Abstract
Uropathogenic E. coli (UPEC)3 is the causative agent for greater than 80% of uncomplicated urinary tract infections (UTIs). UPEC strains express a number of virulence and fitness factors that allow successful colonization of the mammalian bladder. To combat this, the host has distinct mechanisms, not only to prevent adherence to the bladder wall, but to detect and kill UPEC in the event of colonization. In this study, we investigated the role of IL-17A, an innate-adaptive immunomodulatory cytokine, during UTI using a murine model. Splenocytes, isolated from mice infected by the transurethral route, robustly expressed IL-17A in response to in vitro stimulation with UPEC antigens. Transcript expression of IL-17A in the bladders of infected mice correlated with a role in the innate immune response to UTI, and γδ-positive cells appear to be a key source of IL-17A production. While IL-17A appears to be dispensable for the generation of a protective response to UPEC, its importance in innate immunity is demonstrated by a defect in acute clearance of UPEC in IL-17A−/− mice. This clearance defect is likely a result of deficient cytokine and chemokine transcripts and impaired macrophage and neutrophil influx during infection. These results show that IL-17A is a key mediator for the innate immune response to UTI.
Keywords: Rodent, bacterial, cytokine, mucosa
Introduction
Uncomplicated UTI occurs in otherwise healthy individuals that lack any urinary anatomical abnormalities. Bacteria derived from fecal matter access the urinary tract through the urethra and subsequently colonize the bladder, resulting in inflammation and symptoms clinically characterized as cystitis (1). Left untreated, bacteria can ascend the ureters and colonize the kidneys, resulting in a more serious secondary infection termed acute pyelonephritis (2). UTIs accounted for more than 6.8 million physician office visits and 1.3 million emergency room visits in the year 2000, resulting in an estimated cost of 2.4 billion dollars annually (3). Additionally, 40 to 50% of women will experience one or more UTI in their lifetime, and 10 to 15% of these women will experience recurrent infection (4). UPEC is the primary causative agent of uncomplicated UTI (4). This subset of extraintestinal pathogenic E. coli expresses a number of virulence determinants that contributes to successful colonization of the urinary tract. These determinants include factors needed for flagellar motility (5) and an array of adherence organelles and iron uptake systems (6).
Several mechanical forces are thought to function to minimize UTI, including urine flow and voiding, mucus shedding, and epithelial cell sloughing (7, 8). When UPEC overcomes these physical barriers by adhering to the epithelium, a robust innate immune response is generated. TLR4, TLR5, and TLR11 have been shown to be important for UPEC recognition and immune mobilization (9–11). Due to a stop codon in the available human gene sequences, TLR11 may only function in mice (12). A number of secreted factors such as antimicrobial peptides, Tamm-Horsfall protein, cytokines IL-6, TNF-α, IL-1β, G-CSF, IL-17, and chemokines CXCL1, CXCL2, CXCL3, CXCL8, CCL4 are detected in the mammalian bladder upon infection (13–18). Among cellular infiltrate, neutrophils are the most abundant early responders to infected bladders (19). Additionally, antigen-presenting macrophages (20) and dendritic cells (21), and innate-like lymphocytes such as γδ T cells (22) have been implicated in UTI host defense.
IL-17A or the IL-17 receptor has been shown to play a critical role in autoimmune disease (23, 24) and in bacterial (25–31), fungal (32–35), and even viral (36–38) infection. Because of the roles played in both arms of the immune system, IL-17A has emerged as an innate-adaptive immunomodulatory cytokine. Regarding the innate immune response to infection, IL-17A acts by indirectly enhancing neutrophil migration to infected tissue. Specifically, transcripts for cytokines involved in granulopoiesis and chemotaxis in cells treated with IL-17A exhibit enhanced mRNA stability (39–42). Aside from being the signature cytokine secreted by CD4+ Th17 helper cells, other cell types have been reported to secrete IL-17A, including cytotoxic T cells, γδ T cells, iNK T cells, neutrophils, eosinophils, and monocytes (23). Although the exact microbe-associated molecular patterns that trigger secretion of IL-17A are not defined, components of microbial cell wall, host receptors that recognize such components (i.e. the mannose receptor), and TLR-related signaling pathways are implicated (26, 33).
In an effort to characterize the immune response to known UPEC antigenic outer membrane proteins (43), we discovered that IL-17A was secreted by in vitro stimulated splenocytes derived from UPEC iron receptor-vaccinated mice (44). Given the importance of IL-17A in controlling mucosal infection (45), the role of IL-17A during the innate and adaptive immune response to UTI was formally investigated. In the murine model, IL-17A is upregulated by in vivo sensitized secondary lymphoid tissue cells in response to in vitro restimulation, yet IL-17A appears to be dispensable for the generation of protective immunity. IL-17A is also upregulated in the bladder in response to acute infection, and γδ-positive cells are a major source of secreted IL-17A in the bladder tissue. IL-17A appears to play a role in regulating the innate immune response to UTI; mice lacking IL-17A exhibit both deficient cytokine transcript upregulation and cellular responses during acute UTI, resulting in suboptimal clearance of UPEC.
Materials and Methods
Animals
Mice were maintained in specific pathogen-free conditions and all experiments were conducted according to protocols approved by University Committee on the Use and Care of Animals at the University of Michigan. C57BL/6 wild type mice and mice harboring the Tcrdtm1Mom mutation [TCR δ−/−, resulting in deficient γδ TCR expression in all adult lymphoid and epithelial organs (46)], were purchased from Jackson Laboratories (Bar Harbor, ME). Breeding pairs of IL-17A−/− mice were obtained as a gift from Yoichiro Iwakura (The University of Tokyo) (47). All experiments were conducted when animals were 6 to 15 weeks old, and C57BL/6 wild type mice with birth dates within one week of the IL-17A−/− or TCR δ−/− knockout mice (both in the C57BL/6 background) were used. For manipulation, mice were anesthetized with 100 mg ketamine, 10 mg xylazine per kg body weight. When necessary, mice were euthanized using a lethal dose of isoflurane and appropriate organs were harvested for further analysis.
For infections and sensitizations, a 50 µl UPEC suspension in PBS was inoculated transurethrally using a sterile 0.28 mm polyethylene catheter connected to an infusion pump (Harvard Apparatus). All infections, sensitizations, and challenges consisted of 5×107 CFU per mouse administered the transurethral route. To determine organ CFU, bladders were harvested from euthanized animals, weighed, and homogenized in PBS with a GLH homogenizer (Omni International). Homogenates were plated on Luria-Bertani agar containing 0.5 g/L NaCl using the Autoplate 4000 spiral plater (Spiral Biotech). Colonies were enumerated using the QCount (Spiral Biotech), with a limit of detection of 100 CFU/gram tissue.
Bacterial strains and whole cell lysate preparation
Escherichia coli CFT073 was used for all infections. This prototypic UPEC strain was isolated from the urine and blood of a patient with acute pyelonephritis (48) and has been fully sequenced and annotated (49). For whole cell lysate preparation, a single colony of E. coli CFT073 was inoculated into 250 ml sterile human urine (pooled from five healthy donors) and cultured statically at 37°C until late stationary phase. Bacterial cells were harvested by centrifugation (8,000 × g, 5 min, 4°C), washed, resuspended in 10 ml PBS, and incubated at room temperature with 2 µl benzonase solution (10 U/µl, Sigma) for 30 min. Lysis was executed by two passes through a French pressure cell press (American Instrument Company) at a pressure of 20,000 pounds per square inch. Lysate was cleared by centrifugation (8,000 × g, 5 min, 4°C) and sterilized using a 0.22 µm filter (Millipore). Protein in the lysate was quantified using the BCA Protein Assay Kit (Pierce).
Tissue culture
Spleens (1.5×106 cells/well) or the inner inguinal (lumbar) lymph nodes (5×105 cells/well), responsible for draining the pelvic viscera, were harvested and made into single cell suspensions by forcing organs through 40 µm cell strainers (BD Falcon). Lymph nodes from the same groups of animals were pooled and plated in replicate wells. Erythrocytes were lysed using 8.02 mg/ml NH4Cl, 0.84 mg/ml NaHCO3, 0.37 mg/ml EDTA in distilled water. Final cellular suspensions were made in RPMI medium (with L-Glutamine, Gibco) with 1% sodium pyruvate, 1% L-glutamine, 1% penicillin/streptomycin, 1% non-essential amino acids, 10% FBS, 0.001% 50 mM β-mercaptoethanol. Cells were cultured with medium, 5 µg/ml α-CD3 [clone 145-2C11, a gift from Dr. Cheong-Hee Chang (University of Michigan)], or 25 µg/ml E. coli CFT073 whole cell lysate, and incubated at 37°C, 5% CO2 for 72 h at which point supernatants were harvested and stored at −20°C for ELISA.
Cytokine ELISA
Purified IL-17A and corresponding matched antibody pairs were obtained from R&D Systems. Ninety-six well ELISA plates (Corning) were coated with 50 µl of 5 µg/ml capture antibody diluted in borate-buffered saline (7.0 mg/ml NaCl, 3.1 mg/ml H3BO3, 0.64 mg/ml NaOH) overnight at 4°C. Non-specific binding sites were blocked with 2% BSA in PBS at 37°C for 1 h. Dilutions of purified IL-17A or test samples were made in dilution buffer (0.05% Tween 20, 2% FBS in PBS) and 50 µl applied to wells at 37°C for 1 h. Biotinylated detection antibodies were diluted to 0.25 µg/ml in dilution buffer and applied to wells at 37°C for 45 min. Streptavidin-HRP (Southern Biotech) was diluted 1:5000 in dilution buffer and 100 µl was applied to wells at 37°C for 30 min. OPD easy-tablets (2 mg/tablet, Acros Organics) were dissolved (4 tablets, 5 µL H2O2 in 12 ml distilled H2O) and 100 µl applied to wells at room temperature until color developed. The reaction was stopped by addition of 100 µl 6 N H2SO4 and absorbance was measured with a µQuant plate reader (Bio-tek Instruments, Inc.) at 490 nm. Between all steps, plates were washed by flooding each well four times with wash buffer.
