Abstract
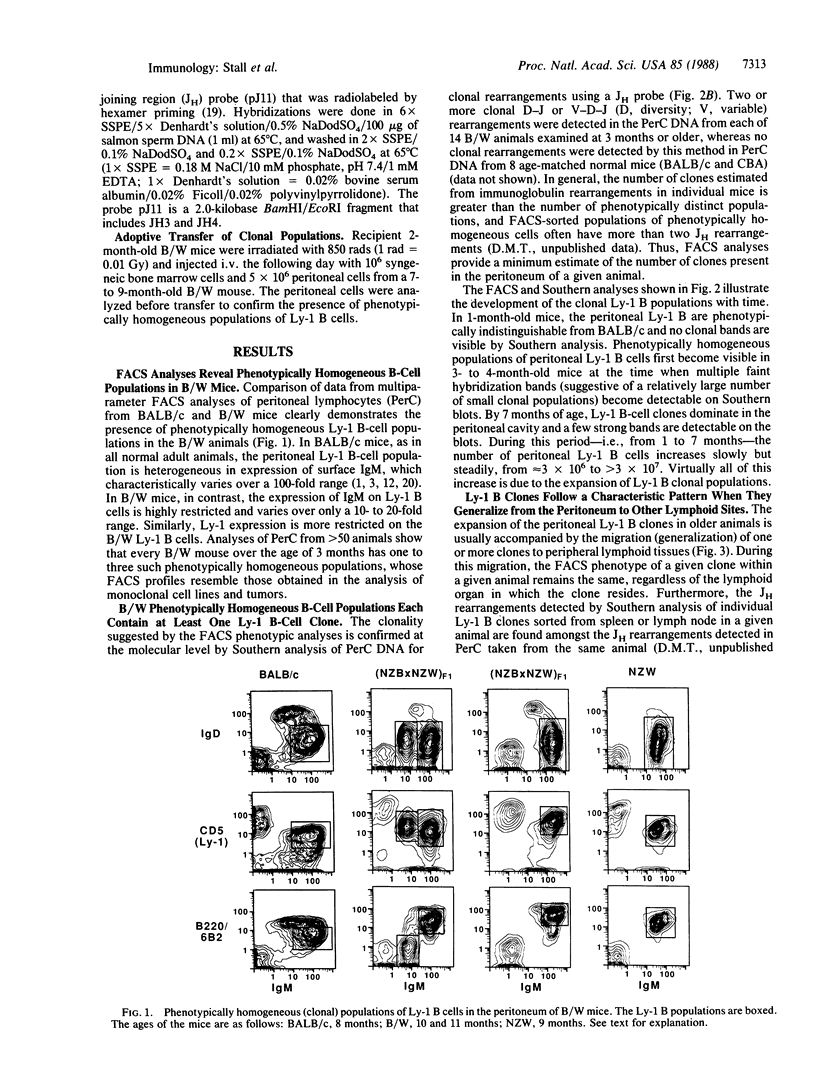
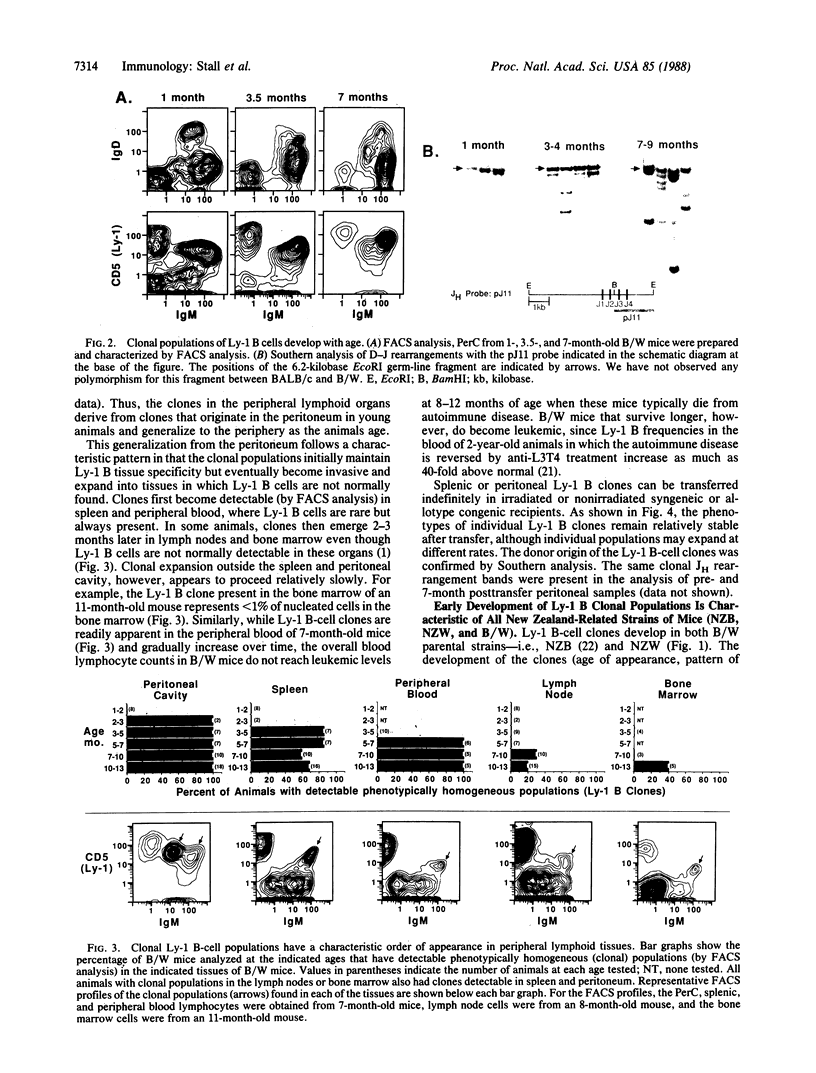
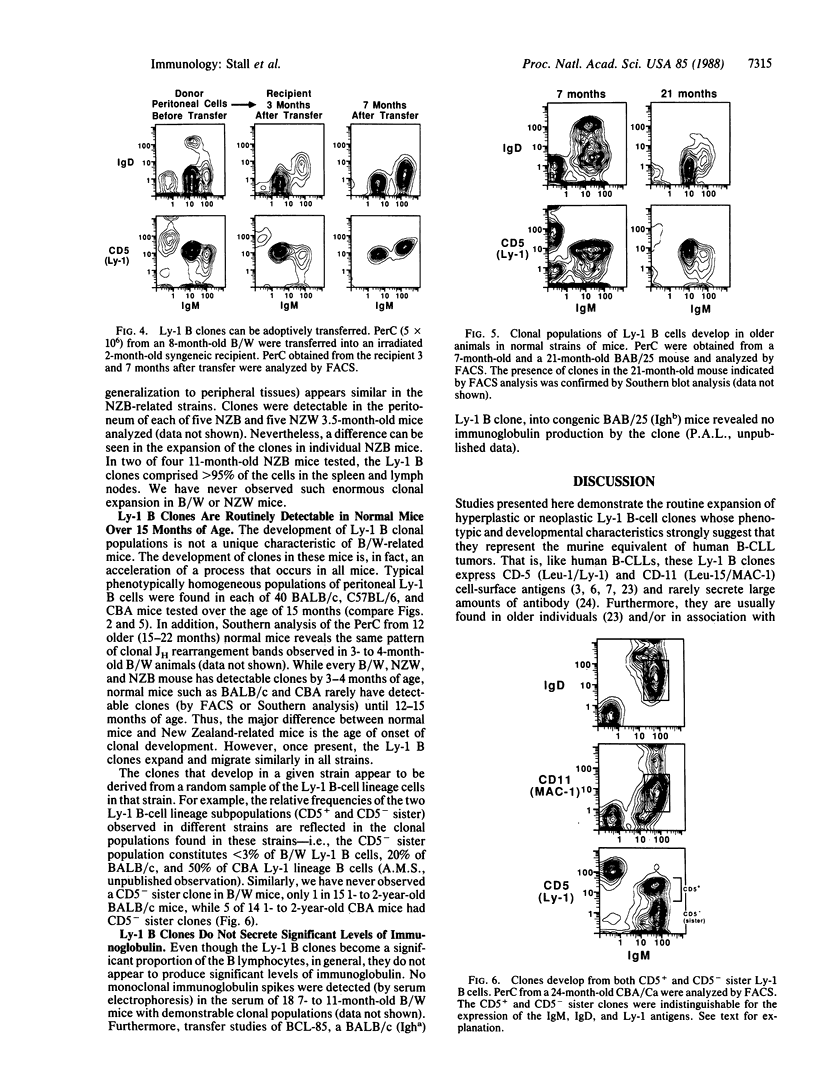
Studies presented here demonstrate that individually expanded clones of murine Ly-1 B cells, perhaps analogous to the expanded neoplastic Leu-1 B-cell clones in human chronic lymphocytic leukemias, are universally detectable in young New Zealand Black (NZB)-related autoimmune mice and in senescent normal mice (greater than 18 months old). These clones are visible as phenotypically homogeneous cell populations in multiparameter fluorescence-activated cell sorter analyses of peritoneal and splenic B cells; they show unique immunoglobulin heavy- and light-chain gene rearrangements in Southern gel analyses of peritoneal and splenic DNA; and, like the self-replenishing Ly-1 B-cell population from which they are drawn, they tend to grow readily in irradiated or unirradiated syngeneic or allotype congenic hosts. Furthermore, they develop and generalize in primary and secondary hosts in a characteristic pattern (peritoneum much greater than spleen greater than lymph node greater than bone marrow) that suggests that their initial growth is controlled by the mechanisms that normally control Ly-1 B-cell distribution in lymphoid organs. The universal emergence of these clones within the Ly-1 B-cell lineage may be explained by the substantially greater opportunity for hyperplastic and neoplastic transformation events in this long-lived self-replenishing Ly-1 B-cell population, which must divide relatively frequently to maintain its normal size throughout adulthood. Repeated exposure to internal or environmental antigens (with which Ly-1 B cells are known to react) may also play a role in driving the development of these clones.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Braun J., Citri Y., Baltimore D., Forouzanpour F., King L., Teheranizadeh K., Bray M., Kliewer S. B-Ly1 cells: immortal Ly-1+ B lymphocyte cell lines spontaneously arising in murine splenic cultures. Immunol Rev. 1986 Oct;93:5–21. doi: 10.1111/j.1600-065x.1986.tb01499.x. [DOI] [PubMed] [Google Scholar]
