Abstract
Males exhibit greater histologic and behavioral impairment after stroke than do age-matched females. However, the contribution of androgens to stroke outcome remains unclear. We compared outcomes from middle cerebral artery occlusion (MCAO) in castrated mice with those in testosterone- or dihydrotestosterone (DHT)-replaced castrated mice. Castrates treated with 1.5 mg testosterone or 0.5 mg DHT before MCAO showed smaller infarct volumes (hemisphere: 27 or 26%) at 24 h after 90 mins MCAO than did untreated castrates (37%), whereas 5 mg testosterone or 1.5 mg DHT exacerbated infarcts (53 or 51%). These outcomes were blocked by the androgen receptor antagonist, flutamide, suggesting that androgen receptors mediate these responses to ischemia. We further evaluated long-term outcomes with a milder 60-min MCAO in castrates treated with the protective 1.5 mg testosterone dose. Consistent with data obtained at 24 h reperfusion, the infarct volume was decreased at 9 days reperfusion. Neurobehavioral analysis showed that motor functional recovery was improved during the first 3 days of reperfusion, but not improved at 7 days. We conclude that testosterone exhibits dose-dependent and time-sensitive effects after ischemia and that testosterone is likely to be an important factor in sex-linked differences in cerebrovascular disease.
Keywords: ischemia, MCAO, neurobehavior, testosterone
Introduction
Male sex is a significant risk factor for cerebrovascular disease and stroke (Foulkes et al, 1988). In experimental stroke and traumatic brain injury, adult male animals sustain consistently more damage relative to age-matched females (Hall et al, 1991; Li et al, 1996; Alkayed et al, 1998; Bramlett and Dietrich, 2001). Nevertheless, it is unclear if androgens account for, or influence, ischemic sensitivity in the male brain. The sparse observations available in the human stroke literature suggest that low levels of the principal circulating androgen, testosterone, are linked to increased morbidity and poor recovery (Jeppesen et al, 1996). However, the removal of endogenous testosterone by castration results in decreased ischemic damage in male rodents (Yang et al, 2002; Cheng et al, 2007). Infarct volume after middle cerebral artery occlusion (MCAO) increases in castrated males when testosterone is replaced, consistent with the concept that endogenous testosterone exacerbates ischemic brain injury in gonadally intact males (Hawk et al, 1998; Yang et al, 2002; Cheng et al, 2007). In contrast, functional outcome after MCAO is improved by testosterone (Pan et al, 2005), perhaps because of the steroid's ability to promote motor axon survival and regeneration (Fargo et al, 2008). Finally, testosterone protects cultured neurons from oxidative stress, β-amyloid toxicity, and serum deprivation through an androgen receptor (AR)-dependent mechanism (Ahlbom et al, 1999, 2001; Hammond et al, 2001; Pike, 2001).
Several factors may be important in explaining these conflicting observations. Earlier studies have not generally addressed the possibility that testosterone and its metabolite, dihydrotestosterone (DHT), have complex dose–response relationships in ischemic stroke. In addition, most studies evaluated androgen effects on histologic outcome within 24 h after ischemia, without considering mature infarct size taken in conjunction with behavioral end points. Therefore, the purpose of this study was to assess two doses of testosterone and its potent metabolite and AR agonist, DHT, for effects on short- and long-term histologic damage and behavioral recovery in male mice. We hypothesized that the effect of these steroids, even within a relatively narrow, physiologically relevant range, is dose dependent and mediated through AR.
Materials and methods
All experiments were carried out in accordance with National Institutes of Health guidelines for research animal care and approved by the Institutional Animal Care and Use Committee at Oregon Health & Science University.
Experimental Groups
All experiments were carried out on male C57Bl/6 mice (Charles River Laboratories, Wilmington, MA, USA), ∼9 to 10 weeks of age and weighing 22 to 30 g. The mice were maintained on a 12/12-h light–dark cycle and permitted ad libitum access to water and standard lab chow. The mice were randomized for surgery and behavioral testing.
The experimental animals were divided into 12 groups using two protocols. Eight groups were analyzed for infarct volume at 24 h reperfusion after 90 mins MCAO: gonadally intact male mice (INTACT, n = 20); untreated castrated male mice (CAST, n = 25); castrated male mice implanted with subcutaneous pellets containing 1.5 mg testosterone (T(1.5), n = 20); 1.5 mg testosterone + 1.5 mg flutamide (T(1.5) + F(1.5), n = 12); 5 mg testosterone (T(5), n = 13); 5 mg testosterone + 10 mg flutamide (T(5) + F(10), n = 13); 0.5 mg DHT (DHT(0.5), n = 11); or with 1.5 mg DHT (DHT(1.5), n = 14). Four additional groups were evaluated for long-term histologic and behavioral outcomes: untreated castrated male mice subjected to 60 mins MCAO (n = 34) or sham MCAO (n = 11) and castrated male mice implanted with 1.5 mg testosterone subjected to 60 mins MCAO (n = 27) or sham MCAO (n = 12).
Castration and Hormone Replacements
Castration and hormone pellet implantation were carried out 1 week before MCAO and sham MCAO. Mice were anesthetized with isoflurane (induction 5.0% and maintenance 1.0% to 2.0%, delivered through a face mask in O2-enriched air), and surgical castration was carried out through a midline scrotal incision to allow bilateral access to the testis. After exposing each testicle, a cautery (ACCU-TEMP, Medtronic Xomed, Jacksonville, FL, USA) was used to remove the testicle and adhere the sac after removal. (Mice in the intact group were anesthetized with isoflurane for 15 mins, but no surgical castration was performed.) Hormone pellets were implanted at the time of surgical castration. Lower abdominal skin was incised, and each pellet was positioned subcutaneously. After each procedure, the skin was closed with 6-0 Vicryl (Ethicon, Somerville, NJ) sutures.
