Abstract
SWAP-70-like adapter of T cells (SLAT; also known as Def6) is a novel guanine nucleotide exchange factor for Rho GTPases that has been previously shown to play a role in CD4+ T cell activation and Th1/Th2 differentiation. However, the role of SLAT/Def6 in autoimmunity and its associated Th1- and Th17-specific responses has not yet been clearly elucidated. We used a prototypical and pathologically relevant Th1/Th17-mediated autoimmune model, that is, experimental autoimmune encephalomyelitis, to assess the role of SLAT/Def6 in autoantigen-specific T cell response. We found that T cell-expressed SLAT/Def6 was critical for experimental autoimmune encephalomyelitis development and pathogenesis, as evidenced by the resistance of Def6-deficient (Def6−/−) mice to clinical signs of the disease associated with a lack of CNS inflammation and demyelination in myelin oligodendrocyte glycoprotein-immunized Def6−/− mice. Moreover, Def6 deficiency resulted in a severely diminished myelin oligodendrocyte glycoprotein-specific CD4+ T cell proliferation as well as a defect in IFN-γ and IL-17 production in secondary lymphoid organs and the CNS. Lastly, Def6−/− CD4+ T cells were grossly deficient in their ability to differentiate into Th17 cells both in vitro and in vivo in a T cell-intrinsic manner. Therefore, our study establishes T cell-expressed SLAT/Def6 as a pivotal positive regulator of Th17 inflammatory responses and, thus, essential in controlling autoimmune and inflammatory diseases.
Experimental autoimmune encephalomyelitis (EAE)4 is a CD4+ T cell-mediated disease of the CNS that is commonly used as an animal model of multiple sclerosis (MS), the most common demyelinating disease of the human CNS. Indeed, EAE shares significant similarities with human MS in its clinical course and pathological features (1), characterized by the invasion of self-reactive Th cells into the CNS, leading to demyelination, axonal loss, and relapsing/remitting or chronic progressive paralysis. Upon activation, CD4+ cells differentiate into three subsets of helper T cells, namely, Th1, Th2 and Th17, based on their distinct cytokine expression profiles and their subsequent immune regulatory functions (2–4). Th1 cells mainly secrete IL-2 and IFN-γ and mediate defense against infection by intracellular pathogens, and an overactive Th1 response can lead to autoimmunity. Th17 cells produce IL-17, IL-17F, IL-21, and IL-22, all of which regulate tissue inflammatory responses, and have been shown to be critical for enhancing host protection against extra-cellular bacteria and fungi (3–7). Furthermore, Th17 cells have been shown to have critical functions in the pathogenesis of a variety of organ-specific autoimmune inflammation, for example, EAE (8, 9), collagen-induced arthritis (10), and psoriasis (11).
EAE has been long considered to be the prototypic Th1-mediated autoimmune disease until studies revealed a significant exacerbation of the disease in IFN-γ-deficient mice (12–15). Several lines of evidence indicate that Th17 cells play a primary role in the induction of several autoimmune diseases and particularly in the onset and maintenance of EAE (6, 9, 16, 17). Furthermore, Th17 cells were also reported to be by far superior to Th1 cells in their capacity to adoptively transfer EAE (9). However, highly purified Th1 cells have also been shown in numerous studies to be highly immunopathogenic and capable of inducing experimental autoimmune disease at low cell numbers (18, 19). Further examination of the T cells found within the CNS after active induction of EAE revealed both IL-17- and IFN-γ-producing cell populations leading to the notion that both Th1 and Th17 cells may be involved in the pathogenic process at different times or at different sites (6, 20–23).
Our recent work has led to the identification and characterization of a novel TCR-regulated guanine nucleotide exchange factor (GEF) for Cdc42 and Rac1, termed SWAP-70-like adapter of T cells (SLAT) (24), based on its abundant expression in Th2 cells and its homology with SWAP-70, a B cell-enriched GEF involved in B cell activation, Ig class switching, and migration to lymphoid organs (25–27). SLAT (also known as Def6 or IBP, hereafter termed SLAT/Def6) is abundant in central and peripheral lymphoid tissues, with high amounts found in thymocytes and peripheral T cells (24, 28, 29), and it translocates to the immunological synapse upon Ag stimulation (24, 30, 31).
Examination of Def6−/− mice on a mixed C57BL/6 (B6) × 129 background revealed spontaneous development of systemic lupus in aged female mice (32). Our more recent study revealed that Def6−/− mice on a homogeneous B6 background are resistant to Th1- and Th2-mediated inflammation in a model of lung inflammation (29). This resistance reflects the essential role of SLAT/Def6 in promoting TCR-induced Ca2+ release from endoplasmic reticulum stores and, hence, subsequent steps in the Ca2+ signaling pathway, including activation of the transcription factor NFAT (29). More recently, we demonstrated that SLAT/Def6 is activated by Lck-dependent phosphorylation of two defined tyrosine residues, an event that targets SLAT/Def6 to the plasma membrane and, more specifically, to the immunological synapse, in Ag-stimulated T cells (31). Furthermore, the Ca2+ signaling function of SLAT/Def6 leading to Th1/Th2 differentiation depends on its GEF activity (31).
Given the emerging importance of Th17 cells in autoimmunity, we have analyzed the development of myelin oligodendrocyte glycoprotein (MOG)-induced EAE in Def6−/− B6 mice. In the present study we show that MOG-primed Def6−/− mice are resistant to EAE development and pathogenesis, as assessed by mononuclear and CD4 T cell infiltration in the CNS, demyelination, MOG-induced proliferation, and levels of IL-17 and IFN-γ in the CNS and draining lymph nodes (dLNs). Additionally, Def6−/− CD4+ T cells were intrinsically deficient in their ability to differentiate in vitro and in vivo into Th17 cells, demonstrating that T cell-expressed SLAT/Def6 positively regulates Th17 inflammatory responses.
Materials and Methods
Mice
Mice were maintained under specific pathogen-free conditions in accordance with guidelines of the Association for Assessment and Accreditation of Laboratory Animal Care International. The studies performed in this paper conform to the principles outlined by the Animal Welfare Act and the National Institutes of Health guidelines for the care and use of animals in biomedical research. Def6−/− mice on a B6 background have been previously described (29). Def6−/− OT-II TCR-transgenic (Tg) mice were generated by intercrossing OT-II TCR-Tg mice and Def6−/− mice (both on a B6 background), and their T cells were used as a source of Vβ5Vα2 CD4+ T cells specific for amino acid residues 323–339 of OVA (OVA323–339). MOG35–55-specific 2D2 TCR-Tg mice were a gift from S. Zamvil (University of California San Francisco, CA). Rag1−/− and B6.SJL (CD45.1+) mice were purchased from The Jackson Laboratory.
EAE induction and adoptive transfers
Groups of 8- to 10-wk-old wild-type (WT) or Def6−/− female B6 mice were injected s.c. in the flank with 150 µg of a peptide consisting of amino acids 35–55 of MOG (MOG35–55; Met-Glu-Val-Gly-Trp-Tyr-Arg-Ser-Pro-Phe-Ser-Arg-Val-Val-His-Leu-Tyr-Arg-Asn-Gly-Lys) purified to >95% purity (Auspep). Immunization was performed by emulsifying the peptide with CFA (Difco Laboratories). Additionally, the mice received 300 ng of pertussis toxin (List Biological Laboratories) i.v. on days 0 and 2. Mice were monitored daily for up to 30 days for signs of disease. They were scored for disease severity using the standard scale: 0, no clinical signs of disease; 1, decreased tail tone; 2, paraperesis (partial hind limb paralysis); 3, paraplegia (complete hind limb paralysis); 4, paraplegia with forelimb weakness or paralysis; 5, moribund or dead. Disease incidence and scores were measured daily. For T cell transfer, 1 × 107 purified CD4+ T cells isolated from the spleen and peripheral lymph nodes (LNs) were injected i.v. into Rag1−/− B6 mice. Twenty-four hours after transfer, the recipient mice were primed as described above to induce EAE.
Assay of T cell function ex vivo
To measure the proliferation of ex vivo-isolated T cells, splenocytes or dLN cells from MOG-immunized mice were collected and, after lysing RBC with ACK lysis buffer, cells were cultured in triplicate for 72 h at 37°C in 200 µl per well of culture medium (RPMI 1640 with 10% FCS, 2 mM l-glutamine, 1% nonessential amino acids, 1 mM sodium pyruvate, and 100 µg/ml penicillin/streptomycin) at a density of 2 × 105 per well in 96-well plates with increasing concentrations of MOG35–55 peptide. After 56 h, cells were pulsed with 1 µCi of tritiated TdR ([3H]TdR; ICN Bio-medicals) for an additional 16 h of culture and then harvested for scintillation counting. Supernatants were harvested after 72 h of in vitro stimulation for measurement of IL-2, IL-17, and IFN-γ using ELISA kits (R&D Systems) according to the manufacturer’s instructions. A standard curve was performed for each plate and used to calculate the absolute concentrations of the indicated cytokines.
Histological analysis
Spinal cords and brains from EAE-induced or control mice transcardially perfused with ice-cold PBS and 10% zinc-buffered formalin were dissected and fixed overnight in 10% formalin. Paraffin-embedded 5- to 10-µm spinal cord and brain sections were stained with H&E or Luxol fast blue and then examined by light microscopy.
