Abstract
Objective
To better understand the dynamics of breast milk HIV shedding and its relation to postnatal HIV transmission, we investigated the temporal and lateral relations of breast milk viral shedding and sodium concentrations in HIV-positive women.
Design
This was a longitudinal cohort study in Lusaka, Zambia.
Method
We examined patterns of HIV shedding in breast milk over the first 4 months of breast-feeding and their correlations with postnatal HIV transmission among 138 breast-feeding mothers. Sodium concentration in breast milk was also examined in the same samples and in breast milk from 23 HIV-negative controls.
Results
Higher breast milk viral load at 1 week, 1 month, and 4 months and consistent viral shedding in breast milk were significantly associated with increased risk of HIV transmission. Elevated breast milk sodium concentration ($13 mmol/L) at 4 months was associated with HIV transmission, low maternal CD4 cell count, and high maternal plasma viral load. Elevated sodium concentration at 1 week postpartum was common and was not associated with any of these parameters.
Conclusions
Consistent viral shedding and high breast milk viral load are strong predictors of mother-to-child HIV transmission. Although sodium concentrations later in breast-feeding correlate with breast milk viral load, increased breast milk sodium is normal in early lactation and does not predict HIV transmission.
Keywords: breast-feeding, breast milk sodium, HIV, lactation, mastitis, mother-to-child transmission, viral load
In low-resource settings in which the HIV/AIDS epidemic predominates, most HIV-infected women breast-feed their children because breast milk alternatives are unavailable, unaffordable, or unsafe.1–3 Short-course regimens of antiretroviral drugs recommended for use in these settings reduce only slightly, if at all, risks of early postnatal HIV transmission.4–6 Improved understanding of the biology of breast-feeding transmission and associated risk factors may strengthen counseling programs, enabling women to make informed choices, and may assist with the design of new strategies to reduce postnatal transmission. These include, for example, new laboratory tests or risk assessment algorithms applied in clinical settings to identify women at highest risk of transmitting HIV to their children.
The quantity of HIV, cell-associated and cell-free virus, detectable in breast milk has been identified as a strong correlate of mother-to-child transmission (MTCT) of HIV.7–13 Although discrepancies between breast milk viral load from left and right breasts have been described,14 there are only limited data on the magnitude and frequency of these differences and their consequence on HIV transmission. There is also evidence that mastitis and other clinical breast pathologic conditions increase the risk of MTCT.15,16 Subclinical mastitis, defined by elevated sodium concentration in mature breast milk, has been correlated with increased breast milk viral shedding17–19 and shown to be a predictor of postnatal transmission.20 Breast-feeding is a dynamic process, with changes in breast milk composition and volume over time as lactation is established; however, little is known about these changes in the presence of HIV.21 To date, no studies have examined lateral or temporal variation of sodium levels in relation to HIV transmission.
To better understand the role of viral shedding, which is defined here as the quantity of HIV RNA detected in breast milk, and elevated sodium in MTCT, we investigated lateral (between breasts) and temporal (over the first 4 months) dynamics of viral load and sodium concentrations in breast milk in relation to HIV transmission. This analysis is possible in our cohort because breast milk samples were simultaneously taken from both breasts during multiple visits, along with sequential and frequent infant dried blood spot samples to determine timing of HIV infection.
METHODS
This study includes the first 171 women recruited as part of the Zambia Exclusive Breast-Feeding Study (ZEBS) who had live births during the first 9 months of the study (April 2001 to January 2002); details of the ZEBS are described elsewhere.22 Briefly, HIV-positive pregnant women were enrolled into a randomized clinical trial designed to assess the impact on HIV transmission and child mortality of exclusive breast-feeding for 4 months with abrupt weaning (group A) versus exclusive breast-feeding for 6 months with normal weaning with the full duration of breast-feeding based on the mother’s informed choice (group B). All women and children were given single-dose nevirapine (NVP) to reduce the risk of intrapartum transmission. Women were randomized to group A or group B and were seen every week to 1 month, every month to 6 months, and then every 3 months until 24 months postpartum. A small number of HIV-negative women were also enrolled. All women signed informed consent forms approved by all the human subjects review boards of the investigators’ institutions.
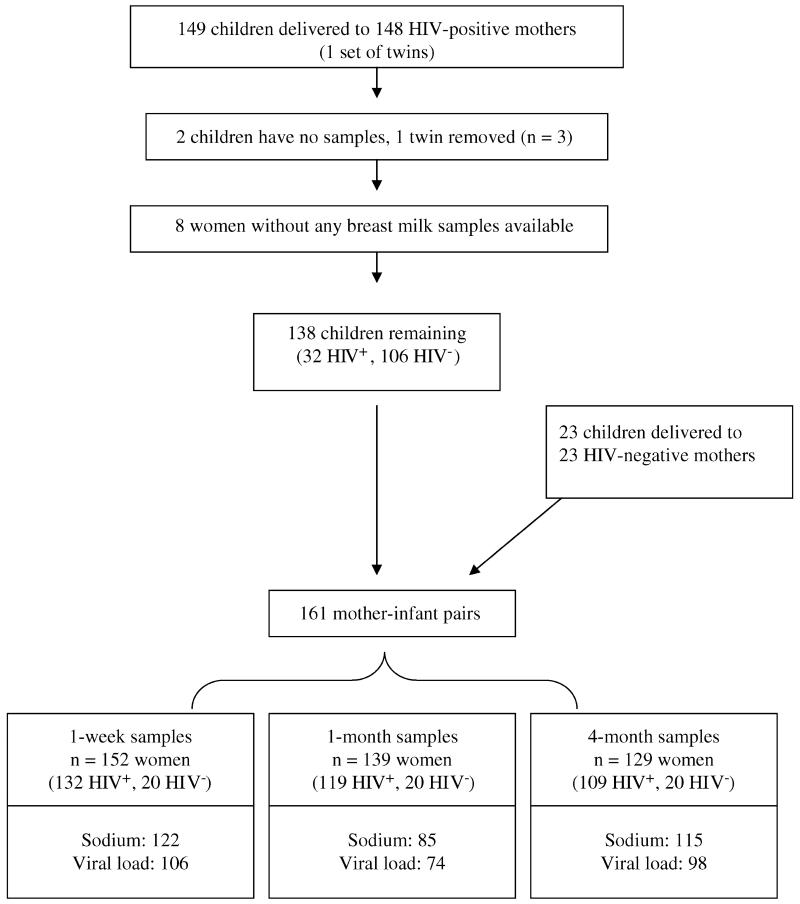
The 171 women included 148 HIV-infected and 23 HIV-uninfected women. Of the 149 children (1 twin pair) born to 148 HIV-infected women, 2 children had no samples for HIV testing, 1 child of the twin pair was selected for inclusion (HIV status of the pair was concordant), and 8 children were excluded because maternal breast milk samples were not collected. Thus, 138 children born to HIV-infected women were included in the analysis. During the same time period, 23 HIV-negative women delivered and completed follow-up (Fig. 1).
