Abstract
The angiotensin type 1 receptor (AT1R) is highly expressed on renal nuclei and stimulates reactive oxygen species (ROS). It is not known whether other functional components of the angiotensin (Ang) system regulate the nuclear Ang II-AT1R-ROS pathway. Therefore, we examined the expression of Ang receptors in nuclei isolated from the kidneys of young adult (1.5 years) and older adult (3–5 years) sheep. Binding studies in renal nuclei revealed the AT2R as the predominant receptor subtype (~80%) in young sheep, with the AT7R (Mas protein) and AT1R antagonists competing for 52% and 25% of nuclear sites, respectively. Conversely, in older sheep, the AT1R accounted for ~85% of nuclear sites while the AT2R and AT7R subtypes comprised ~20% of remaining sites. Ang II increased nuclear ROS to a greater extent in older (97 ± 22%; n = 6) versus young animals (7 ± 2%; p = 0.01, n = 4) and this was abolished by an AT1R antagonist. The AT7R antagonist D-Ala7-Ang-(1-7) increased ROS formation to Ang II approximately two-fold (174 ± 5% vs. 97 ± 22%; p<0.05) in older adults. Immunoblots of renal nuclei revealed protein bands for the AT7R and angiotensin converting enzyme (ACE2) which metabolizes Ang II to Ang-(1-7). The ACE2 inhibitor MLN4760 also exacerbated the Ang II-dependent formation of ROS (156 ± 15%) and abolished the generation of Ang-(1-7) from Ang II. We conclude that an ACE2-Ang-(1-7)-AT7R pathway modulates Ang II-dependent ROS formation within the nucleus, providing a unique protective mechanism against oxidative stress and cell damage.
Keywords: Angiotensin, Reactive Oxygen Species, kidney, angiotensin – (1-7) receptor, intracellular RAS
INTRODUCTION
It is well-established that reactive oxygen species (ROS) play an important role as signaling molecules in a variety of cellular responses 1. Sustained perturbations in redox homeostasis can result in oxidative stress leading to cardiovascular damage and cellular injury. Angiotensin (Ang) II stimulates the generation of ROS through the AT1 receptor isoform 2. Blockade of the renin-angiotensin aldosterone system (RAAS) either by selective AT1 receptor antagonists (ARBs) or inhibition of the formation of Ang II by angiotensin converting enzyme (ACE) inhibitors is the leading therapeutic approach to lower blood pressure and reduce tissue injury in various cardiovascular pathologies. A wealth of experimental evidence reveals that inhibition of the Ang II-generating axis of the RAAS is associated with a reduction in oxidative stress within the kidney and other tissues 3-6.
The Ang II-dependent formation of ROS occurs through stimulating the assembly of the NAD(P)H (NOX) complex associated with the cell membrane. Chronic stimulation by Ang II also promotes the synthesis of several NOX components including p22 phox and p47 phox, as well as attenuates the expression of various scavenging proteins within the cell, resulting in higher levels of intracellular ROS 2, 7, 8. Moreover, increased levels of ROS may stimulate the expression of components of the RAAS favoring Ang II, thus leading to a potentially vicious feedback loop that would exacerbate tissue injury 9. However, there are additional pathways within the RAAS that may functionally antagonize an activated Ang II-AT1 receptor pathway. One alternative product of the RAAS is the peptide Ang-(1-7), which is formed from either Ang I or Ang II. In contrast to the Ang II- AT1 receptor mediated actions, Ang-(1-7) exhibits vasodilatory properties through the stimulation of nitric oxide or prostaglandins, stimulates natriuresis and diuresis, and conveys anti-fibrotic and anti-oxidant actions 10-12.
The inflammatory, fibrotic and pressor actions of Ang II are assumed to originate at the cell surface by activation of the AT1 protein, a prototypic seven-transmembrane G-protein coupled receptor (GPCR); however, we and others find a significant density of intracellular AT1 receptors on isolated nuclei obtained from the renal cortex and medulla of both the rat and sheep 13-16. Indeed, we recently demonstrated Ang II-AT1 receptor dependent stimulation of ROS in purified renal nuclei that was abolished by both protein kinase C (PKC) and phosphoinositol 3-kinase (PI3K) inhibitors 14. Although these findings suggest a functional Ang II- AT1 receptor pathway on renal nuclei, whether the nucleus exhibits alternative RAAS pathways that antagonize or modulate the actions of Ang II is not known. Therefore, the current studies examined the modulatory effects of Ang-(1-7) on Ang II-mediated ROS generation within renal nuclei.
MATERIALS AND METHODS
Animals
Tissues were obtained from 4 young adult (1.5 years of age) and 6 older adult (3-5 years) female mixed-breed sheep pasture-reared then housed in the animal facility of Wake Forest University for one week prior to study. Animals were synchronized by estrus cycles and maintained on a 12:12hr light-dark cycle with access to food and water ad libitum. Kidney cortices were obtained fresh from animals anesthetized with ketamine and isoflurane, and processed immediately for the isolation of cortical nuclei. All procedures were approved by the Institutional Animal Care and Use Committee at Wake Forest University School of Medicine.