RNA isolation, cDNA synthesis, quantitative reverse transcriptase (RT) PCR (qPCR), and RT-PCR
For qPCR analyses, RNA was isolated from bladder tissue using TRIzol Reagent (Invitrogen) according to the manufacturer’s instructions. cDNA was synthesized from 1 µg RNA using the High Capacity cDNA Reverse Transcription Kit (Applied Biosystems). Oligo dT primers (0.5 µg/µl stock) and RNase inhibitor (10 U/µl stock) were obtained from Invitrogen. The cDNA reaction mix consisted of (per 10 reactions): 81 µl RNase-free water, 30 µl 10x buffer, 6 µl oligo dT primers, 12 µl dNTP mix, 6 µl RNase inhibitor, and 15 µl Multiscribe reverse transcriptase. The cDNA reaction conditions were: 25°C for 10 min, 37°C for 120 min, and 85°C for five s in a PTC-200 thermal cycler (MJ Research). Most qPCR reactions consisted of (per 1 reaction): 8.75 µl RNase-free water, 12.5 µl 2x Taqman Universal PCR Master Mix (Applied Biosystems), 1.0 µl 20x target primers and FAM probe (Applied Biosystems), 0.75 µl 20x control GAPDH primers and VIC probe (Applied Biosystems), and 2.0 µl cDNA. For G-CSF, forward (5’-TCCCTGGAGCAAGTGAGGAA-3’) and reverse (5’-GCTTGTAGGTGGCACACAACTG-3’) primers were designed using Taqman Probe and Primer Design Software (Applied Biosystems). This qPCR reaction consisted of: 9.5 µl RNase-free water, 12.5 µl 2x SYBR Green PCR Master Mix, 1 µl each of forward and reverse primer, and 1 µl cDNA. The qPCR reaction conditions were: 50°C for two min, 95°C for 10 min, and 55 cycles of 95°C for 15 s followed by 60°C for 1 min in a M×3000P thermal cycler (Stratagene). Fluorescence was read at 60°C at the end of each cycle. qPCR reactions were conducted in duplicate or triplicate wells with appropriate no template and no RT controls for cDNA and qPCR reactions. Data were analyzed with MxPro Software (Stratagene). Cytokine transcripts were normalized to that of GAPDH (2−ΔCt, where ΔCt equals the cycle threshold of test gene minus the cycle threshold of GAPDH).
For RT-PCR analyses, RNA was isolated from bladder tissue using the RNeasy Mini Kit (Qiagen) according to the manufacturer’s instructions. Genomic DNA was removed from the samples using TURBO DNA-free (Ambion) according to the manufacturer’s instructions. cDNA was synthesized from 1 µg of DNase-treated RNA using the SuperScript First-Strand Synthesis System for RT-PCR (Invitrogen) using gene-specific primers for common γ and δ chains [Cγ (50), Cδ (51)] according to the manufacturer’s instructions. PCR for γ and δ variable chain transcripts was conducted using 2 µl Cγ or Cδ-generated cDNA, respectively, and either Taq Polymerase (Invitrogen) or Phire Hot Start DNA Polymerase (Finnzymes) according to the manufacturer’s recommendations with a melting temperature of 54°C (with the exception of Vγ5 and Vδ8, which were amplified with a melting temperature of 51°C). Primer sets used were as follows: Vγ1.1 with Jγ4 (52); Vγ1.2 with Cγ (53); Vγ2–4 (54) and Vγ5 (5’-cct act tct agc ttt ctt gc-3’) with Jγ1 (5’-ctt acc aga ggg aat tac tat gag-3’); Vδ1–3 and 5–8 (52), and Vδ4 (53) with Jδ1 (51). Nomenclature used in the manuscript is according to Garman (55). As with qPCR, RT-PCR reactions were conducted with appropriate no template and no RT controls for cDNA and qPCR reactions (data not shown).
Histology
Bladder tissue was harvested at necropsy and fixed in 10% neutral buffered formalin for 24 h. Tissues were trimmed and processed by standard histological methods and were stained with hematoxylin and eosin. Light microscopic histopathological assessment was performed by a board-certified veterinary pathologist blinded to the group assignment of the samples. The presence or absence of inflammation and the predominating type and tissue distribution of inflammatory cells was qualitatively assessed. Additional points assessed were the presence/absence of bacteria, subjective assessment of bacterial quantity and distribution, and mucosal changes.
Neutrophil counts
Urine was collected by massaging the mouse abdomen while holding the urethra over a sterile Eppendorf tube. Urine was mixed 10:1 with Turk’s stain (0.05 mg/ml crystal violet, 3% glacial acetic acid in distilled water) and neutrophils were enumerated using a hemacytometer.
Cellular staining and flow cytometry
Bladders, isolated from euthanized mice, were cut into small pieces with a scalpel. Tissue was digested for 50 min at 37°C with agitation in 0.5% heat inactivated FBS, 20 mM HEPES pH 7, 0.057 Kunitz units/µl DNase I (Sigma), and 1 mg/ml collagenase A (Roche) in RMPI medium, with repeated passage through an 18½ gauge needle 25 min into the incubation. Erythrocytes were lysed as described above. Erythrocyte-lysed homogenates were filtered through 40 micron cell strainers and washed once with flow cytometry buffer (1% FBS, 0.01% NaN3 in PBS). After enumeration by hemacytometer, cellular suspensions were treated with mouse anti-CD16/CD32 (clone 2.4G2, eBioscience) for 10 min to block Fc receptors. Surface markers were stained for 30 min, and cells were fixed overnight in a 4% formalin solution. For intracellular staining, fixed cells were permeabilized with 1% FBS, 0.1% saponin in PBS, and stained for 60 min. Data were acquired using a BD FACSCanto flow cytometer and BD FACSDiva software, and analyzed using FlowJo v7.2.4 (Tree Star, Inc.). Mouse anti-CD4-FITC (clone GK1.5), anti-γδ-TCR-FITC (clone eBioGL3), anti-F4/80-FITC (clone BM8), anti-IL-17A-PE (clone eBio17B7), anti-CD8a-PE (clone 53–6.7), anti-CD4-PE-Cy7 (clone GK1.5), anti-Ly-6G(Gr-1)-PE-Cy7 (clone RB6-8C5), anti-CD45R(B220)-APC (clone RA3-6B2), anti-MHC Class II(I-A/I-E) (clone M5/114.15.2), anti-CD8a(Ly-2)-APC-Cy7 (clone 53–6.7), and anti-CD11b-APC-Cy7 (clone M1/70) used staining and calibration controls were obtained from eBioscience.
Statistics
Graphing and statistical analyses were done using GraphPad Prism 5. Data were represented as mean or median values based on the D'Agostino & Pearson omnibus normality test. Where applicable, the Mann-Whitney test, Paired t-test, or Fisher’s exact test was used to determine statistical significance with two-way analysis of variance and 95% confidence intervals.
Results
IL-17A is secreted by spleen and lymph node cells from C57BL/6 mice in response to transurethral infection with UPEC
To determine if IL-17A is secreted adaptively in response to UPEC antigens, wild type C57BL/6 mice were inoculated the transurethral route with either UPEC strain CFT073 or PBS according to the outlined schedules (Fig. 1). Cells harvested from the spleen (Fig. 1A) and the inner inguinal lymph nodes (Fig. 1B) were stimulated in vitro with medium alone, α-CD3 mAb, or UPEC strain CFT073 whole cell lysate and incubated for 72 h before harvesting supernatants for ELISA. As expected, splenocytes treated with α-CD3 mAb had high expression of IL-17A, regardless of whether they originated from PBS- or CFT073-treated animals, and unstimulated cells from either treatment group did not secrete IL-17A (Fig. 1A and 1B). However, in response to in vitro stimulation with UPEC whole cell lysate, only the splenocytes from UPEC-infected mice showed significant secretion of IL-17A compared to unstimulated controls (P=0.0147) (Fig. 1A). Additionally, inner inguinal lymph node cells from UPEC-infected mice secreted significantly higher amounts of IL-17A in response to in vitro lysate stimulation than lymphoid cells from PBS-treated animals (P=0.0105) (Fig. 1B). These results indicate that treatment with UPEC antigens stimulates adaptive secretion of IL-17A from in vivo-sensitized cells of both systemic and local lymphoid origins.
Figure 1. Spleen and inner inguinal lymph node cells from C57BL/6 mice receiving UPEC infection secrete IL-17A.
(A) Mice were sensitized transurethrally with either PBS (open symbols) or UPEC (filled symbols) on days 0, 14, and 28, and were sacrificed on day 33. Upon sacrifice, splenocytes from individual animals (n=10) were stimulated with medium alone, 5 µg/ml α-CD3 mAb, or 25 µg/ml whole cell lysate from UPEC strain CFT073. Each symbol represents an individual animal while bars represent the median values. (B) Mice were sensitized transurethrally with either PBS (open bars) or UPEC (filled bars) on days 0 and 7 and sacrificed 2 days after the second sensitization, day 9. Pooled inguinal lymph node cells from each group of animals (n=6) were plated in replicate wells and stimulated as in (A). Bars and error represent the mean ± SEM of triplicate ELISA wells in a representative experiment. n.d., not detectable.