Isolation of mononuclear cells from the CNS
CNS mononuclear cells were prepared from the brain and spinal cord using gradient centrifugation as previously described (33). Briefly, euthanized mice were perfused through the left cardiac ventricle with cold PBS to eliminate peripheral blood. The forebrain and cerebellum were dissected and the spinal cords flushed out with PBS by hydrostatic pressure. CNS tissue was cut into pieces and digested with collagenase D (300 µg/ml; Roche Diagnostics) for 1 h at 37°C. Mononuclear cells were isolated by passing the tissue through a cell strainer (70 µm), followed by Percoll gradient (70%/30%) centrifugation. Mononuclear cells were removed from the interphase, washed, and resuspended in culture medium for further analysis.
Cell surface and intracellular cytokine staining (ICS)
Mononuclear cells from CNS tissue, spleen, or LN cells were labeled with the following fluorochrome-conjugated Abs (BD Biosciences): anti-CD4 (FITC, PE, PerCP, or allophycocyanin), anti-CD25 (FITC or PE), anti-CD44 (PE), anti-CD45.2 (allophycocyanin), and anti-CD62L (FITC). For intracellular cytokine staining, isolated CNS-infiltrating cells were stimulated with 50 ng/ml PMA and 500 ng/ml ionomycin in the presence of GolgiPlug (BD Biosciences) for 5 h. Cell surfaces were stained with mAb against CD4. Then the cells were washed, fixed, and permeabilized with Cytofix/Cytoperm buffer, and intracellular cytokines were stained with Abs against IL-17 (FITC) or IFN-γ (PE) (BD Biosciences). T cells were subsequently analyzed using a FACSCalibur flow cytometer (BD Biosciences) by gating on the CD4 population. The number of cytokine-producing cells was calculated from the percentage of cytokine-positive CD4+ T cells. We determined the number of CD4+ T cells in the CNS by multiplying the percentage of lineage marker-positive cells by the total number of mononuclear cells isolated from the CNS.
Th17 differentiation
Spleen and peripheral LN naive CD4+ T cells from WT or Def6−/− OT-II mice from were sorted by flow cytometry as CD4+CD44lowCD62Lhigh cells. These CD4+ T cells were stimulated with irradiated (3000 rad) CD4-depleted B6 splenocytes (as an APC source) loaded with OVA323–339 peptide (10 µg/ml; Synthetic Biomolecules) in the absence (neutral conditions) or presence of Th17-polarizing cytokines, that is, IL-6 (1, 5, or 20 ng/ml; PeproTech), TGF-β (5 ng/ml; PeproTech), IL-23 (50 ng/ml; R&D Systems), anti-IL-4 mAb (10 µg/ml; BD Biosciences), and anti-IFN-γ mAb (10 µg/ml; BD Biosciences). Cultures were supplemented with recombinant IL-2 (50 U/ml; PeproTech) on day 2. After 5 days of culture, supernatants were harvested for measurement of IL-17, IL-21, and IL-22 using ELISA kits (R&D Systems) according to the manufacturer’s instructions, and cells were restimulated with 50 ng/ml PMA and 500 ng/ml ionomycin in the presence of GolgiPlug for 5 h; IL-17- and IFN-γ-producing cells were analyzed using ICS as described above.
For in vivo induction of Th17 cells, FACS-sorted naive CD4+ T cells (1 × 106) from WT or Def6−/− OT-II mice (CD45.2+) were transferred i.v. into congenic (CD45.1+) recipients. The next day, recipient mice were immunized s.c. with 100 µg of whole OVA protein emulsified in CFA. Eight days later, lymphoid cells from the dLNs of these mice were stimulated with PMA plus ionomycin, and intracellular IL-17 in CD4-gated CD45.2+ cells was determined as described above.
Induced regulatory T cell (Treg) differentiation and suppression assays
Naive CD4+ T cells were purified from spleens and then stimulated with 1 µg/ml coated anti-CD3 mAb (145-2C11; Biolegend) in the presence of 100 U/ml IL-2 and 2.5 ng/ml TGF-β. Three days later, cells were harvested and resuspended in fresh TGF-β- and IL-2-containing medium for an additional day and then analyzed for CD25 and FoxP3 expression.
Naive responder cells (CD4+CD25−) and natural Tregs (CD4+CD25+) cells were FACS-sorted from spleens of WT or Def6−/− mice. A total of 5 × 104 naive T cells per well were stimulated with 5 µg/ml coated anti-CD3 and soluble anti-CD28 mAbs in the absence or presence or of 5 × 104 Tregs for 72 h. [3H]TdR was added for the last 16 h of culture, and proliferation was determined as described above.
Statistics
Statistical significance was analyzed by Student’s t test. Unless otherwise indicated, data represent the mean ± SD, with p < 0.05 considered statistically significant.
Results
Def6−/− mice are resistant to EAE
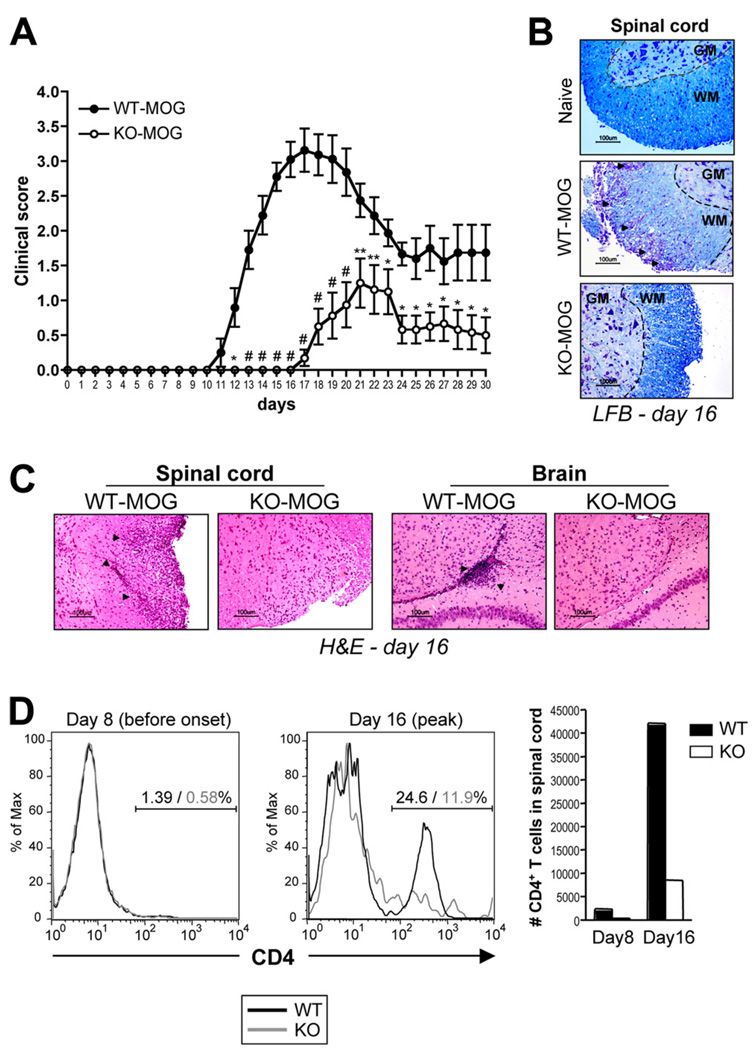
To determine the role of SLAT/Def6 in Th17-mediated immunopathology, we compared the susceptibility of WT and Def6−/− mice to EAE induction. Mice were primed with the encephalitogenic MOG35–55 peptide as described in Materials and Methods and thereafter monitored over time for neurological impairment using a standardized scoring system, and for CNS infiltration by histochemistry. WT mice developed acute EAE characterized by ascending paralysis, starting ~10–14 days after immunization, peaking around day 16, and followed by a remission phase between days ~20 and 30. In striking contrast, MOG-immunized Def6−/− mice consistently displayed minimal clinical signs of disease characterized by delay in day of onset (22.1 vs 12.2) and peak (23.4 vs 16.3) as well as a much lower severity (1.25 vs 3.47) of the disease compared with their WT counterparts (Table I and Fig. 1A). These results clearly show that SLAT/Def6 is required for the initiation and progression of clinical EAE.
Table I.
Clinical parameters of MOG-induced EAE in WT and KO C57BL/6 micea
| Mouse Strain | Mice per Group (n) |
Disease Incidence (%)b | Onset Day (mean ± SEM) |
Maximal Score Day (mean ± SEM) |
Maximal Score (mean ± SEM) |
|---|---|---|---|---|---|
| WT C57BL/6 | 12 | 12/12 (100%) | 12.2 ± 0.6 | 16.3 ± 0.4 | 3.47 ± 0.3 |
| KO C57BL/6 | 12 | 8/12 (67%) | 22.1 ± 2.4 | 23.4 ± 2.4** | 1.25 ± 0.34*** |
Mice were immunized with MOG35–55 peptide emulsified in CFA and injected twice with pertussis toxin. Mice were then monitored daily for the development of EAE. Statistical analysis was performed by comparing the experimental and control groups using a t test.
p < 0.001;
p < 0.0001.
EAE clinical score of ≥1.
