Abstract
Our laboratories have focused on the role of the tetraspanin CD81 in the regulation of mitotic activity. Previously we have shown that antibodies directed against CD81 can block the proliferation of cultured retinal pigment epithelial (RPE) cells. The present study investigates the role of this protein by analyzing the structure of the adult retina in mice with a null mutation of cd81. Adult cd81−/− mice were produced by crossing two inbred strains, NIHS-BC/Tac and 129X1/SvJ, carrying the cd81 mutation as heterozygotes (+/−). Seven cd81−/− mice and 11 wildtype (cd81+/+) littermates were anesthetized and perfused with paraformaldehyde. The eyes were removed and processed for examination by light and electron microscopy. In general, the retinas of the cd81−/− mice appeared normal. However, upon close examination, there was an 18% increase in the number of RPE nuclei in the cd81−/− mice. The photoreceptor layer of the cd81−/− mice was significantly thinner than that of the wild-type mice, even though there was no difference in the total thickness of the retinas in the two groups of mice. At the electron microscopic level we did not observe any differences in cell–cell junctions in the retinas of the cd81−/− mice as compared to their wild-type littermates. These data support a role for CD81 controlling cell-cycle and the number of RPE nuclei in the mouse retina.
Keywords: TAPA, growth regulation, retina, tetraspanin
In the adult retina, proliferation of normally quiescent supportive cells can lead to clinical complications such as proliferative vitreoretinopathy (PVR). This response of the retina is characterized by the formation of cellular membranes formed in part by Müller glial cells [Guidry, 1997; Lewis and Fisher, 2003] and retinal pigment epithelium [Machemer and Laqua, 1975; Liou et al., 2002]. Understanding the molecular mechanism involved in this proliferative response [Dyer and Cepko, 2000] is critical to defining interventions to prevent the deleterious effects and the potential for traction retinal detachments [Saishin et al., 2003]. Our laboratory has focused on the reactive/proliferative response that occurs in the retina with a specific focus on the role of CD81, a member of the tetraspanin family of proteins. CD81 is a small membrane protein that is involved in the regulation of cell growth [Geisert et al., 1996, 2002b]. In the rodent the highest levels of CD81 are found in the CNS and retina [Geisert et al., 1996, 2002a]. Antibody directed against CD81 dramatically decreases the proliferation of cultured glial cells [Geisert et al., 1996], including retinal pigment epithelial (RPE) cells [Geisert et al., 2002b]. CD81, like other members of the tetraspanin family, can form molecular complexes within the plane of the cell membrane and is often associated with adhesion molecules [Ikeyama et al., 1993; Hemler, 1998; Yáñez-Mó et al., 1998; Fitter et al., 1999; Charrin et al., 2001; Stipp et al., 2001]. Current evidence suggests that these tetraspanin complexes link cell adhesion to intracellular signaling cascades [Jennings et al., 1990; Schick et al., 1993; Yatomi et al., 1993; Berditchevski et al., 1997]. By partnering with different membrane proteins [Takahashi et al., 1990; Bradbury et al., 1992; Schick et al., 1993; Yáñez-Mó et al., 1998; Stipp et al., 2001], CD81 can be involved in a variety of different cellular junctions. For example, when CD81 forms a complex with either α4β1 integrin [Mannion et al., 1996] or α3β1 integrin [Stipp and Hemler, 2000] it is directly involved in the regulation of cellular interactions with the extracellular matrix. This interaction has a defined effect on second-messenger systems [Berditchevski et al., 1997; Yauch and Hemler, 2000; Zhang et al., 2001] and, eventually, modulates the mitotic activity of the cell [Oren et al., 1990; Geisert et al., 1996; Hemler, 2001].
One approach to understand the role of CD81 is to examine the changes that occur when the gene is knocked out. A previous study [Geisert et al., 2002a] demonstrates an increase in the numbers of astrocytes and microglia in the brains of mice carrying a cd81-null mutation. The present study examines the effects of this null mutation on the structure and cellular composition of the retina, with a specific emphasis on the structure of the RPE. The development of the retina follows a precise progression of cellular proliferation and differentiation. Cells of the RPE are among the first to differentiate. Early in development, the outer cells of the optic cup undergo extensive proliferation and the cells form a single layer sitting on a basal lamina. These pigmented cells interact with their environment to affect the development of the choroid and retina [Coulombre and Coulombre, 1970; Rapaport et al., 1995; Zhao and Overbeek, 2001]. After birth there is a continued proliferation of the RPE that continues into the second postnatal week [Young, 1976; Bodenstein and Sidman, 1987]. Unlike most cells in the body, the RPE cells go through the cell-cycle but fail to undergo cytokinesis near the end of the proliferative phase, resulting in the presence of many binucleated cells [Bodenstein and Sidman, 1987]. To evaluate the role of CD81 in vivo, we examine the retina in cd81−/− mice, paying particular attention to the number of nuclei in the RPE cell layer.
MATERIALS AND METHODS
Animals
All animal protocols used in this study were approved by the Animal Care and Use Committee of the University of Tennessee, Health Science Center. Adult mice with a cd81−/− mutation [Maecker and Levy, 1997] were back-crossed (minimum of eight) onto the NIHS-BC/Tac and the 129X1/SvJ backgrounds. These backcross progeny are more than 99% homozygous for alleles derived from C57BL/6ByJ and BALB/cByJ. cd81−/− mice from both inbred backgrounds have difficulty breeding and are propagated as CD81 heterozygotes. As observed in our study on the brains of the cd81−/− mice, the effects of the null-mutation are not as prevalent when this mutation is expressed on an inbred strain. We speculate that this effect may be due to recessive interacting loci within the inbred strain. For this analysis we used the progeny from F1 crosses between mice with the cd81+/− genotype on the NIHS-BC/Tac and 129X1/SvJ background. Thus, all of the mice were identical, with one allele from the NIHSBC/Tac strain and one from the 129X1/SvJ strain, eliminating the potential for the effects of recessive loci. We studied 7 cd81−/− mice (4 males, 3 females) and 11 wild-type (cd81+/+) mice (5 males, 6 females), all over the age of 60 days. Of these 18 mice, 7 came from the first mating and 11 from a second mating. Although these breedings resulted in 161 viable pups, only 12 of them were cd81−/− at the time of weaning (21 days), when they were genotyped, far below the expected number. Thus, only 30% of the expected number of cd81−/− mice survived to genotyping. At present, we have no explanation for the low number of surviving cd81−/− mice. The cd81−/− mice may die in utero or shortly after birth. The fact that there were fewer cd81−/− mice than expected indicates that we may be analyzing a subpopulation of the total number of cd81−/− mice conceived.
