Abstract
The human intestine has evolved in the presence of a diverse array of luminal microorganisms. In order to maintain intestinal homeostasis, mucosal immune responses to these microorganisms must be tightly regulated. The intestine needs to be able to respond to pathogenic organisms while at the same time maintain tolerance to normal commensal flora. Toll-like receptors (TLRs) play an important role in this delicate balance. TLRs are transmembrane non-catalytic receptor proteins that induce activation of innate and adaptive immune responses to microorganisms by recognizing structurally conserved molecular patterns of microbes. Expression of TLRs by intestinal epithelial cell is normally down-regulated to maintain immune tolerance to the luminal microorganisms.
One of the challenges of TLR research in the human intestine is that it is difficult for many experimental methods to detect very low expression of TLRs within the intestinal mucosa. Quantitative methods such as PCR are limited in their ability to detect TLR expression by specific cell types within a tissue sample, which can be important when studying the contribution of TLR signaling to pathological conditions. In this regard, immunohistochemistry (IHC) is advantageous in that one can visualize the distribution and localization of target proteins within both normal and pathologic parts of a given tissue sample. We found that a subset of human colorectal cancers over-express TLR4 by means of immunoflourescence (IF) and IHC methods. Localization of TLR4 within cancer tissue often appears to be patchy, making IHC an appropriate way to examine these changes. We will describe our current techniques to detect TLR4 in paraffin-embedded human large intestine sections. Establishing a practical IHC technique that may provide consistent results between laboratories will significantly enhance understanding of the role of TLRs in human intestinal health and disease.
Keywords: Toll-like receptor (TLR), intestine, colon, cancer, inflammation, immunoflourescence, immunohistochemistry, western blot analysis
1. Introduction
Despite extensive technological advances in research methods, there is always the need to improve and refine established qualitative and quantitative research techniques employed in the detection and meaurement of molecular and pathological phenomena. Immunohistochemistry (IHC) is a widely used general research tool that allows for detection of specific targets within tissue samples through the use of labeled antibodies and reagents that can then be visualized with enzymatic reactions. IHC has the advantage of allowing one to visualize the distribution and localization of specific targets within a specimen. The location of an antigen within a cell (organelle, nucleus, membrane, etc) can be determined while simultaneously appreciating anatomical and biological changes within the tissue, such as neoplasia. In fact, IHC is widely used in the diagnosis of cancer. Specific markers detectable with ICH may further characterize types and/or stages of cancer.
The gastrointestinal tract is a unique organ in which the intestinal epithelium is in direct contact with a diverse array of microorganisms. The human intestine has developed to have a dual relationship with the commensal flora. The epithelium serves as a barrier against bacterial invasion while at the same time taking advantage of these bacteria to assist in the maintenance and function of the intestine. In order to maintain intestinal homeostasis, the mucosal immune response to the microorganisms must be tightly controlled. TLRs play important immune and non-immune functions in establishing this delicate balance in the intestinal mucosa. TLRs are members of a conserved interleukin-1 (IL-1) superfamily of transmembrane receptors that recognize pathogen-associated molecular patterns (PAMPs) (1). The interaction of TLR4 with its ligand lipopolysaccharide results in NF-kB activation and multiple gene expression causing rapid immune responses. The mitogen activated protein kinase (MAPK) pathway can also be activated by the TLR4 mediated signaling (2).
Although the expression of TLRs in the gastrointestinal tract has been examined, the expression, localization, and function of individual TLRs are still unclear. Intestinal epithelial cells (IECs) in the human large intestine have been thought to express most of the TLRs. However, in vitro data has shown that TLR expression and signaling in IECs appear to be down-regulated (3–5). In contrast, up-regulation of TLR expression in IECs has been seen in chronic inflammatory conditions. In the intestinal mucosa of patients with inflammatory bowel disease (IBD), one of the typical chronic inflammatory conditions of the intestine, increased expression of TLR4 has been reported by immunofluorescent methods (6). Others, however, have reported that expression of TLR4 and TLR2 is only increased in lamina propria macrophages in IBD by IHC detection (7). Although the reason of this discrepancy is still unclear, both results imply TLR signaling in the intestinal mucosa, especially by TLR4, may be increased in certain situations.
Since expression level of TLR4 is normally very low, assaying for changes in its expression is sometimes difficult because of the limitations of detection methods. Although polymerase chain reaction (PCR) based method has an advantage to detect such fine biological changes, results of intestinal expression of TLR4 by PCR method usually vary within the whole mucosa. Both IEC and infiltrating immune cells potentially express TLR4 making the expression level vary according to how many TLR4 expressing cells exist in each sample. IHC may be a good tool to overcome this problem since we may look at expression of TLR4 in various cell types in a larger tissue sample. We have attempted to detect expression changes of TLR4 by IHC in the human intestine, specifically in colorectal cancer samples. In this chapter, we describe our current techniques used to enhance detection of the extremely low level of TLR4 protein within tissue samples while attempting to reduce nonspecific antibody reactions.
2. Materials
2.1. Immunoflourescence for TLR4
Xylenes, certified A.C.S. (Fisher Scientific, Fair Lawn, NJ). Xylene is flammable and should be stored in an appropriate location at room temperature.
Graded series of ethanol (Pharmco-Aaper, Brookfield, CT) 100%, 95% and 75%. Store with flammables at room temperature.
3% hydrogen peroxide diluted in methanol stored at 4°C. Replace after 10 uses. We create this working concentration by diluting 30% hydrogen peroxide (Fisher Scientific, Fair Lawn, NJ) 1:10 in methanol (Fisher Scientific, Fair Lawn, NJ). Total amount of solution should be adjusted to size of wash basin.
10 mM citrate buffer (pH 6.0): We make a stock solution of 5×10mM citrate buffer pH 6.0 by mixing 19.2g of anhydrous citric acid (VWR International, West Chester, PA) in 2 liters of deionized water adjusted to pH 6.0 and then prepare working concentrations as need by diluting 1:5 with deionized water. Both stock and working solutions can be stored at room temperature. Working solution should be replaced once a significant amount of specimen tissue is seen floating, usually after 5 to 10 uses.