FIGURE 1.
Resistance to EAE development in Def6−/− mice. A, Groups of WT and Def6−/− (KO) mice were immunized s.c. with MOG35–55 in CFA. Pertussis toxin was given i.p. at the time of immunization and 48 h later. Mice were monitored daily for 30 days for clinical signs of EAE and were scored using an arbitrary scale of 0–5 as described in Materials and Methods. Results are the means ± SEM from 12 mice per group. Statistical differences were determined using a two-tailed Student’s t test. *, p < 0.05; **, p < 0.01; #, p < 0.001; WT vs KO mice. B and C, Histopathology of spinal cord and brain tissue sections during the acute (day 16) phase of EAE. Sections were stained with Luxol fast blue (B) or H&E (C). Arrows indicate areas of demyelination of the spinal cord’s white matter (B), as well as perivascular mononuclear cell infiltrates (C). D, Mononuclear cells were isolated from pooled spinal cords and brains of MOG-immunized WT and KO mice 8 or 16 days after immunization, stained for CD4+ expression, and analyzed by flow cytometry. Numbers above bracketed lines (two left panels) indicate the percentage of CD4+ WT (black) and KO (gray) T cells. The right panel shows the absolute number of CD4+ T cells infiltrating the CNS at the indicated times after MOG/CFA immunization.
During EAE progression, MOG-specific CD4+ effector T cells, generated in the local dLNs, must gain access into the CNS to mount their inflammatory response against CNS Ags, resulting in axonal demyelination and paralysis. Consistent with the lack of clinical signs, the CNS inflammation and damage, as evidenced by the spinal cord’s white matter demyelination (Fig. 1B), and the infiltration of mononuclear cells into the CNS (Fig. 1C) were dramatically reduced in Def6−/− mice compared with WT mice. Analysis of the infiltrating mononuclear cells in the CNS revealed that before clinical onset of EAE, a time when T cells first enter the CNS to establish inflammation, the CD4 infiltrate in the spinal cord was, as expected, minimal. However, during active EAE (day 16), WT mice displayed a dramatic increase in the number of infiltrating CD4+ T cells (Fig. 1D). In striking contrast, MOG-immunized Def6−/− mice exhibited a lower proportion of CD4+ T cells than that of WT mice and a 6-fold decrease in the number of CD4+ T cells having accessed the CNS on day 16 (Fig. 1D).
Resistance to EAE in Def6−/− mice is T cell-intrinsic
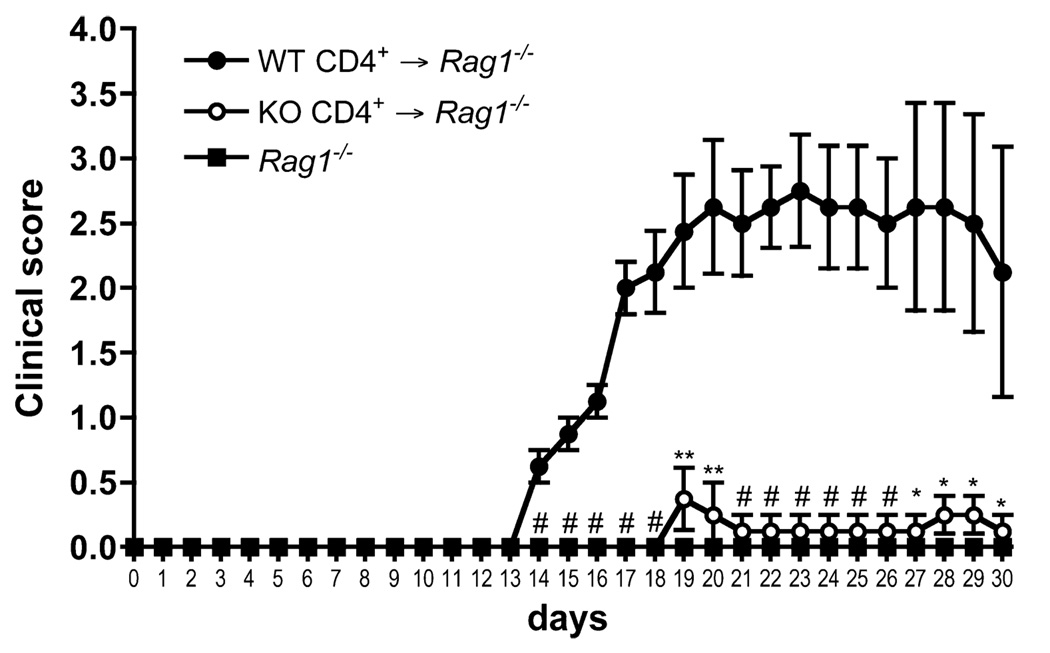
Although many cells contribute to the pathogenesis of EAE, CD4+ T cells are both the initiators and effectors in EAE, and hence the major mediators of the disease (34). Given the importance of T cells in EAE, and the fact that, in addition to its abundant expression in T cells, SLAT/Def6 is also expressed in macrophages (35) and dendritic cells (DCs), we wanted to determine whether the resistance to EAE in Def6−/− mice reflects an intrinsic T cell defect. To address this question, we used an adoptive transfer system to compare the ability of WT vs Def6−/− T cells to cause EAE. We adoptively transferred WT or Def6−/− CD4+ T cells into Rag1−/− recipient mice that cannot develop EAE on their own in the absence of endogenous T cells (Fig. 2), and we induced EAE by MOG/CFA priming. Upon immunization with MOG, mice that received WT T cells developed severe EAE disease. In sharp contrast, Rag1−/− mice that received Def6−/− T cells did not develop any signs of disease (Fig. 2). This result indicates that the resistance to EAE in Def6−/− mice is T cell-intrinsic, although it does not exclude potential contribution by other cell types.
FIGURE 2.
Resistance to EAE in Def6−/− mice is intrinsic to the T cells. CD4+ T cells were harvested from WT and KO LNs and spleen and transferred i.v. into Rag1−/− mice. Twenty-four hours after transfer, the recipient mice were primed to induce EAE, monitored daily for 30 days for clinical signs of EAE, and scored using an arbitrary scale of 0–5 as described in Fig. 1. Results are the means ± SEM from five mice per group. Statistical differences were determined as in Fig. 1.
SLAT/Def6 deficiency results in CD4+ T cell hyporesponsiveness
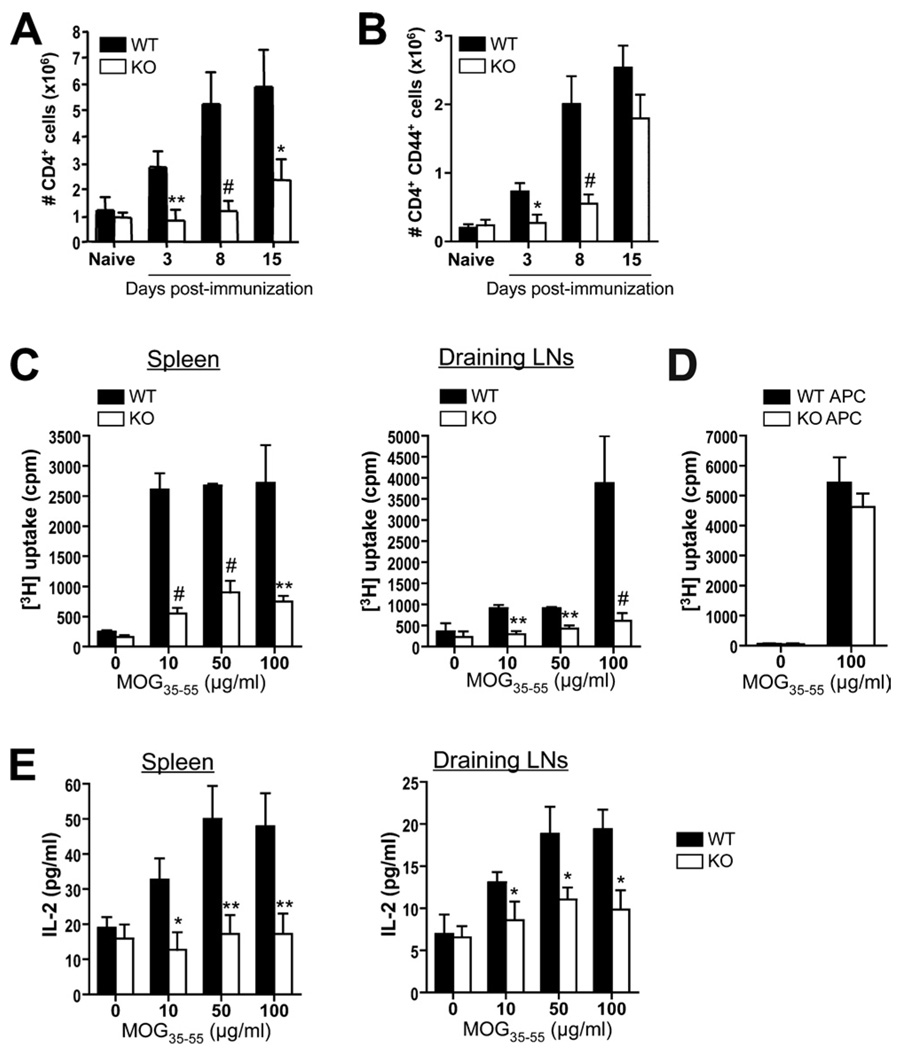
One likely explanation for the EAE resistance of Def6−/− mice is a defect in MOG-specific effector CD4+ T cell responses. We therefore quantified the extent of T cell priming after EAE induction by performing kinetic evaluation of the number and activation state of CD4+ T cells in the dLNs of WT and Def6−/− mice following MOG immunization. At all times examined postimmunization with MOG/CFA (before disease onset and until the peak of disease), the total number of pooled inguinal, axillary, and lumbar dLN CD4+ T cells was significantly decreased in Def6−/− mice in comparison with WT mice (Fig. 3A). Consistently, analysis of the activation status of CD4+ T cells in dLNs, based on their high expression of CD44, also revealed a significantly greater absolute number of CD4+CD44high T cells in the dLNs of MOG-immunized WT mice in comparison with their Def6−/− counterparts (Fig. 3B).