We genotyped mice using polymerase chain reaction (PCR). Genomic DNA was isolated from a 1 mm tail clip, approximately 0.5 μg genomic DNA was used for each PCR reaction. All PCR reagents come from Promega (Taq DNA Polymerase, no. 163466) and performed in an MJ Research PTC-200 DNA engine. In making the transgenic mouse a Neomycin resistant gene was inserted into the CD81 locus. In the screening of the mice with the null-mutation, we take advantage of this genetic marker. The primers identifying the NEO insert, 5′-GCCTTCTTGACGAGTTCTTCTGAG-3′ and 5′-CATTGAAGGCATAAGAGGGCTTAC-3′, result in a 950 bp product. The primers identifying the normal CD81 gene, 5′-CTCAACTGTTGTGGCTCCAAC-3′ and 5′-CCAATGAGGTACAGCTTCCC-3′, result in a 500 bp product. The reactions were run for 30 cycles at 94°C for 30 s, 58°C for 15 s, and 72°C for 1 min. We ran out the PCR products on a 1% agarose gel stained with ethidium bromide and viewed them on an ultraviolet light box. The normal CD81 band runs at approximately 500 bases; the Neo band runs at 950 bases.
Tissue Processing
Mice were anesthetized with a mixture of xylazine (13 mg/kg Rompun) and ketamin (87 mg/kg Ketalar) administered by intraperitoneal injection. We then perfused the mice through the heart with a solution of 0.01 M phosphate-buffered saline (PBS, pH 7.5) followed by 4% paraformaldehyde in 0.1 M phosphate buffer (pH 7.5). We did all of the perfusions at the same time (between 1:00 and 2:00 PM) in a room with standard fluorescent lighting (the range of luminance being between 60 and 65 fc). We removed each brain and weighed it. We then removed the eyes and put them in 4% paraformaldehyde. After 24 h, we removed the cornea, iris, and lens from one eye from each mouse for embedding in plastic. The eye cups from these eyes were postfixed with 1% osmium tetroxide in PBS for 4 h. After a brief rinse in deionized water, the eye cups, en bloc, were stained overnight at 4°C with 2% uranyl acetate in 0.85% sodium chloride. The eye cups were dehydrated by passage through a graded series of alcohol solutions (30% through 100%, 1 h each). The eye cups were infiltrated with 50% Spurr's resin in 100% alcohol overnight at room temperature, then with 100% Spurr's resin for 24 h, during which the resin was changed at least four times. They were cured at 60°C for 2 days. One-micron sections were cut through the retina with a Reichert Ultracut E microtome (Vienna, Austria) and stained with toluidine blue for light microscopy. The areas of interest were selected, sectioned at approximately 75 nm (silver-gray sections), and poststained with lead citrate. The tissues were examined and photographed on a JEOL 2000EX TEM (Tokyo, Japan) at 60 kV.
We left the remaining eyes in 4% paraformaldehyde and phosphate buffer and then the retina was dissected from each of these eyes. The cornea, iris, and lens first were carefully removed to include the ora serrata with the retina. During dissection, the tissues were kept in a pool of physiological saline. The retinas were mounted on glass slides and photographed with an Optronix digital camera mounted on an Olympus SZX12 dissecting microscope. The resulting images were analyzed with the NIH Image Program to determine the total area of the retina (not including any attached ciliary body) from each mouse.
Quantification
The light microscopic measurements of the 1 μm plastic embedded sections were made using a Leitz Orthoplan microscope connected to a Panasonic video camera with a final magnification of 500×. These sections, taken from the dorso-ventral midline of the eye, extended from the dorsal to the ventral attachment of the ora serrata. To estimate the number of RPE cells in a retina, we counted the number of nuclei in the RPE layer from a single section through the retina. We then divided this number by the linear distance of the RPE layer to estimate the linear packing density of the RPE cells. To estimate the total number of RPE cells per retina in each mouse, we used the linear count of RPE nuclei to calculate the number of RPE cells per μm2 of retina. This number was then corrected using the Abercrombie (1946) correction method: N = n (T/T+D), where N is the “true” number of cells, n is the estimated cell number, T is the thickness of the section, and D is the diameter of the nuclei. Then multiplied this number by the total area of the retina from the contralateral eye of the same mouse. We also measured the thickness of each cell layer of the retina at 200 μm intervals along the section. At least 20 measurements were made per section. We measured the thickness of the photoreceptor outer segment layer from the apical layer of the RPE to the external limiting membrane and the thickness of the retina from the apical layer of the RPE to the internal limiting membrane.
We made similar morphologic measurements on electron micrographs using NIH Image software (Version 1.62). We first scanned the negatives of these micrographs using a Polaroid SprintScan 45 PPC scanner (Version 1.03). All the scanned pictures were assembled into a montage using Adobe Photoshop (Version 6.0) and viewed at a final magnification of 4,000×. All the measurements were made blinded to the genotype (cd81−/− or wild type) of the mouse which was revealed after the analysis. We entered all data into Microsoft Excel, then analyzed the differences in measurements using means and standard error of the mean (SE). The student's t-test and regression analysis by Stat View (Version 5.0) were used to compare data sets. For all comparisons, P<0.05 was considered to be a significant difference.
In addition to weighing the brain of each mouse, we tabulated each one's body weight, sex, eye weight, age, and retinal area. We analyzed these variables by a multiple regression analysis to define the factors that affected the number of RPE cells in the cd81−/− mice. The number of RPE cells was the dependent variable; sex, age, body weight, brain weight, and genotype were the independent variables.