Phosphate buffered saline 10× (PBS) (Fisher Scientific, Fair Lawn, NJ). Create 1× (0.1M) working concentration by diluting in deionized water and store at room temperature.
NaBH4 (Sigma-Aldrich, St. Louis, MO) mixed at a concentration of 1mg/ml in PBS buffered to pH 8.0. This solution must be made fresh.
Skim milk diluted 1:20 (5%) in PBS. Can be stored as working concentration stock at 4°C for two weeks.
Primary antibody: Biotin-conjugated mouse anti-human TLR4 monoclonal immunoglobulin (HTA125) (eBioscience, San Diego, CA). Stored at 4°C.
Fluorescein (FITC) conjugated Streptavidin (eBioscience, San Diego, CA). Stored at 4°C protected from light.
0.02% Triton X-100 (working concentration): We create a stock of 10% Triton X-100 (Fisher Scientific, Fair Lawn, NJ) diluted in deionized water and then add the appropriate amount depending on the volume of the wash buffer. For example, we add 460 microliters of 10% Trition X-100 to 230 ml of wash buffer. Store stock at room temperature.
SlowFade Gold antifade reagent (Invitrogen, Carlsbad, CA) stored at 4°C protected from light. Place at room temperature 30 minutes prior to use.
Cover slips 20×40 (Fisher Scientific, Fair Lawn, NJ).
2.2. Immunohistochemitry for TLR4
Xylenes, certified A.C.S. (Fisher Scientific, Fair Lawn, NJ). Xylene is flammable and should be stored in an appropriate location at room temperature.
Graded series of ethanol (Pharmco-Aaper, Brookfield, CT) 100%, 95% and 75%. Store with flammables at room temperature.
10 mM citrate buffer (pH 6.0): We make a stock solution of 5×10mM citrate buffer pH 6.0 by mixing 19.2g of anhydrous citric acid (VWR International, West Chester, PA) in 2 liters of deionized water adjusted to pH 6.0 and then prepare working concentrations as need by diluting 1:5 with deionized water. Both stock and working solutions can be stored at room temperature. Working solution should be replaced once a significant amount of specimen tissue is seen floating, usually after 5 to 10 uses.
3% hydrogen peroxide diluted in methanol stored at 4°C. Replace after 10 uses. We create this working concentration by diluting 30% hydrogen peroxide (Fisher Scientific, Fair Lawn, NJ) 1:10 in methanol (Fisher Scientific, Fair Lawn, NJ). Total amount of solution should be adjusted to size of wash basin.
Phosphate buffered saline 10× solution (PBS) (Fisher Scientific, Fair Lawn, NJ). Create 1× (0.1M) working concentration by diluting in deionized water and store at room temperature.
Avidin/Biotin blocking kit (Zymed, Carlsbad, CA) stored at 4°C. (see Note 1)
- Tyramide Signal Amplification (TSA) Biotin System (Perkin Elmer/ NEN Life Science, Boston MA). The entire kit is stored at 4°C and is stable for 6 months according to the manufacturer. Multiple components of this kit are utilized in this staining methodology (see Note 2):
- Blocking buffer is made, as directed by manufacturer, by adding the blocking reagent supplied in the TSA kit to 0.1M Tris-HCl (VWR International, West Chester, PA), pH 7.5 with 0.15M NaCl (Fisher Scientific, Fair Lawn, NJ) while heating gradually to 60°C. The blocking buffer contains caffeine and high purity casein. The process of making this buffer can take a few hours. Blocking buffer can be stored as single use aliquots at −20°C. Thaw prior to use.
- Streptavidin-horseradish peroxidase (Streptavidin-HRP) is provided with kit but alternative streptavidin-HRP may be substituted.
- Biotinyl Tyramide is provided as a powder that is reconstituted with dimethyl sulfoxide (DMSO) molecular biology grade (Fisher Scientific, Fair Lawn, NJ) as specified by the particular TSA kit, which come in different sizes. Thaw prior to use.
Primary antibody: rabbit anti-human TLR4 polyclonal immunoglobulin (Imgenex, San Diego, CA) stored at 4°C.
1% Albumin, bovine serum (BSA) (Sigma-Aldrich, St. Louis, MO) in PBS. Store at 4°C and discard if white flakes appear in solution or if solution becomes cloudy.
Wash buffer: 0.5M NaCl in PBS (Fisher Scientific, Fair Lawn, NJ). We make 1× PBS (0.1M) first and then add 290 grams of NaCl to 2 liters of 1× PBS and mix for 20 minutes or until dissolved and then add to rest of stock. Store at room temperature.
0.02% Triton X-100 (working concentration): We create a stock of 10% Triton X-100 (Fisher Scientific, Fair Lawn, NJ) diluted in deionized water and then add the appropriate amount depending on the volume of the wash buffer. For example, we add 460 microliters of 10% Trition X-100 to 230 ml of wash buffer. Store stock at room temperature.
Secondary antibody: biotin-conjugated goat F(ab’)2 anti-rabbit monoclonal immunoglobulin (Invitrogen, Carlsbad, CA) stored at 4°C. (see Note 3)
3,3’ diaminobenzidine tetrahydrochloride (DAB) (Sigma-Aldrich, St. Louis, MO). DAB comes as a powder packaged in a 100ml bottle and should be reconstituted by injecting 10ml of deionized water through rubber bottle cap. The solution can then be aliquot and stored as single use aliquots at −20°C. DAB is a suspected carcinogen and during handling direct contact should be avoided.
0.01M sodium azide (N3Na) in 0.05M Tris HCl buffer pH 7.6: dissolve N3Na (Sigma-Aldrich, St. Louis, MO) and Tris HCl (VWR International, West Chester, PA) in deionized water and store at room temperature. (see Note 4)
DAB mixture: mix 2ml 0.01M sodium azide (N3Na) in 0.05M Tris HCl buffer pH 7.6, 2 microliters 30% hydrogen peroxide, and 100 microliters DAB. Vortex. Make fresh immediately prior to visualization reaction.
Hematoxylin (Sigma-Aldrich, St. Louis, MO). Store at room temperature.
Cytoseal XYL (Richard-Allan Scientific, Kalamazoo, MI) stored at room temperature.
Cover slips 20×40 (Fisher Scientific, Fair Lawn, NJ).
