Abstract
Vitamin D deficiency has been correlated with increased rates of infection. Since the early 19th century, both environmental (i.e., sunlight) and dietary sources (cod liver) of vitamin D have been identified as treatments for TB. The recent discovery that vitamin D induces antimicrobial peptide gene expression explains, in part, the ‘antibiotic’ effect of vitamin D and has greatly renewed interest in the ability of vitamin D to improve immune function. Subsequent work indicates that this regulation is biologically important for the response of the innate immune system to wounds and infection and that deficiency may lead to suboptimal responses toward bacterial and viral infections. The regulation of the cathelicidin antimicrobial peptide gene is a human/primate-specific adaptation and is not conserved in other mammals. The capacity of the vitamin D receptor to act as a high-affinity receptor for vitamin D and a low-affinity receptor for secondary bile acids and potentially other novel nutritional compounds suggests that the evolutionary selection to place the cathelicidin gene under control of the vitamin D receptor allows for its regulation under both endocrine and xenobiotic response systems. Future studies in both humans and humanized mouse models will elucidate the importance of this regulation and lead to the development of potential therapeutic applications.
Keywords: antimicrobial peptide, bile acid, cathelicidin, infection, innate immunity, intestinal, steroid-hormone receptor, vitamin D, vitamin D receptor, xenobiotic
Vitamin D insufficiency/deficiency is a worldwide, public health problem in both developed and developing countries. Vitamin D promotes and maintains healthy bones and teeth, but with the near eradication of rickets in the early part of the 20th century by fortification of foods, chronic insufficiency has gone largely unrecognized. However, with the current reemergence of nutritional rickets among infants, recent evidence that low levels of circulating vitamin D are associated with increased risk and mortality from cancer, and evidence of the beneficial effects of vitamin D on multiple sclerosis, rheumatoid arthritis, diabetes, cardiovascular disease and microbial infections, there has been renewed interest in this vitamin. In 2007, Time magazine cited the “benefits of vitamin D” in its list of “Top 10 Medical Breakthroughs”. Although extensive research has been done on vitamin D, the molecular and cellular mechanisms responsible for its many benefits have not been fully elucidated. With the discovery that vitamin D regulates the expression of an important antimicrobial peptide gene, exciting research findings are revealing and characterizing new pathways regulated by vitamin D and its receptor that may be essential for optimal immune function.
Vitamin D, vitamin D receptor & immunity
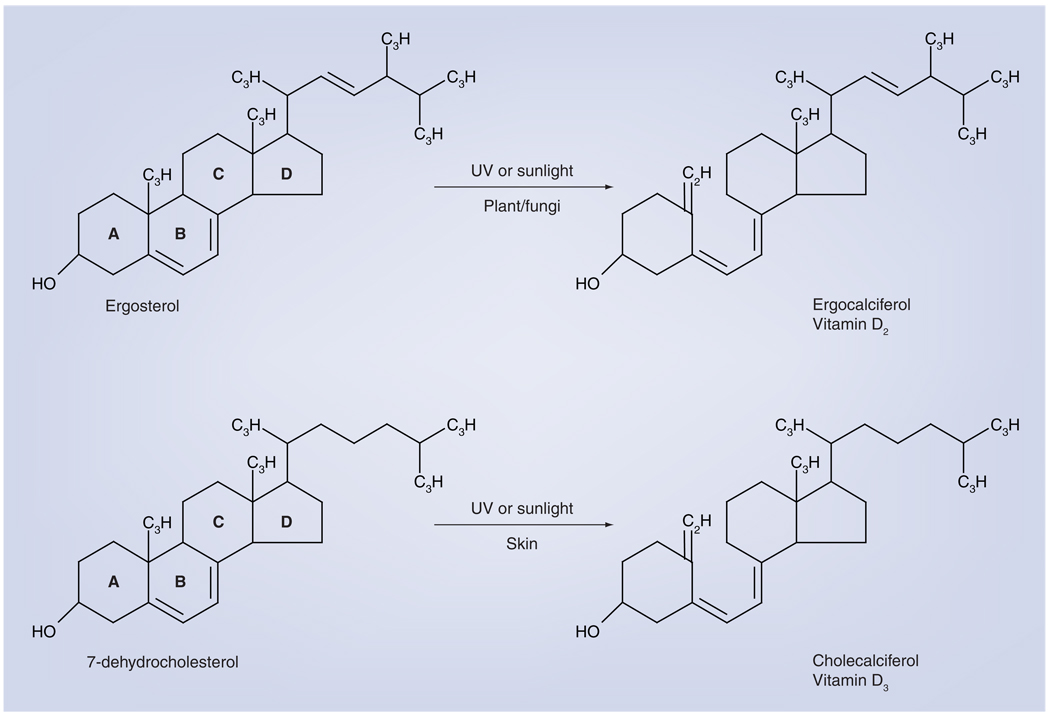
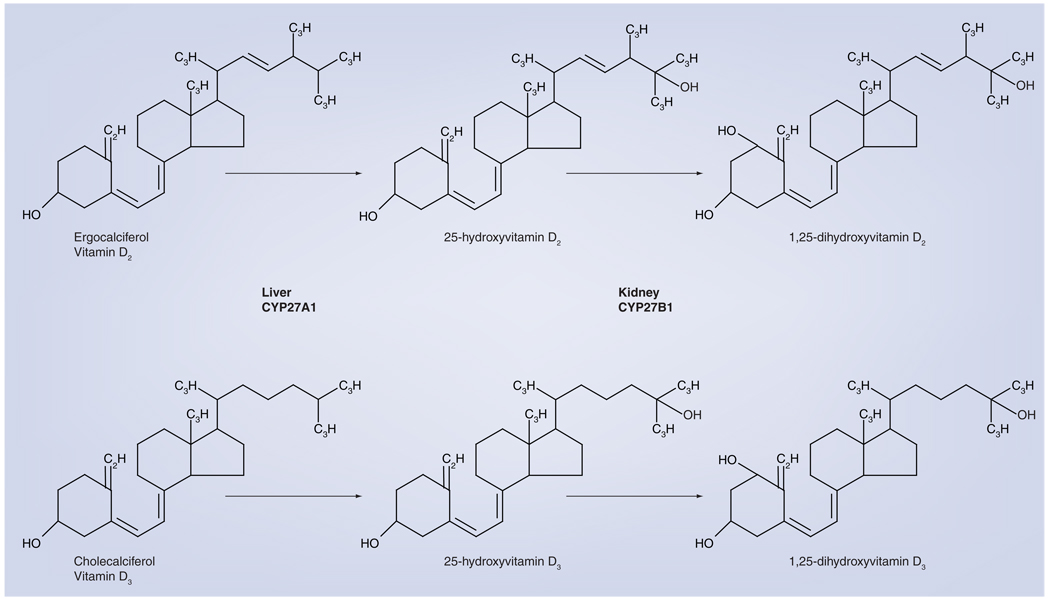
Humans can obtain vitamin D in two different forms: vitamin D3, or cholecalciferol, and vitamin D2, or ergocalciferol. The former is synthesized in the skin by exposure to UVB radiation; the latter is produced in various plant materials, yeast and fungi when they are exposed to UVB radiation (Figure 1) [1]. Humans can obtain both forms in the diet by consumption of either animal or plant products that possess them. Both forms of vitamin D are hydroxylated by the cytochrome P450 enzyme CYP27A1 in the liver to 25-hydroxyvitamin D (25[OH]D) in substrate-dependent reaction (Figure 2) [1]. The 25(OH)D circulates in the blood bound to the vitamin D-binding protein and is a reliable indicator of vitamin D status [2]. To become fully activated, the 25(OH)D is converted into 1,25-dihydroxyvitamin D (1,25[OH]2D) by the mitochondrial 1 α-hydroxylase enzyme (CYP27B1) (Figure 2). The majority of the body’s 1,25(OH)2D is synthesized in the primary renal tubules of the kidney, but synthesis also occurs in numerous extrarenal sites in cells that express CYP27B1 [1].
Figure 1. Photoproduction of vitamin D.
In plants or fungi, sunlight or artificial UVB rays cleave ergosterol in the B-ring to produce ergocalciferol or vitamin D2. In a very similar reaction in skin, 7-dehydrocholesterol is cleaved to produce cholecalciferol or vitamin D3. Both forms can be used as sources of vitamin D.
Figure 2. Conversion of vitamin D2 or vitamin D3 into active vitamin D.
Vitamin D2 or vitamin D3 are hydroxylated in the liver by the enzyme CYP27A1 into 25-hydroxyvitamin D. This form of vitamin D circulates in the blood and is used to determine the vitamin D status of individuals (deficient: ≤20 ng/ml; insufficient: 21–29 ng/ml; sufficient: ≥30 ng/ml). When required by the body, the 25-hydroxyvitamin D form is converted by the CYP27B1 enzyme into the bioactive form, 1,25-dihydroxyvitamin D, which binds to the vitamin D receptor and activates gene expression. This primarily occurs in the kidney, but also occurs locally in cells that express CYP27B1. Immune-activated macrophages produce significant amounts of CYP27B1 and 1,25-dihydroxyvitamin D.
The genomic actions of 1,25(OH)2D are modulated through the vitamin D receptor (VDR), transcription factor belonging to the steroid/hormone receptor family [3]. Target genes contain vitamin D response elements (VDREs) in their promoters, to which heterodimers of VDR and retinoid X receptors (RXRs) can bind and transactivate expression of the target genes [4]. Most dividing cell types, normal and malignant, can express VDR and respond to 1,25(OH)2D, and the VDR is expressed in at least 30 different target tissues [5,6].
Renal production of 1,25(OH)2D occurs in response to decreased levels of circulating Ca2+, which stimulates the production of parathyroid hormone (PTH). PTH induces the production of CYP27B1 by primary renal tubules. As circulating levels of 1,25(OH)2D rise, it suppresses its own production via a negative feedback loop in which the VDR binds to the CYP27B1 promoter to repress its expression. 1,25(OH)2D increases the uptake of Ca2+ by the intestine, which leads to a decrease in PTH levels. In addition, 1,25(OH)2D induces FGF-23 in osteocytes that represses PTH production. Furthermore, vitamin D induces the production of CYP24, a mitochondrial cytochrome P450 enzyme that catabolizes both 1,25(OH)2D and 25(OH)D, thus limiting its own production [7].