RESULTS
The present study is focused on the effects of the cd81-null mutation on the number of nuclei in the RPE cell layer. During this study we made a brief qualitative assessment of the entire retina. During this examination of the retina in these animals, we were particularly interested in any gross morphological anomalies (Fig. 1). At the light microscopic level the retinas of cd81−/− animals appeared to be similar to that of the wild-type eyes. All retinal layers and all cell types appeared to be present. Ganglion cells were present in both retinas. The inner plexiform layer appeared similar in both sets of animals. The normal complement of cells appeared to be present in the inner nuclear layer and there were no obvious differences in cell types within this layer. The outer plexiform layer was normal in the cd81−/− mice and there was no obvious difference in the outer nuclear layer. Two obvious differences were observed. There did appear to be a greater number of RPE cells in the cd81−/− mice. In addition, the photoreceptor layer in the cd81−/− mice appeared to be thinner than that in the wild-type littermates.
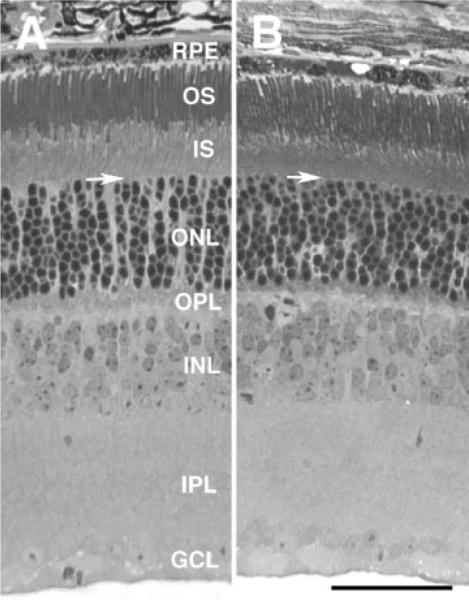
Fig. 1.
Photomicrographs of the retina of a wild-type mouse (A) and a cd81−/− mouse littermate (B). The histological features of both retinas appear similar. All of the cell layers are present in both retinas and there is a normal complement of cells. The thickness of the photoreceptor layer, including the outer and inner segments, is noticeably greater in the retina of the wild-type than that of the cd81−/−. The layers of the retina are indicated in A: Retinal pigment epithelium (RPE); outer segment (OS); inner segment (IS); outer nuclear layer (ONL); outer plexiform layer (OPL); inner nuclear layer (INL); inner plexiform layer (IPL); and ganglion cell layer (GCL). The arrows mark the inner limiting membrane. Both photomicrographs are at the same magnification: the scale bar in B = 50 μm.
The RPE Cell Layer
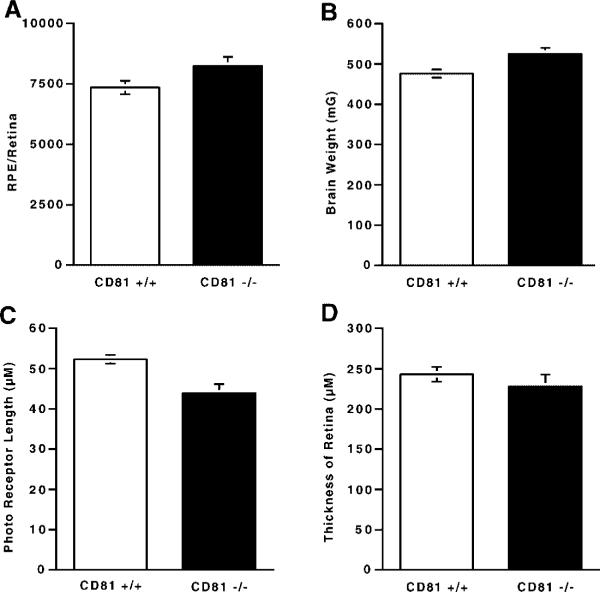
To determine if there was a change in the number of RPE within the cd81−/− retina, the number of nuclei within the RPE layer (Fig. 2) was counted within a single section from ora serrata to ora serrata. The linear distance of the RPE layer was also measured. The number of nuclei was divided by the distance to provide an estimate of the number of RPE nuclei/mm of retina. The number of RPE nuclei in the cd81−/− mice was 24.21±0.5 nuclei/mm (mean±SE, n=7). In wild-type littermates the number of RPE nuclei was 22.55±0.4 nuclei/mm (n=11). This represents a 7% increase in the number of RPE nuclei/mm of retina examined and this increase was significant (P<0.05, Student's t-test). When we measured the surface area of the retina from the contralateral eye of each mouse, we found that the mean surface area for the cd81−/− mice was 14.9 mm2, while that for the wild-type mice was 14.4 mm2. This difference was not significant. Using the linear count from one eye and the surface area from the contralateral eye to calculate the total number of RPE nuclei within the retina of each animal, we found that cd81−/− mice had an average of 43,400±890 RPE nuclei (mean±SE) per retina, whereas their wild-type littermates had an average of 36,900±650 RPE nuclei per retina (Fig. 3). Thus, there were 18% more RPE nuclei in the retinas of the cd81−/− mice, a difference that attained significance (P<0.005).
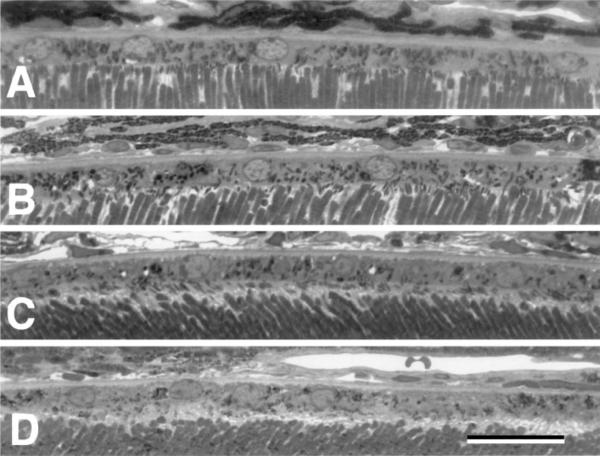
Fig. 2.