Assessment of Flutamide Effectiveness
To confirm the antagonist properties of flutamide in ischemic mouse brain, we evaluated the effectiveness of our flutamide implant in blocking the transcription of an androgen-responsive gene after MCAO. We have shown earlier that Snf1-like kinase (Snf1lk) is robustly induced by androgens in the cortex after MCAO (Cheng et al, 2007). We now evaluated castrated mice (n = 3), castrated mice treated with T (1.5 mg, n = 2), with T + F (1.5 mg, n = 2), or with DHT (0.5 mg, n = 3). All mice were exposed to MCAO (90 mins), and then the peri-infarct cortex was subdissected for measurement of snf1lk. SNF1lk mRNA level was measured by quantitative PCR (commercial Taqman probe and primers, Applied Biosystems, Foster City, CA, USA, Mm00440322_g1) and normalized to 18S RNA (Cheng et al, 2007). The expression of snf1lk in the cortex was evaluated relative to the corresponding cortical site within the nonischemic side and expressed as a ratio of ipsilateral to contralateral snf1lk. We confirmed that T induces snf1lk (ratio 2.8) and DHT (3.4) in the ischemic cortex. Flutamide blocked the T induction of snf1lk (1.7) to an expression level similar to that observed in castrated mice without endogenous androgens (1.5).
Middle Cerebral Artery Occlusion Model
Transient focal cerebral ischemia was induced using reversible MCAO through the intra-luminal filament technique as described earlier (Zhang et al, 2007). Briefly, mice were anesthetized with isoflurane (induction 5.0% and maintenance 1.5% to 2.0%, delivered through a face mask in O2-enriched air). Head temperature was monitored using a probe placed under the left temporal muscle and was maintained at 35.5°C to 37.5°C throughout the MCAO surgery with water pads. A small laser–Doppler probe (Model MBF3D, Moor Instruments, Oxford, England, UK) was affixed to the skull to monitor cortical perfusion and to verify vascular occlusion and reperfusion. A skin incision was made in the middle of the anterior neck. After the right common carotid artery was tightened using 6-0 silk suture (ETHICON, Somerville, NJ, USA), a 6-0 nylon monofilament with a silicone-coated tip was inserted into the right internal carotid artery through the external carotid artery until the laser–Doppler flowmetry (LDF) value decreased to < 35% of baseline. After securing the filament in place, the surgical site was closed with 6-0 Vicryl sutures. Each mouse was then placed in a separate cage, with a warm water pad under the cage and a blanket over the cage. At the end of the period of occlusion, the mice were re-anesthetized, the laser–Doppler probe was re-positioned over the same site on the skull, and the occluding filament was withdrawn for reperfusion. The mice were then allowed to recover and were observed according to the experimental protocol. In the sham MCAO animal group, each procedure was performed in the same way as in the MCAO animal group, except that the common, external, and internal carotid arteries were not disturbed.
Infarct Volume Assessment
After the period of reperfusion, the mice were anesthetized with isoflurane (4.0% to 5.0%), and blood was collected for hormone analysis. The animals were then decapitated for brain removal. If subarachnoid hemorrhage was observed, the mouse was excluded from the study. Each brain was sliced into five 2-mm-thick coronal sections. The sections were placed in a 1.2% solution of 2,3,5-triphenyltetrazolium chloride (TTC, Sigma, St Louis, MO, USA) for 30 mins at 37°C and fixed in 10% formalin for 24 h. Both sides of each stained coronal slice were photographed using a digital camera, and infarction was measured with digital image analysis software (SigmaScan Pro; Jandel, San Rafael, CA, USA) and integrated across all five slices. To account for the effect of edema, the infarcted volume was estimated indirectly and expressed as a percentage of the contralateral structure.
Hormone Assays
Serum levels of total and free testosterone and of DHT were measured by radioimmunoassay (Diagnostic Products, Los Angeles, CA, USA). The lower limits of detection for total and free testosterone and for DHT were 0.01 ng/mL, 0.5 pg/mL, and 0.01 ng/mL, respectively. While calculating mean hormone levels per treatment group, a value of zero was assigned to hormone values less than the lower limits of detection.
Behavioral Evaluations
We selected behavioral tests that have consistently provided reliable evaluations of outcome across experimental ischemia studies in our laboratories (Li et al, 2004; Craft et al, 2006). All mice undergoing behavioral testing were single housed in a 12/12-h light–dark cycle, and all assessments were carried out during the second half of the light cycle (1200 to 1800 hours). The observer who carried out and scored the mouse behavior tests was blind to the treatment group. All equipments were cleaned with 10% ethanol between trials.
Cylinder test
We carried out the cylinder test (Schallert et al, 2000) to analyze forelimb use bias. Each mouse was placed in a transparent cylinder measuring 9 cm in diameter and 15 cm in height. The cylinder was wide enough for the mouse to move freely and small enough to encourage rearing behavior. Four separate video cameras were placed around the cylinder at 90° intervals to record rearing behavior from all angles. We recorded a maximum of two paw touches for one rearing event. If the mouse touched the cylinder wall with the same paw twice, we designated these motions as ‘independent.’ If the mouse touched the cylinder wall the first time with one paw and a second time with the other paw, we designated these motions as ‘independent’ and ‘both,’ respectively. A total of 20 forelimb touches were recorded during the 10-min test and were found to be sufficient to determine bias without habituating the mouse to the apparatus. Mice with fewer than 20 forelimb touches in this time period were excluded from behavioral analysis. The final score was calculated as the percentage of total touches that used the impaired forelimb. Baseline paw preference was assessed 1 day before surgery (Pre). Subsequent assessments were made at 3 and 7 days after surgery (Day 3 and Day 7).
Neurologic deficit score
This score was recorded in each mouse on days 1 and 3 after surgery (Day 1 and Day 3) as described earlier (Li et al, 2004). The graded scoring systems ranged from 0 to 2 to 0 to 5 depending on the behavior assessed, with 0 indicating no deficit and 2 to 5 indicating the most impaired. The behaviors assessed included consciousness (0 to 3), interaction (0 to 2), eye appearance (0 to 2), breathing (0 to 2), food/water intake (0 to 2), ability to grab wire top (0 to 2), motor function (0 to 5), and activity (0 to 2).
