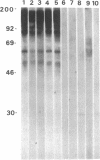
Abstract
Primary biliary cirrhosis is a chronic, destructive autoimmune liver disease of humans. Patient sera are characterized by a high frequency (greater than 95%) of autoantibodies to a Mr 70,000 mitochondrial antigen, a component of the M2 antigen complex. We have identified a human cDNA clone encoding the complete amino acid sequence of this autoantigen. The predicted structure has significant similarity with the dihydrolipoamide acetyltransferase (EC 2.3.1.12) of the Escherichia coli pyruvate dehydrogenase multienzyme complex. The human sequence preserves the Glu-Thr-Asp-Lys-Ala motif of the lipoyl-binding site and has two potential binding sites. Expressed fragments of the cDNA react strongly with sera from patients with primary biliary cirrhosis but not with sera from patients with autoimmune chronic active hepatitis or sera from healthy subjects.
Full text
PDF


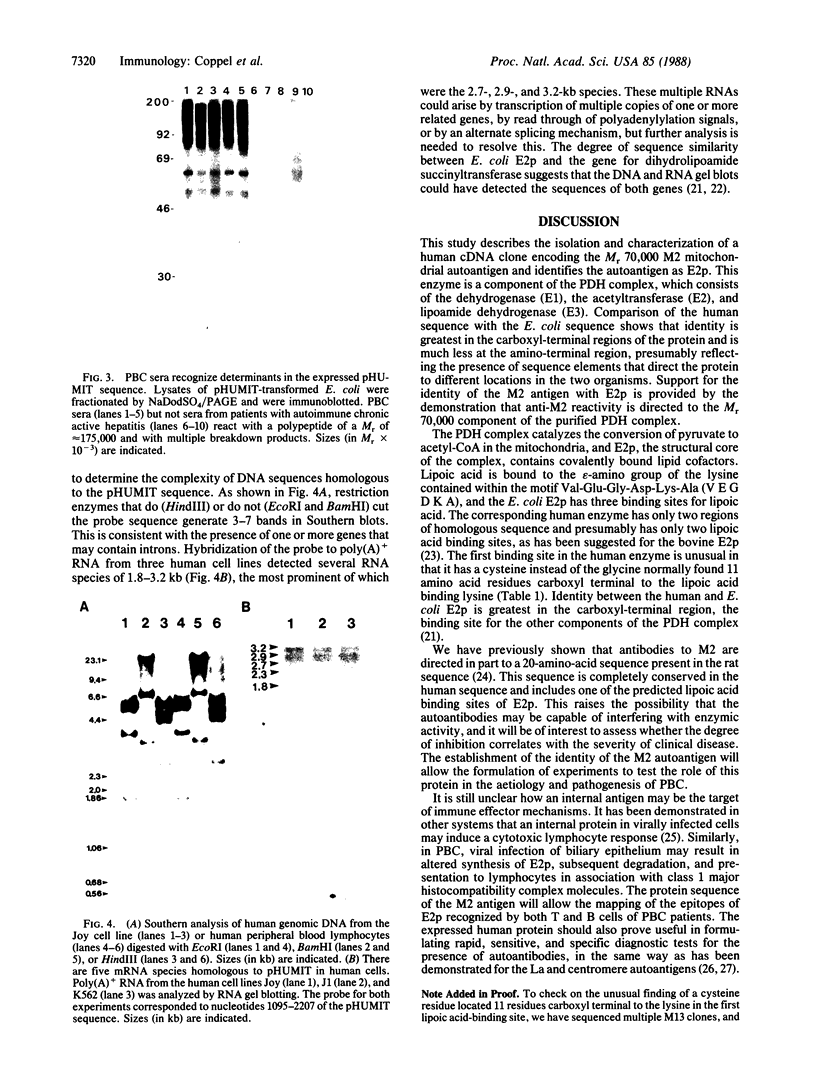

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baum H., Berg P. A. The complex nature of mitochondrial antibodies and their relation to primary biliary cirrhosis. Semin Liver Dis. 1981 Nov;1(4):309–321. doi: 10.1055/s-2008-1040734. [DOI] [PubMed] [Google Scholar]
- Berg P. A., Klein R. Clinical and prognostic relevance of different mitochondrial antibody profiles in primary biliary cirrhosis (PBC). Mol Aspects Med. 1985;8(3):235–247. doi: 10.1016/0098-2997(85)90008-1. [DOI] [PubMed] [Google Scholar]
- Berg P. A., Klein R., Lindenborn-Fotinos J., Klöppel W. ATPase-associated antigen (M2): marker antigen for serological diagnosis of primary biliary cirrhosis. Lancet. 1982 Dec 25;2(8313):1423–1426. doi: 10.1016/s0140-6736(82)91327-7. [DOI] [PubMed] [Google Scholar]
- Bradford A. P., Howell S., Aitken A., James L. A., Yeaman S. J. Primary structure around the lipoate-attachment site on the E2 component of bovine heart pyruvate dehydrogenase complex. Biochem J. 1987 Aug 1;245(3):919–922. doi: 10.1042/bj2450919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Meirleir L., MacKay N., Lam Hon Wah A. M., Robinson B. H. Isolation of a full-length complementary DNA coding for human E1 alpha subunit of the pyruvate dehydrogenase complex. J Biol Chem. 1988 Feb 5;263(4):1991–1995. [PubMed] [Google Scholar]
- Earnshaw W. C., Machlin P. S., Bordwell B. J., Rothfield N. F., Cleveland D. W. Analysis of anticentromere autoantibodies using cloned autoantigen CENP-B. Proc Natl Acad Sci U S A. 1987 Jul;84(14):4979–4983. doi: 10.1073/pnas.84.14.4979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frazer I. H., Mackay I. R., Jordan T. W., Whittingham S., Marzuki S. Reactivity of anti-mitochondrial autoantibodies in primary biliary cirrhosis: definition of two novel mitochondrial polypeptide autoantigens. J Immunol. 1985 Sep;135(3):1739–1745. [PubMed] [Google Scholar]
- Gershwin M. E., Mackay I. R., Sturgess A., Coppel R. L. Identification and specificity of a cDNA encoding the 70 kd mitochondrial antigen recognized in primary biliary cirrhosis. J Immunol. 1987 May 15;138(10):3525–3531. [PubMed] [Google Scholar]