Preparation of nuclei
Cortical nuclei were prepared as previously described 15. Briefly, fresh tissue was homogenized in buffer containing 25 mmol/L KCl, 5 mmol/L MgCl2, 20 mmol/L Tricine-KOH and 25 mmol/L sucrose (pH 7.8), filtered through a 100-μm mesh and centrifuged twice at 1,000 × g (4°C) for 10 min. The pellet was re-suspended in 20% OptiPrep solution (Accurate Chemical and Scientific, Westbury, NY), layered on a discontinuous density gradient column of 10, 20, 25, 30, and 35% OptiPrep solution and centrifuged at 10,000 × g for 20 min (4°C). The enriched fraction of nuclei was recovered at the 30–35% layer interface 13-15.
Characterization of angiotensin receptors in young adult (1.5 yr old) and older adult (3-5 yr old) female sheep kidney
Angiotensin receptor binding was performed as previously described 13, 14. Briefly, isolated nuclei were suspended in Hepes buffer and co-incubated with the radioligand 125I-[Sar1, Thr8]-Ang II (125I-Sarthran) in the presence of losartan (the AT1-receptor antagonist), D-Ala7-(Ang 1-7) [A779 or DALA; the Ang-(1-7) receptor antagonist], PD123319 (the AT2-receptor antagonist) or non-labeled Sarthran, each at a final concentration of 10 μmol/L.
Western Blotting
Nuclei isolated from OptiPrep gradient separation were suspended in PBS and added to Laemmli buffer containing mercaptoethanol. Proteins were separated on 10% SDS polyacrylamide gels and electrophoretically transferred onto polyvinylidene difluoride membranes. Immunoblots were blocked for 1 h with 5% Dry Milk (Bio-Rad) and Tris-buffered saline containing 0.05%Tween, then probed with antibodies against AT1 (1:5000; Alpha Diagnostics, San Antonio, TX), the Ang-(1-7) receptor, Mas* (1:200, Alomone Labs, Jerusalem, Israel), the angiotensin converting enzyme homolog, ACE2 (1:2000; prepared at the Hypertension and Vascular Research Center, No. AN212), NOX2 (gp91phox: 1:1000; BD Transduction Laboratories, San Diego, CA), p47phox (1:200; Cell Signaling, Beverly, MA). *To confirm specificity of the Mas receptor antibody, immunogenic Mas peptide was incubated (1μg peptide per 1μg antibody) in 1% BSA at room temperature for 1 h, then added to immunoblots of purified nuclear extracts and incubated at 4°C overnight. Reactive proteins for all immunoblots were detected with Pierce Super Signal Chemiluminescent substrates and exposed to Amersham Hyperfilm enhanced chemiluminescence (Piscataway, NJ).
Measurement of Reactive Oxygen Species Production
Isolated cortical nuclei were pre-incubated with the fluorescence dye, 5-(and-6)-chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate-acetyl ester (DCF; 20μg/ml) (Molecular Probes, Eugene, OR) in buffer containing 100 mmol/L KH2PO4, 1 mmol/L NaN3, 1 mmol/L EGTA, 100 μmol/L FAD, and 100 μmol/L NADH (pH 7.4) for 30 min at 37°C 17. Nuclei were washed twice in Hepes buffer to remove any unbound dye, then incubated with 1nmol/L Ang II in the presence of losartan (the AT1-receptor antagonist), D-Ala7-(Ang 1-7) [DALA; the Ang-(1-7) receptor antagonist], PD123319 (the AT2-receptor antagonist), the ACE2 inhibitor MLN4760 (MLN) or buffer alone. Increases in DCF fluorescence, as an indicator of ROS production, were measured using a SpectraMax M2e microplate reader (Molecular Devices, Sunnyvale, CA) at wavelengths of 488 nm (excitation) and 510 nm (emission).
Determination of ACE2 activity
ACE2 enzymatic activity was determined in isolated cortical nuclei of older adult sheep kidney. Nuclei were purified by OptiPrep density gradient separation and analyzed at 37°C in 10 mmol/L HEPES, 125 mmol/L NaCl, 10 μmol/L ZnCl2 (pH 7.4), with inhibitors, as previously described 18. Briefly, nuclei were co-incubated with 0.5 nmol/L iodinated 125I-Ang I or 125I-Ang II, washed with ice-cold 1.0% phosphoric acid and filtered before separation by reverse-phase high-performance liquid chromatography (HPLC).
Statistical analysis
Data are represented as means ± SEM. Paired Student's t-test, one-way ANOVA with Tukey's multiple comparison post-hoc and non-linear regression were performed using GraphPad Prism 5.0 plotting and statistical software.
RESULTS
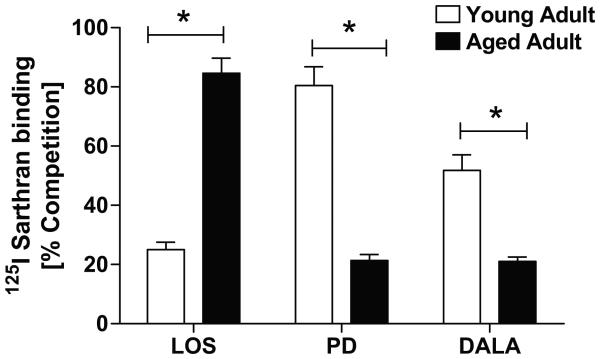
We initially determined the profile of Ang receptor subtypes in nuclei freshly isolated from the renal cortex of younger and older adult female sheep by radioligand binding of the non-selective antagonist 125I-[Sar1,Thr8]-Ang II (125I-Sarthran) and competition of binding with selective isotype antagonists. In 1.5 year old sheep (equivalence of 20-25 years of human age), the AT2 isoform represents the greatest proportion of Ang binding sites in renal cortical nuclei (80 ± 6% competition with PD123319) consistent with our previous results 15, while antagonists for the AT7 and AT1 subtypes competed for (52 ± 5% and 25 ± 2%), respectively (Figure 1). Conversely, the AT1 isoform is the predominant receptor subtype (84 ± 5% competition with losartan) in cortical nuclei isolated from older sheep (3-5 years or 45-60 years of human age). In comparison, both PD123319 (the AT2 subtype antagonist) and DALA (the AT7 receptor antagonist) competed to a similar extent (21 ± 2% vs. 21 ± 1%) for 125I-Sarthran binding.