FIGURE 3.
Defective MOG-induced T cell activation in Def6−/− mice. A and B, Groups of four WT and KO mice were immunized with MOG/CFA. Naive, nonimmunized mice served as controls. On days 3, 8, and 15, dLNs were collected and total CD4+ (A) or CD44high-expressing CD4+ (B) T cells were enumerated. C, Pooled splenocytes and dLN cells (four mice per group) isolated 8 days postimmunization were stimulated for 72 h with increasing MOG35–55 peptide concentrations, and proliferation was analyzed by [3H]TdR uptake. D, Proliferation of purified CD4+ T cells from 2D2 (MOG-specific) TCR-Tg mice cultured with CD4-depleted splenocytes from WT or KO B6 mice in the presence of 100 µg/ml MOG35–55. Results are presented as mean [3H]TdR incorporation (cpm ± SD) from triplicate cultures. E, ELISA measurement of IL-2 production by splenocytes and dLN cells obtained from MOG-immunized WT and KO mice, which were restimulated for 72 h with the indicated MOG35–55 peptide concentrations. Data are means ± SD of triplicate wells. Statistical differences were determined as in Fig. 1.
To further examine the function of WT and Def6−/− MOG-specific T cells, we restimulated splenocytes or dLN cells from MOG/CFA-primed mice in vitro with MOG35–55 peptide and measured T cell proliferation and cytokine production. Splenocytes as well as dLN cells from Def6−/− mice prepared 8 (Fig. 3C), 15, or 24 (supplemental Fig. S1)5 days after immunization displayed a significantly lower degree of in vitro proliferation than did WT cells in response to varying concentrations of MOG peptide, indicating that SLAT/Def6 deficiency resulted in a state of hyporesponsiveness in the responding MOG-specific CD4+ T cells and/or reduced number of splenic or dLN MOG-specific CD4+ T cells.
Since we used in the above experiment unfractionated spleen and dLN cells from intact mice, it was possible that a Def6-associated defect in the function of DCs or other APCs (which also express SLAT/Def6) contributed to the hyporesponsiveness of Def6−/− cells. To address this possibility, we purified MOG35–55-specific TCR-Tg (2D2) CD4+ T cells and stimulated them with MOG35–55 in the presence of CD4-depleted splenocytes from either WT or Def6−/− mice as an APC source. The proliferation of 2D2 CD4+ T cell in response to MOG35–55 peptide stimulation was comparable between cultures containing WT or Def6−/− APCs (Fig. 3D), strongly suggesting that APCs do not contribute to the decreased MOG-specific T cell response in Def6−/− mice, and emphasizing once again the notion that the EAE resistance of Def6−/− mice is T cell-autonomous. As an additional indication of the hyporesponsiveness of Def6−/− T cells, we found that culture supernatants from 8-day-primed Def6−/− splenocytes and dLN cells stimulated in vitro with MOG35–55 peptide displayed a dose-dependent decrease of IL-2 production when compared with their WT counterparts (Fig. 3E). Thus, SLAT/Def6 resulted in impaired proliferation and activation of MOG-specific T cells, thereby contributing in a major way to the EAE resistance of Def6−/− mice.
Absence of SLAT/Def6 leads to impaired MOG-specific Th1 and Th17 responses
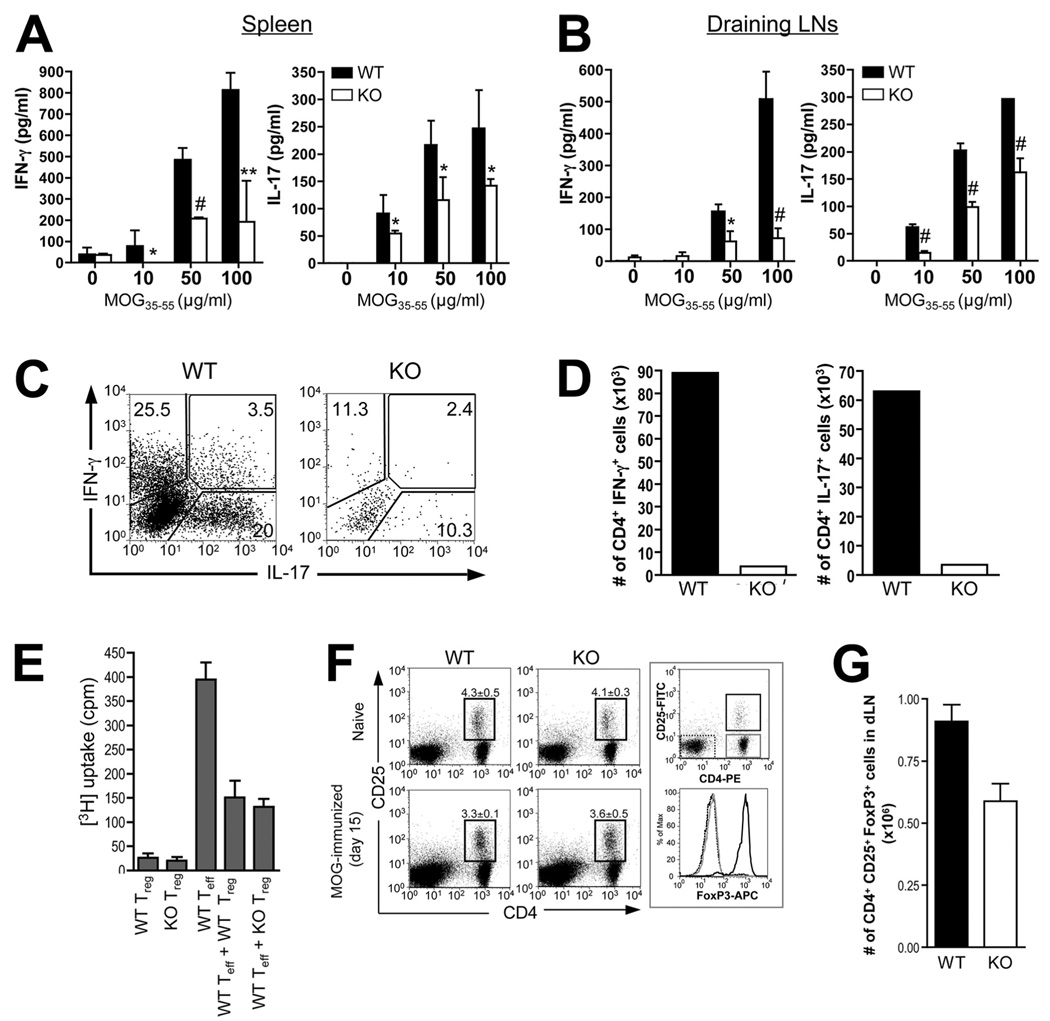
There is compelling evidence that EAE development and pathogenesis is characteristically associated with the overproduction and activity of proinflammatory cytokines, most predominantly IFN-γ and IL-17, by autoreactive inflammatory T cells activated either systemically or at the site of inflammation (i.e., CNS) (22, 23). Moreover, SLAT/Def6 has previously been shown to regulate Th1 differentiation (29), but its role in Th17 differentiation remains elusive (36). Thus, to further evaluate the impact of the Def6 mutation on Th1/Th17 responses in lymphoid organs, we harvested splenocytes or dLN cells from WT and Def6−/− mice on days 8 (before disease onset), 15 (between disease onset and peak), or 24 (remission phase) after MOG/CFA immunization, stimulated them in vitro with MOG35–55 peptide for 3 days, and used an ELISA to measure IL-17 and IFN-γ levels in culture supernatants. The results showed that Def6−/− splenocytes and dLN cells exhibited reduced IL-17 and IFN-γ production compared with WT cells at all of the times tested, and in response to different MOG35–55 concentrations (Fig. 4, A and B, for day 8, and supplemental Fig. S2 for days 15 and 24), demonstrating that the resistance of Def6−/− mice to EAE development correlates with a specific defect in activating autoantigen-specific Th1 and Th17 cells. This reduction of proinflammatory cytokines did not reflect skewing of Def6−/− T cells toward a Th2 lineage since IL-4, IL-5, and IL-13 levels were undetectable (data not shown). Importantly, the reduced production of IFN-γ and IL-17 was also observed when purified CD4+ T cells isolated from the brain and spinal cord of Def6−/− mice 15 days after MOG/CFA immunization were stimulated with phorbol ester (PMA) plus ionomycin, and cytokine expression was assessed by ICS (Fig. 4C). This defect was more drastic when the data were converted into absolute number of IFN-γ- and IL-17-expressing CD4+ T cells (Fig. 4D), considering the gross defect in CNS inflammation in Def6−/− mice (Fig. 1). Thus, the impaired proliferative and cytokine responses of Def6−/− purified CD4+ T cells from primed mice to in vitro MOG35–55 stimulation cleary indicate that the Def6 mutation causes hyperresponsiveness on a per cell basis. Overall, these results demonstrate that, in addition to the observed defect in MOG-specific CD4+ T cell activation, impaired differentiation or function of MOG-specific Th1 and/or Th17 cells contributes to the EAE resistance observed in Def6−/− mice.