- Caligaris-Cappio F., Gobbi M., Bofill M., Janossy G. Infrequent normal B lymphocytes express features of B-chronic lymphocytic leukemia. J Exp Med. 1982 Feb 1;155(2):623–628. doi: 10.1084/jem.155.2.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casali P., Burastero S. E., Nakamura M., Inghirami G., Notkins A. L. Human lymphocytes making rheumatoid factor and antibody to ssDNA belong to Leu-1+ B-cell subset. Science. 1987 Apr 3;236(4797):77–81. doi: 10.1126/science.3105056. [DOI] [PubMed] [Google Scholar]
- Conley C. L., Misiti J., Laster A. J. Genetic factors predisposing to chronic lymphocytic leukemia and to autoimmune disease. Medicine (Baltimore) 1980 Sep;59(5):323–334. doi: 10.1097/00005792-198009000-00001. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- HELYER B. J., HOWIE J. B. Renal disease associated with positive lupus erythematosus tests in a cross-bred strain of mice. Nature. 1963 Jan 12;197:197–197. doi: 10.1038/197197a0. [DOI] [PubMed] [Google Scholar]
- Han T., Ozer H., Gavigan M., Gajera R., Minowada J., Bloom M. L., Sadamori N., Sandberg A. A., Gomez G. A., Henderson E. S. Benign monoclonal B cell lymphocytosis--a benign variant of CLL: clinical, immunologic, phenotypic, and cytogenetic studies in 20 patients. Blood. 1984 Jul;64(1):244–252. [PubMed] [Google Scholar]
- Hardy R. R., Hayakawa K. Development and physiology of Ly-1 B and its human homolog, Leu-1 B. Immunol Rev. 1986 Oct;93:53–79. doi: 10.1111/j.1600-065x.1986.tb01502.x. [DOI] [PubMed] [Google Scholar]
- Hardy R. R., Hayakawa K., Parks D. R., Herzenberg L. A., Herzenberg L. A. Murine B cell differentiation lineages. J Exp Med. 1984 Apr 1;159(4):1169–1188. doi: 10.1084/jem.159.4.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy R. R., Hayakawa K., Shimizu M., Yamasaki K., Kishimoto T. Rheumatoid factor secretion from human Leu-1+ B cells. Science. 1987 Apr 3;236(4797):81–83. doi: 10.1126/science.3105057. [DOI] [PubMed] [Google Scholar]