IL-17A is not necessary for the adaptively-acquired protective immune response to UTI
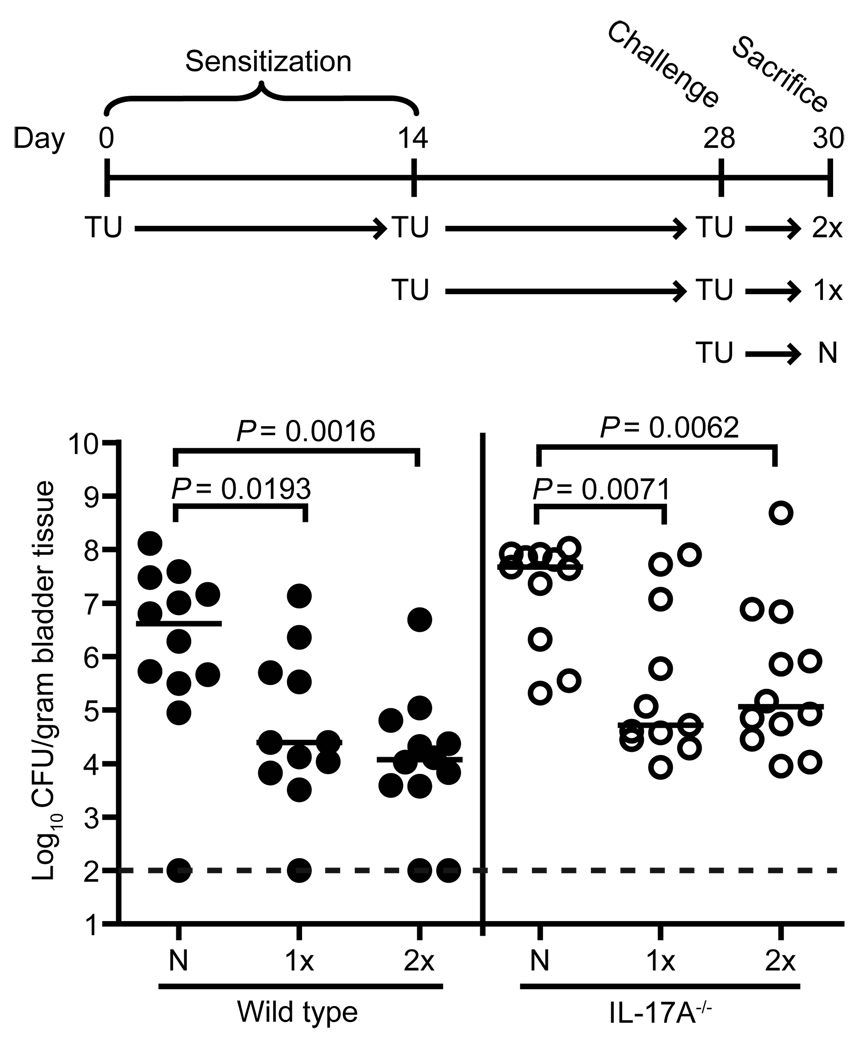
Because IL-17A was secreted in response to UPEC antigens (Fig. 1) and the role that IL-17A plays in bacterial vaccination models (56, 57), we wanted to determine whether IL-17A is required for the generation of a protective immune response to UTI. To do this, we utilized a reinfection model based on one presented by Thumbikat et al. (58). Wild type and IL-17A−/− mice were transurethrally sensitized once (1x), twice (2x), or not at all (N), prior to a 48 h challenge with UPEC (Fig. 2). Both wild type sensitization groups had significantly fewer bacteria in their bladders when compared to wild type naïve mice (P=0.0193 for 1x and P=0.0016 for 2x compared to N) (Fig. 2), consistent with a previously published study (58). Sensitized IL-17A−/− mice also exhibited a similar pattern of accelerated clearance (P=0.0071 for 1x and P=0.0062 for 2x compared to N) (Fig. 2). These results suggest that IL-17A is not required for the generation of a protective immune response to UTI.
Figure 2. IL-17A is not necessary for the protective immune response to UTI.
Cohorts of wild type and IL-17A−/− mice (n=11–12 each) were sensitized by the transurethral route with UPEC strain CFT073 once (1x), twice (2x), or not at all (N for naïve) on the indicated days. Subsequently, all mice were challenged with CFT073 on day 28. Bladder colonization levels 48 h post challenge for wild type (filled symbols) and IL-17A−/− (open symbols) mice are shown. The dotted line indicates the 100 CFU/gram tissue limit of detection. Each symbol represents an individual animal while bars represent the median values. TU=transurethral infection.
IL-17A transcript is upregulated in response to acute bladder infection by UPEC
Since there was no overt defect in the generation of protective immunity in IL-17A−/− mice, we sought to determine if IL-17A played a role in the innate response to UTI. To do this, we first examined IL-17A transcript dynamics during acute infection. Mice were inoculated transurethrally with UPEC and their bladders were collected for qPCR analysis at several time points during a 28-day period. UPEC-infected mice demonstrated a dramatic increase in IL-17A with median values peaking at 48 hours post infection (hpi) (Fig. 3A), suggesting a role for IL-17A in the innate immune response to UTI.
Figure 3. IL-17A transcript dynamics in response to acute infection and peak expression levels in wild type and γδ TCR−/− mice.
(A) Mice (n=4–18 per time point) were transurethrally inoculated with UPEC strain CFT073 and bladders were collected at specified time points for analysis of IL-17A transcript levels by qPCR. (B) Wild type (filled symbols) and γδ TCR−/− (open symbols) mice (n=9 each) were inoculated transurethrally with UPEC and sacrificed at 48 hpi for analysis of bladder mRNA by qPCR. Each symbol represents an individual animal while bars represent the median values.
γδ T cells are a significant source of IL-17A during acute UTI in the mouse model
Since TCR δ−/− mice are more susceptible to UTI (22) and γδ TCR-positive cell populations are known to express IL-17A in the context of bacterial infection (25, 28–30, 59), we wanted to see if TCR δ−/− mice had a deficiency in IL-17A transcript expression upon bladder infection. Wild type C57BL/6 and TCR δ−/− mice were inoculated transurethrally with PBS or UPEC and their bladders were analyzed by qPCR at 48 hpi. Both wild type and TCR δ−/− mice exhibited significant upregulation of IL-17A compared to PBS-treated controls (data not shown). However, the median value of IL-17A expression was 3.7-fold higher in the wild type mice (1.16×10−3 relative to GAPDH for wild type compared to 3.1×10−4 for TCR δ−/− mice) (Fig. 3B). These results demonstrate that mice deficient in the γδ TCR tend to have lower expression of IL-17A at 48 hpi, suggesting that γδ T cells are a source of the IL-17A secreted in response to UPEC bladder colonization.
Because TCR δ−/− mice express less IL-17A in response to experimental UTI, we sought to quantify the level of IL-17A expression by γδ T cells in infected wild type animals by flow cytometry. Bladders were isolated from PBS- or UPEC-inoculated wild type C57BL/6 mice at 48 hpi and made into single cell suspensions for staining and flow cytometric analysis. For comparison, the expression of IL-17A by CD4-positive cells was also analyzed. While the number of infiltrating CD4-positive cells was an order of magnitude higher than that of γδ TCR-positive cells, the increases in both populations were statistically significant (P=0.0021 and P=0.0229, respectively) (Fig. 4A). Each population was then interrogated for IL-17A positivity, as depicted by representative plots (Fig. 4B). Only γδ-positive cells exhibited statistically significant increases in IL-17A positivity after UPEC infection (P=0.0225) (Fig. 4C). In addition, the median frequency of γδ-positive cells also staining positive for IL-17A was ~5%; in some animals, up to 12% of the γδ cell population expressed IL-17A when compared to PBS group (P=0.0317) (Fig. 4D). These results indicate that at 48 hpi, γδ-positive T cells are responsible for the upregulated IL-17A transcripts seen in UPEC-infected mouse bladders.
Figure 4. γδ T cells are a source of IL-17A during acute UTI.
Bladders from mice treated transurethrally with PBS (open symbols, n=7) or UPEC strain CFT073 (filled symbols, n=9) were collected at 48 hpi, made into a single cell suspension, and stained for flow cytometry. Forward- versus side-scatter plots were gated to include all CD4- and γδ TCR-positive cells as determined by backgating. CD4- and γδ TCR-positive gates were then interrogated for IL-17A positivity based on unstained and singly-stained bladder control samples. (A) Total numbers of CD4- and γδ TCR-positive cells. (B) Flow plots showing CD4-positive (left) and γδ TCR-positive (right) cells also staining positive for IL-17A from representative PBS-treated (top) and UPEC-infected (bottom) animals. (C) The total number of CD4- and γδ-positive cells also staining positive for IL-17A. (D) Frequency of γδ TCR-positive cells also positive for IL-17A in PBS treated and UPEC-infected animals. In (A), (C), and (D) each symbol represents an individual animal while bars represent the median values.
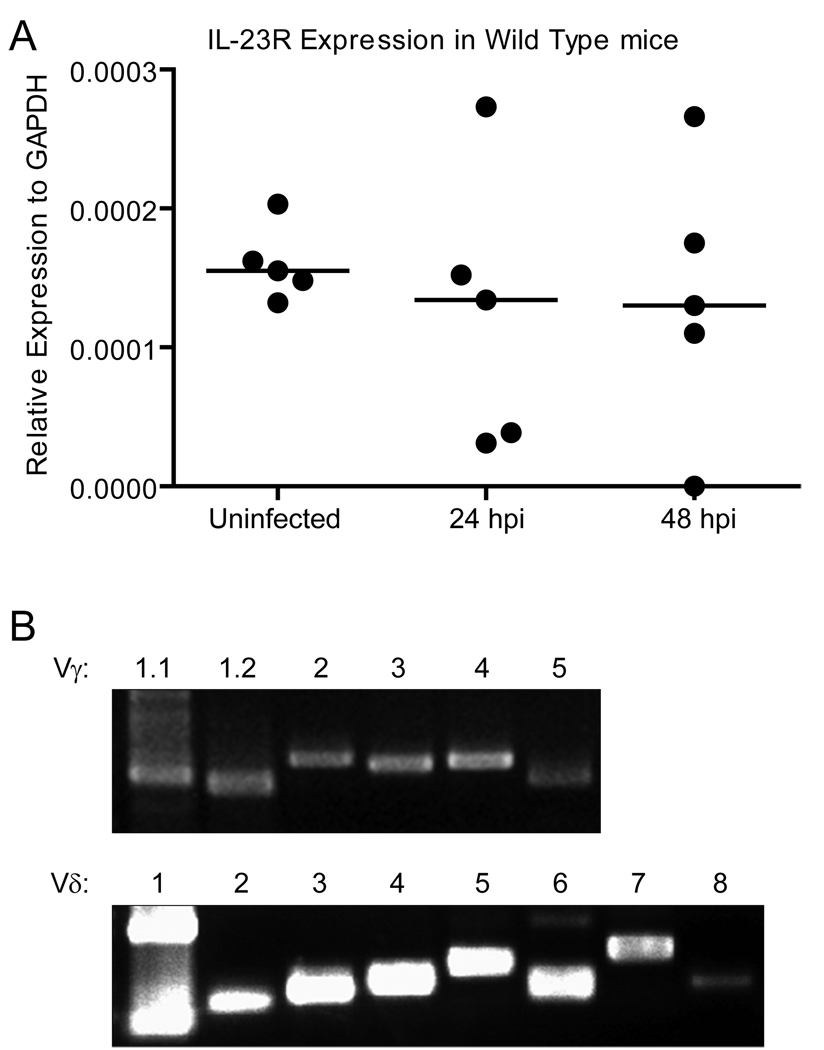
Recently, it has been shown that responsiveness to IL-23 is important for the expression of IL-17A by γδ T cells in a non-T cell receptor-dependent fashion (60). To see if IL-23R was expressed in the bladder and thus could be mediating a role in IL-17A expression by γδ T cells in response to UTI, we quantified IL-23R expression in bladder tissue by qPCR. IL-23R transcript was detected in the bladder of C57BL/6 wild type mice regardless of the state of their infection (Fig. 5A), and levels of IL-23R transcript were similar in wild type and IL-17A−/− mice (data not shown). These results indicate that the IL-23R is present in the bladder tissue of mice and may play a role in the rapid expression of IL-17A in response to UTI.
