Abstract
We examined the hypothesis that nontoxic concentrations of selenium induce apoptosis and growth inhibition selectively in prostate cancer cells but not in benign prostate cells. Nontumorigenic BPH-1 prostate epithelial cells, androgen-sensitive LNCaP, and androgen-independent PC-3 prostate cancer cells were exposed to sodium selenite at 1 to 10 μmol/l for 24 to 72 h. Cell proliferation, viability, and apoptosis were assessed by MTT assay, trypan blue exclusion, flow cytometry, DNA laddering, and caspase activation. BPH-1 cells were more sensitive for cytotoxic selenium effects than malignant prostate cells, whereas LNCaP cells were more sensitive than PC-3 cells. At noncytotoxic selenium concentrations, there was no apoptosis in BPH-1 and PC-3 cells and no growth inhibition of LNCaP and BPH-1 cells. PC-3 cells were refractory to apoptosis induction but were growth inhibited at non-cytotoxic concentrations. LNCaP cells were growth stimulated at 1 μmol/l and sensitive to apoptosis induction at higher noncytotoxic concentrations. Thus, noncytotoxic selenite concentrations did not induce growth inhibition or apoptosis selectively in prostate cancer cells. Growth stimulation of LNCaP cells by low concentrations suggests the possibility of adverse effects of selenium supplementation on hormone sensitive prostate cancer, whereas inhibition of PC-3 cell proliferation at noncytotoxic concentrations suggests potential benefit of selenium in advanced prostate cancer.
INTRODUCTION
Prostate cancer is the most common cancer among U.S. men and is the second leading cause of cancer deaths. Few risk factors offer opportunities for primary prevention of this malignancy (1,2). Therefore, chemoprevention is an attractive and potentially powerful approach to prostate cancer prevention that can be mechanism based (3,4), and selenium compounds are one class of chemoprevention agents that have shown promise in this respect (5). Clark et al. (6) reported a dramatic decrease in the incidence of prostate, lung, and colorectal cancer in skin cancer patients who supplemented their diets with 200 μg of selenium in the form of selenized yeast. However, only subjects with baseline selenium levels in the lower two tertiles benefited from the regimen (7). Selenium supplementation did not prevent the recurrence of skin cancer; in fact, in a recent follow-up, squamous cell carcinoma and total nonmelanoma skin cancer incidence were increased (8), which suggests a potential danger of selenium supplementation.
There are many reports of experimental evidence that selenium supplementation can reduce the incidence of many types of cancer when nontoxic doses are provided to the diet of rodent species (9,10). Epidemiological studies have identified low selenium levels as one of several factors that are associated with an increased risk of prostate cancer (11,12), but not all studies have shown this relationship (6,13). The observed inverse relationship between selenium intake and prostate cancer risk has created a great deal of interest in understanding the mechanisms of prostate cancer chemoprevention by selenium.
Although the precise mechanism of chemoprevention by selenium remains unknown, two theories have evolved. First, selenium metabolites have been postulated to promote apoptosis and inhibit cell proliferation selectively in cancer cells (14,15). Second, it has been proposed that selenium supplementation increases the levels of antioxidant selenoproteins and thereby prevents the DNA damage that could lead to cell transformation (16). Diverse forms of selenium have been shown effects on biological processes important in carcinogenesis including inhibition of cell proliferation, induction of apoptosis, protection against oxidative stress and genetic damage, and inhibition of tumor angiogenesis (17–24). Which of the various anticancer effects of selenium are important most likely depends on baseline selenium status, selenium dose, and form of selenium.
The intracellular production of selenide from selenite is likely to be of importance for the expression of selenoproteins because selenide is a precursor for selenoprotein synthesis (25). Administration of selenite to cells in low (nM) concentrations will stimulate synthesis of selenoproteins, whereas with increasing concentrations (in the μM range), selenium will become increasingly toxic for cancer cells (26). It has been proposed that at such higher selenite concentrations, the redox cycling of selenite with the selenoprotein thioredoxin reductase (TR) and oxygen may kill cancer cells, whereas normal cells may be relatively resistant to that effect (27). Other forms of selenium than selenite, for example, organoselenium compounds such as selenomethionine, need to undergo metabolic “activation” before selenium can be incorporated into selenoproteins (26).
This study was designed to better understand the effects of noncytotoxic levels of selenium on benign and malignant prostate cells and to explore molecular mechanisms of such selenium effects. Our hypothesis was that selenium at relevant, nontoxic concentrations induces apoptosis selectively in cancer cells but not in benign prostate epithelial cells. We tested this hypothesis by comparing the effects of sodium selenite on nontumorigenic and malignant prostate cancer epithelial cells to investigate selenium-induced changes in cell proliferation, cell cycle parameters, apoptosis, and TR expression and activity. Noncytotoxic doses of selenite inhibited proliferation only in androgen-independent PC-3 cells but not in androgen-responsive LNCaP cells or nontumorigenic BPH-1 cells (28), and we unexpectedly observed stimulation of proliferation of LNCaP cells by relatively low concentrations of selenium.
MATERIALS AND METHODS
Chemicals, Reagents, and Antibodies
Sodium selenite (Na2SeO3H2) and all chemicals employed for TR (EC 1.6.4.5) enzyme assay were purchased from Sigma Chemical Co. (St. Louis, MO). RPMI 1640, fetal calf serum, and PBS Dulbecco’s without calcium, magnesium, and sodium bicarbonate were from GIBCO BRL, InVitrogen (Carlsbad, CA). Antibodies for poly (ADP-ribose polymerase (PARP), caspases, and β-actin were obtained from Cell Signaling Technology (Beverly, MA). Prostate apoptosis response-4 (PAR-4) antibody was purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). Anti-TR antibody was from GeneTex, Inc. (San Antonio, TX). Secondary goat antirabbit antibody was from DAKO (Glostrup, Denmark). Apoptotic DNA laddering kit was purchased from Roche Diagnostic Co. (Indianapolis, IN). In all studies, concentrated sodium selenite stock was diluted in PBS to 1 mmol/l immediately before use.
Cell Culture and Treatments
LNCaP (androgen dependent) and PC-3 (androgen independent) prostate cancer cells were obtained from the American Type Cell Culture Collection (Rockville, MD). Immortalized BPH-1 benign prostate epithelial cells (28) were a generous gift from Dr. S. W. Hayward who is at Vanderbilt University Medical Center, Nashville, Tennessee. All cell lines were cultured in RPMI 1640 with L-glutamine supplemented with 10% FBS, and 100 IU/ml penicillin and 100 μg/ml streptomycin at 37°C in a humidified atmosphere of 5% CO2 in air. Cells were cultured in T75 or T25 flasks unless stated otherwise, and the medium was changed when they reached 50–75% confluence.
Cell Proliferation and Cell Viability/Cytotoxicity Assays
To determine the effect of sodium selenite on cell proliferation and cell viability by hemocytometer counting, cells were plated onto 24-well cell culture plates at 5,000 cells/well in 1 ml of culture medium with FBS. Before treatment cells were allowed to adhere to the bottom of the plate for 24 h and treated with 1 to 10 μmol/l sodium selenite. At 24, 48, and 72 h treatment at 37°C, the cells were harvested by trypsin solution. Cell counts were performed in triplicates using a hemocytometer with trypan blue (0.2%) exclusion to identify viable cells. The total numbers of viable and dye-stained cells in each experiment were compared with those of the parallel untreated control cell counts performed simultaneously in three independent experiments.
For the MTT cell proliferation, assay cells were seeded in 96-well plates (5,000 cells/well) and treated with 1 to 10 μmol/l sodium selenite for 24, 48, and 72 h. Cell survival was assayed using Vybrant® MTT Cell Proliferation Assay Kit (InVitrogen). MTT 3-(4, 5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide solution was added as 10 μl to each well and incubated for an additional period of 4 h at 37°C in a humidified incubator. The stop solution (100 μl) was added to solubilize the formazan product, and the absorbance at 570 nm was recorded using microplate reader (Bio-Rad model 550; Bio-Rad Laboratories, Hercules, CA).
Flow Cytometric Analysis
After sodium selenite treatment and trypsinization, 1 × 106 cells were washed with PBS and fixed in 70% ethanol for at least 24 h at −20°C. After spinning cells at 200g for 10 min, the cells were stained in 1 ml of propidium iodide staining solution (20 μg/ml propidium iodide, 0.2 mg/ml DNase-free RNase, in 0.1% Triton X-100). Both cell cycle distribution and apoptotic cells were simultaneously measured in a Beckman Coulter EPICS XL Flow Cytometer (Beckman Coulter, Fullerton, CA) using 488 nm laser excitation.