3. Methods
TLR4 is a key regulator of both inflammation and epithelial homeostasis in the human intestine. TLR4 is normally down-regulated in the gastrointestinal tract in order to prevent continuous activation of the innate immune system. However, under certain pathologic conditions, TLR4 expression appears to be increased. Therefore, the ability to assay for this receptor may impact understanding of an important mechanism in the pathogenesis of gastrointestinal diseases.
The intestine is a complex organ with a diverse array of cell types in addition to the normally high concentration of commensal microorganisms. This presents a unique challenge when assaying for TLR4 in that its expression is typically low and there is the potential for non-specific staining in an environment so rich in antigens. We will describe our current methods to detect TLR4, which usually has a low level of expression, in human intestinal tissues. We have developed both immunoflourescence (IF) and IHC methods. These have similar applications but can be used to complement one another (Table2a, 2b). Our technique includes antigen retrieval, enhancement positive signals, inhibition of nonspecific binding of antibodies, and reduction of general background staining.
Table 2.
|
Table 2a: Advantages and disadvantages of immunohistochemistry | |
|---|---|
| Immunohistochemistry | |
| Advantages | Disadvantages |
| Allows for better | Formalin fixation |
| cellular | can increase |
| localization of | background |
| staining | |
| Difficult to quantify | |
| Able to more | differences in |
| easily archive | staining intensity |
| stained tissue | between samples |
|
Table 2b: Advantages and disadvantages of immunoflourescence | |
|---|---|
| Immunoflourescence | |
| Advantages | Disadvantages |
| Easier to quantify | Slides fade |
| differences in | |
| staining intensity | More difficult to |
| between samples | archive |
3.1. Immunoflourescence for TLR4
-
1.
Fill three wash basins with xylene. Place paraffin-embedded human tissue slides in slide holder and immerse in xylene for 5 minutes a total of 3 times, once in each basin. This step should be done under a hood since xylene gives off a strong odor. Insufficient removal of paraffin may lead to increase in background staining.
-
2.
Fill two wash basins with 100% ethanol, two wash basins with 95% ethanol, and one with 75% ethanol. Transfer slides to first 100% ethanol wash basin. Immerse slides in 100% ethanol for 5 minutes twice, once in each basin, followed by two washes in 95% ethanol, once in each basin for 5 minutes each, and finally one wash in 75% ethanol for 5 minutes. We recommend transferring the slides from xylene to ethanol in the hood. After the final ethanol wash, place slides in a basin filled with deionized water for 5 minutes to wash away ethanol.
-
3.
Fill a 1000ml beaker with 500ml of 10 mM citrate buffer (pH 6) and immerse slides in slide rack in solution. Place beaker in a standard household microwave and bring to a boil at high power. This usually takes 5 minutes. Once solution is brought to a boil, stop the microwave and open door until bubbles disappear. Then restart microwave on high power and bring to a boil again (usually another 15–20 seconds). Stop microwave and open door. Repeat this step for a total of 5 additional boils. Remove beaker from microwave and let cool at room temperature for at least 20 minutes. Slides may cool in citrate buffer for as long as necessary. However, after 20 minutes the beaker may be placed in cool tap water to speed up the cooling process. Once beaker is cool to touch, remove slides and place in wash basin with deionized water for 3 minutes. (see Note 5).
-
4.
Place slides in NaBH4 for 15 minutes and then wash for 3 minutes in PBS. Small bubbles of hydrogen gas should be seen. This step can inhibit autofluorescence caused by formaldehyde.
-
5.
Apply avidin blocker to each slide. Ensure that entire tissue is covered by gently spreading the solution with a pipette. Place slides in a humidified chamber for 10 minutes at room temperature. Then gently rinse slides with deionized water and place in PBS wash twice for 3 minutes each.
-
6.
Apply biotin blocker to each slide. Ensure that entire tissue is covered by gently spreading the solution with a pipette. Place slides in a humidified chamber for 10 minutes at room temperature. Then gently rinse slides with deionized water and place in PBS wash twice for 3 minutes each.
-
7.
Apply 5% milk solution to each slide. Ensure that entire tissue is covered by gently spreading the solution with a pipette. Place slides in a humidified chamber for 30 minutes at room temperature. Following incubation, gently tap side of slide on paper towel to remove excess blocking buffer prior to applying primary antibody. Do not wash slides. To block non-specific binding at this step, 5–10% normal serum corresponding to the animal from which the secondary antibody was made is typically used. However, since we do not use a secondary antibody we utilized milk instead.
-
9.
Apply primary antibody at a 1:500 dilution in 1% BSA in PBS. 100 microliters per slide is usually sufficient. Ensure that entire tissue is covered by gently spreading the solution with a pipette. Place slides in a humidified chamber and incubate overnight at 4°C.
-
10.
The next morning, gently rinse slides with deionized water and wash in PBS 3 times for 5 minutes each.
-
11.
Apply streptavidin-FITC diluted 1:200 in PBS. 100 microliters per slide is usually sufficient. Ensure that entire tissue is covered by gently spreading the solution with a pipette. Place slides in a humidified chamber and incubate for 1.5 hours at room temperature. From this step on, make sure slides are protected from light (incubation chamber should be opaque or wrapped in tin foil as should wash basins).
-
12.
Wash slides in PBS 3 times for 5 minutes each. Then wash slides once in PBS with 0.02% Triton X-100 for 5 minutes. Finally, wash again in PBS 3 times for 5 minutes each.
-
13.
Remove slides and wipe away excess PBS. Apply SlowFade Gold antifade reagent onto each tissue sample and cover with cover slip. Seal sides of cover slip with clear nail polish and protect from light. Visualize with immunofluorescence microscope. We recommend viewing slides the day after mounting with antifade reagent to allow sufficient time for tissue to absorb this reagent. (Figure 1)
Figure 1.