Extrarenal production of vitamin D occurs in bone, epithelial cells of the skin, lung and colon, parathyroid glands and immune cells, especially activated macrophages. Control of its production in macrophages differs from renal tissues. Immune activation of macrophages with Toll-like receptor (TLR) ligands or IFN-γ leads to induction of CYP27B1 and production of 1,25(OH)2D and is dependent on the availability of 25(OH)D. CYP27B1 activity is not regulated by PTH or FGF-23 in macrophages and these cells synthesize an alternative splice variant of CYP24 that leads to the production of a dominant negative-acting protein that is catalytically inactive and prevents the catabolism of 1,25(OH)2D [1,8]. Thus, unlike renal tubules, macrophages do not limit their production of 1,25(OH)2D and their continued activation may lead to accumulation of 1,25(OH)2D and contribute to human disease [8].
The importance of vitamin D and the active metabolite 1,25(OH)2D in immune function became apparent with the discovery of VDR expression in activated inflammatory cells [9,10]. Also, the VDR is expressed in most cells of the immune system [11,12]. Indeed, 1,25(OH)2D3 directly modulates T-cell proliferation and cytokine production, decreases Th1 development, inhibits Th17 development and enhances the frequency of Th2 and regulatory T-cell production [13–17]. A key mechanism in modulation of the adaptive immune system by vitamin receptor agonists involves their effects on myeloid dendritic cells (DCs) [18]. Numerous studies have demonstrated that 1,25(OH)2D impacts DC phenotype and function by downregulating expression of costimulatory molecules (CD40, CD80 and CD86) and cytokine IL-12 and upregulating IL-10 levels (recently reviewed in [18]). The overall effect is to create tolerogenic myeloid DCs, which leads to decreased Th1 cell development, promotion of CD4+ suppressor T-cell activity and enhanced recruitment of regulatory T cells via increased expression of chemokine CCL22 [19–21]. Protolerogenic, plasmacytoid DCs are negligibly affected by VDR agonists; thus, their tolerogenic potential is less likely to be modified [18]. The ability of 1,25(OH)2D to promote tolerance in DCs and T cells has prompted investigators to explore possible therapeutic treatments for a number of human autoimmune diseases [11,21–23]. Vitamin D inhibits proliferation and production of immunoglobulin and slows differentiation of B-cell precursors into plasma cells [24]. In addition to regulating their response to VDR agonists, macrophages, DCs and T cells can regulate the production and degradation of 1,25(OH)2D, which suggests an important biological role for 1,25(OH)2D in regulating innate and adaptive immunity [25–30]. Suppression of the adaptive immune system and the inflammatory action of the nuclear factor (NF)-κB pathway [31–33] by vitamin D are probably beneficial for conditions that involve autoimmunity [34]; however, it could prove detrimental for some infections [35,36].
Contrary to its suppressive effects on adaptive immunity, vitamin D has been known to be important for protecting against infection. Prior to the development of antibiotics, cod liver oil, sunlight (both sources of vitamin D) and pharmacologic doses of vitamin D were used to treat TB [37]. This fell out of favor following the development of very effective antibiotic therapy. Recently, however, interest in using pharmacologic doses of vitamin D has been rekindled by discoveries made by our group and others that 1α,25-dihydroxyvitamin D can profoundly boost the innate immune system to combat pathogenic infections in vitro. The ‘antibiotic’ effect of vitamin D appears to be mediated, in part, by the induction of the human antimicrobial peptide genes [25,38–41].
Vitamin D is critical for the regulation of both the CAMP and DEFB4 genes in both normal and transformed epithelial and hematopoietic cells [38–40,42–44]. This regulation is biologically important for the response of the innate immune system to wounds and infection [25,43]. Also, it may provide a mechanism to boost the innate immune system and counter the suppression of the adaptive immune system as described earlier.
Cathelicidins, defensins & innate immunity
Cathelicidins are synthesized as a prepropeptide consisting of an N-terminal signal peptide, a conserved prosequence (cathelin domain) and a highly variable C-terminal cationic AMP [45]. In neutrophils, proteolytic cleavage generates the mature AMP [46]. While some mammals express numerous cathelicidins, humans possess a single cathelicidin gene [47,48] called CAMP, also known as hCAP18/LL-37/FALL39 (humans) and CRAMP/CNLP/MCLP (mice). Throughout this paper the gene and mRNA is referred to as CAMP, the proprotein as cathelicidin or hCAP18 (human) or CRAMP (mouse) and the processed peptide as LL-37 (human).
The hCAP18/CRAMP protein is synthesized and secreted in significant amounts by those tissues constantly exposed to environmental microbes [49], as well as other tissues [50–53] and fluids [50,52,54–56], and is expressed by a wide array of immune cells [57–59]. Other lines of evidence similarly indicate that hCAP18/CRAMP has important functions in host defense [60–62]. Mice deficient in CRAMP are more susceptible to skin infection than wild-type mice [63], and CRAMP secretion is induced by bacteria and protects the murine urinary tract against invasive bacterial infection [64]. Decreased expression of hCAP18 is reported in human diseases whose common denominator is enhanced susceptibility to infection [65–67]. Collectively, these data strongly implicate a key role for CAMP in maintaining adequate host defenses.
The defensins are antimicrobial peptides with a characteristic β-sheet-rich fold resulting from a framework of six disulfide-linked cysteines [68]. They are expressed by leukocytes and various epithelial cells that come in contact with environmental microbes. Expression is both constitutive and inducible depending on the tissue [68]. Both cathelicidins and defensins disrupt the integrity of the bacterial cell membrane, resulting in the death of the microbe [68].
Vitamin D, TB & other infectious diseases
Since the 1840s, both environmental (i.e., sunlight) and dietary sources (cod liver oil, eggs and liver) of vitamin D have been identified as treatments for TB [37]. By the 1930s, vitamin D had been isolated from cod-liver oil and it was possible to begin using pharmacologic doses of vitamin D for treatment [37]. Effective chemotherapeutic treatments decreased the interest in using cod-liver oil, sunlight and vitamin D for TB treatment, but studies over the last two decades have demonstrated that vitamin D deficiency is a risk factor for active TB and impaired antimycobacterial activity [25,69–71].
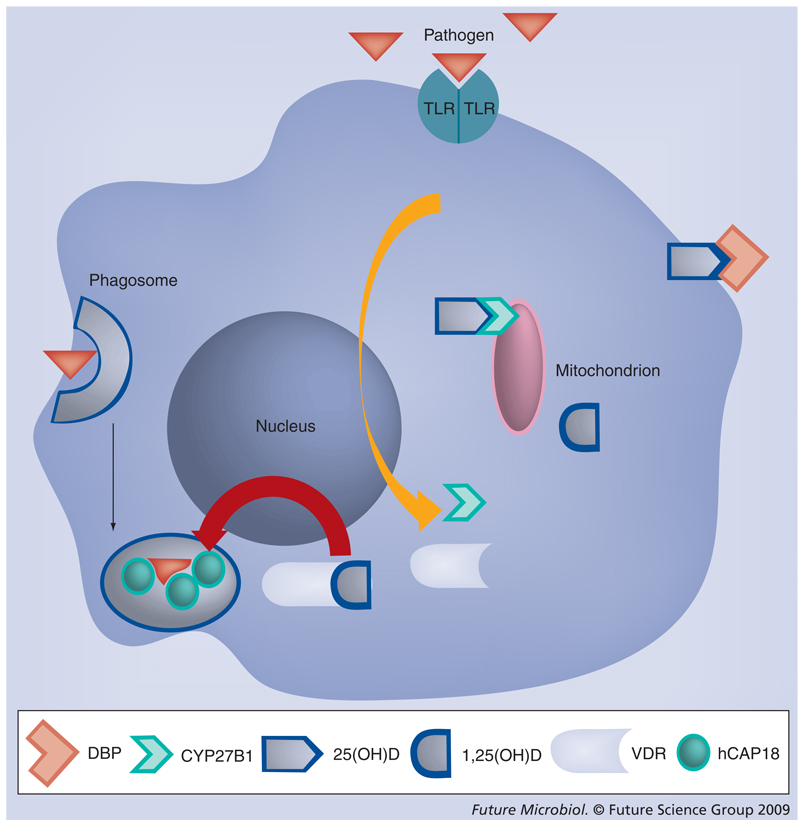
The mechanism of immune modulation that boosted antimycobacterial acitivity had remained an enigma. In the mid 1980s, vitamin D was found to boost the antimicrobial activity of human monocytes/macrophages against Mycobacterium tuberculosis [72]. Most recently, we and others discovered that vitamin D strongly upregulated antimicrobial peptide gene expression [38,39]. Subsequently, it was demonstrated in vitro that TLR2/1 signaling by a synthetic 19-kD M. tuberculosis-derived lipopeptide enhances the antimicrobial capacity of monocytes via a vitamin D and VDR-dependent pathway [25]. This involved induction of the CAMP gene and its protein [25,37,73]. In the model proposed, TLR2/1 activation of monocyte/macrophages induces the expression of CYP27B1 (25-hydroxyvitamin D-1α-hydroxylase) which, in turn, leads to production of bioactive 1,25(OH)2D from circulating inactive 25(OH)D [25]. Also, the expression of VDR is increased and in the presence of locally high levels of 1,25(OH)2D it activates the CAMP gene (Figure 3) [25].
Figure 3. Vitamin D-dependent Toll-like receptor-activation of cathelicidin gene expression.
The current model proposes that when a pathogen is detected by its respective TLR, VDR and CYP27B1 gene expression are induced. This leads to 1-α-hydroxylation of 25(OH)D, which is taken up from the blood (in a complex with the D-binding protein) and subsequent binding of 1,25(OH)2D to the VDR. The cathelicidin gene is activated and the protein (hCAP18/LL-37) is synthesized for use against the pathogen that has been engulfed in the phagosome of the macrophage.
TLR: Toll-like receptor; VDR: Vitamin D receptor.
While there is growing evidence that vitamin D boosts antimycobacterial immunity in vitro, it is essential to elucidate the mechanism for immune modulation in vivo. In a review of three randomized controlled trials and ten case series in which vitamin D had been given to patients with pulmonary TB, it was concluded that the studies to date were flawed methodologically [74]. Two small, randomized studies have suggested beneficial effects of vitamin D on treatment of TB [75,76]. Also, administration of a single oral dose of vitamin D (2.5 mg) to individuals significantly enhanced the ability of their whole blood to restrict bacillus Calmette–Guérin-lux luminescence in vitro without affecting antigen-stimulated IFN-γ responses [77]. In a larger recent double-blind, randomized, placebo-controlled trial it was found that vitamin D did not improve clinical outcome among patients with TB and no overall effect on mortality in patients with TB was observed [78]. The authors went on to conclude that it is possible that the dose used was insufficient. Indeed, both the placebo and treated groups had similar levels of vitamin D at the start of the trial and at 2 and 8 months after the start of treatment [78]. Further controlled, randomized, clinical trials need to be appropriately powered to detect modest effects and investigators should consider dose escalation, dosing frequency schedule and targeting populations that are severely deficient in vitamin D [79].