FIGURE 1.
Flow diagram of mother-infant pairs included in the analysis of breast milk viral load and sodium at 1 week, 1 month, and 4 months postpartum.
Of the 138 children born to HIV-infected women, 32 (23.2%) tested positive for HIV-1 DNA by polymerase chain reaction (PCR) assay on dried blood spots collected through 24 months of age. Seven children had positive results within 48 hours of birth and were presumed to be infected in utero.23 Twenty had their first positive test result by 70 days of age, with an earlier HIV-negative result (range: 0 to 35 days) and were presumed to be infected intrapartum or in the early postnatal period. Five children had their first positive test result at >71 days of age and were presumed to be infected through breast-feeding; the last HIV-negative result occurred for these 5 children at 3 months, 4 months, 6 months (2 children), and 15 months.
Maternal blood samples were collected at study enrollment during pregnancy and were tested for HIV-1 RNA levels (Amplicor 1.5 RNA Kit; Roche Molecular Systems, Branchburg, NJ), CD4 cell count (FacsCount; Becton Dickinson, San Jose, CA), and hemoglobin (β-Hemoglobin System; HemoCue, Lake Forest, CA). Breast milk samples were collected at every clinic visit while the mother continued to breast-feed; this analysis focuses on samples collected at 1 week, 1 month, and 4 months postpartum. Breast milk HIV-1 RNA quantification was performed using the Amplicor 1.5 RNA Kit, with a lower limit of detection of 50 copies/mL.24 Median levels of HIV reported here are calculated from the samples with detectable virus. Sodium concentrations in breast milk were assessed with an ion-selective electrode (Beckman Coulter Synchron LX 20; Beckman Coulter, Fullerton, CA). Conventionally, the lower limit of detection of the sodium assay is 13 mmol/L; however, Neville et al25 validated the ion-selective electrode method with flame photometry to a lower limit of 5 mmol/L in breast milk. Because of changes in laboratory reporting, exact sodium levels <13 mmol/L were not available on 10 (8.2%) women at 1 week, on 17 (20%) women at 1 month, and on 5 (4.3%) women at 4 months. The median value (<13 mmol/L) for the appropriate time point was used in place of the 32 samples missing exact sodium levels.
Of all women in this analysis who completed the relevant visits, 80.2% (122 of 152) of 1-week, 61.2% (85 of 139) of 1-month, and 89.1% (115 of 129) of 4-month samples had sodium measured. Of the HIV-infected women who completed visits, 80.3% (106 of 132) of 1-week, 62.2% (74 of 119) of 1-month, and 89.9% (98 of 109) of 4-month samples had viral load measured. Breast milk from the right breast was selected if available for each time point. A subset of milk samples from both breasts was tested for viral load and sodium to examine laterality (viral load: 101 pairs at 1 week, 64 pairs at 1 month, and 17 pairs at 4 months; sodium: 118 pairs at 1 week, 75 pairs at 1 month, and 18 pairs at 4 months).
Categoric variables were compared across groups using χ2 tests; continuous variables were compared with Kruskal-Wallis tests. Spearman rank order correlations were used to describe relations between continuous variables. Multivariate analysis of variables measured at 1 point in time only was performed using linear or logistic regression, as appropriate. If variables were measured at more than 1 time point in the same individual, generalized estimating equations (GEEs; proc genmod in SAS) were used. Data analysis was completed using SAS version 9.1 (SAS Institute, Cary, NC).
RESULTS
Descriptive Characteristics
The study included 138 HIV-infected and 23 HIV-uninfected mothers and their infants. HIV-uninfected women were of similar age but had lower parity than HIV-infected women. Among HIV-infected women at study enrollment, the median CD4 count was 367 cells/μL (interquartile range [IQR]: 227 to 530 cells/μL) and the median plasma viral load (pVL) was 45,418 copies/mL (IQR: 10,254 to 142,345 copies/mL). Transmitting (n = 32) and nontransmitting (n = 106) HIV-infected women had similar clinical characteristics; however, transmitters were more likely to have CD4 counts <200 cells/μL(P < 0.0001), hemoglobin <10 g/dL (P = 0.001), and pVL ≥100,000 copies/mL compared with nontransmitters (P = 0.0002) (Table 1).
TABLE 1.
Descriptive Characteristics of 138 HIV-Positive Women (32 Who Transmitted and 106 Who Did Not) and 23 HIV-Negative Women Recruited in Lusaka, Zambia
| Transmitters (n = 32) | Nontransmitters (n = 106) | P* | Controls (n = 23) | P† | |
|---|---|---|---|---|---|
| Maternal age (y) | |||||
| <20 | 3 (9.4) | 13 (12.3) | 6 (26.1) | ||
| 20 to 29 | 19 (59.4) | 76 (71.7) | 12 (52.2) | ||
| ≥30 | 10 (31.3) | 17 (16.0) | 0.16 | 5 (21.7) | 0.14 |
| Parity | |||||
| First child | 5 (15.6) | 19 (17.9) | 9 (39.1) | ||
| Second or third child | 21 (65.6) | 70 (66.0) | 10 (43.5) | ||
| Fourth or greater child | 6 (18.8) | 17 (16.0) | 0.91 | 4 (17.4) | 0.046 |
| Low hemoglobin (<10 g/dL) | 18 (56.3) | 27 (25.5) | 0.001 | 2 (8.7) | 0.02 |
| Low BMI at 1 mo postpartum (<18.5 kg/m2) | 5 (15.6) | 16 (15.1) | 0.97 | 3 (13.0) | 0.85 |
| Low birth weight (<2500 g) | 5 (15.6) | 10 (9.4) | 0.27 | 4 (17.4) | 0.39 |
| Among HIV+ women only | |||||
| CD4 count (cells/μL) | |||||
| <200 | 14 (43.8) | 15 (14.2) | |||
| 200 to 349 | 12 (37.5) | 24 (22.6) | |||
| 350 to 499 | 4 (12.5) | 29 (27.4) | |||
| ≥500 | 2 (6.3) | 38 (35.9) | <0.0001 | ||
| Median (IQR) | 224 (136 to 324) | 413 (290 to 565) | <0.0001 (KW test) | ||
| pVL (copies/mL) | |||||
| <10,000 | 3 (9.4) | 30 (28.3) | |||
| 10,000 to 99,999 | 9 (28.1) | 51 (48.0) | |||
| ≥100,000 | 20 (62.5) | 25 (23.6) | 0.0002 | ||
| Median (IQR) | 136,782 (41,551 to 213,508) | 33,201 (7638 to 86,022) | 0.0004 | ||
| HIV/AIDS stage III‡ | 17 (53.3) | 49 (30.4) | 0.29 | ||
| Received NVP at delivery | 29 (90.6) | 100 (94.3) | 0.46 | ||
Comparing transmitting and nontransmitting HIV-positive mothers.