FIGURE 4.
Reduced MOG-specific Th1 and Th17 responses, but normal Treg cell compartment, in Def6−/− mice. A and B, Splenocytes (A) or dLN cells (B) from WT and KO mice isolated 8 days postimmunization were challenged in vitro with the indicated concentrations of MOG35–55 peptide, and culture supernatants collected after 72 h were analyzed for IFN-γ (left panel) and IL-17 (right panel) concentration by ELISA. Statistical differences were determined as in Fig. 1. C, Cytokine production by CNS-derived, MOG35–55-stimulated CD4+ T cells. CD4+ T cells were isolated from CNS mononuclear cells 16 days after disease induction. The cells were stimulated for 5 h in vitro with PMA plus ionomycin in the presence of GolgiStop and analyzed for IFN-γ and IL-17 expression by ICS. D, Absolute number of IFN-γ- or IL-17-producing CD4+ T cells in CNS on day 16 after MOG/CFA immunization. For C and D, cells from three mice per group were pooled for analysis. Data are representative of three experiments. E, Suppressive activity of WT and KO Treg cells. CD4+CD25+ cells (Tregs) were purified from the spleens and LNs of WT or KO mice and were added (or not) to cultures of purified CD4+CD25− WT T cells (responders). Proliferation of WT responder T cells stimulated by anti-CD3 and anti-CD28 mAbs (5 µg/ml each) was determined by [3H]TdR incorporation, and cultures of Tregs alone served as a control. Data are means ± SD of triplicate wells. The results are representative of two independent experiments. F and G, Flow cytometric analysis (F) and calculated number (G) CD4+CD25+FoxP3+ Treg cells in WT and KO mice before (naive) and 15 days after MOG/CFA immunization. dLN cells were stained for CD4, CD25, and intracellular FoxP3 expression. The right panel in F shows that CD4+CD25+ cells are mostly FoxP3+, whereas CD4+CD25− and CD4−CD25− cells are FoxP3−.
Given that conditions leading to Treg vs Th17 development are mutually exclusive (37, 38), and that Tregs have been shown to provide protection against EAE through their suppressive action on effector CD4+CD25− T cells (39, 40), we investigated next whether an increased number of Tregs or an enhanced suppressive function of Def6−/− Tregs could contribute to the impaired Th17 response in Def6−/− mice. However, we did not find any difference between WT and Def6−/− mice in the (1) frequency of CD4+CD25+FoxP3+ naturally occurring or induced (in the presence of TGF-β) Tregs (supplemental Fig. S3); (2) suppressive function of Tregs toward responder T cell proliferation in naive mice, as evidenced in an in vitro suppression assay (Fig. 4E); (3) IL-10 production by induced Tregs (supplemental Fig. S3); or (4) frequency of Tregs in dLNs during the course of EAE (Fig. 4, F and G). If anything, fewer CD4+CD25+FoxP3+ cells were found in the dLNs of MOG-immunized Def6−/− mice as compared with their WT counterparts. Altogether, these data suggest that SLAT/Def6 plays a role in driving Th1 and Th17 responses in the EAE model, but does not substantially affect Treg cell development or function.
SLAT/Def6 is intrinsically required for Th17 differentiation
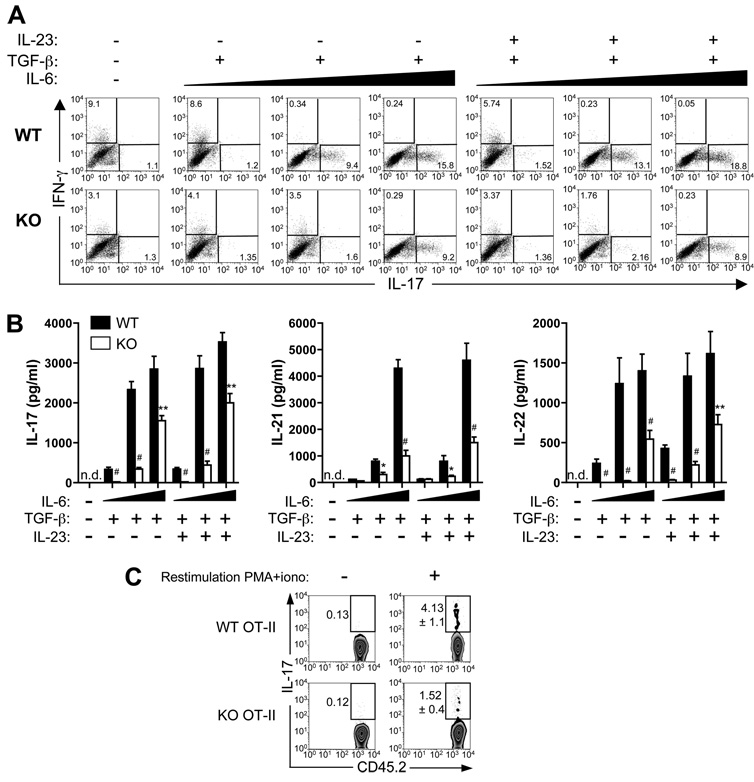
In addition to the role of SLAT/Def6 in Th1 differentiation, which we previously documented (29), our data also point to a role of SLAT/Def6 in promoting MOG-specific Th17 cells in vivo (Fig. 4). Thus, we wanted to determine whether SLAT/Def6 is also required for Th17 differentiation in vitro. FACS-sorted naive (CD62LhighCD44low) OT-II (OVA-specific) TCR-Tg CD4+ T cells from WT or Def6−/− mice were cultured under Th17-inducing conditions (TGF-β plus IL-6) in the presence of irradiated OVA323–339-loaded WT APCs, and IL-17 vs IFN-γ expression was determined by ICS. Several pertinent points can be made from the results of this experiment (Fig. 5). First, under neutral culture conditions, and as expected, we could detect an IFN-γ (but not IL-17) response, which was reduced by ~3-fold in Def6−/− T cells, consistent with our previous findings (29); however, this Th1 response was abolished in the presence of TGF-β and higher IL-6 concentrations (Fig. 5A). Second, Th17 differentiation of Def6−/− T cells in the presence of TGF-β plus IL-6 was impaired in comparison with WT T cells. This impairment was most prominent (~80% reduction) when using an intermediate concentration of IL-6 (5 ng/ml) (Fig. 5A). Third, addition of IL-23, which is required for the terminal differentiation and expansion of already committed Th17 cells (9, 41, 42), to the Th17-polarizing cytokine “cocktail” did not reverse or ameliorate the defective Th17 differentiation of Def6−/− T cells (Fig. 5A). Similarly, when analyzed by ELISA, the production of three Th17 signature cytokines (i.e., IL-17, IL-21 and IL-22) was consistently decreased in culture supernatants of Def6−/− cells (Fig. 5B), confirming that SLAT/Def6 plays a crucial role in Th17 differentiation in vitro. Moreover, since the APCs used in this experiment are SLAT/Def6-sufficient, these results are consistent with data shown above demonstrating the T cell-intrinsic nature of the Def6 defect.
FIGURE 5.
Defective in vitro and in vivo Th17 differentiation of Def6−/− T cells. A and B, FACS-sorted naive CD4+CD62LhighCD44low OT-II T cells from WT or KO mice were stimulated for 5 days with irradiated, OVA323–339-pulsed WT splenic APCs, and cultured in the absence or presence of TGF-β (5 ng/ml) and increasing concentrations (1, 5, or 20 ng/ml; indicated by a black wedge) of IL-6. IL-17- and IFN-γ-expressing CD4+ T cells were measured by ICS (A). The production of IL-17, IL-21, and IL-22 in culture supernatants was measured by an ELISA (B). Data represent means ± SD of triplicate wells and are representative of three independent experiments with similar results. C, FACS-sorted naive CD45.2+ T cells from WT or KO OT-II mice were transferred i.v. into CD45-congenic (CD45.1+) mice. The recipients (three mice per group) were immunized with OVA in CFA. Eight days later, dLN cells were stimulated with PMA and ionomycin for 5 h, and IL-17-expressing CD4+ T cells were measured by ICS after gating on CD45.2+CD4+ T cells. Numbers adjacent to the gates indicate the percentage of IL-17-positive cells among the transferred cells. Statistical differences were determined as in Fig. 1.
