Abstract
Our previous works have elucidated that the 12-lipoxygenase (12-Lox) pathway is directly implicated in glutamate-induced neural cell death, and that such that toxicity is prevented by nM concentrations of the natural vitamin E α-tocotrienol (TCT). In the current study we tested the hypothesis that phospholipase A2 (PLA2) activity is sensitive to glutamate and mobilizes arachidonic acid (AA), a substrate for 12-Lox. Furthermore, we examined whether TCT regulates glutamate-inducible PLA2 activity in neural cells. Glutamate challenge induced the release of [3H]AA from HT4 neural cells. Such response was attenuated by calcium chelators (EGTA and BAPTA), cPLA2-specific inhibitor (AACOCF3) as well as TCT at 250 nM. Glutamate also caused the elevation of free polyunsaturated fatty acid (AA and docosahexaenoic acid) levels and disappearance of PL-esterified AA in neural cells. Furthermore, glutamate induced a time-dependent translocation and enhanced serine phosphorylation of cPLA2 in the cells. These effects of glutamate on fatty acid levels and on cPLA2 were significantly attenuated by nM TCT. The observations that AACOCF3, transient knock-down of cPLA2 as well as TCT significantly protected against the glutamate-induced death of neural cells implicate cPLA2 as a TCT-sensitive mediator of glutamate induced neural cell death. This work presents first evidence recognizing glutamate-induced changes in cPLA2 as a novel mechanism responsible for neuroprotection observed in response to nanomolar concentrations of TCT.
Keywords: Glutamate, Neurotoxicity, α-Tocotrienol, vitamin E
INTRODUCTION
Natural vitamin E is a generic term for eight congeners including four tocopherols and four tocotrienols which qualitatively exhibit the biological activity of α-tocopherol (Sen et al. 2007b; Harvard Health Publications 2008). Compared to tocopherols, tocotrienols have been poorly studied (Sen et al. 2006; Sen et al. 2007b; Miyamoto et al. 2009). It is clear, however, that members of the vitamin E family are not redundant with respect to their biological functions. α-Tocotrienol,γ–tocopherol, and δ-tocotrienol have emerged as vitamin E molecules with functions in health and disease that are clearly distinct from that of α-tocopherol (Hensley et al. 2004; Sen et al. 2006; Sen et al. 2007b; Miyamoto et al. 2009). At concentrations 25–50 μM, α-tocopherol uniquely regulates specific signal transduction pathways by mechanisms that are independent of its antioxidant properties (Boscoboinik et al. 1994). Micromolar amounts of tocotrienol, not tocopherol, suppress the activity of hydroxy-3-methylglutaryl coenzyme A reductase (Pearce et al. 1992; Pearce et al. 1994). Tocotrienols possess anti-cancer and cholesterol lowering properties that are often not exhibited by tocopherols (Theriault et al. 1999; Packer et al. 2001; Schaffer et al. 2005; Sen et al. 2007a; Miyazawa et al. 2009). Structurally, tocotrienols differ from tocopherols by possessing a farnesyl (isoprenoid) rather than a saturated phytyl side chain. Ten years ago we have reported first evidence demonstrating that at nanomolar concentration, α-tocotrienol, not α-tocopherol, prevents stroke-related neurodegeneration (Sen et al. 2000). During the last decade our laboratory has published a series of reports characterizing the molecular mechanisms that explain such potent unique neuroprotective activity of tocotrienol (Khanna et al. 2003; Khanna et al. 2005a; Khanna et al. 2006; Khanna et al. 2007; Park et al. 2009) and demonstrating that tocotrienol protects against stroke in vivo (Khanna et al. 2005b). On a concentration basis, this finding represents the most potent of all biological functions exhibited by any natural vitamin E molecule. Recent studies from several laboratories have consistently reported the potent unique neuroprotective properties of tocotrienol in several different experimental settings (Osakada et al. 2004; Shichiri et al. 2007).
Murine HT hippocampal neural cell line, lacking the intrinsic excitotoxicity-pathway, represent an useful model to characterize redox-sensitive pathways involved in neurotoxicity (Schubert and Piasecki 2001; Tan et al. 2001; Dargusch and Schubert 2002; Khanna et al. 2003; Khanna et al. 2006; Khanna et al. 2007; Xu et al. 2007). Our studies on HT cells have recognized the 12-Lox pathway as a glutamate-inducible mechanism that is directly implicated in neural cell death and inhibited by tocotrienol (Khanna et al. 2003; Park et al. 2009). The primary substrate of the 12-Lox pathway, a key mediator of neural cell death (Li et al. 1997), is arachidonic acid (AA) mobilized from the cell membrane. Phospholipase A2 (PLA2) is an important membrane phospholipid-hydrolyzing enzyme that cleaves membrane phospholipids at the sn-2 position to release AA and other free unsaturated fatty acid and lysophospholipid (Dennis et al. 1991). PLA2s present in mammalian cells have been broadly categorized under three main groups such as cytosolic PLA2 (cPLA2), secretory PLA2 (sPLA2), and intracellular PLA2 (iPLA2) depending on their substrate specificities, requirement for calcium, and lipid modification (Chakraborti 2003). PLA2, especially the cPLA2 form, is emerging as a key player in neurotoxicity and neurodegenerative diseases associated with ischemia-reperfusion and oxidant injury (Sapirstein and Bonventre 2000; Sun et al. 2004; Adibhatla and Hatcher 2008). Brain contains predominantly two types of polyunsaturated fatty acids (PUFAs), namely arachidonic and docosahexanoic acids as esters in the sn-2 position of phospholipids which are released upon hydrolysis by PLA2 for subsequent conversion of those PUFAs into eicosanoids (hydroperoxyeicosatetraenoic acid, HPETE) by Lox including 12-Lox (Tassoni et al. 2008). Arachidonic acid-derived eicosanoids are directly implicated in neurodegenerative diseases (Tassoni et al. 2008). We therefore sought to examine whether glutamate-induced PLA2 function in neural cells is sensitive to tocotrienol. This study provides evidence demonstrating that in neural cells glutamate may induce the release of AA via activation of cPLA2. Furthermore, this study recognizes inducible PLA2 as a target of tocotrienol action in the degenerating neural cell.
MATERIALS and METHODS
MATERIALS
L-glutamic acid monosodium salt, dimethyl sulfoxide (DMSO), 1,2-bis-(o-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid tetra-(acetoxymethyl) ester (BAPTA-AM), ethyleneglycol-bis-(β-aminoethylether)-N,N,N′,N′-tetraacetic acid (EGTA), and lactate dehydrogenase (LDH) cytotoxicity assay kit were obtained from Sigma Chemical Co. (St. Louis, MO). α-Tocotrienol (TCT, 90%) was obtained from Carotech Inc, NJ. For cell culture, Dulbecco’s Modified Eagle Medium (DMEM), fetal calf serum, and antibiotics (penicillin and streptomycin) were purchased from Invitrogen Corporation (Carlsbad, CA). Cell culture dishes were obtained from Nunc (Denmark). [3H]Arachidonic acid (AA) was from American Radiolabeled Chemicals, Inc. (St. Louis, MO). AACOCF3 was procured from Cayman Chemical (Ann Arbor, MI). cPLA2α inhibitor was obtained from Calbiochem. Primary rabbit polyclonal antibodies developed against cPLA2 and phosphoserine (Ser-505)-cPLA2 were obtained from Cell Signaling Technology, Inc (Danvers, MA). Secondary anti-Rabbit IgG was obtained from Amersham Biosciences.
Cell Culture & Treatments
Murine hippocampal HT4 neural cells, originally provided by D.E. Koshland Jr., University of California, Berkeley, were grown in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal calf serum, penicillin (100 units/ml), and streptomycin (100 μg/ml) at 37°C in a humidified atmosphere containing 95% air and 5% CO2 (Sen et al. 2000). Confluent cells were trypsinized and sub-cultured at specified density under the standard culture conditions as described before (Khanna et al. 2003; Khanna et al. 2005b; Khanna et al. 2006; Khanna et al. 2007). The cells were then exposed to 10 mM of L-glutamate for the specified duration. Wherever pre-treatment with the chosen pharmacological agent was necessary, the cells were pre-treated with the pharmacological agent alone for the specified length of time, following which the cells were subjected to treatment with the experimental compound. Stock solutions of TCT were prepared in ethanol. Respective controls were treated with an equal volume (0.1%, v/v) of ethanol. TCT (at a final concentration of 250 nM) was added to the culture dishes 10 min before glutamate challenge.