Apoptosis Detection
In addition to the flow cytometry, 4′,6-diamidine-phenylindole dihydrochloride (DAPI) staining, DNA laddering, and identification of the caspases were performed as apoptosis detection methods. For DAPI staining, after trypsinization, 1 × 104 cells were washed with PBS and fixed in 10% formaldehyde for 15 min. After incubating the cells with 0.1% Triton X for 15 min, 4 mol/l HCL for 5 min, and 0.1 mol/l sodium tetraborate for 5 min, they were treated with DAPI for 30 min. After washing with PBS, they were fixed in formalin and examined with a fluorescence microscope. For apoptotic DNA laddering analysis, DNA isolation and gel electrophoresis were done according to the instructions of the manufacturer (Roche Diagnostic Co.). Briefly, cells were scraped in PBS and harvested by centrifugation at 500 g for 5 min at room temperature and then lysed in 400 μl lysis buffer for 10 min at room temperature. Following an addition of 100 μl isoproponoal, the lysate was centrifuged through a filter and washed with the washing buffer. Genomic DNA was eluted with 100 μl elution buffer. DNA samples were loaded onto a 1.5% agarose gel electrophoresis containing 1 mg/ml ethidium bromide and electrophorized at 50 V.
Western Blotting
Cell pellets were resuspended in lysis buffer (50 mmol/l HEPES, pH 7.5, 150 mmol/l NaCl, 1 mmol/l EGTA, 10% glycerol, 1% Triton, 25 mmol/l NaF, 1 mmol/l EDTA, and 10 μmol/l ZnCl2) followed by addition of 100 μl/ml protease inhibitor cocktail (Sigma Chemical Co. St. Louis, MO) and 10 μl/ml of 100 mmol/l Na Orthovanadate on ice. Then cells were scraped off with rubber policeman and transferred to microcentrifuge tubes. After freezing-thawing at −80°C, they were spun down at 10,000 g at 4°C. Protein was quantitated by Bradford analysis and measured at 595 nm with a microplate reader using the Bio-Rad Protein Assay kit (Bio-Rad Laboratories, Hercules, CA). Fifty μg protein/lane was resolved by 12.5% SDS-PAGE (Bio-Rad) and transferred to PVDF membranes and blocked with 5% milk/Tris buffered saline (TBS; 50 mmol/l Tris, 150 mmol/l NaCl adjusted pH 7.5 with HCl, 0.1% Tween) or 5% BSA/Tris buffered saline (50 mmol/l Tris, 150 mmol/l NaCl adjusted pH 7.5 with HCl, 0.1% Tween). After incubating the membrane with the primary antibody at the appropriate dilution overnight at 4°C or at room temperature for 1 or 3 h, secondary antibodies were conjugated to horseradish peroxidase and ECL Western blotting detection reagents (Amersham Biosciences, Piscataway, NJ) to show antibody-bound bands.
mRNA Expression Analysis of TR
Total RNA was extracted from cells using an RNAgents Total RNA Isolation System (Promega Co., Madison, WI) and stored at −80°C. RNA quantities were determined by measuring absorbance at 260 and 280 nm in a spectrophotometer (NanoDrop; Thermo Scientific, Waltham, MA). A ratio of >1.8 of A260/A280was considered a suitable purity. After isolating total RNA, cDNA was synthesized using SuperScript RT III (InVitrogen). TR1 mRNA was determined by real-time RT-PCR (TaqMan Assay) using the Applied Biosystems 7300 Real-Time PCR System (Applied Biosystems, Foster City, CA). Primers and the fluorogenic TaqMan probes were designed for the human TR1 and β-actin (housekeeping gene) sequences by the manufacturer. The probes were labeled with a 5′ reporter dye, FAM (6-carboxyfluoroscein), and 3′ quencher dye, TAMRA (6-carboxytetramethylrhodamine). RT-PCR reactions were carried out in a 96-well plate using TaqMan one-step RT-PCR master mix reagent kit in a total volume of 25 μl/well consisting of 100 nmol/l probe, 200 to 300 nmol/l forward and reverse primers, and 10 ng of total RNA. TaqMan RT-PCR conditions were 48°C for 30 min, 95°C for 10 min, and then 40 cycles of 95°C for 15 s and 60°C for 1 min. Data were analyzed according to the Applied Biosystems calculation method. TaqMan threshold cycle numbers (Ct) were normalized into fold of relative induction using the equation of ΔCt method. Reactions were carried out in triplicates.
TR Activity Assay
The activity of TR in prostate cells was determined using insulin disulfides as substrate essentially as described by Holmgren and Bjornstedt (29). Protein concentration was quantified employing the Bradford Method (Bio-Rad). A volume corresponding to 100 μg protein from each cell extract was incubated with 80 mmol/l HEPES (pH 7.5), 0.9 mg/ml NADPH, 6 mmol/l EDTA, 2 mg/ml insulin, and 10 μmol/l E. coli thioredoxin (Trx) at 37°C for 20 min in a final volume of 120 μl. By the addition of 500 μl DTNB (0.4 mg/ml) in 6 M guanidine hydrochloride/0.2 M Tris-Cl (pH 8.0), the reaction was terminated. A blank sample, containing everything except Trx, was incubated and treated in the same manner as each unknown sample. The absorbance at 412 nm was measured and the blank value subtracted from the corresponding absorbance value of the sample. A standard curve was prepared by using purified calf thymus TR with a defined specific activity.
Statistical Analysis
The data were analyzed using Student’s t -test for two sample comparisons and with a one-way ANOVA followed by an appropriate post hoc test (Dunnett’s or Tukey’s test).
RESULTS
Dose- and Time-Dependent Inhibitory Effect of Selenium on Cell Growth and Viability
The minimally required noncytotoxic dose of selenium to cause maximal growth inhibition was determined, as well as the effect of exposure duration, comparing LNCaP (androgen sensitive) and PC-3 (androgen insensitive) prostate cancer cells and nontumorigenic BPH-1 prostate epithelial cells. For these studies, we applied two widely used methods, the MTT assay and hemocytometer counting with trypan blue dye exclusion, that assess fundamentally different endpoints: The MTT assay estimates a combination of a measure of the number of metabolically active cells and the level of their metabolic activity, whereas hemocytometer counting determines the actual number of cells, and when combined with trypan blue dye exclusion, it provides an estimate of the percentage of structurally intact (viable) cells. Viability (trypan blue exclusion) of all three cell lines at baseline exceeded 95% (Figs. 1–3).
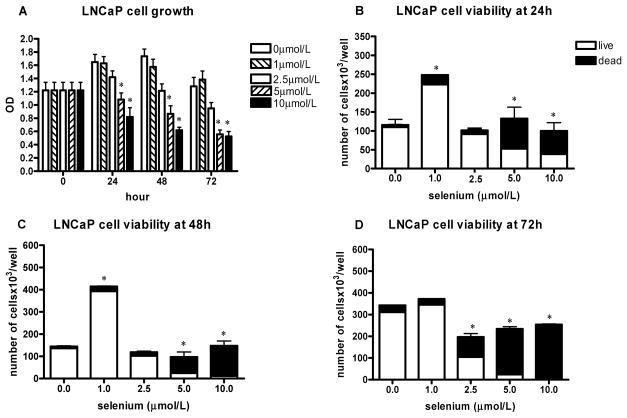
FIG. 1.
The effect of sodium selenite on LNCaP cell growth and viability. The data represent the mean ± SD of three experiments. A: Cell growth measured by the MTT assay presented as absorbance read by microplate reader at 570 nm at different time intervals; *P < 0.05 (two-sided) for difference with control cells. OD, optical density. B–D: The number of viable cells and cell viability determined by hemocytometer counting and trypan blue exclusion after 24 h (B), 48 h (C), and 72 h (D); *P < 0.001 for difference with control cells. Where error bars are not visible, the variation was very small.
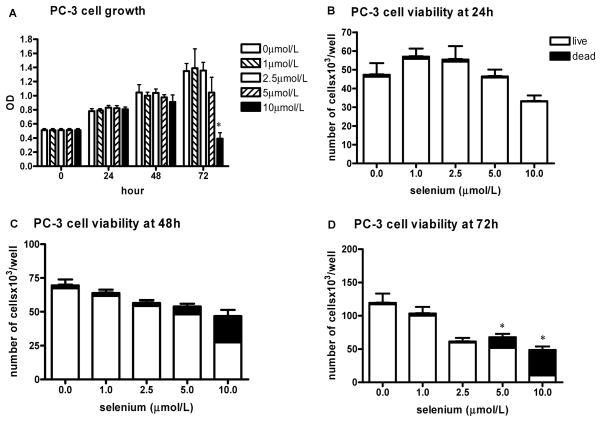
FIG. 3.
The effect of sodium selenite on PC-3 cell growth and viability. The data represent the mean ± SD of three experiments. A: Cell growth measured by the MTT assay presented as absorbance read by microplate reader at 570 nm at different time intervals; *P < 0.001 (two-sided) for difference with control cells. OD, optical density. B–D: The number of viable cells and cell viability determined by hemocytometer counting and trypan blue exclusion after 24 h (B), 48 h (C), and 72 h (D); *P < 0.001 (two-sided) for difference with control cells.