To directly determine whether a similar defect in the Th17 differentiation of Def6−/− cells is also observed in vivo, we transferred naive OT-II TCR-Tg CD4+ T cells from either WT or Def6−/− mice (CD45.2+) into B6.SJL (CD45.1+) mice and immunized the recipients with OVA in CFA. After 8 days, we harvested dLN cells and restimulated them in vitro with PMA plus ionomycin to assess IL-17 expression of donor (CD4+CD45.2+) T cells by ICS. As shown in Fig. 5C, the frequency of IL-17-producing Def6−/− OT-II CD4+ T cells was greatly reduced (~3-fold) when compared with WT CD4+ T cells. Taken together, these data indicate that SLAT/Def6 positively regulates Th17 differentiation both in vivo and in vitro in a T cell-intrinsic manner.
Discussion
In this report, we assessed the role of SLAT/Def6 in a well-characterized and highly relevant autoimmune disorder, namely, EAE, to substantiate the importance of SLAT/Def6 in autoimmunity. We demonstrate that SLAT/Def6 plays a prominent role in EAE development and pathogenesis and in the generation of encephalitogenic Th1 and Th17 cells, which are responsible for the clinical signs of the disease. MOG-immunized Def6−/− mice displayed minimal signs of CNS inflammation, as evidenced by the lack of inflammatory cells in the brain and spinal cord (including CD4+ T cells), as well as an absence of demyelination of the spinal cord’s white matter following MOG immunization, leading ultimately to delayed onset and minimal severity of the disease. Analysis of CD4+ T cells isolated from peripheral lymphoid organs of Def6−/− mice indicated reduced accumulation of activated T cells and an impaired ability to proliferate, and to produce IL-2, as well as the effector Th1 and Th17-signature cytokines, IFN-γ and IL-17, respectively, at different times after immunization. We also provide evidence for a drastic defect in IFN-γ and IL-17 cytokine production in the target organ, that is, the CNS tissue, contributing to the EAE resistance observed in the absence of SLAT/Def6. Lastly, we show that SLAT/Def6 controls the differentiation/expansion of naive CD4+ T cells into Th17 cell lineage in vitro and in vivo.
The pathogenesis of EAE/MS is generally viewed as a complex process involving activation of APCs, differentiation of myelin-reactive T cells (particularly Th1 and Th17 CD4+ effector T cells), and secretion of inflammatory cytokines in the CNS (43–45). Our findings show that resistance of Def6−/− mice to EAE correlates with Ag-specific hyporesponsiveness of Def6−/− CD4+ T cells obtained from MOG-immunized mice. Although SLAT/Def6 protein expression is for the most part limited to cells of the lymphoid system, in particular thymocytes and peripheral T cells, SLAT/Def6 is also expressed, albeit at a lower level, in DCs and myeloid cells, raising the possibility that the effects of Def6 deletion on EAE development were not entirely T cell-restricted (e.g., reflecting a defect in Ag presentation by APCs, or the lack of SLAT/Def6 in other cell types that participate in the inflammatory response). Our findings that (1) purified Def6−/− CD4+ T cells display impaired proliferation, IL-2 production, and Ca2+ signaling in response to anti-CD3/CD28 costimulation (29), and (2) Def6−/− APCs are as effective as WT APCs in inducing CD4+ T cell proliferation of 2D2 (MOG-specific) TCR-Tg WT CD4+ T cells upon in vitro MOG35–55 stimulation strongly suggest an intrinsic T cell defect and seem to rule out a role for an APC defect in the impaired T cell proliferation. The finding that WT CD4+ T cells, but not of Def6−/− CD4+ T cells, can transfer EAE susceptibility to Rag1−/− mice unambigously indicates that the resistance to EAE in Def6−/− mice is intrinsic to the T cells (albeit not excluding a potential role of other, extrinsic factors).
Typically, EAE development is mediated by the activation of MOG-specific T cells, which expand in lymphoid tissues and differentiate in Th1 and Th17 CD4+ effector T cells, before migrating across the blood-brain barrier and entering the CNS in a highly specialized process involving a specific set of adhesion molecules, integrins, and chemokine receptors. Once in the CNS, the effector T cells mount the inflammatory response against CNS Ags, resulting in axonal demyelination and paralysis. Thus, the Ag (MOG)-specific hyporesponsive state of CD4+ T cells observed during the course of EAE pathology in Def6−/− mice may be attributed, in a mutually nonexclusive manner, to defects in different phases of CD4+ T cell response, including T cell activation, proliferation/expansion, survival, Th1/Th17 differentiation, and migration to lymphoid organs and the CNS. This latter notion, that is, the potential role of SLAT/Def6 in migration of autoreactive T cells, arises primarily from the finding that the SLAT/Def6 homolog, SWAP-70, has a known role in migration of B cells to lymphoid organs in an inflammatory context (27). Furthermore, SLAT/Def6 has GEF activity toward the small GTPases, Cdc42 and Rac1 (30, 31, 46), which regulate the actin cytoskeleton, a process-critical component for the polarization and migration of T lymphocytes. Therefore, the lack of inflammation in the CNS during EAE development may reflect a defect in migration of Def6−/− T cells, which could contribute to the EAE resistance of Def6−/− mice. These findings raise the possibility that SLAT/Def6 may transduce chemokine receptor signals involved in the selective migration of effector T cells to the inflammatory sites.
The defective Th1 response observed in LN and CNS from MOG-immunized Def6−/− animals is consistent with our previous study revealing a critical role of SLAT/Def6 in Th1 responses in vitro and in a lung inflammatory model (29). In addition to the well-known Th1 and Th2 CD4+ T cell subsets, Th17 cells have recently emerged as a third Th subset, which has been reported to play a critical role in the pathogenesis of a variety of organ-specific autoimmune and inflammatory diseases (10, 11), including EAE (6, 9, 16, 17). An important observation from our studies is the demonstration that SLAT/Def6 also regulates Th17 response in vitro and in vivo in the context of EAE induction, thereby emphasizing the emerging role of SLAT/Def6 as an important positive regulator of Th1, Th2, and Th17 responses in different experimental models of inflammatory diseases.
In contrast to our study, a recent paper reported that Def6−/− BALB/c mice crossed to DO11.10 TCR-Tg mice expressing an MHC class II-restricted OVA-specific TCR spontaneously and rapidly develop rheumatoid arthritis-like joint disease and large vessel vasculitis associated with enhanced IL-17 and IL-21 production (47). The deregulated production of IL-17 and IL-21 by Def6−/− DO11.10 mice was traced to the inability of SLAT/Def6 to sequester, and negatively regulate, IRF-4, a transcription factor recently found to control Th17 differentiation (48). Nevertheless, these observations, made in the setting of a restricted TCR repertoire (i.e., in the DO11.10 TCR Tg background), have not been confirmed in mice expressing an intact polyclonal TCR repertoire since young, non-TCR-Tg Def6−/− BALB/c mice did not develop obvious signs of arthritis (47). Thus, the physiological and pathological relevance of findings based on the study of Def6−/− mice on a TCR-Tg background remains unclear (36).
SLAT/Def6 is essential for TCR-stimulated Ca2+ release from intracellular stores in naive CD4+ T cells, the first step in the Ca2+/NFAT signaling pathway (29). As a result, Def6−/− T cells display a severe defect in Ca2+ influx and NFAT activation (but not in AP-1 or NF-κB activation), which translates into impaired Th1-, Th2- (29), and, likely, Th17-mediated inflammatory diseases. In fact, multiple lines of evidence support an emerging role for NFAT in Th17 differentiation and function. In particular, treatment with high doses of cyclosporin A, which blocks NFAT activation by inhibiting the Ca2+/calmodulin-dependent phosphatase, calcineurin, has been shown to suppress clinical signs of EAE in different species, including rats, guinea pigs, and monkeys (49). Additionally, the proximal promoter of the human il-17a gene contains two cis-acting NFAT-binding sites, which appear to be important for IL-17 expression (50). Lastly, NR2F6, a nuclear zinc-finger orphan receptor, has been shown to interfere with the DNA binding of NFAT-AP-1 (but not NF-κB) and, consequently, to inhibit transcriptional activity of the NFAT-dependent il-17a cytokine promoter (51). Whether the importance of SLAT in EAE induction and pathogenesis reflects its essential function in mediating TCR-induced Ca2+/NFAT signaling, however, remains to be determined.
It has been shown that naive T cells develop reciprocally into pathogenic Th17 or protective Treg subsets depending on the presence or absence of IL-6, respectively, in the local cytokine milieu (38). CD4+CD25+ Treg cells can provide protection by suppressing proliferation of effector CD4+CD25− T cells. They have been shown to play a crucial role in maintaining self-tolerance and in protecting against autoimmune disease (40). Thus, considering the reciprocal regulation of Treg and Th17 development and the altered Th17 response in the absence of SLAT/Def6, one would perhaps have expected enhanced development and/or regulatory function of Tregs in Def6−/− mice. On the other hand, considering the role of SLAT/Def6 in controlling NFAT activation and the promoting role of NFAT in Treg function (reflecting its cooperation with FoxP3) (52), impaired Treg function in Def6−/− mice could also be conceivable. However, the absolute requirement of NFAT-FoxP3 cooperation for Treg differentiation is complex since NFAT1/4 double-deficiency does not interfere with the development and function of Tregs (53). Our data showed no apparent role of SLAT/Def6 in Treg development or function in naive mice, or in Treg expansion in the context of EAE induction, thereby tending to rule out the possibility that enhanced development and/or regulatory function of FoxP3+CD4+CD25+ cells in Def6−/− mice may account for their EAE resistance. Future studies are needed to clarify the specific signaling pathways involved in SLAT/Def6-mediated Ca2+/NFAT activation that promotes Th differentiation, while seemingly not altering Treg differentiation and function.