Vitamin D deficiency has been correlated with increased rates of other types of infection. Epidemiological studies demonstrate a link between vitamin D deficiency and increased rates of respiratory infections [80–83]. It has been hypothesized that epidemic influenza may be the result of vitamin D deficiency [84]. Maternal vitamin D deficiency is associated with bacterial vaginosis in the first trimester of pregnancy [85]. Low levels of vitamin D are described in HIV-infected individuals and this may have an impact on the progression of the disease [86]. Consistent with the epidemiological data for respiratory infection, activation of the vitamin D pathway in response to respiratory syncitial virus infection occurs in lung cells via TLR3 signaling, resulting in the induction of the CAMP gene [87]. Deficiency in vitamin D could impair this response by cells in the lung.
Regulation of CAMP gene expression by vitamin D is a primate-specific adaptation
We demonstrated that regulation of the CAMP gene by VDR and its ligand 1,25(OH)2 vitamin D3 is not evolutionarily conserved in mice, rats or dogs because the promoters of their CAMP genes lack a VDRE [38]. The VDRE was found to be present in an AluSx short interspersed element present in the CAMP promoter of both chimps and humans, suggesting that this immune response is a primate-specific adaptation [38]. Recently, we provided evidence for the evolutionary conservation of this regulation in humans, apes, Old World monkeys and New World monkeys, but not prosimians [88]. This demonstrates that exaptation or co-option of vitamin D-mediated gene regulation by an AluSx short interspersed element provided a novel, biologically important innate immune response that is conserved in humans and nonhuman primates, but not other mammalian species. It is a convincing example of an evolutionarily-fixed, Alu-mediated divergence in steroid hormone nuclear receptor gene regulation between humans/primates and other mammals. This evolutionary conservation provides strong evidence that the TLR2/1–vitamin D–cathelicidin pathway evolved as a biologically important immune response mechanism for protecting human and nonhuman primates against infection [88].
What is the selective advantage of placing the primate CAMP gene under the regulation of the vitamin D pathway? The human CAMP gene is not induced consistently by pro-inflammatory stimuli [38,89–93]. Additionally, infection of human macrophages with M. tuberculosis and other cell types with pathogens leads to the repression of the CAMP gene, whereas the murine CAMP gene is induced [37,94,95]. Acquisition of the VDRE by ancestral primates that likely possessed high levels of vitamin D, similar to today’s nonhuman primates [96], would have provided a pathway for induction of the CAMP gene in cells such as macrophages or epithelial barrier cells that are capable of activating the vitamin D pathway in response to infection or wounding [25,43]. The activation of the vitamin D pathway provides a way for human macrophages to prevent the suppression of the CAMP gene when activated with TLR2 or TLR4 ligands [97]. Thus, induction of the CAMP gene by 1,25(OH)2D3 provides a possible mechanism for primates to counteract pathogen-mediated suppression and modulate the immune response.
The mouse model is a powerful tool for understanding biological processes. The lack of vitamin D regulation of the cathelicidin gene makes it a poor model to study this mechanism. Our group is developing a ‘humanized’ mouse that carries a portion of the human CAMP gene locus that allows for proper levels of CAMP expression in the appropriate tissues. In addition, the mouse will lack the murine CAMP gene [63]. This mouse model will provide opportunities to study the importance of the vitamin D–cathelicidin pathway in infectious disease models while leveraging the power of crossing into other murine transgenics or knockouts.
Convergence of additional signal pathways with vitamin D modulate antimicrobial peptide gene expression
Activation of TLRs promotes the release of immunomodulatory cytokines that play a role in the innate immune response. Once such cytokine, IL-15, was shown to be required for TLR2/1 induction of cathelicidin and also found to be sufficient to activate the vitamin D pathway in macrophages [98]. The in vivo importance of IL-15 remains to be elucidated, but it may provide a mechanism to activate the vitamin D pathway in neighboring monocytes and macrophages, thus amplifying the innate immune response [98].
In conditions such as psoriasis, lesions contain significant levels of inflammatory cytokines. IL-17A is present at high levels in psoriatic lesions owing to an enrichment of Th17 cells, and LL-37 and HBD2 are present at high levels in psoriatic lesions and may contribute to the development of the disease [61,99]. Recently, it was described that the LL-37 peptide converts self-DNA and self-RNA from dying host cells into a trigger of TLR9, -7 and -8 in human DCs, thus providing new insights into the mechanism that drives the autoimmune responses in psoriasis [99,100]. In human keratinocytes it was found that IL-17A enhances expression of the CAMP gene via activation of Act1 and MEK–ERK in a process that is dependent on the presence of 1,25(OH)2D [101]. HBD2 and HBD3 have been shown to be regulated by IL-22 and -17A via JAK-STAT and NF-κB pathways [102,103]. These findings elucidate a mechanism for cytokine-mediated inflammation in psoriatic skin through increased antimicrobial peptide expression. Interestingly, vitamin D analogs are used to successfully treat psoriasis and do not exacerbate the disease. Elucidation of the effects of vitamin D analogs on the cytokine milieu of the lesion or on keratinocyte differentiation may provide clues that explain this apparent paradox. Peric et al. recently reported that topical application of vitamin D and vitamin D analogs reduced expression of IL-17A, IL-17F and IL-8 in psoriatic lesions as well as the antimicrobial peptides HBD2 and HBD3 [104]. The vitamin D analogs blocked HBD2 induction by IL-17 by inhibition of NF-κB signaling [104]. By contrast, levels of CAMP mRNA were increased in the lesions. In human primary keratinocytes in vitro, the unprocessed 18-kD form of the protein, hCAP18, was induced by vitamin D and its analogs, but the processed peptide LL-37, which plays a critical role in the development of psoriasis, was not detected by western blot analysis [104]. The authors postulated that the induction of hCAP18 may not be accompanied by an increase in the active LL-37 peptide owing to a lack of processing. It remains to be determined if hCAP18 processing into LL-37 is altered in psoriatic skin lesions treated with active vitamin D and its analogs, but the hypothesis is testable and may explain why vitamin D does not exacerbate psoriasis.
While some cytokines may boost CAMP gene expression, others may inhibit it. In atopic dermatitis patients, the expression level of CAMP is found to be relatively low when compared with psoriasis patients [61]. The reduced expression may be due to Th2-type cytokines, such as IL-4, -10 and -13, which are abundant in atopic dermatitis skin, inducing Bcl-3, a transcriptional regulator that represses CAMP gene expression [105].
The involvement of additional signaling pathways in the induction of antimicrobial peptide gene expression is especially important for induction of the DEFB4 gene. The focus on cathelicidin gene expression has been due, in large part, to its robust and easily detected expression by 1,25(OH)2D treatment alone. This was not the case for the DEFB4 gene [Gombart AF, Unpublished Data]. A recent report demonstrated in vitro that induction of the DEFB4 gene in macrophages requires TLR activation and the convergence of the IL-1β and vitamin D pathways [97].
The VDR & beyond vitamin D: other ligands
The VDR is a member of the steroid/hormone nuclear receptor superfamily that includes 48 ligand-activated transcription factors [3]. These factors bind to conserved elements in the promoters of genes as hetero- or homodimers through a zinc-finger motif and the adjacent ligand-binding domain activates or represses transcription upon binding to a small diffusible ligand. This allows nuclear receptors to function as intracellular detectors for endocrine hormones, dietary lipids and xenobiotic metabolites or compounds.
The nuclear receptor superfamily has been split into four groups based on class of ligand bound and whether DNA binding involves a hetero- or homodimer complex [106,107]. The first class is the endocrine receptors that function as homodimers and bind steroid hormones produced by endocrine tissues, and include androgen, estrogen, progesterone, mineralocorticoid and glucocorticoid. The second group forms heterodimers with RXRs and ligands include dietary lipids, xenobiotics and various cholesterol metabolites. The third group heterodimerize with RXRs and include the thryroid hormone, retinoic-acid (vitamin A) and vitamin D receptors. The fourth group are those receptors that have unknown ligands and are referred to as orphan nuclear receptors.
Interestingly, the VDR was shown to possess characteristics of both the second and third groups with the discovery of its ability to function as an intestinal secondary bile acid receptor [108]. Members of the second group include receptors for primary bile acids such as the farnesoid X receptor (FXR) and the xenobiotic receptors pregnane X receptor (PXR) and constitutive androstane receptor (CAR) [106]. Primary bile acids are major metabolites of cholesterol that are produced in the liver, excreted in the bile and subsequently reabsorbed in the intestine. Bile acids that are not reabsorbed are converted to secondary bile acids by the intestinal microflora [109]. Secondary bile acids are toxic and must undergo detoxification by the body. FXR and PXR are receptors for secondary bile acids and promote their metabolism in the liver [108,110,111]. VDR functions as a sensor for secondary bile acid lithocholic acid (LCA) and appears to be important for the detoxification of LCA in both the liver and intestine via the induction of CYP3A, a cytochrome P450 and known target gene of vitamin D, which mediates the catabolism of LCA [107–109,112,113]. The importance of these nuclear receptors in maintaining a functional detoxification system in the intestinal tract has been nicely reviewed [107].
