Abstract
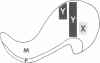
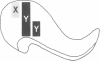
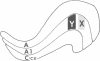
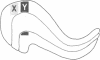
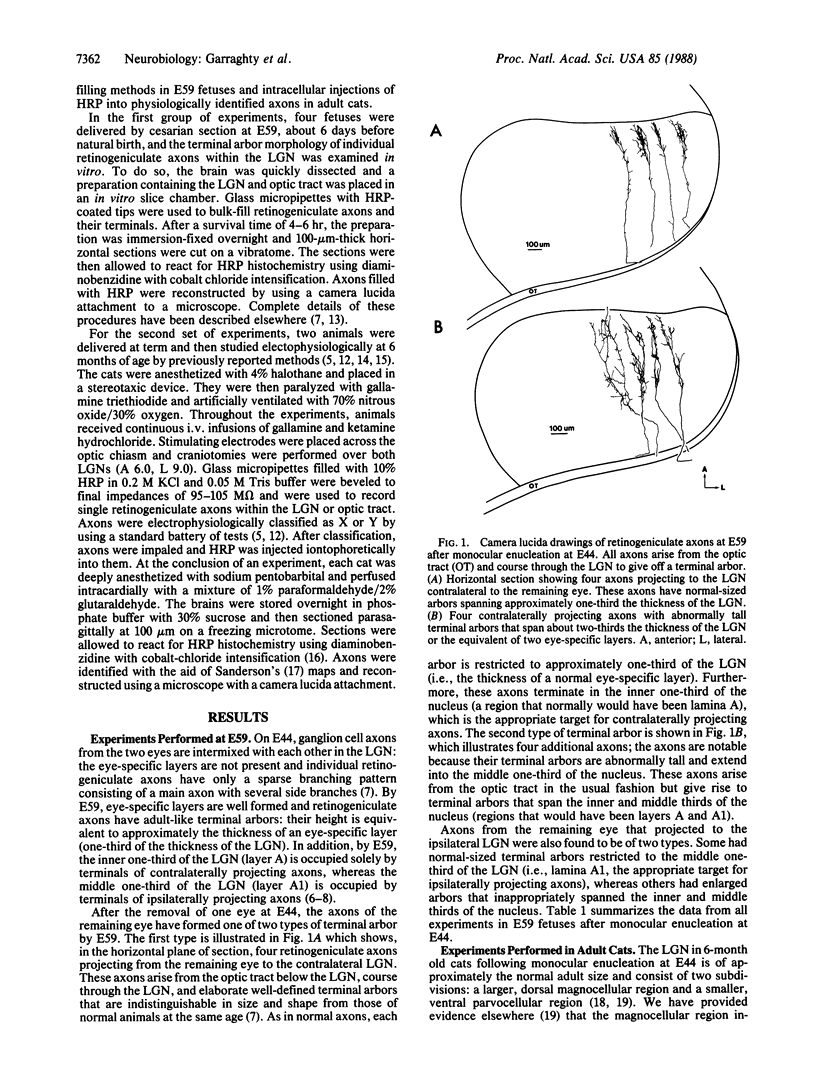
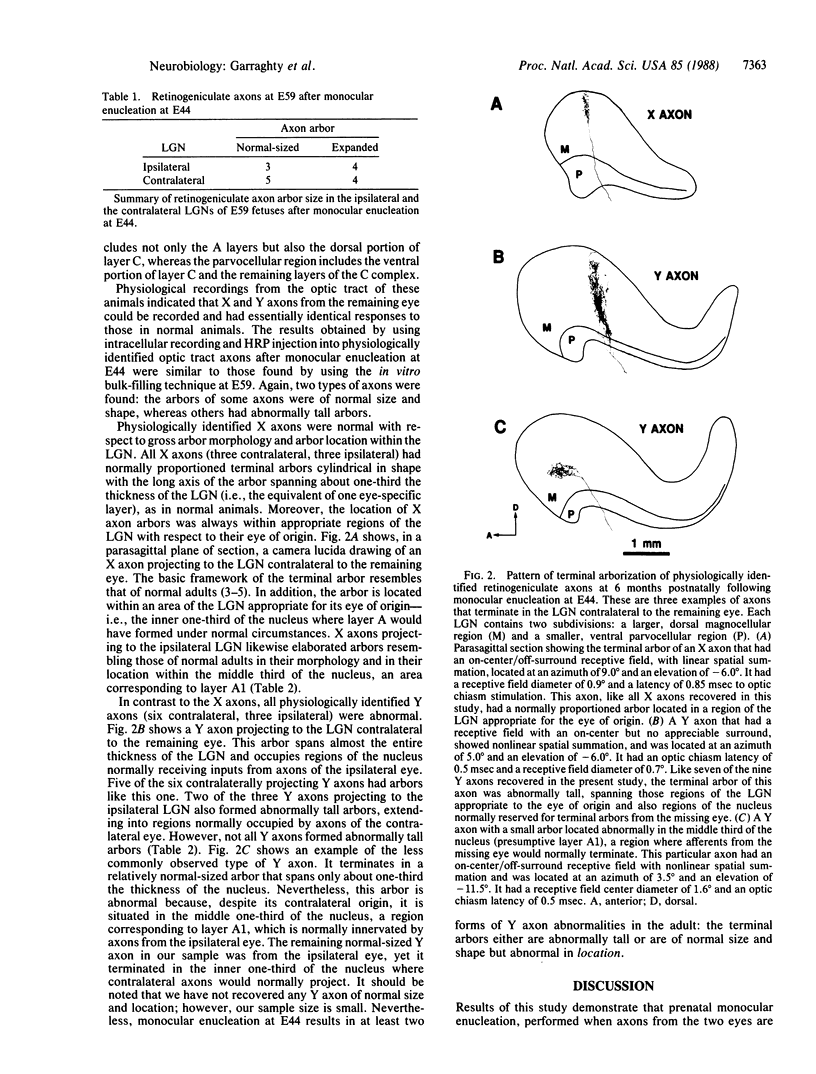
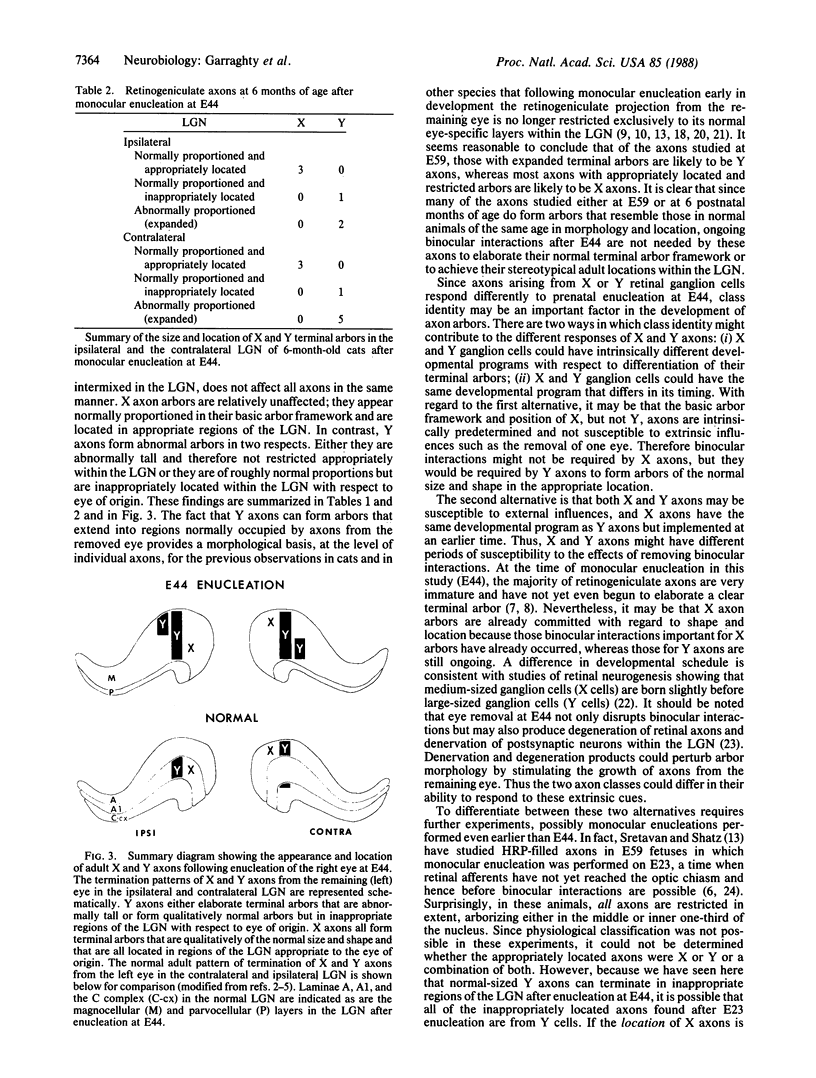
In the mammalian visual system, the terminal arbors of retinal ganglion cell axons from the two eyes are restricted to mutually exclusive territories within their thalamic target, the lateral geniculate nucleus (LGN). Here we have investigated some of the factors that determine the adult morphology of terminal arbors in the cat's retinogeniculate system. Removal of one eye during prenatal life at a time when retinogeniculate axons from the two eyes are extensively intermixed within the LGN perturbs the subsequent morphological development of some but not all axons from the remaining eye. The presence of terminal arbors qualitatively normal in size, shape, and location within the LGN suggests that for some retinal axons, ongoing binocular interactions throughout prenatal life are not needed for the development of normal arbor morphology. However, many of the axons form arbors of abnormal size or location, suggesting that such features of axon morphology are not intrinsically determined for these axons but may be susceptible to external influences. Electrophysiological studies reveal that the abnormal arbors all belong to the functionally distinct Y class of retinal ganglion cells, whereas the normal arbors all belong to X cells. The different responses of X and Y axons to prenatal enucleation demonstrate that during development subsets of a single neuronal population projecting to the same target in the central nervous system can be under different developmental controls for axon arbor differentiation.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams J. C. Heavy metal intensification of DAB-based HRP reaction product. J Histochem Cytochem. 1981 Jun;29(6):775–775. doi: 10.1177/29.6.7252134. [DOI] [PubMed] [Google Scholar]