In summary, our findings establish a pivotal role of SLAT/Def6, which is obligatory for TCR-induced Ca2+/NFAT signaling in T cells, in the development of Th17- and Th1-mediated organ-specific autoimmunity. Further work seeking to explore the role of SLAT/Def6 in distinct aspects of myelin-specific CD4+ T cell responses as well as to determine the role of Ca2+/NFAT signaling in EAE pathogenesis will be critical to fully define the relationship between SLAT/Def6 and Th1/Th17-mediated inflammatory responses. These studies might have important implications in the basic scientific arena by elucidating new Th1/Th17-regulating pathways, and also in a clinical setting by establishing SLAT/Def6 as a promising drug target for T cell-mediated autoimmunity and inflammation.
Supplementary Material
Acknowledgments
We thank all of the members of the Cell Biology Division for helpful comments, C. Kim and K. C. Van Gunst for assistance with flow cytometry and cell sorting. This is manuscript number 1204 from the La Jolla Institute for Allergy and Immunology.
Footnotes
This work was supported by National Institutes of Health Grant AI68320 (to A.A.) and fellowships from the Fondation pour la Recherche Medicale (to S.B.), the Diabetes & Immune Disease National Research Institute (to S.B.), and the Philippe Foundation (to S.B.).
Abbreviations used in this paper: EAE, experimental autoimmune encephalomyelitis; MS, multiple sclerosis; GEF, guanine nucleotide exchange factor; SLAT, SWAP-70-like adapter of T cells; MOG, myelin oligodendrocyte glycoprotein; dLN, draining lymph node; Tg, transgenic; WT, wild type; ICS, intracellular cytokine staining; Treg, regulatory T cell.
The online version of this article contains supplemental material.
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Steinman L, Zamvil SS. Virtues and pitfalls of EAE for the development of therapies for multiple sclerosis. Trends Immunol. 2005;26:565–571. doi: 10.1016/j.it.2005.08.014. [DOI] [PubMed] [Google Scholar]
- 2.Mosmann TR, Coffman RL. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu. Rev. Immunol. 1989;7:145–173. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- 3.McGeachy MJ, Cua DJ. Th17 cell differentiation: the long and winding road. Immunity. 2008;28:445–453. doi: 10.1016/j.immuni.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 4.Dong C. Th17 cells in development: an updated view of their molecular identity and genetic programming. Nat. Rev. Immunol. 2008;8:337–348. doi: 10.1038/nri2295. [DOI] [PubMed] [Google Scholar]
- 5.Harrington LE, Mangan PR, Weaver CT. Expanding the effector CD4 T-cell repertoire: the Th17 lineage. Curr. Opin. Immunol. 2006;18:349–356. doi: 10.1016/j.coi.2006.03.017. [DOI] [PubMed] [Google Scholar]
- 6.Weaver CT, Hatton RD, Mangan PR, Harrington LE. IL-17 family cytokines and the expanding diversity of effector T cell lineages. Annu. Rev. Immunol. 2007;25:821–852. doi: 10.1146/annurev.immunol.25.022106.141557. [DOI] [PubMed] [Google Scholar]
- 7.Ouyang W, Kolls JK, Zheng Y. The biological functions of T helper 17 cell effector cytokines in inflammation. Immunity. 2008;28:454–467. doi: 10.1016/j.immuni.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Park H, Li Z, Yang XO, Chang SH, Nurieva R, Wang YH, Wang Y, Hood L, Zhu Z, Tian Q, Dong C. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat. Immunol. 2005;6:1133–1141. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Langrish CL, Chen Y, Blumenschein WM, Mattson J, Basham B, Sedgwick JD, McClanahan T, Kastelein RA, Cua DJ. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J. Exp. Med. 2005;201:233–240. doi: 10.1084/jem.20041257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nakae S, Nambu A, Sudo K, Iwakura Y. Suppression of immune induction of collagen-induced arthritis in IL-17-deficient mice. J. Immunol. 2003;171:6173–6177. doi: 10.4049/jimmunol.171.11.6173. [DOI] [PubMed] [Google Scholar]
- 11.Chan JR, Blumenschein W, Murphy E, Diveu C, Wiekowski M, Abbondanzo S, Lucian L, Geissler R, Brodie S, Kimball AB, et al. IL-23 stimulates epidermal hyperplasia via TNF and IL-20R2-dependent mechanisms with implications for psoriasis pathogenesis. J. Exp. Med. 2006;203:2577–2587. doi: 10.1084/jem.20060244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ferber IA, Brocke S, Taylor-Edwards C, Ridgway W, Dinisco C, Steinman L, Dalton D, Fathman CG. Mice with a disrupted IFN-γ gene are susceptible to the induction of experimental autoimmune encephalomyelitis (EAE) J. Immunol. 1996;156:5–7. [PubMed] [Google Scholar]
- 13.Furlan R, Brambilla E, Ruffini F, Poliani PL, Bergami A, Marconi PC, Franciotta DM, Penna G, Comi G, Adorini L, Martino G. Intrathecal delivery of IFN-γ protects C57BL/6 mice from chronic-progressive experimental autoimmune encephalomyelitis by increasing apoptosis of central nervous system-infiltrating lymphocytes. J. Immunol. 2001;167:1821–1829. doi: 10.4049/jimmunol.167.3.1821. [DOI] [PubMed] [Google Scholar]
- 14.Willenborg DO, Fordham SA, Staykova MA, Ramshaw IA, Cowden WB. IFN-γ is critical to the control of murine autoimmune encephalomyelitis and regulates both in the periphery and in the target tissue: a possible role for nitric oxide. J. Immunol. 1999;163:5278–5286. [PubMed] [Google Scholar]
- 15.Willenborg DO, Fordham S, Bernard CC, Cowden WB, Ramshaw IA. IFN-γ plays a critical down-regulatory role in the induction and effector phase of myelin oligodendrocyte glycoprotein-induced autoimmune encephalomyelitis. J. Immunol. 1996;157:3223–3227. [PubMed] [Google Scholar]
- 16.Cua DJ, Sherlock J, Chen Y, Murphy CA, Joyce B, Seymour B, Lucian L, To W, Kwan S, Churakova T, et al. Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature. 2003;421:744–748. doi: 10.1038/nature01355. [DOI] [PubMed] [Google Scholar]
- 17.Murphy CA, Langrish CL, Chen Y, Blumenschein W, McClanahan T, Kastelein RA, Sedgwick JD, Cua DJ. Divergent pro- and anti-inflammatory roles for IL-23 and IL-12 in joint autoimmune inflammation. J. Exp. Med. 2003;198:1951–1957. doi: 10.1084/jem.20030896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Katz JD, Benoist C, Mathis D. T helper cell subsets in insulin-dependent diabetes. Science. 1995;268:1185–1188. doi: 10.1126/science.7761837. [DOI] [PubMed] [Google Scholar]
- 19.Lafaille JJ, Keere FV, Hsu AL, Baron JL, Haas W, Raine CS, Tonegawa S. Myelin basic protein-specific T helper 2 (Th2) cells cause experimental autoimmune encephalomyelitis in immunodeficient hosts rather than protect them from the disease. J. Exp. Med. 1997;186:307–312. doi: 10.1084/jem.186.2.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kroenke MA, Carlson TJ, Andjelkovic AV, Segal BM. IL-12- and IL-23-modulated T cells induce distinct types of EAE based on histology, CNS chemokine profile, and response to cytokine inhibition. J. Exp. Med. 2008;205:1535–1541. doi: 10.1084/jem.20080159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Luger D, Silver PB, Tang J, Cua D, Chen Z, Iwakura Y, Bowman EP, Sgambellone NM, Chan CC, Caspi RR. Either a Th17 or a Th1 effector response can drive autoimmunity: conditions of disease induction affect dominant effector category. J. Exp. Med. 2008;205:799–810. doi: 10.1084/jem.20071258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lees JR, Iwakura Y, Russell JH. Host T cells are the main producers of IL-17 within the central nervous system during initiation of experimental autoimmune encephalomyelitis induced by adoptive transfer of Th1 cell lines. J. Immunol. 2008;180:8066–8072. doi: 10.4049/jimmunol.180.12.8066. [DOI] [PubMed] [Google Scholar]
- 23.O’Connor RA, Prendergast CT, Sabatos CA, Lau CW, Leech MD, Wraith DC, Anderton SM. Cutting edge: Th1 cells facilitate the entry of Th17 cells to the central nervous system during experimental autoimmune encephalomyelitis. J. Immunol. 2008;181:3750–3754. doi: 10.4049/jimmunol.181.6.3750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tanaka Y, Bi K, Kitamura R, Hong S, Altman Y, Matsumoto A, Tabata H, Lebedeva S, Bushway PJ, Altman A. SWAP-70-like adapter of T cells, an adapter protein that regulates early TCR-initiated signaling in Th2 lineage cells. Immunity. 2003;18:403–414. doi: 10.1016/s1074-7613(03)00054-2. [DOI] [PubMed] [Google Scholar]