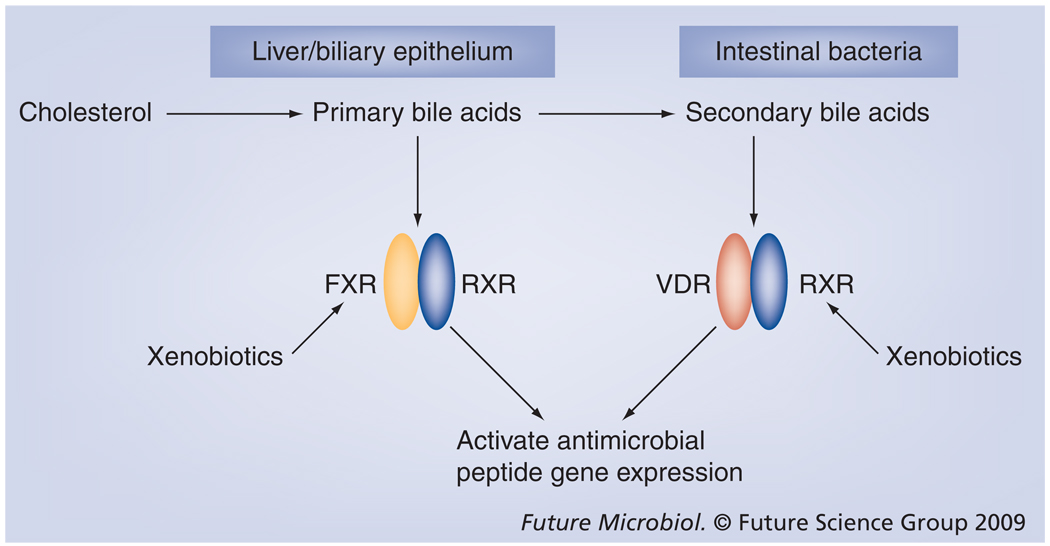
It is becoming clear that there is overlap both in the ligands that activate PXR, FXR, CAR and VDR and the genes that are regulated by them. Because vitamin D is an important immune regulator one may hypothesize that these other receptors could regulate some aspects of immunity, particularly gut immunity. Obstruction of bile flow leads to bacterial overgrowth in the small intestine, mucosal injury, barrier disruption and systemic infection. Oral administration of bile acids ameliorates the problems associated with bile-acid deficiency [114]. Recently, in studies using mice, it was demonstrated that FXR plays a critical role in preventing bacterial overgrowth and maintaining the epithelial barrier in the intestine by upregulating several genes involved in mucosal defense of the gut [115]. In addition, it was shown that bile salts may contribute to biliary tract sterility in humans by regulating the expression of the cathelicidin gene through the nuclear receptors VDR and FXR (Figure 4) [116].
Figure 4. A role for enteric receptors in regulating the innate immune system by inducing antimicrobial gene expression.
Intestinal detoxification receptors and the VDR share ligands and target genes. It has been shown that primary bile acids are capable of inducing cathelicidin gene expression in biliary epithelial cells through the FXR. Secondary bile acids that are byproducts of metabolism of primary bile acids by intestinal bacteria induce the cathelicidin gene via the VDR. The extensive crosstalk by these receptors possibly creates a system where bile acids, xenobiotic compounds and microbial byproducts can maintain critical gut immunity by inducing antimicrobial gene expression.
FXR: Farnesoid X receptor; VDR: Vitamin D receptor; RXR: Retinoid X receptor.
The accumulating evidence suggests that in the intestinal tract, the byproducts of gut microbes could be important for communicating with the epithelial cells, thus modulating the expression of the cathelicidin gene. This may be important for establishing a mucosal barrier to prevent contact of microbes and pathogens with the intestinal epithelium. This is nicely demonstrated by the ability of short-chain fatty acids such as sodium butyrate that are produced by fermentation of fiber by microbes in the colon to induce expression of the cathelicidin [92]. The vitamin D pathway has been implicated in this induction [117]. The production of secondary bile acids by microbes may modulate cathelicidin expression in the colon via the VDR. One could speculate that the selective force for placing the cathelicidin gene under the regulation of the VDR was so its expression could be regulated by both vitamin D and xenobiotic factors (Figure 4).
A recent study indicates that the VDR may act as a receptor for additional nutritional ligands, including curcumin and polyunsaturated fats such as α-linolenic acid, docosahexaenoic acid, eicosapentaenoic acid and arachidonic acid [118]. The in vivo relevance of these findings remains to be elucidated, but it is intriguing to consider that numerous nutritional compounds may modulate the expression of VDR target genes such as antimicrobial peptides.
Therapeutic applications
Increasing endogenous cathelicidin (hCAP18/LL-37) expression may offer advantages over simple administration of synthetic antimicrobial peptides; transient expression of hCAP18 protein by adenovirus was tenfold more effective than synthetic peptide in preventing bacterial growth on second-degree burns in a mouse model [119]. Also, induction of CAMP with butyrate treatment in Shigella-infected rabbits improved their outcome [120,121]. Thus, hCAP18 may aid in clearance of bacterial infection and protect against sepsis. Administration of synthetic hCAP18/LL-37 may be beneficial, but because butyrate- [120] or adenoviral vector-induced increases in endogenous hCAP18 produced markedly improved outcomes, we propose that interventions that increase endogenous hCAP18 levels may be particularly useful in the treatment of infections.
Our own research has shown that high levels of hCAP18 in the blood of hemodialysis patients at the start of their treatment was indicative of a significant decrease in 1-year mortality [122]. There was modest correlation with 1,25(OH)2D levels, but not with 25(OH)D levels [122]. In a recent study with sepsis patients, an association was found between severe illness and lower 25(OH)D, DBP and cathelicidin levels as compared with healthy controls [123]. A positive correlation between 25(OH)D and cathelicidin levels was observed in all subjects [123]. The question of whether increased supplementation of vitamin D-deficient individuals with 25(OH)D or therapeutic treatment of patients with active analogs of 1,25(OH)2D will boost plasma levels of cathelicidin remains to be seen.
A study in atopic dermatitis patients demonstrated that supplementation with 4000 international units (IU)/day of oral vitamin D for 21 days increased cathelicidin expression in the lesional skin with a mild increase in the normal skin [124]. We found that supplementation with 50,000 IU two times per week for 5 weeks had no effect on circulating levels of cathelicidin; however, it should be noted that the individuals in this study were relatively vitamin D replete and no patients were deficient. Thus, it is possible that circulating cathelicidin levels may be more dependent on vitamin D status at very low levels of serum 25(OH)D [97]. In this same study, we did demonstrate that the ability of human macrophages to induce cathelicidin expression in response to TLR activation was directly proportional to serum vitamin D status. Cathelicidin induction was enhanced in vitamin D-insufficient patients given supplementary vitamin D [97]. The studies to date would argue that it is important for individuals to have sufficient serum levels of 25(OH)D to allow cells in the body to synthesize cathelicidin when needed.
Conclusion
Deficiency in vitamin D is associated with numerous health conditions ranging from bone health to cancer, but with the discovery of antimicrobial peptide gene regulation by the vitamin D pathway a renewed interest in its impact on the immune system has ensued. It is particularly attractive to realize that adequate serum levels (>30 ng/ml) throughout life may alleviate many of the chronic ills that befall us as we age.
During the past 30 years or so, research has demonstrated the importance of vitamin D for optimal immune function, but many pieces of the puzzle are yet to be discovered and put in place. Both cathelicidin and defensin have activities beyond killing of microbes and can act as signaling molecules to activate the immune system [125,126]. Numerous studies have established that α-defensins and cathelicidin act as chemo-attractants for a variety of leukocytes, including DCs, T cells, monocytes and neutrophils [126]. In addition, these antimicrobial peptides induce the expression of numerous cytokines and chemokines [126]. This may provide another mechanism for vitamin D to exert additional effects on the adaptive immune system.
The overlap in the ligands and target genes of the VDR, FXR, PXR and CAR may reflect an important evolutionary redundancy that is critical for the detoxification and immunity of the gut. It also offers an abundance of new compounds that can be modified to produce potent ligands for the VDR. The therapeutic use of active vitamin D has been hampered by the toxic side effects of hypercalcemia. The development of synthetic analogs seeks to reduce the side effects while boosting the benefits. LCA can perform the in vivo functions of vitamin D by elevating serum calcium in rats [127] and there is an interest in developing LCA derivatives that act as selective VDR ligands without inducing hypercalcemia [128].
The therapeutic use of vitamin D to boost immunity is an exciting possibility. While it is still not clear that we can increase the levels of cathelicidin in the blood with vitamin D or its synthetic analogs, we believe that higher levels of cathelicidin in the blood will be beneficial. We are particularly interested in testing this with the ‘humanized’ mouse model that we have developed.
Executive summary
Vitamin D, vitamin D receptor & immunity
Renal production of active vitamin D is important for bone health by maintaining proper serum calcium and phosphorus levels.
Extrarenal production of active vitamin D in immune cells is important for optimal immune response.
Vitamin D modulates T-cell responses and has anti-inflammatory properties, but boosts innate immune responses by induction of the human gene for cathelicidin, CAMP.
Cathelicidins, defensins & innate immunity
Cathelicidins and defensins are small peptides with amphipathic structures that allow them to disrupt the integrity of the pathogen cell membrane, resulting in its death.
These proteins are expressed by most immune cells or those epithelial cells that are in contact with the environment.
Deficiency in these peptides results in increased susceptibility to infection.
Regulation of gene expression is not fully understood.
Vitamin D, TB & other infectious diseases
Vitamin D deficiency is a risk factor for TB and, historically, sources of vitamin D were identified as treatments for TB.
Vitamin D boosts the ability of macrophages to kill Mycobacterium tuberculosis via the vitamin D–cathelicidin pathway.
Supplementation of humans with vitamin D has shown mixed results and future randomized, placebo-controlled trials need to be appropriately powered, consider dose escalation and frequency, and target severely deficient populations.
Vitamin D deficiency has been correlated with increased respiratory tract infections, influenza and bacterial vaginosis. Cause and effect is unknown.
Regulation of CAMP gene expression by vitamin D is a primate-specific adaptation
A human/primate-specific transposable element provided a vitamin D receptor binding site in the promoter of the cathelicidin gene, thus conferring regulation of the cathelicidin gene by vitamin D to humans, apes, Old World and New World monkeys.
This regulation is lacking in other mammals.
This provides a possible mechanism for primates to counteract pathogen-mediated suppression and modulate the immune response.
Our group is generating a humanized mouse model for understanding the biological importance of this regulation.
Convergence of additional signal pathways with vitamin D modulate antimicrobial peptide gene expression
Activation of the cathelicidin gene by vitamin D is potentially modulated by cytokine signaling pathways.
Vitamin D alone is not sufficient for induction of the human defensin β2, but requires TLR activation and IL-1β.
The importance of additional signals needs to be examined.
The vitamin D receptor & beyond vitamin D: other ligands
The vitamin D receptor (VDR) functions as a receptor for both vitamin D and secondary bile acids.
The vitamin D receptor activates important detoxification genes that are targets of xenobiotic receptors.
Xenobiotic receptors potentially activate VDR target genes and this cross-talk may play an important role in gut immunity.
Evolutionary selection to place the cathelicidin gene under the regulation of the VDR potentially allows both endocrine and xenobiotic factors to regulate its expression.