Treatment of LNCaP cells with sodium selenite resulted in significant dose-related reduction in cell proliferation and viability using the MTT assay after 24 h in 96-well plates at concentrations of 2.5 μmol/l and higher (Fig. 1A). This reduction became more marked at later time points. The number of viable cells was decreased, and the number of dead cells increased at all three time points at doses of 5 and 10 μmol/l using hemocytometer counting and trypan blue dye exclusion with cells cultured in 6-well plates (Figs. 1B–1D). The 2.5 μmol/l dose was minimally cytotoxic, as a reduction in viable cell number did not become apparent until 72 h. Interestingly, at the 1 μmol/l dose, there was a stimulatory effect of selenite on LNCaP cell proliferation using hemocytometer counting, but no difference compared to control was seen with the MTT assay.
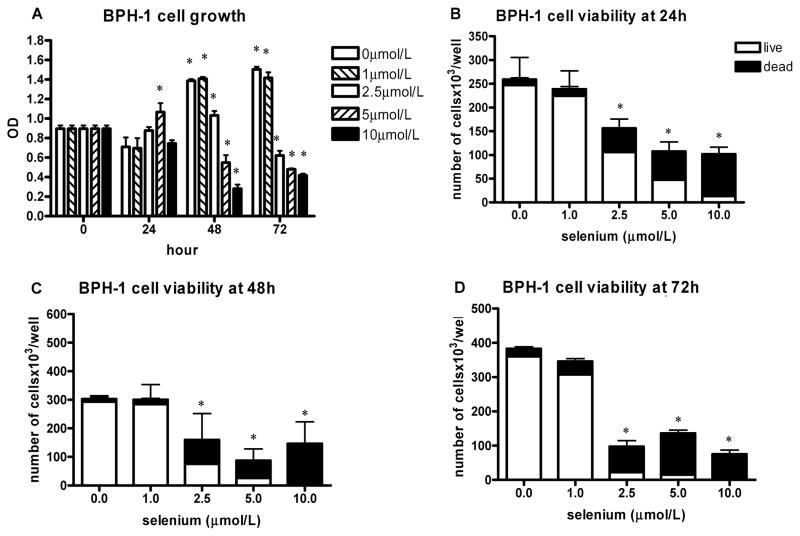
BPH-1 cells were more sensitive than LNCaP cells to the cytotoxic and growth-inhibitory effects of selenite. A significant dose-dependent growth inhibition starting at 48 h was found using the MTT assay (Fig. 2A). After 24 h, there was slight growth stimulation at 5 μmol/l, but this was not apparent using hemocytometer counting of cells grown in 6-well plates. Using the latter method, the viability and total number of BPH-1 cells appeared to be decreased in both a dose- and time-dependent manner at concentrations of 2.5 μmol/l and higher (Figs. 2B–2D).
FIG. 2.
The effect of sodium selenite on BPH-1 cell growth and viability. The data represent the mean ± SD of three experiments. A: Cell growth measured by the MTT assay presented as absorbance read by microplate reader at 570 nm at different time intervals; *P < 0.001 (two-sided) for difference with control cells. OD, optical density. B–D: The number of viable cells and cell viability determined by hemocytometer counting and trypan blue exclusion after 24 h (B), 48 h (C), and 72 h (D); *P < 0.001 (two-sided) for difference with control cells.
PC-3 cells were much more resistant to the treatment with sodium selenite than the LNCaP and BPH-1 cells. The viability of PC-3 cells decreased in both a dose- and time-dependent manner at concentrations of 5 μmol/l and higher with the hemocytometer method, and the total number of cells was slightly decreased in a dose-dependent manner at the noncytotoxic dose of 2.5 μmol/l and higher cytotoxic concentrations (Figs. 3B–3D). With the MTT assay, there was an inhibitory effect only after 72 h and only at the 10 μmol/l dose (Fig. 3A).
In summary (see Table 1), for all three cell lines, the 5 and 10 μmol/l doses were cytotoxic in the trypan blue exclusion assay. The 1 μmol/l dose was not cytotoxic in any cell line, and it was growth stimulatory in LNCaP cells but not the two other cell lines. Growth inhibition occurred only at cytotoxic concentrations in BPH-1 cells and, less so, in LNCaP cells. Growth inhibition of PC-3 cells was found at both noncytotoxic and cytotoxic concentrations. However, the MTT assay results were not in total agreement with those obtained by hemocytometer counting and trypan blue exclusion; in particular, no growth stimulation of LNCaP cells was observed at the 1 μmol/l selenite dose with the MTT assay.
TABLE 1.
Summary of the overall effects on LNCaP, BPH-1, and PC-3 cellsa
| Sodium Selenite (μmol/l) | Number of Viable Cells |
Cytotoxicityb |
Cell in S-Phasec | Apoptotic Cells (Flow Cytometry)c | DNA Ladderingd | Caspase Activatione | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| 24 h | 48 h | 72h | 24 h | 48 h | 72 h | |||||
| LNCaP | ||||||||||
| 1 | ↑ ↑ | ↑ ↑ ↑ | ~ – ↑ | ±/~ | ~ | ~ | ↑ ↑ | ± | ± | ± |
| 2.5 | ~ | ~ | ↓ ↓ | ~ | ~ | ++ | ↑ | ~ | + | +± |
| 5 | ↓ ↓ | ↓ ↓ ↓ | ↓ ↓ ↓ | ++ | + + + | + + + | ↑ ↑ ↑ | ++ | ++ | ++ |
| 10 | ↓ ↓ | ↓ ↓ ↓ | ↓ ↓ ↓ | ++ | + + + | + + + | ↑ | + + + | + + + | ++± |
| BPH-1 | ||||||||||
| 1 | ~ – ↓ | ~ | ↓ | ~ | ~ | ± | ~ – ↑ | ~ | ~ | + |
| 2.5 | ↓ ↓ | ↓ ↓ | ↓ ↓ ↓ | + | ++ | + + + | ~ | ~ | ~ | +± |
| 5 | ↓ ↓ ↓ | ↓ ↓ ↓ | ↓ ↓ ↓ | ++ | + + + | + + + | ~ – ↓ | ++ | + | ++ |
| 10 | ↓ ↓ ↓ | ↓ ↓ ↓ | ↓ ↓ ↓ | + + + | + + + | + + + | ↓ | + + + | ++ | ++± |
| PC-3 | ||||||||||
| 1 | ~ – ↑ | ~ | ↓ | ~ | ~ | ~ | ~ | ~ | ~ | ~ |
| 2.5 | ~ – ↑ | ↓ | ↓ ↓ | ~ | ~ | ~ | ~ | ~ | ~ | ~ |
| 5 | ~ | ↓ | ↓ ↓ | ~ | ± | + | ~ | ± | ~ | ~ |
| 10 | ↓ | ↓ ↓ | ↓ ↓ ↓ | ~ | ++ | + + + | ~ – ↑ | ± | ~ | ~ |
Abbreviations are as follows: LNCaP, lymph node carcinoma of the prostate; BPH-1, benign prostatic hyperplasia-1; PC-3, prostate cancer-3. ↑, ↑ ↑, ↑ ↑ ↑ = slightly, moderately, markedly increased, respectively; ↓, ↓ ↓, ↓ ↓ ↓ = slightly, moderately, markedly decreased, respectively; ±, +, ++, + + + = minimal, slight, moderately, markedly increased, respectively; ~= no change. Shaded cells indicate cytotoxicity.
Trypan blue leaking cells.
At 48 h.
At 72 h.
At 24 h.