High power photomicrographs of the RPE cell layer in a wild-type mouse (A, B) and a cd81−/− mouse littermate (C, D). Notice that the RPE cell layer appears similar. The RPE sits on a well-defined basal lamina Bruch's membrane and the apical surface is intimate contact with the outer segments. All photomicrographs are taken at the same magnification and the scale bar in D = 25 μm.
Fig. 3.
We compared cd81+/+ and cd81−/− mice with respect to the number of RPE nuclei per retina (A), brain weight (B), thickness of the photoreceptor layer (C), and total thickness of the retina (D). In cd81−/− retinas, there were 18% more RPE cells. This was a significantly greater number of cells than were present in wild-type littermates (A). The thickness of the photoreceptor layer in cd81−/− mice was significantly thinner than that of their wild-type littermates (C), while the total thickness of the retina did not differ in the two groups of mice (D). As we have shown previously, the brain weight of cd81−/− mice is significantly greater than that of their wild-type littermates. The differences illustrated in A, B, and C are statistically significant (P < 0.05).
The differences we observed in the number of RPE nuclei in the retina may be related to specific characteristics of the cd81−/− mice. Previously, we found that the lack of CD81 can have profound effects on brain weight, the number of astrocytes and microglia in the brain [Geisert et al., 2002a]. In this study we examined several characteristics of the mice. We measured the brain weights in cd81−/− mice (526.0±9.2 mg) and wild-type littermates (476.6±6.6 mg) (Fig. 3D). The cd81−/− mice had significantly larger brains (P<0.001). Several other measures were taken, including body weight, sex, eye weight, retinal area and age. These variables were tabulated and used to define the factors that affected the number of RPE cells in the cd81−/− mice. The data were analyzed by a multiple regression analysis. The number of RPE nuclei was the dependent variable, and sex, age, body weight, brain weight, and genotype were the independent variables. The only independent variable that significantly affected RPE nuclear number was genotype (P<0.01). These data indicate that the differences observed in the number of RPE nuclei within the retina were primarily due to the lack of CD81.
The Photoreceptor Layer
The inner and outer segments of the photoreceptor layer of the retinas from cd81−/− mice appeared to be thinner than those in the retinas of wild-type mice (Fig. 1). We therefore measured the thickness of the total photoreceptor layer and its two component layers to determine if lack of CD81 caused any systematic difference. The average thickness of the photoreceptor layer in the cd81−/− mice was 43.9±2.2 μm, while that in wild-type littermates was 52.3±1.1 μm (Fig. 2C), a significant difference (P<0.01). To determine if this difference was the result of a change in either the inner or outer segment, we measured the thickness of each layer and compared the measurements in the two groups. The average length of the inner segment layer was 14.8±0.2 μm in cd81−/− mice and 18.8±0.3 μm in wild-type mice; the length of the outer segment layer was 23.1±1 μm in cd81−/− mice and 28.7±0.2 μm in their wild-type littermates. Thus, both layers were significantly shorter (P<0.01) in the cd81−/− mice. In a final comparison, we determined the ratio of the length of the inner segments to that of the outer segments in the two groups of mice. These ratios were not significantly different. The ratio for the cd81−/− mice was 1.57±0.1, while that for their wild-type littermates was 1.53±0.02. Other than the difference between the thickness of the retinal layers, no abnormalities were visible at the light microscopic level (Fig. 1).
Since there was a clear difference in the number of RPE cells and the photoreceptor layer was thinner in the retinas of cd81−/− mice, we examined the total thickness of the retina to define the extent of the differences. Initial examination of the sections through the retina suggested no obvious difference between cd81−/− and wild-type mice in the total thickness of the retina. To examine the possibility that there was a change in the thickness of the retina, we also measured the total thickness of the retina in each mouse (Fig. 3D). In the cd81−/− animals, the mean thickness of the retina from the RPE-photoreceptor junction to the internal limiting membrane was 228 μm (SE=14.7 μm). This measurement was not statistically different from that in the wild-type littermates, which was 243 μm (SE=9.1 μm). Because there was no difference in the total thickness of the retina in the two groups of mice, while the photoreceptor layer was significantly thinner in mice lacking CD81, we expected that the cellular layers of the retina would be thicker in cd81−/− mice. However, when we subtracted thickness of the photoreceptor layer from the total thickness of the retina for each animal, we found no significant difference between cd81−/− mice (184.0±13.8 μm) and wild-type littermates (190.7±7.9 μm). Thus, in spite of its statistical significance, the relative thinness of the photoreceptor layer in cd81−/− mice is not significant when compared to the total thickness of the retina.
Electron Microscopic Morphology
The electron micrographs showed no obvious abnormalities in the outer retina of cd81−/− mice relative to their wild-type littermates, with the obvious exception of the thickness of the inner and outer segments. The general structures of the inner and outer segments were normal. The outer segment was contained mitochondria and the connecting stalk and associated cilium. Surrounding the outer segments were the microvilli extending from the Müller cell pedicle. Adherens junctions were present between Müller cells in both cd81−/− and wild-type mice. The ultrastructural morphology of the junctions were similar in both groups of animals, as well as their overall length. The cells in the RPE cell layer in cd81−/− mice appeared normal in every respect (Fig. 4). Pigment granules were present in both groups of mice with approximately the same frequency. Mitochondria in the cd81−/− mice had a similar morphology as those in the wild-type mice and the numbers were approximately the same. There was no obvious difference in the extent or structure of the basal enfolding and Bruch's membrane was of a similar appearance. The junctional complexes between the RPE were similar in structure and extent (see arrow in Fig. 3A and B). Junctional complexes in both cells contained the obvious zonula adherens attached to the underlying cytoskeletal network. The close apposition of the zonula occludens was also easily observed. In both groups of mice, the apical projections of the RPE interacted with the outer segments. The microvilli extending form the surface of the cells were observed in close proximity to the stacked layers of disks within the outer segments of the rod photoreceptors. Although the thickness of the RPE cell layer was the same, more cells were present per unit of length in cd81−/− mice. Thus, there were no obvious abnormalities that could be identified at the ultrastructural level in the cd81−/− mice relative to their wild-time littermates.