- Bowling D. B., Michael C. R. Projection patterns of single physiologically characterized optic tract fibres in cat. Nature. 1980 Aug 28;286(5776):899–902. doi: 10.1038/286899a0. [DOI] [PubMed] [Google Scholar]
- Bowling D. B., Michael C. R. Terminal patterns of single, physiologically characterized optic tract fibers in the cat's lateral geniculate nucleus. J Neurosci. 1984 Jan;4(1):198–216. doi: 10.1523/JNEUROSCI.04-01-00198.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalupa L. M., Williams R. W. Organization of the cat's lateral geniculate nucleus following interruption of prenatal binocular competition. Hum Neurobiol. 1984;3(2):103–107. [PubMed] [Google Scholar]
- Garraghty P. E., Frost D. O., Sur M. The morphology of retinogeniculate X- and Y-cell axonal arbors in dark-reared cats. Exp Brain Res. 1987;66(1):115–127. doi: 10.1007/BF00236208. [DOI] [PubMed] [Google Scholar]
- Garraghty P. E., Shatz C. J., Sur M. Prenatal disruption of binocular interactions creates novel lamination in the cat's lateral geniculate nucleus. Vis Neurosci. 1988;1(1):93–102. doi: 10.1017/s0952523800001048. [DOI] [PubMed] [Google Scholar]
- Garraghty P. E., Sur M., Sherman S. M. Role of competitive interactions in the postnatal development of X and Y retinogeniculate axons. J Comp Neurol. 1986 Sep 8;251(2):216–239. doi: 10.1002/cne.902510207. [DOI] [PubMed] [Google Scholar]
- Garraghty P. E., Sur M., Weller R. E., Sherman S. M. Morphology of retinogeniculate X and Y axon arbors in monocularly enucleated cats. J Comp Neurol. 1986 Sep 8;251(2):198–215. doi: 10.1002/cne.902510206. [DOI] [PubMed] [Google Scholar]
- Guillery RW THE LAMINAR D. a new interpretation. J Comp Neurol. 1970 Mar;138(3):339–366. doi: 10.1002/cne.901380307. [DOI] [PubMed] [Google Scholar]