Flow Cytometric Analysis of Sodium Selenite Effects
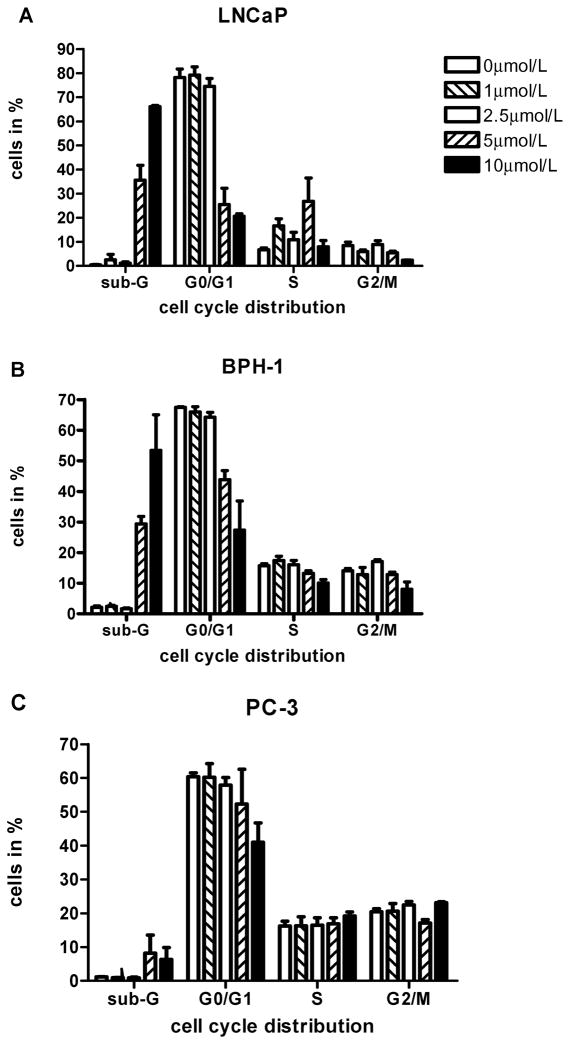
To determine whether the inhibition of cell growth was due to apoptosis and/or cell cycle arrest, cell cycle analyses were carried out using flow cytometry of asynchronously growing LNCaP, BPH-1, and PC-3 cells treated with sodium selenite for 48 h in T25 flasks (Figs. 4A–4C). This time point was selected because this was the latest time point at which in LNCaP and PC-3 cells cytotoxicity had not yet developed at low concentrations, 24 h usually being too early for observing cell cycle effects. Control cells of all three cell lines had virtually no DNA from apoptotic and necrotic cells in the sub G0/G1 area, and these cells displayed a distribution of cells in the G0/G1, S, and G2/M phases typical of proliferating cancer cells. There was evidence of a growth arrest of LNCaP cells after sodium selenite treatment for 48 h at 5 μmol/l, less so at 1 and 2.5 μmol/l, which was suggestive of an S-phase block or S/G2-phase transition block at these cytotoxic concentrations. A moderate amount of DNA in the sub-G0/G1 range reflective of apoptosis and/or cytotoxicity at 5 μmol/l was detected in these cells as well, and there was evidence of massive apoptosis and/or cytotoxicity at 10 μmol/l. In BPH-1 cells, treatment with sodium selenite did not lead to a cell cycle arrest, but at 5 and 10 μmol/l, a concentration-dependent decrease of the cell number in G0/G1 phase was found compared to controls because of considerable amount of DNA in the sub-G0/G1 range at 5 μmol/l and massive amount of sub-G0/G1 DNA at 10 μmol/l. In PC-3 cells, there was also no clear evidence of a cell cycle arrest at any of the selenium concentrations examined despite the growth inhibition seen with other methods, and there was a little evidence of DNA in the sub-G0/G1 range at 5 and 10 μmol/l, suggestive of a slight increase in apoptosis and/or cytotoxicity.
FIG. 4.
Panels represent summaries of the results of three separate analyses (means ± SD) of cell cycle distribution (sub-G0/G1, G0/G1, S, and G2/M phase, respectively) after treatment of increasing concentrations of sodium selenite (0, 1, 2.5, 5, and 10 μmol/l) for 48 h in A) LNCaP cells, B) BPH-1 cells, and C) PC-3 cells. Where error bars are not visible, the variation was very small.
Induction of Apoptosis by Sodium Selenite
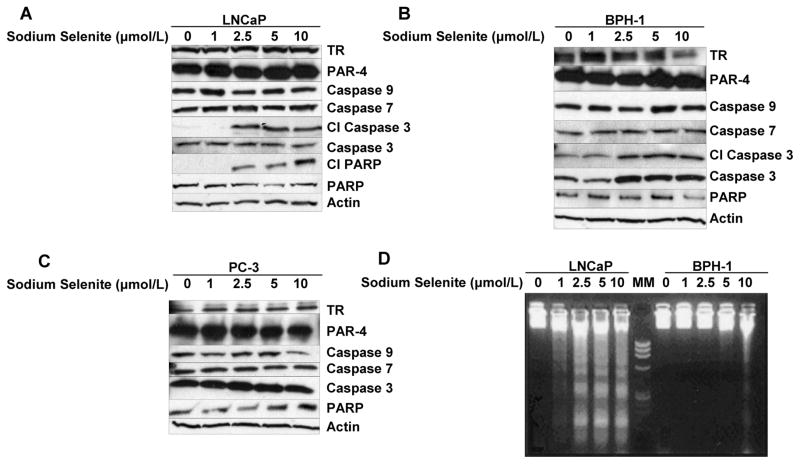
Exposure of LNCaP cells for 72 h to sodium selenite in T25 flasks resulted in DNA laddering indicative of nucleosomal DNA fragmentation typical of late-stage apoptosis in a concentration-dependent manner, with the first signs of apoptosis apparent at 1 μmol/l (Fig. 5D). BPH-1 cells, on the other hand, showed only a modest apoptotic laddering response to sodium selenite exposure for 72 h at 5 μmol/l and displayed frank laddering only at 10 μmol/l (Fig. 5D). Exposure of LNCaP and BPH-1 cells to 5 and 10 μmol/l selenite led to detachment of individual cells from the culture vessels after 24 and 48 h of exposure. At these time points and selenite concentrations, most, but not all, LNCaP and BPH-1 cells and detached cells (floaters) displayed a typical apoptotic morphology (prominent cytoplasmic vacuoles, cellular shrinkage, and nuclear chromatin condensation) following DAPI staining, but selenite-treated PC-3 cells did not exhibit such apoptotic morphology, and no DNA laddering was detected (data not shown).
FIG. 5.
A–C: Western blot analysis of the effect of sodium selenite on cleavage of PARP and selected pro-caspases after 24 h exposure to increasing concentrations of sodium selenite (0, 1, 2.5, 5, and 10 μmol/l) in A) LNCaP cells, B) BPH-1 cells, and C) PC-3 cells. D) Agarose (1.5%) gel electrophoretic detection of nucleosomal DNA fragmentation (laddering) in LNCaP (left 5 lanes) and BPH-1 cells (right 5 lanes) after 72 h exposure to increasing concentrations of sodium selenite. The middle lane, which is indicated as MM, was loaded with 100-bp DNA size markers. TR, thioredoxin reductase; CI, cleaved; PAR-4, Prostate apoptosis response-4.
Caspase Activation and PARP Cleavage Induced by Sodium Selenite
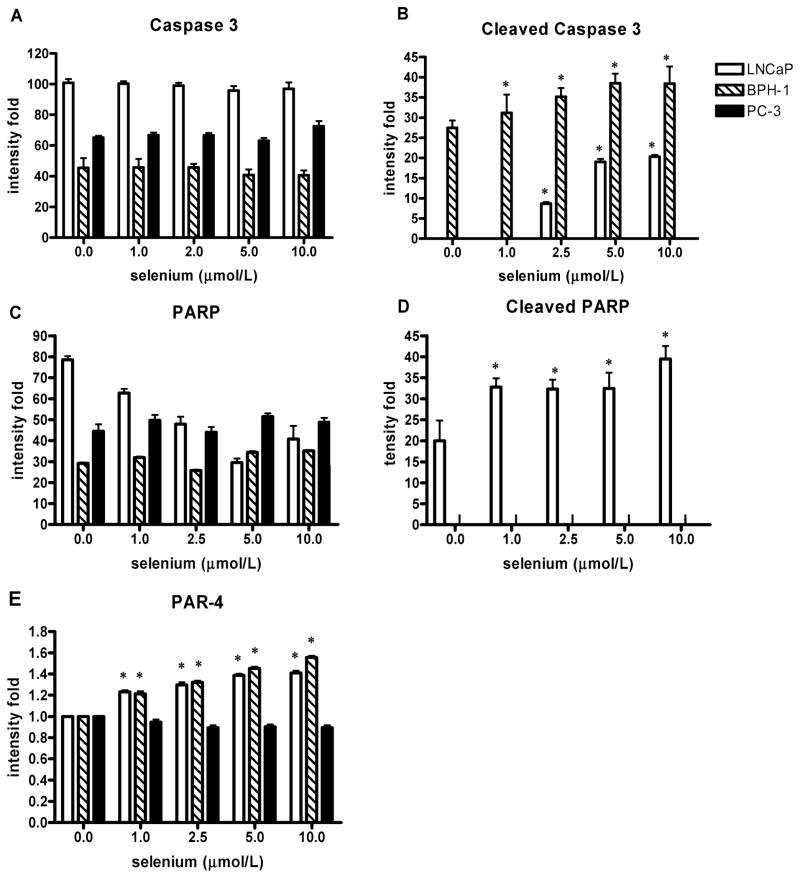
To further characterize the early stage of apoptotic process, immunoblot identification of caspase activation was performed after 24 h of selenite exposure. Because PARP is a substrate of the caspase-3 protease during apoptosis, cell lysates after selenium treatment were also examined by Western analysis for PARP cleavage. Expression of the intact 116 kDa PARP molecule was observed in all cell lines (Figs. 5A–5C and Fig. 6C). In LNCaP cells, exposure to selenite at 2.5 μmol/l and higher, which led to apoptosis, resulted in a dose-dependent cleavage of PARP as indicated by an increased amount of 86 kDa cleavage product and a decrease in full-length PARP expression (Fig. 5A, Figs. 6C and 6D). Thus, PARP cleavage was involved in apoptosis execution induced by selenite in this cell line. To define which caspases were involved in PARP cleavage during selenite-induced apoptosis in LNCaP cells, additional Western analyses were performed that indicated that caspase-3 had undergone cleavage in the selenite-treated LNCaP cells at concentrations of 2.5 μmol/l and higher in a dose-dependent manner. Exposure of BPH-1 cells to selenite only resulted in cleavage of caspase-3, but not PARP, at concentrations of 1 μmol/l and higher in a dose-dependent manner (Figs. 5B and 6B). In contrast, there was no cleavage of either PARP or caspase-3 in selenite treated PC-3 cells (Fig. 5C). Expression of caspases 7 and 9 was not changed by selenite in any of the cell lines tested (Fig. 6C), and their cleaved products were not detectable (data not shown). These results provide solid evidence for the involvement of caspase-3 activation in apoptosis induced by sodium selenite exposure in both LNCaP and BPH-1 cells. Furthermore, BPH-1 cells were resistant to induction of PARP cleavage by selenite, whereas LNCaP cells showed clear evidence of PARP cleavage. The absence of cleavage of PARP and the caspases examined in PC-3 cells after selenite exposure was in line with the lack of detectable apoptosis in this cell line. The loading control, β-actin, was not different among the 3 cell lines tested at any of the selenite doses studied.