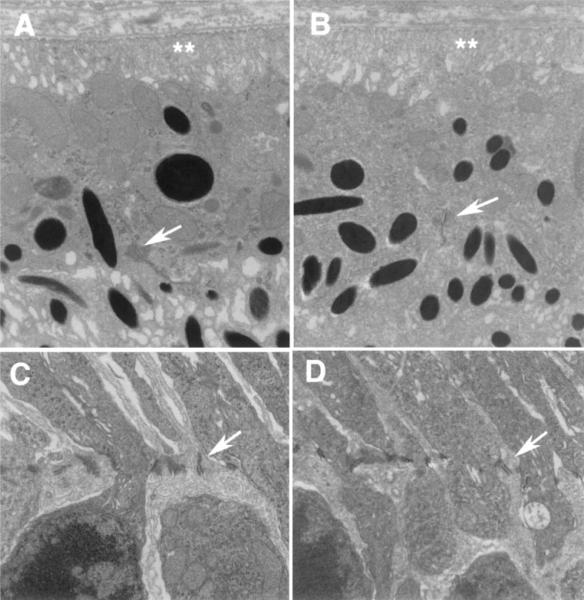
Fig. 4.
Comparison of the ultrastructure of the outer retina of a wild-type mouse (A, C) and a cd81−/− mouse (B, D) reveals similar structure of the junctional complexes cells. No differences were observed in the junctional complexes between RPE cells (arrows in A and B) or in the basal enfolding of the RPE (double asterisks). At the outer limiting membrane there was no observable difference between the two groups of mice with respect to the junctional complexes between Müller cells (arrows in C and D).A and B are at the same magnifications (20,000×), as are C and D (10,000×).
DISCUSSION
CD81 is a member of the tetraspanin family of proteins [Levey et al., 1998; Boucheix and Rubinstein, 2001; Hemler, 2001]. These proteins form a molecular complex within the plane of the cell membrane that associates with other molecules such as cell adhesion molecules [Boucheix and Rubinstein, 2001]. These complexes appear to regulate cell behaviors, including: adhesion, migration, and proliferation, [Hemler, 2001] by linking events occurring on the external surface of the cell to second- messenger systems [Schick et al., 1993; Berditchevski et al., 1997; Yauch and Hemler, 2000]. In our attempt to understand the functional role of tetraspanins, one functional theme is emerging: tetraspanins are involved in the control of cell growth and migration.
Originally, CD81 was termed a target of an antiproliferative antibody, TAPA-1 [Oren et al., 1990]. As that name implies, antibodies directed against an extracellular epitope on CD81 block cell-cycle progression in cultured cells [Oren et al., 1990; Geisert et al., 1996; Kelic et al., 2001; Geisert et al., 2002b]. Further proof of the role of CD81 in the regulation of cell growth comes from studies demonstrating that the brains of cd81−/− mice are larger than those of wild-type mice, and contain significantly more astrocytes and microglial cells, which are known to express CD81 [Geisert et al., 1996; Dijkstra et al., 2001; Geisert et al., 2002b]. The phenotype of cd81−/− mice is affected by the genetic background. To limit these effects, we have chosen to study only mice from an F1 cross (SW/129-F1) of the inbred strains 129/SvJ and NIHS-BC/Tac.
The retinas of cd81−/− mice had photoreceptor layers that were on average thinner than those observed in the wild-type mice. There is no immediate explanation for the difference in the photoreceptor layer. We know that the photo-receptors are continuously renewed with the outer segment being phagocytosis by the RPE [Steinberg et al., 1977]. This process is associated with integrins found on the apical surface of the RPE [Brem et al., 1994]. The integrins expressed on the apical surface of the RPE include αvβ5 [Finnemann and Rodriguez-Boulan, 1999], β1 [Chen et al., 1997], β2, and α4 [Brem et al., 1994]. CD81 is expressed on the RPE microvilli surrounding the outer segment [Geisert et al., 2002b], raising the possibility that it may be involved in the process of phagocytosis. However, at the present time there is no direct evidence to support this speculation.
Another possible explanation for the thinner photoreceptor layer in the cd81−/− mice may be altered interactions with Müller cells, which also express CD81 [Clarke and Geisert, 1998]. Müller cells play an important role in photoreceptor cell development during the final stage of retinal maturation [Young, 1985]. It has been demonstrated that Müller cells and photoreceptors descend from a single retinal progenitor cell [Turner and Cepko, 1987; Reichenbach et al., 1993]. The Müller cells surround photoreceptors and provide metabolic and trophic support [Reichenbach et al., 1993; Newman and Reichenbach, 1996; Cao et al., 1997]. A lack of CD81 on the surface of Müller cells may affect the interaction of these cells with the outer segments in cd81−/− mice.