Latency to move
Each mouse was placed in the center of a 12-cm-diameter circle on a flat surface and the time required for the mouse to move outside the circle was recorded at Day 1 and Day 3, as described earlier (Li et al, 2004).
Physiologic Measurements
In a separate, nonsurviving cohort of animals, LDF, mean arterial blood pressure, arterial blood gases (pH, PaO2, PaCO2), head temperature, and blood glucose values were measured just before occlusion and at 45 and 90 mins during MCAO in castrates (n = 3) and in castrates implanted with 1.5 mg testosterone (n = 3) to ensure consistency of occlusion and equivalency of physiologic variables between these two groups.
Statistics
All data are expressed as mean ± s.e.m. Infarct volumes and hormone assay data were analyzed using one-way analysis of variance (ANOVA) with the post hoc Student–Newman–Keuls multiple range test or Dunn's method for multiple groups as indicated, and the unpaired t-test when comparing two groups. Physiologic data were analyzed by two-way ANOVA with a post hoc Student–Newman–Keuls test to correct for multiple comparisons. Behavior testing was analyzed by two-way ANOVA with a post hoc Newman–Keuls test to correct for multiple comparisons and also one-way ANOVA repeated measurement with the post hoc Newman–Keuls test to correct for multiple comparisons for paw preference test in each group. Survival rates were analyzed by log-rank test in behavior testing. P < 0.05 was considered statistically significant. All statistical analyses were carried out using SigmaStat Statistical Software (Version 3.0; SPSS, Chicago, IL, USA).
Results
Infarct Volumes in Untreated and Androgen-Replaced Castrates
Gonadally intact and castrated male C57Bl/6 mice and castrated male C57Bl/6 mice implanted with subcutaneous androgens were subjected to 90 mins MCAO. Premature mortality was observed in two mice in the INTACT group, in five in CAST, in zero in T(1.5), in one in T(1.5) + F(1.5), in one in T(5), in zero in T(5) + F(10), in zero in DHT(0.5), and in one in the DHT(1.5) group. Mice excluded due to intra-ischemic LDF≥35% were zero in all groups, except for one in the T(5) + F(10) group and one in the D(1.5) group. No differences in intra-ischemic physiologic parameters and LDF reduction were observed among experimental groups (Table 1).
Table 1.
LDF and temporalis muscle temperature in 90 mins MCAO+24 h reperfusion
| Group | LDF (%) | Temporalis muscle temperature (°C) | |||
|---|---|---|---|---|---|
| MCAO 85 mins | Reperfusion 5 mins | Pre MCAO | MCAO 85 mins | Reperfusion 5 mins | |
| INTACT (n = 18) | 16 ± 1 | 93 ± 4 | 36.8 ± 0.1 | 36.3 ± 0.1 | 36.3 ± 0.1 |
| CAST (n = 20) | 16 ± 1 | 89 ± 2 | 36.8 ± 0.1 | 36.5 ± 0.1 | 36.5 ± 0.1 |
| T(1.5) (n = 20) | 13 ± 1 | 90 ± 4 | 36.8 ± 0.1 | 36.5 ± 0.1 | 36.6 ± 0.1 |
| T(1.5)+F(1.5) (n = 11) | 15 ± 1 | 91 ± 2 | 36.9 ± 0.1 | 36.7 ± 0.1 | 36.6 ± 0.1 |
| T(5) (n = 12) | 15 ± 2 | 91 ± 4 | 36.9 ± 0.1 | 36.7 ± 0.1 | 36.7 ± 0.1 |
| T(5)+F(10) (n = 12) | 14 ± 1 | 92 ± 4 | 37.0 ± 0.1 | 36.7 ± 0.1 | 36.7 ± 0.1 |
| DHT(0.5) (n = 11) | 11 ± 2 | 88 ± 4 | 36.5 ± 0.1 | 36.7 ± 0.1 | 36.7 ± 0.1 |
| DHT(1.5) (n = 12) | 13 ± 1 | 89 ± 4 | 36.7 ± 0.1 | 36.7 ± 0.1 | 36.8 ± 0.1 |
CAST, untreated castrated male mice; DHT, dihydrotestosterone; F, flutamide; INTACT, gonadally intact male mice; LDF, laser–Doppler flowmetry; MCAO, middle cerebral artery occlusion; T, testosterone.
Values are mean ± s.e.m.
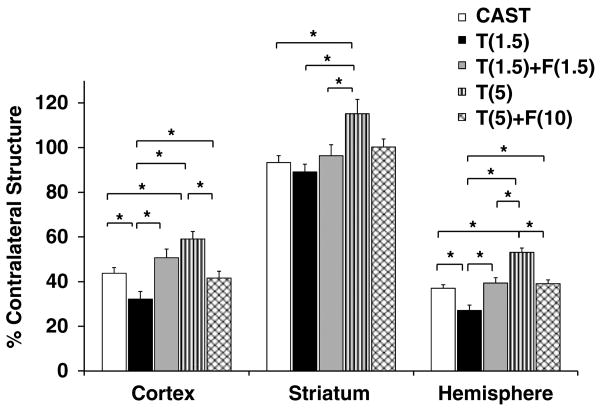
Castrated mice had significantly smaller infarct volumes than did intact male mice (37 ± 2% in CAST versus 48 ± 3% in INTACT total hemisphere), a pattern that was also apparent in the cortex (44 ± 3% in CAST versus 56 ± 3% in INTACT) and in the striatum (94 ± 3% in CAST versus 100 ± 5% in INTACT). Castrated mice implanted with low-dose (1.5 mg) testosterone sustained smaller infarct volumes compared with castrated mice (Figure 1). However, castrated mice implanted with high-dose testosterone sustained larger infarct volumes as compared with castrates. Damage in the T(5) group was not different from that in intact males.