Figure 1.
Characterization of angiotensin receptor subtypes in isolated renal cortical nuclei of young (1.5 years of age) and older (3-5 years of age) female sheep isolated by OptiPrep density gradient. Competition binding was carried out using 0.5 nmol/L 125I-Sarthran and receptor antagonists (LOS – losartan; PD – PD123319; DALA – [D-Ala7]-Ang-(1-7)) or unlabeled Sarthran at a final concentration of 10 μmol/L. Data are expressed as means ± SEM (n = 4; *p <0.05 vs. young).
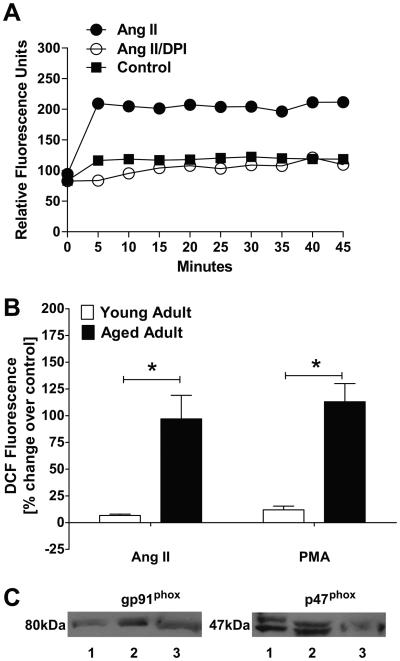
We next assessed the generation of ROS in response to Ang II in freshly isolated cortical nuclei from both younger and older adult females using the fluorescent dye dichloroflurorescein (DCF). As shown in the fluorescent tracing of Figure 2A, Ang II (1 nmol/L) stimulated a sustained increase in DCF fluorescence over control nuclei (buffer alone) from older sheep that was abolished by the NADPH oxidase inhibitor diphenyleneiodonium chloride (DPI, 10μmol/L). Basal level ROS production in older adult sheep was 7.3-fold higher than that of young adult sheep [P<0.01] (Data not shown). Moreover, in Figure 2B, Ang II stimulated the DCF signal to a greater extent in the cortical nuclei isolated from the older sheep in comparison to the young adult sheep [97 ± 22% vs.7 ± 1%, p<0.05; n = 4 young, n = 6 older]. In the older sheep, DPI abolished the increase in DCF fluorescence to Ang II (−15 ± 5%; n = 6). Similar to the response to Ang II, the PKC agonist phorbol 12-myristate 13-acetate (PMA, 1μmol/L) also increased DCF fluorescence to a greater extent in the nuclei isolated from the older animals (Figure 2B).
Figure 2.
Ang II stimulation of ROS in renal nuclei. Renal cortical nuclei were freshly isolated by OptiPrep density gradient separation and pre-incubated with the fluorescent dye, dichlorofluorescein (DCF). Isolated nuclei were stimulated with Ang II (1 nmol/L), Ang II + the NOX inhibitor, DPI (10 μmol/L) or buffer alone. A: Representative tracing of DCF fluorescence. B: Comparison of ROS generation in nuclei from younger (n = 4) and older (n = 6) animals. Nuclei were stimulated with Ang II (1 nmol/L), the PKC agonist PMA (1 μmol/L) or buffer alone. Values represented are expressed as % change in fluorescence intensity over control (baseline) measured at 45 minutes. Data are the means ± SEM (*p = 0.014 vs. young). C: Immunoblots of three distinct preparations of purified nuclei from older animals with antibodies directed against gp91phox (NOX2) and p47phox, the cytosolic subunit of the NOX2 complex required for activation.