Cell Viability Assay
Viability of cells was assessed by measuring cellular LDH content and release from cells into the medium following glutamate challenge using a in vitro toxicology assay kit (Sigma Chemical Co.) as previously described (Khanna et al. 2003; Khanna et al. 2005b; Khanna et al. 2006; Khanna et al. 2007).
Cell Morphology Examination
Morphology of HT4 cells following treatments was determined by phase-contrast microscopic examination on Zeiss Axiovert 200M microscope. Digital images of cells grown on cell culture plates were captured at 10X magnification.
PLA2 Assay in Intact Cells
PLA2 activity in the intact HT4 cells was assayed by determining the release of AA into the medium (Verity et al. 1994; Lin et al. 1996; Mazerik et al. 2007). The release of [3H]AA into the medium from cells pre-labeled overnight with [3H]AA (carrier free, 0.5 μCi/dish), as an index of PLA2 activity, following treatments with DMEM alone or DMEM containing the chosen pharmacological agent(s) alone or glutamate (10 mM) alone or chosen pharmacological agent(s) + glutamate (10 mM) for the desired length of time. At the end of the experiment, culture medium was collected, centrifuged at 1000 g, and radioactivity in the medium was determined on a liquid scintillation counter. PLA2 activity was expressed as the [3H]AA released into the medium in DPM/dish (3 × 104 cells).
Determination of Fatty Acids
Following treatments of HT4 neuronal cells at different designated times, cellular lipids were extracted by the Folch extraction method with 2:1 chloroform and methanol (vol/vol) and the lipid extracts were taken to dryness under a stream of nitrogen. Once dry, lipid extracts were immediately re-dissolved in a small amount of chloroform:methanol (10:1) and capped under nitrogen. Total phospholipid (PLs) and free fatty acids (FFAs) were then isolated by thin layer chromatography (TLC). Briefly, under N2 environment, lipid extracts were applied to high performance thin layer silica G plates and developed in distilled petroleum ether, diethyl ether, acetic acid (80:20:1 v/v/v). Lipid classes were visualized by dichlororfluorescein spray under UV light. Corresponding bands for polar lipids (total PL) and FFAs were scraped into screw cap tubes. Isolated lipid bands were then dissolved in chloroform and derivatized with 10% boron trifluoride in excess methanol at 70°C. The resulting fatty acid methyl esters were analyzed by capillary gas chromatography employing a Shimadzu GC20 with a 50 m polar capillary column (Quadrex 007-FFAP New Haven CT). Fatty acids were identified by comparison to authentic controls and data were expressed in percent (mol%) composition.
Glutathione Assay
Cellular glutathione levels were determined using the GSH-Glo glutathione assay kit (Promega, Madison, WI). After 8h of glutamate exposure, cells were washed twice with PBS and harvested. Next, cells were centrifuged at 200 × g for 5 min in 4°C, and 110 μl PBS was added to the pellet. The supernatant (25 μl) was used to detect glutathione levels and protein content. For analysis of total protein content, cells were pelleted, lysed and subjected to BCA protein assay.
Subcellular Fractionation
Cells (1×106 cells/100mm dish) were cultured for 24h before exposure to glutamate (10 mM) challenge. After 30 min of glutamate exposure, cells were washed twice with PBS and harvested using cell lifter. Subcellular fractionation of cells was performed using the Qproteome cell compartment kit (Qiagen, Valencia, CA).
Western Blot Analysis
cPLA2 and phosphoserine-cPLA2 in HT4 cells following glutamate treatment were detected by SDS–polyacrylamidegel electrophoresis (SDS-PAGE) and Western blotting analysis as described previously (Sen et al. 2000) utilizing the rabbit primary anti-cPLA2 and anti-phosphoserine-cPLA2 antibodies. Following exposure of cells to DMEM or DMEM containing glutamate (10 mM) for the desired lengths of time, cells were rinsed twice with ice-cold PBS, scraped in 200 μl of lysis buffer containing 20 mM Tris–HCl (pH, 7.4), 150 mM NaCl, 2 mM EGTA, 5 mM glycerophosphate, 1 mM MgCl2, 1% Triton X-100, 1 mM sodium orthovanadate, 10 μg/ml protease inhibitors, 1 μg/ml aprotinin, 1 μg/ml leupeptin, 1 mM phenylmethylsulfonyl fluoride, and 1 μg/ml pepstatin, incubated at 4° C for 10 min and were cleared by centrifugation in a microfuge at 10,000×g for 5 min at 4° C. After determination of the total protein in the lysates, 5× Laemmli sample buffer was added to cell lysates (20–40μg of protein/lane) and boiled for 5 min. Proteins were separated on 4–12% gels by SDS–PAGE and transferred onto polyvinylidene difluoride (PVDF) membranes. After blocking in 10% blotting-grade blocker non fat dry milk for 2.5h at room temperature, membranes were incubated with Tris-Buffered Saline Tween-20 (TBST) containing 3% milk for 12 h at 4° C with rabbit primary anti-cPLA2, anti-phosphoserine-cPLA2 antibodies (1:1000 dilution). To evaluate loading efficiency, membranes were probed with anti-GAPDH (cytosolic), anti-LAMIN A (nuclear protein) or anti-β-actin (plasma membrane protein) antibodies.
Immunofluorescence microscopy of cPLA2 and phosphoserine-cPLA2
Formation of phosphoserine-cPLA2 was analyzed by immunofluorescence microscopy as described previously (Varadharaj et al. 2006). Cells grown on sterile glass coverslips were treated with DMEM alone or DMEM containing glutamate (10 mM) for the desired length of time and then rinsed three times with PBS, and fixed with 3.7% formaldehyde in PBS for 10 min at room temperature. The cells were then rinsed three times with PBS and permeabilized with 0.25 % Triton X-100 prepared in TBS containing 0.01% Tween-20 (TBST) for 5 min. Next, the cells were washed three times with TBST, and treated with TBST containing 1 % BSA blocking buffer for 30 min at room temperature. The cells were incubated for 1 h at room temperature with the primary rabbit antibodies [cPLA2 and phosphoserine-cPLA2 antibodies (1:200 dilution)] in 1% BSA solution in TBST. After rinsing three times with TBST, the cells were treated with AlexaFluor 488 (1:200 dilution) in 1% BSA in TBST for 1 h. Immunofluorescent images were captured using a Zeiss Axiovert 200M microscope supported with a AxioVision™ imaging system.
siRNA knock-down of cPLA2 gene expression
HT4 cells (0.1 × 106 cells/well in 12-well plate) were cultured in antibiotic-free medium for 24 h prior to transfection. DharmaFECT™ 1 transfection reagent (Dharmacon RNA technologies, Lafayette, CO) was used to transfect the cells with 100nM siGENOME SMARTpool (Dharmacon RNA technologies, Lafayette, CO) for 72 h according to the manufacturer’s protocol. The SMARTpool technology reduces false negatives by targeting four mRNA regions at once with a single, highly functional reagent. For control cells, siControl non-targeting siRNA pool (mixture of 4 siRNAs designed to have ≥4 mismatches with the corresponding mouse gene) was used. A transfection efficiency of >90% was achieved. Cells were then harvested and seeded and 12 h after culturing, the medium was changed followed by treatment with glutamate. For determination of mRNA and protein expression, samples were collected 72 h after siRNA transfection. Total RNA was isolated from cells using the Absolutely RNA® Miniprep kit (Stratagene, La Jolla, CA). The abundance of mRNA for cPLA2 was determined using the real-time PCR. The double-stranded DNA binding dye SYBR green-I was used. The following primer sets based on the NM_008869, Mus musculus phospholipase A2, group IVA (cytosolic, calcium-dependent) (Pla2g4a) mRNA were used:
m_GAPDH F: 5′- ATG ACC ACA GTC CAT GCC ATC ACT -3′
m_GAPDH R: 5′- TGT TGA AGT CGC AGG AGA CAA CCT -3′
m_cPLA2 IVA F: 5′- AAG GCT CTA CAA TGG AAG AGG AAT T -3′
m_cPLA2 IVA R: 5′- ACG TCC TTC TCG GGT ATT GAA TAA -3′
cPLA2 protein expression in the transfected cells was determined by subjecting the cell lysates (20 μg of protein/lane) to the SDS-PAGE (4–12% gel) and Western blotting with primary rabbit anti-cPLA2 antibody as described above. To validate the protein loading efficiency, PVDF membranes were probed with anti-β-actin antibody.
