Abstract
Background:
Diagnosis of behavioral variant frontotemporal dementia (bvFTD) relies on criteria that are constraining and potentially ambiguous. Some features are open to clinical interpretation and their prevalence unknown. This study investigated the sensitivity of current diagnostic criteria in a large group of patients with bvFTD.
Methods:
Forty-five patients with clear evidence of bvFTD as judged by progressive clinical decline (>3 years) with marked frontal features and significant frontal brain atrophy on brain MRI were included. Thirty-two have died; pathologic confirmation of frontotemporal lobar degeneration was found in all 18 coming to autopsy. We established the prevalence of core and supportive diagnostic features at presentation and with disease progression.
Results:
Only 25/45 patients (56%) showed all five core features necessary for a diagnosis of bvFTD at initial presentation and 33/45 (73%) as their disease progressed. Two core features, emotional blunting and loss of insight, were never observed in 25% and 13% of cases. Executive dysfunction, hyperorality, mental inflexibility, and distractibility were the only supportive features present in >50% of cases at initial presentation. Although not a diagnostic feature, impaired activities of daily living was present in 33/45 patients (73%).
Conclusions:
Strict application of the criteria misses a significant proportion of patients. Many supportive features have low prevalence and are clinically not useful. Revision of the criteria to include level of certainty (definite, probable, possible) dependent on the number of features present and the presence of ancillary information (e.g., brain atrophy, neuropsychological abnormalities, impaired activities of daily living) is encouraged.
GLOSSARY
- ACE
= Addenbrooke’s Cognitive Examination;
- ADL
= activities of daily living;
- bvFTD
= behavioral variant frontotemporal dementia;
- MMSE
= Mini-Mental State Examination.
Diagnosis of behavioral variant frontotemporal dementia (bvFTD) in life remains a challenge.1–3 Patients present with marked behavior and personality changes, as well as cognitive deficits, and differentiation from other dementia syndromes and psychiatric disorders is not without difficulty. Currently, most clinicians and research investigations rely on the Consensus Criteria published in 1998 by Neary and colleagues.4 These criteria were a comprehensive attempt to establish clinical diagnostic criteria for FTD, based on the burgeoning research focusing on non–Alzheimer disease dementia syndromes. Since their publication, these criteria have become the de facto clinical standard in the diagnosis and in the recruitment of participants for clinical research on FTD. They are, however, not without limitations. While they aim to capture clinical changes pathognomonic of the disease, the core clinical features are poorly operationalized.5 The quantitative changes required to satisfy presence or absence of a feature are not specified and are open to clinical interpretation. The use of the supportive features is ambiguous and their prevalence is unknown. Some may be rarely endorsed except in patients with advanced disease.
Over recent years, an explosion of research on clinical characteristics,6,7 pathology,8,9 neuroimaging,10,11 and genetics12,13 in FTD syndromes has markedly improved our understanding of the disease. In a previous study, however, up to two thirds of cases did not conform to the consensus criteria at presentation,14 and the evolution of symptoms in pathologically confirmed cases of bvFTD is unknown. As such, features originally included in the clinical diagnostic criteria may not be appropriate for early diagnosis and other features, not originally considered, may contribute or enhance the sensitivity of the diagnosis. As recently discussed by an international group,5 a revision of the clinical criteria for FTD diagnosis is long overdue. In addition, diagnostic accuracy is complicated by recent reports of patients with features of bvFTD but who show little or no progression over many years.11,15 The inclusion of these so-called “phenocopy” cases may dramatically change the sensitivity of the diagnostic criteria.
Our study on the sensitivity of the criteria for bvFTD takes into account comprehensive longitudinal (at least 3 years) clinical and neuropsychological investigations, with pathologic confirmation in a high proportion of cases, and extends, therefore, existing findings in important ways. One previous investigation retrospectively examined the sensitivity of the diagnostic criteria at presentation, and after 2 years, in a small group of patients with FTD without pathologic confirmation, and did not report cognitive data.14 A second study examined how well Alzheimer disease and FTD cases, with postmortem confirmation, could be differentiated in life using the criteria for bvFTD.16 That study, however, did not explore the utility of the consensus criteria and did not take into account longitudinal changes.