Comparing HIV-positive and HIV-negative mothers.
World Health Organization clinical criteria stage III disease: diarrhea, cough, fever >30 days, tuberculosis, oral candidiasis, and severe weight loss.
BMI indicates body mass index; KW, Kruskal-Wallis.
Laterality of Breast Milk Viral Shedding
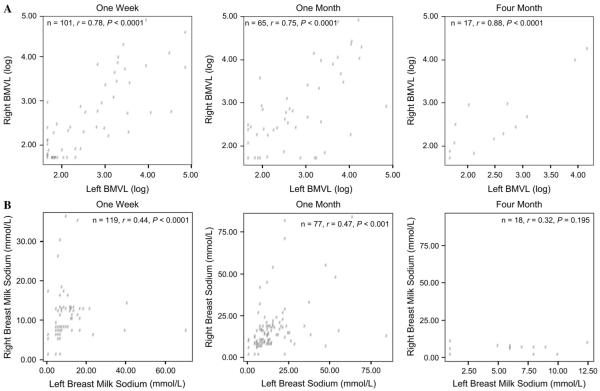
To compare breast milk composition between breasts, we examined breast milk viral load and sodium levels between left and right breasts. The consistency of viral shedding across left and right breasts within individual women at each visit was investigated (Table 2A; Fig. 2A). In HIV-infected women, breast milk viral loads of the right and left breasts were significantly correlated at all visits (1 week: n = 101, ρ = 0.78, P < 0.0001; 1 month: n = 65, ρ = 0.75, P < 0.0001; 4 months: n = 17, ρ = 0.88, P < 0.0001). At all visits, viral load in either breast was significantly correlated with maternal pVL and CD4 cell count at enrollment (data not shown). Detectable viral load was found in both breasts (laterally concordant viral shedders [CSs]) in 34.7% of women at 1 week, in 56.9% at 1 month, and in 64.7% at 4 months, whereas approximately a third of all women at each time point had a nondetectable viral load (viral load <50 copies/mL) in both breasts (concordant viral nonshedders [CNSs]). The proportion of women who had detectable virus in only 1 breast (discordant viral shedders [DSs]) was 19.8% at 1 week, 15.4% at 1 month, and 5.9% at 4 months. Median breast milk viral loads were similar across time in those who had detectable viral loads in 1 or both breasts (see Table 2A).
TABLE 2.
Laterality of Breast Milk HIV Viral Load (A) and Breast Milk Sodium Levels (B), Right Versus Left Breast, at 1 Week, 1 Month, and 4 Months Postpartum
| 1 Wk (n = 101) |
1 Mo (n = 65) |
4 Mo (n = 17) |
||||
|---|---|---|---|---|---|---|
| Detectable Viral Load | n (%) | Median (R, L) Copies/mL | n (%) | Median (R, L) Copies/mL | n (%) | Median (R, L) Copies/mL |
| Both breasts | 35 (34.7) | 544, 1241 | 37 (56.9) | 666, 1136 | 11 (64.7) | 289, 464 |
| Single breast | 20 (19.8) | 118, 74 | 10 (15.4) | 253, 429 | 1 (5.9) | 71 |
| Neither breast | 46 (45.4) | — | 18 (27.7) | — | 5 (29.4) | — |
| 1 Wk (n = 119) |
1 Mo (n = 77) |
4 Mo (n = 18) |
||||
|---|---|---|---|---|---|---|
| Elevated Sodium (mmol/L) | n (%) | Median (R, L) | n (%) | Median (R, L) | n (%) | Median (R, L) |
| Both breasts | 31 (26.1) | 18, 21 | 3 (5.9) | 14, 16 | ||
| Single breast | 38 (31.9) | 13,13 | 25 (32.5) | 13, 13 | ||
| Neither breast | 50 (42.0) | 8, 7 | 49 (63.6) | 7, 7 | 18 (100) | 5.5, 6 |
L indicates left; R, right.
FIGURE 2.
Laterality of breast milk HIV viral load (A) and breast milk sodium levels (B), right versus left breast, at 1 week, 1 month, and 4 months postpartum.
Laterally CSs were compared with 2 groups: DSs and CNSs. At 1 week, CSs had a lower CD4 count (<200 cells/μL; P = 0.022) and were more likely to have a maternal pVL > 100,000 copies/mL (P = 0.009) than DSs. Similar but nonsignificant associations comparing CSs with DSs with respect to pVL and CD4 cell count were observed at 1 and 4 months. CSs were more likely than CNSs to have a low CD4 count (<200 cells/μL) and high pVL (>100,000 copies/mL) at 1 week (CD4 cell count, P < 0.0001; pVL, P < 0.0001), 1 month (CD4 cell count, P = 0.0046; pVL, P = 0.017), and 4 months (CD4 cell count, P = 0.06; pVL, P = 0.005).
Among a subgroup of HIV-infected and uninfected women, sodium levels were significantly correlated across breasts at the 1-week and 1-month visits (see Table 2B; see Fig. 2B). Only 18 sodium levels from both breasts were available at the 4-month visit, limiting our capacity to distinguish if lateral correlation was present at this time point.