Statistical Analysis
Statistical analysis of data was performed using SPSS Statistics software (v17.0). All data are reported as mean ± S.D. Comparison between groups were tested using Student’s t test or one-way analysis of variance with Tukey’s post-hoc test as indicated in respective figure legends. p<0.05 was considered statistically significant.
RESULTS
Glutamate induced release of arachidonic acid
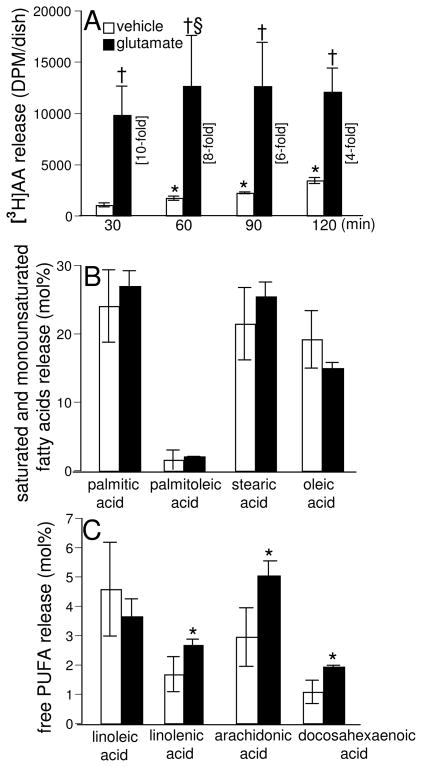
Previously we have reported that glutamate induces the activation of 12-Lox in neural cells (Khanna et al. 2003; Khanna et al. 2005b; Khanna et al. 2006). As PLA2 provides free arachidonic acid from the membrane phospholipids as the substrate for Lox for the formation of HPETES and leukotrienes, here we investigated whether glutamate would induce the release of arachidonic acid in HT4 cells. Release of arachidonic acid from the sn-2 position of the cell membrane phospholipids upon hydrolysis mediated by PLA2 serves as an index of PLA2 activity in intact cells. Hence, we examined the release of [3H] arachidonic acid from HT4 cells prelabeled with [3H] arachidonic acid as an index of PLA2 activation. In the control untreated cells, there was a steady and significant increase in the release of arachidonic acid in a time-dependent fashion representing residual cellular lipase activity under resting conditions (Fig. 1A). In glutamate-treated cells, as compared to the control untreated cells at 30 min of treatment, a significant enhancement (10-fold) of arachidonic acid release was observed. Upon prolonging the time of treatment with glutamate up to 60 min, a further significant increase in the extent of arachidonic acid release was evident. No further increase in arachidonic acid release was noted during 60–120 mins. Overall, these results demonstrated that glutamate challenge significantly induces the release of arachidonic acid from neural cells, suggesting the activation of PLA2 by glutamate.
Fig. 1. Glutamate induces release of arachidonic acid (AA) and formation of free polyunsaturated fatty acids (PUFA).
After labeling with [3H]AA (0.5 μCi/dish) overnight, cells were treated with L-glutamate (10 mM; closed bars) or not (open bars) in DMEM for 30–120 min, following which release of [3H]AA into medium was measured [A]. Free saturated and monounsaturated fatty acid levels [B] and free PUFA levels [C] in cells following 30min of glutamate exposure were determined by GC as described in Materials & Methods. Experiments were conducted in triplicates and data represent means ± S.D. of three independent experiments. *, p < 0.05 as compared to the untreated control cells at 30min of treatment; †, p < 0.05 as compared to the untreated control cells at 30min of treatment. §, p<0.05 as compared to glutamate-treated cells at 30min of treatment. Statistical significance was determined by ANOVA.
Glutamate induced formation of free polyunsaturated fatty acids (PUFA)
Another complementary approach of determining the PLA2 activation is following the formation of free PUFAs due to their release from the cell membrane phospholipids that is mediated by the action of PLA2. GC analysis of fatty acids formed in HT4 cells treated with glutamate demonstrated that the extent of release of saturated (palmitic and stearic) and monounsaturated fatty acids (palmitoleic and oleic) from the glutamate-challenged cells was not altered as compared to control untreated cells under identical conditions, suggesting that glutamate did not induce the hydrolysis of fatty acids esterified in the sn-1 position of the phospholipids and the monounsaturated fatty acids esterified in the sn-2 position (Fig. 1B). On the other hand, the extent of formation of PUFAs (linolenic, arachidonic, and docosahexaenoic) was significantly elevated in the glutamate-treated cells (Fig. 1C), suggesting that glutamate preferentially induced the hydrolysis of PUFAs esterified at the sn-2 position of the HT4 cell membrane phospholipids through PLA2 activation.
Calcium chelators attenuate glutamate-induced arachidonic acid release
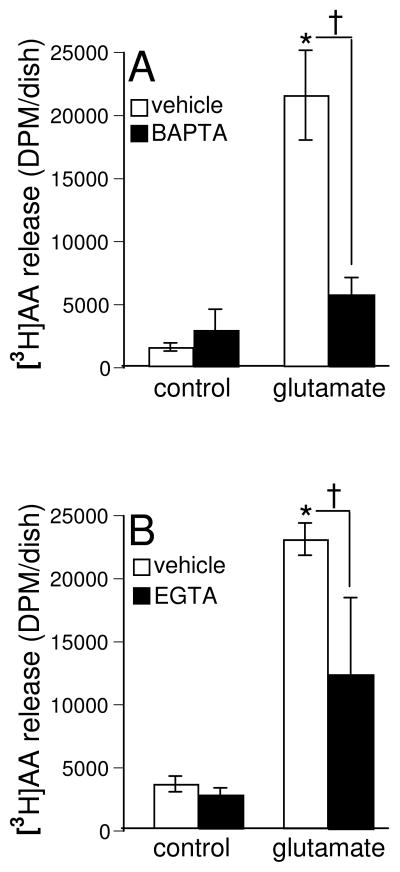
Cytosolic calcium-dependent PLA2 (cPLA2) is known to be activated by a wide variety of agonists, then causes the hydrolysis of sn-2 esterified PUFAs in the membrane phospholipids, and sets them free (Hirabayashi and Shimizu 2000; Nito et al. 2008). Therefore, we were led to investigate the role of calcium-dependent cPLA2 in the glutamate-induced release of arachidonic acid from cells by utilizing the calcium-chelating agents. The intracellular calcium chelator, BAPTA, caused a significant attenuation (70% decrease) of the arachidonic acid release in HT4 cells treated with glutamate as compared to the cells treated with glutamate alone under identical conditions (Fig. 2A). However, the extracellular calcium chelator, EGTA, was also effective in causing a significant decrease of arachidonic acid release from the glutamate-treated cells as compared to the same in the cells treated with glutamate alone (45% decrease) under identical conditions (Fig. 2B). These results revealed that intracellular calcium played a greater role in causing the glutamate-induced release of arachidonic acid, suggesting the activation of cPLA2 in HT4 cells. Under resting conditions, neural cells are known to actively limit [Ca2+]i to nM levels(Ross 1989). High [Ca2+]i (μM-mM) is required to activate cPLA2 (Yoshihara and Watanabe 1990). Thus, it is not surprising that the basal level of [3H] AA release in control was not sensitive to Ca2+ chelators. Basal [3H] AA release in control cells are expected to be contributed by Ca2+-independent phospholipases (Lee et al. 2007; Kurusu et al. 2008)as well as diacylglycerol lipase (Yoshida et al. 2006).
Fig. 2. Calcium chelators attenuate glutamate-induced arachidonic acid release.
After labeling with [3H]AA (0.5 μCi/dish) overnight cells were pre-treated with BAPTA (100 nM, closed bars) and then treated with L-glutamate (10 mM) [A] for 30 min or treated with EGTA (1 mM, closed bars) and L-glutamate (10 mM) [B] for 30 min in DMEM. At the end of incubation, release of [3H]AA into medium was measured as described in Materials & Methods. Experiments were conducted in triplicates and data represent means ± S.D. of threeindependent experiments. *, p < 0.05 as compared to the untreated control cells at 30 min of treatment; †, p < 0.05 as compared to the cells treated with L-glutamate alone for 30 min. Statistical significance was determined by ANOVA.