The current study investigated the prevalence of the core and supporting diagnostic features at presentation and during disease progression in a large sample of patients with bvFTD seen by the same investigators, many with pathologic confirmation of the disease. We also examined whether additional clinical features, not part of the original consensus criteria, could contribute to the clinical diagnosis of bvFTD.
METHODS
Participants.
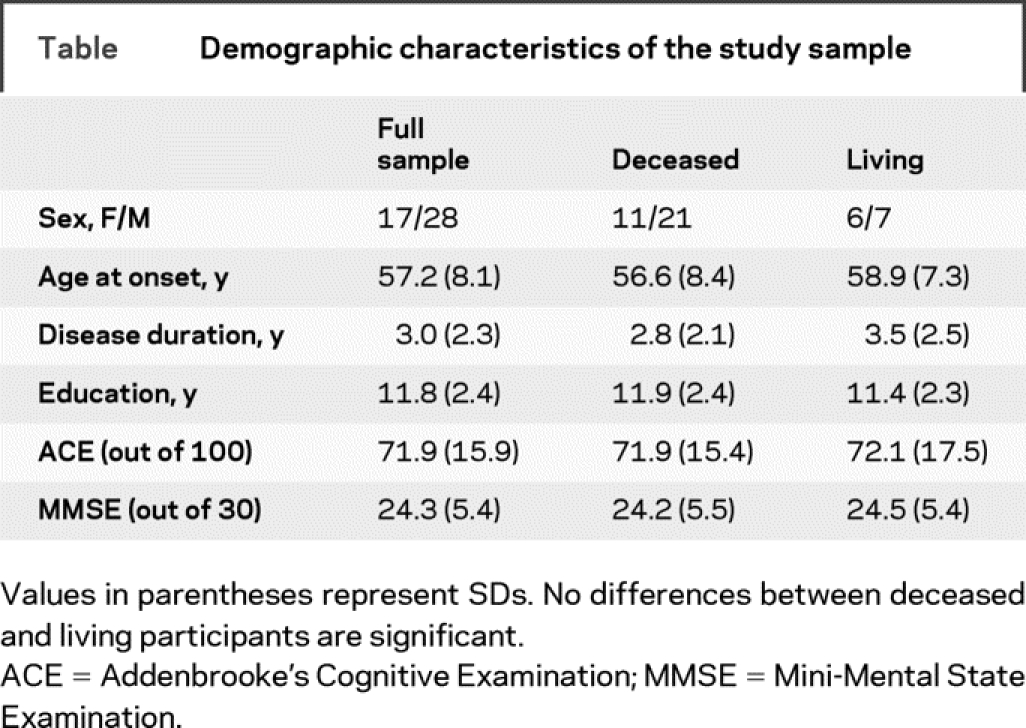
Review of the Cambridge Memory and Early Onset Dementia Clinics established in 1990 and 1997 identified 123 patients who had received a putative diagnosis of bvFTD. We included patients who met the following conditions: referral by a general practitioner, or by a specialist who had made a diagnosis of possible FTD within the previous 12 months; living at home without institutional support at the time of diagnosis; able to participate in neuropsychological examination and imaging; and showing clear evidence of bvFTD as judged by progression to advanced dementia with marked frontal features or death. A total of 78 patients were excluded for the following reasons: limited clinical information (no informant or English as a second language), follow-up of less than 3 years, or no subsequent objective evidence of progression despite prolonged follow-up typically accompanied by normal structural MR brain imaging (i.e., nonprogressors).11,15 A total of 45 patients with bvFTD were included in the final study cohort. All 45 had undergone a comprehensive neurologic, psychiatric, and cognitive assessment from a multidisciplinary team to exclude other neurologic and psychiatric disorders, showed significant brain atrophy on neuroimaging at presentation, and had progressive decline for at least 3 years. All had been seen by a highly experienced behavioral neurologist (J.R.H.) and the diagnosis established at a consensus meeting, based on clinical history, caregiver information, medical and neurologic investigations, and formal cognitive assessment. All caregivers had been interviewed using a standardized systematic proforma17 to enquire about behavioral changes and all confirmed a distinct, yet insidious, change in personality and behavior in the patients. Postmortem examination was conducted in 18 of the 32 deceased cases (56% of the deceased cases and 40% of the total sample) and confirmed the clinical diagnosis of FTD in all autopsied cases. Eight cases showed positive tau inclusions, whereas eight revealed Tar DNA binding protein-43 depositions. The remaining two cases showed dementia lacking distinctive histopathology. Thirteen patients were still alive. Clinical presentation, disease progression, and demographic characteristics did not differ significantly between living and deceased cases or between deceased cases with or without postmortem investigations (table).