In this study, there was a significant increase in RPE nuclei in cd81−/− mice compared to their wild-type littermates. The cell-cycle of the RPE is controlled by many factors. Growth factors modulate DNA synthesis [Leschey et al., 1991] and stimulate cell proliferation [Campochiaro and Glaser, 1986], causing changes in cell-adhesion molecules, migratory behavior, and cell survival. Several studies have shown that the effects of growth factors can interact synergistically with extracellular matrix components [Smith-Thomas et al., 1996; Hinton et al., 1998; Jin et al., 2000]. Growth factors also elicit in RPE cells a complex signal-transduction cascade [Bornfeldt et al., 1995] that can be affected by cell adhesion. These cell adhesions are particularly complex in RPE cells with at least three distinct surfaces to consider. The innermost surface interacts with the outer segments of the photoreceptors with integrins [Brem et al., 1994; Chen et al., 1997; Finnemann and Rodriguez-Boulan, 1999]. At the regions of contact between RPE cells specialized junctions are known to exist expressing a variety of adhesion molecules [McKay et al., 1997; Kaida et al., 2000]. Finally the RPE interacts with Bruch's membrane and may use a β1 integrin [Anderson et al., 1990; Chu and Grunwald, 1991]. Our initial studies [Geisert et al., 2002a] suggest that CD81 is present on all three surfaces, which would allow CD81 to form molecular complexes with a number of different proteins. Since cell-cycle progression of cultured RPE can be altered by antibodies directed against CD81 it is tempting to speculate that the CD81 protein complexes on the different surfaces of RPE cells may differentially regulate phases of RPE cell-cycle. The present study demonstrates that CD81 is important in regulating the number of RPE nuclei within the retina. Since many of these cells may be binucleated [Bodenstein and Sidman, 1987] we cannot definitively comment on the number of RPE cells—mononucleated or binucleated. Future studies will focus on the issue of cell number and binucleation. In addition, we will continue to define the role CD81 plays in the complex biology of RPE and the retina.
ACKNOWLEDGMENTS
The authors thank Dr. Dianna Johnson for her constructive comments, Katherine Troughton for help in preparing sections and electron microscopy, William Orr for statistical analysis, Jeanne Cole for her editorial assistance, and Vivian Simon for assistance in article preparation.
Grant sponsor: PHS; Grant numbers: RO1 EY12369 (to EEG), 3P30 EY13080 (to EEG); Grant sponsor: Research to Prevent Blindness (to EEG); Grant number: AI45900 (to SL); Grant sponsor: Wonkwang University (to B.K.S.).
REFERENCES
- Abercrombie M. Estimation of nuclear populations from microtome sections. Anat Rec. 1946;94:239–247. doi: 10.1002/ar.1090940210. [DOI] [PubMed] [Google Scholar]
- Anderson DH, Guerin CJ, Matsumoto B, Pfeffer BA. Identification and localization of a beta-1 receptor from the integrin family in mammalian retinal pigment epithelia cells. Invest Ophthalmol Vis Sci. 1990;31(1):81–93. [PubMed] [Google Scholar]
- Berditchevski F, Tolias KF, Wong K, Carpenter CL, Hemler ME. A novel link between integrins, transmembrane-4 superfamily proteins (CD63 and CD81), and phosphatidylinositol 4-kinase. J Biol Chem. 1997;272:2595–2598. doi: 10.1074/jbc.272.5.2595. [DOI] [PubMed] [Google Scholar]
- Bodenstein L, Sidman RL. Growth and development of mouse retinal pigment epithelium. Dev Biol. 1987;121:192–204. doi: 10.1016/0012-1606(87)90152-7. [DOI] [PubMed] [Google Scholar]
- Bornfeldt KE, Raines EW, Graves LM, Skinner MP, Krebs EG, Ross R. Platelet-derived growth factor. Distinct signal transduction pathways associated with migration versus proliferation. Ann NY Acad Sci. 1995;766:416–430. doi: 10.1111/j.1749-6632.1995.tb26691.x. [DOI] [PubMed] [Google Scholar]
- Boucheix C, Rubinstein E. Tetraspanins. Cell Mol Life Sci. 2001;58:1189–1205. doi: 10.1007/PL00000933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradbury LE, Kansas GS, Levy S, Evans RL, Tedder TF. The CD19/CD21 signal transducing complex of human B lymphocytes includes the target of antiproliferative antibody-1 and Leu-13 molecules. J Immunol. 1992;149:2841–2850. [PubMed] [Google Scholar]
- Brem RB, Robbins SG, Wilson DJ, O'Rourke LM, Mixon RN, Robertson JE, Planck SR, Rosenbaum JT. Immunolocalization of integrins in the human retina. Invest Ophthalmol Vis Sci. 1994;35:3466–3474. [PubMed] [Google Scholar]
- Campochiaro PA, Glaser BM. Mechanisms involved in retinal pigment epithelial cell chemotaxis. Arch Ophthalmol. 1986;104:277–280. doi: 10.1001/archopht.1986.01050140135036. [DOI] [PubMed] [Google Scholar]
- Cao W, Wen R, Li F, Cheng T, Steinberg RH. Induction of basic fibroblast growth factor mRNA by basic fibroblast growth factor in Muller cells. Invest Ophthalmol Vis Sci. 1997;38:1358–1366. [PubMed] [Google Scholar]
- Charrin S, Le Naour F, Oualid M, Billard M, Faure G, Hanash SM, Boucheix C, Rubinstein E. The major CD9 and CD81 molecular partner. Identification and characterization of the complexes. J Biol Chem. 2001;276:14329–14337. doi: 10.1074/jbc.M011297200. [DOI] [PubMed] [Google Scholar]
- Chen W, Joos TO, Defoe DM. Evidence for beta 1-integrins on both apical and basal surfaces of Xenopus retinal pigment epithelium. Exp Eye Res. 1997;64:73–84. doi: 10.1006/exer.1996.0183. [DOI] [PubMed] [Google Scholar]