- Hickey T. L. Translaminar growth of axons in the kitten dorsal lateral geniculate nucleus following removal of one eye. J Comp Neurol. 1975 Jun 1;161(3):359–382. doi: 10.1002/cne.901610307. [DOI] [PubMed] [Google Scholar]
- Rakic P. Development of visual centers in the primate brain depends on binocular competition before birth. Science. 1981 Nov 20;214(4523):928–931. doi: 10.1126/science.7302569. [DOI] [PubMed] [Google Scholar]
- Robson J. A. Abnormal axonal growth in the dorsal lateral geniculate nucleus of the cat. J Comp Neurol. 1981 Jan 20;195(3):453–476. doi: 10.1002/cne.901950306. [DOI] [PubMed] [Google Scholar]
- Robson J. A., Mason C. A., Guillery R. W. Terminal arbors of axons that have formed abnormal connections. Science. 1978 Aug 18;201(4356):635–637. doi: 10.1126/science.675248. [DOI] [PubMed] [Google Scholar]
- Sanderson K. J. The projection of the visual field to the lateral geniculate and medial interlaminar nuclei in the cat. J Comp Neurol. 1971 Sep;143(1):101–108. doi: 10.1002/cne.901430107. [DOI] [PubMed] [Google Scholar]
- Shatz C. J., Kirkwood P. A. Prenatal development of functional connections in the cat's retinogeniculate pathway. J Neurosci. 1984 May;4(5):1378–1397. doi: 10.1523/JNEUROSCI.04-05-01378.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shatz C. J. The prenatal development of the cat's retinogeniculate pathway. J Neurosci. 1983 Mar;3(3):482–499. doi: 10.1523/JNEUROSCI.03-03-00482.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- So K. F., Woo H. H., Jen L. S. The normal and abnormal postnatal development of retinogeniculate projections in golden hamsters: an anterograde horseradish peroxidase tracing study. Brain Res. 1984 Feb;314(2):191–205. doi: 10.1016/0165-3806(84)90042-7. [DOI] [PubMed] [Google Scholar]