Table Demographic characteristics of the study sample
Procedure.
One neurologist (B.P.S.), who had not been involved in the initial diagnosis and who had no prior knowledge of the patients, reviewed the clinical files. Clinical data were categorized according to the clinical diagnostic criteria described by Neary and colleagues4: core features, supportive features (behavioral disorder, speech and language, physical signs), and excluding features using a study proforma to determine their prevalence at presentation and on subsequent follow-up. The detailed descriptors from each of the core and supportive features were listed (e.g., for emotional blunting, the study proforma included inappropriate emotional shallowness, unconcern, loss of emotional warmth, loss of empathy or sympathy, and marked indifference to others). Each feature was rated as present if at least one of its descriptors listed was present. In addition, a series of features believed to be characteristic of patients with frontal lobe dysfunction but not explicitly included in the criteria were also rated. The full proforma is available from the authors.
The research program was approved by the Addenbrooke’s Hospital local research ethics committee.
RESULTS
Core features.
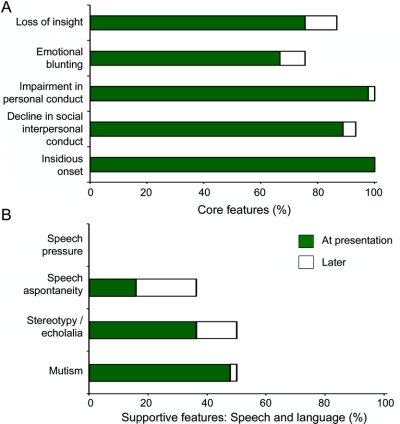
At presentation, all cases showed insidious onset and gradual progression. The frequency for the other core features ranged between 67% (emotional blunting) and 98% (decline in personal conduct) (figure 1). In this sample, only 25 patients (56%) exhibited all five core features at initial presentation and another 12 four of the five features (total: 37/45 or 82%). With disease progression, all core features were present in 33 cases (73%) and four features in an additional 6 cases (total: 39/45 cases or 87%). Emotional blunting and loss of insight were not seen at any point during the course of the disease in 25% and 13% of cases (figure 1). Given the variable prevalence of the core features, we analyzed clusters of four and three core features that best identified FTD cases: removing emotional blunting improved the diagnostic sensitivity and detected 32/45 cases (71%) of the sample. When relaxing the number of core features necessary for a diagnosis of FTD to three, the combination of insidious onset and gradual progression, decline in personal conduct, and decline in social interpersonal conduct detected 39/45 cases (87%).
Figure 1 Prevalence of core features (A) and speech and language supportive features (B) of clinical behavioral variant frontotemporal dementia at initial presentation and with disease progression
Among the core features, loss of insight includes lack of awareness, or denial of mental symptoms; emotional blunting includes loss of empathy and sympathy; impairment in personal conduct includes inertia and passivity or overactivity such as pacing; decline in social interpersonal conduct includes disinhibited behavior, tactlessness, and loss of social graces.
Supportive features.