FIG. 6.
A–E: Effects of sodium selenite treatment of LNCaP, BPH-1, and PC-3 cells for 24 h on caspase 3 and PARP cleavage and Prostate apoptosis response-4 (PAR-4 protein expression. A: Caspase 3. B: Cleaved caspase 3. C: PARP. D: Cleaved PARP. E: PAR-4. Data are expressed as intensity and represent the means ± SD of three independent experiments; *P < 0.05 (two-sided) for difference with control cells. Where error bars are not visible, the variation was very small; and where bars are not visible, there was no detectable protein.
Immunoblot Detection of Prostate Apoptosis Response-4 (PAR-4) Protein
The prostate apoptosis response (PAR) proteins-1, -3, -4, and -5 represent an apoptosis response gene program common to both androgen-dependent and -independent prostate cells (30). Induction of PAR-4 is apoptosis specific, and it is not induced by inducers of growth, oxidative stress and necrosis, or growth arrest in prostate cells (30). To study whether the PAR-4 protein is induced by exposure to sodium selenite, we examined expression of the PAR-4 protein by Western blot analysis (Figs. 5A–5C). There was a significant dose-related induction of PAR-4 protein in LNCaP and BPH-1 cells at all doses of selenium treatment, whereas there was not any significant PAR-4 protein expression in selenium-induced apoptosis resistant PC-3 cells (Fig. 6E).
Effects of Sodium Selenite on Expression and Activity of the Selenoprotein Thioredoxin Reductase (TR)
The activity and expression of TR was measured after 24 h of selenite exposure (data not shown). In LNCaP cells, TR activity was not affected significantly. In BPH-1 and PC-3 cells, TR activity was not significantly affected at 1 and 2.5 μmol/l, but enzyme activity was significantly decreased at 5 and 10 μmol/l. Selenite exposure did not change the TR protein level in LNCaP cells significantly at any dose. In BPH-1 cells, TR protein levels were significantly increased in all concentrations compared to control, but this effect was not dose related. TR protein level in PC-3 cells was not affected significantly at 2.5 to 10 μmol/l, but it was slightly but significant decreased at 1 μmol/l compared to control. TR mRNA levels were not significantly affected by selenite in LNCaP, BPH-1, and PC-3 cells except that at 10 μmol/l TR, mRNA expression was not detectable in LNCaP cells (data not shown). Thus, selenite effects on TR activity did not appear to correlate in any way with the effects of selenite on cell growth and viability in the three cell lines.
DISCUSSION
This study addressed two questions: First, we examined whether selenium in its inorganic form as sodium selenite inhibits proliferation and induces apoptosis in prostate cancer cells at noncytotoxic exposure levels. Second, we examined whether selenite had effects on nontumorigenic prostate epithelial cells that are different from those on cancerous prostate cells with regard to cell growth and apoptosis in order to determine possible selectivity for malignant cells of such anticancer selenium effects. Our results indicate that substantive growth inhibition and apoptosis induction are not induced by selenite at relevant noncytotoxic concentrations in any of the three cell lines examined, nontumorigenic benign BPH-1 prostate epithelial cells, androgen-sensitive LNCaP cells, and androgen-independent PC-3 cells. Noncytotoxic selenite doses induced modest apoptosis only in LNCaP cells, but not BPH-1 and PC-3 cells, and caused modest growth inhibition only in PC-3 cells but not BPH-1 and LNCaP cells. In addition, nontumorigenic BPH-1 cells were more sensitive for the cytotoxic effects of selenium than the malignant cells as also reported for benign HPV-immortalized RPE-1 cells (31), whereas LNCaP cells were more sensitive in this regard than PC-3 prostate cancer cells. Thus, selenium did indeed have differential effects in the prostate cancer cell lines we tested vs. nontumorigenic prostate epithelial cells (BPH-1), but we did not find that the prostate cancer cells were selectively sensitive to growth inhibition by selenite compared to benign prostate cells. This is the first report of selenium effects on cell growth and apoptosis of BPH-1 cells.
Surprising was our observation that proliferation of LNCaP cells was actually stimulated at a noncytotoxic concentration of 1 μmol/l selenium, the lowest concentration tested. The nontumorigenic BPH-1 cells and PC-3 cancer cells were not growth-inhibited or -stimulated at this noncytotoxic concentration. The observation of stimulation of proliferation of LNCaP cells at the 1 μmol/l concentration is of some concern and has some precedent in studies by Ghosh (32) who consistently found stimulation of LNCaP cell proliferation after 72 h of 0.5 μmol/l selenite exposure and inhibition at concentrations of 1.5 μmol/l and higher using an MTT-like assay. These findings, if confirmed in repeat studies with other androgen-responsive cell lines and other prostate cancer models, suggest the possibility of a biphasic response to selenium of untreated, early stage prostate cancer for which LNCaP cells may be a model. Some in vivo support for this possibility comes from a study by Novoselov et al. (33) with selenium in TGFα-c-Myc transgenic mice that develop liver cancer. Compared to both selenium-deficient mice and mice fed high nutritional selenium levels (2.25 ppm), far more mice developed liver tumors at sharply increased multiplicity when fed 0.4 ppm selenium, the lowest dose that maximally induced selenoproteins including GPx1 and TR1. Mitotic activity was also highest at 0.4 ppm and the apoptotic index reduced. Although these findings can be interpreted as inhibition of liver carcinogenesis by selenium deficiency and high selenium levels, it could also indicate stimulation of tumorigenesis by low adequate dietary selenium levels. There are other studies in which in vitro stimulation of proliferation by low concentrations of selenite (0.1–1.0 μmol/l selenium) was found for other types of cancer cells. However, these studies were conducted in serum-free medium (34–36), which creates selenium deficiency. Of note, studies using serum-containing medium have also been conducted under selenium-deficient or -marginal conditions, because most serum used in tissue culture contains no more than a few hundred nmol/l selenium. Thus, our results of cell proliferation of LNCaP cells grown in 1.0 μmol/l selenium could be interpreted as reflective of a “normal selenium status.” However, the selenoprotein TR was not induced over control values by any selenium concentration in any of the cell lines. This would suggest that selenoprotein expression in control cells was maximally induced, reflective of a nutritionally adequate selenium status. Furthermore, it is remarkable that we did not observe a stimulatory effect on cell proliferation in the two other cell lines studied. Despite these uncertainties and the limitations inherent to extrapolating the results of cell culture studies, our data suggest that there is some potential for an adverse effect on prostate cancer risk of selenium supplementation in humans (and rodent models), which is likely to be dependent on tumor characteristics such as, for example, androgen dependence, baseline selenium status (8) and on selenium dose and form and selenoprotein genotype (26) as well as potentially confounding factors such as vitamin E intake (13).
Sensitivity of LNCaP cells to selenite-induced apoptosis similar to our findings has been reported by others (14,32,37). Zhong and Oberley (37) also found cytotoxicity at similar selenium concentrations using both MTT assay and reduced growth by hemocytometer counting. However, our findings are not in agreement with the lack of selenium effects on normal prostate epithelial cells (Clonetics cells, not BPH-1 cells) observed by Ghosh (32) and by Menter et al. (38). In our study, PC-3 cells treated with selenite did not show apoptosis induction even at 10 μmol/l, which is in conflict with the findings of Menter et al. (38) who used that dose. Perhaps these differences can be explained by differences in passage number of the cell lines used or variations in culture conditions and other methods used. Because PC-3 cells were derived from advanced stage prostate cancer metastasis, they grow very rapidly and have a tendency to change as they are passaged in culture and may respond differently to selenium with increasing passage number.