Figure 5. IL-23R and all of the known γ and δ TCR variable chains are expressed in the murine bladder.
Wild type mice were left uninfected or transurethrally infected with UPEC. (A) At 24 or 48 hpi, bladders were harvested for RNA isolation and analysis of IL-23R expression by qPCR. Each symbol represents an individual animal while bars represent the median values. (B) Bladders were harvested for RNA isolation and analysis of γ and δ variable chain expression by RT-PCR. Top panel: Vγ chain expression; Bottom panel: Vδ chain expression. Primer pairs are listed in the Materials and Methods. The presence of two products in the Vδ1 lane may reflect the junctional diversity capabilities of δ chain rearrangements (76).
In addition to IL-23R expression, we wanted to probe the expression of γ and δ variable chains to determine if a particular subset of γδ T cells is responsible for secretion of IL-17A in response to UTI. Bladders from C57BL/6 mice that were transurethrally infected with CFT073 for 48 h were harvested and prepared for RT-PCR. cDNA was first synthesized using gene specific primers for the common γ or common δ chain, and PCR for six Vγ and eight Vδ chains was performed on the corresponding products. We detected expression of all of the Vγ [with the exception of the Vγ1.3 pseudogene (61)] and Vδ chains tested in the bladder tissue of infected mice (Fig. 5B). A similar pattern of expression was observed in uninfected mice (data not shown), indicating that variable chain expression did not appear to change in response to infection.
IL-17A plays a role in defending the urinary tract from acute UPEC colonization
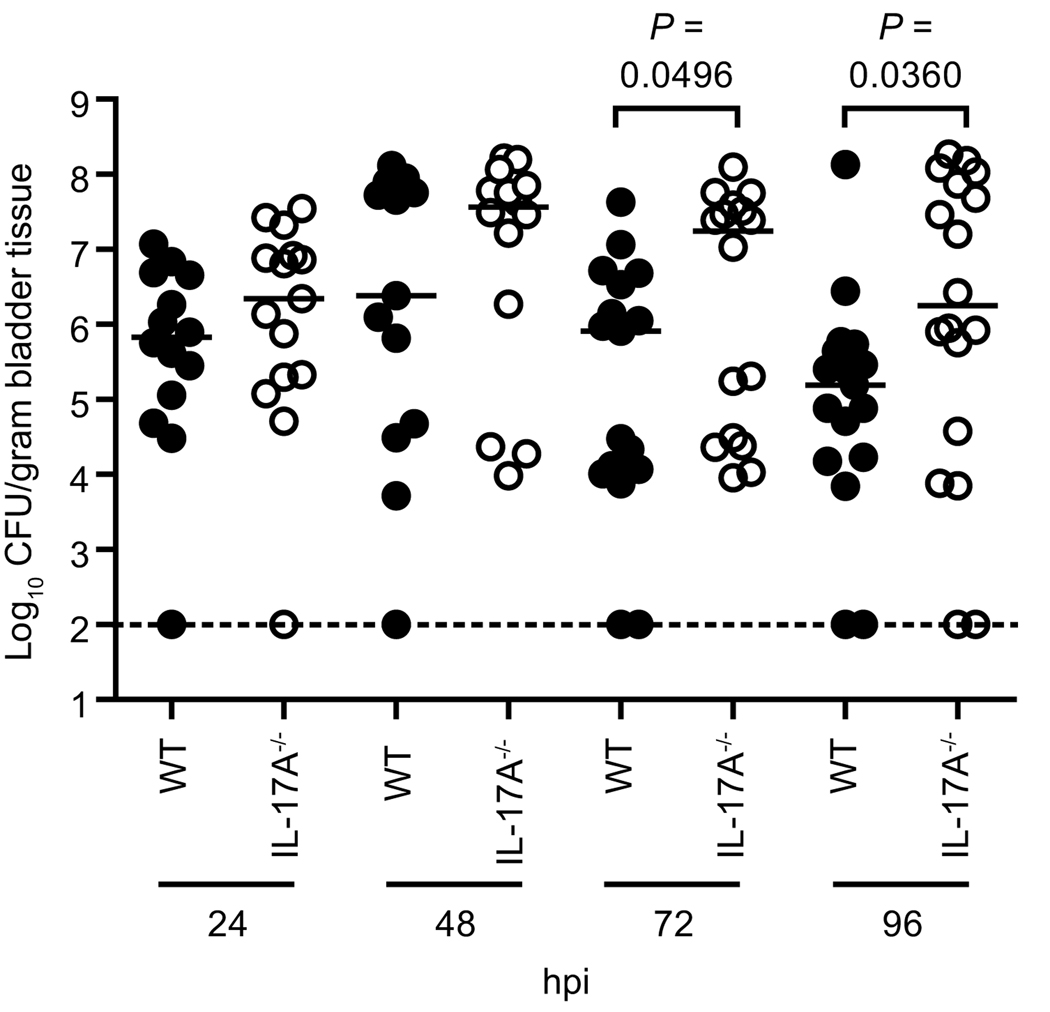
To examine the role of IL-17A in innate control of UTI, bladder and kidney homogenates from wild type and IL-17A−/− mice were cultured at the reported peak of bacterial colonization in C57BL/6 mice, 24 hpi (14). At this time point, IL-17A−/− mice had a 3-fold higher median CFU/gram bladder tissue (Fig. 6). By 48 hpi, the peak of IL-17A transcript expression in the bladder (Fig. 3A), this trend increased ten-fold to a 35-fold higher median CFU/ gram tissue in the bladders of IL-17A−/− mice (Fig. 6). While these trends were reproducible, we sought to investigate later time points, presuming the colonization phenotypes resulting from the lack of IL-17A may be exacerbated. Indeed, at both 72 and 96 hpi IL-17A−/− mice had significantly more bacteria colonizing their bladders (P < 0.05) (Fig. 6). These results indicate that IL-17A−/− mice are more susceptible to cystitis than isogenic wild type mice.
Figure 6. Colonization of UPEC-infected wild type and IL-17A−/− mice.
Wild type (WT, filled symbols) and IL-17A−/− (open symbols) mice were infected transurethrally with UPEC strain CFT073. At 24, 48, 72, and 96 hpi mice were sacrificed and bladder homogenates were plated on LB agar to determine the bacterial burden (n=14–19 mice per group per time point). The dotted line indicates the 100 CFU/gram tissue limit of detection. Each symbol represents an individual animal while bars represent the median values.
IL-17A is necessary for proinflammatory transcript upregulation in response to UTI
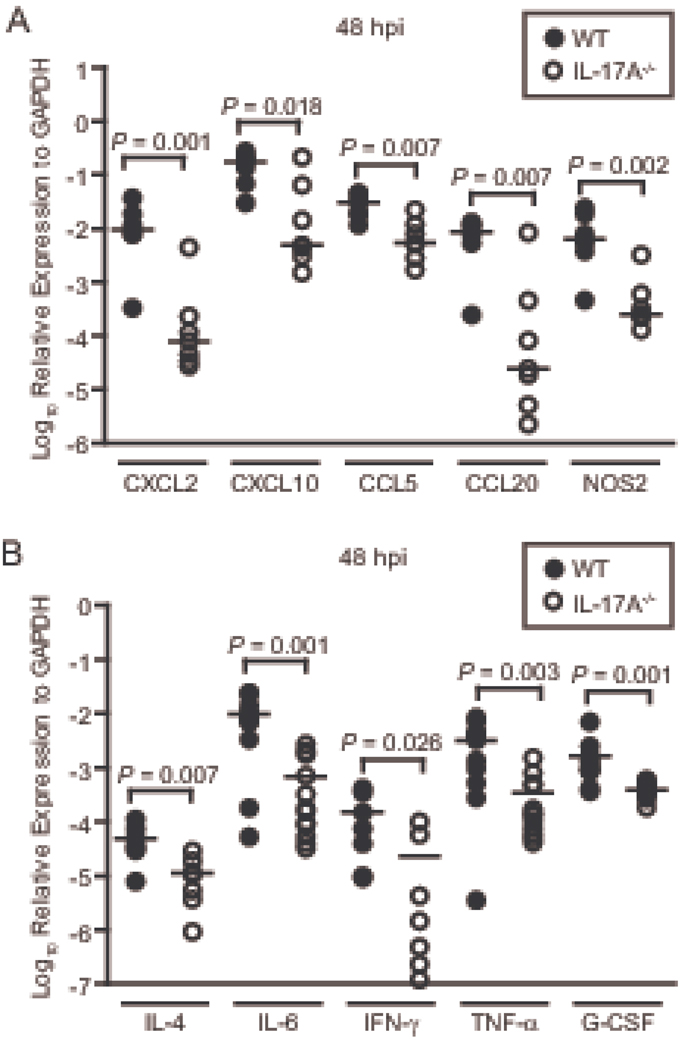
Given that IL-17A mediates inflammatory responses largely by influencing mRNA levels of key cytokines and chemokines posttranscriptionally (39–42), we wanted to determine if such effects were present in the context of UTI. Wild type and IL-17A−/− mice were inoculated transurethrally with UPEC and their bladders were collected at 48 hpi for transcript analysis by qPCR. We measured mRNA levels of a panel of chemokines and one antimicrobial effector protein, inducible nitric oxide synthase (iNOS), (Fig. 7A) and cytokines (Fig. 7B) previously shown to be affected by IL-17A expression (62). Strikingly, transcripts for all of the genes investigated were expressed at a significantly lower level in IL-17A−/− mice compared to their wild type counterparts (Fig. 7A and 7B), indicating animals lacking IL-17A−/− signaling are not able to efficiently upregulate the appropriate mRNA transcripts in infected bladder.
Figure 7. Proinflammatory transcript upregulation in response to acute UTI is defective in IL-17A−/− mice.
Wild type (filled symbols) and IL-17A−/− (open symbols) mice were inoculated transurethrally with UPEC strain CFT073. Mice (n=7–12) were sacrificed at 48 hpi and their bladders were harvested for qPCR analysis of (A) chemokines CXCL2, CXCL10, CCL5, CCL20 and the antimicrobial innate effector iNOS and (B) cytokines IL-4, IL-6, IFN-γ, TNF-α, and G-CSF. Each symbol represents an individual animal while bars represent the median values.