Breast Milk Viral Shedding Over Time
Of the 138 HIV-infected women, breast milk viral load was detectable in 44.3% of 1-week samples (n = 106, median = 487), 67.6% of 1-month samples (n = 74, median = 774), and 57.1% of 4-month samples (n = 98, median = 364). Breast milk viral shedding at 1 week was correlated with shedding at 1 month (n = 50, ρ = 0.67, P < 0.0001) and 4 months (n = 71, ρ = 0.51, P < 0.0001), and 1-month viral shedding correlated with shedding at 4 months (n = 54, ρ = 0.69, P < 0.0001). Maternal CD4 cell count but not pVL was significantly correlated with breast milk viral load at 1 week (CD4 cell count: ρ = −0.21, P = 0.03; pVL: ρ = −0.038, P = 0.70). Both factors were significantly associated with breast milk viral load at 1 month (CD4 cell count: ρ = −0.38, P = 0.0008; pVL: ρ = 0.25, P = 0.03), however, and most strongly at 4 months (CD4 cell count: ρ = −0.30, P = 0.0025; pVL: ρ = 0.33, P = 0.0010).
In regression models, after adjusting for pVL, CD4 cell count predicted breast milk viral load at 1 week (P = 0.058) and 1 month (P = 0.0007). After adjustment for maternal CD4 cell count, pVL predicted breast milk viral load at 1 month (P = 0.0181) and 4 months (P < 0.0001).
Of the 104 HIV-infected women with multiple breast milk viral load measurements over time, 39.4% had detectable virus in every breast milk sample, 30.8% had inconsistent viral shedding patterns, and 29.8% had an undetectable viral load in all the samples tested. Breast milk viral load from temporally consistent shedders was higher (median = 523 copies/mL at 1 week, median = 5775 copies/mL at 1 month, and median = 609 copies/mL at 4 months) than from inconsistent shedders (median = 180 copies/mL at 1 week [P = 0.25], median = 324 copies/mL at 1 month [P = 0.01], and median = 322 copies/mL at 4 months [P = 0.15]). Compared with inconsistent shedders, women who consistently shed virus in breast milk were more likely to have CD4 counts <200 cells/μL (46.3% vs. 12.5%; P = 0.0042) and pVLs >100,000 copies/mL (58.5% vs. 21.9%; P = 0.013). This was even more pronounced when comparing consistent shedders with consistent nonshedders (CD4 count <200 cells/μL [46.3% vs. 0%], P < 0.0001; pVL >100,000 copies/mL [58.5% vs. 4.4%], P < 0.0001).
Elevated Sodium in Breast Milk
Among 161 (HIV-infected and HIV-uninfected) women, the proportion with elevated breast milk sodium ($13 mmol/L) declined sharply from 41.8% at 1 week to 20.0% at 1 month and further to 8.7% at 4 months. During the first week of lactation, sodium levels <16 mmol/L are considered normal; at this threshold, 30.3% had elevated sodium levels at 1 week (Table 3).26 Sodium levels at 1 week and 1 month were positively correlated with 4-month levels (1 week to 4 months: n = 85, ρ = 0.30, P = 0.04; 1 month to 4 months: n = 61, ρ = 0.32, P = 0.01). Sodium levels at 1 week were positively correlated with 1-month levels (n = 57, ρ = 0.27, P = 0.04).
TABLE 3.
Predictors of Elevated Sodium in Breast Milk of 138 HIV-Positive and 23 HIV-Negative Breast-Feeding Women
| n (%) in Each Category With Elevated Sodium |
||||
|---|---|---|---|---|
| 1 Wk, n = 122 |
||||
| ≥13 mmol/L, n (%) | <16 mmol/L, n (%) | 1 Mo, n = 85 (>13 mmol/L), n (%) | 4 Mo, n = 115 (>13 mmol/L), n (%) | |
| Maternal HIV status | ||||
| Positive | 43/106 (40.6) | 30/106 (28.3) | 15/74 (20.3) | 7/97 (7.2) |
| Negative | 8/16 (50) | 7/16 (43.8) | 2/11 (18.2) | 3/18 (16.7) |
| Breast problems | ||||
| Yes | 8/16 (56.2) | 7/16 (43.8) | 3/8 (37.5) | 1/2 (50) |
| No | 43/106 (40.6) | 30/106 (28.3) | 14/77 (18.2) | 9/113 (7.9)* |
| HIV-Positive Mothers Only |
||||
|---|---|---|---|---|
| n = 106 | n = 106 | n = 74 | n = 97 | |
| CD4 count (cells/μL) | ||||
| <200 | 11/28 (39.3) | 7/28 (25.0) | 4/12 (33.3) | 1/19 (5.3) |
| 200 to 349 | 7/24 (29.2) | 6/24 (25.0) | 6/22 (27.3) | 6/25 (24.0) |
| 350 to 499 | 12/25 (48.0) | 8/25 (32.0) | 4/16 (25.0) | 0/22 (0) |
| ≥500 | 13/29 (44.8) | 9/29 (31.0) | 1/24 (4.2) | 0/31 (0)* |
| pVL (copies/mL) | ||||
| <10,000 | 13/26 (50.0) | 9/26 (34.6) | 0/18 (0) | 0/23 (0) |
| 10,000 to 99,999 | 18/44 (40.9) | 12/44 (27.3) | 7/36 (19.4) | 2/43 (4.7) |
| ≥100,000 | 12/36 (33.3) | 9/36 (25.0) | 8/20 (40.0)† | 5/31 (16.1)* |
| Breast milk viral load (copies/mL)‡ | ||||
| <50 | 18/58 (31.0) | 10/58 (17.2) | 2/24 (8.3) | 0/42 (0) |
| ≥50 | 25/46 (54.3)* | 20/46 (43.5)* | 12/45 (26.7) | 7/55 (12.7)* |
| Parity | ||||
| First child | 7/17 (41.2) | 4/17 (23.5) | 4/9 (44.4) | 3/12 (25.0) |
| Second or third child | 33/74 (44.6) | 24/74 (32.4) | 11/51 (21.6) | 4/65 (6.2) |
| Fourth or greater child | 3/15 (20.0) | 2/15 (13.3) | 0/14 (0)† | 0/20 (0)† |
| Breast problems§ | ||||
| Yes | 6/13 (46.2) | 4/13 (30.7) | 2/7 (28.6) | 1/2 (50.0) |
| No | 37/93 (39.8) | 26/93 (28.0) | 13/67 (19.4) | 6/95 (6.3)† |
P < 0.05
P < 0.01.