- Lindenborn-Fotinos J., Baum H., Berg P. A. Mitochondrial antibodies in primary biliary cirrhosis: species and nonspecies specific determinants of M2 antigen. Hepatology. 1985 Sep-Oct;5(5):763–769. doi: 10.1002/hep.1840050510. [DOI] [PubMed] [Google Scholar]
- Mendel-Hartvig I., Frostell A., Nelson B. D. Primary biliary cirrhosis: assessment of the quantitative importance of the adenine nucleotide translocator protein as a major mitochondrial antigen. Clin Exp Immunol. 1986 Nov;66(2):399–405. [PMC free article] [PubMed] [Google Scholar]
- Mendel-Hartvig I., Nelson B. D., Löf L., Tötterman T. H. Primary biliary cirrhosis: further biochemical and immunological characterization of mitochondrial antigens. Clin Exp Immunol. 1985 Nov;62(2):371–379. [PMC free article] [PubMed] [Google Scholar]
- Messing J., Vieira J. A new pair of M13 vectors for selecting either DNA strand of double-digest restriction fragments. Gene. 1982 Oct;19(3):269–276. doi: 10.1016/0378-1119(82)90016-6. [DOI] [PubMed] [Google Scholar]
- Rüther U., Müller-Hill B. Easy identification of cDNA clones. EMBO J. 1983;2(10):1791–1794. doi: 10.1002/j.1460-2075.1983.tb01659.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D. B., Johnson K. S. Single-step purification of polypeptides expressed in Escherichia coli as fusions with glutathione S-transferase. Gene. 1988 Jul 15;67(1):31–40. doi: 10.1016/0378-1119(88)90005-4. [DOI] [PubMed] [Google Scholar]
- Spencer M. E., Darlison M. G., Stephens P. E., Duckenfield I. K., Guest J. R. Nucleotide sequence of the sucB gene encoding the dihydrolipoamide succinyltransferase of Escherichia coli K12 and homology with the corresponding acetyltransferase. Eur J Biochem. 1984 Jun 1;141(2):361–374. doi: 10.1111/j.1432-1033.1984.tb08200.x. [DOI] [PubMed] [Google Scholar]
- Spithill T. W., Samaras N. Genomic organization, chromosomal location and transcription of dispersed and repeated tubulin genes in Leishmania major. Mol Biochem Parasitol. 1987 May;24(1):23–37. doi: 10.1016/0166-6851(87)90112-5. [DOI] [PubMed] [Google Scholar]
- Stephens P. E., Darlison M. G., Lewis H. M., Guest J. R. The pyruvate dehydrogenase complex of Escherichia coli K12. Nucleotide sequence encoding the dihydrolipoamide acetyltransferase component. Eur J Biochem. 1983 Jul 1;133(3):481–489. doi: 10.1111/j.1432-1033.1983.tb07490.x. [DOI] [PubMed] [Google Scholar]
- Townsend A. R. Recognition of influenza virus proteins by cytotoxic T lymphocytes. Immunol Res. 1987;6(1-2):80–100. doi: 10.1007/BF02918106. [DOI] [PubMed] [Google Scholar]
- Van de Water J., Gershwin M. E., Leung P., Ansari A., Coppel R. L. The autoepitope of the 74-kD mitochondrial autoantigen of primary biliary cirrhosis corresponds to the functional site of dihydrolipoamide acetyltransferase. J Exp Med. 1988 Jun 1;167(6):1791–1799. doi: 10.1084/jem.167.6.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittingham S., Naselli G., McNeilage L. J., Coppel R. L., Sturgess A. D. Serological diagnosis of primary Sjögren's syndrome by means of human recombinant La (SS-B) as nuclear antigen. Lancet. 1987 Jul 4;2(8549):1–3. [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- von Heijne G. Mitochondrial targeting sequences may form amphiphilic helices. EMBO J. 1986 Jun;5(6):1335–1342. doi: 10.1002/j.1460-2075.1986.tb04364.x. [DOI] [PMC free article] [PubMed] [Google Scholar]