Abstract
Recent evidence has implicated neurokinin B (NKB) in the complex neuronal network mediating the effects of gonadal steroids on the regulation of gonadotrophin-releasing hormone (GnRH) secretion. Since the neurokinin 3 receptor (NK3R) is thought to mediate the effects of NKB at the cellular level, we determined the distribution of immunoreactive NK3R in the septal region, preoptic area (POA) and hypothalamus of the ewe. NK3R cells and/or fibres were found in areas including the bed nucleus of the stria terminalis, POA, anterior hypothalamic and perifornical areas, dopaminergic A15 region, dorsomedial and lateral hypothalamus, arcuate nucleus (ARC) and the ventral premammillary nucleus. We also used dual-label immunocytochemistry to determine whether a neuroanatomical basis for direct modulation of GnRH neurones by NKB was evident. No GnRH neurones at any rostral-caudal level were observed to contain NK3R immunoreactivity, although GnRH neurones and fibres were in proximity to NK3R-containing fibres. Because NKB fibres formed close contacts with NKB neurones in the ARC, we determined whether these NKB neurones also contained immunoreactive NK3R. In luteal-phase ewes, 64% ± 11 of NKB neurones colocalised NK3R. In summary, NK3R is distributed in areas of the sheep preoptic area and hypothalamus known to be involved in the control of reproductive neuroendocrine function. Colocalization of NK3R in NKB neurones of the ARC suggests a potential mechanism of autoregulation of this subpopulation; however, the lack of NK3R in GnRH neurones suggests that the actions of NKB on GnRH neurosecretory activity in the ewe are mediated indirectly via other neurones and/or neuropeptides.
Keywords: neuroendocrinology, reproduction, oestrous cycle, GnRH, NKB
Introduction
The pattern of secretion of gonadotrophin-releasing hormone (GnRH) and luteinizing hormone (LH) during the oestrous cycle is regulated largely by ovarian hormones. The actions of gonadal steroids are generally considered to be mediated by afferent inputs to GnRH neurones because few, if any, GnRH cells contain progesterone receptor or oestrogen receptor-α in rats (1), sheep (2 – 4), and primates (5). Although oestrogen receptor-β is found in GnRH neurones (6), it probably plays little or no role in oestradiol feedback (7). Hence, the precise neural populations and pathways that mediate the influence of gonadal steroids on GnRH neurosecretion are still under investigation. The recent report that loss-of-function mutations in either the gene encoding neurokinin B (NKB), or its receptor, produce gonadotrophin deficiencies in humans (8) strongly points to NKB as a key regulator of GnRH.
Neurokinin B is a product of the preprotachykinin B gene and, in the hypothalamus, is expressed predominantly within neurones in the arcuate nucleus (ARC; 9 – 13). Oestrogen receptor-α is present in a large percentage of NKB neurones in the ARC of the ovine hypothalamus (11). In rats (10) and monkeys (13), the expression of the preprotachykinin B gene is increased after ovariectomy, and oestradiol replacement decreases preprotachykinin B mRNA in ovariectomised rats (10). In the sheep, NKB is also regulated by gonadal hormones, with short-term oestradiol treatment decreasing the expression of preprotachykinin B gene in the ARC in ewes (14). Moreover, NKB expression is sexually dimorphic in this species, with fewer immunoreactive NKB cells present in the ARC of male and androgenised female sheep compared to normal females (11). In the ewe, ARC NKB neurones also contain the endogenous opioid peptide, dynorphin (15), the RF-amide peptide, kisspeptin (16), and progesterone receptors (17). Kisspeptin is a potent stimulator of GnRH that has been implicated in oestrogen feedback in a number of species (18, 19), and dynorphin appears to mediate progesterone negative feedback in ewes (15, 20). We have recently hypothesized that the balance of neuropeptide expression within kisspeptin/dynorphin cells of the ARC, and release at the level of GnRH neurons, is important in the regulation of steroid feedback control of GnRH (21). Therefore, the NKB in these same ARC neurones may well participate in mediating the actions of gonadal steroids on the regulation of GnRH secretion.
The actions of NKB at the cellular level are mediated through tachykinin receptors. The neurokinin-3 receptor (NK3R) is the tachykinin receptor that binds NKB with highest binding affinity among the known tachykinins (22). The NK3R belongs to the family of G-protein coupled receptors (23) and its distribution has been reported in the rat (24, 25), human (25), and guinea pig (26) brain. Although evidence in the rat suggests that Senktide, a NK3R agonist, decreases circulating concentrations of LH (27), recent studies in the ewe indicates that Senktide delivered into the third ventricle dramatically stimulates LH release during the follicular phase of the oestrous cycle (28). It is unknown whether the influence of NKB on LH secretion in the ewe is mediated directly upon GnRH neurones. Although direct contacts are present between NKB fibres and ovine GnRH cells (11), whether these GnRH neurones contain NK3R remains to be determined; only 16% of GnRH neurones in the rat contain immunoreactive NK3R suggesting the involvement of interneurones in conveying the influence of NKB onto GnRH neurones (29). Therefore, in the present study, we sought to determine the distribution of NK3R immunoreactivity in the ovine septal region, POA and hypothalamus. Because responsiveness of the hypothalamic-gonadotropic axis to Senktide differs depending on endocrine milieu (28), we specifically determined whether NK3R is colocalised within GnRH neurones in tissues obtained from ewes during the luteal and follicular phases of the oestrous cycle. In addition, since dynorphin/NKB neurones of the sheep arcuate nucleus possess reciprocal contacts with each other (15), we examined the NKB cells of the ARC for possible colocalization of NK3R immunoreactivity.
Materials and methods
Animals and experimental protocols
Adult, black-faced ewes of mixed breeding were maintained in an open barn with free access to water and fed once daily with a maintenance regimen silage. Ewes were moved to an indoor facility 3–7 d before procedures and housed 2/pen under artificial lighting that mimicked the natural environment photoperiod for the season. Experiments were performed during the breeding season (October through January) with ewes demonstrating regular oestrous cycles determined by monitoring oestrous behaviour with a vasectomised ram.
To determine the distribution and pattern of NK3R immunoreactivity, and examine whether GnRH and NKB neurones contain NK3R immunoreactivity, tissue sections from ewes sacrificed during the luteal phase (day 9 of the oestrous cycle, n=3) were used. Because Senktide, a NK3R agonist, stimulates the release of LH during the follicular phase of the estrous cycle in ewes (28), we sought to investigate further whether NK3R immunoreactivity is present in GnRH neurons during the follicular phase. For this experiment, 3 cycling ewes were treated with 2 injections of 5 mg prostaglanding F2α (Lutalyse, Pharmacia & Upjohn, Kalamazoo MI) 3 h apart during mid luteal phase (days 7 to 12 post oestrus) to regress the corpus luteum, and euthanised 24 h after the first prostaglandin injection. The stage of the oestrous cycle was characterised based on observation of at least 1 previous normal-length oestrous cycle and confirmed by the presence of at least 1 large corpus luteum on the ovaries of luteal phase ewes, and presence of corpus albicans and medium to large follicles on the ovaries of follicular phase ewes. Animal procedures were conducted at West Virginia University - Morgantown, WV, USA (luteal phase) and at Texas A&M University - College Station, TX, USA (follicular phase). All procedures involving animals were approved by the West Virginia University Animal Care and Use Committee or the Institutional Agricultural Animal Care and Use Committee of the Texas A&M University System.
Tissue collection
Ewes were injected intravenously twice at 10-min intervals with 25,000 U of heparin i.v. (Abraxiz Pharmaceutical Products, Schaumburg IL) and euthanised with approximately 2 g i.v. sodium pentobarbital (Sigma Aldrich, St. Louis MO). Heads were removed and perfused via both internal carotid arteries with 6 liters of 4% paraformaldehyde in 0.1 M sodium phosphate buffer (PB) containing 10 U/ml heparin and 0.1% sodium nitrite. Following perfusion, the brain was removed and a tissue block containing the septal region, preoptic area, and hypothalamus dissected. Tissue blocks were incubated in 4% paraformaldehyde at 4° C overnight and then placed in 30% sucrose at 4° C until tissue had sunk, indicating sucrose infiltration. Thick (50 µm) frozen coronal sections were cut using a freezing microtome into 4 parallel series and stored at −20° C in a cryopreservative solution (30) until processed for immunohistochemistry.
Immunocytochemistry
All procedures were performed at room temperature under gentle agitation, except when noted.