Breast milk viral load information is missing for 2 1-week samples and 5 1-month samples.
Defined as engorgement, red/shiny breasts, cracked/bleeding nipples, painful breast(s), blocked duct(s), abscess, and/or symptoms of Candida.
Regardless of sampling time, breast milk sodium levels did not differ by maternal HIV infection status (see Table 3). The slightly higher proportion of HIV-negative women with elevated sodium at 4 months was probably explained by a small excess of HIV-negative mothers with first-born children. In our cohort, lower parity was associated with increased risk of elevated sodium in breast milk.
Among HIV-infected women (n = 138), breast milk sodium concentrations were moderately correlated with breast milk viral load at all visits (1 week: n = 104, ρ = 0.29, P = 0.003; 1 month: n = 69, ρ = 0.35, P = 0.003), with the strongest correlation observed at the 4-month visit (n = 97, ρ = 0.42, P < 0.0001). Lower CD4 cell count and higher pVL were associated with elevated sodium in breast milk at 1 and 4 months, but neither was at 1 week (see Table 3).
Breast problems (defined as engorgement, red/shiny breasts, cracked/bleeding nipples, painful breast[s], blocked duct[s], abscess, and/or symptoms of Candida) were detected in 15 (11.3%) of 132 HIV-positive women at the 1-week postpartum visit. Breast problems were unilateral in 10 of 15 women at 1 week and in all women at 1 month (n = 11) and 4 months (n = 3). Of these HIV-infected women with breast problems, 13 of 15 had their sodium level assessed at 1 week, 7 of 11 at 1 month, and 2 of 3 at 4 months. At 1 and 4 months postpartum, elevated sodium was more common (20.3% at 1 month and 7.2% at 4 months) than reported or observed breast problems (9.5% at 1 month and 2.8% at 4 months) (see Table 3). Strong associations were observed between elevated sodium and clinical breast problems at 4 months, but there were no significant associations at the earlier visits.
Mother-to-Child Transmission of HIV
Breast milk viral shedding at all visits was significantly related to MTCT of HIV (Table 4). In 3 separate multivariate logistic regression models for each visit adjusting for CD4 cell count and pVL, detectable breast milk viral load at 1 week (odds ratio [OR] = 3.50, 95% confidence interval [CI]: 1.71 to 7.17), at 1 month (OR = 3.22, 95% CI: 1.52 to 6.80), and at 4 months (OR = 5.49, 95% CI: 2.13 to 14.23) was associated with HIV transmission. CD4 cell count was also significantly associated with transmission after adjusting for breast milk viral load at 1 week and 4 months (1 week: β = −0.004, P = 0.013; 4 months: β = −0.0052, P = 0.037). pVL was not associated with transmission after adjusting for CD4 cell count and breast milk viral load in these models.
TABLE 4.
Breast Milk HIV Viral Load and Sodium Concentration at 1 Week, 1 Month, and 4 Months as Predictors of MTCT of HIV Among 138 HIV-Infected Breast-Feeding Women
| 1 Wk |
1 Mo |
4 Mo |
||||
|---|---|---|---|---|---|---|
| Breast Milk Characteristic | n (%) Total |
n (%) Who Transmitted |
n (%) Total |
n (%) Who Transmitted |
n (%) Total |
n (%) Who Transmitted |
| Breast milk RNA | ||||||
| <50 copies/mL | 59 (56) | 6 (10.1) | 24 (68) | 3 (12.5) | 42 (43) | 1 (2.4) |
| ≥50 copies/mL | 47 (44) | 22 (46.8)* | 50 (32) | 19 (38.0)‡ | 56 (57) | 21 (37.5)* |
| Lateral consistency | ||||||
| Breast milk viral load detectable in both breasts | 35 (35) | 18 (51.4) | 37 (57) | 11 (29.7) | 11 (65) | 5 (45.5) |
| Only 1 | 20 (20) | 5 (25.0) | 10 (15) | 2 (20.0) | 1 (6) | 0 (0) |
| Neither | 46 (47) | 3 (6.5)* | 18 (28) | 1 (5.6)‡ | 5 (29) | 0 (0) |
| Breast milk sodium | ||||||
| <13 mmol/L | 63 (59) | 16 (25.4) | 59 (80) | 12 (20.4) | 90 (93) | 16 (17.8) |
| ≥13 mmol/L | 43 (41) | 12 (27.9) | 15 (20) | 5 (33.3) | 7 (7) | 5 (71.4)* |
| Breast problems | ||||||
| Unilateral | 10 (9) | 5 (50.0) | 11 (9) | 4 (36.4) | 3 (3) | 2 (66.7) |
| Bilateral | 5 (4) | 1 (20.0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| None | 117 (87) | 25 (21.4) | 108 (91) | 26 (24.1) | 104 (97) | 25 (24.0) |
| Detectable breast milk viral load in both breasts at >1 time point | ||||||
| Yes | 15 (11) | 8 (53.3) | — | — | — | — |
| No | 123 (89) | 24 (19.5)† | ||||
| Breast milk viral shedding (over time) | ||||||
| Never detectable viral load | 31 (30) | 2 (6.5) | — | — | — | — |
| Intermittently detectable viral load | 32 (31) | 5 (15.6) | ||||
| Consistently detectable viral load | 41 (39) | 20 (48.8)* | ||||
P < 0.0001
P < 0.01
P < 0.05
Consistency of viral shedding laterally across breasts and temporally across visits was associated with increased risk of transmission (see Table 4). Women with detectable viral loads in both breasts at more than 1 time point were more likely to transmit HIV to their children (OR = 3.4, 95% CI: 1.08 to 10.79; P = 0.03). In a regression model, including multiple time points, detectable breast milk viral load at more than 1 time point was predictive of transmission (OR = 3.35, β = 1.21; P = 0.0393) independent of breast milk viral load at individual time points.
Elevated breast milk sodium at 4 months was related to HIV transmission (P = 0.001), but elevated sodium at 1 week and 1 month was not. Excluding women with breast problems, the significant association between elevated sodium at 4 months and transmission remained (P = 0.002). There was a nonsignificant trend toward increased HIV transmission with unilateral breast problems.