The possible importance of methods and cell culture conditions is illustrated by our observation that the MTT assay results were not totally identical to those obtained by hemocytometer counting and trypan blue exclusion. In particular, no growth stimulation of LNCaP cells was observed at 1 μmol/lM selenite with the MTT assay. However, selenium effects on growth of BPH-1 and PC-3 cells were in general similar with the two methods as were the selenium effects on viability in all three cell lines. Because the MTT assay and similar methods provide not only an estimate of the number of viable cells, but also measure the metabolic activity of these cells, the hemocytometer counting and trypan blue exclusion may be a more realistic approach to determining effects on growth and viability. In fact, some reports have mistakenly referred to reduced MTT readings at concentrations above 1 μmol/l selenite as indicative of growth inhibition, whereas our results clearly indicate that these are cytotoxic concentrations that are not experimentally realistic or biologically relevant to study. Another possible explanation for differences between experiments with these two assays may be differences in the types of culture vessels used in MTT-like assays and the trypan blue-hemocytometer assay, 96-well plates and 6-well plates, respectively, potentially affecting the conditions to grow for cells.
Differences in the results of various studies are probably also in part due to the diversity in doses and forms of selenium used (39). Selenium supplementation of humans is given in various forms, most often as selenomethionine or selenized yeast, but also as sodium selenite, which has been shown to have biological effects in human subjects at nutritionally relevant low doses (40). The selenium doses used in some other studies ranged from 10 to 500 μmol/l, which are toxic to extremely toxic levels of selenite that induce DNA-strand breaks and necrosis. In our study and some other studies in the literature, the doses of sodium selenite have ranged from 1 to 10 μmol/l, which are within the range of physiologic serum concentrations and have been shown to inhibit cell growth, induce apoptosis, and provide prooxidant effects especially in a variety of rapidly dividing cells with a high intracellular redox state (41). In a recent epidemiologic study by Peters et al. (13), the mean serum selenium level was 141 ng/ml (range = 51–253 ng/ml) in a U.S. male population, which amounts to approximately 1.8 μmol/l with a range of 0.63 μmol/l to 3.2 μmol/l. Vogt et al. (42) found similar serum selenium levels (means of 119–126 ng/ml) in the third NHANES study. In the Clark trial, in which selenium supplementation in yeast reduced prostate cancer risk, the average plasma selenium level at baseline was 114 ng/ml, with a range of 42 to 220 ng/ml (43), and only 6 of the 1,112 subjects randomized had a selenium level below 80 ng/ml (equivalent to 1 μmol/l), which is considered physiologically adequate and results in maximal induction of most if not all selenoproteins such as thioredoxin reductase (TR) and glutathione peroxidase (GPx) (44,45). However, although it is not certain what the exact tissue selenium levels are in the human prostate, reported tissue levels have been similar to or approximately two times higher than serum levels (46–48). Thus, the lower concentrations used in our study, 1–5 μmol/l, encompass the upper and lower ends of the normal range of serum and possibly tissue levels, but they do not include subadequate selenium concentrations.
Our data indicate that treatment with selenite resulted in a modest S-phase arrest in LNCaP cells and in BPH-1 cells and a slight G2/M arrest in PC-3 cells. These results are consistent with those of others including Menter et al. (38) who showed that sodium selenite caused greater S-phase arrest than G2-M arrest in both LNCaP and PC-3 cells. Although these reports and our observations suggest that one of the mechanisms by which selenite may act to inhibit proliferation of prostate cancer cells is cell cycle impairment, this appeared to occur mostly at nonrelevant cytotoxic selenium concentrations.
Apoptosis plays a crucial role in eliminating mutated neoplastic and hyperproliferating cells and is considered a protective mechanism against cancer development and progression. Our results indicate that treatment of LNCaP and BPH-1 cells with selenite resulted in significant induction of apoptosis only at cytotoxic concentrations and that this effect was not seen in PC-3 cells. Noncytotoxic selenium concentrations induced apoptosis only in LNCaP cells, as we indicated earlier. We obtained solid evidence for the involvement of activation of caspase-3, but not caspase-7 and -9, in the apoptosis induced by sodium selenite exposure in both LNCaP and BPH-1 cells. BPH-1 cells were resistant to induction of PARP cleavage by sodium selenite, whereas LNCaP cells showed clear evidence of PARP cleavage. These findings are not in agreement with those others who have found that selenite treatment resulted in cleavage of caspase-7 and -9 in LNCaP cells (14) and PARP cleavage in LNCaP (and PC-3 cells) (38). Differences in time points and selenite concentrations may be the reason for these inconsistencies, illustrating the difficulties of comparing studies that do not use the exact same procedures. Because this is the first selenium study with BPH-1 cells, we could not compare our results with those of others, but the response of these immortalized cells was different from the Clonetics primary prostate epithelial cells used by Menter et al. (38).
Although PC-3 cells were totally refractory to apoptosis induction by selenite up to a concentration of 10 μmol/l, they were growth inhibited at the highest noncytotoxic concentration of 2.5 μmol/l. In advanced hormone-refractory prostate cancer, cancer cells become resistant to induction of apoptosis and cytotoxicity by most chemotherapeutic agents (49), similar to what we found for the insensitivity to selenium of PC-3 cells. However, our results suggest that there may be therapeutic benefit of selenium at high (but nontoxic) doses against advanced hormone-refractory prostate cancer through growth-inhibitory activity. LNCaP cells, on the other hand, began to show signs of apoptosis (by DNA laddering) even at a noncytotoxic concentration of 1 μmol/l when they were also growth stimulated. BPH-1 cells began to undergo apoptosis at cytotoxic concentrations of 2.5 μmol/l and, with frank DNA laddering, 5 μmol/l, which is near the upper limit of the physiological range of selenium concentrations. Thus, the reduced numbers of cells observed after selenite treatment are probably due to a combination of cell cycle arrest, apoptotic cell death, and cytotoxicity (necrosis); but at noncytotoxic selenium concentrations, reduction of cell proliferation is very modest at best, and apoptosis appears to play a minimal, if any, role.
PAR-4 is an apoptosis-specific protein in prostate cells (30). There was a significant dose-related induction of PAR-4 protein expression in both LNCaP and BPH-1 cells over the entire range of selenium concentrations. There was not any significant PAR-4 protein expression in PC-3 cells, consistent with the observed absence of apoptosis in PC-3 cells after selenium exposure. There was an excellent correlation between the PAR-4 protein upregulation and the activation of caspase-3 in BPH-1 and LNCaP cells and PARP activation in LNCaP cells. Thus, PAR-4 may be an appropriate marker of apoptosis in prostate (cancer) cells.
The selenoprotein TR, which is part of thioredoxin (Trx) system that also includes NADPH and Trx, is known to be tightly regulated, and large overexpression of Trx in transfected cells appears to be toxic (50). The redox cycling of selenite with TR and oxygen may give rise to oxidative stress, leading to killing of cancer cells by necrosis and apoptosis at high selenite concentrations (27). Therefore, it is conceivable that selenium-induced increases in TR activity are related to cancer preventive effects of selenium in prostate cancer through the induction of apoptotic cell death. There was overall no evidence of induction of TR by selenite compared to control cultures in any of the three cell lines used. This would indicate that TR and possibly other selenoproteins were maximally induced in control cultures in the absence of selenium supplementation. At 5 μmol/l, TR activity was decreased in BPH-1 and PC-3 cells; and at 10 μmol/l, there was a decrease in TR activity in all three cells compared to controls, which is consistent with a study by Nilsonne et al. (51). Cells with lower TR activity may be more prone to oxidative damage (52), which may explain some but not most of the cytotoxicity seen at these selenium concentrations because there was a very poor correlation between TR activity reduction and induction of cytotoxicity with increasing selenite dose. It has been suggested that TR may induce caspase-3 activity in prostate cancer cells (37), and Lindner et al. (53) showed that an increase in TR in estrogen responsive cells was required to cause caspase-3 activation. This finding is consistent with our results on caspase-3 in nontumorigenic BPH-1, but not LNCaP, cancer cells. However, TR activity stimulation by various doses of selenium can vary greatly between different cell lines (51).