Glutamate-induced arachidonic acid release is cPLA2-dependent
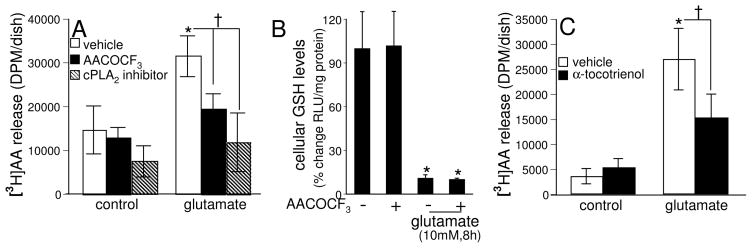
To test whether glutamate-induced arachidonic acid release from neural cells was mediated by cPLA2 activation we utilized two widely used cPLA2-specific inhibitors, AACOCF3 and a cPLA2α inhibitor. Both AACOCF3 as well as the cPLA2α inhibitor significantly attenuated the glutamate-induced arachidonic acid release from cells compared to the same in the cells treated with glutamate alone for 30 min (Fig. 3A). These results revealed that glutamate induced release of cellular arachidonic acid was cPLA2 dependent. Of note in this context is the observation that inhibition of cPLA2 using AACOCF3 did not influence glutamate-induced cellular GSH loss suggesting that cPLA2 is not implicated in such outcome (Fig. 3B).
Fig. 3. cPLA2-specific inhibitors and α-tocotrienol attenuate glutamate-induced arachidonic acid release.
Cells, after labeling with [3H]AA (0.5 μCi/dish) overnight, were pre-treated with [A] AACOCF3 (5 μM) or cPLA2α inhibitor (5 μM) for 1 h or [C] α-tocotrienol (250 nM) for10 min and then treated with L-glutamate (10 mM) for 30 min in DMEM. At the end of incubation, release of [3H]AA into medium was measured as described in Materials & Methods. The cPLA2 inhibitor AACOCF3 does not prevent glutamate induced GSH loss in HT4 cells [B]. Cells (1 × 106 cells/100 mm dish) were pre-treated with AACOCF3 (5 μM) for 2 h and then treated with L-glutamate (10 mM) for 8 h, following which GSH levels were measured. Experiments were conducted in triplicates and data represent means ± S.D. of three independent experiments. *, p < 0.05 as compared to the untreated control cells at 30 min of treatment; †, p < 0.05 as compared to the cells treated with L-glutamate alone for 30 min. Statistical significance was determined by ANOVA.
α-Tocotrienol attenuates glutamate-induced arachidonic acid release
Previously we have reported that TCT inhibits the glutamate-induced activation of 12-Lox (Khanna et al. 2003; Khanna et al. 2006). It is also established that arachidonic acid release from membrane phospholipids upon hydrolysis by PLA2 acts as a substrate for Lox towards the formation of HPETEs and leukotrienes(Hirabayashi and Shimizu 2000). Therefore we investigated whether TCT would attenuate the glutamate-induced release of arachidonic acid from HT4 cells. As shown in Fig. 3C, nanomolar TCT significantly attenuated glutamate-induced release of arachidonic acid from cells as compared to the same in cells treated with glutamate alone under identical conditions. This observation leads to the hypothesis that TCT attenuated the glutamate-induced cPLA2 activation in HT4 cells.
α-Tocotrienol attenuates glutamate-induced formation of free polyunsaturated fatty acids (PUFA)
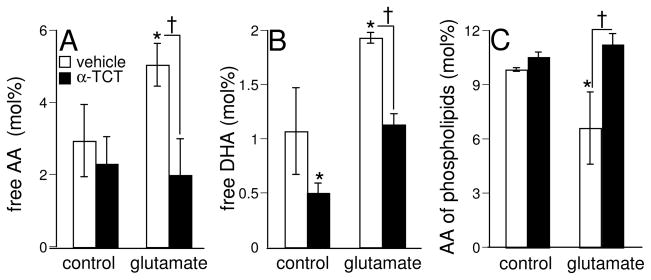
Hydrolysis of membrane phospholipids results in mobilization of free PUFA in cells. We were therefore led to test whether TCT attenuates the formation of free PUFAs in HT4 cells challenged with glutamate. TCT significantly attenuated the glutamate-induced formation of free arachidonic acid (60% decrease) and docosahexaenoic acid (40% decrease) as compared to the same in the cells treated with glutamate alone under identical conditions (Figs. 4A and 4B). These results suggest that glutamate-induced PLA2 activation is sensitive to TCT.
Fig. 4. α-Tocotrienol attenuates glutamate-induced formation of free polyunsaturated fatty acids (PUFA) and loss of arachidonic acid from cellular phospholipids.
Cells were pre-treated with α-tocotrienol (250 nM, closed bars) or not (open bars) for 10 min and then treated with L-glutamate (10 mM) in DMEM for 30 min, following which free PUFA levels (arachidonic acid [A], docosahexaenoic acid [B]) in the cells or arachidonic acid levels in cellular phospholipids [C] were determined by GC as described in Materials & Methods. Experiments were conducted in triplicates and data represent means ± S.D. of three independent experiments. *, p < 0.05 as compared to the untreated control cells at 30 min of treatment; †, p < 0.05 as compared to the cells treated with L-glutamate alone for 30 min. Statistical significance was determined by ANOVA.
α-Tocotrienol attenuates glutamate-induced loss of arachidonic acid from phospholipids
Loss of PUFAs from membrane phospholipids, especially arachidonic acid at the sn-2 position, can serve as an index of PLA2 activation. Following the treatment of cells with glutamate for 30 min, a significant decrease in arachidonic acid in the total phosopholipid pool was observed as compared to the same in the control untreated cells under identical conditions (Fig. 4C). Also, TCT treatment offered a significant and complete attenuation of the glutamate-induced decrease in arachidonic acid levels of cellular phospholipids. These results demonstrate that the glutamate-induced loss of arachidonic acid content of cellular phospholipids, presumably mediated by PLA2, was attenuated by TCT.
Glutamate induced translocation and serine phosphorylation of cPLA2
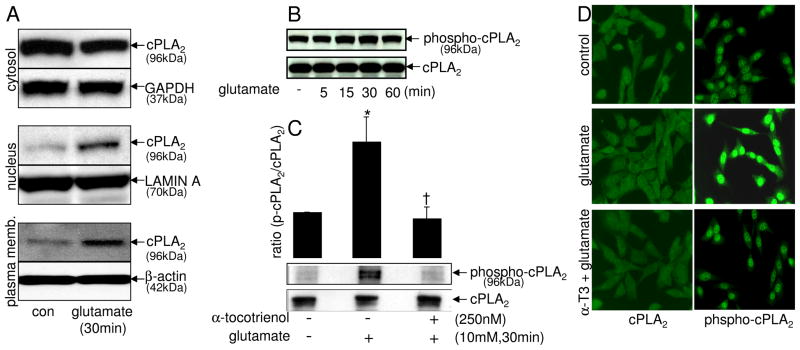
Activation of cPLA2 is known to be associated with cellular translocation and serine phosphorylation (Hirabayashi and Shimizu 2000; Nito et al. 2008). Also, our previous experiments of the current study revealed that cPLA2-specific inhibitors attenuated the glutamate-induced release of arachidonic acid from cells, suggesting that cPLA2 as a target for glutamate-induced activation. Therefore, we investigated whether glutamate would induce the translocation and serine phosphorylation of cPLA2. Glutamate challenge induced translocation of cPLA2 as determined by Western blot of subcellular fractions (Fig. 5A). Under non-challenged basal conditions cPLA2 was primarily localized in the cytosol of neural cells as expected (Kishimoto et al. 1999). Challenge by glutamate resulted in rapid translocation of cPLA2 to the nucleus and plasma membrane. Nuclear translocation of cPLA2 is known to occur by a [Ca2+]i dependent mechanism (Schievella et al. 1995; Sheridan et al. 2001). The association of cPLA2 with intracellular membranes is central to the generation of free arachidonic acid (Jupp et al. 2003; Hastings et al. 2009). cPLA2 is known to be activated by phosphorylation at serine-505 (Pavicevic et al. 2008). Consistent with the rapid translocation of cPLA2 that took place within the first 30 mins of glutamate challenge, glutamate-induced serine-505 phosphorylation of cPLA2 was noted 15 and 30 mins after challenge. This response was transient and subsided after 1h of glutamate challenge (Fig. 5B).