Single-label immunocytochemistry for NK3R
One series of sections from luteal phase ewes containing every fourth section (200 µm apart) was processed for immunoperoxidase detection of NK3R. Free-floating tissue sections were washed in 0.1 M PB containing 0.9% NaCl (PBS) for several hours to remove cryopreservative. After washing, the sections were incubated with PBS containing 1% hydrogen peroxide (Sigma, St. Louis, MO, USA) for 10 min to remove endogenous peroxidase activity. After further washing, the sections were incubated with PBS containing 0.4% Triton X-100 (PBSTX; Sigma) and 4% normal goat serum (NGS; Jackson Laboratories, Inc., West Grove, PA, USA) for 1 h. Sections were then incubated in rabbit polyclonal antibody against NK3R (1:25,000; Novus Biologicals, Littleton, CO, USA; Cat. # NB 300-102) diluted in PBSTX containing 4% NGS for 16 h. This antibody was raised against a synthetic peptide corresponding to the C-terminus of the rat NK3R (amino acid residues 438–452), and antiserum from same commercial source has been used to localise NK3R immunoreactivity in several tissues, including guinea-pig (26) and rat (29) brains. The NK3R peptide antigen does not share significant sequence identity to other receptors or proteins in mammalian CNS (29). Following incubation with primary antibody, sections were washed and then incubated for 1 h in a solution containing biotinylated goat anti-rabbit IgG (1:400; Vector Laboratories, Burlingame, CA, USA) in PBSTX + 4% NGS. The sections were then washed and incubated in PBS containing avidin-biotin-horseradish peroxidase complex (1:400; Vectastain Elite ABC, Vector Laboratories). NK3R was visualised using 3,3’-diaminobenzidine (DAB; 0.2 mg/ml) with hydrogen peroxide (0.012%; Sigma) in PB as substrate. Immunohistochemical controls included omission of the primary antibody from the immunostaining protocol and preabsorption of antibody solution for 24 hrs at 4° C with nanomolar concentrations of synthetic immunogen peptide provided by the supplier. Both procedures completely eliminated all immunostaining.
In addition, alternate series of sections were processed by immunofluorescence for confocal microscopic analysis of the subcellular location of NK3R immunoreactivity. Sections were processed as described above except that primary antibody was detected using fluorophore conjugate following tyramide amplification. Briefly, sections were incubated with rabbit polyclonal antibody against NK3R (1:150,000; Novus Biologicals) diluted in PBSTX + 4% NGS for 16 h, biotinylated goat anti-rabbit IgG (1:400; Vector Laboratories) for 1 h, ABC (1:400;, Vector Laboratories) for 1 h, and PBS containing biotinyl-tyramide (1:250; NEN Life Sciences, Boston, MA, USA) and hydrogen peroxide (0.003%; Sigma) for 10 min. Next, sections were incubated for 30 min with Alexa 555 conjugated streptavidin (1:250; Molecular Probes, Eugene, OR, USA) and counterstained for 30 min with fluorescent Nissl (1:250, NeuroTrace; Molecular Probes; Cat. # N-21480) to aid in the delineation of cytoplasmic/nuclear boundaries. Sections were then mounted on glass slides, air dried, and coverslipped using gelvatol.
Double-label immunofluorescence for GnRH and NK3R
One series of sections (400 µm apart) throughout the POA and hypothalamus from three luteal phase and three early follicular phase ewes were processed for dual immunofluorescence for GnRH and NK3R. First, free-floating sections were incubated in PBS containing 1% hydrogen peroxide (Sigma) for 10 min and incubated with PBSTX and 4% NGS for 1 h. Sections were then incubated for 16 h in a solution of PBSTX + 4% NGS containing both primary antibodies, a rabbit polyclonal antibody against NK3R (1:200,000; Novus Biologicals) and a mouse monoclonal antibody against GnRH (1:400, Sternberger Monoclonals Inc., Lutherville, MD, USA; SMI 41) as reported previously (31). Next, sections were incubated in biotinylated goat anti-rabbit (1:400; Vector Laboratories) for 1 h, ABC (1:500;Vector Laboratories) for 1 hr, biotinyl-tyramide (1:250; NEN Life Sciences) in PBS containing hydrogen peroxide (0.003%) for 10 minutes and Alexa 555 conjugated streptavidin (1:250; Molecular Probes) for 30 min to visualise NK3R. Finally, sections were incubated for 1 h in PBSTX + 4% NGS containing Alexa 488 conjugated goat anti-mouse IgG (1:200; Molecular Probes) to visualise GnRH. Sections were then mounted on glass slides, dried, and coverslipped with gelvatol.
Double-label immunofluorescence for NKB and NK3R
One series of sections (400 µm apart) throughout the POA and hypothalamus from 3 luteal phase ewes were processed for dual immunofluorescence for NKB and NK3R. Since the available primary antibodies recognizing these antigens were both raised in rabbits, a modification of a protocol described by Hunyady et al. (32) was used to eliminate the possibility of cross reactivity and false colocalization of the antigens. Briefly, the first antigen is visualized using a very low concentration of primary antibody followed by tyramide amplification and the second primary antibody was visualised using indirect detection with fluorophore-conjugated secondary antibody. This procedure has been used successfully by our laboratory to detect a number of antigens in ovine tissue (16, 33).
Free-floating sections were incubated in PBS containing 1% hydrogen peroxide (Sigma) for 10 min, incubated with PBSTX and 4% NGS for 1 h, and then incubated in rabbit polyclonal antibody against NKB (1:10,000; Penninsula Laboratories, San Carlos, CA, USA; IHC 7357) diluted in PBSTX containing 4% NGS for 16 h as reported previously (34). Next, sections were incubated in biotinylated goat anti-rabbit (1:400; Vector Laboratories) for1 h, ABC (1:400;Vector Laboratories) for 1 h, biotinyl-tyramide (1:250; NEN Life Sciences) in PBS containing hydrogen peroxide (0.003%) for 10 min and Alexa 488 conjugated streptavidin (1:200; Molecular Probes) for 30 min. The sections were then incubated for 16 h in rabbit polyclonal antibody against NK3R (1:5,000; Novus Biologicals) diluted in PBSTX containing 4% NGS and Alexa 555 conjugated goat anti-rabbit IgG (1:400; Molecular Probes) in PBSTX + 4% NGS for 1 h. Sections were mounted on glass slides, dried, and coverslipped with gelvatol.
Controls for the dual immunfluorescent procedures included omission of each of the primary antibodies from the immunostaining protocol, the absence of which completely eliminated staining for the corresponding antigen. In addition, pre-absorption of diluted antibodies against NKB or NK3R for 24 h at 4° C with nanomolar concentrations of corresponding purified peptide against which antibody was raised eliminated all staining for that antigen, while preabsorption with the other peptide had no effect.
Analysis
For single-label NK3R, the distribution of immunoreactive cells and fibres was examined in a series of every fourth section through the septal region, POA and hypothalamus of each ewe. NK3R-ir cells were identified by the presence of dense reaction product that labelled their somas and dendrites. Drawings of representative sections were made using a camera lucida attached to a Nikon Optiphot microscope (Nikon Corporation, Tokyo, Japan). Images of labelled material were captured using a digital camera (Magnafire; Optronics, Goleta, CA, USA) attached to a Leica microscope (Leica Corp., Deerfield, IL, USA) and imported into Adobe Photoshop 7.0 (Adobe Systems, San Jose, CA, USA). Images were not altered in any way except for minor adjustments of brightness and contrast.
For determination of cellular location of NK3R immunoreactivity, images from sections processed for single-label immunofluorescence for NK3R and counterstained with fluorescent Nissl were captured using a Zeiss LSM-510 laser-scanning confocal microscope system (Zeiss, Heidelberg, Germany). Z stacks of 1 µm optical sections through labelled cells were captured for analysis. Of each stack of images, a single optical section through the middle of the neurone (identified as a section containing a clear Nissl-stained nucleole) was used to determine the number of NK3R-ir particles clearly dissociated from the cytoplasmic membrane. Immunoreactive NK3R neurones containing 3 or more cytosolic particles were considered “internalised” cells (35). Otherwise, labelled neurones were considered to contain mainly membrane-bound receptors.
For dual-label studies, the number of single- and double-labelled cells was counted in each section using a Nikon Microphot-FX microscope (Nikon) equipped with epi-fluorescent attachment. Alexa 555 (red) was visualised with a 610 nm emission filter and Alexa 488 was visualised with a 535 emission filter. To verify accuracy of cell counting, the number of single-and double label cells were determined in images of representative tissue sections captured using a Zeiss LSM-510 laser-scanning confocal microscope system (Zeiss). Alexa 555 fluorescence (red) was imaged with a 567 nm emission filter and a HeNe laser. Alexa 488 fluorescence (green) was imaged with a 505 nm emission filter and Argon laser. Results from these counts (56 NKB and 92 NK3R cells) were compared to the number of single- and double-labelled cells obtained by direct visual observation of same tissue sections using an epi-fluorescent microscope. Because the two methods produced identical results, the number of single- and double label cells was determined for the remainder of the study using direct visual observation of cells in the epi-fluoerescent microscope. A total of 309 NKB and 521 NK3R cells were analysed. Cell counts were used to calculate the mean percentage of NK3R-ir cells that colocalised NKB, as well as the percentage of NKB cells in the arcuate nucleus that were double-labelled for NK3R. For examining colocalization of GnRH and NK3R immunoreactivity, a total of 517 GnRH-ir neurones were analysed in tissue sections obtained from 3 luteal-phase (n = 256 GnRH neurones) and 3 follicular-phase ewes (n=261 GnRH neurones). For determination of NKB-ir fibre terminals in close contact with NK3R-ir neurones, 10 cells in at least 2 sections per brain region for each ewe were analysed.