Infected wild type and IL-17A−/− exhibit qualitatively similar responses to UTI when examined histologically
Since IL-17A−/− mice had decreased cytokine and chemokine expression, we wanted to examine the bladders of wild type and IL-17A−/− histologically to determine if there were any gross pathological or qualitative differences in inflammation. Longitudinal sections of bladders from wild type and IL-17A−/− mice that were either uninfected or infected for 48 hours (the peak of IL-17A expression) were stained with hematoxylin and eosin and visualized microscopically. The sections revealed similar histopathological effects in response to UTI both backgrounds (Fig. 8A–H). More specifically, bladders from the uninfected mice were histologically within normal limits, without inflammation or other alteration (Fig. 8A, 8B, 8E, 8F). Bladders from the infected mice, however, had expansion of the lamina propria (LP) by edema fluid accompanied by perivascular and interstitial inflammation (Fig. 8C, 8D, 8G, 8H, black filled arrowheads). These occurrences ranged from mild to severe in both wild type and IL-17A−/− mice. Additionally, umbrella cell sloughing was also apparent in the infected animals (compare apical surface of the transitional epithelium (TE) in Fig.8E and 8F to that in Fig. 8G and 8H; UC=umbrella cell). Inflammatory infiltrates consisted of primarily neutrophils; although, sometimes a mixed monocytic and neutrophilic infiltrate was observed (high power images not shown). Occasional intraepithelial inflammation was also noted in both backgrounds (Fig. 8H, white open arrowhead). Small numbers of adherent bacteria were observed in slides from infected animals while larger numbers of both adherent and intraluminal bacteria were present in sections from IL-17A−/− mice (Fig. 8H, “B” with arrow), indicative of a decreased ability to eliminate bacteria and consistent with higher bacterial loads in the knockout animals.
Figure 8. Wild type and IL-17A−/− exhibit similar histological profiles in response to UTI.
Wild type (A, C, E, G) and IL-17A−/− (B, D, F, H) mice were left untreated (A, B, E, F) or infected with UPEC for 48 hours (C, D, G, H). Subsequently, bladders were harvested and prepared for histological examination. (A–D) 40x magnification, bar=500 µm; (E–H) 200x magnification, bar=100 µm. L=lumen, TE=transitional epithelium, LP=lamina propria, BV=blood vessel, M=muscularis, UC=umbrella cell, B=bacteria, open arrowhead=intraepithelial inflammation, closed arrowheads= perivascular and interstitial inflammation.
IL-17A is required for optimal macrophage and neutrophil infiltration in response to UTI
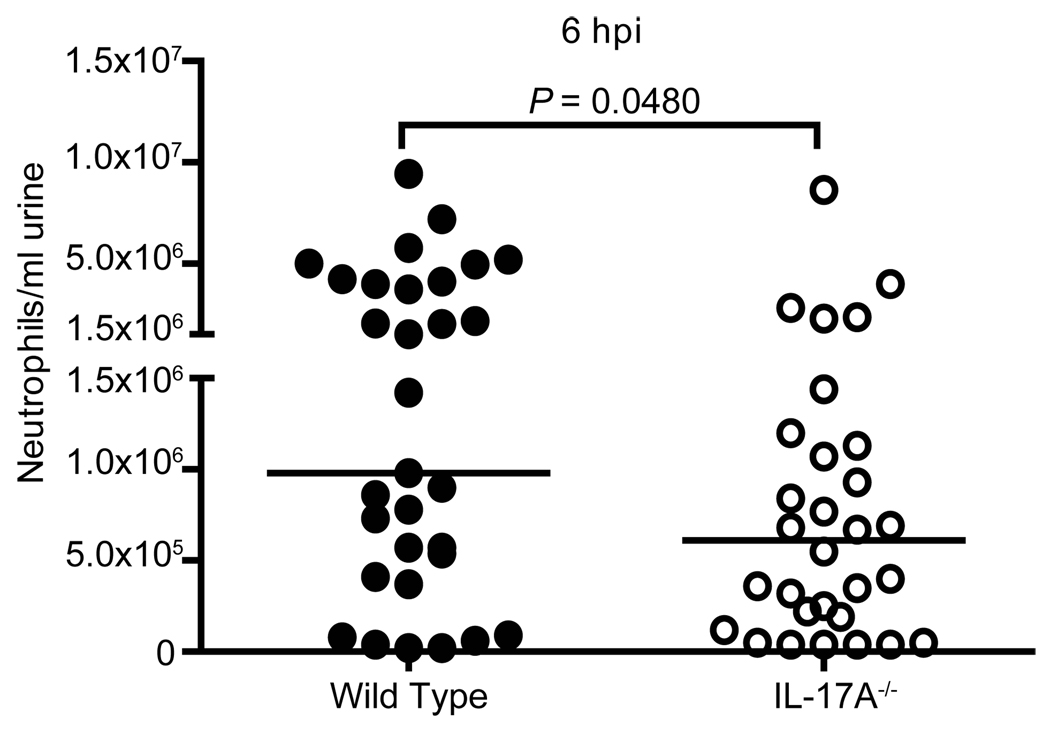
Neutrophils are the first cell type to migrate to the bladder in the event of UTI, and they are crucial for controlling infection at early time points (10, 19, 63). Since no differences in neutrophil numbers were seen histologically, a more quantitative approach to determine the neutrophil response in wild type and IL-17A−/− mice was taken. We counted the number of neutrophils in the urine after infection using a hemacytometer; the peak of this measurement being 6 hpi in both mice and humans (19, 64). At 6 hpi, IL-17A−/− mice had significantly fewer neutrophils in their urine (P=0.0480) (Fig.9). Additionally, IL-17A−/− mice lacked a population of “high-responder” mice present in the wild type cohort; these animals had greater than 1.5×106 neutrophils per ml present in their urine at 6 hpi (compare 45% of wild type animals to 17% of IL-17A−/− animals, P = 0.0262) (Fig. 9). Since IL-17A transcript and protein are detectable in the bladder at this early time point (Fig. 3A) (14), these results indicate that IL-17A may be important for very early neutrophil migration to the bladder in response to UPEC infection.
Figure 9. Early neutrophil infiltration is deficient in IL-17A−/− mice.
Wild type (filled symbols) and IL-17A−/− (open symbols) mice were transurethrally infected with UPEC strain CFT073. At 6 hpi, urine was collected from live mice (n=30–31) by gentle abdomen massage. Cells in the excretion were stained and neutrophils were counted by hemacytometer. Each symbol represents an individual animal while bars represent the median values.
To investigate the innate cellular response to UTI in the absence of IL-17A at a later time point (48 hpi), we quantified the number of macrophages and neutrophils in wild type and IL-17A−/− mice localized to the bladder tissue using flow cytometry. Macrophages were determined by interrogating the bladder for F4/80+MHC Class II+ cells while neutrophils were defined as Ly-6G+CD11b+MHC Class II− cells. Representative plots showing the gating for macrophages and neutrophils in wild type and IL-17A−/− bladder cells are shown (Fig. 10A and 10C). After quantification, both the macrophage (P=0.0145) (Fig. 10B) and the neutrophil (P=0.0031) (Fig. 10D) cell populations were significantly lower in IL-17A−/− mice as compared to wild type. These data reveal that IL-17A plays an important role in the recruitment of both macrophages and neutrophils to the bladder in response to UPEC infection.
Figure 10. Macrophage and neutrophil infiltration is deficient in IL-17A−/− mice in response to UTI.
Wild type (filled symbols) and IL-17A−/− (open symbols) mice were inoculated with UPEC strain CFT073 via the transurethral route. Mice (n=14) were sacrificed at 48 hpi and their bladders were harvested and prepared for analysis of innate cellular infiltrate by flow cytometry. Forward- versus side-scatter plots were gated to include all MHC Class II+F4/80+ and Ly-6G+CD11b+ cells as determined by backgating. The Ly-6G+CD11b+ population was further interrogated for MHC Class II negatively to enumerate neutrophils. (A) Representative flow plots showing MHC Class II+F4/80+ cells from wild type (left) and IL-17A−/− animals (right). (B) The total number of MHC Class II+F4/80+ cells. (C) Representative flow plots showing Ly-6G+CD11b+ cells from wild type (left) and IL-17A−/− animals (right). The bottom plots show MHC Class II expression by the boxed population in the top plots. (D) The total number of Ly-6G+CD11b+MHC Class II− cells. In (A) and (C) numbers are the percent of the parent population and in (B) and (D) each symbol represents an individual animal while bars represent the median values.
Discussion
With the exception of asymptomatic bacteriuria (65), the bladder mucosa has been widely accepted as a sterile environment. Mammalian hosts employ a number of mechanisms to keep this niche microbe-free, as infection by bacterial and fungal pathogens can lead to serious clinical consequences. Here we characterized the role of the cytokine IL-17A during UTI using a murine model. IL-17A was upregulated specifically in response to UPEC antigens by secondary lymphoid tissue cells from UPEC-infected C57BL/6 mice. IL-17A transcripts were also highly upregulated in the bladders of acutely infected mice, γδ T cells being a major source. Although no role for IL-17A in vaccination-induced UTI protection was observed, we noted a deficiency in bladder neutrophil influx during the very early stages of acute cystitis, and higher bacterial burdens in IL-17A−/− mice. Knockout mice also had impaired proinflammatory transcript expression and fewer macrophages and neutrophils infiltrating the bladder tissue. Taken together, these results define IL-17A as an important factor in the innate immune response to UPEC-mediated UTI.
Although the presence of pathogen-specific antibodies in the urine and serum of infected humans and experimental animals has been documented for decades (66), the cascade of immunological events that occurs during the generation of adaptive immunity during UTI has not been established. Although cells from UPEC-sensitized mice highly upregulate IL-17A in vitro, IL-17A was not necessary for the generation of vaccine-induced protective immunity (Fig. 2). This result is in contrast to vaccination models for Streptococcus pneumoniae, Bordetella pertussis, and Pseudomonas aeruginosa, where IL-17A was required for a protective immunity (56, 57, 67). As the immune system features redundant pathways, it is unclear if there is a compensatory factor acting in bladder or lymphoid tissue or if the downstream effects of IL-17A are indeed not necessary for a protective response to UTI.