Therapeutic applications
The cathelicidin protein is present in the blood at significant levels and correlates with reduced mortality from infectious causes in hemodialysis patients.
Severe sepsis patients have low vitamin D and cathelicidin levels.
Supplementation with vitamin D increases cathelicidin expression in the lesions of atopic dermatitis patients and increases activated macrophage production of cathelicidin.
The therapeutic potential of vitamin D and synthetic analogs against infection remain unexplored.
Future perspective
The future for the sunshine vitamin looks bright. Creative approaches involving animal models, human studies and genomic approaches to identify new targets of the VDR promise to shed even more light on the mechanisms by which vitamin D mediates its beneficial effects. Because vitamin D-mediated regulation of the antimicrobial peptide genes is a human/primate-specific process, it will be important to realize that animal models may not always reflect the human situation. This will require the generation of creative animal models and verification in human systems.
Many epithelial tissues such as the oral mucosa, intestinal tract, skin, urinary tract and reproductive organs are constantly exposed to the environment and the importance of the vitamin D–cathelicidin pathway in providing protection against pathogens in these tissues is already a major focus. Additional studies should reveal just how important vitamin D is in barrier defense of the body.
Considering that most people have insufficient levels of vitamin D and that nearly 1 billion people worldwide are deficient [129], properly designed supplementation studies in humans will be important for determining the benefits from raising serum levels of vitamin D on immune system function. It will be particularly interesting to determine if sufficient vitamin D levels will aid in treating patients with TB and HIV infection. These are classically deficient populations and vitamin D supplementation could be a potentially cheap adjuvant therapy for these conditions and is particularly attractive for impoverished countries where these diseases are rampant.
Acknowledgements
The author would like to thank members of the laboratory for their useful discussions and apologizes to those authors whose interesting data could not be included owing to space constraints.
Footnotes
Financial & competing interests disclosure
This work was supported by grant 5R01AI065604–04 to the author from the National Institute of Allergy and Infectious Diseases at the NIH. The author has received a patent covering the use of vitamin D and its analogs to induce the cathelicidin gene. The author has no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Bibliography
Papers of special note have been highlighted as:
▪ of interest
▪▪ of considerable interest
- 1.Bikle D. Nonclassic actions of vitamin D. J. Clin. Endocrinol. Metab. 2009;94(1):26–34. doi: 10.1210/jc.2008-1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Holick MF, Chen TC. Vitamin D deficiency: a worldwide problem with health consequences. Am. J. Clin. Nutr. 2008;87(4):1080S–1086S. doi: 10.1093/ajcn/87.4.1080S. [DOI] [PubMed] [Google Scholar]
- 3. Mangelsdorf DJ, Thummel C, Beato M, et al. The nuclear receptor superfamily: the second decade. Cell. 1995;83(6):835–839. doi: 10.1016/0092-8674(95)90199-x. ▪ Excellent review on nuclear receptors.
- 4.Christakos S, Raval-Pandya M, Wernyj RP, Yang W. Genomic mechanisms involved in the pleiotropic actions of 1,25-dihydroxyvitamin D3. Biochem. J. 1996;316(Pt 2):361–371. doi: 10.1042/bj3160361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bouillon R, Garmyn M, Verstuyf A, Segaert S, Casteels K, Mathieu C. Paracrine role for calcitriol in the immune system and skin creates new therapeutic possibilities for vitamin D analogs. Eur. J. Endocrinol. 1995;133(1):7–16. doi: 10.1530/eje.0.1330007. [DOI] [PubMed] [Google Scholar]
- 6.James SY, Williams MA, Newland AC, Colston KW. Leukemia cell differentiation: cellular and molecular interactions of retinoids and vitamin D. Gen. Pharmacol. 1999;32(1):143–154. doi: 10.1016/s0306-3623(98)00098-6. [DOI] [PubMed] [Google Scholar]
- 7.Zierold C, Darwish HM, Deluca HF. Two vitamin D response elements function in the rat 1,25-dihydroxyvitamin D 24-hydroxylase promoter. J. Biol. Chem. 1995;270(4):1675–1678. doi: 10.1074/jbc.270.4.1675. [DOI] [PubMed] [Google Scholar]
- 8.Ren S, Nguyen L, Wu S, Encinas C, Adams JS, Hewison M. Alternative splicing of vitamin D-24-hydroxylase: a novel mechanism for the regulation of extrarenal 1,25-dihydroxyvitamin D synthesis. J. Biol. Chem. 2005;280(21):20604–20611. doi: 10.1074/jbc.M414522200. [DOI] [PubMed] [Google Scholar]
- 9.Provvedini D, Tsoukas C, Deftos L, Manolagas S. 1,25-dihydroxyvitamin D3 receptors in human leukocytes. Science. 1983;221(4616):1181–1183. doi: 10.1126/science.6310748. [DOI] [PubMed] [Google Scholar]
- 10.Bhalla AK, Amento EP, Clemens TL, Holick MF, Krane SM. Specific high-affinity receptors for 1,25-dihydroxyvitamin D3 in human peripheral blood mononuclear cells: presence in monocytes and induction in T lymphocytes following activation. J. Clin. Endocrinol. Metab. 1983;57(6):1308–1310. doi: 10.1210/jcem-57-6-1308. [DOI] [PubMed] [Google Scholar]
- 11.Deluca HF, Cantorna MT. Vitamin D: its role and uses in immunology. FASEB J. 2001;15(14):2579–2585. doi: 10.1096/fj.01-0433rev. [DOI] [PubMed] [Google Scholar]
- 12.Adorini L, Giarratana N, Penna G. Pharmacological induction of tolerogenic dendritic cells and regulatory T cells. Semin. Immunol. 2004;16(2):127–134. doi: 10.1016/j.smim.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 13.Lemire JM, Archer DC, Beck L, Spiegelberg HL. Immunosuppressive actions of 1,25-dihydroxyvitamin D3: preferential inhibition of Th1 functions. J. Nutr. 1995;125 Suppl. 6:1704S–1708S. doi: 10.1093/jn/125.suppl_6.1704S. [DOI] [PubMed] [Google Scholar]
- 14.Boonstra A, Barrat FJ, Crain C, Heath VL, Savelkoul HF, O’Garra A. 1α,25-dihydroxyvitamin D3 has a direct effect on naive CD4+ T cells to enhance the development of Th2 cells. J. Immunol. 2001;167(9):4974–4980. doi: 10.4049/jimmunol.167.9.4974. [DOI] [PubMed] [Google Scholar]
- 15.Penna G, Adorini L. 1 α,25-dihydroxyvitamin D3 inhibits differentiation, maturation, activation, and survival of dendritic cells leading to impaired alloreactive T cell activation. J. Immunol. 2000;164(5):2405–2411. doi: 10.4049/jimmunol.164.5.2405. [DOI] [PubMed] [Google Scholar]
- 16.Staeva-Vieira TP, Freedman LP. 1,25-dihydroxyvitamin D3 inhibits IFN-γ and IL-4 levels during in vitro polarization of primary murine CD4+ T cells. J. Immunol. 2002;168(3):1181–1189. doi: 10.4049/jimmunol.168.3.1181. [DOI] [PubMed] [Google Scholar]
- 17.Daniel C, Sartory NA, Zahn N, Radeke HH, Stein JM. Immune modulatory treatment of trinitrobenzene sulfonic acid colitis with calcitriol is associated with a change of a T helper (Th) 1/Th17 to a Th2 and regulatory T cell profile. J. Pharmacol. Exp. Ther. 2008;324(1):23–33. doi: 10.1124/jpet.107.127209. [DOI] [PubMed] [Google Scholar]
- 18.Adorini L, Penna G. Dendritic cell tolerogenicity in immunomodulation by vitamin D receptor agonists. Hum. Immunol. 2009 doi: 10.1016/j.humimm.2009.01.016. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 19.Adorini L, Penna G. Dendritic cell tolerogenicity: a key mechanism in immunomodulation by vitamin D receptor agonists. Hum. Immunol. 2009;70(5):345–352. doi: 10.1016/j.humimm.2009.01.016. [DOI] [PubMed] [Google Scholar]
- 20.Adorini L, Penna G, Giarratana N, et al. Dendritic cells as key targets for immunomodulation by vitamin D receptor ligands. J. Steroid Biochem. Mol. Biol. 2004;89–90(1–5):437–441. doi: 10.1016/j.jsbmb.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 21.Adorini L. Intervention in autoimmunity: the potential of vitamin D receptor agonists. Cell Immunol. 2005;233(2):115–124. doi: 10.1016/j.cellimm.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 22.Mathieu C, Adorini L. The coming of age of 1,25-dihydroxyvitamin D(3) analogs as immunomodulatory agents. Trends Mol. Med. 2002;8(4):174–179. doi: 10.1016/s1471-4914(02)02294-3. [DOI] [PubMed] [Google Scholar]
- 23.Adorini L, Penna G. Control of autoimmune diseases by the vitamin D endocrine system. Nat. Clin. Pract. Rheumatol. 2008;4(8):404–412. doi: 10.1038/ncprheum0855. [DOI] [PubMed] [Google Scholar]
- 24.Chen S, Sims GP, Chen XX, Gu YY, Lipsky PE. Modulatory effects of 1,25-dihydroxyvitamin D3 on human B cell differentiation. J. Immunol. 2007;179(3):1634–1647. doi: 10.4049/jimmunol.179.3.1634. [DOI] [PubMed] [Google Scholar]
- 25. Liu PT, Stenger S, Li H, et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006;311(5768):1770–1773. doi: 10.1126/science.1123933. ▪▪ Demonstrated vitamin D-dependent activation of cathelicidin by Toll-like receptor signaling and established importance of vitamin D induction of cathelicidin in response to Mycobacterium tuberculosis.
