Springer; 2005. [Google Scholar]
- Maina JN, West JB. Thin and strong! The bioengineering dilemma in the structural and functional design of the blood-gas barrier: comparative and evolutionary perspectives. Physiol. Rev. 2005;85:811–844. doi: 10.1152/physrev.00022.2004. [DOI] [PubMed] [Google Scholar]
- Mead J, Takishima T, Leith D. Stress distribution in lungs: a model of pulmonary elasticity. J. Appl. Physiol. 1970;28:596–608. doi: 10.1152/jappl.1970.28.5.596. [DOI] [PubMed] [Google Scholar]
- Pattle RE. Lung surfactant and lining in birds. In: Piiper J, editor. Respiratory Function in Birds Adult and Embryonic. Berlin: Springer-Verlag; 1978. pp. 23–32. [Google Scholar]
- Scheuermann DW, Klika E, De Groodt-Lasseel MHA, Bazantova I, Switka A. An electron microscopic study of the parabronchial epithelium in the mature lung of four bird species. Anat. Rec. 1997;249:213–225. doi: 10.1002/(SICI)1097-0185(199710)249:2<213::AID-AR8>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Watson RR, Fu Z, West JB. Morphometry of the extremely thin pulmonary blood-gas barrier in the chicken lung. Am. J. Physiol. Lung Cell. Mol. Physiol. 2007;292:769–777. doi: 10.1152/ajplung.00355.2006. [DOI] [PubMed] [Google Scholar]
- Watson RR, Fu Z, West JB. Minimal distensibility of pulmonary capillaries in avian lungs compared with mammalian lungs. Resp. Physiol. Neuro. 2008;160:208–214. doi: 10.1016/j.resp.2007.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weibel ER. Morphological basis of alveolar-capillary gas exchange. Physiol. Rev. 1973;53:419–495. doi: 10.1152/physrev.1973.53.2.419. [DOI] [PubMed] [Google Scholar]
- Weidner JW, Selna LA, McClure DE, DeFouw DO. Effect of extracellular fluid volume expansion on avian lung balance. Resp. Physiol. 1993;91:125–136. doi: 10.1016/0034-5687(93)90094-q. [DOI] [PubMed] [Google Scholar]
- West JB. Comparative physiology of the pulmonary blood-gas barrier: the unique avian solution. Am. J. Physiol. Reg. Integrat. Comp. Physiol. 2009 doi: 10.1152/ajpregu.00459.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West JB, Dollery CT, Naimark A. Distribution of blood flow in isolated lung; relation to vascular and alveolar pressures. J. Appl. Physiol. 1964;19:713–724. doi: 10.1152/jappl.1964.19.4.713. [DOI] [PubMed] [Google Scholar]
- Woodward JD, Maina JN. A 3D digital reconstruction of the components of the gas exchange tissue of the lung of the muscovy duck, Cairina moschata. J. Anat. 2005;206:477–492. doi: 10.1111/j.1469-7580.2005.00413.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward JD, Maina JN. Study of the structure of the air and blood capillaries of the gas exchange tissue of the avian lung by serial section three-dimensional reconstruction. J. Microscop. 2008;230:84–93. doi: 10.1111/j.1365-2818.2008.01958.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
SM1. Electron tomograph showing the pulmonary capillary with its three bridges that make up Fig. 1A. By rotating the capillary in three dimensions, the junctions between the bridges and the capillary wall are more clearly seen.
SM2. Electron tomograph showing a ribbon of epithelial bridge running between two capillaries Rotation of the image shows the nature of the junctions between the bridge and the capillary walls clearly.
SM3. Serial block-face SEM study showing an epithelial plate surrounded by blood capillaries. The plate has been colored green while the capillaries are blue. This was done by tracing around the periphery of the plate with a video pen. In part of the clip it is possible to see structures through the plate because it is so thin. In a later part of the clip the capillaries have removed by the operator and the plate is well seen both in plane view and side view.
SM4. Serial block-face SEM study showing the blood and air capillaries in a block of tissue 30 µm thick. The configuration of the two sets of capillaries can be seen as the section is rotated.
SM5. Serial block-face SEM study in which we appear to move through a 30 µm thick tissue block in a series of small steps. As we do this and concentrate on a single capillary, its thickness changes and eventually we see the connecting epithelial bridge. For some of the capillaries there is no bridge on the far side but simply the empty air capillary.