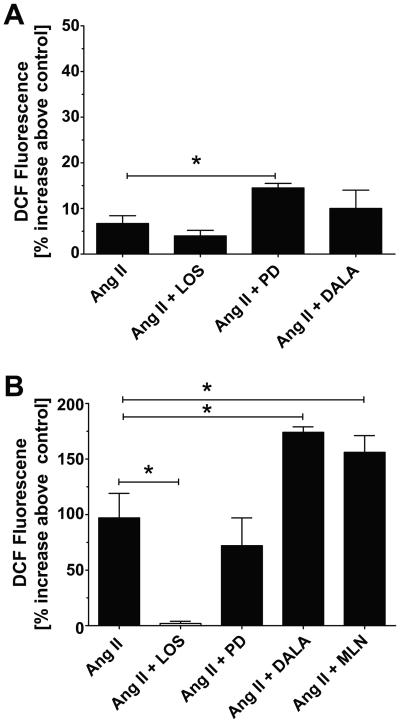
To assess whether NOX components are expressed in the sheep nuclei, we performed Western blot analysis on purified nuclear extracts from the renal cortex of older animals. In the blot of Figure 2C, we show a single immunoreactive band at ~80 kDa for gp91phox (NOX2), a membrane bound glycoprotein component of the NAD(P)H oxidase complex that functions in electron transport. Moreover, we demonstrate doublet bands of 47 and 50 kDa for p47phox, the cytosolic component of the NOX complex that is required for activation of NOX2. To identify the Ang receptor subtypes that elicit the formation of ROS, we pre-incubated DCF-loaded nuclei from younger and older sheep with losartan, PD123319 or DALA (1 μmol/L each). As shown in Figure 3A, the AT1 antagonist losartan tended to reduce the small increase in Ang II-stimulated DCF fluorescence. However, pre-treatment with the AT2 antagonist PD123319 significantly enhanced the DCF response to Ang II [14 ± 1% vs. 7 ± 1%, p = 0.014] while the AT7 receptor antagonist DALA had no effect. In the nuclei of older animals, losartan essentially abolished the Ang II-dependent increase in DCF (Figure 3B). In contrast to the ROS-enhancing effects in younger animals, PD123319 did not alter the Ang II response in older sheep; however, DALA increased the Ang II stimulation of ROS approximately 2-fold [174 ± 5% vs. 97 ± 22%, p<0.05] in the latter group. The addition of the Ang II or Ang-(1-7) receptor antagonists alone to DCF-loaded nuclei had no effect on control DCF levels in older animals (data not shown). Treatment of nuclei with the specific ACE2 inhibitor MLN4760 increased Ang II-dependent DCF fluorescence to a similar extent as that of DALA [174 ± 5% vs. 156 ± 15%, p >0.05] (Figure 3B).
Figure 3.
Influence of angiotensin receptor antagonists on Ang II stimulation of ROS in renal nuclei. Measurement of Ang II-stimulated ROS (increase in DCF fluorescence) in the presence of losartan (LOS), PD123319 (PD), D-Ala7-(Ang 1-7) (DALA) or the ACE2 inhibitor MLN4760 (MLN) in nuclei from younger [n=4] (A) or older [n=6] (B) animals. All antagonists or inhibitors are at 1 μmol;/L final concentration. Data are expressed as means ± SEM; *p<0.05 vs. Ang II.
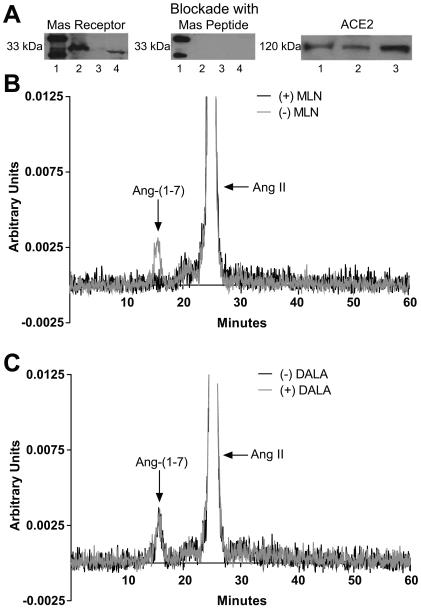
In purified nuclei from the sheep renal cortex, immunoblots reveal the presence of the AT7 /Mas receptor protein (Figure 4A). Pre-incubation of the immunoblot with the Mas peptide blocked this immunoreactive response. Western blot analysis of purified nuclei also revealed a single 120 kDa band for ACE2 (Figure 4A). Lastly, we demonstrated ACE2 activity in purified nuclei by the conversion of 125I-Ang II to 125I-Ang-(1-7) [17 ± 4 fmol min−1 mg protein−1, n = 3]. Nuclear ACE2 activity was abolished by the ACE2 inhibitor MLN4760 (Figure 4B), but not by the addition of the DALA peptide (Figure 4C).
Figure 4.
Immunoblots reveal the Ang-(1-7) receptor Mas, blockade of Mas immunoreactivity by pre-incubation with the Mas peptide, as well as the enzyme ACE2 in three distinct preparations of purified nuclei from older animals (A). Demonstration of ACE2 activity in purified cortical nuclei by the conversion of 125I-Ang II to 125I-Ang-(1-7) as detected by HPLC (B and C). ACE2 activity was abolished by the ACE2 inhibitor MLN (B), but not by the AT7 receptor antagonist DALA (C).
DISCUSSION
Evidence for the intracellular expression of the RAAS within the kidney is becoming increasingly apparent, particularly the functional expression of the AT1 receptor on the cell nucleus 22-24. We previously reported that the AT1 receptor stimulates ROS formation in isolated nuclei of the rat renal cortex 14. In contrast, nuclei prepared from the renal cortex of young adult sheep expressed primarily the AT2 receptor subtype, which is functionally linked to the generation of NO 15. The present studies demonstrate age-dependent changes in angiotensin receptors where an increase in AT1 and a corresponding decrease in AT2 and AT7 receptor subtypes are expressed in renal nuclei of older adult sheep. Associated with increased AT1 receptor expression, ROS levels following Ang II or PMA stimulation were significantly higher in the nuclei obtained from the kidneys of the older sheep. Moreover, blockade of the AT7 receptor or ACE2 enhanced the Ang II-dependent stimulation of ROS. To our knowledge, these studies are the first to demonstrate a regulatory role of Ang-(1-7) that originates from the proteolytic conversion of Ang II by ACE2 within the nucleus. Demonstration of the nuclear expression for both the AT7 receptor and ACE2 suggests that Ang-(1-7) may well function as an endogenous buffer within the cell to modulate the actions of Ang II in the production of ROS. Indeed, these data support previous findings that exogenous Ang-(1-7) attenuates either Ang II- or hyperglycemic-induced increases in ROS 25, 26.