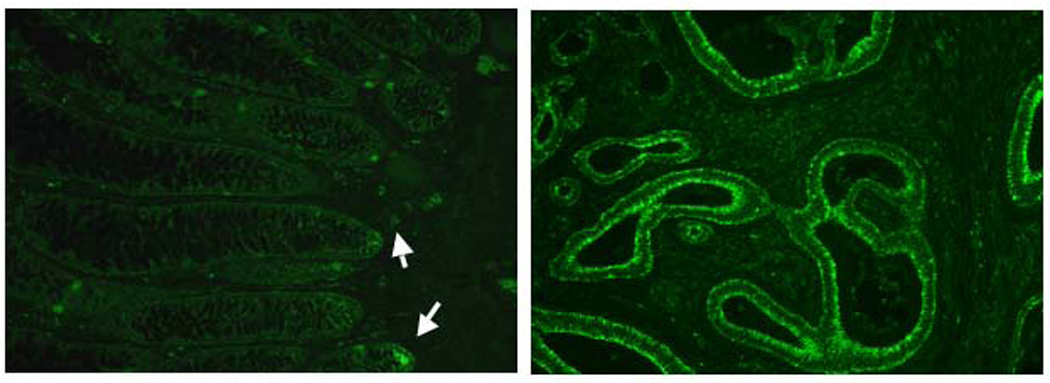
Normal IEC (left) are weakly positive for TLR4 at the base of crypts (arrow). Several scattered lamina propria cells are also positive for TLR4 in the normal large intestine. Several colon cancer specimens showed strong positivity of TLR4 in IEC (right).
3.2 Immunohistochemistry for TLR4
Fill three wash basins with xylene. Place paraffin-embedded human tissue slides in slide holder and immerse in xylene for 5 minutes a total of 3 times, once in each basin. This step should be done under a hood since xylene gives off a strong odor. Insufficient removal of paraffin may lead to increase in background staining.
Fill two wash basins with 100% ethanol, two wash basins with 95% ethanol, and one with 75% ethanol. Transfer slides to first 100% ethanol wash basin. Immerse slides in 100% ethanol for 5 minutes twice, once in each basin, followed by two washes in 95% ethanol, once in each basin for 5 minutes each, and finally one wash in 75% ethanol for 5 minutes. We recommend transferring the slides from xylene to ethanol in the hood. After the final ethanol wash, place slides in a basin filled with deionized water for 5 minutes to wash away ethanol.
Fill a 1000ml beaker with 500ml of 10 mM citrate buffer (pH 6) and immerse slides in slide rack in solution. Place beaker in a standard household microwave and bring to a boil at high power. This usually takes 5 minutes. Once solution is brought to a boil, stop the microwave and open door until bubbles disappear. Then restart microwave on high power and bring to a boil again (usually another 15–20 seconds). Stop microwave and open door. Repeat this step for a total of 5 additional boils. Remove beaker from microwave and let cool at room temperature for at least 20 minutes. Slides may cool in citrate buffer for as long as necessary. However, after 20 minutes the beaker may be placed in cool tap water to speed up the cooling process. Once beaker is cool to touch, remove slides and place in wash basin with deionized water for 3 minutes.
Place slides in 3% hydrogen peroxide diluted in methanol for 15 minutes then rinse away hydrogen peroxide from slides by dipping in basin filled with deionized water and wash in PBS three times for 3 minutes each. (see Note 6)
Apply avidin blocker to each slide. Ensure that entire tissue is covered by gently spreading the solution with a pipette. Place slides in a humidified chamber for 10 minutes at room temperature. Then gently rinse slides with deionized water and place in PBS wash twice for 3 minutes each.
Apply biotin blocker to each slide. Ensure that entire tissue is covered by gently spreading the solution with a pipette. Place slides in a humidified chamber for 10 minutes at room temperature. Then gently rinse slides with deionized water and place in PBS wash twice for 3 minutes each.
Apply blocking buffer to each slide, 100 microliters per slide is usually sufficient. Ensure that entire tissue is covered by gently spreading the solution with a pipette. Place slides in a humidified chamber for 30 minutes at room temperature. Following incubation, gently tap side of slide on paper towel to remove excess blocking buffer prior to applying primary antibody. Do not wash slides.
Apply primary antibody at a 1:2000 dilution in 1% BSA in PBS. 100 microliters per slide is usually sufficient. Ensure that entire tissue is covered by gently spreading the solution with a pipette. Place slides in a humidified chamber and incubate overnight at 4°C. The next morning, prior to rinsing and washing slides, let stand for at room temperature for 30 minutes. If slides appear dry, increase amount of primary antibody applied in subsequent experiments. Gently rinse slides with PBS and place in wash solution 3 times for 5 minutes each. The second of the 3 washes should include 0.02% Triton X-100. (see Notes 7, 8)
Apply biotin-conjugated secondary antibody at a 1:1000 dilution in 1% BSA in PBS. 100 microliters per slide is usually sufficient. Ensure that entire tissue is covered by gently spreading the solution with a pipette. Place slides in a humidified chamber and incubate at room temperature for 30 minutes. Gently rinse slides with PBS and place in wash solution 3 times for 5 minutes each. The second of the 3 washes should include 0.02% Triton X-100.
Apply streptavidin-HRP at a 1:100 dilution in 1% BSA in PBS. 100 microliters per slide is usually sufficient. Ensure that entire tissue is covered by gently spreading the solution with a pipette. Place slides in a humidified chamber and incubate at room temperature for 30 minutes. Gently rinse slides with deionized water and place in wash solution 3 times for 5 minutes each. The second of the 3 washes should include 0.02% Triton X-100.
Apply tyramide at a 1:50 dilution in amplification diluent supplied in TSA biotin system kit. 100 microliters per slide is usually sufficient. Ensure that entire tissue is covered by gently spreading the solution with a pipette. Place slides in a humidified chamber and incubate at room temperature for 10 minutes. Gently rinse slides with deionized water and place in wash solution 3 times for 5 minutes each. The second of the 3 washes should include 0.02% Triton X-100.
Apply streptavidin-HRP again at a 1:100 dilution in 1% BSA in PBS. 100 microliters per slide is usually sufficient. Ensure that entire tissue is covered by gently spreading the solution with a pipette. Place slides in a humidified chamber and incubate at room temperature for 30 minutes. Gently rinse slides with deionized water and place in wash solution 3 times for 5 minutes each. The second of the 3 washes should include 0.02% Triton X-100.