Results
Distribution of NK3R immunoreactive (ir) cells in the septal region, POA and hypothalamus
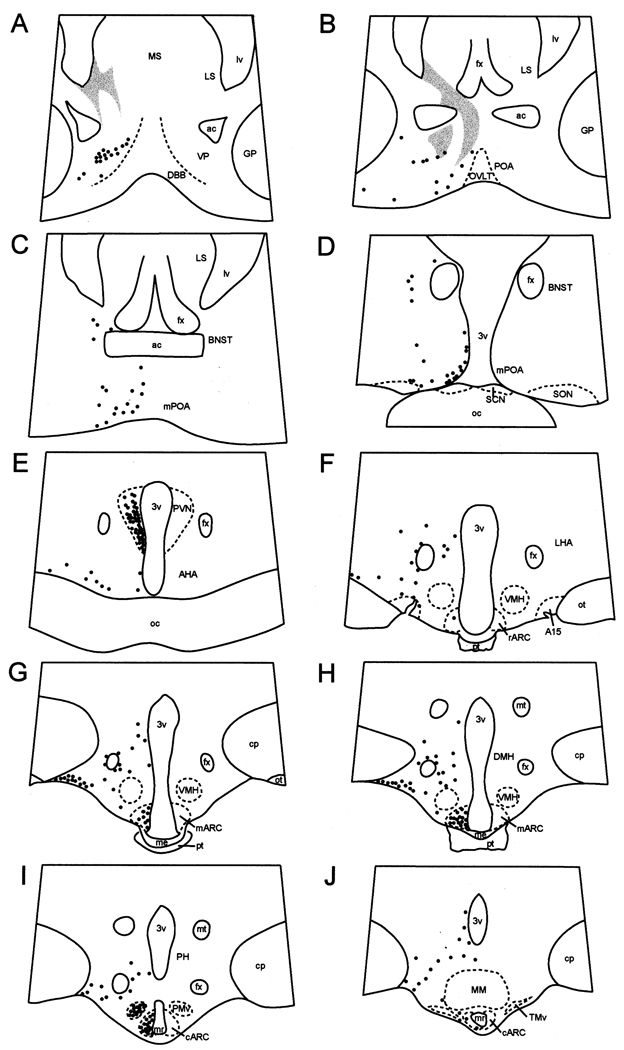
A regional distribution of NK3R cells (Fig. 1) and morphologically distinct NK3R-ir cell types were observed. Multipolar, magnocellular neurones (somal diameter of 20 ± 0.5 µm) were observed in the bed nucleus of the stria terminalis (BNST), perifornical area, dopaminergic A15 region, dorsomedial nucleus of the hypothalamus (DMH), and ventral portion of the premammillary nucleus of the hypothalamus (PMv). Bipolar or multipolar parvicellular neurons (somal diameter of 12 ± 0.3 µm) were observed in the ventral pallidum, POA, paraventricular nucleus (PVN), anterior hypothalamic area (AHA), ARC, and PMv. Within the POA, NK3R-ir cells were parvicellular, bipolar neurones, with the highest density of cells observed in ventromedial POA (Fig. 1B–D, 2A, 2D) adjacent to the organum vasculosum of the lamina terminalis (OVLT). NK3R-ir cells in the POA extended dorsally and laterally, but fewer cells were observed in lateral portions of the POA. NK3R-ir neurones were also observed in the post-commissural division of the lateral BNST (Fig. 1C–D, 3A).
Fig. 1.
Camera lucida drawings of coronal sections depicting the distribution of NK3R-immunoreactive (solid circles) cells in the POA area and hypothalamus of ewes. Shaded areas on panels A and B represent areas of dense fibre labelling in the septum that extends towards the dorsal aspect of the preoptic area. A15, dopaminergic A15 area; ac, anterior commissure; AHA, anterior hypothalamic area; mARC, middle arcuate nucleus; rARC, rostral arcuate nucleus; BNST; bed nucleus of stria terminalis; cp, cerebral peduncle; DBB, diagonal band of Broca; DMH, dorso-medial hypothalamus; fx, fornix; GP, globus pallidus; LHA, lateral hypothalamic area; LS, lateral septum; lv; lateral ventricle; me, median eminence; MM, mammillary body; mr, mammillary recess of the third ventricle; MS, medial septum; mt, mammillothalamic tract; oc, optic chiasma; ot; optic tract; OVLT, organum vasculosum of lamina terminalis; PH, posterior hypothalamus; PMv; ventral premammillary nucleus; POA, preoptic area; mPOA, medial preoptic area; pt, pars tuberalis of the adenohypophysis; PVN, paraventricular nucleus; SCN, suprachiasmatic nucleus; SON, supraoptic nucleus; TMv, ventral tuberomammillary nucleus; VMH, ventromedial hypothalamus; VP, ventral pallidum; 3v, third ventricle.
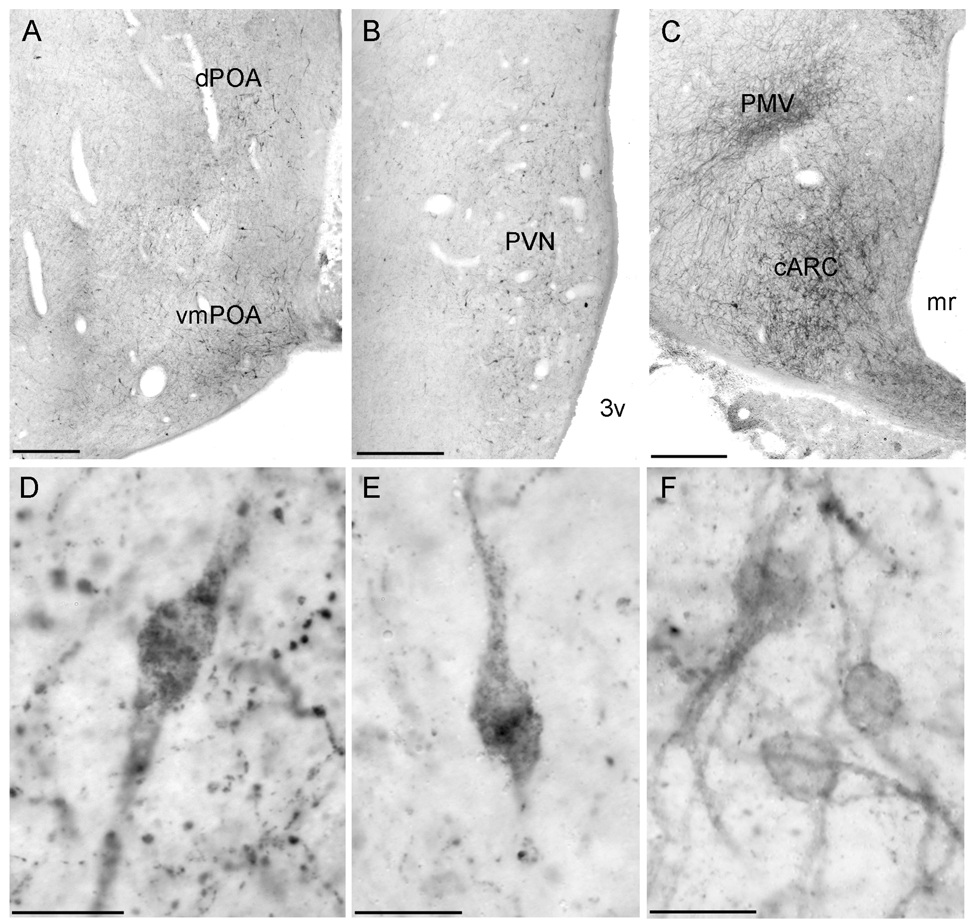
Fig. 2.
Low power photomicrograph demonstrating the distribution of NK3R-immunoreactive cells and fibres in the preoptic area (A) and paraventricular (B), and arcuate and ventral premammillary (C) nucleus. High power photomicrographs showing NK3R-immunoreactive neurones in the POA (D), PVN (E), and ARC (F). Scale bars, 500 µm (A–C); 20 µm (D–F).
Fig. 3.
High power photomicrographs of individual NK3R-immunoreactive neurones in the BNST (A), dopaminergic A15 area (B), and ventral premammillary nucleus (C). Scale bars, 20 µm.
Numerous NK3R-ir cells were observed in the PVN of the hypothalamus. These were parvicellular, bipolar neurones and observed initially in the anterior hypothalamus as a small, ventral group of cells that extended caudally and dorsally along the third ventricle in the periventricular zone. At most caudal aspects of the PVN, NK3R-ir cells were distributed in a wing shape (Fig. 1E, 2B, 2E). Scattered, multipolar, parvicellular NK3R-ir neurones were observed in the anterior hypothalamus outside the PVN and concentrated mainly in ventrolateral portions of this region (Fig. 1E); however, few periventricular cells were observed at this level.