- Sretavan D. W., Shatz C. J. Prenatal development of cat retinogeniculate axon arbors in the absence of binocular interactions. J Neurosci. 1986 Apr;6(4):990–1003. doi: 10.1523/JNEUROSCI.06-04-00990.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sretavan D. W., Shatz C. J. Prenatal development of retinal ganglion cell axons: segregation into eye-specific layers within the cat's lateral geniculate nucleus. J Neurosci. 1986 Jan;6(1):234–251. doi: 10.1523/JNEUROSCI.06-01-00234.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sretavan D., Shatz C. J. Prenatal development of individual retinogeniculate axons during the period of segregation. 1984 Apr 26-May 2Nature. 308(5962):845–848. doi: 10.1038/308845a0. [DOI] [PubMed] [Google Scholar]
- Sur M. Development and plasticity of retinal X and Y axon terminations in the cat's lateral geniculate nucleus. Brain Behav Evol. 1988;31(4):243–251. doi: 10.1159/000116592. [DOI] [PubMed] [Google Scholar]
- Sur M., Esguerra M., Garraghty P. E., Kritzer M. F., Sherman S. M. Morphology of physiologically identified retinogeniculate X- and Y-axons in the cat. J Neurophysiol. 1987 Jul;58(1):1–32. doi: 10.1152/jn.1987.58.1.1. [DOI] [PubMed] [Google Scholar]
- Sur M., Sherman S. M. Retinogeniculate terminations in cats: morphological differences between X and Y cell axons. Science. 1982 Oct 22;218(4570):389–389. doi: 10.1126/science.7123239. [DOI] [PubMed] [Google Scholar]
- Walsh C., Polley E. H., Hickey T. L., Guillery R. W. Generation of cat retinal ganglion cells in relation to central pathways. Nature. 1983 Apr 14;302(5909):611–614. doi: 10.1038/302611a0. [DOI] [PubMed] [Google Scholar]
- Williams R. W., Bastiani M. J., Lia B., Chalupa L. M. Growth cones, dying axons, and developmental fluctuations in the fiber population of the cat's optic nerve. J Comp Neurol. 1986 Apr 1;246(1):32–69. doi: 10.1002/cne.902460104. [DOI] [PubMed] [Google Scholar]