Freshly prepare DAB mixture (while slides are undergoing final wash). Remove slides one by one from wash basin, dry back of slide and apply DAB. 100 microliters per slide is usually sufficient. Ensure that entire tissue is covered by gently spreading the solution with a pipette. Place slide under a light microscope to monitor for development of dark brown reaction product. Once tissue has developed to desired intensity, interrupt staining by gently rinsing with deionized water over waste cup. Place in a separate wash basin with deionized water. Repeat for all slides. Make sure to dispose of remaining DAB mixture and contents of waste cup in an appropriate organic waste container. (see Note 9)
Place slide rack with developed slides in a larger basin and place under running tap water for 1–2 minutes. The basin should be large enough so that the stream of water does not directly touch the slides.
Place slides in hematoxylin for 40 seconds and rinse under tap water until water is running clear of hematoxylin. (see Note 10)
Dip slides 2–3 times in 0.5% acid alcohol to remove excess hematoxylin and rinse under tap water again for 15–30 seconds.
Dip slides 8–10 times in ammonia water to set hematoxylin blue and rinse under tap water again for 15–30 seconds.
Transfer slides to wash basin filled with 95% ethanol twice, one minute each. Then place slides in 100% ethanol twice, one minute each. Lastly, place slides in xylene basin three times, one minute each.
Remove slides individually from xylene and wipe away xylene on front and back of slide (do not touch tissue sample). Place two drops of cytoseal at base of slide, apply cover slip at an angle so as to begin to spread the cytoseal, and slowly lower the cover slip onto the tissue while cytoseal spreads underneath. Repeat for all slides. (see Note 11) (Figure 2).
Figure 2.
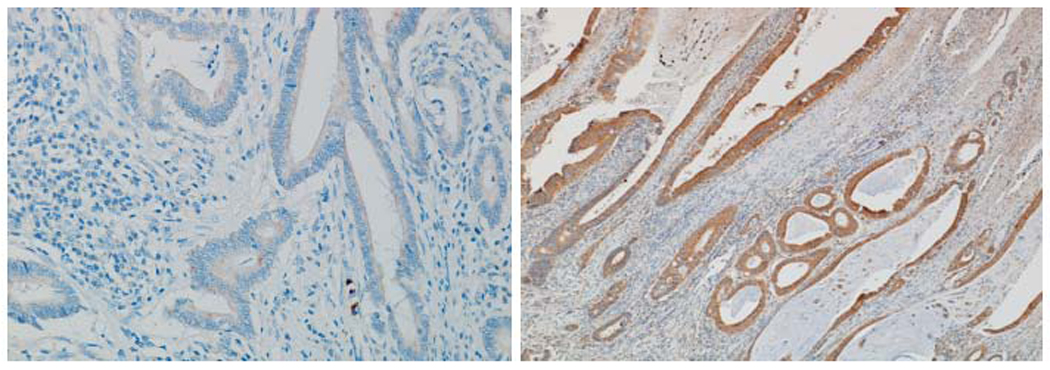
Comparison between streptavidin-HRP method (left) with tyramide amplification method (right) in a colon cancer specimen. Notice the marked difference in intensity of staining.
4. Notes
We have found it necessary to use an avidin and biotin blockers because endogenous biotin can cause false positives in immunohistochemistry methods that utilize streptavidin to bind biotin-conjugated antibodies. As detailed by Mount, et al, endogenous biotin is present in many human tissues with abundant mitochondria (8). This was of importance in our colon cancer studies because many cancers have high levels of endogenous biotin (Figure 3).
We have found the TSA Biotin System to significantly increase the sensitivity of detecting antigen. Multiple tyramide molecules deposit at sites where HRP is present, such as where streptavidin-HRP is bound to biotin-conjugated secondary antibodies. Since the tyramide is biotinylated, this results in greater amounts of biotin at sites of immunreactivity (9). (Figures 4a, b). A study by Toda, et al reported a ten-fold signal enhancement compared to unamplified slides without an increase in background (10). However, we have found that tyramide can increase background when using higher concentrations of antibodies. This effect may also be due to insufficient blockade of endogenous peroxidase in the tissue. In fact, one of the advantages of tyramide is that it allows for conservation of expensive antibody since lower concentrations can provide a strong signal. Because the tyramide is subject to repeated freeze-thaw cycles, we have suspected that it may become ineffective after many uses. If staining suddenly stops working, one potential problem may be the activity of the tyramide stock.
Using an F(ab’)2 antibody fragment instead of a full antibody reduces the chance of non-specific binding and in our experience results in less background staining. The Fc portion of antibodies may react non-specifically and thus increase chance increased background and false positives (Figure 5).
Using 0.01M sodium azide (N3Na) in 0.05M Tris HCl buffer pH 7.6 as the diluent for the DAB mixture used in the visualization step results in reduced background staining by inhibiting development of excess HRP. Adding a very small amount of sodium azide may decease background created by endogenous peroxidase (11).
This step is used for antigen retrieval. During the fixation process, target proteins or antigens may “shrivel” and become inaccessible to detection antibodies. Antigen retrieval denatures the protein enough to expose enough of the molecule to allow binding. We prefer microwave antigen retrieval to using enzymes such as 0.1% trypsin, 0.05% pronase, 0.4% pepsin because it allows for greater preservation of tissue integrity.
Placing the slides in hydrogen peroxide serves to block endogenous peroxidase activity, which is important in any system that utilizes horseradish peroxidase conjugated reagents to avoid false positives. Furthermore, this step is of particular importance in the intestine where there are high levels of endogenous peroxidase. In our experience with human intestine tissue, 15 minutes is ample time to block endogenous peroxidase activity while avoiding tissue damage.
We recommend titrating the dilution of primary and secondary antibodies to optimize balance between strength of primary signal and background staining. As an alternative to this polyclonal primary antibody we have tried the monoclonal antibody utilized for the immunoflourescence method described above at a dilution of 1:50 (manufacturer recommends titrating antibody in range of 1–10 µg/ml). Instead of incubating overnight at 4°C we have found that the signal is stronger when incubating overnight at 20°C with minimal change in background. In this case, it is important to ensure that the slides do not dry out and it is often necessary to apply extra antibody. However, we have had inconsistent results with the monoclonal antibody in the immunhistochemistry method with greater batch-to-batch variability. We suspect that this antibody is more labile when incubated at 20°C. To confirm specificity of primary antibodies, we recommend using primary antibody in Western blot analysis. If unexpected bands are seen by this method, consider possibility of false positive reaction in the staining (Figure 6).