- 26.Overbergh L, Decallonne B, Waer M, et al. 1α,25-dihydroxyvitamin D3 induces an autoantigen-specific T-helper 1/T-helper 2 immune shift in NOD mice immunized with GAD65 (p524-543) Diabetes. 2000;49(8):1301–1307. doi: 10.2337/diabetes.49.8.1301. [DOI] [PubMed] [Google Scholar]
- 27.Koeffler HP, Reichel H, Bishop JE, Norman AW. γ-interferon stimulates production of 1,25-dihydroxyvitamin D3 by normal human macrophages. Biochem. Biophys. Res. Commun. 1985;127(2):596–603. doi: 10.1016/s0006-291x(85)80202-3. [DOI] [PubMed] [Google Scholar]
- 28.Hewison M, Kantorovich V, Liker HR, et al. Vitamin D-mediated hypercalcemia in lymphoma: evidence for hormone production by tumor-adjacent macrophages. J. Bone Miner. Res. 2003;18(3):579–582. doi: 10.1359/jbmr.2003.18.3.579. [DOI] [PubMed] [Google Scholar]
- 29.Cadranel J, Hance AJ, Milleron B, Paillard F, Akoun GM, Garabedian M. Vitamin D metabolism in tuberculosis. Production of 1,25(OH)2D3 by cells recovered by bronchoalveolar lavage and the role of this metabolite in calcium homeostasis. Am. Rev. Respir. Dis. 1988;138(4):984–989. doi: 10.1164/ajrccm/138.4.984. [DOI] [PubMed] [Google Scholar]
- 30.Reichel H, Koeffler HP, Norman AW. Synthesis in vitro of 1,25-dihydroxyvitamin D3 and 24,25-dihydroxyvitamin D3 by interferon-γ-stimulated normal human bone marrow and alveolar macrophages. J. Biol. Chem. 1987;262(23):10931–10937. [PubMed] [Google Scholar]
- 31.Yu XP, Bellido T, Manolagas SC. Down-regulation of NF-κb protein levels in activated human lymphocytes by 1,25-dihydroxyvitamin D3. Proc. Natl Acad. Sci. USA. 1995;92(24):10990–10994. doi: 10.1073/pnas.92.24.10990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Harant H, Andrew PJ, Reddy GS, Foglar E, Lindley IJ. 1α,25-dihydroxyvitamin D3 and a variety of its natural metabolites transcriptionally repress nuclear-factor-κb-mediated interleukin-8 gene expression. Eur. J. Biochem. 1997;250(1):63–71. doi: 10.1111/j.1432-1033.1997.00063.x. [DOI] [PubMed] [Google Scholar]
- 33.Sun J, Kong J, Duan Y, et al. Increased NF-κb activity in fibroblasts lacking the vitamin D receptor. Am. J. Physiol. Endocrinol. Metab. 2006;291(2):E315–E322. doi: 10.1152/ajpendo.00590.2005. [DOI] [PubMed] [Google Scholar]
- 34.Van Etten E, Mathieu C. Immunoregulation by 1,25-dihydroxyvitamin D3: basic concepts. J. Steroid Biochem. Mol. Biol. 2005;97(1–2):93–101. doi: 10.1016/j.jsbmb.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 35.Rajapakse R, Mousli M, Pfaff AW, et al. 1,25-dihydroxyvitamin D3 induces splenocyte apoptosis and enhances BALB/C mice sensitivity to toxoplasmosis. J. Steroid Biochem. Mol. Biol. 2005;96(2):179–185. doi: 10.1016/j.jsbmb.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 36.Ehrchen J, Helming L, Varga G, et al. Vitamin D receptor signaling contributes to susceptibility to infection with Leishmania major. FASEB J. 2007;21(12):3208–3218. doi: 10.1096/fj.06-7261com. [DOI] [PubMed] [Google Scholar]
- 37. Martineau AR, Wilkinson KA, Newton SM, et al. IFN-γ- and TNF-independent vitamin D-inducible human suppression of mycobacteria: the role of cathelicidin LL-37. J. Immunol. 2007;178(11):7190–7198. doi: 10.4049/jimmunol.178.11.7190. ▪ Provided further evidence that induction of cathelicidin is important for suppression of M. tuberculosis growth.
- 38. Gombart AF, Borregaard N, Koeffler HP. Human cathelicidin antimicrobial peptide (CAMP) gene is a direct target of the vitamin D receptor and is strongly up-regulated in myeloid cells by 1,25-dihydroxyvitamin D3. FASEB J. 2005;19(9):1067–1077. doi: 10.1096/fj.04-3284com. ▪▪ One of the first papers to demonstrate induction of cathelicidin by vitamin D particularly in macrophages.
- 39. Wang TT, Nestel FP, Bourdeau V, et al. Cutting edge: 1,25-dihydroxyvitamin D3 is a direct inducer of antimicrobial peptide gene expression. J. Immunol. 2004;173(5):2909–2912. doi: 10.4049/jimmunol.173.5.2909. ▪ First report of vitamin D inducing cathelicidin and defensin β2/DEFB4.
- 40. Weber G, Heilborn JD, Chamorro Jimenez CI, Hammarsjo A, Torma H, Stahle M. Vitamin D induces the antimicrobial protein hCAP18 in human skin. J. Invest. Dermatol. 2005;124(5):1080–1082. doi: 10.1111/j.0022-202X.2005.23687.x. ▪▪ First report showing that topical application of vitamin D induces cathelicidin in human skin.
- 41.Zasloff M. Fighting infections with vitamin D. Nat. Med. 2006;12(4):388–390. doi: 10.1038/nm0406-388. [DOI] [PubMed] [Google Scholar]
- 42.Gombart AF, O’Kelly J, Saito T, Koeffler HP. Regulation of the CAMP gene by 1,25(OH)2D3 in various tissues. J. Steroid Biochem. Mol. Biol. 2007;103(3–5):552–557. doi: 10.1016/j.jsbmb.2006.12.095. [DOI] [PubMed] [Google Scholar]
- 43. Schauber J, Dorschner RA, Coda AB, et al. Injury enhances TLR2 function and antimicrobial peptide expression through a vitamin D-dependent mechanism. J. Clin. Invest. 2007;117(3):803–811. doi: 10.1172/JCI30142. ▪▪ Demonstrates that Toll-like receptor induction of cathelicidin in wounded skin requires a vitamin D pathway.
- 44. Liu PT, Schenk M, Walker VP, et al. Convergence of IL-1β and VDR activation pathways in human TLR2/1-induced antimicrobial responses. PLoS One. 2009;4(6):e5810. doi: 10.1371/journal.pone.0005810. ▪▪ Demonstrates the importance of convergent signaling pathways to induce defensin β2/DEFB4 by the vitamin D pathway.
- 45.Zanetti M, Gennaro R, Romeo D. Cathelicidins: a novel protein family with a common proregion and a variable C-terminal antimicrobial domain. FEBS Lett. 1995;374(1):1–5. doi: 10.1016/0014-5793(95)01050-o. [DOI] [PubMed] [Google Scholar]
- 46.Sorensen OE, Follin P, Johnsen AH, et al. Human cathelicidin, hCAP-18, is processed to the antimicrobial peptide LL-37 by extracellular cleavage with proteinase 3. Blood. 2001;97(12):3951–3959. doi: 10.1182/blood.v97.12.3951. [DOI] [PubMed] [Google Scholar]
- 47.Lehrer RI, Ganz T. Cathelicidins: a family of endogenous antimicrobial peptides. Curr. Opin. Hematol. 2002;9(1):18–22. doi: 10.1097/00062752-200201000-00004. [DOI] [PubMed] [Google Scholar]
- 48.Zanetti M, Gennaro R, Scocchi M, Skerlavaj B. Structure and biology of cathelicidins. Adv. Exp. Med. Biol. 2000;479:203–218. doi: 10.1007/0-306-46831-X_17. [DOI] [PubMed] [Google Scholar]
- 49.Frohm Nilsson M, Sandstedt B, Sorensen O, Weber G, Borregaard N, Stahle-Backdahl M. The human cationic antimicrobial protein (hCAP18), a peptide antibiotic, is widely expressed in human squamous epithelia and colocalizes with interleukin-6. Infect. Immun. 1999;67(5):2561–2566. doi: 10.1128/iai.67.5.2561-2566.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bals R, Wang X, Zasloff M, Wilson JM. The peptide antibiotic LL-37/hCAP-18 is expressed in epithelia of the human lung where it has broad antimicrobial activity at the airway surface. Proc. Natl Acad. Sci. USA. 1998;95(16):9541–9546. doi: 10.1073/pnas.95.16.9541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Murakami M, Ohtake T, Dorschner RA, Gallo RL. Cathelicidin antimicrobial peptides are expressed in salivary glands and saliva. J. Dent. Res. 2002;81(12):845–850. doi: 10.1177/154405910208101210. [DOI] [PubMed] [Google Scholar]
- 52.Murakami M, Ohtake T, Dorschner RA, Schittek B, Garbe C, Gallo RL. Cathelicidin anti-microbial peptide expression in sweat, an innate defense system for the skin. J. Invest. Dermatol. 2002;119(5):1090–1095. doi: 10.1046/j.1523-1747.2002.19507.x. [DOI] [PubMed] [Google Scholar]
- 53.Agerberth B, Gunne H, Odeberg J, Kogner P, Boman HG, Gudmundsson GH. Fall-39, a putative human peptide antibiotic, is cysteine-free and expressed in bone marrow and testis. Proc. Natl Acad. Sci. USA. 1995;92(1):195–199. doi: 10.1073/pnas.92.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Malm J, Sorensen O, Persson T, et al. The human cationic antimicrobial protein (hCAP-18) is expressed in the epithelium of human epididymis, is present in seminal plasma at high concentrations, and is attached to spermatozoa. Infect. Immun. 2000;68(7):4297–4302. doi: 10.1128/iai.68.7.4297-4302.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hammami-Hamza S, Doussau M, Bernard J, et al. Cloning and sequencing of SOB3, a human gene coding for a sperm protein homologous to an antimicrobial protein and potentially involved in zona pellucida binding. Mol. Hum. Reprod. 2001;7(7):625–632. doi: 10.1093/molehr/7.7.625. [DOI] [PubMed] [Google Scholar]
- 56.Andersson E, Sorensen OE, Frohm B, Borregaard N, Egesten A, Malm J. Isolation of human cationic antimicrobial protein-18 from seminal plasma and its association with prostasomes. Hum. Reprod. 2002;17(10):2529–2534. doi: 10.1093/humrep/17.10.2529. [DOI] [PubMed] [Google Scholar]
- 57.Agerberth B, Charo J, Werr J, et al. The human antimicrobial and chemotactic peptides LL-37 and α-defensins are expressed by specific lymphocyte and monocyte populations. Blood. 2000;96(9):3086–3093. [PubMed] [Google Scholar]
- 58.Di Nardo A, Vitiello A, Gallo RL. Cutting edge: mast cell antimicrobial activity is mediated by expression of cathelicidin antimicrobial peptide. J. Immunol. 2003;170(5):2274–2278. doi: 10.4049/jimmunol.170.5.2274. [DOI] [PubMed] [Google Scholar]
- 59.Sorensen O, Arnljots K, Cowland JB, Bainton DF, Borregaard N. The human antibacterial cathelicidin, hCAP-18, is synthesized in myelocytes and metamyelocytes and localized to specific granules in neutrophils. Blood. 1997;90(7):2796–2803. [PubMed] [Google Scholar]
- 60.Dorschner RA, Pestonjamasp VK, Tamakuwala S, et al. Cutaneous injury induces the release of cathelicidin antimicrobial peptides active against group A Streptococcus. J. Invest. Dermatol. 2001;117(1):91–97. doi: 10.1046/j.1523-1747.2001.01340.x. [DOI] [PubMed] [Google Scholar]
- 61.Ong PY, Ohtake T, Brandt C, et al. Endogenous antimicrobial peptides and skin infections in atopic dermatitis. N. Engl. J. Med. 2002;347(15):1151–1160. doi: 10.1056/NEJMoa021481. [DOI] [PubMed] [Google Scholar]
- 62.Frohm M, Agerberth B, Ahangari G, et al. The expression of the gene coding for the antibacterial peptide LL-37 is induced in human keratinocytes during inflammatory disorders. J. Biol. Chem. 1997;272(24):15258–15263. doi: 10.1074/jbc.272.24.15258. [DOI] [PubMed] [Google Scholar]
- 63. Nizet V, Ohtake T, Lauth X, et al. Innate antimicrobial peptide protects the skin from invasive bacterial infection. Nature. 2001;414(6862):454–457. doi: 10.1038/35106587. ▪▪ Development of cathelicidin knockout mouse and demonstration of its susceptibility to skin infection.