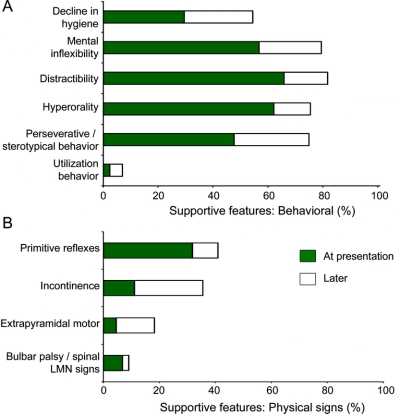
Over 80% of cases experienced disease onset before age 65 years. Frequencies of the other supportive features at presentation or later in the disease are shown in figure 2. Among these, mental inflexibility distractibility and hyperorality were the only features observed in at least half of the cases, with perseverative/stereotypical behavior and personal hygiene disturbance becoming more common over time (figure 2). Utilization behavior was rarely encountered. Physical signs were infrequent, apart from the primitive reflexes, and were present in fewer than 20% of patients at presentation. Language disturbance was frequent in this group (figure 1). Sixteen cases had intact language and of the remaining 29 cases, 17 exhibited one feature, 9 showed two features, and 3 cases three language features. Mutism was most common, followed by stereotypical speech. Speech pressure was never observed in this sample. Finally, 27 cases (60%) had executive dysfunction on cognitive testing at presentation, and an additional 9 cases showed similar deficit subsequently (total: 36/45 or 80%).
Figure 2 Prevalence of behavioral supportive features (A) and physical signs (B) in patients with behavioral variant frontotemporal dementia at initial presentation and with disease progression
Frequencies of the core and supportive clinical features in cases with postmortem confirmation did not differ from the cases without confirmation or from the patients who were still alive.
Exclusion features.
In line with previous reports,2 5 cases (11%) had severe amnesia at presentation (plus an additional patient who developed amnesia subsequently). Examining the prevalence of the relative diagnostic exclusion features, vascular risk factors were observed in 4 cases (9%), and 2 cases had a history of alcohol abuse.
Features not part of the classification criteria.
Impaired activities of daily living (ADL) were observed in 18 cases at presentation, with an additional 15 cases (total: 33/45 or 73%) exhibiting difficulty in this domain with disease progression. Carer distress was also common: 8/45 (18%) carers reported experiencing significant distress by the time the patient presented to the clinic. This feature was endorsed by almost two thirds of caregivers as the disease progressed.
DISCUSSION
In a group of clinically well-characterized patients with bvFTD presentation, 18 with confirmed postmortem pathology, just over half (58%) of the cases exhibited all five core clinical features necessary for a clinical diagnosis of bvFTD when examined for the first time. Although common, core features were far from ubiquitous, with prevalence ranging between 70% and 100%. This finding is in keeping with another study of patients with bvFTD showing that a third exhibited all core features at presentation, although they indicated that most patients with FTD would show the core features over the course of the disease.14 In contrast, only two (insidious onset and progression, decline in personal conduct) of the five core features were present in all of our patients as they progressed. At presentation, some degree of insight was maintained in a fifth of patients and emotional blunting was not observed in a similar proportion of cases over the course of the disease.
Using a more lenient threshold that employed the “best” clusters of either four or three core features improved case detection considerably at initial presentation, although it still missed 15% of cases in this sample. Importantly, of the six cases who showed two or three core features over the entire course of the disease, four had neuropathologically confirmed FTD changes. Postmortem examination was not conducted on the other two cases. These findings indicate that strict application of the clinical diagnostic criteria will miss a significant proportion of patients who harbor FTD pathology. From a clinical viewpoint, any delay in establishing the correct diagnosis raises the risk of inappropriate management or administration of therapeutic intervention that may at best be ineffective and at worst have significant adverse effects. These findings support the removal of the distinction between core and supportive features in favor of a probable/possible diagnostic hierarchy5 similar to clinical diagnostic criteria currently applied to other dementia syndromes.18–20
Recent evidence points to the existence of patients who show typical bvFTD clinical behavioral changes but who do not show progression over many years. Current diagnostic criteria do not differentiate well between true bvFTD and phenocopy cases, although impairment on tests of executive function,21 prominent atrophy on MRI,11,15 and impaired ADL appear to be the best discriminators. A recent analysis21 showed that a cluster of frontal executive tasks (Trails B, verbal fluency, digit span backward, and Hayling test of inhibitory control) could identify true bvFTD from phenocopy cases. In addition, high-quality MR T1 images acquired in the coronal plane are recommended when a diagnosis of bvFTD is suspected.11,15
Our study also confirmed that disruption in ADL is common in bvFTD. ADL disturbance was present in 40% of cases at presentation but three quarters experienced decline in this area with disease progression. These findings are consistent with those from Cambridge showing that decline in ADL proficiency was much more common in bvFTD compared to the other subtypes of FTD (i.e., semantic dementia and progressive nonfluent aphasia) and affected basic, as well as instrumental (complex), ADLs at an early stage.22
The current clinical criteria contain a large number of supportive features, many of which have a low prevalence. At presentation, only mental inflexibility, distractibility, and hyperorality were present in at least half of the patients. Over time, decline in hygiene and perseverative or stereotypical behavior also became more common. In contrast, some features such as utilization behavior were rare even with increasing disease severity. In other words, absence of these features does not rule out a diagnosis of bvFTD.