In addition to secretion in a recall response setting, IL-17A is upregulated in an innate fashion (Fig. 3A). Similarly, airway IL-17A peaked innately in response to intranasal infection with Chlamydia muridarum, another mucosal pathogen, and this was dependent on bacterial replication (26). In experimental UTI, IL-17A upregulation was also dependent on the ability of UPEC to successfully colonize the urinary tract, as a fecal strain that is unable to colonize the bladder efficiently, EFC4 (48), does not induce IL-17A transcript (data not shown). A recent study by Ingersoll and colleagues surveyed cytokine and chemokine protein in the bladder of mice during a two-week experimental UTI (14). While most of the cytokines examined returned to near baseline levels one week post infection, IL-17 remained elevated (above control mice) throughout the experiment (14). Our transcript data agreed with this and the fact that IL-17A is highly upregulated in response to UTI, with peak levels attained only days after infection.
A number of cell types have been shown to secrete IL-17A (23). Unlike classical αβ T cells, which recognize antigen that is processed and presented in the context of self MHC molecules, γδ T cells harbor the ability to directly recognize cognate antigen, allowing for rapid production of effector molecules (61, 68). Therefore, because of the early upregulation of IL-17A in the bladder (Fig. 3A) (14), we reasoned that CD4-positive Th17 cells are not the principal source of IL-17A. Despite being in the T cell minority (Fig. 4A), intracellular staining and flow cytometric analyses demonstrated that γδ T cells were a major source of IL-17A during UTI (Fig. 4B–D). Of note, TCR δ−/− mice were still able to generate some IL-17A transcript over background (Fig. 3B), demonstrating that additional cell types make IL-17A in the bladder of UPEC-infected mice. These results suggest that γδ-positive cells recognize UPEC by a currently unidentified ligand and secrete IL-17A in response to UPEC infection.
In the context UTI, IL-17A appears to play a role in optimal restriction of bacterial burden (Fig. 6). Infection models for Listeria monocytogenes, disseminated E. coli, Klebsiella pneumoniae, oral and systemic Candida albicans, oral Toxoplasma gondii, C. muridarum, Bacillus subtilis, and others also show that IL-17A signaling is required for acute clearance of the invading organism (25, 26, 29, 31, 32, 34, 69, 70). This collection of data demonstrates the breadth and versatility of IL-17A-mediated pathways in handling various classes of microbes. In contrast, IL-17A has been shown to be dispensable for clearance in infection models for systemic Salmonella enterica serovar Enteritidis and pulmonary Mycobacterium bovis bacille Calmette-Guérin (28, 30, 71). The factors determining whether or not IL-17A signaling is important for clearance in a particular infection model are not clear. They may depend on anatomical location, inherent qualities of the infectious agent, or innate immune signaling in response to pathogen recognition.
IL-17A has a well-documented role in affecting the level of cytokine, chemokine, and antimicrobial expression (62). Thus, it was not surprising that proinflammatory bladder transcripts probed in infected IL-17A−/− mice were not expressed as well as in wild type counterparts (Fig. 7). The lack of chemokines important for the infiltration of neutrophils (CXCL2 and weakly CCL20), T cells (CXCL10, CCL5), and dendritic cells (CXCL10, CCL20) may contribute to the defect in bacterial clearance seen in the IL-17A−/− animals. Additionally, cytokines crucial for activation and mobilization of innate immune responses (IL-6 and IFN-γ) were also lacking in this background. While deficiency in these transcripts may be the result of inadequate stimulation of epithelial cells and resident macrophages, lower iNOS levels may be a reflection of impaired neutrophil infiltrate in the IL-17A−/− mice (Fig. 10D). Unexpectedly, IL-4 was also significantly lower in the bladders of IL-17A−/− mice. IL-4 is a canonical Th2 cytokine (72), and given that IL-4 knockout mice do not have an acute UPEC clearance defect (22), a function in UTI immunity has not been defined. Nonetheless, the reported role of IL-4 in B cell activation (73) suggests that IL-4 may be upregulated to stimulate B cell antibody generation in an adaptive response to UTI. In total, it appears that there is a general inflammatory defect in IL-17A−/− mice, and that genes of both appreciated and unknown importance are affected by the absence of IL-17A during UTI.
Since IL-17A orchestrates neutrophil recruitment to infected tissue, there is precedence for an innate immune mechanism involving rapid secretion of large amounts of IL-17A to bolster neutrophil killing of UPEC (19, 74). A lack of early neutrophil infiltrate in IL-17A−/− mice (Fig. 9) may be the result of both impaired neutrophil exit from the bone marrow and migration to the bladder tissue, as growth factor G-CSF and neutrophil-specific chemokine transcripts were deficient in infected IL-17A−/− animals. Interestingly, antibody-mediated knockdown of G-CSF rendered mice more resistant to UTI, and the authors suggested that macrophage activation status is responsible for this surprising phenotype (14). Although lacking some G-CSF expression, IL-17A−/− mice do not exhibit an enhanced clearance phenotype (Fig. 6), possibly due to the pleiotropic effects of IL-17A deficiency or signaling by the existing G-CSF. We also examined neutrophil levels in wild type and IL-17A−/− mice at later time points during infection. Macrophages were included in the analysis to see if their total numbers varied with neutrophils. Upon UPEC infection, both populations increased several fold over PBS mock-infected mice; however, in the IL-17A−/− mice, both neutrophil and macrophage counts were lower compared to wild type (Fig. 10B and 10D). These data reveal that the defect IL-17A−/− mice exhibit in UPEC clearance may be due to less macrophages and neutrophils present in the bladder to execute bacterial clearance.
Collectively, these data demonstrate that IL-17A plays a role in the innate immune response to experimental UTI in a mouse model. As many of the genes influenced by IL-17A have similar function during UTI in mice and men (75), we expect that IL-17A also plays a role in controlling the bacterial burdens early during UTI in humans. IL-17A accomplishes such control by enhancing the presence of mRNA transcripts important for the infiltration of neutrophils and other inflammatory mediators. The presence of such cell types is crucial to the defense of the urinary tract from epithelial cell adherence and subsequent invasion by UPEC.
Acknowledgements
Histology was performed by the Pathology Cores for Animal Research (PCAR) in the Unit for Laboratory Animal Medicine at the University of Michigan. Histopathological assessment was performed by PCAR pathologist Dr. Ingrid Bergin, VMD, MS, DACLAM, DACVP. The authors would also like to thank Dr. Cheong-Hee Chang, Dr. Phillip D. King, Dr. Nick Lukacs, and Dr. Mary O’Riordan for reagents; Dr. Timothy Bauler, Pamela Lincoln, and Dr. M. Hanief Sofi for technical support; Dr. Yoichiro Iwakura for the gift of IL-17A−/− mice; and members of the Mobley Lab for critical review of the manuscript.
Footnotes
This work was supported in part by Public Health Service Grant AI043363 from the National Institutes of Health.
Abbreviations used in this paper: UPEC, uropathogenic Escherichia coli; UTI, urinary tract infection; TCR δ−/−, Tcrdtm1Mom targeted mutation knockout mice; RT, reverse transcriptase; qPCR, quantitative RT-PCR; hpi, hours post infection; WT, wild type; iNOS, inducible nitric oxide synthase
References
- 1.Kaper JB, Nataro JP, Mobley HL. Pathogenic Escherichia coli. Nat. Rev. Microbiol. 2004;2:123–140. doi: 10.1038/nrmicro818. [DOI] [PubMed] [Google Scholar]
- 2.Brown P, Ki M, Foxman B. Acute pyelonephritis among adults: cost of illness and considerations for the economic evaluation of therapy. Pharmacoeconomics. 2005;23:1123–1142. doi: 10.2165/00019053-200523110-00005. [DOI] [PubMed] [Google Scholar]
- 3.Litwin MS, Saigal CS, Yano EM, Avila C, Geschwind SA, Hanley JM, Joyce GF, Madison R, Pace J, Polich SM, Wang M. Urologic diseases in America Project: analytical methods and principal findings. J. Urol. 2005;173:933–937. doi: 10.1097/01.ju.0000152365.43125.3b. [DOI] [PubMed] [Google Scholar]
- 4.Foxman B, Barlow R, D'Arcy H, Gillespie B, Sobel JD. Urinary tract infection: self-reported incidence and associated costs. Ann. Epidemiol. 2000;10:509–515. doi: 10.1016/s1047-2797(00)00072-7. [DOI] [PubMed] [Google Scholar]
- 5.Lane MC, Alteri CJ, Smith SN, Mobley HL. Expression of flagella is coincident with uropathogenic Escherichia coli ascension to the upper urinary tract. Proc. Natl. Acad. Sci. USA. 2007;104:16669–16674. doi: 10.1073/pnas.0607898104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Johnson JR. Virulence factors in Escherichia coli urinary tract infection. Clin. Microbiol. Rev. 1991;4:80–128. doi: 10.1128/cmr.4.1.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aronson M, Medalia O, Amichay D, Nativ O. Endotoxin-induced shedding of viable uroepithelial cells is an antimicrobial defense mechanism. Infect. Immun. 1988;56:1615–1617. doi: 10.1128/iai.56.6.1615-1617.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Orikasa S, Hinman F., Jr Reaction of the vesical wall to bacterial penetration: resistance to attachment, desquamation, and leukocytic activity. Invest. Urol. 1977;15:185–193. [PubMed] [Google Scholar]