At rostral levels of the mediobasal hypothalamus, a few NK3R-ir cells were observed scattered in the periventricular zone. Multipolar, magnocellular NK3R-ir neurones were observed surrounding the fornix and in the vicinity of and within the A15 area (Fig. 1F, 3B), but none were seen in the ventromedial hypothalamus (VMH; Fig. 1F–H) NK3R-ir neurons extended from rostral to caudal levels of the lateral hypothalamus along its ventral surface (Fig. 1E–J). These cells were large (< 20 µm in diameter) and exhibited multiple processes.
NK3R-ir cells were observed throughout the rostral-caudal extent of the ARC. These were parvicellular neurones and the highest density was observed in the middle and caudal portions of the ARC (Fig. 1G–J, 2C, 2F). The caudal ARC extended into the premammillary region and fewer NK3R-ir neurones were observed towards most caudal portion of the caudal ARC. Occasionally, cells in immediately adjacent regions, including within the median eminence, were observed. Multipolar, magnocellular NK3R-ir neurones were observed throughout the DMH, dorsomedially to the fornix (Fig. 1H). NK3R cells extended caudally in the hypothalamus and were observed in large numbers in the PMv (Fig. 1I, 2C, 3C). These cells were mostly magnocellular, multipolar neurones. The distribution of NK3R-ir cells was also investigated in tissue collected from follicular phase ewes (data not shown). Overall, the distribution of NK3R-ir cells in the septal region, POA and hypothalamus, and the number of cells on those areas were identical in tissues obtained from ewes during the luteal and follicular phases.
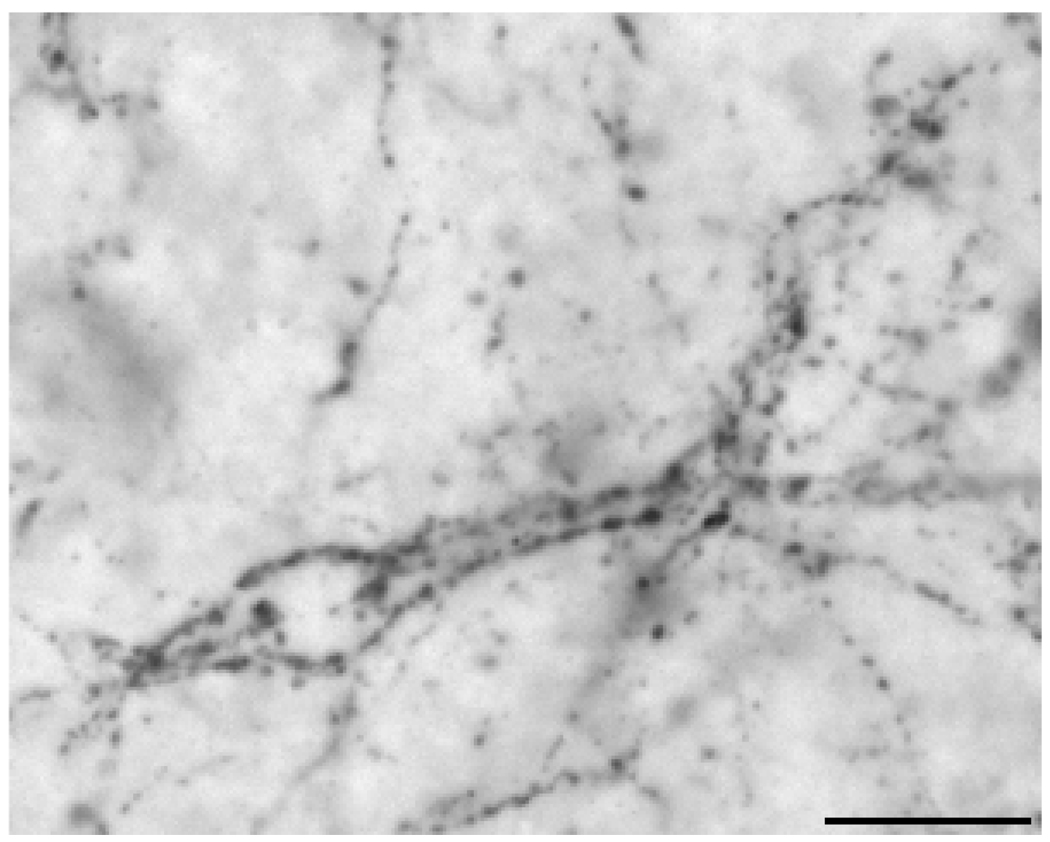
Fibres containing NK3R immunoreactivity were observed widely throughout the septal region, POA and hypothalamus. An area of dense fibre labelling, but devoid of NK3R-ir soma, was observed in the ventromedial and lateral portions of the lateral septum (Fig. 1A–B). These fibres appear to form close contacts with cells within this area, because NK3R-ir fibres could be observed outlining cells in this region (Fig. 4). This pattern of fibre labelling was observed to form a continuum extending ventrally towards ventral portions of the BNST and dorsal POA (Fig. 1B). In addition, a high density of NK3R-ir fibres was observed in the medial portion of the external zone of the median eminence and basement membrane in its interface with the pars tuberalis of the adenohypophysis. These fibres extended laterally at more caudal levels of the median eminence.
Fig. 4.
Photomicrograph demonstrating NK3R-immunoreactive fibres in close contact outlining cells in the lateral septal nuclei. Scale bar, 20 µm.
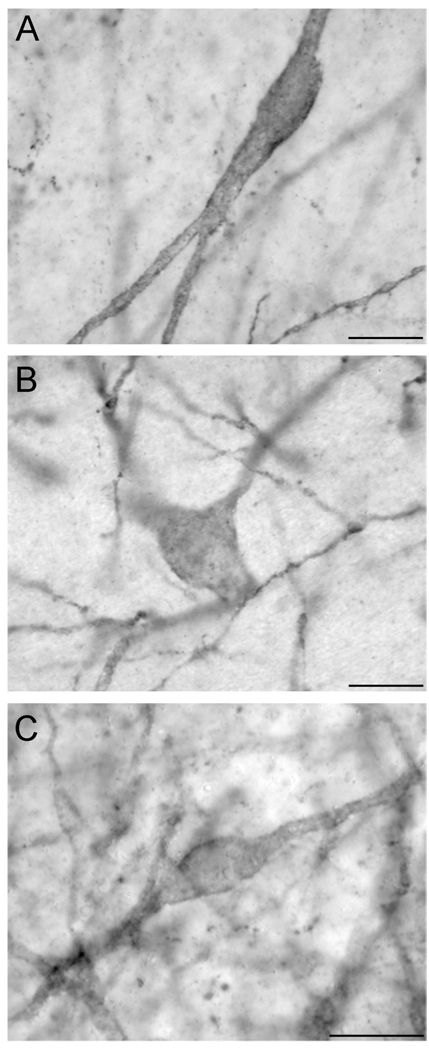
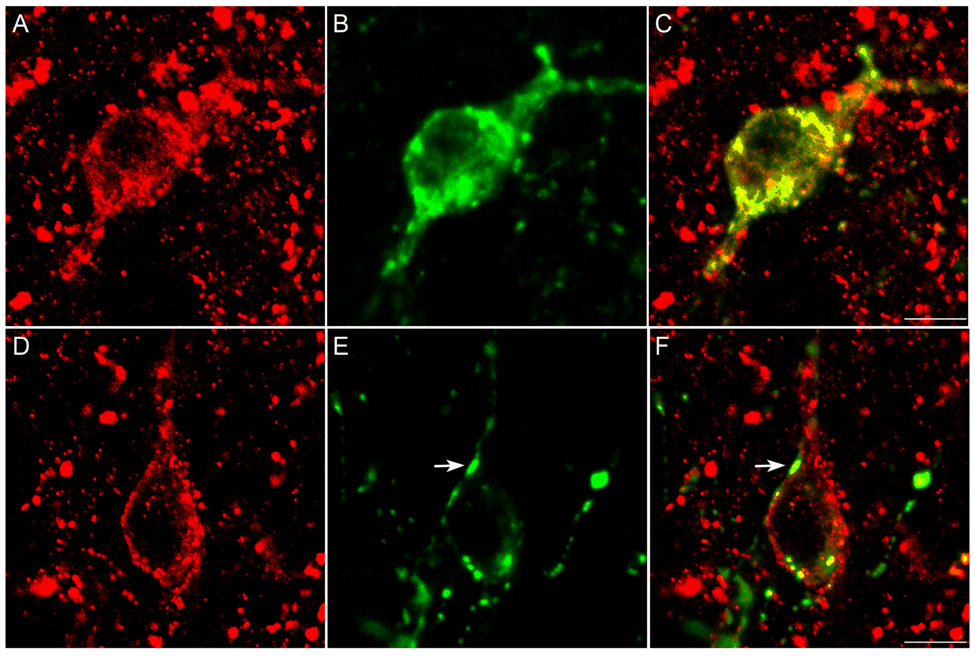
Two patterns of immunoperoxidase labelling were observed in NK3R-ir neurones in the POA and hypothalamus. The first was an accumulation of a reaction product that appeared within the cytoplasm of the cell (Fig. 2D–E). The second pattern of labelling was reaction product that was mainly associated with the surface of neurones, suggesting predominant labelling at the cell membrane (Fig. 2F, 3A–C). To verify this sub-cellular location, selected sections through the POA and hypothalamus of luteal-phase ewes were processed for immunfluorescence detection of NK3R. Confocal analysis of NK3R-ir cells confirmed that some cells displayed fluorescence signal predominantly associated with the cell surface (membrane-bound receptors; Fig. 5A–B) whereas other cells contained fluorescent particles that were clearly localized within the cytoplasm (Fig. 5C–D). In general, NK3R-ir neurones in the BNST, A15, DMH, ARC, and PMv were observed predominantly with cell surface labelling, while the majority of NK3R-ir neurones in the POA and PVN had cytosolic immunofluorescent particles.