One limitation of our study is the absence of a comparison group with another non-FTD form of dementia, which is required to evaluate specificity as well as sensitivity. Unfortunately, the Cambridge clinic was heavily oriented toward FTD syndromes particularly after 1997. Since the cohort was seen over a long time period, we did not have the benefit of consistent evaluation of ADL, which should be included in future prospective studies. The only other large study examining clinical features in pathologically verified cases compared Alzheimer disease with bvFTD, semantic dementia, and progressive nonfluent aphasia, but did not have longitudinal clinical data; it was, therefore, not entirely comparable to the present work.16 Another possible limitation is that presence of clinical features was established retrospectively by examining existing information (i.e., clinical files, correspondence, questionnaires, and other instruments). When a feature was not mentioned, it was rated absent, which may have underestimated the prevalence of some of the features. Importantly, however, the fact that a comprehensive and systematic assessment was conducted using a standardized approach17 at initial presentation and during subsequent visits supports the view that absence of a feature is likely to reflect true absence rather than simple omission of recording the feature.
When first assessed in the clinic, only a proportion of patients with definite bvFTD will fulfill all current core criteria. This may reflect difficulty in the interpretation or the application of some features, notably emotional blunting and the presence, or absence, of insight. In addition, some of the supportive features (hyperorality, mental inflexibility, distractibility, and stereotypic behavior) are sufficiently common to consider their inclusion in revised diagnostic criteria, as recently suggested.5,23
AUTHOR CONTRIBUTIONS
Statistical analyses were conducted by O. Piguet.
Supplementary Material
Address correspondence and reprint requests to Prof. John Hodges, Prince of Wales Medical Research Institute, Barker St, Randwick NSW 2031 Australia j.hodges@powmri.edu.au
Funded in part by an MRC program grant to J.R.H. J.R.H. is supported by an Australian Research Council Federation Fellowship. O.P. is supported by a National Health and Medical Research Council of Australia Clinical Career Development Award Fellowship (#510184).
Disclosure: The authors report no disclosures.
Received August 3, 2008. Accepted in final form November 12, 2008.
REFERENCES
- 1.Bozeat S, Gregory CA, Ralph MA, Hodges JR. Which neuropsychiatric and behavioural features distinguish frontal and temporal variants of frontotemporal dementia from Alzheimer’s disease? J Neurol Neurosurg Psychiatry 2000;69:178–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Graham A, Davies R, Xuereb J, et al. Pathologically proven frontotemporal dementia presenting with severe amnesia. Brain 2005;128:597–605. [DOI] [PubMed] [Google Scholar]
- 3.Kramer JH, Jurik J, Sha SJ, et al. Distinctive neuropsychological patterns in frontotemporal dementia, semantic dementia, and Alzheimer disease. Cogn Behav Neurol 2003;16:211–218. [DOI] [PubMed] [Google Scholar]
- 4.Neary D, Snowden JS, Gustafson L, et al. Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology 1998;51:1546–1554. [DOI] [PubMed] [Google Scholar]
- 5.Rascovsky K, Hodges JR, Kipps CM, et al. Diagnostic criteria for the behavioral variant of frontotemporal dementia (bvFTD): current limitations and future directions. Alzheimer Dis Assoc Disord 2007;21:S14–18. [DOI] [PubMed] [Google Scholar]