- 64.Chromek M, Slamova Z, Bergman P, et al. The antimicrobial peptide cathelicidin protects the urinary tract against invasive bacterial infection. Nat. Med. 2006;12(6):636–641. doi: 10.1038/nm1407. [DOI] [PubMed] [Google Scholar]
- 65.Putsep K, Carlsson G, Boman HG, Andersson M. Deficiency of antibacterial peptides in patients with morbus Kostmann: an observation study. Lancet. 2002;360(9340):1144–1149. doi: 10.1016/S0140-6736(02)11201-3. [DOI] [PubMed] [Google Scholar]
- 66.Gombart AF, Koeffler HP. Neutrophil specific granule deficiency and mutations in the gene encoding transcription factor c/ebp(ε) Curr. Opin. Hematol. 2002;9(1):36–42. doi: 10.1097/00062752-200201000-00007. [DOI] [PubMed] [Google Scholar]
- 67.Yang YH, Zheng GG, Li G, Zhang B, Song YH, Wu KF. Expression of LL-37/hCAP-18 gene in human leukemia cells. Leuk. Res. 2003;27(10):947–950. doi: 10.1016/s0145-2126(03)00020-1. [DOI] [PubMed] [Google Scholar]
- 68.Ganz T. Defensins: antimicrobial peptides of vertebrates. C. R. Biol. 2004;327(6):539–549. doi: 10.1016/j.crvi.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 69.Davies PD. A possible link between vitamin D deficiency and impaired host defence to Mycobacterium tuberculosis. Tubercle. 1985;66(4):301–306. doi: 10.1016/0041-3879(85)90068-6. [DOI] [PubMed] [Google Scholar]
- 70.Wilkinson RJ, Llewelyn M, Toossi Z, et al. Influence of vitamin D deficiency and vitamin D receptor polymorphisms on tuberculosis among Gujarati Asians in West London: a case–control study. Lancet. 2000;355(9204):618–621. doi: 10.1016/S0140-6736(99)02301-6. [DOI] [PubMed] [Google Scholar]
- 71.Nnoaham KE, Clarke A. Low serum vitamin D levels and tuberculosis: a systematic review and meta-analysis. Int. J. Epidemiol. 2008;37(1):113–119. doi: 10.1093/ije/dym247. [DOI] [PubMed] [Google Scholar]
- 72.Rook GA, Steele J, Fraher L, et al. Vitamin D3, γ-interferon, and control of proliferation of Mycobacterium tuberculosis by human monocytes. Immunology. 1986;57(1):159–163. [PMC free article] [PubMed] [Google Scholar]
- 73. Liu PT, Stenger S, Tang DH, Modlin RL. Cutting edge: vitamin D-mediated human antimicrobial activity against Mycobacterium tuberculosis is dependent on the induction of cathelicidin. J. Immunol. 2007;179(4):2060–2063. doi: 10.4049/jimmunol.179.4.2060. ▪ Uses siRNA approaches to demonstrate that antimycobacterial activity induced by vitamin D is due, in part, to cathelicidin expression.
- 74.Martineau AR, Honecker FU, Wilkinson RJ, Griffiths CJ. Vitamin D in the treatment of pulmonary tuberculosis. J. Steroid Biochem. Mol. Biol. 2007;103(3–5):793–798. doi: 10.1016/j.jsbmb.2006.12.052. [DOI] [PubMed] [Google Scholar]
- 75.Nursyam EW, Amin Z, Rumende CM. The effect of vitamin D as supplementary treatment in patients with moderately advanced pulmonary tuberculous lesion. Acta Med. Indones. 2006;38(1):3–5. [PubMed] [Google Scholar]
- 76.Morcos MM, Gabr AA, Samuel S, et al. Vitamin D administration to tuberculous children and its value. Boll. Chim. Farm. 1998;137(5):157–164. [PubMed] [Google Scholar]
- 77.Martineau AR, Wilkinson RJ, Wilkinson KA, et al. A single dose of vitamin D enhances immunity to mycobacteria. Am. J. Respir. Crit. Care Med. 2007;176(2):208–213. doi: 10.1164/rccm.200701-007OC. [DOI] [PubMed] [Google Scholar]
- 78.Wejse C, Gomes VF, Rabna P, et al. Vitamin D as supplementary treatment for tuberculosis: a double-blind, randomized, placebo-controlled trial. Am. J. Respir. Crit. Care Med. 2009;179(9):843–850. doi: 10.1164/rccm.200804-567OC. [DOI] [PubMed] [Google Scholar]
- 79.Martineau AR, Nanzer AM, Satkunam KR, et al. Influence of a single oral dose of vitamin D(2) on serum 25-hydroxyvitamin D concentrations in tuberculosis patients. Int. J. Tuberc. Lung Dis. 2009;13(1):119–125. [PubMed] [Google Scholar]
- 80.Laaksi I, Ruohola JP, Tuohimaa P, et al. An association of serum vitamin D concentrations < 40 nmol/l with acute respiratory tract infection in young Finnish men. Am. J. Clin. Nutr. 2007;86(3):714–717. doi: 10.1093/ajcn/86.3.714. [DOI] [PubMed] [Google Scholar]
- 81.Karatekin G, Kaya A, Salihoglu O, Balci H, Nuhoglu A. Association of subclinical vitamin D deficiency in newborns with acute lower respiratory infection and their mothers. Eur. J. Clin. Nutr. 2009;63(4):473–477. doi: 10.1038/sj.ejcn.1602960. [DOI] [PubMed] [Google Scholar]
- 82.Walker VP, Modlin RL. The vitamin D connection to pediatric infections and immune function. Pediatr. Res. 2009 doi: 10.1203/PDR.0b013e31819dba91. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ginde AA, Mansbach JM, Camargo CA., Jr Vitamin D, respiratory infections, and asthma. Curr. Allergy Asthma Rep. 2009;9(1):81–87. doi: 10.1007/s11882-009-0012-7. [DOI] [PubMed] [Google Scholar]
- 84.Cannell JJ, Vieth R, Umhau JC, et al. Epidemic influenza and vitamin D. Epidemiol. Infect. 2006;134(6):1129–1140. doi: 10.1017/S0950268806007175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bodnar LM, Krohn MA, Simhan HN. Maternal vitamin D deficiency is associated with bacterial vaginosis in the first trimester of pregnancy. J. Nutr. 2009;139(6):1157–1161. doi: 10.3945/jn.108.103168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Villamor E. A potential role for vitamin D on HIV infection? Nutr. Rev. 2006;64(5 Pt 1):226–233. doi: 10.1301/nr.2006.may.226-233. [DOI] [PubMed] [Google Scholar]
- 87.Hansdottir S, Monick MM, Hinde SL, Lovan N, Look DC, Hunninghake GW. Respiratory epithelial cells convert inactive vitamin D to its active form: potential effects on host defense. J. Immunol. 2008;181(10):7090–7099. doi: 10.4049/jimmunol.181.10.7090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Gombart AF, Saito T, Koeffler HP. Exapation of an ancient ALU short interspersed element provides a highly conserved vitamin D-mediated innate immune response in humans and primates. BMC Genomics. 2009;10(1):321. doi: 10.1186/1471-2164-10-321. ▪▪ Demonstrated that vitamin D regulation of cathelicidin is human/primate specific and conserved during for 50–60 million years of primate evolution.
- 89.Hase K, Eckmann L, Leopard JD, Varki N, Kagnoff MF. Cell differentiation is a key determinant of cathelicidin LL-37/human cationic antimicrobial protein 18 expression by human colon epithelium. Infect. Immun. 2002;70(2):953–963. doi: 10.1128/iai.70.2.953-963.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Erdag G, Morgan JR. Interleukin-1α and interleukin-6 enhance the antibacterial properties of cultured composite keratinocyte grafts. Ann. Surg. 2002;235(1):113–124. doi: 10.1097/00000658-200201000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sorensen OE, Cowland JB, Theilgaard-Monch K, Liu L, Ganz T, Borregaard N. Wound healing and expression of antimicrobial peptides/polypeptides in human keratinocytes, a consequence of common growth factors. J. Immunol. 2003;170(11):5583–5589. doi: 10.4049/jimmunol.170.11.5583. [DOI] [PubMed] [Google Scholar]
- 92.Schauber J, Svanholm C, Termen S, et al. Expression of the cathelicidin LL-37 is modulated by short chain fatty acids in colonocytes: relevance of signalling pathways. Gut. 2003;52(5):735–741. doi: 10.1136/gut.52.5.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Schauber J, Dorschner RA, Yamasaki K, Brouha B, Gallo RL. Control of the innate epithelial antimicrobial response is cell-type specific and dependent on relevant microenvironmental stimuli. Immunology. 2006;118(4):509–519. doi: 10.1111/j.1365-2567.2006.02399.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Islam D, Bandholtz L, Nilsson J, et al. Downregulation of bactericidal peptides in enteric infections: a novel immune escape mechanism with bacterial DNA as a potential regulator. Nat. Med. 2001;7(2):180–185. doi: 10.1038/84627. [DOI] [PubMed] [Google Scholar]
- 95.Chakraborty K, Ghosh S, Koley H, et al. Bacterial exotoxins downregulate cathelicidin (hCAP-18/LL-37) and human β-defensin 1 (HBD-1) expression in the intestinal epithelial cells. Cell Microbiol. 2008;10(12):2520–2537. doi: 10.1111/j.1462-5822.2008.01227.x. [DOI] [PubMed] [Google Scholar]
- 96.Vieth R. What is the optimal vitamin D status for health? Prog. Biophys. Mol. Biol. 2006;92(1):26–32. doi: 10.1016/j.pbiomolbio.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 97. Adams JS, Ren S, Liu PT, et al. Vitamin D-directed rheostatic regulation of monocyte antibacterial responses. J. Immunol. 2009;182(7):4289–4295. doi: 10.4049/jimmunol.0803736. ▪ Proposes that vitamin D regulation may prevent pathogen-mediated repression of cathelicidin gene expression.