Breast milk viral load was elevated among all transmitters regardless of the route of transmission (Table 5). There was a suggestion that the association between breast milk viral shedding and intrauterine transmission was slightly weaker than that observed for intrapartum and postnatal transmission. Nearly all (93% to 100%) transmitting mothers whose children had negative results by PCR at birth had high levels of HIV in breast milk at 1 and 4 months postpartum.
TABLE 5.
Breast Milk Viral Shedding by Timing of Infant Infection in a Cohort of 138 HIV-Infected Zambian Breast-Feeding Women
| Breast Milk Viral Shedding (HIV RNA Copies/mL) |
||||||
|---|---|---|---|---|---|---|
| 1 Wk, n = 106 |
1 Mo, n = 74 |
4 Mo, n = 98 |
||||
| n (%) >50 of Those Tested* |
Median† | n (%) >50 of Those Tested* |
Median† | n (%) >50 of Those Tested* |
Median† | |
| All infected | 22/28 (78.6) | 1830 | 19/22 (86.4) | 18,277 | 21/22 (95.5) | 4037 |
| In utero‡ | 3/6 (50.0) | 11,274 | 3/5 (60.0) | 18,277 | 4/4 (100) | 4968 |
| Intrapartum/early postpartum§ | 15/17 (88.2) | 694 | 14/15 (93.3) | 21,437 | 12/13 (92.3) | 2227 |
| Postpartum∥ | 4/5 (80.0) | 5394 | 2/2 (100) | 2218 | 5/5 (100) | 10,570 |
| Uninfected | 25/78 (32.1) | 235 | 31/52 (59.6) | 314 | 35/76 (46.1) | 189 |
| P value* | <0.001 | 0.028 | 0.024 | <0.0001 | <0.001 | <0.0001 |
Comparing “all infected” with “uninfected” using χ2 test to compare number detectable and Kruskal-Wallis test to calculate median breast milk viral load.
Median calculated among samples with detectable virus only.
In utero indicates HIV-positive result within 48 hours of birth.
Intrapartum/early postpartum indicates first HIV test by 70 days, with HIV-negative result at earlier time point (range: 0 to 35 days).
Postpartum indicates first positive HIV test result at 71 days or older and HIV-negative test result at earlier time point.
DISCUSSION
Our data examining breast milk viral shedding and sodium concentrations at multiple visits over the first 4 months of breast-feeding provide a more refined understanding of the role of elevated sodium concentrations and the influence of breast milk viral load in postnatal HIV transmission. Many studies have identified the importance of breast milk viral load on the risk of postnatal MTCT of HIV, demonstrating that high viral load increases the risk of transmission.9,11,13,20 This analysis has the added benefit of assessing the temporal and lateral dynamics of breast milk HIV RNA and sodium levels, however. Breast milk composition and breast permeability change dramatically from the early breast-feeding period to mature milk; understanding these changes in relation to HIV over time is important to the development of appropriate counseling and risk analysis.
In our cohort, breast milk HIV viral load was strongly correlated between breasts and consistent viral shedding was the most predictive marker of transmission. Because of the relatively common occurrence of intermittent viral shedding even among transmitting women, however, the clinical utility of a single breast milk viral load measurement is limited.
Breast milk sodium levels reflect epithelial permeability and have been shown to be elevated when milk volumes are low, such as during the first week of lactation, weaning, and with breast inflammation. More than 40% of women reported here had sodium concentrations >13 mmol/L at 1 week postpartum (30.3% at the 16-mmol/L cutoff). Because of the dynamic nature of breast milk composition during the first days of lactation, sodium at 1 week was not associated with maternal CD4 cell count, maternal pVL, or HIV transmission. As breast-feeding stabilizes, sodium levels recede and become strongly associated with breast milk viral shedding and HIV transmission at 4 months, irrespective of CD4 cell count and pVL. In mature milk, sodium levels average 6 mmol/L and persistently elevated concentrations during later breast-feeding signify inflammatory processes that may manifest as clinically detectable breast pathologic conditions in some women.21,25 We observed elevated sodium levels in the absence of observed or recently reported breast problems in a substantial proportion of women, indicating the presence of subclinical mastitis. Thus, breast milk sodium measurement at later postnatal ages may be a sensitive, more rapid, and less costly surrogate marker of viral shedding in milk than direct HIV viral load testing. The pattern of breast milk sodium at 1 month was intermediate between the 1-week and 4-month observations, suggesting that sodium levels are less useful potential surrogates for subclinical mastitis, increased viral load, and risk of HIV transmission.
We expected to observe increased breast milk viral shedding at 1 week with increased mammary gland permeability during the establishment of lactation, but we did not. Because concentrations of innate immune factors in breast milk tend to be highest in early versus later milk,27 these anti-infective soluble factors may help to counteract the increased permeability that exists at this time, which would ordinarily result in higher breast milk viral load.
Alternatively, low viral load at 1 week might be explained by the continued antiviral activity of short-course NVP taken during labor on breast milk viral load at 1 week. NVP (half-life of 6 days) was consumed during labor by 93% of the HIV-infected women in the cohort, thus suppressing the 1-week breast milk viral load sample.28 Long-term highly active antiretroviral therapy (HAART) has been shown to reduce breast milk viral load, and breast milk viral load reductions from single-dose NVP have been reported in a study in Kenya.29–31 Although these data do not establish the impact of NVP on breast milk viral load at the 1-week visit, we observed similar proportions of undetectable breast milk viral load compared with other studies among similar populations in the absence of NVP exposure.7,12,32
Studies in Malawi and South Africa have reported associations between elevated breast milk sodium levels and increased risk of HIV transmission; however, they did not report the temporal variations.12,20 In the Malawi study, only sodium levels from 1 point in time (6 weeks postpartum) were available and clinical data on breast problems were not reported.20 In the South African study, sodium levels were aggregated over the first 14 weeks of breast-feeding without distinguishing between earlier and later samples.12 Our data refine these observations to highlight the developmental specificity of sodium as a marker in breast milk in relation to HIV transmission. Elevated sodium in early milk (1) reflects the physiologic onset of normal lactation, (2) is a poor predictor of HIV transmission, and (3) may correlate with soluble anti-infective factors of breast milk. Elevated sodium in later milk likely reflects clinical or subclinical mucosal pathologic change linked to an increased risk of HIV transmission. Later but not earlier measurements of sodium may be a useful and simple predictor of increased risk of postnatal HIV transmission.