In conclusion, the results of this study indicate that that selenite at noncytotoxic concentrations does not induce growth inhibition or apoptosis selectively in prostate cancer cells compared to nontumorigenic prostate epithelial cells. Androgen-independent PC-3 cells were refractory to apoptosis induction by selenite up to a concentration of 10 μM but were growth inhibited at lower noncytotoxic selenium concentrations. Androgen responsive LNCaP cells, on the other hand, were growth stimulated at a physiological concentration of 1 μM and were sensitive to apoptosis induction at low selenium concentrations. At noncytotoxic selenium concentrations, there was no induction of apoptosis in BPH-1 and PC-3 cells and no growth inhibition of LNCaP and BPH-1 cells. The observation of growth stimulation by low, nutritionally adequate, selenium concentrations selectively in LNCaP cells raises the possibility of adverse effects of selenium supplementation on prostate cancer risk. The inhibition of PC-3 cell proliferation at noncytotoxic selenium concentrations suggests a possible benefit of selenium in advanced prostate cancer.
Acknowledgments
This work was supported in part by NIH Grant No. R01 CA104334 and by Environmental Health Science Center Core Grant No. ES00260 and Cancer Center Support Grant CA13343. The authors want to thank Alan Diamond for his encouragement and help in preparing the article and Ryan Deaton for his help with preparing the figures.
Footnotes
Publisher's Disclaimer: Full terms and conditions of use: http://www.informaworld.com/terms-and-conditions-of-access.pdf
This article may be used for research, teaching and private study purposes. Any substantial or systematic reproduction, re-distribution, re-selling, loan or sub-licensing, systematic supply or distribution in any form to anyone is expressly forbidden.
The publisher does not give any warranty express or implied or make any representation that the contents will be complete or accurate or up to date. The accuracy of any instructions, formulae and drug doses should be independently verified with primary sources. The publisher shall not be liable for any loss, actions, claims, proceedings, demand or costs or damages whatsoever or howsoever caused arising directly or indirectly in connection with or arising out of the use of this material.
Contributor Information
Nur Özten Kandaş, Department of Pathology, University of Illinois at Chicago, Chicago, Illinois, USA.
Carla Randolph, Department of Environmental Medicine, New York University School of Medicine, Tuxedo, New York, USA.
Maarten C. Bosland, Department of Pathology, University of Illinois at Chicago and Departments of Environmental Medicine and Urology, New York University School of Medicine, New York and Tuxedo, New York, USA
References
- 1.Platz EA, Giovannucci E. The epidemiology of sex steroid hormones and their signaling and metabolic pathways in the etiology of prostate cancer. J Steroid Biochem Mol Biol. 2004;92:237–253. doi: 10.1016/j.jsbmb.2004.10.002. [Epub 2005 January 5] [DOI] [PubMed] [Google Scholar]
- 2.Bosland MC. The role of steroid hormones in prostate carcinogenesis. J Natl Cancer Inst Monogr. 2000;27:39–66. doi: 10.1093/oxfordjournals.jncimonographs.a024244. [DOI] [PubMed] [Google Scholar]
- 3.Klein EA. Chemoprevention of prostate cancer. Ann Rev Med. 2006;57:49–63. doi: 10.1146/annurev.med.57.121304.131435. [DOI] [PubMed] [Google Scholar]
- 4.Bosland MC, McCormick DL, Melamed J, Walden PD, Zeleniuch-Jacquotte A, et al. Chemoprevention strategies for prostate cancer. Eur J Cancer Prev. 2002;11(2 Suppl):S18–S27. [PubMed] [Google Scholar]
- 5.Sinha R, El-Bayoumy K. Apoptosis is a critical cellular event in cancer chemoprevention and chemotherapy by selenium compounds. Curr Cancer Drug Targets. 2004;4:13–28. doi: 10.2174/1568009043481614. [DOI] [PubMed] [Google Scholar]
- 6.Clark LC, Combs GF, Jr, Turnbull BW, et al. Effects of selenium supplementation for cancer prevention in patients with carcinoma of the skin: a randomized controlled trial. Nutritional Prevention of Cancer Study Group. JAMA. 1996;276:1957–1963. [Erratum in JAMA 277, 1520, 1997] [PubMed] [Google Scholar]
- 7.Duffield-Lillico AJ, Reid ME, Turnbull BW, et al. Baseline characteristics and the effect of selenium supplementation on cancer incidence in a randomized clinical trial: a summary report of the Nutritional Prevention of Cancer Trial. Cancer Epidemiol Biomarkers Prev. 2002;11:630–639. [PubMed] [Google Scholar]
- 8.Duffield-Lillico AJ, Slate EH, Reid ME, et al. Selenium supplementation and secondary prevention of nonmelanoma skin cancer in a randomized trial. J Natl Cancer Inst. 2003;95:1477–1481. doi: 10.1093/jnci/djg061. [DOI] [PubMed] [Google Scholar]
- 9.El-Bayoumy K, Upadhyaya P, Date V, Sohn OS, Fiala ES, et al. Metabolism of [14C] benzyl selenocyanate in the F344 rat. Chem Res Toxicol. 1991;4:560–565. doi: 10.1021/tx00023a012. [DOI] [PubMed] [Google Scholar]
- 10.El-Bayoumy K, Das A, Narayanan B, Fiala ES, Desai D, et al. Molecular targets of the chemopreventive agent 1,4-phenylenebis (methylene)-selenocyanate in human non-small cell lung cancer. Carcinogenesis. 2006;27:1369–1376. doi: 10.1093/carcin/bgi328. [Epub 2006 January 6] [DOI] [PubMed] [Google Scholar]
- 11.Brinkman M, Reulen RC, Kellen E, Buntinx F, Zeegers MP. Are men with low selenium levels at increased risk of prostate cancer? Eur J Cancer. 2006;42:2463–2471. doi: 10.1016/j.ejca.2006.02.027. [Epub 2006 September 1] [DOI] [PubMed] [Google Scholar]
- 12.Li H, Stampfer MJ, Giovannucci EL, et al. A prospective study of plasma selenium levels and prostate cancer risk. J Natl Cancer Inst. 2004;96:696–703. doi: 10.1093/jnci/djh125. [DOI] [PubMed] [Google Scholar]
- 13.Peters U, Foster CB, Chatterjee N, et al. Serum selenium and risk of prostate cancer-a nested case-control study. Am J Clin Nutr. 2007;85:209–217. doi: 10.1093/ajcn/85.1.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jiang C, Hu H, Malewicz B, Wang Z, Lu J. Selenite-induced p53 Ser-15 phosphorylation and caspase-mediated apoptosis in LNCaP human prostate cancer cells. Mol Cancer Ther. 2004;3:877–884. [PubMed] [Google Scholar]
- 15.Ip C, Thompson HJ, Zhu Z, Ganther HE. In vitro and in vivo studies of methylseleninic acid: evidence that a monomethylated selenium metabolite is critical for cancer chemoprevention. Cancer Res. 2000;60:2882–2886. [PubMed] [Google Scholar]
- 16.Diwadkar-Navsariwala V, Diamond AM. The link between selenium and chemoprevention: a case for selenoproteins. J Nutr. 2004;134:2899–2902. doi: 10.1093/jn/134.11.2899. [DOI] [PubMed] [Google Scholar]
- 17.Ip C. Lessons from basic research in selenium and cancer prevention. J Nutr. 1998;128:1845–1854. doi: 10.1093/jn/128.11.1845. [DOI] [PubMed] [Google Scholar]
- 18.Combs GF., Jr Considering the mechanisms of cancer prevention by selenium. Adv Exp Med Biol. 2001;492:107–117. doi: 10.1007/978-1-4615-1283-7_9. [DOI] [PubMed] [Google Scholar]
- 19.El-Bayoumy K. Overview: the late Larry C. Clark showed the bright side of the moon element (selenium) in a clinical cancer prevention trial. Nutr Cancer. 2001;40:4–5. doi: 10.1207/S15327914NC401_3. [DOI] [PubMed] [Google Scholar]
- 20.Fleming J, Ghose A, Harrison PR. Molecular mechanisms of cancer prevention by selenium compounds. Nutr Cancer. 2001;40:42–49. doi: 10.1207/S15327914NC401_9. [DOI] [PubMed] [Google Scholar]
- 21.Ganther HE. Selenium metabolism and mechanisms of cancer prevention. Adv Exp Med Biol. 2001;492:119–130. doi: 10.1007/978-1-4615-1283-7_10. [DOI] [PubMed] [Google Scholar]