- Haughton G., Arnold L. W., Bishop G. A., Mercolino T. J. The CH series of murine B cell lymphomas: neoplastic analogues of Ly-1+ normal B cells. Immunol Rev. 1986 Oct;93:35–51. doi: 10.1111/j.1600-065x.1986.tb01501.x. [DOI] [PubMed] [Google Scholar]
- Hayakawa K., Hardy R. R., Herzenberg L. A., Herzenberg L. A. Progenitors for Ly-1 B cells are distinct from progenitors for other B cells. J Exp Med. 1985 Jun 1;161(6):1554–1568. doi: 10.1084/jem.161.6.1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayakawa K., Hardy R. R., Herzenberg L. A. Peritoneal Ly-1 B cells: genetic control, autoantibody production, increased lambda light chain expression. Eur J Immunol. 1986 Apr;16(4):450–456. doi: 10.1002/eji.1830160423. [DOI] [PubMed] [Google Scholar]
- Hayakawa K., Hardy R. R., Honda M., Herzenberg L. A., Steinberg A. D., Herzenberg L. A. Ly-1 B cells: functionally distinct lymphocytes that secrete IgM autoantibodies. Proc Natl Acad Sci U S A. 1984 Apr;81(8):2494–2498. doi: 10.1073/pnas.81.8.2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayakawa K., Hardy R. R., Parks D. R., Herzenberg L. A. The "Ly-1 B" cell subpopulation in normal immunodefective, and autoimmune mice. J Exp Med. 1983 Jan 1;157(1):202–218. doi: 10.1084/jem.157.1.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayakawa K., Hardy R. R., Parks D. R., Herzenberg L. A. The "Ly-1 B" cell subpopulation in normal immunodefective, and autoimmune mice. J Exp Med. 1983 Jan 1;157(1):202–218. doi: 10.1084/jem.157.1.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayakawa K., Hardy R. R., Stall A. M., Herzenberg L. A., Herzenberg L. A. Immunoglobulin-bearing B cells reconstitute and maintain the murine Ly-1 B cell lineage. Eur J Immunol. 1986 Oct;16(10):1313–1316. doi: 10.1002/eji.1830161021. [DOI] [PubMed] [Google Scholar]