- 98. Krutzik SR, Hewison M, Liu PT, et al. IL-15 links TLR2/1-induced macrophage differentiation to the vitamin D-dependent antimicrobial pathway. J. Immunol. 2008;181(10):7115–7120. doi: 10.4049/jimmunol.181.10.7115. ▪ Provides evidence that implicates cytokine expression important in vitamin D-mediated induction of cathelicidin.
- 99.Lande R, Gregorio J, Facchinetti V, et al. Plasmacytoid dendritic cells sense self-DNA coupled with antimicrobial peptide. Nature. 2007;449(7162):564–569. doi: 10.1038/nature06116. [DOI] [PubMed] [Google Scholar]
- 100.Ganguly D, Chamilos G, Lande R, et al. Self-RNA-antimicrobial peptide complexes activate human dendritic cells through TLR7 and TLR8. J. Exp. Med. 2009;206(9):1983–1994. doi: 10.1084/jem.20090480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Peric M, Koglin S, Kim SM, et al. IL-17α enhances vitamin D3-induced expression of cathelicidin antimicrobial peptide in human keratinocytes. J. Immunol. 2008;181(12):8504–8512. doi: 10.4049/jimmunol.181.12.8504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wolk K, Kunz S, Witte E, Friedrich M, Asadullah K, Sabat R. IL-22 increases the innate immunity of tissues. Immunity. 2004;21(2):241–254. doi: 10.1016/j.immuni.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 103.Kao CY, Chen Y, Thai P, et al. IL-17 markedly up-regulates β-defensin-2 expression in human airway epithelium via JAK and NF-κb signaling pathways. J. Immunol. 2004;173(5):3482–3491. doi: 10.4049/jimmunol.173.5.3482. [DOI] [PubMed] [Google Scholar]
- 104.Peric M, Koglin S, Dombrowski Y, et al. Vitamin D analogs differentially control antimicrobial peptide/“Alarmin” expression in psoriasis. PLoS One. 2009;4(7):e6340. doi: 10.1371/journal.pone.0006340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Buchau AS, Macleod DT, Morizane S, Kotol PF, Hata T, Gallo RL. Bcl-3 acts as an innate immune modulator by controlling antimicrobial responses in keratinocytes. J. Invest. Dermatol. 2009;129(9):2148–2155. doi: 10.1038/jid.2009.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Chawla A, Repa JJ, Evans RM, Mangelsdorf DJ. Nuclear receptors and lipid physiology: opening the X-files. Science. 2001;294(5548):1866–1870. doi: 10.1126/science.294.5548.1866. [DOI] [PubMed] [Google Scholar]
- 107. Schmidt DR, Mangelsdorf DJ. Nuclear receptors of the enteric tract: guarding the frontier. Nutr. Rev. 2008;66(10) Suppl. 2:S88–S97. doi: 10.1111/j.1753-4887.2008.00092.x. ▪ Excellent review of nuclear receptors and protection of the digestive system.
- 108. Makishima M, Okamoto AY, Repa JJ, et al. Identification of a nuclear receptor for bile acids. Science. 1999;284(5418):1362–1365. doi: 10.1126/science.284.5418.1362. ▪▪ First evidence that lithocholic acid is a ligand for the vitamin D receptor.
- 109.Adachi R, Honma Y, Masuno H, et al. Selective activation of vitamin D receptor by lithocholic acid acetate, a bile acid derivative. J. Lipid Res. 2005;46(1):46–57. doi: 10.1194/jlr.M400294-JLR200. [DOI] [PubMed] [Google Scholar]
- 110.Staudinger JL, Goodwin B, Jones SA, et al. The nuclear receptor PXR is a lithocholic acid sensor that protects against liver toxicity. Proc. Natl Acad. Sci. USA. 2001;98(6):3369–3374. doi: 10.1073/pnas.051551698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Xie W, Radominska-Pandya A, Shi Y, et al. An essential role for nuclear receptors SXR/PXR in detoxification of cholestatic bile acids. Proc. Natl Acad. Sci. USA. 2001;98(6):3375–3380. doi: 10.1073/pnas.051014398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Matsubara T, Yoshinari K, Aoyama K, et al. Role of vitamin D receptor in the lithocholic acid-mediated CYP3a induction in vitro and in vivo. Drug Metab. Dispos. 2008;36(10):2058–2063. doi: 10.1124/dmd.108.021501. [DOI] [PubMed] [Google Scholar]
- 113.Ogura M, Nishida S, Ishizawa M, et al. Vitamin D3 modulates the expression of bile acid regulatory genes and represses inflammation in bile duct-ligated mice. J. Pharmacol. Exp. Ther. 2009;328(2):564–570. doi: 10.1124/jpet.108.145987. [DOI] [PubMed] [Google Scholar]
- 114.Lorenzo-Zuniga V, Bartoli R, Planas R, et al. Oral bile acids reduce bacterial overgrowth, bacterial translocation, and endotoxemia in cirrhotic rats. Hepatology. 2003;37(3):551–557. doi: 10.1053/jhep.2003.50116. [DOI] [PubMed] [Google Scholar]
- 115.Inagaki T, Moschetta A, Lee YK, et al. Regulation of antibacterial defense in the small intestine by the nuclear bile acid receptor. Proc. Natl Acad. Sci. USA. 2006;103(10):3920–3925. doi: 10.1073/pnas.0509592103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.D’aldebert E, Biyeyeme Bi Mve MJ, Mergey M, et al. Bile salts control the antimicrobial peptide cathelicidin through nuclear receptors in the human biliary epithelium. Gastroenterology. 2009;136(4):1435–1443. doi: 10.1053/j.gastro.2008.12.040. [DOI] [PubMed] [Google Scholar]
- 117.Schwab M, Reynders V, Shastri Y, Loitsch S, Stein J, Schroder O. Role of nuclear hormone receptors in butyrate-mediated up-regulation of the antimicrobial peptide cathelicidin in epithelial colorectal cells. Mol. Immunol. 2007;44(8):2107–2114. doi: 10.1016/j.molimm.2006.09.016. [DOI] [PubMed] [Google Scholar]
- 118.Jurutka PW, Bartik L, Whitfield GK, et al. Vitamin D receptor: key roles in bone mineral pathophysiology, molecular mechanism of action, and novel nutritional ligands. J. Bone Miner. Res. 2007;22 Suppl. 2:V2–V10. doi: 10.1359/jbmr.07s216. [DOI] [PubMed] [Google Scholar]
- 119.Jacobsen F, Mittler D, Hirsch T, et al. Transient cutaneous adenoviral gene therapy with human host defense peptide hCAP-18/LL-37 is effective for the treatment of burn wound infections. Gene Ther. 2005;12(20):1494–1502. doi: 10.1038/sj.gt.3302568. [DOI] [PubMed] [Google Scholar]
- 120.Raqib R, Sarker P, Bergman P, et al. Improved outcome in shigellosis associated with butyrate induction of an endogenous peptide antibiotic. Proc. Natl Acad. Sci. USA. 2006;103(24):9178–9183. doi: 10.1073/pnas.0602888103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zasloff M. Inducing endogenous antimicrobial peptides to battle infections. Proc. Natl Acad. Sci. USA. 2006;103(24):8913–8914. doi: 10.1073/pnas.0603508103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Gombart AF, Bhan I, Borregaard N, et al. Low plasma level of cathelicidin antimicrobial peptide (hCAP18) predicts increased infectious disease mortality in patients undergoing hemodialysis. Clin. Infect. Dis. 2009;48(4):418–424. doi: 10.1086/596314. ▪▪ First paper to demonstrate that circulating cathelicidin may provide protection from infection and/or sepsis.
- 123.Jeng L, Yamshchikov AV, Judd SE, et al. Alterations in vitamin D status and anti-microbial peptide levels in patients in the intensive care unit with sepsis. J. Transl. Med. 2009;7:28. doi: 10.1186/1479-5876-7-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Hata TR, Kotol P, Jackson M, et al. Administration of oral vitamin D induces cathelicidin production in atopic individuals. J. Allergy Clin. Immunol. 2008;122(4):829–831. doi: 10.1016/j.jaci.2008.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Oppenheim JJ, Tewary P, De La Rosa G, Yang D. Alarmins initiate host defense. Adv. Exp. Med. Biol. 2007;601:185–194. doi: 10.1007/978-0-387-72005-0_19. [DOI] [PubMed] [Google Scholar]
- 126.Yang D, De La Rosa G, Tewary P, Oppenheim JJ. Alarmins link neutrophils and dendritic cells. Trends Immunol. 2009 doi: 10.1016/j.it.2009.07.004. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Nehring JA, Zierold C, Deluca HF. Lithocholic acid can carry out in vivo functions of vitamin D. Proc. Natl Acad. Sci. USA. 2007;104(24):10006–10009. doi: 10.1073/pnas.0703512104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ishizawa M, Matsunawa M, Adachi R, et al. Lithocholic acid derivatives act as selective vitamin D receptor modulators without inducing hypercalcemia. J. Lipid Res. 2008;49(4):763–772. doi: 10.1194/jlr.M700293-JLR200. [DOI] [PubMed] [Google Scholar]
- 129.Holick MF. The vitamin D deficiency pandemic and consequences for nonskeletal health: mechanisms of action. Mol. Aspects Med. 2008;29(6):361–368. doi: 10.1016/j.mam.2008.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]