- 9.Andersen-Nissen E, Hawn TR, Smith KD, Nachman A, Lampano AE, Uematsu S, Akira S, Aderem A. Cutting edge: TLR5−/− mice are more susceptible to Escherichia coli urinary tract infection. J. Immunol. 2007;178:4717–4720. doi: 10.4049/jimmunol.178.8.4717. [DOI] [PubMed] [Google Scholar]
- 10.Shahin RD, Engberg I, Hagberg L, Svanborg Eden C. Neutrophil recruitment and bacterial clearance correlated with LPS responsiveness in local gram-negative infection. J. Immunol. 1987;138:3475–3480. [PubMed] [Google Scholar]
- 11.Zhang D, Zhang G, Hayden MS, Greenblatt MB, Bussey C, Flavell RA, Ghosh S. A toll-like receptor that prevents infection by uropathogenic bacteria. Science. 2004;303:1522–1526. doi: 10.1126/science.1094351. [DOI] [PubMed] [Google Scholar]
- 12.Lauw FN, Caffrey DR, Golenbock DT. Of mice and man: TLR11 (finally) finds profilin. Trends Immunol. 2005;26:509–511. doi: 10.1016/j.it.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 13.Chromek M, Slamova Z, Bergman P, Kovacs L, Podracka L, Ehren I, Hokfelt T, Gudmundsson GH, Gallo RL, Agerberth B, Brauner A. The antimicrobial peptide cathelicidin protects the urinary tract against invasive bacterial infection. Nat. Med. 2006;12:636–641. doi: 10.1038/nm1407. [DOI] [PubMed] [Google Scholar]
- 14.Ingersoll MA, Kline KA, Nielsen HV, Hultgren SJ. G-CSF induction early in uropathogenic Escherichia coli infection of the urinary tract modulates host immunity. Cell. Microbiol. 2008;10:2568–2578. doi: 10.1111/j.1462-5822.2008.01230.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saemann MD, Weichhart T, Horl WH, Zlabinger GJ. Tamm-Horsfall protein: a multilayered defence molecule against urinary tract infection. Eur. J. Clin. Invest. 2005;35:227–235. doi: 10.1111/j.1365-2362.2005.01483.x. [DOI] [PubMed] [Google Scholar]
- 16.Svanborg C, Bergsten G, Fischer H, Godaly G, Gustafsson M, Karpman D, Lundstedt AC, Ragnarsdottir B, Svensson M, Wullt B. Uropathogenic Escherichia coli as a model of host-parasite interaction. Curr. Opin. Microbiol. 2006;9:33–39. doi: 10.1016/j.mib.2005.12.012. [DOI] [PubMed] [Google Scholar]
- 17.Hiratsuka T, Nakazato M, Ihi T, Minematsu T, Chino N, Nakanishi T, Shimizu A, Kangawa K, Matsukura S. Structural analysis of human beta-defensin-1 and its significance in urinary tract infection. Nephron. 2000;85:34–40. doi: 10.1159/000045627. [DOI] [PubMed] [Google Scholar]
- 18.Samuelsson P, Hang L, Wullt B, Irjala H, Svanborg C. Toll-like receptor 4 expression and cytokine responses in the human urinary tract mucosa. Infect. Immun. 2004;72:3179–3186. doi: 10.1128/IAI.72.6.3179-3186.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haraoka M, Hang L, Frendeus B, Godaly G, Burdick M, Strieter R, Svanborg C. Neutrophil recruitment and resistance to urinary tract infection. J. Infect. Dis. 1999;180:1220–1229. doi: 10.1086/315006. [DOI] [PubMed] [Google Scholar]
- 20.Hirose T, Kumamoto Y, Matsukawa M, Yokoo A, Satoh T, Matsuura A. Study on local immune response in Escherichia coli-induced experimental urinary tract infection in mice--infiltration of Ia-positive cells, macrophages, neutrophils, T cells and B cells. Kansenshogaku Zasshi. 1992;66:964–973. doi: 10.11150/kansenshogakuzasshi1970.66.964. [DOI] [PubMed] [Google Scholar]
- 21.Engel D, Dobrindt U, Tittel A, Peters P, Maurer J, Gutgemann I, Kaissling B, Kuziel W, Jung S, Kurts C. Tumor necrosis factor alpha- and inducible nitric oxide synthase-producing dendritic cells are rapidly recruited to the bladder in urinary tract infection but are dispensable for bacterial clearance. Infect. Immun. 2006;74:6100–6107. doi: 10.1128/IAI.00881-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jones-Carson J, Balish E, Uehling DT. Susceptibility of immunodeficient gene-knockout mice to urinary tract infection. J. Urol. 1999;161:338–341. [PubMed] [Google Scholar]
- 23.Bettelli E, Korn T, Oukka M, Kuchroo VK. Induction and effector functions of T(H)17 cells. Nature. 2008;453:1051–1057. doi: 10.1038/nature07036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mills KH. Induction, function and regulation of IL-17-producing T cells. Eur. J. Immunol. 2008;38:2636–2649. doi: 10.1002/eji.200838535. [DOI] [PubMed] [Google Scholar]
- 25.Hamada S, Umemura M, Shiono T, Tanaka K, Yahagi A, Begum MD, Oshiro K, Okamoto Y, Watanabe H, Kawakami K, Roark C, Born WK, O'Brien R, Ikuta K, Ishikawa H, Nakae S, Iwakura Y, Ohta T, Matsuzaki G. IL-17A produced by γδ T cells plays a critical role in innate immunity against Listeria monocytogenes infection in the liver. J. Immunol. 2008;181:3456–3463. doi: 10.4049/jimmunol.181.5.3456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang X, Gao L, Lei L, Zhong Y, Dube P, Berton MT, Arulanandam B, Zhang J, Zhong G. A MyD88-dependent early IL-17 production protects mice against airway infection with the obligate intracellular pathogen Chlamydia muridarum. J. Immunol. 2009;183:1291–1300. doi: 10.4049/jimmunol.0803075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ishigame H, Kakuta S, Nagai T, Kadoki M, Nambu A, Komiyama Y, Fujikado N, Tanahashi Y, Akitsu A, Kotaki H, Sudo K, Nakae S, Sasakawa C, Iwakura Y. Differential roles of Interleukin-17A and -17F in host defense against mucoepithelial bacterial infection and allergic responses. Immunity. 2009;30:108–119. doi: 10.1016/j.immuni.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 28.Schulz SM, Kohler G, Holscher C, Iwakura Y, Alber G. IL-17A is produced by Th17, γδ T cells and other CD4- lymphocytes during infection with Salmonella enterica serovar Enteritidis and has a mild effect in bacterial clearance. Int. Immunol. 2008;20:1129–1138. doi: 10.1093/intimm/dxn069. [DOI] [PubMed] [Google Scholar]
- 29.Shibata K, Yamada H, Hara H, Kishihara K, Yoshikai Y. Resident Vδ1+ γδ T cells control early infiltration of neutrophils after Escherichia coli infection via IL-17 production. J. Immunol. 2007;178:4466–4472. doi: 10.4049/jimmunol.178.7.4466. [DOI] [PubMed] [Google Scholar]
- 30.Umemura M, Yahagi A, Hamada S, Begum MD, Watanabe H, Kawakami K, Suda T, Sudo K, Nakae S, Iwakura Y, Matsuzaki G. IL-17-mediated regulation of innate and acquired immune response against pulmonary Mycobacterium bovis bacille Calmette-Guerin infection. J. Immunol. 2007;178:3786–3796. doi: 10.4049/jimmunol.178.6.3786. [DOI] [PubMed] [Google Scholar]
- 31.Ye P, Rodriguez FH, Kanaly S, Stocking KL, Schurr J, Schwarzenberger P, Oliver P, Huang W, Zhang P, Zhang J, Shellito JE, Bagby GJ, Nelson S, Charrier K, Peschon JJ, Kolls JK. Requirement of Interleukin 17 receptor signaling for lung CXC chemokine and granulocyte colony-stimulating factor expression, neutrophil recruitment, and host defense. J. Exp. Med. 2001;194:519–528. doi: 10.1084/jem.194.4.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huang W, Na L, Fidel PL, Schwarzenberger P. Requirement of interleukin-17A for systemic anti-Candida albicans host defense in mice. J. Infect. Dis. 2004;190:624–631. doi: 10.1086/422329. [DOI] [PubMed] [Google Scholar]
- 33.van de Veerdonk FL, Marijnissen RJ, Kullberg BJ, Koenen HJPM, Cheng S-C, Joosten I, van den Berg WB, Williams DL, van der Meer JWM, Joosten LAB, Netea MG. The macrophage mannose receptor induces IL-17 in response to Candida albicans. Cell Host Microbe. 2009;5:329–340. doi: 10.1016/j.chom.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 34.Conti HR, Shen F, Nayyar N, Stocum E, Sun JN, Lindemann MJ, Ho AW, Hai JH, Yu JJ, Jung JW, Filler SG, Masso-Welch P, Edgerton M, Gaffen SL. Th17 cells and IL-17 receptor signaling are essential for mucosal host defense against oral candidiasis. J. Exp. Med. 2009;206:299–311. doi: 10.1084/jem.20081463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rudner XL, Happel KI, Young EA, Shellito JE. Interleukin-23 (IL-23)-IL-17 cytokine axis in murine Pneumocystis carinii infection. Infect. Immun. 2007;75:3055–3061. doi: 10.1128/IAI.01329-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hamada H, Garcia-Hernandez MdlL, Reome JB, Misra SK, Strutt TM, McKinstry KK, Cooper AM, Swain SL, Dutton RW. Tc17, a Unique Subset of CD8 T Cells That Can Protect against Lethal Influenza Challenge. J. Immunol. 2009;182:3469–3481. doi: 10.4049/jimmunol.0801814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hou W, Kang HS, Kim BS. Th17 cells enhance viral persistence and inhibit T cell cytotoxicity in a model of chronic virus infection. J. Exp. Med. 2009;206:313–328. doi: 10.1084/jem.20082030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kawakami Y, Tomimori Y, Yumoto K, Hasegawa S, Ando T, Tagaya Y, Crotty S, Kawakami T. Inhibition of NK cell activity by IL-17 allows vaccinia virus to induce severe skin lesions in a mouse model of eczema vaccinatum. J. Exp. Med. 2009;206:1219–1225. doi: 10.1084/jem.20082835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hartupee J, Liu C, Novotny M, Li X, Hamilton T. IL-17 enhances chemokine gene expression through mRNA stabilization. J. Immunol. 2007;179:4135–4141. doi: 10.4049/jimmunol.179.6.4135. [DOI] [PubMed] [Google Scholar]
- 40.Hartupee J, Liu C, Novotny M, Sun D, Li X, Hamilton TA. IL-17 signaling for mRNA stabilization does not require TNF Receptor-Associated Factor 6. J. Immunol. 2009;182:1660–1666. doi: 10.4049/jimmunol.182.3.1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hata K, Andoh A, Shimada M, Fujino S, Bamba S, Araki Y, Okuno T, Fujiyama Y, Bamba T. IL-17 stimulates inflammatory responses via NF-kappaB and MAP kinase pathways in human colonic myofibroblasts. Am. J. Physiol. Gastrointest. Liver Physiol. 2002;282:G1035–G1044. doi: 10.1152/ajpgi.00494.2001. [DOI] [PubMed] [Google Scholar]