Several studies that have measured viral shedding in mucosal compartments, including breast milk and genital tract secretions, have observed stronger associations between viral shedding at the mucosa and MTCT of HIV than between systemic pVL and transmission.33–35 In addition, others have shown cell-associated virus to be a stronger predictor of transmission.13 In our cohort, breast milk viral shedding at 1 week, 1 month, and 4 months was associated with increased risk of HIV transmission through all routes: in utero, intrapartum, and postpartum transmission. High breast milk viral loads may be an indicator of greater mucosal shedding at other sites. It is perhaps for this reason that breast milk viral load correlated more strongly than pVL with intrapartum HIV transmission, which is also thought to be attributable to exposure to HIV in mucosal fluids.36–38
Our findings demonstrate the capacity of the breast epithelium to prevent entry of HIV into breast milk in approximately one third of untreated women at individual times. If present, however, HIV remains in milk at relatively stable levels across breasts and over time. Breast milk viral load levels were highly concordant across breasts at each time point, suggesting the influence of systemic factors rather than local factors or inflammation in the regulation of breast epithelial cell permeability to HIV. Similarly, sodium levels were correlated across breasts but not strongly, suggesting the impact of local factors on sodium level. Our data confirm previous findings of escalation of HIV transmission with increased breast milk viral loads. Elevated sodium concentration in breast milk during established breast-feeding but not early (<1 month) breast-feeding was also a significant predictor of HIV transmission. It is important to investigate interventions, such as good lactation practices, that may reduce the likelihood of inflammatory breast pathologic findings associated with elevated sodium and viral load. Simple methods to determine sodium concentrations in breast milk at later postnatal ages may also be helpful to guide in counseling breast-feeding HIV-infected women.
ACKNOWLEDGMENTS
The authors thank the staff of the ZEBS for their tireless efforts, the study participants for their dedication to the study, and Don Decker for his technical assistance in the laboratory assays.
This research was supported by the National Institute for Child Health and Human Development, Bethesda, MD (RO1 HD39611 and R01 HD 40777) for the Zambia Exclusive Breast-Feeding Study. G. M. Aldrovandi is an Elizabeth Glaser Pediatric AIDS Foundation Scientist.
REFERENCES
- 1.De Cock KM, Fowler MG, Mercier E, et al. Prevention of mother-to-child HIV transmission in resource-poor countries: translating research into policy and practice. JAMA. 2000;283:1175–1182. doi: 10.1001/jama.283.9.1175. [DOI] [PubMed] [Google Scholar]
- 2.WHO. UNICEF. UNAIDS. UNFPA . HIV and Infant Feeding. Guidelines for Decision-Makers. World Health Organization; Geneva, Switzerland: 2003. [Google Scholar]
- 3.Labbok MH, Clark D, Goldman AS. Breastfeeding: maintaining an irreplaceable immunological resource. Nat Rev Immunol. 2004;4:565–572. doi: 10.1038/nri1393. [DOI] [PubMed] [Google Scholar]
- 4.Jackson JB, Musoke P, Fleming T, et al. Intrapartum and neonatal single-dose nevirapine compared with zidovudine for prevention of mother-to-child transmission of HIV-1 in Kampala, Uganda: 18-month follow-up of the HIVNET 012 randomised trial. Lancet. 2003;362:859–868. doi: 10.1016/S0140-6736(03)14341-3. [DOI] [PubMed] [Google Scholar]
- 5.John-Stewart G, Mbori-Ngacha D, Ekpini R, et al. Breast-feeding and transmission of HIV-1. J Acquir Immune Defic Syndr. 2004;35:196–202. doi: 10.1097/00126334-200402010-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leroy V, Karon JM, Alioum A, et al. Postnatal transmission of HIV-1 after a maternal short-course zidovudine peripartum regimen in West Africa. AIDS. 2003;17:1493–1501. doi: 10.1097/00002030-200307040-00010. [DOI] [PubMed] [Google Scholar]
- 7.Pillay K, Coutsoudis A, York D, et al. Cell-free virus in breast milk of HIV-1–seropositive women. J Acquir Immune Defic Syndr. 2000;24:330–336. doi: 10.1097/00126334-200008010-00006. [DOI] [PubMed] [Google Scholar]
- 8.Hoffman IF, Martinson FE, Stewart PW, et al. Human immunodeficiency virus type 1 RNA in breast-milk components. J Infect Dis. 2003;188:1209–1212. doi: 10.1086/378414. [DOI] [PubMed] [Google Scholar]
- 9.Lewis P, Nduati R, Kreiss JK, et al. Cell-free human immunodeficiency virus type 1 in breast milk. J Infect Dis. 1998;177:34–39. doi: 10.1086/513816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rousseau CM, Nduati RW, Richardson BA, et al. Association of levels of HIV-1-infected breast milk cells and risk of mother-to-child transmission. J Infect Dis. 2004;190:1880–1888. doi: 10.1086/425076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rousseau CM, Nduati RW, Richardson BA, et al. Longitudinal analysis of human immunodeficiency virus type 1 RNA in breast milk and of its relationship to infant infection and maternal disease. J Infect Dis. 2003;187:741–747. doi: 10.1086/374273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Willumsen JF, Filteau SM, Coutsoudis A, et al. Breastmilk RNA viral load in HIV-infected South African women: effects of subclinical mastitis and infant feeding. AIDS. 2003;17:407–414. doi: 10.1097/00002030-200302140-00015. [DOI] [PubMed] [Google Scholar]
- 13.Koulinska IN, Villamor E, Chaplin B, et al. Transmission of cell-free and cell-associated HIV-1 through breast-feeding. J Acquir Immune Defic Syndr. 2006;41:93–99. doi: 10.1097/01.qai.0000179424.19413.24. [DOI] [PubMed] [Google Scholar]