Fig. 5. Glutamate induces translocation and serine phosphorylation of cPLA2 in a tocotrienol-sensitive manner.
cPLA2 translocation in subcellular fractions (cytosol, nucleus & plasma membrane) was detected by Western blots [A] and normalized against house keeping proteins as described in Materials & Methods. Cells were treated with L-glutamate (10 mM) for 0–60 min [B] or were pre-treatedwith α-tocotrienol (250 nM) for 10 min and then were exposed to glutamate (10 mM) for 30 min [C], following which cPLA2 and phosphoserine-cPLA2 were detected in the cellular proteins by Western blots [B & C] and in situ by immunofluorescence microscopy [D] as described in Materials & Methods. *, p < 0.05 as compared to the untreated control cells at 30 min of treatment; †, p < 0.05 as compared to the cells treated with L-glutamate alone for 30 min. Magnification 20×.
α-Tocotrienol attenuated glutamate-induced serine-505 phosphorylation of cPLA2
Of striking interest is our observation that nanomolar TCT can inhibit glutamate-inducible serine-505 phosphorylation of cPLA2 (Fig. 5C). The key significance of this finding lies in the fact that this phosphorylation is recognized as being critical in enabling the catalytic function of cPLA2 (Pavicevic et al. 2008). Given that extracellular regulated kinase 1/2 is responsible for this phosphorylation (Pavicevic et al. 2008), our finding is consistent with our previous report demonstrating that nanomolar TCT inhibits glutamate-induced activation of extracellular regulated kinase 1/2 (Sen et al. 2000). Pre-treatment of cells with TCT significantly attenuated serine-505 phosphorylation of cPLA2 in cells exposed to glutamate for 30 min as revealed by Western blot (Fig. 5C) and immunofluorescence microscopy (Fig. 5D).
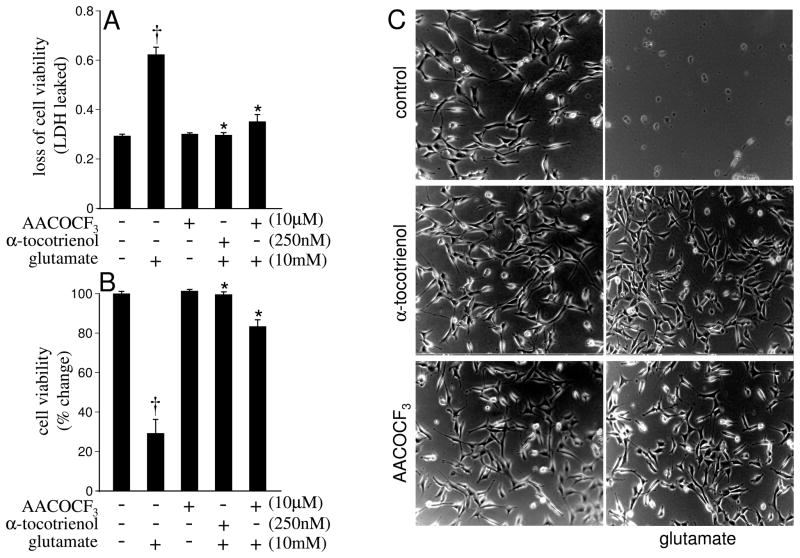
Inhibition of glutamate-induced cPLA2 activity was neuroprotective
To determine the functional significance of glutamate-induced cPLA2 activation on associated neurotoxicity, the effects of AACOCF3 (a known inhibitor of cPLA2) and that of TCT (inhibits glutamate-induced cPLA2, results of this study) were tested. Glutamate alone, 24 h after treatment, caused massive loss of cell viability. Treatment of cells with TCT or AACOCF3 offered significant protection (Figs. 6A–C). These results indicate that cPLA2 activation by glutamate directly contributes to cell death and that the inducible cPLA2 inhibitory effects of TCT contribute towards its neuroprotective properties.
Fig. 6. cPLA2-specific inhibitor AACOCF3 and α-tocotrienol protect against glutamate-induced neurotoxicity.
Cells (0.1 × 106 cells/well in 12 well plate) without or with pretreatment with AACOCF3 (10 μM) or α-tocotrienol (250 nM) for 2 h were challenged with L-glutamate (10 mM) for 24 h. At the end of the treatment, cell viability was determined by assaying the release of LDH from cells [A], LDH content in the cells [B] and examination of cell morphology [C] as described in Materials & Methods. Experiments were conducted in triplicates and datarepresent means ± S.D. of three independent experiments. †, p < 0.05 as compared to the untreated control cells at 24 h of treatment; *, p < 0.05 as compared to the cells treated with L-glutamate alone for 24 h. Statistical significance was determined by Student’s t test. Magnification 10×.
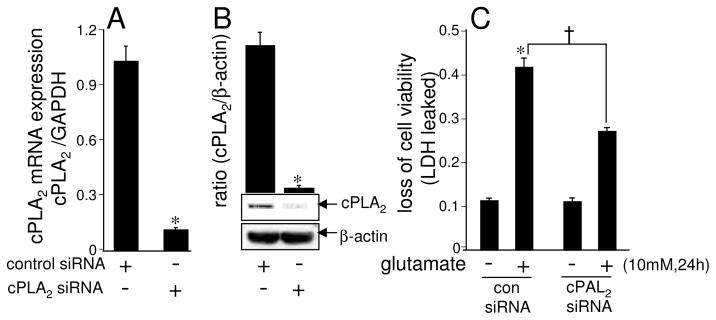
Knock-down of cPLA2 attenuated glutamate-induced neurotoxicity
Experiments to establish the procedure of transient knock-down of cPLA2 expression in HT4 cells showed a significant 90% decrease in the cPLA2 mRNA expression and 96% decrease in the cPLA2 protein expression, respectively following the transfection of cells with the cPLA2-directed siRNA (Figs. 7A–B). Glutamate-induced loss of cell viability was significantly attenuated in cells subjected to knock-down of cPLA2 (Fig. 7C). This line of observation, taken together with results from studies employing pharmacological inhibitor, establishes that cPLA2 plays a critical role in the glutamate-induced neurotoxicity.
Fig. 7. cPLA2 knock-down attenuates glutamate-induced neurotoxicity.
Cells (0.1 × 106 cells/well in 12 well plates) were transfected with 100 nM siControl non-targeting siRNA or cPLA2 siRNA for 72 h. After achieving >90% transfection efficiency, cPLA2 mRNA expression [A], cPLA2 protein expression [B], and glutamate-induced cytotoxicity [C] were determined as described in Materials & Methods. Experiments were conducted in triplicates and data represent means ± S.D. of three independent experiments. *, p < 0.05 as compared to the siControl non-targeting siRNA-transfected cells [A], [B] and [C]; †, p< 0.05 in cPLA2 siRNA-transfected cells as compared to the siControl non-targeting siRNA-transfected cells treated with L-glutamate alone for 24 h [C]. Statistical significance was determined by Student’s t test.
DISCUSSION
Members of the natural vitamin E family possess overlapping as well as unique functional properties. Among the natural vitamin E molecules, d-α-tocopherol (RRR-α-tocopherol) is best studied. So far, all major clinical trials have only tested α-tocopherol and outcomes have been less than satisfactory (Friedrich 2004; Greenberg 2005). Non-α-tocopherol forms of natural vitamin E have thus drawn growing attention (Sen et al. 2004; Sen et al. 2007b). The tocotrienol subfamily of natural vitamin E possesses powerful neuroprotective, anticancer, and cholesterol-lowering properties that are often not exhibited by tocopherols. Current developments in vitamin E research clearly indicate that members of the vitamin E family are not redundant with respect to their biological functions. TCT, γ-tocopherol, and δ-tocotrienol have emerged as vitamin E molecules with functions in health and disease that are clearly distinct from that of α-tocopherol (Sen et al. 2004; Sen et al. 2007b). We have originally reported that at nanomolar concentration, TCT, not α-tocopherol, prevents neurodegeneration (Sen et al. 2000). On a concentration basis, this finding represents the most potent of all biological functions exhibited by any natural vitamin E molecule. Our subsequent work has therefore focused on understanding the mechanisms by which nanomolar concentrations of TCT exerts its neuroprotective effects. This work provides first evidence in recognizing inducible cPLA2 activity as a key target of TCT in protecting against glutamate-induced neurotoxicity.