Figure 1.
Dose-dependent effects of testosterone and the androgen receptor antagonist, flutamide, on infarct volumes. Infarct volumes (% contralateral structure = total infarct volume of ipsilateral structure/total volume contralateral structure) were assessed in untreated castrated mice (CAST, n = 20) and in castrates implanted with 1.5 mg testosterone (T(1.5), n = 20), with 1.5 mg testosterone and 1.5 mg flutamide (T(1.5) + F(1.5), n = 11), with 5 mg testosterone (T(5), n = 12), or with 5 mg testosterone and 10 mg flutamide (T(5) + F(10), n = 12). Values are mean ± s.e.m. *P < 0.05.
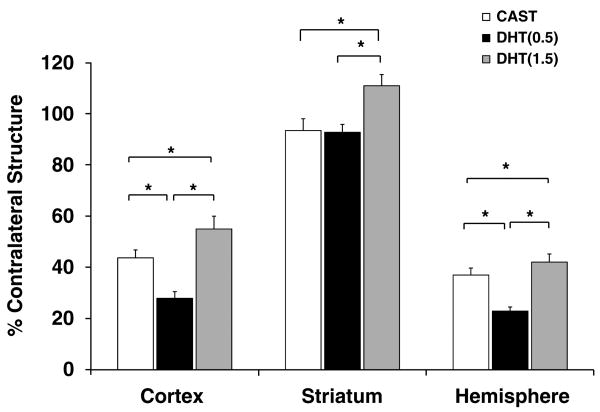
Effects of low- and high-dose testosterone on infarct size were blocked by concurrent exposure to flutamide (Figure 1). To further assess whether ARs play a role in mediating ischemic damage, we treated castrated mice with DHT, an AR agonist that is more potent than testosterone and cannot be aromatized to estrogen. Compared with castrated mice, castrates treated with low-dose (0.5 mg) DHT had significantly smaller infarct volumes, whereas castrates implanted with high-dose (1.5 mg) DHT had significantly larger infarct volumes in the cortex and hemisphere (Figure 2).
Figure 2.
Dose-dependent effects of DHT on infarct volumes. Infarct volumes (% contralateral structure = total infarct volume of ipsilateral structure/total volume contralateral structure) were assessed in untreated castrated mice (CAST, n = 20) and in castrates implanted with 0.5 mg DHT (DHT(0.5), n = 11) or with 1.5 mg DHT (DHT(1.5), n = 12). Values are mean ± s.e.m. * P < 0.05.
Baseline and Intra-Ischemic Arterial Blood Pressure and Blood Gases
To further evaluate the physiologic mechanisms by which low-dose testosterone could reduce ischemic injury, we measured blood pressure and arterial oxygenation in castrated and T(1.5) nonsurvival cohorts. There were no differences between the groups; mean arterial blood pressure, arterial blood gases, and glucose remained within physiologic limits (Table 2).
Table 2.
Physiologic parameters in CAST and T(1.5) groups during MCAO
| Group | CAST (n = 3) | T(1.5) (n = 3) | ||||
|---|---|---|---|---|---|---|
| Pre-MCAO | MCAO | Pre-MCAO | MCAO | |||
| 45 mins | 90 mins | 45 mins | 90 mins | |||
| MABP (mm Hg) | 78 ± 1 | 77 ± 3 | 78 ± 2 | 82 ± 1 | 82 ± 5 | 77 ± 4 |
| LDF (%) | 100 | 17 ± 4 | 19 ± 4 | 100 | 14 ± 2 | 12 ± 3 |
| Temp (°C) | 37.0 ± 0.2 | 37.1 ± 0.1 | 36.7 ± 0.3 | 36.4 ± 0.1 | 37.2 ± 0.1 | 37.1 ± 0.3 |
| pH | 7.36 ± 0.02 | 7.35 ± 0.01 | 7.33 ± 0.02 | 7.41 ± 0.02 | 7.39 ± 0.02 | 7.36 ± 0.02 |
| PaO2 (mm Hg) | 148 ± 1 | 152 ± 3 | 149 ± 6 | 154 ± 3 | 145 ± 2 | 156 ± 6 |
| PaCO2 (mm Hg) | 37 ± 4 | 36 ± 4 | 37 ± 2 | 31 ± 2 | 32 ± 2 | 34 ± 2 |
| Glucose (mg/dL) | 134 ± 23 | 162 ± 16 | 166 ± 7 | 138 ± 14 | 149 ± 25 | 177 ± 10 |
CAST, untreated castrated male mice; LDF, laser–Doppler flowmetry; MABP, mean arterial blood pressure; MCAO, middle cerebral artery occlusion; T, testosterone; Temp, temporalis muscle temperature.
Values are mean ± s.e.m.
Hormone Levels
In this study, total and free testosterone and DHT levels in each mouse were within the range of those in naive male mice (n = 12) in our laboratory (Table 3). In untreated castrates, free serum testosterone was undetectable; but in testosterone-replaced castrates, both total and free testosterone levels increased relative to the dose of testosterone implanted. Dihydrotestosterone replacement had no effect on total or free testosterone, but increased serum DHT (Table 3).
Table 3.
Serum hormone levels in naive male mice and mice treated with 90 mins MCAO+24 h reperfusion
| Group | Testosterone | DHT | |
|---|---|---|---|
| Total | Free (pg/mL) | (ng/mL) (ng/mL) | |
| INTACT (n = 18) | 0.8 ± 0.1 | 0.5 ± 0.4 | NM |
| CAST (n = 20) | 0.3 ± 0.0 | 0.0 ± 0.0 | NM |
| T(1.5) (n = 20) | 11.0 ± 0.7 | 27.3 ± 1.2 | NM |
| T(1.5)+F(1.5) (n = 11) | 8.5 ± 0.8 | 23.2 ± 2.3 | NM |
| T(5) (n = 12) | 21.9 ± 2.3 | 43.4 ± 2.6 | NM |
| T(5)+F(10) (n = 12) | 18.6 ± 2.7 | 48.8 ± 1.8 | NM |
| DHT(0.5) (n = 11) | 0.1 ± 0.0 | 0.0 ± 0.0 | 0.4 ± 0.1 |
| DHT(1.5) (n = 12) | 0.2 ± 0.1 | 0.0 ± 0.0 | 0.9 ± 0.1 |
| Naïve (n = 12) | 5.7 ± 2.0 [0.1–21.7] | 15.7 ± 5.7 [0.0–55.1] | 0.9 ± 0.3 [0.0–3.0] |
CAST, untreated castrated male mice; DHT, dihydrotestosterone; F, flutamide; INTACT, gonadally intact male mice; MCAO, middle cerebral artery occlusion; NM, not measured; [], range; T, testosterone.