- 22.Kim YS, Milner J. Molecular targets for selenium in cancer prevention. Nutr Cancer. 2001;40:50–54. doi: 10.1207/S15327914NC401_10. [DOI] [PubMed] [Google Scholar]
- 23.Lu J, Jiang C. Antiangiogenic activity of selenium in cancer chemoprevention: metabolite-specific effects. Nutr Cancer. 2001;40:64–73. doi: 10.1207/S15327914NC401_12. [DOI] [PubMed] [Google Scholar]
- 24.Youn BW, Fiala ES, Sohn OS. Mechanisms of organoselenium compounds in chemoprevention: effects on transcription factor-DNA binding. Nutr Cancer. 2001;40:28–33. doi: 10.1207/S15327914NC401_7. [DOI] [PubMed] [Google Scholar]
- 25.Patrick L. Selenium biochemistry and cancer: a review of the literature. Altern Med Rev. 2004;9:239–258. [PubMed] [Google Scholar]
- 26.Rayman MP. Selenium in cancer prevention: a review of the evidence and mechanism of action. Proc Nutr Soc. 2005;64:527–542. doi: 10.1079/pns2005467. [DOI] [PubMed] [Google Scholar]
- 27.Arner ES, Holmgren A. The thioredoxin system in cancer. Semin Cancer Biol. 2006;16:420–426. doi: 10.1016/j.semcancer.2006.10.009. [Epub 2006 October 28] [DOI] [PubMed] [Google Scholar]
- 28.Hayward SW, Dahiya R, Cunha GR, Bartek J, Deshpande N, et al. Establishment and characterization of an immortalized but non-transformed human prostate epithelial cell line: BPH-1. In Vitro Cell Dev Biol Anim. 1995;31:14–24. doi: 10.1007/BF02631333. [DOI] [PubMed] [Google Scholar]
- 29.Holmgren A, Bjornstedt M. Thioredoxin and thioredoxin reductase. Methods Enzymol. 1995;252:199–208. doi: 10.1016/0076-6879(95)52023-6. [DOI] [PubMed] [Google Scholar]
- 30.Sells SF, Wood DP, Jr, Joshi-Barve SS, et al. Commonality of the gene programs induced by effectors of apoptosis in androgen-dependent and -independent prostate cells. Cell Growth Differ. 1994;5:457–466. [PubMed] [Google Scholar]
- 31.Rebsch CM, Penna FJ, III, Copeland PR. Selenoprotein expression is regulated at multiple levels in prostate cells. Cell Res. 2006;16:940–948. doi: 10.1038/sj.cr.7310117. [Erratum in Cell Res 17, 272, 2007] [DOI] [PubMed] [Google Scholar]
- 32.Ghosh J. Rapid induction of apoptosis in prostate cancer cells by selenium: reversal by metabolites of arachidonate 5-lipoxygenase. Biochem Biophys Res Commun. 2004;315:624–635. doi: 10.1016/j.bbrc.2004.01.100. [DOI] [PubMed] [Google Scholar]
- 33.Novoselov SV, Calvisi DF, Labunskyy VM, et al. Selenoprotein deficiency and high levels of selenium compounds can effectively inhibit hepatocarcinogenesis in transgenic mice. Oncogene. 2005;24:8003–8011. doi: 10.1038/sj.onc.1208940. [DOI] [PubMed] [Google Scholar]
- 34.Nano JL, Czerucka D, Menguy F, Rampal P. Effect of selenium on the growth of three human colon cancer cell lines. Biol Trace Elem Res. 1989;20:31–43. doi: 10.1007/BF02919096. [DOI] [PubMed] [Google Scholar]
- 35.Zhu S, Gray TE, Nettesheim P. The effect of sodium selenite on cell proliferation and transformation of primary rat tracheal epithelial cells. Carcinogenesis. 1992;13:1725–1729. doi: 10.1093/carcin/13.10.1725. [DOI] [PubMed] [Google Scholar]
- 36.Lee YC, Tang YC, Chen YH, Wong CM, Tsou AP. Selenite-induced survival of HuH7 hepatoma cells involves activation of focal adhesion kinase-phosphatidylinositol 3-kinase-Akt pathway and Rac1. J Biol Chem. 2003;278:39615–39624. doi: 10.1074/jbc.M304095200. [DOI] [PubMed] [Google Scholar]
- 37.Zhong W, Oberley TD. Redox-mediated effects of selenium on apoptosis and cell cycle in the LNCaP human prostate cancer cell line. Cancer Res. 2001;61:7071–7078. [PubMed] [Google Scholar]
- 38.Menter DG, Sabichi AL, Lippman SM. Selenium effects on prostate cell growth. Cancer Epidemiol Biomarkers Prev. 2000;9:1171–1182. [PubMed] [Google Scholar]
- 39.Zhou N, Xiao H, Li TK, Nur-E-Kamal A, Liu LF. DNA damage-mediated apoptosis induced by selenium compounds. J Biol Chem. 2003;278:29532–29537. doi: 10.1074/jbc.M301877200. [Epub 2003 May 24] [DOI] [PubMed] [Google Scholar]
- 40.Pagmantidis V, Méplan C, van Schothorst EM, Keijer J, Hesketh JE. Supplementation of healthy volunteers with nutritionally relevant amounts of selenium increases the expression of lymphocyte protein biosynthesis genes. Am J Clin Nutr. 2008;87:181–189. doi: 10.1093/ajcn/87.1.181. [DOI] [PubMed] [Google Scholar]
- 41.Bjorkhem-Bergman L, Jonsson K, Eriksson LC, et al. Drug-resistant human lung cancer cells are more sensitive to selenium cytotoxicity: effects on thioredoxin reductase and glutathione reductase. Biochem Pharmacol. 2002;63:1875–1884. doi: 10.1016/s0006-2952(02)00981-4. [Erratum in Biochem Pharmacol 64, 159, 2002] [DOI] [PubMed] [Google Scholar]
- 42.Vogt TM, Ziegler RG, Patterson BH, Graubard BI. Racial differences in serum selenium concentration: analysis of US population data from the Third National Health and Nutrition Examination Survey. Am J Epidemiol. 2007;166:280–288. doi: 10.1093/aje/kwm075. [Epub 2007 June 8] [DOI] [PubMed] [Google Scholar]
- 43.Reid ME, Duffield-Lillico AJ, Garland L, Turnbull BW, Clark LC, et al. Selenium supplementation and lung cancer incidence: an update of the nutritional prevention of cancer trial. Cancer Epidemiol Biomarkers Prev. 2002;11:1285–1291. [PubMed] [Google Scholar]
- 44.Hill KE, Chittum HS, Lyons PR, Boeglin ME, Burk RF. Effect of selenium on selenoprotein P expression in cultured liver cells. Biochim Biophys Acta. 1996;1313:29–34. doi: 10.1016/0167-4889(96)00047-x. [DOI] [PubMed] [Google Scholar]
- 45.Neve J. Human selenium supplementation as assessed by changes in blood selenium concentration and glutathione peroxidase activity. J Trace Elem Med Biol. 1995;9:65–73. doi: 10.1016/S0946-672X(11)80013-1. [DOI] [PubMed] [Google Scholar]
- 46.Gianduzzo TR, Holmes EG, Tinggi U, Shahin M, Mactaggart P, et al. Prostatic and peripheral blood selenium levels after oral supplementation. J Urol. 2003;170:870–873. doi: 10.1097/01.ju.0000081052.51707.cf. [DOI] [PubMed] [Google Scholar]
- 47.Nyman DW, Suzanne Stratton M, Kopplin MJ, Dalkin BL, Nagle RB, et al. Selenium and selenomethionine levels in prostate cancer patients. Cancer Detect Prev. 2004;28:8–16. doi: 10.1016/j.cdp.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 48.Zachara BA, Szewczyk-Golec K, Tyloch J, et al. Blood and tissue selenium concentrations and glutathione peroxidase activities in patients with prostate cancer and benign prostate hyperplasia. Neoplasma. 2005;52:248–254. [PubMed] [Google Scholar]
- 49.Pilat MJ, Kamradt JM, Pienta KJ. Hormone resistance in prostate cancer. Cancer Metastasis Rev. 1999;17:373–381. doi: 10.1023/a:1006166511344. [DOI] [PubMed] [Google Scholar]
- 50.Gallegos A, Berggren M, Gasdaska JR, Powis G. Mechanisms of the regulation of thioredoxin reductase activity in cancer cells by the chemopreventive agent selenium. Cancer Res. 1997;57:4965–4970. [PubMed] [Google Scholar]
- 51.Nilsonne G, Sun X, Nystrom C, et al. Selenite induces apoptosis in sarcomatoid malignant mesothelioma cells through oxidative stress. Free Radic Biol Med. 2006;41:874–885. doi: 10.1016/j.freeradbiomed.2006.04.031. [Epub 2006 May 10] [DOI] [PubMed] [Google Scholar]
- 52.Zhang J, Svehlikova V, Bao Y, Howie AF, Beckett GJ, et al. Synergy between sulforaphane and selenium in the induction of thioredoxin reductase 1 requires both transcriptional and translational modulation. Carcinogenesis. 2003;24:497–503. doi: 10.1093/carcin/24.3.497. [DOI] [PubMed] [Google Scholar]
- 53.Lindner DJ, Hofmann ER, Karra S, Kalvakolanu DV. The interferon-beta and tamoxifen combination induces apoptosis using thioredoxin reductase. Biochim Biophys Acta. 2000;1496:196–206. doi: 10.1016/s0167-4889(00)00021-5. [DOI] [PubMed] [Google Scholar]