- 25.Borggrefe T, Wabl M, Akhmedov AT, Jessberger R. A B-cell-specific DNA recombination complex. J. Biol. Chem. 1998;273:17025–17035. doi: 10.1074/jbc.273.27.17025. [DOI] [PubMed] [Google Scholar]
- 26.Shinohara M, Terada Y, Iwamatsu A, Shinohara A, Mochizuki N, Higuchi M, Gotoh Y, Ihara S, Nagata S, Itoh H, et al. SWAP-70 is a guanine-nucleotide-exchange factor that mediates signalling of membrane ruffling. Nature. 2002;416:759–763. doi: 10.1038/416759a. [DOI] [PubMed] [Google Scholar]
- 27.Pearce G, Angeli V, Randolph GJ, Junt T, von Andrian U, Schnittler HJ, Jessberger R. Signaling protein SWAP-70 is required for efficient B cell homing to lymphoid organs. Nat. Immunol. 2006;7:827–834. doi: 10.1038/ni1365. [DOI] [PubMed] [Google Scholar]
- 28.Gupta S, Lee A, Hu C, Fanzo J, Goldberg I, Cattoretti G, Pernis AB. Molecular cloning of IBP, a SWAP-70 homologous GEF, which is highly expressed in the immune system. Hum. Immunol. 2003;64:389–401. doi: 10.1016/s0198-8859(03)00024-7. [DOI] [PubMed] [Google Scholar]
- 29.Becart S, Charvet C, Canonigo Balancio AJ, De Trez C, Tanaka Y, Duan W, Ware C, Croft M, Altman A. SLAT regulates Th1 and Th2 inflammatory responses by controlling Ca2+/NFAT signaling. J. Clin. Invest. 2007;117:2164–2175. doi: 10.1172/JCI31640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gupta S, Fanzo JC, Hu C, Cox D, Jang SY, Lee AE, Greenberg S, Pernis AB. T cell receptor engagement leads to the recruitment of IBP, a novel guanine nucleotide exchange factor, to the immunological synapse. J. Biol. Chem. 2003;278:43541–43549. doi: 10.1074/jbc.M308960200. [DOI] [PubMed] [Google Scholar]
- 31.Becart S, Balancio AJ, Charvet C, Feau S, Sedwick CE, Altman A. Tyrosine-phosphorylation-dependent translocation of the SLAT protein to the immunological synapse is required for NFAT transcription factor activation. Immunity. 2008;29:704–719. doi: 10.1016/j.immuni.2008.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fanzo JC, Yang W, Jang SY, Gupta S, Chen Q, Siddiq A, Greenberg S, Pernis AB. Loss of IRF-4-binding protein leads to the spontaneous development of systemic autoimmunity. J. Clin. Invest. 2006;116:703–714. doi: 10.1172/JCI24096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chu CQ, Wittmer S, Dalton DK. Failure to suppress the expansion of the activated CD4 T cell population in interferon γ-deficient mice leads to exacerbation of experimental autoimmune encephalomyelitis. J. Exp. Med. 2000;192:123–128. doi: 10.1084/jem.192.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kuchroo VK, Anderson AC, Waldner H, Munder M, Bettelli E, Nicholson LB. T cell response in experimental autoimmune encephalomyelitis (EAE): role of self and cross-reactive antigens in shaping, tuning, and regulating the autopathogenic T cell repertoire. Annu. Rev. Immunol. 2002;20:101–123. doi: 10.1146/annurev.immunol.20.081701.141316. [DOI] [PubMed] [Google Scholar]
- 35.Mehta H, Glogauer M, Becart S, Altman A, Coggeshall KM. Adaptor protein SLAT modulates Fcγ receptor-mediated phagocytosis in murine macrophages. J. Biol. Chem. 2009;284:11882–11891. doi: 10.1074/jbc.M809712200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Altman A, Becart S. Does Def6 deficiency cause autoimmunity? Immunity. 2009;31:1–2. doi: 10.1016/j.immuni.2009.06.013. author reply 2–3. [DOI] [PubMed] [Google Scholar]
- 37.Mangan PR, Harrington LE, O’Quinn DB, Helms WS, Bullard DC, Elson CO, Hatton RD, Wahl SM, Schoeb TR, Weaver CT. Transforming growth factor-β induces development of the Th17 lineage. Nature. 2006;441:231–234. doi: 10.1038/nature04754. [DOI] [PubMed] [Google Scholar]
- 38.Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector Th17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 39.Kohm AP, Carpentier PA, Anger HA, Miller SD. Cutting edge: CD4+CD25+ regulatory T cells suppress antigen-specific autoreactive immune responses and central nervous system inflammation during active experimental autoimmune encephalomyelitis. J. Immunol. 2002;169:4712–4716. doi: 10.4049/jimmunol.169.9.4712. [DOI] [PubMed] [Google Scholar]
- 40.Hori S, Takahashi T, Sakaguchi S. Control of autoimmunity by naturally arising regulatory CD4+ T cells. Adv. Immunol. 2003;81:331–371. doi: 10.1016/s0065-2776(03)81008-8. [DOI] [PubMed] [Google Scholar]
- 41.McGeachy MJ, Chen Y, Tato CM, Laurence A, Joyce-Shaikh B, Blumenschein WM, McClanahan TK, O’Shea JJ, Cua DJ. The interleukin 23 receptor is essential for the terminal differentiation of interleukin 17-producing effector T helper cells in vivo. Nat. Immunol. 2009;10:314–324. doi: 10.1038/ni.1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. TGFβ in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24:179–189. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 43.Bitsch A, Bruck W. Differentiation of multiple sclerosis subtypes: implications for treatment. CNS Drugs. 2002;16:405–418. doi: 10.2165/00023210-200216060-00004. [DOI] [PubMed] [Google Scholar]
- 44.Zamvil SS, Steinman L. The T lymphocyte in experimental allergic encephalomyelitis. Annu. Rev. Immunol. 1990;8:579–621. doi: 10.1146/annurev.iy.08.040190.003051. [DOI] [PubMed] [Google Scholar]
- 45.Steinman L, Martin R, Bernard C, Conlon P, Oksenberg JR. Multiple sclerosis: deeper understanding of its pathogenesis reveals new targets for therapy. Annu. Rev. Neurosci. 2002;25:491–505. doi: 10.1146/annurev.neuro.25.112701.142913. [DOI] [PubMed] [Google Scholar]
- 46.Mavrakis KJ, McKinlay KJ, Jones P, Sablitzky F. DEF6, a novel PH-DH-like domain protein, is an upstream activator of the Rho GTPases Rac1, Cdc42, and RhoA. Exp. Cell Res. 2004;294:335–344. doi: 10.1016/j.yexcr.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 47.Chen Q, Yang W, Gupta S, Biswas P, Smith P, Bhagat G, Pernis AB. IRF-4-binding protein inhibits interleukin-17 and interleukin-21 production by controlling the activity of IRF-4 transcription factor. Immunity. 2008;29:899–911. doi: 10.1016/j.immuni.2008.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brustle A, Heink S, Huber M, Rosenplanter C, Stadelmann C, Yu P, Arpaia E, Mak TW, Kamradt T, Lohoff M. The development of inflammatory TH-17 cells requires interferon-regulatory factor 4. Nat. Immunol. 2007;8:958–966. doi: 10.1038/ni1500. [DOI] [PubMed] [Google Scholar]
- 49.Bolton C, Borel JF, Cuzner ML, Davison AN, Turner AM. Immunosuppression by cyclosporin A of experimental allergic encephalomyelitis. J. Neurol. Sci. 1982;56:147–153. doi: 10.1016/0022-510x(82)90138-1. [DOI] [PubMed] [Google Scholar]
- 50.Liu XK, Lin X, Gaffen SL. Crucial role for nuclear factor of activated T cells in T cell receptor-mediated regulation of human interleukin-17. J. Biol. Chem. 2004;279:52762–52771. doi: 10.1074/jbc.M405764200. [DOI] [PubMed] [Google Scholar]
- 51.Hermann-Kleiter N, Gruber T, Lutz-Nicoladoni C, Thuille N, Fresser F, Labi V, Schiefermeier N, Warnecke M, Huber L, Villunger A, et al. The nuclear orphan receptor NR2F6 suppresses lymphocyte activation and T helper 17-dependent autoimmunity. Immunity. 2008;29:205–216. doi: 10.1016/j.immuni.2008.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wu Y, Borde M, Heissmeyer V, Feuerer M, Lapan AD, Stroud JC, Bates DL, Guo L, Han A, Ziegler SF, et al. FOXP3 controls regulatory T cell function through cooperation with NFAT. Cell. 2006;126:375–387. doi: 10.1016/j.cell.2006.05.042. [DOI] [PubMed] [Google Scholar]
- 53.Bopp T, Palmetshofer A, Serfling E, Heib V, Schmitt S, Richter C, Klein M, Schild H, Schmitt E, Stassen M. NFATc2 and NFATc3 transcription factors play a crucial role in suppression of CD4+ T lymphocytes by CD4+CD25+ regulatory T cells. J. Exp. Med. 2005;201:181–187. doi: 10.1084/jem.20041538. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.