- Chu PG, Grunwald GB. Functional inhibition of retinal pigment epithelial cell-substrate adhesion with a monoclonal antibody against the beta 1 subunit of integrin. Invest Ophthalmol Vis Sci. 1991;32:1763–1769. [PubMed] [Google Scholar]
- Clarke K, Geisert EE., Jr. The target of the anti-proliferative antibody (TAPA) in the normal and injured rat retina. Mol Vis. 1998;4:3. [PubMed] [Google Scholar]
- Coulombre JL, Coulombre AJ. Influence of mouse neural retina on regeneration of chick neural retina from chick embryonic pigmented epithelium. Nature. 1970;228:559–560. doi: 10.1038/228559a0. [DOI] [PubMed] [Google Scholar]
- Dijkstra S, Geisert EE, Jr., Dijkstra CD, Bar PR, Joosten EA. CD81 and microglial activation in vitro: Proliferation, phagocytosis and nitric oxide production. J Neuroimmuol. 2001;114:151–159. doi: 10.1016/s0165-5728(01)00240-5. [DOI] [PubMed] [Google Scholar]
- Dyer M, Cepko CL. Control of Muller glial cell proliferation and activation following retinal injury. Nat Neurosci. 2000;3:873–880. doi: 10.1038/78774. [DOI] [PubMed] [Google Scholar]
- Finnemann SC, Rodriguez-Boulan E. Macrophage and retinal pigment epithelium phagocytosis: Apoptotic cells and photoreceptors compete for alphavbeta3 and alphavbeta5 integrins, and protein kinase C regulates alphavbeta5 binding and cytoskeletal linkage. J Exp Med. 1999;190:861–874. doi: 10.1084/jem.190.6.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitter S, Sincock PM, Jolliffe CN, Ashman LK. Transmembrane 4 superfamily protein CD151 (PETA-3) associates with beta1 and alphaIIb beta3 integrins in haemopoietic cell lines and modulates cell–cell adhesion. Biochem J. 1999;338:61–70. [PMC free article] [PubMed] [Google Scholar]
- Geisert EE, Jr., Yang L, Irwin MH. Astrocyte growth, reactivity, and the target of the antiproliferative antibody, TAPA. J Neurosci. 1996;16:5478–5487. doi: 10.1523/JNEUROSCI.16-17-05478.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisert EE, Jr., Abel HJ, Fan L, Geisert GR. Retinal pigment epithelium of the rat express CD81, the target of the anti-proliferative antibody (TAPA) Invest Ophthalmol Vis Sci. 2002a;43:274–280. [PubMed] [Google Scholar]
- Geisert EE, Jr., Williams RW, Geisert GR, Fan L, Asbury AM, Maecker HT, Deng J, Levy S. Increased brain size and glial cell number in CD81-null mice. J Comp Neurol. 2002b;453:22–32. doi: 10.1002/cne.10364. [DOI] [PubMed] [Google Scholar]
- Guidry C. Tractional force generation by porcine Muller cells. Invest Ophthalmol Vis Sci. 1997;38:456–468. [PubMed] [Google Scholar]
- Hemler ME. Integrin associated proteins. Curr Opin Cell Biol. 1998;10:578–585. doi: 10.1016/s0955-0674(98)80032-x. [DOI] [PubMed] [Google Scholar]
- Hemler ME. Specific tetraspanin functions. J Cell Biol. 2001;155:1103–1107. doi: 10.1083/jcb.200108061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinton DR, He S, Graf K, Yang D, Hsueh WA, Ryan SJ, Law RE. Mitogen-activated protein kinase activation mediates PDGF-directed migration of RPE cells. Exp Cell Res. 1998;239:11–15. doi: 10.1006/excr.1997.3873. [DOI] [PubMed] [Google Scholar]
- Ikeyama S, Koyama M, Yamaoko M, Sasada R, Miyake M. Suppression of cell motility and metastasis by transfection with human motility-related protein (MRP-1/CD9) DNA. J Exp Med. 1993;177:1231–1237. doi: 10.1084/jem.177.5.1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings LK, Fox CF, Kouns WC, McKay CP, Ballou LR, Schultz HE. The activation of human platelets mediated by anti-human platelet p24/CD9 monoclonal antibodies. J Biol Chem. 1990;265:3815–3822. [PubMed] [Google Scholar]
- Jin M, He S, Worpel V, Ryan SJ, Hinton DR. Promotion of adhesion and migration of RPE cells to provisional extracellular matrices by TNF-alpha. Invest Ophthalmol Vis Sci. 2000;41:4324–4332. [PubMed] [Google Scholar]
- Kaida M, Cao F, Skumatz CMB, Irving P, Burke JM. Time at confluence for human RPE cells: Effects on the adherens junction and in vitro wound closure. Invest Ophthalmol Vis Sci. 2000;41:3215–3224. [PubMed] [Google Scholar]
- Kelic S, Levy S, Suarez C, Weinstein DE. CD81 regulates neuron-induced astrocyte cell-cycle exit. Mol Cell Neurosci. 2001;17(3):551–560. doi: 10.1006/mcne.2000.0955. [DOI] [PubMed] [Google Scholar]
- Leschey KH, Hines J, Singer JH, Hackett SF, Campochiaro PA. Inhibition of growth factor effects in retinal pigment epithelial cells. Invest Ophthalmol Vis Sci. 1991;32:1770–1778. [PubMed] [Google Scholar]
- Levey S, Todd SC, Maecker HT. CD81 (TAPA-1): A molecule involved in signal transduction and cell adhesion in the immune system. Annu Rev Immunol. 1998;16:89–109. doi: 10.1146/annurev.immunol.16.1.89. [DOI] [PubMed] [Google Scholar]
- Lewis GP, Fisher SK. Up-regulation of glial fibrillary acidic protein in response to retinal injury: its potential role in glial remodeling and a comparison to vimentin expression. Int Rev Cytol. 2003;230:263–290. doi: 10.1016/s0074-7696(03)30005-1. [DOI] [PubMed] [Google Scholar]
- Liou GI, Pakalnis VA, Matragoon S, Samuel S, Behzadian MA, Baker J, Khalil IE, Roon P, Caldwell RB, Hunt RC, Marcus DM. HGF regulation of RPE proliferation in an IL-1beta/retinal hole-induced rabbit model of PVR. Mol Vis. 2002;8:494–501. [PubMed] [Google Scholar]
