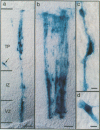
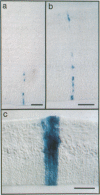
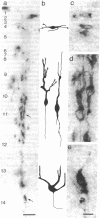
Abstract
A recently described method for lineage analysis in rodents uses a recombinant retrovirus to insert a foreign gene into the genome of a precursor cell; the gene's product is later detected histochemically in the infected cell's progeny. We have adapted this method for use in chicken embryos and used it to study the disposition of clonally related cells in the optic tectum. We report that descendants of a single precursor form narrow, radially oriented columns that span the thickness of the developing tectum. Analysis of embryos injected with virus at different stages suggests a developmental scheme in which early-born progeny are displaced laterally, late-born progeny are displaced radially, and cell mixing is limited. Many clones remain radially arrayed as laminae form and contribute neurons of diverse types of several laminae. In light of previous studies showing physiological relationships among vertically arrayed neurons, our results suggest that neurons descended from a single precursor are richly interconnected and functionally related.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BISHOP P. O., KOZAK W., LEVICK W. R., VAKKUR G. J. The determination of the projection of the visual field on to the lateral geniculate nucleus in the cat. J Physiol. 1962 Oct;163:503–539. doi: 10.1113/jphysiol.1962.sp006991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnerot C., Rocancourt D., Briand P., Grimber G., Nicolas J. F. A beta-galactosidase hybrid protein targeted to nuclei as a marker for developmental studies. Proc Natl Acad Sci U S A. 1987 Oct;84(19):6795–6799. doi: 10.1073/pnas.84.19.6795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dräger U. C., Hubel D. H. Responses to visual stimulation and relationship between visual, auditory, and somatosensory inputs in mouse superior colliculus. J Neurophysiol. 1975 May;38(3):690–713. doi: 10.1152/jn.1975.38.3.690. [DOI] [PubMed] [Google Scholar]
- Fujita S. Transitory differentiation of matrix cells and its functional role in the morphogenesis of the developing vertebrate CNS. Curr Top Dev Biol. 1986;20:223–242. doi: 10.1016/s0070-2153(08)60666-3. [DOI] [PubMed] [Google Scholar]
- HUBEL D. H., WIESEL T. N. Receptive fields, binocular interaction and functional architecture in the cat's visual cortex. J Physiol. 1962 Jan;160:106–154. doi: 10.1113/jphysiol.1962.sp006837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt C. E., Bertsch T. W., Ellis H. M., Harris W. A. Cellular determination in the Xenopus retina is independent of lineage and birth date. Neuron. 1988 Mar;1(1):15–26. doi: 10.1016/0896-6273(88)90205-x. [DOI] [PubMed] [Google Scholar]
- Hughes C. P., Pearlman A. L. Single unit receptive fields and the cellular layers of the pigeon optic tectum. Brain Res. 1974 Nov 22;80(3):365–377. doi: 10.1016/0006-8993(74)91023-3. [DOI] [PubMed] [Google Scholar]
- Knudsen E. I. Auditory and visual maps of space in the optic tectum of the owl. J Neurosci. 1982 Sep;2(9):1177–1194. doi: 10.1523/JNEUROSCI.02-09-01177.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaVail J. H., Cowan W. M. The development of the chick optic tectum. I. Normal morphology and cytoarchitectonic development. Brain Res. 1971 May 21;28(3):391–419. doi: 10.1016/0006-8993(71)90053-9. [DOI] [PubMed] [Google Scholar]
- LaVail J. H., Cowan W. M. The development of the chick optic tectum. II. Autoradiographic studies. Brain Res. 1971 May 21;28(3):421–441. [PubMed] [Google Scholar]
- MOUNTCASTLE V. B. Modality and topographic properties of single neurons of cat's somatic sensory cortex. J Neurophysiol. 1957 Jul;20(4):408–434. doi: 10.1152/jn.1957.20.4.408. [DOI] [PubMed] [Google Scholar]
- Miller A. D., Buttimore C. Redesign of retrovirus packaging cell lines to avoid recombination leading to helper virus production. Mol Cell Biol. 1986 Aug;6(8):2895–2902. doi: 10.1128/mcb.6.8.2895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters A., Feldman M. The cortical plate and molecular layer of the late rat fetus. Z Anat Entwicklungsgesch. 1973;141(1):3–37. doi: 10.1007/BF00523363. [DOI] [PubMed] [Google Scholar]
- Price J., Turner D., Cepko C. Lineage analysis in the vertebrate nervous system by retrovirus-mediated gene transfer. Proc Natl Acad Sci U S A. 1987 Jan;84(1):156–160. doi: 10.1073/pnas.84.1.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakic P. Genesis of the dorsal lateral geniculate nucleus in the rhesus monkey: site and time of origin, kinetics of proliferation, routes of migration and pattern of distribution of neurons. J Comp Neurol. 1977 Nov 1;176(1):23–52. doi: 10.1002/cne.901760103. [DOI] [PubMed] [Google Scholar]
- Rakic P. Mode of cell migration to the superficial layers of fetal monkey neocortex. J Comp Neurol. 1972 May;145(1):61–83. doi: 10.1002/cne.901450105. [DOI] [PubMed] [Google Scholar]
- Rakic P. Specification of cerebral cortical areas. Science. 1988 Jul 8;241(4862):170–176. doi: 10.1126/science.3291116. [DOI] [PubMed] [Google Scholar]
- Sanes J. R., Rubenstein J. L., Nicolas J. F. Use of a recombinant retrovirus to study post-implantation cell lineage in mouse embryos. EMBO J. 1986 Dec 1;5(12):3133–3142. doi: 10.1002/j.1460-2075.1986.tb04620.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senut M. C., Alvarado-Mallart R. M. Development of the retinotectal system in normal quail embryos: cytoarchitectonic development and optic fiber innervation. Brain Res. 1986 Sep;394(1):123–140. doi: 10.1016/0165-3806(86)90088-x. [DOI] [PubMed] [Google Scholar]
- Turner D. L., Cepko C. L. A common progenitor for neurons and glia persists in rat retina late in development. Nature. 1987 Jul 9;328(6126):131–136. doi: 10.1038/328131a0. [DOI] [PubMed] [Google Scholar]
- Wetts R., Fraser S. E. Multipotent precursors can give rise to all major cell types of the frog retina. Science. 1988 Mar 4;239(4844):1142–1145. doi: 10.1126/science.2449732. [DOI] [PubMed] [Google Scholar]