HT neuronal cells have used by several laboratories as a standard model to study the oxytosis component of glutamate induced cytotoxicity (Sen et al. 2000; Tirosh et al. 2000; Dargusch and Schubert 2002; Khanna et al. 2007; Xu et al. 2007). Our recent studies of HT cells have identified inducible c-Src (Sen et al. 2000; Khanna et al. 2007) and 12-Lox (Khanna et al. 2003; Khanna et al. 2005b; Park et al. 2009) as key glutamate-inducible yet TCT-sensitive mediators of neurotoxicity. We proposed that cSrc-regulated 12-Lox activation lead to the formation of cytotoxic 12-HPETE which, under the conditions, is lethal for cells (Khanna et al. 2005b; Khanna et al. 2007). This work recognizes glutamate-inducible PLA2 as an early event the activation of which results in free AA within the cell which in turn feeds the above-said Src-Lox death pathway. PLA2 present in mammalian cells, including the neurons, is broadly divided into three classes: (1) cytosolic PLA2 (cPLA2, calcium-dependent), (2) secretory PLA2 (sPLA2, calcium-dependent), and (3) calcium-independent PLA2 (iPLA2) (Hirabayashi and Shimizu 2000; Chakraborti 2003). cPLA2-catalyzed hydrolysis and release of free arachidonic acid from the membrane phospholipids represents the initial and rate-limiting regulatory step for the subsequent lipoxygenation of arachidonic acid by 12-Lox towards the formation of HPETEs (Hirabayashi and Shimizu 2000). Results of this study provide first evidence demonstrating that glutamate activates cPLA2 in neurons in a calcium-dependent manner leading to the hydrolysis of phospholipids and release of free arachidonic acid. This observation is consistent with reports demonstrating accumulation of free calcium in cells following challenge by glutamate (Tirosh et al. 2000; Nishizawa 2001). Activation of calcium-dependent cPLA2 by glutamate noted in the current study thus represents a down-stream effect of the elevation of intracellular free calcium in glutamate-challenged cells.
The mechanism of regulation of cPLA2 is complex reflecting a tightly controlled hydrolysis of phosopholipids and maintenance of levels of free arachidonic acid in the cell. Calcium-induced cellular translocation of cPLA2 from cytosol to membrane regulates the accessibility of the enzyme to the substrate for catalysis and release of free arachidonic acid and regulation of substrate availability for the action of downstream lipid oxygenases (Hirabayashi and Shimizu 2000; Leslie 2004). In addition to cellular translocation, phosphorylation of the protein functions as an additional arm of regulation of cPLA2 activity (Chakraborti 2003; Leslie 2004). The role of mitogen-activated protein kinases (MAPKs) in the activation of cPLA2 has become evident and it is established that p44 MAPK enhances the activity of cPLA2 through phosphorylation in animal cells and tissues (Sano et al. 2001; Zhu et al. 2001; Chakraborti 2003). MAPK-mediated phosphorylation of serine residue(s), especially ser505, has been identified as the key regulator in the activation of cPLA2 followed by the elevation in the intracellular calcium levels, translocation of the enzyme to the cell membrane, and proper binding with the phospholipid substrate sites leading ultimately to the release of arachidonic acid (Chakraborti 2003; Nito et al. 2008). Results of the current study demonstrated that glutamate induces the said phosphorylation as well as translocation of cPLA2 from cytosol to membrane. Importantly, both phosphorylation and translocation of cPLA2 were attenuated with nanomolar concentrations of TCT. This work recognizes glutamate-induced cPLA2 activation as being rapid. The response is prominent within 30 minutes of glutamate challenge at a time when cellular GSH loss is marginal. We have previously reported that even after 90 mins of glutamate insult cellular GSH loss is not significant (Sen et al. 2000). Thus, it is reasonable to rule out glutamate-induced cellular GSH loss as a cause of the reported cPLA2 activation. Given that glutamate results in rapid rise of [Ca2+]i (Tan et al. 1998; Sen et al. 2000; Ha and Park 2006)and the observation of this work that Ca2+-chelators are effective in significantly inhibiting glutamate-induced AA release we are led to hypothesize that glutamate-induced rise of [Ca2+]i is implicated in the reported cPLA2 activation.
Experimental evidence from studies conducted in cellular, tissue, and animal models corroborate that the group IV cPLA2 plays an essential catalytic role in hydrolyzing the sn-2 esterified PUFA of the membrane phospholipids and releasing arachidonic acid for the synthesis of eicosanoids (Hirabayashi and Shimizu 2000). Arachidonic acid-derived eicosanoids, under the regulation of specific PLA2s, have been recognized to play crucial roles in the function of brain (Tassoni et al. 2008). In addition, PLA2 is being recognized as an important player in the regulation of normal physiological functions and pathological states of the central nervous system (Sun et al. 2004). Activation of PLA2, especially that of group IV cPLA2, has been distinctly attributed to play a major role in the neurotoxicity encountered during brain ischemia(Sapirstein and Bonventre 2000). In the primary cultured cerebellar neurons and hippocampal slices of rat, glutamate-induced arachidonic acid release through activation of PLA2, has been shown to be mediated by N-methyl-D-aspartate (NMDA) receptor activation which is also calcium-dependent, and sensitive to a general PLA2 inhibitor, quinacrine (Lazarewicz et al. 1992). Therefore, the observed cPLA2-dependent neuroprotective effects of TCT may be relevant to a wide range of neurodegenerative diseases including stroke.
Acknowledgments
This work was supported by National Institutes of Health grant RO1 NS42617. The expert technical assistance provided by Ms. Jessica N. Mazerik and Ms. Emily Steinhour is greatly appreciated.