Values are mean ± s.e.m.
Naive indicates male mice without any treatments or surgeries in our laboratory.
Androgen levels were also measured in the long-term survival cohorts used in behavioral studies. Total and free testosterone and DHT in each group were, respectively, CAST + MCAO: 0.1 ± 0.0 ng/mL, 0.0 ± 0.0 pg/mL, and 0.1 ± 0.0 ng/mL; CAST + sham MCAO: 0.1 ± 0.0 ng/mL, 0.0 ± 0.0 pg/mL, and 0.1 ± 0.0 ng/mL; T(1.5) + MCAO: 4.2 ± 0.3 ng/mL, 10.6 ± 0.9 pg/mL, and 0.4 ± 0.0 ng/mL; T(1.5) + sham MCAO: 3.1 ± 0.2 ng/mL, 8.0 ± 0.9 pg/mL, and 0.4 ± 0.0 ng/mL.
Behavioral Testing
Neurobehavioral testing was carried out on 9-day survival cohorts of castrated C57Bl/6 mice and C57Bl/6 castrates implanted with 1.5 mg testosterone, which had been subjected to 60 mins MCAO or sham MCAO. The survival rate in each group was as follows: CAST MCAO, 52% (18/34 in total); T(1.5) MCAO, 74% (20/27 in total); CAST sham MCAO, 90% (10/11 in total); and T(1.5) sham MCAO, 91% (11/12 in total). There was no significant difference between castrates and low-dose (1.5 mg) testosterone-implanted castrates with regard to survival rate after MCAO (P = 0.069). No mice were excluded due to premature death or owing to failure to induce effect ischemia as measured by intra-ischemic LDF≥35%.
Infarct volume was assessed at the conclusion of behavioral testing (after 9-day survival). Total hemisphere infarct volume was significantly smaller in low-dose (1.5 mg) testosterone-replaced castrates (20 ± 1%) than in castrated mice (30 ± 1%). As expected, no infarction was detected in the surgical sham groups.
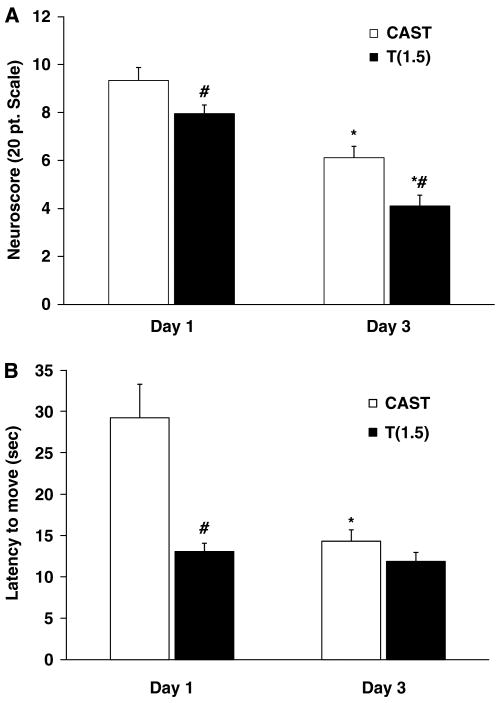
Neuroscore and latency to move were assessed at Day 1 and Day 3 as an indicator of general health. Figure 3A shows that both castrated and low-dose (1.5 mg) testosterone-implanted castrated groups showed impairment on Day 1 (neuroscores in the 8 to 9 range) and significant improvement by Day 3 (neuroscores in the 4 to 6 range). Interestingly, neuroscore performance was significantly better at both time points in castrates with 1.5-mg testosterone replacement compared with that in untreated castrates. Similarly, these testosterone-replaced castrates also exhibited significantly shorter latency to move on Day 1 than did untreated castrates. By Day 3, latency to move was similar for these two experimental groups (Figure 3B).
Figure 3.
General health recovery improved at an early time point with low-dose testosterone. Neuroscore (A) and latency to move (B) were assessed 1 day after MCAO (Day 1) and 3 days after MCAO (Day 3) in untreated castrated mice (n = 18) and in castrates implanted with 1.5 mg testosterone (n = 20). Values are mean ± s.e.m. *P < 0.05 compared with the same group on Day 1; #P < 0.05 compared with castrates.
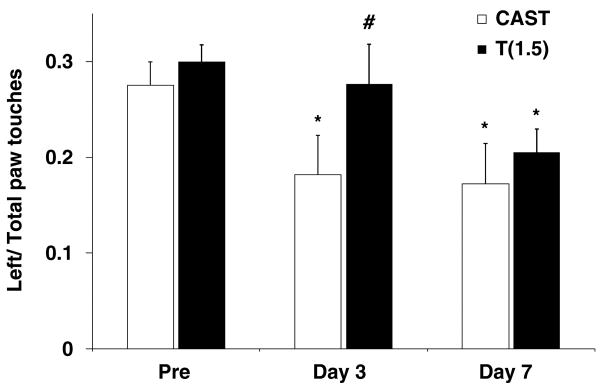
The cylinder test was used to assess motor dysfunction on the affected, contralateral side (left paw) after MCAO. No asymmetries were observed in pre-surgery tests (Figure 4) or in sham-operated animals (data not shown). However, contralateral forelimb use was significantly reduced in castrated MCAO-treated mice at Day 3 (P < 0.05) but not in 1.5-mg testosterone-replaced castrates. By Day 7, impairment was observed in both untreated castrates and testosterone-implanted castrates.