The binding studies in isolated nuclei revealed a shift in the angiotensin receptor profile from the AT2 and AT7 subtypes to the AT1 isoform with age. The AT2 antagonist PD123319 enhanced Ang II stimulation of ROS while the AT7 antagonist had no effect in younger animals. The PD compound may shunt more Ang II to the AT1 receptor or inhibit an AT2 receptor-dependent pathway that normally attenuates ROS, particularly given the predominance of the AT2 subtype at the younger age. In the presence of PD123319, Ang II would preferably bind to the AT1 rather than the AT7 receptor, which may account for the lack of an effect of the AT7 antagonist. In older animals, the AT7 antagonist DALA, but not PD12319, enhanced the Ang II - AT1 effect on ROS despite the similar extent of competition for Sarthran binding with both antagonists. In this case, the AT2 receptor may no longer be functionally coupled to attenuate AT1-dependent stimulation of ROS in older animals. Ang-(1-7), formed by ACE2 processing of Ang II, may preferentially bind to the AT7 rather than the AT2 subtype to antagonize the Ang II-AT1 receptor pathway within the nucleus. Pre-treatment of nuclei with the ACE2 inhibitor MLN exacerbated Ang II-induced ROS formation. This could be attributed to increasing the availability of Ang II to the AT1 receptor by preventing metabolism to Ang-(1-7), but blockade of the AT7 receptor with DALA yielded identical results. Pihneiro et al.,27 recently reported that Mas knock-out mice exhibit increased AT1 receptor expression; however, to our knowledge, there are no reports that acute treatment with DALA increases AT1 receptor expression. Thus, this counter-regulation of Ang II on ROS production is likely mediated by the direct actions of Ang-(1-7) on nuclear AT7 receptors.
The pathways that mediate the AT1-dependent stimulation of ROS or the attenuation of this effect by Ang-(1-7) within the nucleus are not known. We initially reported the functional involvement of both PKC and PI3K in ROS generation by Ang II in rat renal nuclei consistent with studies in intact cells on ROS formation, as well as evidence for a phospholipid signaling pathway within the nucleus 28-30. The increase in ROS with the PKC agonist PMA in sheep nuclei suggests that PKC may contribute to the nuclear actions of Ang II, although we did not attempt to block the Ang II response with PKC inhibitors nor determine whether the effects of PMA and Ang II are additive. PMA responsiveness was also greater in the older animals, which may reflect an increase in PKC in the cortical nuclei. Lokhandwala and colleagues report that older Fisher 344 rats express higher levels of PKC-β and PKC-δ in the kidney cortex 31. Moreover, these investigators find that chronic antioxidant treatment reduces the elevated PKC activity in the proximal tubules of the older rat kidney 32. Although it is not clear from the current studies what mechanism contributes to the alteration in angiotensin receptor subtypes in renal nuclei of older animals, the enhanced ROS response to Ang II likely reflects the participation of additional pathways in conjunction with the increased expression of AT1 receptor subtype.
In regards to Ang-(1-7), exogenous treatment with this peptide reduced the increase in ROS and phosphorylation of c-Src kinase by Ang II in intact endothelial cells 25. The Ang-(1-7)-dependent reduction in ROS was associated with an increase in the interaction of SHP-2 phosphatase with c-Src. Moreover, knockdown of SHP-2 abolished the inhibitory influence of Ang-(1-7). In LLC-PK proximal tubule cells, Ang-(1-7) reduced both MAP kinase activation and the increase in TGF-β in response to high glucose conditions 33. The Ang-(1-7) response in the proximal tubule cells was associated with an increase in the tyrosine phosphatase SHP-1 and the phosphatase inhibitor phenylarsine oxide reversed the inhibitory actions of Ang-(1-7) 33. Finally, Gallagher et al. demonstrated that Ang-(1-7) stimulated a MAP kinase phosphatase that prevented the downregulation of ACE2 by Ang II in cardiomyocytes 34. In lieu of these data in intact cells, we speculate that the acute response to the Ang-(1-7) antagonist or the ACE2 inhibitor may involve a reduction in “phosphatase tone” that would normally attenuate the kinase-dependent Ang II activation of ROS, as various phosphatases have been shown to localize to the cell nucleus 35, 36. Alternatively, our preliminary studies demonstrate that Ang-(1-7) stimulates nuclear production of nitric oxide, which may contribute to its capacity to buffer Ang II-induced ROS 37. However, the identity of the nuclear pathway responsible for the inhibitory actions of Ang-(1-7) is not currently known and is the focus of ongoing investigation.
The present studies provide evidence for the expression of an ACE2-Ang-(1-7)-AT7 receptor pathway on nuclei of the sheep kidney. One potential function of this pathway may be to attenuate the activity of the Ang II-AT1 receptor axis and the accumulation of ROS within the nuclear environment. In the perfused kidney and LLC-PK cells, Ang II-induced DNA damage was attenuated by AT1 receptor blockade, as well as the antioxidants N-acetyl cysteine and α-tocopherol 38, 39. Associated with the increase in DNA damage, Ang II increased intracellular DCF fluorescence 2-fold, which is similar to the increase in fluorescence observed in the present study, albeit the Ang II concentration in the intact cells was 170-fold greater than that used in the nuclei 38. Moreover, treatment with exogenous H2O2, the primary ROS detected by DCF, increased DNA damage to a similar extent as that for Ang II in the proximal tubule cells 38. Although mitochondria are considered the predominant source of cellular ROS 40, the localized increase in ROS within the cell nucleus could potentially contribute to Ang II-dependent DNA damage and cell senescence 41. The exact role(s) of the nuclear RAAS in cellular damage and senescence are not currently known. Indeed, further studies are required to determine if certain transcriptional factors are up-regulated in response to activation of the nuclear angiotensin receptors. However, evidence for reduced tissue levels of Ang-(1-7) with increasing age may mitigate the counter-regulatory actions of the heptapeptide on Ang II-mediated ROS within the nuclear compartment of the cell 42.