Fig. 5.
Confocal images of representative sections of the ARC (A, B) and POA (C, D). Images represent 1 µm optical sections of tissue processed for immunofluorescent detection of NK3R (red) and counterstained with fluorescent Nissl (green). Arrows indicate fluorescent particles dissociated from the cell membrane. Arrow-heads indicate Nissl-stained nucleolus. Scale bars, 10 µm.
Dual-label immunocytochemistry for GnRH and NK3R in the POA and hypothalamus
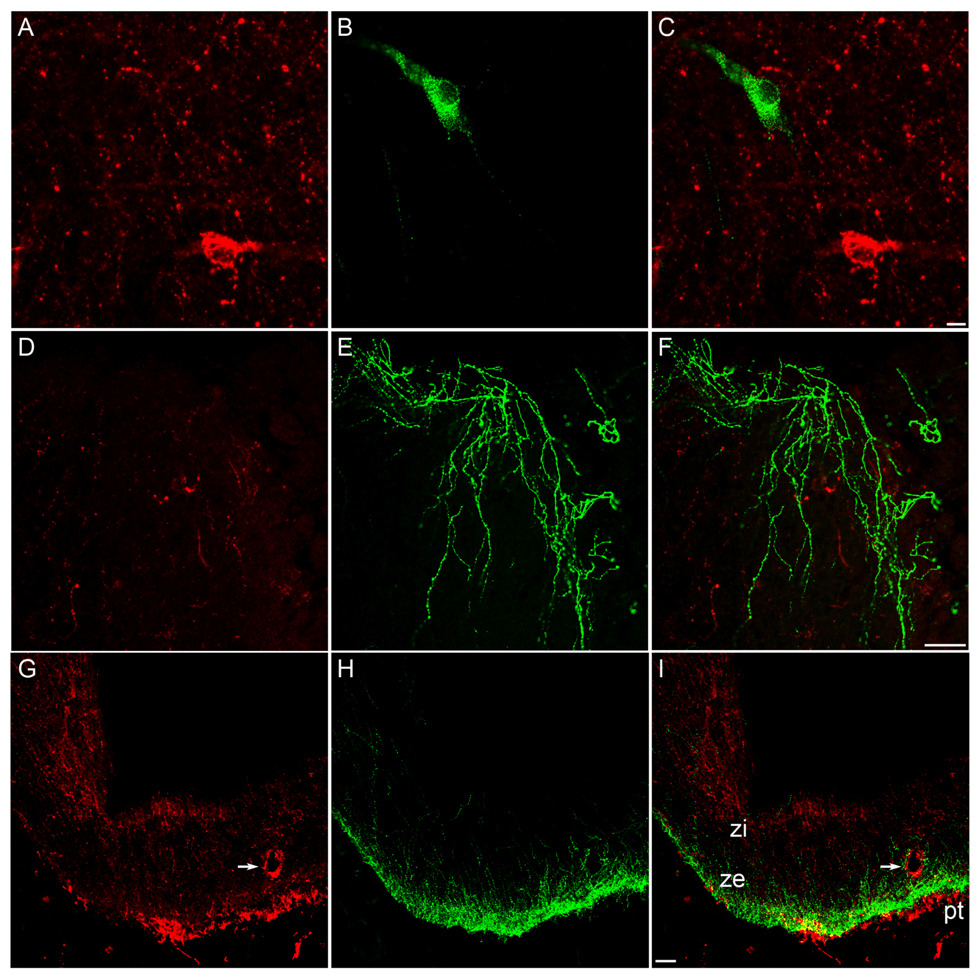
In agreement with previous reports (36), immunoreactive GnRH neurones were observed in a rostral to caudal distribution from the diagonal band of Broca to medial portions of the basal hypothalamus, with the highest concentration of cells in the POA at the level of the OVLT. GnRH-ir cells and fibres were observed intermingled with NK3R-ir cells and fibres in the POA (Fig. 6A–C) and hypothalamus. However, we found no instances of colocalization of GnRH- and NK3R-positive cells (Fig. 6A–C) or fibres (Fig. 6D–F). NK3R-ir axon terminals were observed in close proximity to GnRH cells (Fig. 6C). There was also a lack of colocalization of GnRH and NK3R fibres and terminals at the level of the median eminence (Fig. 6G–I), where the NK3R-ir was sometimes associated with blood vessels in the external zone.
Fig. 6.
Confocal images of representative sections of the POA (A–F) and ME (G–I). Tissue was processed for dual-label immunofluorescent detection of GnRH (green) and NK3R (red). Images represent 1.5 µm optical sections of individual neurones (A–C), 2.5 µm optical sections of fibres in the POA at the level of the OVLT (D–E) and 15 µm optical sections of fibres at the level of the ME (G–I). Panels C, F, and I represent overlays of images on the left. Arrows indicate NK3R-immunoreactivity associated with vasculature. Scale bars, 10 µm (C); 50 µm (F and I).
Dual-label immunocytochemistry for NKB and NK3R in the POA and hypothalamus
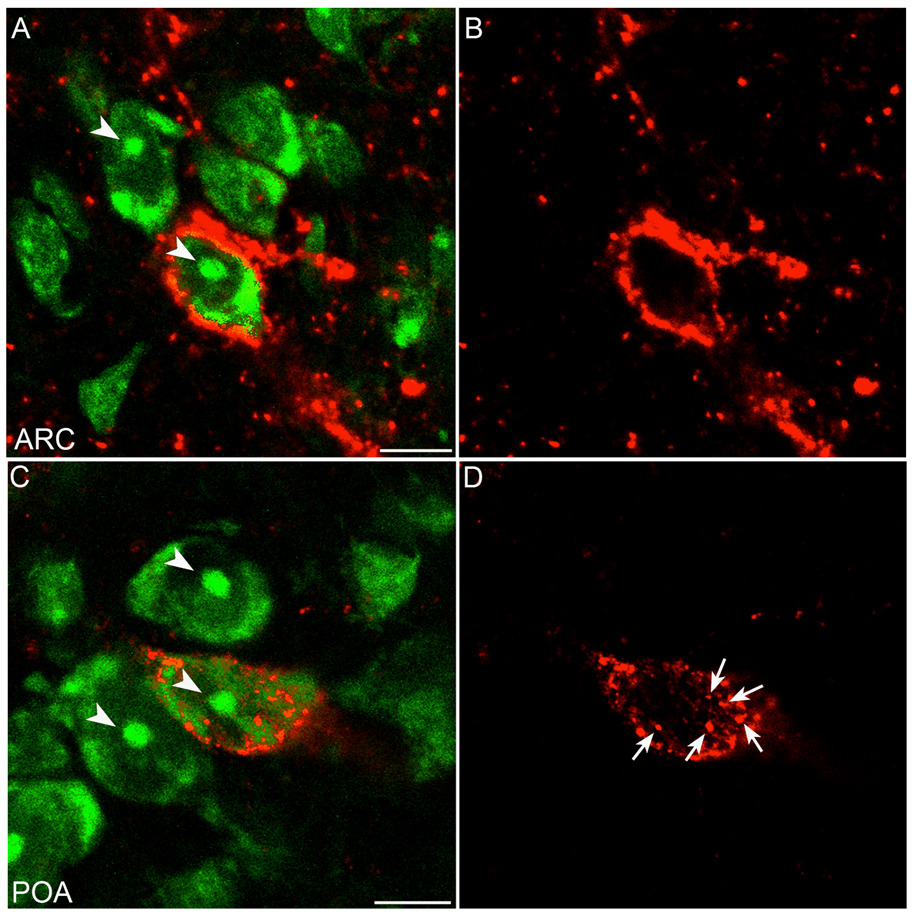
Cells that were immunoreactive for NKB were observed only in the ARC and stalk of the median eminence, although NKB fibres were observed throughout the POA, hypothalamus, and median eminence, in agreement with previous reports (11, 34). The highest concentrations of NKB cells were observed in the middle and caudal portions of the ARC. Cells that were double labelled for both NKB and NK3R (Fig. 7A–C) were observed throughout the ARC with the highest concentrations in the middle and caudal regions. The mean (±SEM) number of NKB and NK3R neurones in the ARC was 103 ± 32 and 174 ± 64, respectively. Overall, 64% ± 11 of the NKB-ir neurones were also immunoreactive for NK3R. In contrast, only 39% ± 2 of NK3R-ir cells in the ARC were immunoreactive for NKB. Close contacts between NKB-ir fibre terminals and NK3R-ir cellswere observed frequently in the ARC (Fig. 7D–F), but not elsewhere, and included the NKB/NK3R neurones. Rare close associations of NKB-containing fibres with NK3R-ir cells were observed in the PVN, A15, perifornical area, DMH and PMv. In the ventral pallidum, POA, BNST and lateral hypothalamus, no close contacts between NKB fibres and NK3R-ir cells were observed. Although both NKB- and NK3R-positive fibres were observed in the internal and external zones of the median eminence, no colocalization of the neurotransmitter and receptor was observed in fibres in this region.