- 42.Henness S, van Thoor E, Ge Q, Armour CL, Hughes JM, Ammit AJ. IL-17A acts via p38 MAPK to increase stability of TNF-α-induced IL-8 mRNA in human airway smooth muscle. Am. J. Physiol. Lung Cell. Mol. Physiol. 2006;290:L1283–L1290. doi: 10.1152/ajplung.00367.2005. [DOI] [PubMed] [Google Scholar]
- 43.Hagan EC, Mobley HL. Uropathogenic Escherichia coli outer membrane antigens expressed during urinary tract infection. Infect. Immun. 2007;75:3941–3949. doi: 10.1128/IAI.00337-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Alteri CJ, Hagan EC, Sivick KE, Smith SN, Mobley HLT. Mucosal immunization with iron receptor antigens protects against urinary tract infection. PLoS Pathog. 2009;5:e1000586. doi: 10.1371/journal.ppat.1000586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dubin PJ, Kolls JayK. Th17 cytokines and mucosal immunity. Immunological Reviews. 2008;226:160–171. doi: 10.1111/j.1600-065X.2008.00703.x. [DOI] [PubMed] [Google Scholar]
- 46.Itohara S, Mombaerts P, Lafaille J, Iacomini J, Nelson A, Clarke AR, Hooper ML, Farr A, Tonegawa S. T cell receptor δ gene mutant mice: Independent generation of αβ T cells and programmed rearrangements of γδ TCR genes. Cell. 1993;72:337–348. doi: 10.1016/0092-8674(93)90112-4. [DOI] [PubMed] [Google Scholar]
- 47.Nakae S, Komiyama Y, Nambu A, Sudo K, Iwase M, Homma I, Sekikawa K, Asano M, Iwakura Y. Antigen-specific T cell sensitization is impaired in IL-17-deficient mice, causing suppression of allergic cellular and humoral responses. Immunity. 2002;17:375–387. doi: 10.1016/s1074-7613(02)00391-6. [DOI] [PubMed] [Google Scholar]
- 48.Mobley HL, Green DM, Trifillis AL, Johnson DE, Chippendale GR, Lockatell CV, Jones BD, Warren JW. Pyelonephritogenic Escherichia coli and killing of cultured human renal proximal tubular epithelial cells: role of hemolysin in some strains. Infect. Immun. 1990;58:1281–1289. doi: 10.1128/iai.58.5.1281-1289.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Welch RA, Burland V, Plunkett G, 3rd, Redford P, Roesch P, Rasko D, Buckles EL, Liou SR, Boutin A, Hackett J, Stroud D, Mayhew GF, Rose DJ, Zhou S, Schwartz DC, Perna NT, Mobley HL, Donnenberg MS, Blattner FR. Extensive mosaic structure revealed by the complete genome sequence of uropathogenic Escherichia coli. Proc. Natl. Acad. Sci. U.S.A. 2002;99:17020–17024. doi: 10.1073/pnas.252529799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Matsuzaki G, Hiromatsu K, Yoshikai Y, Muramori K, Nomoto K. Characterization of T-cell receptor γδ T cells appearing at the early phase of murine Listeria monocytogenes infection. Immunology. 1993;78:22–27. [PMC free article] [PubMed] [Google Scholar]
- 51.Rajasekar R, Sim GK, Augustin A. Self heat shock and γδ T-cell reactivity. Proc. Natl. Acad. Sci. U S A. 1990;87:1767–1771. doi: 10.1073/pnas.87.5.1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xiong N, Kang C, Raulet DH. Positive selection of dendritic epidermal γδ T cell precursors in the fetal thymus determines expression of skin-homing receptors. Immunity. 2004;21:121–131. doi: 10.1016/j.immuni.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 53.Matsukawa M, Kumamoto Y, Hirose T, Matsuura A. Tissue gamma/delta T cells in experimental urinary tract infection relationship between other immuno-competent cells. Kansenshogaku Zasshi. 1994;68:1498–1511. doi: 10.11150/kansenshogakuzasshi1970.68.1498. [DOI] [PubMed] [Google Scholar]
- 54.Goldman JP, Spencer DM, Raulet DH. Ordered rearrangement of variable region genes of the T cell receptor γ locus correlates with transcription of the unrearranged genes. J. Exp. Med. 1993;177:729–739. doi: 10.1084/jem.177.3.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Garman RD, Doherty PJ, Raulet DH. Diversity, rearrangement, and expression of murine T cell gamma genes. Cell. 1986;45:733–742. doi: 10.1016/0092-8674(86)90787-7. [DOI] [PubMed] [Google Scholar]
- 56.Higgins SC, Jarnicki AG, Lavelle EC, Mills KH. TLR4 mediates vaccine-induced protective cellular immunity to Bordetella pertussis: role of IL-17-producing T cells. J. Immunol. 2006;177:7980–7989. doi: 10.4049/jimmunol.177.11.7980. [DOI] [PubMed] [Google Scholar]
- 57.Lu YJ, Gross J, Bogaert D, Finn A, Bagrade L, Zhang Q, Kolls JK, Srivastava A, Lundgren A, Forte S, Thompson CM, Harney KF, Anderson PW, Lipsitch M, Lipsitch M, Malley R. Interleukin-17A mediates acquired immunity to pneumococcal colonization. PLoS Pathog. 2008;4:e1000159. doi: 10.1371/journal.ppat.1000159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Thumbikat P, Waltenbaugh C, Schaeffer AJ, Klumpp DJ. Antigen-specific responses accelerate bacterial clearance in the bladder. J. Immunol. 2006;176:3080–3086. doi: 10.4049/jimmunol.176.5.3080. [DOI] [PubMed] [Google Scholar]
- 59.Lockhart E, Green AM, Flynn JL. IL-17 production is dominated by γδ T cells rather than CD4 T cells during Mycobacterium tuberculosis infection. J. Immunol. 2006;177:4662–4669. doi: 10.4049/jimmunol.177.7.4662. [DOI] [PubMed] [Google Scholar]
- 60.Sutton CE, Lalor SJ, Sweeney CM, Brereton CF, Lavelle EC, Mills KHG. Interleukin-1 and IL-23 induce innate IL-17 production from γδ T Cells, amplifying Th17 responses and autoimmunity. Immunity. 2009;31:331–341. doi: 10.1016/j.immuni.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 61.Carding SR, Egan PJ. γδ T cells: functional plasticity and heterogeneity. Nat. Rev. Immunol. 2002;2:336–345. doi: 10.1038/nri797. [DOI] [PubMed] [Google Scholar]
- 62.Gaffen SL. An overview of IL-17 function and signaling. Cytokine. 2008;43:402–407. doi: 10.1016/j.cyto.2008.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Agace WW, Hedges SR, Ceska M, Svanborg C. Interleukin-8 and the neutrophil response to mucosal gram-negative infection. J. Clin. Invest. 1993;92:780–785. doi: 10.1172/JCI116650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Agace WW. The role of the epithelial cell in Escherichia coli induced neutrophil migration into the urinary tract. Eur. Respir. J. 1996;9:1713–1728. doi: 10.1183/09031936.96.09081713. [DOI] [PubMed] [Google Scholar]
- 65.Warren JW. Clinical presentation and epidemiology of urinary tract infections. In: Mobley HLT, Warren JW, editors. Urinary tract infections: molecular pathogenesis and clinical management. Washington, D.C: ASM Press; 1996. pp. 3–27. [Google Scholar]
- 66.Uehling DT, Johnson DB, Hopkins WJ. The urinary tract response to entry of pathogens. World J. Urol. 1999;17:351–358. doi: 10.1007/s003450050160. [DOI] [PubMed] [Google Scholar]
- 67.Priebe GP, Walsh RL, Cederroth TA, Kamei A, Coutinho-Sledge YS, Goldberg JB, Pier GB. IL-17 is a critical component of vaccine-induced protection against lung infection by lipopolysaccharide-heterologous strains of Pseudomonas aeruginosa. J. Immunol. 2008;181:4965–4975. doi: 10.4049/jimmunol.181.7.4965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chien Y-h, Jores R, Crowley MP. Recognition by γδ T cells. Ann. Rev. Immunol. 1996;14:511–532. doi: 10.1146/annurev.immunol.14.1.511. [DOI] [PubMed] [Google Scholar]
- 69.Simonian PL, Roark CL, Wehrmann F, Lanham AM, Born WK, O'Brien RL, Fontenot. AP. IL-17A-expressing T cells are essential for bacterial clearance in a murine model of hypersensitivity pneumonitis. J. Immunol. 2009;182:6540–6549. doi: 10.4049/jimmunol.0900013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kelly MN, Kolls JK, Happel K, Schwartzman JD, Schwarzenberger P, Combe C, Moretto M, Khan IA. Interleukin-17/interleukin-17 receptor-mediated signaling is important for generation of an optimal polymorphonuclear response against Toxoplasma gondii infection. Infect. Immun. 2005;73:617–621. doi: 10.1128/IAI.73.1.617-621.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Schulz SM, Kohler G, Schutze N, Knauer J, Straubinger RK, Chackerian AA, Witte E, Wolk K, Sabat R, Iwakura Y, Holscher C, Muller U, Kastelein RA, Alber G. Protective immunity to systemic infection with attenuated Salmonella enterica serovar Enteritidis in the absence of IL-12 Is associated with IL-23-dependent IL-22, but not IL-17. J. Immunol. 2008;181:7891–7901. doi: 10.4049/jimmunol.181.11.7891. [DOI] [PubMed] [Google Scholar]
- 72.O'Garra A, Arai N. The molecular basis of T helper 1 and T helper 2 cell differentiation. Trends Cell Biol. 2000;10:542–550. doi: 10.1016/s0962-8924(00)01856-0. [DOI] [PubMed] [Google Scholar]
- 73.Kopf M, Le Gros G, Coyle AJ, Kosco-Vtlbois M, Brombacher F, Kohler G. Immune responses of IL-4, IL-5, IL-6 deficient mice. Immunol. Rev. 1995;148:45–69. doi: 10.1111/j.1600-065x.1995.tb00093.x. [DOI] [PubMed] [Google Scholar]
- 74.Kolls JK, Linden A. Interleukin-17 family members and inflammation. Immunity. 2004;21:467–476. doi: 10.1016/j.immuni.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 75.Ragnarsdóttir B, Fischer H, Godaly G, Grönberg-Hernandez J, Gustafsson M, Karpman D, Lundstedt AC, Lutay N, Rämisch S, Svensson ML, Wullt B, Yadav M, Svanborg C. TLR- and CXCR1-dependent innate immunity: insights into the genetics of urinary tract infections. Eur. J. Clin. Invest. 2008;38:12–20. doi: 10.1111/j.1365-2362.2008.02004.x. [DOI] [PubMed] [Google Scholar]
- 76.Olive C. Expression of the T cell receptor δ-chain repertoire in mouse lymph node. Immunol. Cell Biol. 1996;74:313–317. doi: 10.1038/icb.1996.56. [DOI] [PubMed] [Google Scholar]