- 6.Liu W, Miller BL, Kramer JH, et al. Behavioral disorders in the frontal and temporal variants of frontotemporal dementia. Neurology 2004;62:742–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wittenberg D, Possin KL, Rascovsky K, Rankin KP, Miller BL, Kramer JH. The early neuropsychological and behavioral characteristics of frontotemporal dementia. Neuropsychol Rev 2008;18:91–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cairns NJ, Bigio EH, Mackenzie IR, et al. Neuropathologic diagnostic and nosologic criteria for frontotemporal lobar degeneration: consensus of the Consortium for Frontotemporal Lobar Degeneration. Acta Neuropathol (Berl) 2007;114:5–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hodges JR, Davies RR, Xuereb JH, et al. Clinicopathological correlates in frontotemporal dementia. Ann Neurol 2004;56:399–406. [DOI] [PubMed] [Google Scholar]
- 10.Jeong Y, Cho SS, Park JM, et al. 18F-FDG PET findings in frontotemporal dementia: an SPM analysis of 29 patients. J Nucl Med 2005;46:233–239. [PubMed] [Google Scholar]
- 11.Kipps CM, Davies RR, Mitchell J, Kril JJ, Halliday GM, Hodges JR. Clinical significance of lobar atrophy in frontotemporal dementia: application of an MRI visual rating scale. Dement Geriatr Cogn Disord 2007;23:334–342. [DOI] [PubMed] [Google Scholar]
- 12.Cruts M, Gijselinck I, van der Zee J, et al. Null mutations in progranulin cause ubiquitin-positive frontotemporal dementia linked to chromosome 17q21. Nature 2006;442:920–924. [DOI] [PubMed] [Google Scholar]
- 13.Wilhelmsen KC, Clark LN, Miller BL, Geschwind DH. Tau mutations in frontotemporal dementia. Dement Geriatr Cogn Disord 1999;10 suppl 1:88–92. [DOI] [PubMed] [Google Scholar]
- 14.Mendez MF, Perryman KM. Neuropsychiatric features of frontotemporal dementia: evaluation of consensus criteria and review. J Neuropsychiatry Clin Neurosci 2002;14:424–429. [DOI] [PubMed] [Google Scholar]
- 15.Davies RR, Kipps CM, Mitchell J, Kril JJ, Halliday GM, Hodges JR. Progression in frontotemporal dementia: identifying a benign behavioral variant by magnetic resonance imaging. Arch Neurol 2006;63:1627–1631. [DOI] [PubMed] [Google Scholar]
- 16.Rosen HJ, Hartikainen KM, Jagust W, et al. Utility of clinical criteria in differentiating frontotemporal lobar degeneration (FTLD) from AD. Neurology 2002;58:1608–1615. [DOI] [PubMed] [Google Scholar]
- 17.Hodges JR. Cognitive Assessment for Clinicians: A Practical Guide. Oxford: Oxford University Press; 2007. [Google Scholar]
- 18.Roman GC, Tatemichi TK, Erkinjuntti T, et al. Vascular dementia: diagnostic criteria for research studies: report of the NINDS-AIREN International Workshop. Neurology 1993;43:250–260. [DOI] [PubMed] [Google Scholar]
- 19.McKeith IG, Dickson DW, Lowe J, et al. Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology 2005;65:1863–1872. [DOI] [PubMed] [Google Scholar]
- 20.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA work group under the auspices of Department of Health and Human Services task force on Alzheimer’s disease. Neurology 1984;34:939–944. [DOI] [PubMed] [Google Scholar]
- 21.Hornberger M, Piguet O, Kipps CM, Hodges JR. Executive function in progressive and non-progressive behavioral variant frontotemporal dementia. Neurology 2008;71:1481–1488. [DOI] [PubMed] [Google Scholar]
- 22.Mioshi E, Kipps CM, Dawson K, Mitchell J, Graham A, Hodges JR. Activities of daily living in frontotemporal dementia and Alzheimer disease. Neurology 2007;68:2077–2084. [DOI] [PubMed] [Google Scholar]
- 23.Kipps CM, Nestor PJ, Fryer TD, Hodges JR. Behavioural variant frontotemporal dementia: not all it seems? Neurocase 2007;13:237–247. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.