Abbreviations
- AA
arachidonic acid
- cPLA2
cytosolic cPLA2
- FFAs
free fatty acids
- iPLA2
intracellular PLA2
- HPETE
hydroperoxyeicosatetraenoic acid
- LDH
lactate dehydrogenase
- 12-Lox
12-lipoxygenase
- NMDA
N-methyl-D-aspartate
- PLA2
phospholipase A2
- PUFAs
polyunsaturated fatty acids
- PVDF
polyvinylidene difluoride
- PLs
phospholipid
- sPLA2
secretory PLA2
- SDS-PAGE
SDS–polyacrylamide gel electrophoresis
- TCT
α-tocotrienol
- TLC
thin layer chromatography
References
- Adibhatla RM, Hatcher JF. Altered lipid metabolism in brain injury and disorders. Subcell Biochem. 2008;49:241–268. doi: 10.1007/978-1-4020-8831-5_9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boscoboinik DO, Chatelain E, Bartoli GM, Stauble B, Azzi A. Inhibition of protein kinase C activity and vascular smooth muscle cell growth by d-alpha-tocopherol. Biochim Biophys Acta. 1994;1224:418–426. doi: 10.1016/0167-4889(94)90277-1. [DOI] [PubMed] [Google Scholar]
- Chakraborti S. Phospholipase A(2) isoforms: a perspective. Cell Signal. 2003;15:637–665. doi: 10.1016/s0898-6568(02)00144-4. [DOI] [PubMed] [Google Scholar]
- Dargusch R, Schubert D. Specificity of resistance to oxidative stress. Journal of Neurochemistry. 2002;81:1394–1400. doi: 10.1046/j.1471-4159.2002.00950.x. [DOI] [PubMed] [Google Scholar]
- Dennis EA, Rhee SG, Billah MM, Hannun YA. Role of phospholipase in generating lipid second messengers in signal transduction. Faseb J. 1991;5:2068–2077. doi: 10.1096/fasebj.5.7.1901288. [DOI] [PubMed] [Google Scholar]
- Friedrich MJ. To “E” or not to “E,” vitamin E’s role in health and disease is the question. Jama. 2004;292:671–673. doi: 10.1001/jama.292.6.671. [DOI] [PubMed] [Google Scholar]
- Greenberg ER. Vitamin E supplements: good in theory, but is the theory good? Ann Intern Med. 2005;142:75–76. doi: 10.7326/0003-4819-142-1-200501040-00112. [DOI] [PubMed] [Google Scholar]
- Ha JS, Park SS. Glutamate-induced oxidative stress, but not cell death, is largely dependent upon extracellular calcium in mouse neuronal HT22 cells. Neurosci Lett. 2006;393:165–169. doi: 10.1016/j.neulet.2005.09.056. [DOI] [PubMed] [Google Scholar]
- Harvard Health Publications. Vitamin E: separate and unequal? Harvard Health Letter; Boston: 2008. [Google Scholar]
- Hastings AD, Herbert SP, Gawler D, Walker JH. Association with actin mediates the EGTA-resistant binding of cytosolic phospholipase A2-alpha to the plasma membrane of activated platelets. Cell Biol Int. 2009;33:83–91. doi: 10.1016/j.cellbi.2008.09.010. [DOI] [PubMed] [Google Scholar]
- Hensley K, Benaksas EJ, Bolli R, Comp P, Grammas P, Hamdheydari L, Mou S, Pye QN, Stoddard MF, Wallis G, Williamson KS, West M, Wechter WJ, Floyd RA. New perspectives on vitamin E: gamma-tocopherol and carboxyelthylhydroxychroman metabolites in biology and medicine. Free Radic Biol Med. 2004;36:1–15. doi: 10.1016/j.freeradbiomed.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Hirabayashi T, Shimizu T. Localization and regulation of cytosolic phospholipase A(2) Biochim Biophys Acta. 2000;1488:124–138. doi: 10.1016/s1388-1981(00)00115-3. [DOI] [PubMed] [Google Scholar]
- Jupp OJ, Vandenabeele P, MacEwan DJ. Distinct regulation of cytosolic phospholipase A2 phosphorylation, translocation, proteolysis and activation by tumour necrosis factor-receptor subtypes. Biochem J. 2003;374:453–461. doi: 10.1042/BJ20030705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khanna S, Roy S, Park HA, Sen CK. Regulation of c-Src activity in glutamate-induced neurodegeneration. J Biol Chem. 2007;282:23482–23490. doi: 10.1074/jbc.M611269200. [DOI] [PubMed] [Google Scholar]
- Khanna S, Patel V, Rink C, Roy S, Sen CK. Delivery of orally supplemented alpha-tocotrienol to vital organs of rats and tocopherol-transport protein deficient mice. Free Radic Biol Med. 2005a;39:1310–1319. doi: 10.1016/j.freeradbiomed.2005.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khanna S, Roy S, Parinandi NL, Maurer M, Sen CK. Characterization of the potent neuroprotective properties of the natural vitamin E alpha-tocotrienol. J Neurochem. 2006;98:1474–1486. doi: 10.1111/j.1471-4159.2006.04000.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khanna S, Roy S, Ryu H, Bahadduri P, Swaan PW, Ratan RR, Sen CK. Molecular basis of vitamin E action: tocotrienol modulates 12-lipoxygenase, a key mediator of glutamate-induced neurodegeneration. J Biol Chem. 2003;278:43508–43515. doi: 10.1074/jbc.M307075200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khanna S, Roy S, Slivka A, Craft TK, Chaki S, Rink C, Notestine MA, DeVries AC, Parinandi NL, Sen CK. Neuroprotective properties of the natural vitamin E alpha-tocotrienol. Stroke. 2005b;36:2258–2264. doi: 10.1161/01.STR.0000181082.70763.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishimoto K, Matsumura K, Kataoka Y, Morii H, Watanabe Y. Localization of cytosolic phospholipase A2 messenger RNA mainly in neurons in the rat brain. Neuroscience. 1999;92:1061–1077. doi: 10.1016/s0306-4522(99)00051-2. [DOI] [PubMed] [Google Scholar]
- Kurusu S, Matsui K, Watanabe T, Tsunou T, Kawaminami M. The cytotoxic effect of bromoenol lactone, a calcium-independent phospholipase A2 inhibitor, on rat cortical neurons in culture. Cell Mol Neurobiol. 2008;28:1109–1118. doi: 10.1007/s10571-008-9287-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarewicz JW, Salinska E, Wroblewski JT. NMDA receptor-mediated arachidonic acid release in neurons: role in signal transduction and pathological aspects. Adv Exp Med Biol. 1992;318:73–89. doi: 10.1007/978-1-4615-3426-6_7. [DOI] [PubMed] [Google Scholar]
- Lee LY, Ong WY, Farooqui AA, Burgunder JM. Role of calcium-independent phospholipase A2 in cortex striatum thalamus cortex circuitry-enzyme inhibition causes vacuous chewing movements in rats. Psychopharmacology (Berl) 2007;195:387–395. doi: 10.1007/s00213-007-0912-y. [DOI] [PubMed] [Google Scholar]
- Leslie CC. Regulation of the specific release of arachidonic acid by cytosolic phospholipase A2. Prostaglandins Leukot Essent Fatty Acids. 2004;70:373–376. doi: 10.1016/j.plefa.2003.12.012. [DOI] [PubMed] [Google Scholar]
- Li Y, Maher P, Schubert D. A role for 12-lipoxygenase in nerve cell death caused by glutathione depletion. Neuron. 1997;19:453–463. doi: 10.1016/s0896-6273(00)80953-8. [DOI] [PubMed] [Google Scholar]
- Lin TH, Huang YL, Huang SF. Lipid peroxidation in liver of rats administrated with methyl mercuric chloride. Biol Trace Elem Res. 1996;54:33–41. doi: 10.1007/BF02785318. [DOI] [PubMed] [Google Scholar]
- Mazerik JN, Hagele T, Sherwani S, Ciapala V, Butler S, Kuppusamy ML, Hunter M, Kuppusamy P, Marsh CB, Parinandi NL. Phospholipase A2 activation regulates cytotoxicity of methylmercury in vascular endothelial cells. Int J Toxicol. 2007;26:553–569. doi: 10.1080/10915810701707759. [DOI] [PubMed] [Google Scholar]
- Miyamoto K, Shiozaki M, Shibata M, Koike M, Uchiyama Y, Gotow T. Very-high-dose alpha-tocopherol supplementation increases blood pressure and causes possible adverse central nervous system effects in stroke-prone spontaneously hypertensive rats. J Neurosci Res. 2009;87:556–566. doi: 10.1002/jnr.21851. [DOI] [PubMed] [Google Scholar]
- Miyazawa T, Shibata A, Sookwong P, Kawakami Y, Eitsuka T, Asai A, Oikawa S, Nakagawa K. Antiangiogenic and anticancer potential of unsaturated vitamin E (tocotrienol) J Nutr Biochem. 2009;20:79–86. doi: 10.1016/j.jnutbio.2008.09.003. [DOI] [PubMed] [Google Scholar]
- Nishizawa Y. Glutamate release and neuronal damage in ischemia. Life Sci. 2001;69:369–381. doi: 10.1016/s0024-3205(01)01142-0. [DOI] [PubMed] [Google Scholar]