- Herzenberg L. A., Stall A. M., Braun J., Weaver D., Baltimore D., Herzenberg L. A., Grosschedl R. Depletion of the predominant B-cell population in immunoglobulin mu heavy-chain transgenic mice. Nature. 1987 Sep 3;329(6134):71–73. doi: 10.1038/329071a0. [DOI] [PubMed] [Google Scholar]
- Herzenberg L. A., Stall A. M., Lalor P. A., Sidman C., Moore W. A., Parks D. R., Herzenberg L. A. The Ly-1 B cell lineage. Immunol Rev. 1986 Oct;93:81–102. doi: 10.1111/j.1600-065x.1986.tb01503.x. [DOI] [PubMed] [Google Scholar]
- Kipps T. J., Vaughan J. H. Genetic influence on the levels of circulating CD5 B lymphocytes. J Immunol. 1987 Aug 15;139(4):1060–1064. [PubMed] [Google Scholar]
- Plater-Zyberk C., Maini R. N., Lam K., Kennedy T. D., Janossy G. A rheumatoid arthritis B cell subset expresses a phenotype similar to that in chronic lymphocytic leukemia. Arthritis Rheum. 1985 Sep;28(9):971–976. doi: 10.1002/art.1780280903. [DOI] [PubMed] [Google Scholar]
- Royston I., Majda J. A., Baird S. M., Meserve B. L., Griffiths J. C. Human T cell antigens defined by monoclonal antibodies: the 65,000-dalton antigen of T cells (T65) is also found on chronic lymphocytic leukemia cells bearing surface immunoglobulin. J Immunol. 1980 Aug;125(2):725–731. [PubMed] [Google Scholar]
- Seldin M. F., Conroy J., Steinberg A. D., D'Hoosteleare L. A., Raveche E. S. Clonal expansion of abnormal B cells in old NZB mice. J Exp Med. 1987 Nov 1;166(5):1585–1590. doi: 10.1084/jem.166.5.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Theofilopoulos A. N., Dixon F. J. Murine models of systemic lupus erythematosus. Adv Immunol. 1985;37:269–390. doi: 10.1016/s0065-2776(08)60342-9. [DOI] [PubMed] [Google Scholar]
- Wofsy D., Chiang N. Y. Proliferation of Ly-1 B cells in autoimmune NZB and (NZB x NZW)F1 mice. Eur J Immunol. 1987 Jun;17(6):809–814. doi: 10.1002/eji.1830170612. [DOI] [PubMed] [Google Scholar]