Fig. 7.
Confocal images of individual cells of the ARC. Tissue was processed for dual-label immunofluorescent detection of NK3R (red) and NKB (green). Images represent 1.5 µm optical sections of a neurone double-labelled for NK3R and NKB (A–C) and a single-labelled NK3R-immunoreactive neurone (D–F) with close contacts of NKB-containing fibres (arrow). Scale bars, 10 µm.
Discussion
In the present study we determined the distribution of NK3R-ir cells and fibres in the septal region, POA, and hypothalamus of the female sheep brain and examined whether GnRH and NKB neurones contained NK3R immunoreactivity. The distribution of NK3R-ir cells in these areas was, in general, similar to that reported previously for the rat (25), guinea pig (26) and human (25), and consistent to the location of NK3R mRNA in the hypothalamus of the rat (37). However, some differences seem to exist and include the lack of immunoreactivity in magnocellular neurones in the PVN and SON, as reported in the rat (25). Furthermore, NK3R-ir cells were abundant in areas where GnRH neurones are found; however, no examples of colocalization of GnRH and NK3R were observed. In contrast, over 60% of the NKB neurones in the ARC contained NK3R and nearly all NK3R-positive cells examined in the ARC were in close contact with NKB fibre terminals. Therefore, our results suggest that, in the ewe, the regulation of the GnRH neurone by NKB is not direct and instead may be mediated indirectly by other neurones and/or neurotransmitters.
One possibility is that the influence of NKB upon GnRH secretion in the sheep may be mediated by other neuropeptides expressed by the NKB cells of the sheep. Specifically, NKB is colocalised with kisspeptin (16) and dynorphin (15) in a subset of ARC neurones of the sheep, and possibly other mammals including humans (9, 38). Dynorphin cells of the sheep ARC have been shown to provide direct input to GnRH cells (20) and these terminals, like their cells of origin, colocalise dynorphin, NKB and kisspeptin (Lehman, unpublished observations). As noted above, a majority of NKB cells of the ARC examined in the current study also colocalised NK3R. This is consistent with observations in the rat, where NKB neurones have been shown to colocalise dynorphin, and a majority of prodynorphin immunoreactive neurones in the ARC colocalise NK3R (39). In addition, close contacts were frequent between NKB terminals and NKB/NK3R cells in the ARC suggesting a substrate for reciprocal interactions among this cell population. Therefore, it is conceivable that the effects of the NKB agonist are to activate the entire population of NKB/dynorphin/kisspeptin neurones in the ARC, which then convey this stimulatory effect to GnRH neurones via release of kisspeptin (40). Alternatively, it may be that the effects of NKB on GnRH neurones may be conveyed indirectly by a separate population of NK3R-positive neurones, such as those in the POA. It is also possible that NKB may act upon GnRH neurones via tachykinin receptors other than NK3R. However, transcripts for neither NK1 or NK2 receptors were reported to be found in GnRH neurones (41). Further work is necessary to distinguish between these possibilities.
In contrast to the complete absence of NK3R in GnRH neurones in the sheep, NK3R immunoreactivity was observed in approximately 16% of GnRH-neurone somata and in numerous GnRH process in the median eminence and OVLT of the rat (29). The disparity between the studies in the rat and in the ewe might reflect differences in the gonadal steroid milieu. This, however, seems unlikely because there was no effect of stage of the oestrous cycle in the ewe and Krajewski et al. (29) examined tissues from pro-oestrous rats, with high oestradiol levels, as well as ovariectomised rats with low oestradiol concentrations. Another possibility is that, in the sheep, NK3R is transported rapidly to axon terminals of GnRH neurones, becoming undetectable at the level of the soma. However, the presence of only sparse NK3R fibre immunoreactivity in lateral portions of the median eminence, a region containing dense GnRH fibres and terminals, argues against this possibility. Therefore, it seems most likely that species differences exist between sheep and rats in the colocalization of GnRH with NK3R. This hypothesis is consistent with species differences in the effects of the NK3R agonist, Senktide on LH secretion. Senktide is inhibitory in rats (27) and stimulatory in ewes (27). Nevertheless, NK3R transcripts have been detected in GnRH neurones in the mouse (41). Therefore, methodological differences between the studies performed in rodents (29; 41) and our studies in the sheep cannot be excluded and may, in part, account for the disparity in localization of NK3R immunoreactivity in GnRH neurones between rodents and sheep.
A high proportion of NKB neurones of the ARC contain oestrogen receptor-α (11) and progesterone receptors (21), suggesting direct regulation of these neurones by gonadal steroids. As noted above, the high density of close associations between NKB fibres and NK3R-containing neurones in the ARC suggests a role in coordinating the activity of NKB/dynorphin/kisspeptin neurones of the ARC, perhaps in response to gonadal steroids. Given that activation of NK3R appears to be excitatory (42) and NKB agonist stimulates the release of LH in ewes during the follicular phase of the oestrous cycle (28), an increase in NKB release may coordinate activation of NK3R/NKB/kisspeptin neurones during the follicular phase leading to the generation of the preovulatory GnRH/LH surge. A caveat for this hypothesis is that ovariectomy increases the mRNA encoding NKB in rats (10, 27), and oestradiol treatment of ovariectomised rats (10) and ewes (14) decreases the expression of NKB gene. However, as noted above there appear to be species differences in the effects of NKB on LH secretion. Moreover, in the studies by Pillon et al. (14), tissue was collected from ewes 4 h after oestradiol treatment, a time more consistent with oestrogen negative feedback than positive feedback. When tissue was collected during the oestrogen-induced surge, there was no difference in mRNA levels encoding NKB between oestrogen-treated and control ewes (11). Thus the role of NKB in initiation of the LH surge in ewes remains to be determined.
The NK3R belongs to the family of G-protein coupled receptors (43), that upon binding with ligand, are activated and internalised into endosomes (44). Immunolabelling of NK3R was observed in two sub-cellular locations: associated with cell membrane and within the cytoplasm. In our studies using tissue collected from ewes during the luteal phase of the oestrous cycle, NK3R immunoreactivity was most frequently associated with the cell membrane in all areas examined, except the POA and PVN, in which immunoreactive particles were observed within the cytoplasm of the cell bodies. Internalization of G-protein coupled receptors can be used as a marker for receptor activation by endogenous or exogenous ligand. For example, internalization of NK3R in the supraoptic nucleus is observed after injection of the NK3R agonist, Senktide (45, 46). In addition, internalization of the mu-opioid receptor is induced by oestradiol (47) or mating (35), and the latter is blocked by pretreatment with mu receptor antagonist. If the NK3R distribution within neurones indeed reflects ligand-induced endocytosis, it appears that NKB release in the ARC is limited during the luteal phase, as NK3R-ir in the ARC neurones of luteal-phase ewes was commonly associated with cell membrane. In contrast, NK3R cells containing cytosolic immunoreactivity were abundant in the POA and PVN, suggesting high levels of ligand release in both of these areas at a time of elevated progesterone. We are currently investigating whether the proportion of cells containing cytosolic NK3R-ir particles in the POA and hypothalamus changes during the various phases of the oestrous cycle, in association with alterations in the gonadal steroid milieu. Preliminary analysis suggests that cytosolic distribution of NK3R is increased in the ARC during the follicular phase (48). However, the proportion of cells containing cytosolic NK3R particles in the POA appears independent of phase of the oestrous cycle. Because little or no close contacts between NKB fibres and NK3R-ir cells were seen in the POA and PVN, it is possible that NK3R endocytosis in these areas were induced by a neurotransmitter other than NKB. Neurokinin A and substance P also bind to NK3R, although with lower affinity than NKB (43). Alternatively, NKB released from remote sites may diffuse and bind to NK3R in the POA and/or PVN via a mode of non-synaptic communication (49). Thorough examination of NK3R activation during the oestrous cycle may help us to understand of the physiological contributions of the various populations of NK3R-containing neurones in the POA and hypothalamus, and the role of NKB in the regulation of gonadotrophin release.