- Nito C, Kamada H, Endo H, Niizuma K, Myer DJ, Chan PH. Role of the p38 mitogen-activated protein kinase/cytosolic phospholipase A2 signaling pathway in blood-brain barrier disruption after focal cerebral ischemia and reperfusion. J Cereb Blood Flow Metab. 2008;28:1686–1696. doi: 10.1038/jcbfm.2008.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osakada F, Hashino A, Kume T, Katsuki H, Kaneko S, Akaike A. Alpha-tocotrienol provides the most potent neuroprotection among vitamin E analogs on cultured striatal neurons. Neuropharmacology. 2004;47:904–915. doi: 10.1016/j.neuropharm.2004.06.029. [DOI] [PubMed] [Google Scholar]
- Packer L, Weber SU, Rimbach G. Molecular aspects of alpha-tocotrienol antioxidant action and cell signalling. J Nutr. 2001;131:369S–373S. doi: 10.1093/jn/131.2.369S. [DOI] [PubMed] [Google Scholar]
- Park HA, Khanna S, Rink C, Gnyawali S, Roy S, Sen CK. Glutathione disulfide induces neural cell death via a 12-lipoxygenase pathway. Cell Death Differ. 2009 doi: 10.1038/cdd.2009.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavicevic Z, Leslie CC, Malik KU. cPLA2 phosphorylation at serine-515 and serine-505 is required for arachidonic acid release in vascular smooth muscle cells. J Lipid Res. 2008;49:724–737. doi: 10.1194/jlr.M700419-JLR200. [DOI] [PubMed] [Google Scholar]
- Pearce BC, Parker RA, Deason ME, Qureshi AA, Wright JJ. Hypocholesterolemic activity of synthetic and natural tocotrienols. Journal of Medicinal Chemistry. 1992;35:3595–3606. doi: 10.1021/jm00098a002. [DOI] [PubMed] [Google Scholar]
- Pearce BC, Parker RA, Deason ME, Dischino DD, Gillespie E, Qureshi AA, Volk K, Wright JJ. Inhibitors of cholesterol biosynthesis. 2. Hypocholesterolemic and antioxidant activities of benzopyran and tetrahydronaphthalene analogues of the tocotrienols. Journal of Medicinal Chemistry. 1994;37:526–541. doi: 10.1021/jm00030a012. [DOI] [PubMed] [Google Scholar]
- Ross WN. Changes in intracellular calcium during neuron activity. Annu Rev Physiol. 1989;51:491–506. doi: 10.1146/annurev.ph.51.030189.002423. [DOI] [PubMed] [Google Scholar]
- Sano H, Zhu X, Sano A, Boetticher EE, Shioya T, Jacobs B, Munoz NM, Leff AR. Extracellular signal-regulated kinase 1/2-mediated phosphorylation of cytosolic phospholipase A2 is essential for human eosinophil adhesion to fibronectin. J Immunol. 2001;166:3515–3521. doi: 10.4049/jimmunol.166.5.3515. [DOI] [PubMed] [Google Scholar]
- Sapirstein A, Bonventre JV. Phospholipases A2 in ischemic and toxic brain injury. Neurochem Res. 2000;25:745–753. doi: 10.1023/a:1007583708713. [DOI] [PubMed] [Google Scholar]
- Schaffer S, Muller WE, Eckert GP. Tocotrienols: constitutional effects in aging and disease. J Nutr. 2005;135:151–154. doi: 10.1093/jn/135.2.151. [DOI] [PubMed] [Google Scholar]
- Schievella AR, Regier MK, Smith WL, Lin LL. Calcium-mediated translocation of cytosolic phospholipase A2 to the nuclear envelope and endoplasmic reticulum. J Biol Chem. 1995;270:30749–30754. doi: 10.1074/jbc.270.51.30749. [DOI] [PubMed] [Google Scholar]
- Schubert D, Piasecki D. Oxidative glutamate toxicity can be a component of the excitotoxicity cascade. Journal of Neuroscience. 2001;21:7455–7462. doi: 10.1523/JNEUROSCI.21-19-07455.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen CK, Khanna S, Roy S. Tocotrienol: the natural vitamin E to defend the nervous system? Ann N Y Acad Sci. 2004;1031:127–142. doi: 10.1196/annals.1331.013. [DOI] [PubMed] [Google Scholar]
- Sen CK, Khanna S, Roy S. Tocotrienols: Vitamin E beyond tocopherols. Life Sci. 2006;78:2088–2098. doi: 10.1016/j.lfs.2005.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen CK, Khanna S, Roy S. Tocotrienols in health and disease: the other half of the natural vitamin E family. Mol Aspects Med. 2007a;28:692–728. doi: 10.1016/j.mam.2007.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen CK, Khanna S, Roy S, Packer L. Molecular basis of vitamin E action. Tocotrienol potently inhibits glutamate-induced pp60(c-Src) kinase activation and death of HT4 neuronal cells. J Biol Chem. 2000;275:13049–13055. doi: 10.1074/jbc.275.17.13049. [DOI] [PubMed] [Google Scholar]
- Sen CK, Khanna S, Rink C, Roy S. Tocotrienols: the emerging face of natural vitamin E. Vitam Horm. 2007b;76:203–261. doi: 10.1016/S0083-6729(07)76008-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheridan AM, Sapirstein A, Lemieux N, Martin BD, Kim DK, Bonventre JV. Nuclear translocation of cytosolic phospholipase A2 is induced by ATP depletion. J Biol Chem. 2001;276:29899–29905. doi: 10.1074/jbc.M103758200. [DOI] [PubMed] [Google Scholar]
- Shichiri M, Takanezawa Y, Uchida K, Tamai H, Arai H. Protection of cerebellar granule cells by tocopherols and tocotrienols against methylmercury toxicity. Brain Res. 2007;1182:106–115. doi: 10.1016/j.brainres.2007.08.084. [DOI] [PubMed] [Google Scholar]
- Sun GY, Xu J, Jensen MD, Simonyi A. Phospholipase A2 in the central nervous system: implications for neurodegenerative diseases. J Lipid Res. 2004;45:205–213. doi: 10.1194/jlr.R300016-JLR200. [DOI] [PubMed] [Google Scholar]
- Tan S, Schubert D, Maher P. Oxytosis: A novel form of programmed cell death. Current Topics in Medicinal Chemistry. 2001;1:497–506. doi: 10.2174/1568026013394741. [DOI] [PubMed] [Google Scholar]
- Tan S, Sagara Y, Liu Y, Maher P, Schubert D. The regulation of reactive oxygen species production during programmed cell death. J Cell Biol. 1998;141:1423–1432. doi: 10.1083/jcb.141.6.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tassoni D, Kaur G, Weisinger RS, Sinclair AJ. The role of eicosanoids in the brain. Asia Pac J Clin Nutr. 2008;17(Suppl 1):220–228. [PubMed] [Google Scholar]
- Theriault A, Chao JT, Wang Q, Gapor A, Adeli K. Tocotrienol: a review of its therapeutic potential. Clin Biochem. 1999;32:309–319. doi: 10.1016/s0009-9120(99)00027-2. [DOI] [PubMed] [Google Scholar]
- Tirosh O, Sen CK, Roy S, Packer L. Cellular and mitochondrial changes in glutamate-induced HT4 neuronal cell death. Neuroscience. 2000;97:531–541. doi: 10.1016/s0306-4522(00)00028-2. [DOI] [PubMed] [Google Scholar]
- Varadharaj S, Steinhour E, Hunter MG, Watkins T, Baran CP, Magalang U, Kuppusamy P, Zweier JL, Marsh CB, Natarajan V, Parinandi NL. Vitamin C-induced activation of phospholipase D in lung microvascular endothelial cells: regulation by MAP kinases. Cell Signal. 2006;18:1396–1407. doi: 10.1016/j.cellsig.2005.10.019. [DOI] [PubMed] [Google Scholar]
- Verity MA, Sarafian T, Pacifici EH, Sevanian A. Phospholipase A2 stimulation by methyl mercury in neuron culture. J Neurochem. 1994;62:705–714. doi: 10.1046/j.1471-4159.1994.62020705.x. [DOI] [PubMed] [Google Scholar]
- Xu X, Chua CC, Kong J, Kostrzewa RM, Kumaraguru U, Hamdy RC, Chua BH. Necrostatin-1 protects against glutamate-induced glutathione depletion and caspase-independent cell death in HT-22 cells. J Neurochem. 2007;103:2004–2014. doi: 10.1111/j.1471-4159.2007.04884.x. [DOI] [PubMed] [Google Scholar]
- Yoshida T, Fukaya M, Uchigashima M, Miura E, Kamiya H, Kano M, Watanabe M. Localization of diacylglycerol lipase-alpha around postsynaptic spine suggests close proximity between production site of an endocannabinoid, 2-arachidonoyl-glycerol, and presynaptic cannabinoid CB1 receptor. J Neurosci. 2006;26:4740–4751. doi: 10.1523/JNEUROSCI.0054-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshihara Y, Watanabe Y. Translocation of phospholipase A2 from cytosol to membranes in rat brain induced by calcium ions. Biochem Biophys Res Commun. 1990;170:484–490. doi: 10.1016/0006-291x(90)92117-i. [DOI] [PubMed] [Google Scholar]
- Zhu X, Sano H, Kim KP, Sano A, Boetticher E, Munoz NM, Cho W, Leff AR. Role of mitogen-activated protein kinase-mediated cytosolic phospholipase A2 activation in arachidonic acid metabolism in human eosinophils. J Immunol. 2001;167:461–468. doi: 10.4049/jimmunol.167.1.461. [DOI] [PubMed] [Google Scholar]