Figure 4.
Functional recovery improved at an early but not at a later time point with low-dose testosterone. Contralateral paw use in untreated castrated mice (n = 18) and in castrates implanted with 1.5 mg testosterone (n = 20) was tested 1 day before MCAO (Pre), 3 days after MCAO (Day 3), and 7 days after MCAO (Day 7). Values are mean ± s.e.m. *P < 0.05 compared with the same group Pre; #P < 0.05 compared with castrates.
Discussion
This study provided three important findings. First, both testosterone and DHT replacements at low doses reduce early infarct size, whereas higher dose steroid repletion exacerbates damage. Second, a signaling mechanism involving AR is likely to be involved; this conclusion is based on the observation that the specific AR antagonist, flutamide, reverses all testosterone effects on infarct size and that DHT, a non-aromatizable androgen that acts specifically at AR, mimics testosterone's actions. The third important finding is that low-dose testosterone reduces mature infarct size. However, benefit to the animal's functional outcome was no longer apparent 7 days after recovery from MCAO.
Earlier studies in rats, and now in mice, agree that surgical castration or testosterone withdrawal reduces ischemic damage (Hawk et al, 1998; Toung et al, 1998; Yang et al, 2002; Cheng et al, 2007). However, experiments using androgen repletion in vivo have produced relatively little agreement on the hypothesis that androgens are damaging agents. Our data show that, depending on dose, testosterone and DHT can promote either neuroprotection or neurodegeneration. These dose-dependent effects on ischemic damage in mouse offer an explanation for disagreement among reports that androgens exacerbate (Hawk et al, 1998; Yang et al, 2002; Cheng et al, 2007), improve, (Pan et al, 2005), or have no effect (Toung et al, 1998) on injury in rat. All of these studies used subcutaneous testosterone implants that yielded physiologic serum steroid levels, but they also each studied only a single dose. Given the opposing effects of testosterone and DHT at different doses shown here, it is not surprising that single-dose studies have produced such varied outcomes.
Consistent with the concept that testosterone can be neuroprotective in vivo, most evaluations of testosterone or DHT in vitro show protective properties in animal (Pike, 2001; Nguyen et al, 2007; Orlando et al, 2007) and human neurons (Hammond et al, 2001; Zhang et al, 2004), in cerebellar granule cells (Ahlbom et al, 2001) and in astrocytes (Gatson and Singh, 2007). In mixed cortical neuronal cultures, testosterone has a U-shaped dose–response curve with protection against excitotoxicity at nmol/L concentrations, amplification of death at íM concentrations, and no effect in between (Orlando et al, 2007). The finding of androgen signaling that switches from protection to injury exacerbation in vivo is in agreement with these observations.
Our observation that in vivo AR signaling integrity is a likely first step in engaging downstream molecular mechanisms has not been shown earlier, but is consistent with the in vitro studies cited above. Coadministration of the specific AR antagonist, flutamide, with testosterone eliminated the ability of steroid to increase or decrease infarct volume. We used the well-characterized, nonsteroidal, antiandrogen flutamide because of its pure antiandrogenicity, high specificity for AR, and for its ability to cross the blood–brain barrier (Neri et al, 1972). Furthermore, it has been widely used in rodent experiments (for review, see Singh et al, 2000) and as a clinical therapy for prostate cancer. We also evaluated the effects of DHT to further implicate AR, because DHT cannot be metabolized to estradiol by the P450 aromatase and interacts directly as an AR agonist. Dihydrotestosterone clearly showed dose-dependent properties that mirrored those of testosterone.
The present data do not elucidate the mechanisms downstream from AR by which testosterone or DHT ameliorates (or exacerbates) ischemic injury. Androgens are believed to signal through a cognate receptor coded by an X-chromosomal locus. This concept was formulated in part through the observation that a naturally occurring single gene mutation in humans and animals results in a complete loss of androgen-associated function (i.e., testicular feminizing syndrome; for review, see Couse and Korach, 1998). Through classical steroid genomic mechanisms, AR acts as a transcriptional factor for androgen-responsive genes, potentially increasing or decreasing gene products involved in cell survival. We have shown earlier, using microarray and confirmatory quantitative PCR, that DHT alters a wide variety of RNA transcripts after MCAO, which are involved in inflammation, cell signaling, and blood–brain barrier regulation (Cheng et al, 2007). More recently, it has become clear that androgen–AR signaling involves several nonclassical paradigms (for review, see Kampa et al, 2008). Nongenomic mechanisms have been described in vitro wherein androgens rapidly activate phosphoinositide 3-kinase/AKT or MAPK pathways associated with cytoprotection (Gatson et al, 2006; Pike et al, 2008). Finally, two isoforms of classical AR have been described in bone, skin fibroblasts, and in fetal reproductive tissue, including an N-terminal truncated form that may oppose actions of the full-length protein (Wilson and McPhaul, 1996; Liegibel et al, 2003). Furthermore, a plasma membrane-associated receptor has been postulated, through which androgens may signal in non-neuronal tissue and alter cell survival (for reviews, see Heinlein and Chang, 2002, 2004). Further work is essential to determine whether classical AR and nongenomic mechanisms versus transcriptional mechanisms are involved in the ability of androgens to signal by differential means and alter ischemic damage.