PERSPECTIVES
Long-term blockade of the RAAS by ACE inhibitors or AT1 receptor antagonists reduces oxidative stress and deters age-associated cellular or tissue damage 43, 44. Indeed, AT1 receptor deficient mice exhibit a 20% increase in life span associated with reduced tissue levels of nitrotyrosine 45. In addition to inhibiting the Ang II-AT1 receptor axis, RAAS blockade with ACE inhibitors or ARBs increases the levels of Ang-(1-7). The present studies suggest that the activation of the intracellular ACE2-Ang-(1-7)-AT7 receptor may constitute a pathway to convey additional therapeutic benefit in the treatment of cardiovascular disease.
ACKNOWLEDGEMENTS
The authors would like to thank Mr. Brian Westwood and Mrs. Nancy Pirro for their expert technical assistance in these studies. We especially thank Dr. Kathryne Stabile, Department of Orthopedic Surgery, for donation of animal tissue.
SOURCE(S) OF FUNDING
This work was supported by grants from the National Institutes of Health HD17644, HD47584, HL-56973, HL51952, HL-56972 and RR018370, as well as unrestricted grants from the Unifi Corporation (Greensboro, NC) and the Farley-Hudson Foundation (Jacksonville, NC).
Footnotes
CONFLICT(S) OF INTEREST/DISCLOSURE(S)
NONE
REFERENCES
- 1.Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev. 2007;87:245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- 2.Garrido AM, Griendling KK. NADPH oxidases and angiotensin II receptor signaling. Mol Cell Endocrinol. 2008;302:148–158. doi: 10.1016/j.mce.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wolf G. Role of reactive oxygen species in angiotensin II-mediated renal growth, differentiation, and apoptosis. Antioxid Redox Signal. 2005;7:1337–1345. doi: 10.1089/ars.2005.7.1337. [DOI] [PubMed] [Google Scholar]
- 4.Lopez-Real A, Rey P, Soto-Otero R, Mendez-Alvarez E, Labandeira-Garcia JL. Angiotensin-converting enzyme inhibition reduces oxidative stress and protects dopaminergic neurons in a 6-hydroxydopamine rat model of Parkinsonism. J Neurosci Res. 2005;81:865–873. doi: 10.1002/jnr.20598. [DOI] [PubMed] [Google Scholar]
- 5.Khaper N, Singal PK. Modulation of oxidative stress by a selective inhibition of angiotensin II type 1 receptors in MI rats. J Am Coll Cardiol. 2001;37:1461–1466. doi: 10.1016/s0735-1097(01)01126-3. [DOI] [PubMed] [Google Scholar]
- 6.Chen X, Patel K, Connors SG, Mendonca M, Welch WJ, Wilcox CS. Acute antihypertensive action of Tempol in the spontaneously hypertensive rat. Am J Physiol Heart Circ Physiol. 2007;293:H3246–H3253. doi: 10.1152/ajpheart.00957.2007. [DOI] [PubMed] [Google Scholar]
- 7.Touyz RM, Schiffrin EL. Reactive oxygen species and hypertension: a complex association. Antioxid Redox Signal. 2008;10:1041–1044. doi: 10.1089/ars.2007.2012. [DOI] [PubMed] [Google Scholar]
- 8.Li JM, Shah AM. Mechanism of endothelial cell NADPH oxidase activation by angiotensin II. Role of the p47phox subunit. J Biol Chem. 2003;278:12094–12100. doi: 10.1074/jbc.M209793200. [DOI] [PubMed] [Google Scholar]
- 9.Singh VP, Le B, Khode R, Baker KM, Kumar R. Intracellular angiotensin II production in diabetic rats is correlated with cardiomyocyte apoptosis, oxidative stress, and cardiac fibrosis. Diabetes. 2008;57:3297–3306. doi: 10.2337/db08-0805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chappell MC. Emerging evidence for an ACE2-Ang-(1-7)-Mas receptor axis: more than blood pressure regulation? Hypertension. 2007;50:596–599. doi: 10.1161/HYPERTENSIONAHA.106.076216. [DOI] [PubMed] [Google Scholar]
- 11.Ferrario CM. Angiotensin-converting enzyme 2 and angiotensin-(1-7): an evolving story in cardiovascular regulation. Hypertension. 2006;47:515–521. doi: 10.1161/01.HYP.0000196268.08909.fb. [DOI] [PubMed] [Google Scholar]
- 12.Santos RA, Ferreira AJ, Simoes e Silva AC. Recent advances in the angiotensin-converting enzyme 2-angiotensin (1-7)-Mas axis. Exp Physiol. 2008;93:519–527. doi: 10.1113/expphysiol.2008.042002. [DOI] [PubMed] [Google Scholar]
- 13.Pendergrass KD, Averill DB, Ferrario CM, Diz DI, Chappell MC. Differential expression of nuclear AT1 receptors and angiotensin II within the kidney of the male congenic mRen2.Lewis rat. Am J Physiol Renal Physiol. 2006;290:F1497–F1506. doi: 10.1152/ajprenal.00317.2005. [DOI] [PubMed] [Google Scholar]