We have found that using the 0.5 NaCl in PBS wash in combination with 0.02% Triton X-100 leads to a much cleaner stain by decreasing the amount of dust and other contaminating particles adhering to the tissue. Hypertonic saline may block nonspecific binding of antibodies by electronic charge within the tissue. This technique appears to be more effective at washing away reagents in between steps leading to reduced background and edge staining.
During this step, each slide must be carefully monitored so that once the intensity of staining is sufficient the DAB mixture is rinsed off. Leaving DAB on the slide for too long may create extra background. We have generally found that strongly positive slides will develop within 5 minutes. However, there can be much tissue-to-tissue variability. One factor to be aware of is that formalin-fixed tissue that was left in formalin for an extended period of time will be more prone to increased non-specific staining and these slides will need to have DAB removed more quickly.
Counterstaining can be adjusted to create desired contrast between staining and non-staining tissue as well as for cellular localization.
Let slides sit to dry under a hood. Moving slides prior to drying of cytoseal may cause cover slips to become displaced and may damage underlying tissue. For this immunohistochemistry method, we recommend using it as a qualitative measure of TLR4 expression instead of as a quantitative measure. This is because certain steps in the staining process can lead to batch-to-batch variability in staining intensity. For example, leaving DAB on longer in one batch may make the same slide appear to be more intensely positive than when stained in a previous batch. Therefore, this is a good method of establishing whether a tissue is positive or negative for TLR4 but it is difficult to subsequently quantify the staining intensity.
Figure 3.
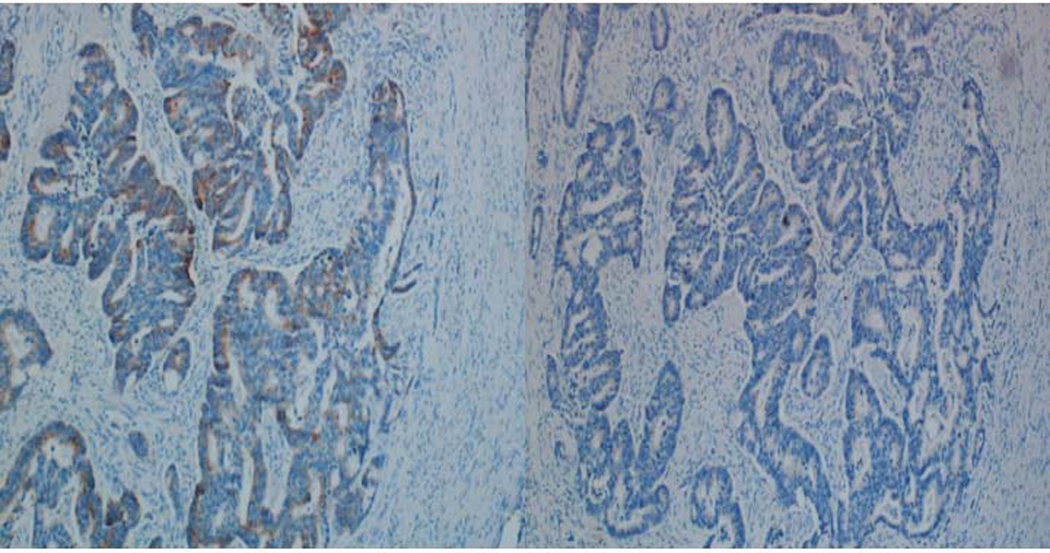
Comparison between slides omitting primary antibody without avidin and biotin blockers (left) and with avidin and biotin blockers (right).
Figure 4.
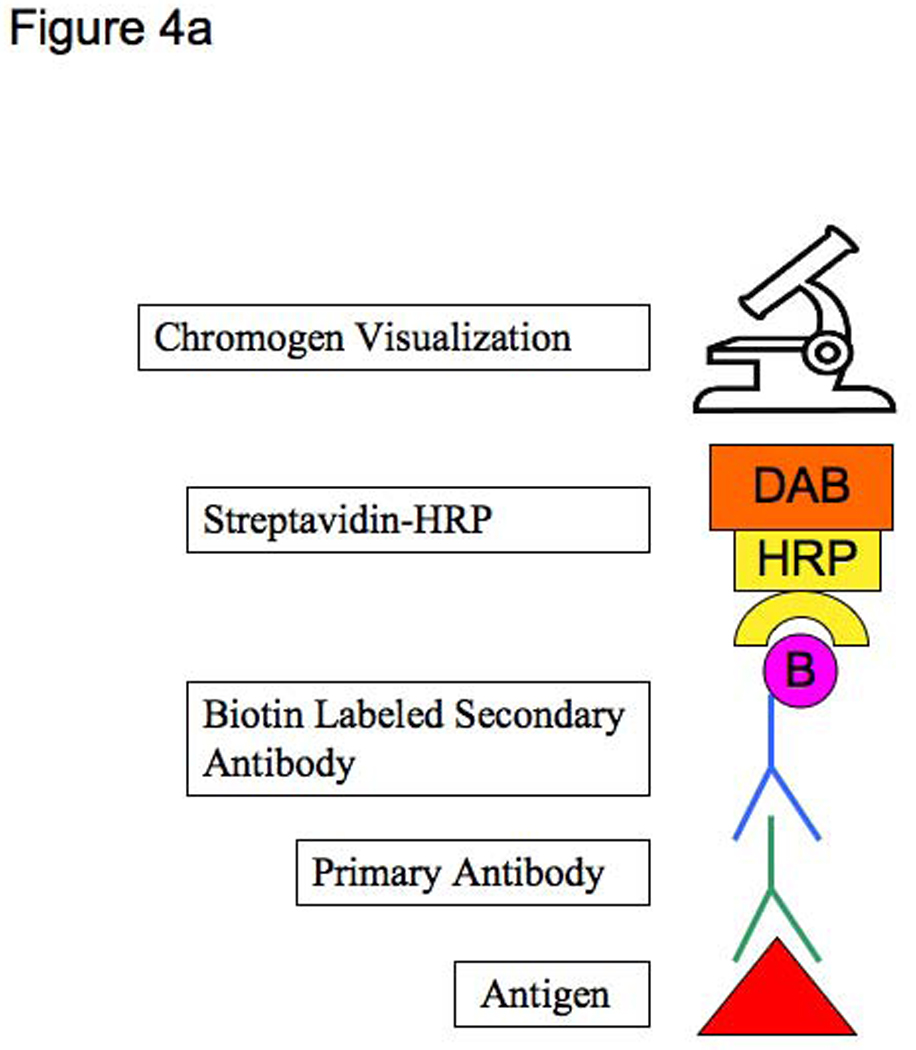
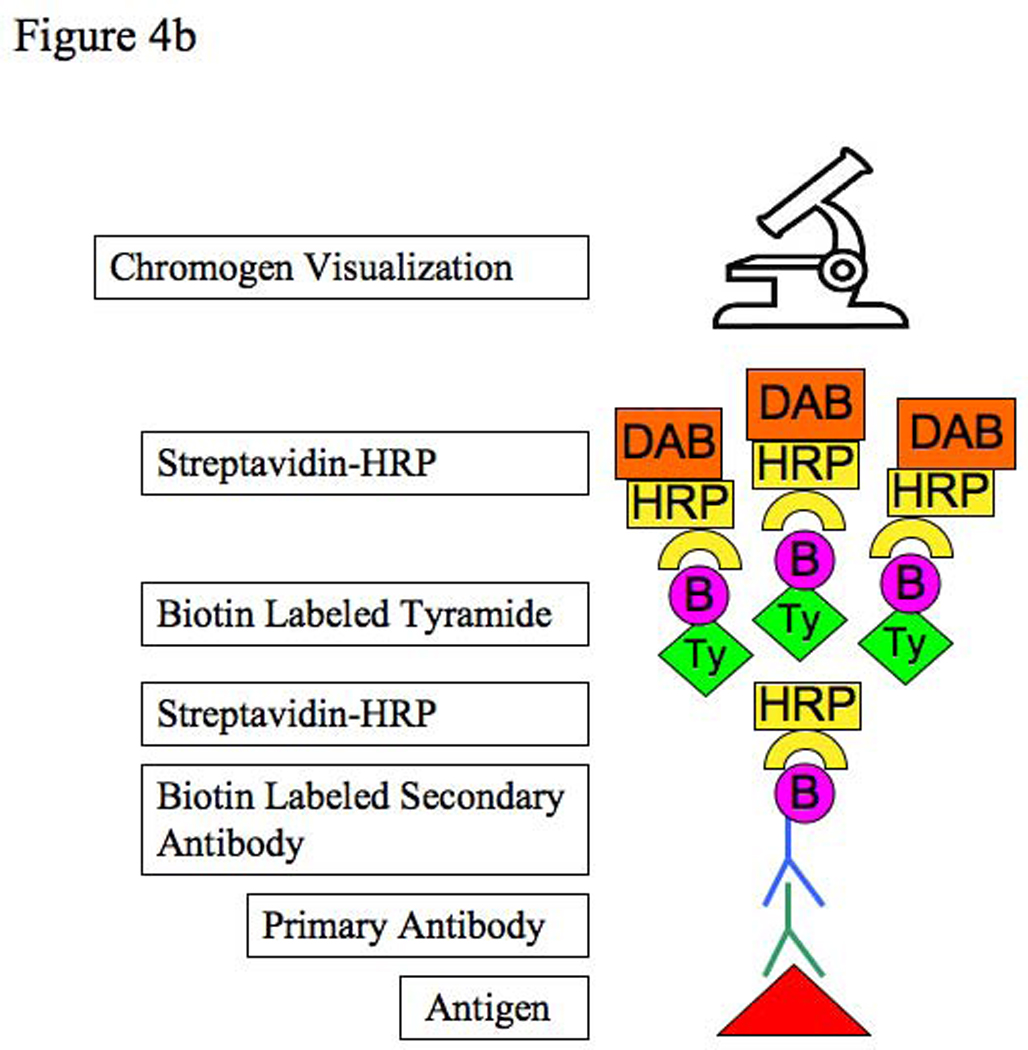
Figure 4a: Standard streptavidin-HRP immunohistochemistry method
Figure 4b: Tyramide amplification immunohistochemistry method
Figure 5.
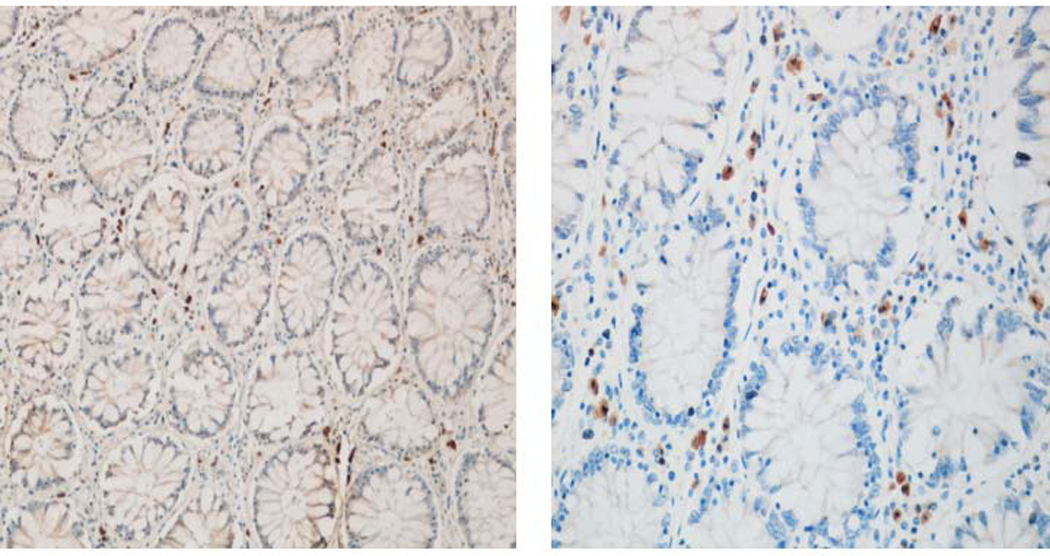
Comparison of full IgG secondary antibody (left) with an F(ab’)2 secondary antibody (right) with reduction in background staining.