We used a battery of behavioral tasks to assess functional outcome early after MCAO (1 to 3 days) and at a later time point (1 week) to extend our initial finding that low-dose testosterone improves histologic outcome. We used a slightly less severe ischemic duration (60 mins) to increase survival in long-term experiments, and again observed that histologic damage was smaller in testosterone-replaced than in untreated mice at 9 days after injury. Although there was no statistical difference in survival rate among treatment groups (P = 0.069), it seems close to being significant. Moreover, the improved neuroscore after MCAO in testosterone-replaced mice suggests that integrated health status was improved. Similarly, the latency to move (LTM) task indicates that testosterone replacement diminishes early impairment after MCAO. We used the cylinder test, which measures contralateral forelimb impairment, because it has been shown to detect impairment for several weeks after MCAO in mice (Li et al, 2004). Similar to the findings with neuroscore and LOM, testosterone treatment prevented the contralateral forelimb impairment observed at 3 days after MCAO in castrates. However, this positive outcome was no longer present by 7 days. Therefore, we conclude that low-dose testosterone replacement is beneficial at early time points after cerebral ischemia, but may not improve long-term deficits.
In conclusion, androgens exhibit dose-dependent and time-sensitive effects after experimental stroke in mice, suggesting that they play an important role in sex-linked differences in cerebrovascular disease. Our pharmacological evidence implicates androgen–AR signaling as being instrumental to both favorable and deleterious effects on tissue outcome, but further work is required to evaluate whether AR transactivation or repression of androgen-responsive genes is involved. Finally, further studies of behavioral outcomes are needed to fully chart the time course of the actions of androgen on recovering neural pathways.
Acknowledgments
The authors thank Ms Kathy Gage, Grants and Publications Writer for the Department of Anesthesiology and Perioperative Medicine, OHSU, for her outstanding editorial work in the preparation of this paper. They also gratefully acknowledge Administrative Coordinators, Ashley Branch, and John Todd, for expert paper preparation.
This study was supported by the grants from the National Institutes of Health (NS49210, NR03521).
Footnotes
Conflict of interest: The authors declare no conflict of interest.
References
- Ahlbom E, Prins GS, Ceccatelli S. Testosterone protects cerebellar granule cells from oxidative stress-induced cell death through a receptor mediated mechanism. Brain Res. 2001;892:255–62. doi: 10.1016/s0006-8993(00)03155-3. [DOI] [PubMed] [Google Scholar]
- Ahlbom E, Grandison L, Bonfoco E, Zhivotovsky B, Ceccatelli S. Androgen treatment of neonatal rats decreases susceptibility of cerebellar granule neurons to oxidative stress in vitro. Eur J Neurosci. 1999;11:1285–91. doi: 10.1046/j.1460-9568.1999.00529.x. [DOI] [PubMed] [Google Scholar]
- Alkayed NJ, Harukuni I, Kimes AS, London ED, Traystman RJ, Hurn PD. Gender-linked brain injury in experimental stroke. Stroke. 1998;29:159–66. doi: 10.1161/01.str.29.1.159. [DOI] [PubMed] [Google Scholar]
- Bramlett HM, Dietrich WD. Neuropathological protection after traumatic brain injury in intact female rats versus males or ovariectomized females. J Neurotrauma. 2001;18:891–900. doi: 10.1089/089771501750451811. [DOI] [PubMed] [Google Scholar]
- Cheng J, Alkayed NJ, Hurn PD. Deleterious effects of dihydrotestosterone on cerebral ischemic injury. J Cereb Blood Flow Metab. 2007;27:1553–62. doi: 10.1038/sj.jcbfm.9600457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couse JF, Korach KS. Exploring the role of sex steroids through studies of receptor deficient mice. J Mol Med. 1998;76:497–511. doi: 10.1007/s001090050244. [DOI] [PubMed] [Google Scholar]
- Craft TK, Zhang N, Glasper ER, Hurn PD, Devries AC. Neonatal factors influence adult stroke outcome. Psychoneuroendocrinology. 2006;31:601–13. doi: 10.1016/j.psyneuen.2006.01.002. [DOI] [PubMed] [Google Scholar]
- Fargo KN, Galbiati M, Foecking EM, Poletti A, Jones KJ. Androgen regulation of axon growth and neurite extension in motoneurons. Horm Behav. 2008;53:716–28. doi: 10.1016/j.yhbeh.2008.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foulkes MA, Wolf PA, Price TR, Mohr JP, Hier DB. The Stroke Data Bank: design, methods, and baseline characteristics. Stroke. 1988;19:547–54. doi: 10.1161/01.str.19.5.547. [DOI] [PubMed] [Google Scholar]
- Gatson JW, Singh M. Activation of a membrane-associated androgen receptor promotes cell death in primary cortical astrocytes. Endocrinology. 2007;148:2458–64. doi: 10.1210/en.2006-1443. [DOI] [PubMed] [Google Scholar]
- Gatson JW, Kaur P, Singh M. Dihydrotestosterone differentially modulates the mitogen-activated protein kinase and the phosphoinositide 3-kinase/Akt pathways through the nuclear and novel membrane androgen receptor in C6 cells. Endocrinology. 2006;147:2028–34. doi: 10.1210/en.2005-1395. [DOI] [PubMed] [Google Scholar]
- Hall ED, Pazara KE, Linseman KL. Sex differences in postischemic neuronal necrosis in gerbils. J Cereb Blood Flow Metab. 1991;11:292–8. doi: 10.1038/jcbfm.1991.61. [DOI] [PubMed] [Google Scholar]