- 14.Pendergrass KD, Gwathmey TM, Michalek RD, Grayson JM, Chappell MC. The angiotensin II-AT1 receptor stimulates reactive oxygen species within the cell nucleus. Biochem Biophys Res Commun. 2009;384:149–154. doi: 10.1016/j.bbrc.2009.04.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gwathmey TM, Shaltout HA, Pendergrass KD, Pirro NT, Figueroa JP, Rose JC, Diz DI, Chappell MC. Nuclear angiotensin II type 2 (AT2) receptors are functionally linked to nitric oxide production. Am J Physiol Renal Physiol. 2009;296:F1484–F1493. doi: 10.1152/ajprenal.90766.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li XC, Zhuo JL. Intracellular angiotensin II induces in vitro transcription of TGF-β1, MCP-1 and NHE3 mRNAs in rat renal cortical nuclei via activation of nuclear AT1 receptors. Am J Physiol Cell Physiol. 2008;294:C1034–C1045. doi: 10.1152/ajpcell.00432.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kuroda J, Nakagawa K, Yamasaki T, Nakamura K, Takeya R, Kuribayashi F, Imajoh-Ohmi S, Igarashi K, Shibata Y, Sueishi K, Sumimoto H. The superoxide-producing NAD(P)H oxidase Nox4 in the nucleus of human vascular endothelial cells. Genes Cells. 2005;10:1139–1151. doi: 10.1111/j.1365-2443.2005.00907.x. [DOI] [PubMed] [Google Scholar]
- 18.Shaltout HA, Westwood B, Averill DB, Ferrario CM, Figueroa J, Diz DI, Rose JC, Chappell MC. Angiotensin metabolism in renal proximal tubules, urine and serum of sheep: Evidence for ACE2-dependent processing of Angiotensin II. Am J Physiol Renal Physiol. 2006;292:F82–F91. doi: 10.1152/ajprenal.00139.2006. [DOI] [PubMed] [Google Scholar]
- 19.Seshiah PN, Weber DS, Rocic P, Valppu L, Taniyama Y, Griendling KK. Angiotensin II stimulation of NAD(P)H oxidase activity: upstream mediators. Circ Res. 2002;91:406–413. doi: 10.1161/01.res.0000033523.08033.16. [DOI] [PubMed] [Google Scholar]
- 20.Choi H, Leto TL, Hunyady L, Catt KJ, Bae YS, Rhee SG. Mechanism of angiotensin II-induced superoxide production in cells reconstituted with angiotensin type 1 receptor and the components of NADPH oxidase. J Biol Chem. 2008;283:255–267. doi: 10.1074/jbc.M708000200. [DOI] [PubMed] [Google Scholar]
- 21.Zhang M, Kho AL, Anilkumar N, Chibber R, Pagano PJ, Shah AM, Cave AC. Glycated proteins stimulate reactive oxygen species production in cardiac myocytes: involvement of Nox2 (gp91phox)-containing NADPH oxidase. Circulation. 2006;113:1235–1243. doi: 10.1161/CIRCULATIONAHA.105.581397. [DOI] [PubMed] [Google Scholar]
- 22.Kumar R, Singh VP, Baker KM. The intracellular renin-angiotensin system: a new paradigm. Trends Endocrinol Metab. 2007;18:208–214. doi: 10.1016/j.tem.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 23.Zhuo JL, Li XC. Novel roles of intracrine angiotensin II and signaling mechanisms in kidney cells. J Renin Angiotensin Aldosterone Syst. 2007;8:23–33. doi: 10.3317/jraas.2007.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Re R. Intracellular renin-angiotensin system: the tip of the intracrine physiology iceberg. Am J Physiol Heart Circ Physiol. 2007;293:H905–H1006. doi: 10.1152/ajpheart.00552.2007. [DOI] [PubMed] [Google Scholar]
- 25.Sampaio WO, Henrique de CC, Santos RA, Schiffrin EL, Touyz RM. Angiotensin-(1-7) counterregulates angiotensin II signaling in human endothelial cells. Hypertension. 2007;50:1093–1098. doi: 10.1161/HYPERTENSIONAHA.106.084848. [DOI] [PubMed] [Google Scholar]
- 26.Benter IF, Yousif MH, Dhaunsi GS, Kaur J, Chappell MC, Diz DI. Angiotensin-(1-7) prevents activation of NADPH oxidase and renal vascular dysfunction in diabetic hypertensive rats. Am J Nephrol. 2008;28:25–33. doi: 10.1159/000108758. [DOI] [PubMed] [Google Scholar]
- 27.Pinheiro SV, Ferreira AJ, Kitten GT, da Silveira KD, da Silva DA, Santos SH, Gava E, Castro CH, Magalhaes JA, da Mota RK, Botelho-Santos GA, Bader M, Alenina N, Santos RA, Simoes e Silva AC. Genetic deletion of the angiotensin-(1-7) receptor Mas leads to glomerular hyperfiltration and microalbuminuria. Kid Int. 2009;75:1184–1193. doi: 10.1038/ki.2009.61. [DOI] [PubMed] [Google Scholar]