Figure 6.
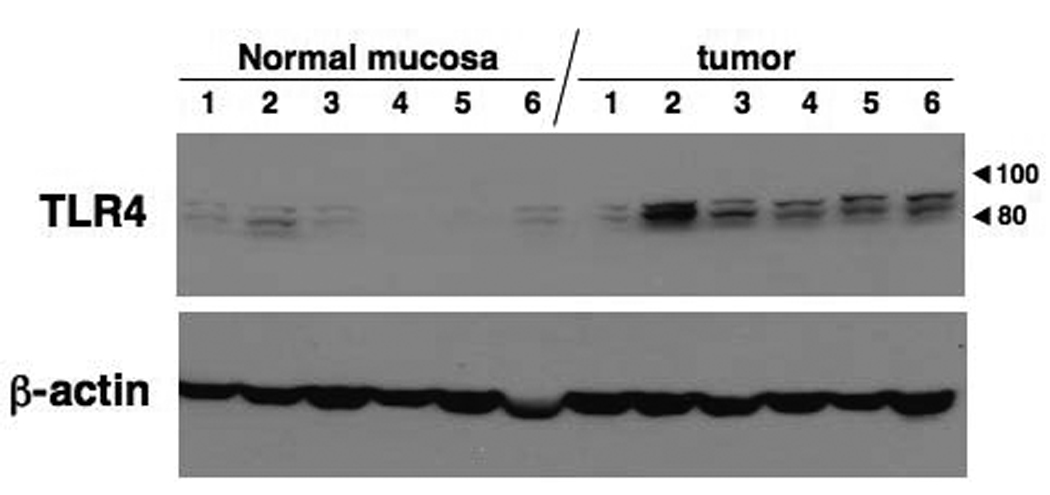
Western blot analysis for human large intestinal samples (6 each normal portion and cancer portion). Molecular weight of TLR4 is 90–100 kDa according to attachment of its accessory molecule MD2. Two bands are expected by TLR4 and TLR4/MD2 complex.
Table 1.
Comparison of Immunohistochemistry and Immunoflourscence Major Steps
| Immunohistochemistry Method | Immunoflourescence Method |
|---|---|
|
|
Note: perform appropriate rinses and washes in between these main steps.
Acknowledgements
We would like to thank Anli Chen for her technical assistance.
References
- 1.Medzhitov R, Preston-Hurlburt P, Kopp E, Stadlen A, Chen C, Ghosh S, Janeway CA., Jr MyD88 is an adaptor protein in the hToll/IL-1 receptor family signaling pathways. Mol Cell. 1998;2:253–258. doi: 10.1016/s1097-2765(00)80136-7. [DOI] [PubMed] [Google Scholar]
- 2.O'Neill LA, Dunne A, Edjeback M, Gray P, Jefferies C, Wietek C. Mal and MyD88: adapter proteins involved in signal transduction by Toll-like receptors. J Endotoxin Res. 2003;9:55–59. doi: 10.1179/096805103125001351. [DOI] [PubMed] [Google Scholar]
- 3.Melmed G, Thomas LS, Lee N, Tesfay SY, Lukasek K, Michelsen KS, Zhou Y, Hu B, Arditi M, Abreu MT. Human intestinal epithelial cells are broadly unresponsive to Toll-like receptor 2-dependent bacterial ligands: implications for host-microbial interactions in the gut. J Immunol. 2003;170:1406–1415. doi: 10.4049/jimmunol.170.3.1406. [DOI] [PubMed] [Google Scholar]
- 4.Otte JM, Cario E, Podolsky DK. Mechanisms of cross hyporesponsiveness to Toll-like receptor bacterial ligands in intestinal epithelial cells. Gastroenterology. 2004;126:1054–1070. doi: 10.1053/j.gastro.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 5.Abreu MT, Vora P, Faure E, Thomas LS, Arnold ET, Arditi M. Decreased expression of Toll-like receptor-4 and MD-2 correlates with intestinal epithelial cell protection against dysregulated proinflammatory gene expression in response to bacterial lipopolysaccharide. J Immunol. 2001;167:1609–1616. doi: 10.4049/jimmunol.167.3.1609. [DOI] [PubMed] [Google Scholar]
- 6.Cario E, Podolsky DK. Differential alteration in intestinal epithelial cell expression of toll-like receptor 3 (TLR3) and TLR4 in inflammatory bowel disease. Infect Immun. 2000;68:7010–7017. doi: 10.1128/iai.68.12.7010-7017.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hausmann M, Kiessling S, Mestermann S, Webb G, Spottl T, Andus T, Scholmerich J, Herfarth H, Ray K, Falk W, Rogler G. Toll-like receptors 2 and 4 are up-regulated during intestinal inflammation. Gastroenterology. 2002;122:1987–2000. doi: 10.1053/gast.2002.33662. [DOI] [PubMed] [Google Scholar]
- 8.Mount SL, Cooper K. Beware of biotin: a source of false-positive immunohistochemistry. Current Diagnostic Pathology. 2001;7:161–167. [Google Scholar]
- 9.King G, Payne S, Walker F, Murray GI. A highly sensitive detection method for immunohistochemistry using biotinylated tyramine. J Pathol. 1997;183:237–241. doi: 10.1002/(SICI)1096-9896(199710)183:2<237::AID-PATH893>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 10.Toda Y, Kono K, Abiru H, Kokuryo K, Endo M, Yaegashi H, Fukumoto M. Application of tyramide signal amplification system to immunohistochemistry: a potent method to localize antigens that are not detectable by ordinary method. Pathol Int. 1999;49:479–483. doi: 10.1046/j.1440-1827.1999.00875.x. [DOI] [PubMed] [Google Scholar]
- 11.Tabata H, Yamakage A, Yamazaki S. Cutaneous localization of endothelin-1 in patients with systemic sclerosis: immunoelectron microscopic study. Int J Dermatol. 1997;36:272–275. doi: 10.1046/j.1365-4362.1997.00171.x. [DOI] [PubMed] [Google Scholar]