- Hammond J, Le Q, Goodyer C, Gelfand M, Trifiro M, LeBlanc A. Testosterone-mediated neuroprotection through the androgen receptor in human primary neurons. J Neurochem. 2001;77:1319–26. doi: 10.1046/j.1471-4159.2001.00345.x. [DOI] [PubMed] [Google Scholar]
- Hawk T, Zhang YQ, Rajakumar G, Day AL, Simpkins JW. Testosterone increases and estradiol decreases middle cerebral artery occlusion lesion size in male rats. Brain Res. 1998;796:296–8. doi: 10.1016/s0006-8993(98)00327-8. [DOI] [PubMed] [Google Scholar]
- Heinlein CA, Chang C. Androgen receptor (AR) coregulators: an overview. Endocr Rev. 2002;23:175–200. doi: 10.1210/edrv.23.2.0460. [DOI] [PubMed] [Google Scholar]
- Heinlein CA, Chang C. Androgen receptor in prostate cancer. Endocr Rev. 2004;25:276–308. doi: 10.1210/er.2002-0032. [DOI] [PubMed] [Google Scholar]
- Jeppesen LL, Jorgensen HS, Nakayama H, Raaschou HO, Olsen TS, Winther K. Decreased serum testosterone in men with acute ischemic stroke. Arterioscler Thromb Vasc Biol. 1996;16:749–54. doi: 10.1161/01.atv.16.6.749. [DOI] [PubMed] [Google Scholar]
- Kampa M, Pelekanou V, Castanas E. Membrane-initiated steroid action in breast and prostate cancer. Steroids. 2008;73:953–60. doi: 10.1016/j.steroids.2007.12.009. [DOI] [PubMed] [Google Scholar]
- Li K, Futrell N, Tovar S, Wang LC, Wang DZ, Schultz LR. Gender influences the magnitude of the inflammatory response within embolic cerebral infarcts in young rats. Stroke. 1996;27:498–503. doi: 10.1161/01.str.27.3.498. [DOI] [PubMed] [Google Scholar]
- Li X, Blizzard KK, Zeng Z, DeVries AC, Hurn PD, McCullough LD. Chronic behavioral testing after focal ischemia in the mouse: functional recovery and the effects of gender. Exp Neurol. 2004;187:94–104. doi: 10.1016/j.expneurol.2004.01.004. [DOI] [PubMed] [Google Scholar]
- Liegibel UM, Sommer U, Boercsoek I, Hilscher U, Bierhaus A, Schweikert HU, Nawroth P, Kasperk C. Androgen receptor isoforms AR-A and AR-B display functional differences in cultured human bone cells and genital skin fibroblasts. Steroids. 2003;68:1179–87. doi: 10.1016/j.steroids.2003.08.016. [DOI] [PubMed] [Google Scholar]
- Neri R, Florance K, Koziol P, Van Cleave S. A biological profile of a nonsteroidal antiandrogen, SCH 13521 (4′-nitro-3′trifluoromethylisobutyranilide) Endocrinology. 1972;91:427–37. doi: 10.1210/endo-91-2-427. [DOI] [PubMed] [Google Scholar]
- Nguyen TV, Yao M, Pike CJ. Flutamide and cyproterone acetate exert agonist effects: induction of androgen receptor-dependent neuroprotection. Endocrinology. 2007;148:2936–43. doi: 10.1210/en.2006-1469. [DOI] [PubMed] [Google Scholar]
- Orlando R, Caruso A, Molinaro G, Motolese M, Matrisciano F, Togna G, Melchiorri D, Nicoletti F, Bruno V. Nanomolar concentrations of anabolic-androgenic steroids amplify excitotoxic neuronal death in mixed mouse cortical cultures. Brain Res. 2007;1165:21–9. doi: 10.1016/j.brainres.2007.06.047. [DOI] [PubMed] [Google Scholar]
- Pan Y, Zhang H, Acharya AB, Patrick PH, Oliver D, Morley JE. Effect of testosterone on functional recovery in a castrate male rat stroke model. Brain Res. 2005;1043:195–204. doi: 10.1016/j.brainres.2005.02.078. [DOI] [PubMed] [Google Scholar]
- Pike CJ, Nguyen TV, Ramsden M, Yao M, Murphy MP, Rosario ER. Androgen cell signaling pathways involved in neuroprotective actions. Horm Behav. 2008;53:693–705. doi: 10.1016/j.yhbeh.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pike CJ. Testosterone attenuates beta-amyloid toxicity in cultured hippocampal neurons. Brain Res. 2001;919:160–5. doi: 10.1016/s0006-8993(01)03024-4. [DOI] [PubMed] [Google Scholar]
- Schallert T, Leasure JL, Kolb B. Experience-associated structural events, subependymal cellular proliferative activity, and functional recovery after injury to the central nervous system. J Cereb Blood Flow Metab. 2000;20:1513–28. doi: 10.1097/00004647-200011000-00001. [DOI] [PubMed] [Google Scholar]
- Singh SM, Gauthier S, Labrie F. Androgen receptor antagonists (antiandrogens): structure–activity relationships. Curr Med Chem. 2000;7:211–47. doi: 10.2174/0929867003375371. [DOI] [PubMed] [Google Scholar]
- Toung TJ, Traystman RJ, Hurn PD. Estrogen-mediated neuroprotection after experimental stroke in male rats. Stroke. 1998;29:1666–70. doi: 10.1161/01.str.29.8.1666. [DOI] [PubMed] [Google Scholar]
- Wilson CM, McPhaul MJ. A and B forms of the androgen receptor are expressed in a variety of human tissues. Mol Cell Endocrinol. 1996;120:51–7. doi: 10.1016/0303-7207(96)03819-1. [DOI] [PubMed] [Google Scholar]
- Yang SH, Perez E, Cutright J, Liu R, He Z, Day AL, Simpkins JW. Testosterone increases neurotoxicity of glutamate in vitro and ischemia–reperfusion injury in an animal model. J Appl Physiol. 2002;92:195–201. doi: 10.1152/jappl.2002.92.1.195. [DOI] [PubMed] [Google Scholar]
- Zhang W, Koerner IP, Noppens R, Grafe M, Tsai HJ, Morisseau C, Luria A, Hammock BD, Falck JR, Alkayed NJ. Soluble epoxide hydrolase: a novel therapeutic target in stroke. J Cereb Blood Flow Metab. 2007;27:1931–40. doi: 10.1038/sj.jcbfm.9600494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Champagne N, Beitel LK, Goodyer CG, Trifiro M, LeBlanc A. Estrogen and androgen protection of human neurons against intracellular amyloid beta1-42 toxicity through heat shock protein 70. J Neurosci. 2004;24:5315–21. doi: 10.1523/JNEUROSCI.0913-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]