In summary, NK3R neurone cell bodies and fibres are distributed widely in the septal region, POA, and hypothalamus of the ewe, and include areas known to be involved in the control of neuroendocrine functions. The lack of localization of NK3R in GnRH neurons suggests that in the ewe, NK3R does not mediate the effects of tachykinins directly onto GnRH neurones but rather indirectly via other interneurones and/or neurotransmitters. In particular, the presence of NK3R in NKB neurones in the ARC, along with growing evidence that this subpopulation plays a key role in gonadal hormone feedback to GnRH neurones (16), suggests that NKB may coordinate activity among this population which then conveys its influence to GnRH neurones via other co-expressed neuropeptides.
Table 1.
Comparison of the Distribution of NK3R-ir Cells and Fibres in the Ovine Septal Region, POA, and Hypothalamus.
| Area | NK3R-ir Cells | NK3R-ir fibres |
|---|---|---|
| Lateral septum | − | ++++ |
| Ventral pallidum | ++ | +++ |
| POA | +++ | +++ |
| BNST | + | ++ |
| PVN | ++++ | +++ |
| A15 area | + | ++ |
| Lateral hypothalamus | ++ | + |
| Perifornical area | + | ++ |
| VMH | − | + |
| DMH | + | +++ |
| ARC | ++++ | ++++ |
| PMv | ++++ | ++ |
| Median eminence | − | ++++ |
++++, dense; +++ high; ++, moderate; +, low; −, no labelling.
Acknowledgements
These studies were supported by NIH HD39916 to M.N.L and R.L.G., and TEX09202 to M.A.
References
- 1.Herbison AE, Theodosis DT. Localization of oestrogen receptors in preoptic neurons containing neurotensin but not tyrosine hydroxylase, cholecystokinin, or luteinizing hormone-releasing hormone in the male and female rat. Neuroscience. 1992;50:283–298. doi: 10.1016/0306-4522(92)90423-y. [DOI] [PubMed] [Google Scholar]
- 2.Lehman MN, Karsch FJ. Do gonadotropin-releasing hormone, tyrosine hydroxylase and β-endorphin-immunoreactive neurons contain estrogen receptors? A double-label immunocytochemical study in the Suffolk ewe. Endocrinology. 1993;133:887–895. doi: 10.1210/endo.133.2.8102098. [DOI] [PubMed] [Google Scholar]
- 3.Herbison AE, Robinson JE, Skinner DC. Distribution of estrogen receptor-immunoreactive cells in the preoptic area of the ewe: co-localization with glutamic acid decarboxylase but not luteinizing hormone-releasing hormone. Neuroendocrinology. 1993;57:751–759. doi: 10.1159/000126433. [DOI] [PubMed] [Google Scholar]
- 4.Skinner DC, Caraty A, Allingham R. Unmasking the progesterone receptor in the preoptic area and hypothalamus of the ewe: no colocalization with gonadotropin-releasing neurons. Endocrinology. 2001;142:573–579. doi: 10.1210/endo.142.2.7956. [DOI] [PubMed] [Google Scholar]
- 5.Sullivan KA, Witkin JW, Ferin M, Silverman AJ. Gonadotropin-releasing hormone neurons in the rhesus macaque are non-immunoreactive for the estrogen receptor. Brain Res. 1995;685:198–200. doi: 10.1016/0006-8993(95)00352-q. [DOI] [PubMed] [Google Scholar]
- 6.Skinner DC, Dufourny L. Oestrogen receptor β-immunoreactive neurones in the ovine hypothalamus: distribution and colocalization with gonadotropin-releasing hormone. J Neuroendocrinol. 2005;17:29–39. doi: 10.1111/j.1365-2826.2005.01271.x. [DOI] [PubMed] [Google Scholar]
- 7.Dorling AA, Todman MG, Korach KS, Herbison AE. Critical role for estrogen receptor alpha in negative feedback regulation of gonadotropin-releasing hormone mRNA expression in the female mouse. Neuroendocrinology. 2003;78:204–209. doi: 10.1159/000073703. [DOI] [PubMed] [Google Scholar]
- 8.Topaloglu AK, Reimann F, Guclu M, Yalin AS, Kotan LD, Porter KM, Serin A, Mungan NO, Cook JR, Ozbek MN, Imamoglu S, Akalin NS, Yuksel B, O’Rahilly S, Semple RK. TAC3 and TAC3R mutations in familial hypogonadotropic hypogonadism reveal a key role for neurokinin B in the central control of reproduction. Nat Genet. 2009;41:354–358. doi: 10.1038/ng.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rance NE, Young WX., 3rd Hypertrophy and increased gene expression of neurons containing neurokinin-B and substance-P messenger ribonucleic acids in the hypothalami of postmenopausal women. Endocrinology. 1991;128:2239–2247. doi: 10.1210/endo-128-5-2239. [DOI] [PubMed] [Google Scholar]
- 10.Rance NE, Bruce TR. Neurokinin B gene expression is increased in the arcuate nucleus of ovariectomized rats. Neuroendocrinol. 1994;60:337–345. doi: 10.1159/000126768. [DOI] [PubMed] [Google Scholar]
- 11.Goubillon ML, Forsdike RA, Robinson JE, Ciofi P, Caraty A, Herbison AE. Identification of neurokinin B-expressing neurons as an highly estrogen-receptive, sexually dimorphic cell group in the ovine arcuate nucleus. Endocrinology. 2000;141:4218–4225. doi: 10.1210/endo.141.11.7743. [DOI] [PubMed] [Google Scholar]
- 12.Dellovade T, Merchenthaler I. Estrogen regulation of neurokinin B gene expression in the mouse arcuate nucleus is mediated by estrogen receptor α. Endocrinology. 2004;143:736–742. doi: 10.1210/en.2003-0894. [DOI] [PubMed] [Google Scholar]
- 13.Sandoval-Guzman T, Stalcup ST, Krajewski SJ, Voytko ML, Rance NE. Effects of ovariectomy on the neuroendocrine axes regulating reproduction and energy balance in young cynomolgus macaques. J Neuroendocrinol. 2004;16:146–153. doi: 10.1111/j.0953-8194.2004.01143.x. [DOI] [PubMed] [Google Scholar]
- 14.Pillon D, Caraty A, Fabre-Nys C, Bruneau G. Short-term effect of oestradiol on neurokinin B mRNA expression in the infundibular nucleus of ewes. J Neuroendocrinol. 2003;15:749–753. doi: 10.1046/j.1365-2826.2003.01054.x. [DOI] [PubMed] [Google Scholar]
- 15.Foradori CD, Goodman RL, Adams VL, Valent M, Lehman MN. Progesterone increases dynorphin A concentrations in cerebrospinal fluid and preprodynorphin messenger ribonucleic acid levels in a subset of dynorphin neurons in the sheep. Endocrinology. 2005;146:1835–1842. doi: 10.1210/en.2004-1326. [DOI] [PubMed] [Google Scholar]
- 16.Goodman RL, Lehman MN, Smith JT, Coolen LM, de Oliveira CV, Jafarzadehshirazi MR, Pereira A, Iqbal J, Caraty A, Ciofi P, Clarke IJ. Kisspeptin neurons in the arcuate nucleus of the ewe express both dynorphin A and neurokinin B. Endocrinology. 2007;148:5752–5760. doi: 10.1210/en.2007-0961. [DOI] [PubMed] [Google Scholar]
- 17.Foradori CD, Coolen LM, Fitzgerald ME, Skinner DC, Goodman RL, Lehman MN. Colocalization of progesterone receptors in parvicellular dynorphin neurons of the ovine preoptic area and hypothalamus. Endocrinology. 2002;143:4366–4374. doi: 10.1210/en.2002-220586. [DOI] [PubMed] [Google Scholar]
- 18.Dungan HM, Clifton DK, Steiner RA. Minireview: kisspeptin neurons as central processors in the regulation of gonadotropin-releasing hormone secretion. Endocrinology. 2006;147:1154–1158. doi: 10.1210/en.2005-1282. [DOI] [PubMed] [Google Scholar]
- 19.Clarke IJ, Smith JT, Caraty A, Goodman RL, Lehman MN. Kisspeptin and seasonality in sheep. Peptides. 2009;30:154–163. doi: 10.1016/j.peptides.2008.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goodman RL, Coolen IM, Anderson GM, Hardy SL, Valent M, Connors JM, Fitzgerald ME, Lehman MN. Evidence that dynorphin plays a major role in mediating progesterone negative feedback on gonadotropin-releasing hormone neurons in sheep. Endocrinology. 2004;145:2959–2967. doi: 10.1210/en.2003-1305. [DOI] [PubMed] [Google Scholar]
- 21.Cheng G, Coolen LM, Padmanabhan V, Goodman RL, Lehman MN. The kisspeptin/neurokinin B/dynorphin (KNDY) cell population of the arcuate nucleus: sex differences and effects of prenatal testosterone in the sheep. Endocrinology. 2009 doi: 10.1210/en.2009-0541. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khawaja AM, Rogers DF. Tachykinins: receptor to effector. Int J Biochem Cell Biol. 1996;28:721–738. doi: 10.1016/1357-2725(96)00017-9. [DOI] [PubMed] [Google Scholar]