- 28.Deleris P, Gayral S, Breton-Douillon M. Nuclear Ptdlns(3,4,5)P3 signaling: an ongoing story. J Cell Biochem. 2006;98:469–485. doi: 10.1002/jcb.20695. [DOI] [PubMed] [Google Scholar]
- 29.Irvine RF. Nuclear lipid signaling. Nat Rev Mol Cell Biol. 2003;4:349–360. doi: 10.1038/nrm1100. [DOI] [PubMed] [Google Scholar]
- 30.Neri LM, Borgatti P, Capitani S, Martelli AM. The nuclear phosphoinositide 3-kinase/AKT pathway: a new second messenger system. Biochim Biophys Acta. 2002;1584:73–80. doi: 10.1016/s1388-1981(02)00300-1. [DOI] [PubMed] [Google Scholar]
- 31.Asghar M, Hussain T, Lokhandwala MF. Overexpression of PKC-βI and -δ contributes to higher PKC activity in the proximal tubules of old Fischer 344 rats. Am J Physiol Renal Physiol. 2003;285:F1100–F1107. doi: 10.1152/ajprenal.00198.2003. [DOI] [PubMed] [Google Scholar]
- 32.Asghar M, Lokhandwala MF. Antioxidant tempol lowers age-related increases in insulin resistance in Fischer 344 rats. Clin Exp Hypertens. 2006;28:533–541. doi: 10.1080/10641960600798697. [DOI] [PubMed] [Google Scholar]
- 33.Gava E, Samad-Zadeh A, Zimpelmann J, Bahramifarid N, Kitten GT, Santos RA, Touyz RM, Burns KD. Angiotensin-(1-7) activates a tyrosine phosphatase and inhibits glucose-induced signaling in proximal tubular cells. Nephrol Dial Transplant. 2009;24:1766–1773. doi: 10.1093/ndt/gfn736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gallagher PE, Ferrario CM, Tallant EA. MAP kinase/phosphatase pathway mediates the regulation of ACE2 by angiotensin peptides. Am J Physiol Cell Physiol. 2008;295:C1169–C1174. doi: 10.1152/ajpcell.00145.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Planchon SM, Waite KA, Eng C. The nuclear affairs of PTEN. J Cell Sci. 2008;121:249–253. doi: 10.1242/jcs.022459. [DOI] [PubMed] [Google Scholar]
- 36.Keyse SM. Dual-specificity MAP kinase phosphatases (MKPs) and cancer. Cancer Metastasis Rev. 2008;27:253–261. doi: 10.1007/s10555-008-9123-1. [DOI] [PubMed] [Google Scholar]
- 37.Gwathmey TM, Pendergrass KP, Rose JC, Diz DI, Chappell MC. Nuclear angiotensin-(1-7) receptors mediate the production of nitric oxide. Hypertension. 2009;54:e74. Abstract. [Google Scholar]
- 38.Schupp N, Schmid U, Rutkowski P, Lakner U, Kanase N, Heidland A, Stopper H. Angiotensin II-induced genomic damage in renal cells can be prevented by angiotensin II type 1 receptor blockage or radical scavenging. Am J Physiol Renal Physiol. 2007;292:F1427–F1234. doi: 10.1152/ajprenal.00458.2006. [DOI] [PubMed] [Google Scholar]
- 39.Schmid U, Stopper H, Schweda F, Queisser N, Schupp N. Angiotensin II induces DNA damage in the kidney. Cancer Res. 2008;68:9239–9246. doi: 10.1158/0008-5472.CAN-08-1310. [DOI] [PubMed] [Google Scholar]
- 40.Madamanchi NR, Runge MS. Mitochondrial dysfunction in atherosclerosis. Circ Res. 2007;100:460–473. doi: 10.1161/01.RES.0000258450.44413.96. [DOI] [PubMed] [Google Scholar]
- 41.Ben-Porath I, Weinberg RA. The signals and pathways activating cellular senescence. Int J Biochem Cell Biol. 2005;37:961–976. doi: 10.1016/j.biocel.2004.10.013. [DOI] [PubMed] [Google Scholar]
- 42.Sakima A, Averill DB, Gallagher PE, Kasper SO, Tommasi EN, Ferrario CM, Diz DI. Impaired heart rate baroreflex in older rats: role of endogenous angiotensin-(1-7) at the nucleus tractus solitarii. Hypertension. 2005;46:333–340. doi: 10.1161/01.HYP.0000178157.70142.33. [DOI] [PubMed] [Google Scholar]
- 43.Ferder LF, Inserra F, Basso N. Advances in our understanding of aging: role of the renin-angiotensin system. Current Opinion in Pharmacology. 2002;2:189–194. doi: 10.1016/s1471-4892(02)00139-x. [DOI] [PubMed] [Google Scholar]
- 44.Basso N, Cini R, Pietrelli A, Ferder L, Terragno NA, Inserra F. Protective effect of long-term angiotensin II inhibition. Am J Physiol Heart Circ Physiol. 2007;293:H1351–1358. doi: 10.1152/ajpheart.00393.2007. [DOI] [PubMed] [Google Scholar]
- 45.Benigni A, Corna D, Zoja C, Sonzogni A, Latini R, Salio M, Conti S, Rottoli D, Longaretti L, Cassis P, Morigi M, Coffman TM, Remuzzi G. Disruption of the Ang II type 1 receptor promotes longevity in mice. J Clin Invest. 2009;119:524–530. doi: 10.1172/JCI36703. [DOI] [PMC free article] [PubMed] [Google Scholar]