- Machemer R, Laqua H. Pigment epithelium proliferation in retinal detachment (massive periretinal proliferation) Am J Ophthalmol. 1975;80:1. doi: 10.1016/0002-9394(75)90862-4. [DOI] [PubMed] [Google Scholar]
- Maecker HT, Levy S. Normal lymphocyte development but delayed humoral immune response in CD81-null mice. J Exp Med. 1997;185:1505–1510. doi: 10.1084/jem.185.8.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannion BA, Berditchevski F, Kraeft SK, Chen LB, Hemler ME. Transmembrane-4 superfamily proteins CD81 (TAPA-1), CD82, CD63, and CD53 specifically associated with integrin alpha 4 beta 1(CD49d/CD29) J Immunol. 1996;157:2039–2047. [PubMed] [Google Scholar]
- McKay BS, Irving PE, Skumatzx CMB, Burke JM. Cell–cell adhesion molecules and the development of an epithelial phenotype in cultured human retinal pigment epithelial cells. Exp Eye Res. 1997;65:661–671. doi: 10.1006/exer.1997.0374. [DOI] [PubMed] [Google Scholar]
- Newman E, Reichenbach A. The Muller cell: A functional element of the retina. Trends Neurosci. 1996;19:307–312. doi: 10.1016/0166-2236(96)10040-0. [DOI] [PubMed] [Google Scholar]
- Oren R, Takahashi S, Doss C, Levy R, Levy S. TAPA-1, the target of an antiproliferative antibody, defines a new family of transmembrane proteins. Mol Cell Biol. 1990;10:4007–4015. doi: 10.1128/mcb.10.8.4007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapaport DH, Rakic P, Yasamura D, LaVail MM. Genesis of the retinal pigment epithelium in the macaque monkey. J Comp Neurol. 1995;363:359–376. doi: 10.1002/cne.903630303. [DOI] [PubMed] [Google Scholar]
- Reichenbach A, Stolzenburg JU, Eberhardt W, Chao TI, Dettmer D, Hertz L. What do retinal muller (glial) cells do for their neuronal `small siblings'? J Chem Neuroanat. 1993;6:201–213. doi: 10.1016/0891-0618(93)90042-3. [DOI] [PubMed] [Google Scholar]
- Saishin Y, Saishin Y, Takhashi K, Seo MS, Melia M, Campochiaro PA. The kinase inhibitor PKC412 suppresses epiretinal membrane formation and retinal detachment in mice with proliferative retinopathies. Invest Ophthalmol Vis Sci. 2003;44:3656–3662. doi: 10.1167/iovs.02-1143. [DOI] [PubMed] [Google Scholar]
- Schick MR, Nguyen VQ, Levy S. Anti-TAPA-1 antibodies induce protein tyrosine phosphorylation that is prevented by increasing intracellular thiol levels. J Immunol. 1993;151:1918–1925. [PubMed] [Google Scholar]
- Smith-Thomas L, Richardson P, Parsons MA, Rennie IG, Benson M, MacNeil S. Additive effects of extra cellular matrix proteins and platelet derived mitogens on human retinal pigment epithelial cell proliferation and contraction. Curr Eye Res. 1996;15:739–748. doi: 10.3109/02713689609003457. [DOI] [PubMed] [Google Scholar]
- Steinberg RH, Wood I, Hogan MJ. Pigment epithelial ensheathment and phagocytosis of extrafoveal cones in human retina. Philos Trans R Soc Lond B Biol Sci. 1977;277:459–474. doi: 10.1098/rstb.1977.0028. [DOI] [PubMed] [Google Scholar]
- Stipp CS, Hemler ME. Transmembrane-4-super-family proteins CD151 and CD81 associate with alpha3 beta1 integrin, and selectively contribute to alpha3 beta1-dependent neurite outgrowth. J Cell Sci. 2000;113:1871–1882. doi: 10.1242/jcs.113.11.1871. [DOI] [PubMed] [Google Scholar]
- Stipp CS, Kolesnikova TV, Hemler ME. EWI-2 is a major CD9 and CD81 partner and member of a novel Ig protein subfamily. J Biol Chem. 2001;276:40545–40554. doi: 10.1074/jbc.M107338200. [DOI] [PubMed] [Google Scholar]
- Takahashi S, Doss C, Levy S, Levy R. TAPA-1, the target of an antiproliferative antibody, is associated on the cell surface with the Leu-13 antigen. J Immunol. 1990;145:2207–2213. [PubMed] [Google Scholar]
- Turner DL, Cepko CL. A common progenitor for neurons and glia persists in rat retina late in development. Nature. 1987;328:131–136. doi: 10.1038/328131a0. [DOI] [PubMed] [Google Scholar]
- Yatomi Y, Ozaki Y, Satoh K, Kume S. Anti-CD9 monoclonal antibody elicits staurosporine inhibitable phosphatidylinositol 4,5-bisphosphate hydrolysis, phosphatidylinositol 3,4-bisphosphate synthesis, and protein-tyrosine phosphorylation in human platelets. FEBS Lett. 1993;322:285–290. doi: 10.1016/0014-5793(93)81587-p. [DOI] [PubMed] [Google Scholar]
- Yauch RL, Hemler ME. Specific interactions among transmembrane 4 superfamily (TM4SF) proteins and phosphoinositide 4-kinase. Biochem J. 2000;351:629–637. [PMC free article] [PubMed] [Google Scholar]
- Young RW. Visual cells and the concept of renewal. Invest Ophthalmol Vis Sci. 1976;15:700–725. [PubMed] [Google Scholar]
- Young RW. Cell differentiation in the retina of the mouse. Anat Rec. 1985;212:199–205. doi: 10.1002/ar.1092120215. [DOI] [PubMed] [Google Scholar]
- Yáñez-Mó M, Alfranca A, Cabañas C, Marazuela M, Tejedor R, Angela Ursa M, Ashman LK, de Landázuri MO. Regulation of endothelial cell motility by complexes of tetraspan molecules CD81/TAPA-1 and CD151/PETA-3 with alpha3beta1 integrin localized at endothelial lateral junctions. J Cell Biol. 1998;141:791–804. doi: 10.1083/jcb.141.3.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang XA, Bontrager AL, Hemler ME. Transmembrane-4 superfamily proteins associate with activated protein kinase C (PKC) and link PKC to specific beta(1) integrins. J Bio Chem. 2001;276:25005–25013. doi: 10.1074/jbc.M102156200. [DOI] [PubMed] [Google Scholar]
- Zhao S, Overbeek PA. Regulation of choroid development by the retinal pigment epithelium. Mol Vis. 2001;7:277–282. [PubMed] [Google Scholar]