- 23.Shigemoto R, Yokota Y, Tsuchida K, Nakanishi S. Cloning and expression of a rat neuromedin K receptor cDNA. J Biol Chem. 1990;265:623–628. [PubMed] [Google Scholar]
- 24.Ding YQ, Shigemoto R, Takada M, Ohishi H, Nakanishi S, Mizuno N. Localization of the neuromedin K receptor (NK3) in the central nervous system of the rat. J Comp Neurol. 1996;364:290–310. doi: 10.1002/(SICI)1096-9861(19960108)364:2<290::AID-CNE8>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 25.Mileusnic D, Lee JM, Magnuson DJ, Hejna MJ, Krause JE, Lorens JB, Lorens SA. Neurokinin-3 receptor distribution in rat and human brain: an immunohistochemical study. Neuroscience. 1999;89:1269–1290. doi: 10.1016/s0306-4522(98)00349-2. [DOI] [PubMed] [Google Scholar]
- 26.Yip J, Chahl LA. Localization of NK1 and NK3 receptors in guinea-pig brain. Regulatory Peptides. 2001;98:55–62. doi: 10.1016/s0167-0115(00)00228-7. [DOI] [PubMed] [Google Scholar]
- 27.Sandoval-Guzman T, Rance NE. Central injection of senktide, an NK3 receptor agonist, or neuropeptide Y inhibits LH secretion and induces different patterns of Fos expression in the rat hypothalamus. Brain Res. 2004;1026:307–312. doi: 10.1016/j.brainres.2004.08.026. [DOI] [PubMed] [Google Scholar]
- 28.McManus CJ, Valent M, Connors JM, Goodman RL, Lehman MN. A neurokinin B agonist stimulates LH secretion in follicular, but not luteal phase ewes. Ann Meeting Soc Neurosc. 2005 Abstr. 760.8. [Google Scholar]
- 29.Krajewski SJ, Anderson MJ, Iles-Shih L, Chen KJ, Urbanski HF, Rance NE. Morphologic evidence that neurokinin B modulates gonadotropin-releasing hormone secretion via NK3 receptors in the rat median eminence. J Comp Neurol. 2005;489:372–386. doi: 10.1002/cne.20626. [DOI] [PubMed] [Google Scholar]
- 30.Watson RE, Jr, Wiegand SJ, Clough RW, Hoffman GE. Use of cryoprotectant to maintain long-term peptide immunoreactivity and tissue morphology. Peptides. 1986;7:155–159. doi: 10.1016/0196-9781(86)90076-8. [DOI] [PubMed] [Google Scholar]
- 31.Foradori CD, Amstalden M, Coolen LM, Singh SR, McManus CJ, Handa RJ, Goodman RL, Lehman MN. Orphanin FQ: evidence for a role in the control of the reproductive neuroendocrine system. Endocrinology. 2007;148:4993–5001. doi: 10.1210/en.2007-0011. [DOI] [PubMed] [Google Scholar]
- 32.Hunyady B, Krempels K, Harta G, Mezey E. Immunohistochemical signal amplification by catalyzed reporter deposition and its application in double immunostaining. J Histochem Cytochem. 1996;44:1353–1362. doi: 10.1177/44.12.8985127. [DOI] [PubMed] [Google Scholar]
- 33.Smith JT, Coolen LM, Kriegsfeld LJ, Sari IP, Jaafarzadehshirazi MR, Maltby M, Bateman K, Goodman RL, Tilbrook AJ, Ubuka T, Bentley GE, Clarke IJ, Lehman MN. Variation in kisspeptin and RFamide-related peptide (RFRP) expression and terminal connections to gonadotropin-releasing hormone neurons in the brain: a novel medium for seasonal breeding in the sheep. Endocrinology. 2008;149:5770–5782. doi: 10.1210/en.2008-0581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Foradori CD, Amstalden M, Goodman RL, Lehman MN. Colocalization of dynorphin A and neurokinin B immunoreactivity in the arcuate nucleus and median eminence of the sheep. J Neuroendocrinol. 2006;18:534–541. doi: 10.1111/j.1365-2826.2006.01445.x. [DOI] [PubMed] [Google Scholar]
- 35.Coolen LM, Fitzgerald ME, Yu L, Lehman MN. Activation of μ opioid receptors in the medial preoptic area following copulation in male rats. Neuroscience. 2004;124:11–21. doi: 10.1016/j.neuroscience.2003.10.045. [DOI] [PubMed] [Google Scholar]
- 36.Lehman MN, Robinson JE, Karsch FJ, Silverman AJ. Immunocytochemical localization of luteinizing hormone-releasing hormone (LHRH) pathways in the sheep brain during anestrus and the mid-luteal phase of the estrous cycle. J Comp Neurol. 1986;244:19–35. doi: 10.1002/cne.902440103. [DOI] [PubMed] [Google Scholar]
- 37.Shughrue PJ, Lane MV, Merchenthaler I. In situ hybridization analysis of the distribution of neurokinin-3 mRNA in the rat central nervous system. J Comp Neurol. 1996;372:395–414. doi: 10.1002/(SICI)1096-9861(19960826)372:3<395::AID-CNE5>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 38.Rometo AM, Krajewski SJ, Voytko ML, Rance NE. Hypertrophy and increased kisspeptin gene expression in the hypothalamic infundibular nucleus of postmenopausal women and ovariectomized monkeys. J Clin Endocrin Metab. 2007;92:2744–2750. doi: 10.1210/jc.2007-0553. [DOI] [PubMed] [Google Scholar]
- 39.Burke MC, Letts PA, Krajewski SJ, Rance NE. Coexpression of dynorphin and neurokinin B immunoreactivity in the rat hypothalamus: morphologic evidence of interrelated function within the arcuate nucleus. J Comp Neurol. 2006;498:712–726. doi: 10.1002/cne.21086. [DOI] [PubMed] [Google Scholar]
- 40.Caraty A, Smith JT, Lomet D, Saïd S, Morrissey A, Cognie J, Doughton B, Baril G, Briant C, Clarke IJ. Kisspeptin synchronizes preovulatory surges in cyclical ewes and causes ovulation in seasonally acyclic ewes. Endocrinology. 2007;148:5258–5267. doi: 10.1210/en.2007-0554. [DOI] [PubMed] [Google Scholar]
- 41.Todman MG, Han SK, Herbison AE. Profiling neurotransmitter receptor expression in mouse gonadotropin-releasing hormone neurons using green fluorescent protein-promoter transgenics and microarrays. Neuroscience. 2005;132:703–712. doi: 10.1016/j.neuroscience.2005.01.035. [DOI] [PubMed] [Google Scholar]
- 42.Rekling JC. NK-3 receptor activation depolarizes and induces an after-depolarization in pyramidal neurons in gerbil cingulated cortex. Brain Res Bull. 2004;63:85–90. doi: 10.1016/j.brainresbull.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 43.Maggi CA. The mammalian tachykinin receptors. Gen Pharmac. 1995;26:911–944. doi: 10.1016/0306-3623(94)00292-u. [DOI] [PubMed] [Google Scholar]
- 44.Ferguson SSG. Evolving concepts in G-protein coupled receptor endocytosis: the role in receptor desensitization and signaling. Pharmacol Rev. 2001;53:1–24. [PubMed] [Google Scholar]
- 45.Howe HE, Somponpun SJ, Sladek CD. Role of neurokinin 3 receptors in supraoptic vasopressin and oxytocin neurons. J Neurosci. 2004;24:10103–10110. doi: 10.1523/JNEUROSCI.3164-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Haley GE, Flynn FW. Agonist and hypertonic saline-induced trafficking of the NK3-receptors on vasopressin neurons within the paraventricular nucleus of the hypothalamus. Am J Physiol Regul Integr Comp Physiol. 2006;290:R1242–R1250. doi: 10.1152/ajpregu.00773.2005. [DOI] [PubMed] [Google Scholar]
- 47.Eckersell CB, Popper P, Micevych PE. Estrogen-induced alteration of μ-opioid receptor immunoreactivity in the medial preoptic nucleus and medial amygdala. J Neurosc. 1998;18:3967–3976. doi: 10.1523/JNEUROSCI.18-10-03967.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Amstalden M, Spell KM, Williams GL, Goodman RL, Lehman RL. Neurokinin 3 receptor internalization in the arcuate nucleus is increased during the follicular phase of the estrous cycle in the ewe. Ann Meeting Soc Neurosc. 2008 Abstr 81.2. [Google Scholar]
- 49.Agnati LF, Fuxe K, Zoli M, Ozini I, Toffano G, Ferraguti F. A correlation analysis of the regional distribution of central enkephalin and beta-endorphin immunoreactive terminals and of opiate receptors in adult and old male rats. Evidence for the existence of two main types of communication in the central nervous system: the volume transmission and the wiring transmission. Acta Physiol Scand. 1986;128:201–207. doi: 10.1111/j.1748-1716.1986.tb07967.x. [DOI] [PubMed] [Google Scholar]