- 14.Willumsen JF, Newell ML, Filteau SM, et al. Variation in breastmilk HIV-1 viral load in left and right breasts during the first 3 months of lactation. AIDS. 2001;15:1896–1898. doi: 10.1097/00002030-200109280-00026. [DOI] [PubMed] [Google Scholar]
- 15.Embree JE, Njenga S, Datta P, et al. Risk factors for postnatal mother-child transmission of HIV-1. AIDS. 2000;14:2535–2541. doi: 10.1097/00002030-200011100-00016. [DOI] [PubMed] [Google Scholar]
- 16.Fawzi W, Msamanga G, Spiegelman D, et al. Transmission of HIV-1 through breastfeeding among women in Dar es Salaam, Tanzania. J Acquir Immune Defic Syndr. 2002;31:331–338. doi: 10.1097/00126334-200211010-00010. [DOI] [PubMed] [Google Scholar]
- 17.Semba RD, Kumwenda N, Taha TE, et al. Mastitis and immunological factors in breast milk of lactating women in Malawi. Clin Diagn Lab Immunol. 1999;6:671–674. doi: 10.1128/cdli.6.5.671-674.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gomo E, Filteau SM, Tomkins AM, et al. Subclinical mastitis among HIV-infected and uninfected Zimbabwean women participating in a multimicronutrient supplementation trial. Trans R Soc Trop Med Hyg. 2003;97:212–216. doi: 10.1016/s0035-9203(03)90124-6. [DOI] [PubMed] [Google Scholar]
- 19.Willumsen JF, Filteau SM, Coutsoudis A, et al. Subclinical mastitis as a risk factor for mother-infant HIV transmission. Adv Exp Med Biol. 2000;478:211–223. doi: 10.1007/0-306-46830-1_19. [DOI] [PubMed] [Google Scholar]
- 20.Semba RD, Kumwenda N, Hoover DR, et al. Human immunodeficiency virus load in breast milk, mastitis, and mother-to-child transmission of human immunodeficiency virus type 1. J Infect Dis. 1999;180:93–98. doi: 10.1086/314854. [DOI] [PubMed] [Google Scholar]
- 21.Neville MC, Neifert M, editors. Lactation: Physiology, Nutrition, and Breast-Feeding. Plenum Press; New York: 1983. [Google Scholar]
- 22.Thea DM, Vwalika C, Kasonde P, et al. Issues in the design of a clinical trial with a behavioral intervention—the Zambia exclusive breast-feeding study. Control Clin Trials. 2004;25:353–365. doi: 10.1016/j.cct.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 23.Bryson YJ, Luzuriaga K, Sullivan JL, et al. Proposed definitions for in utero versus intrapartum transmission of HIV-1. N Engl J Med. 1992;327:1246–1247. doi: 10.1056/NEJM199210223271718. [DOI] [PubMed] [Google Scholar]
- 24.Ghosh MK, Kuhn L, West J, et al. Quantitation of human immunodeficiency virus type 1 in breast milk. J Clin Microbiol. 2003;41:2465–2470. doi: 10.1128/JCM.41.6.2465-2470.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Neville MC, Keller RP, Seacat J, et al. Studies on human lactation. I. Within-feed and between-breast variation in selected components of human milk. Am J Clin Nutr. 1984;40:635–646. doi: 10.1093/ajcn/40.3.635. [DOI] [PubMed] [Google Scholar]
- 26.Allen JC, Keller RP, Archer P, et al. Studies in human lactation: milk composition and daily secretion rates of macronutrients in the first year of life. Am J Clin Nutr. 1991;54:69–80. doi: 10.1093/ajcn/54.1.69. [DOI] [PubMed] [Google Scholar]
- 27.Kuhn L, Trabattoni D, Kankasa C, et al. Alpha-defensins in the prevention of HIV transmission among breastfed infants. J Acquir Immune Defic Syndr. 2005;39:138–142. [PMC free article] [PubMed] [Google Scholar]
- 28.Bennetto-Hood C, Aldrovandi GM, King JR, et al. Persistence of nevirapine in breast milk after discontinuation of treatment nevirapine in breast milk. Clin Infect Dis. 2007;45:391–394. doi: 10.1086/519427. [DOI] [PubMed] [Google Scholar]
- 29.Shapiro RL, Holland DT, Capparelli E, et al. Antiretroviral concentrations in breast-feeding infants of women in Botswana receiving antiretroviral treatment. J Infect Dis. 2005;192:720–727. doi: 10.1086/432483. [DOI] [PubMed] [Google Scholar]
- 30.Shapiro RL, Ndung’u T, Lockman S, et al. Highly active antiretroviral therapy started during pregnancy or postpartum suppresses HIV-1 RNA, but not DNA, in breast milk. J Infect Dis. 2005;192:713–719. doi: 10.1086/432489. [DOI] [PubMed] [Google Scholar]
- 31.Chung MH, Kiarie JN, Richardson BA, et al. Breast milk HIV-1 suppression and decreased transmission: a randomized trial comparing HIVNET 012 nevirapine versus short-course zidovudine. AIDS. 2005;19:1415–1422. doi: 10.1097/01.aids.0000181013.70008.4d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Musoke P, Guay LA, Bagenda D, et al. A phase I/II study of the safety and pharmacokinetics of nevirapine in HIV-1-infected pregnant Ugandan women and their neonates (HIVNET 006) AIDS. 1999;13:479–486. doi: 10.1097/00002030-199903110-00006. [DOI] [PubMed] [Google Scholar]
- 33.Semba RD, Kumwenda N, Taha TE, et al. Mastitis and immunological factors in breast milk of human immunodeficiency virus-infected women. J Hum Lact. 1999;15:301–306. doi: 10.1177/089033449901500407. [DOI] [PubMed] [Google Scholar]
- 34.Panther LA, Tucker L, Xu C, et al. Genital tract human immunodeficiency virus type 1 (HIV-1) shedding and inflammation and HIV-1 env diversity in perinatal HIV-1 transmission. J Infect Dis. 2000;181:555–563. doi: 10.1086/315230. [DOI] [PubMed] [Google Scholar]
- 35.Tuomala RE, O’Driscoll PT, Bremer JW, et al. Cell-associated genital tract virus and vertical transmission of human immunodeficiency virus type 1 in antiretroviral-experienced women. J Infect Dis. 2003;187:375–384. doi: 10.1086/367706. [DOI] [PubMed] [Google Scholar]
- 36.Al Harthi L, Landay A. HIV in the female genital tract: viral shedding and mucosal immunity. Clin Obstet Gynecol. 2001;44:144–153. doi: 10.1097/00003081-200106000-00005. [DOI] [PubMed] [Google Scholar]
- 37.Mbori-Ngacha D, Richardson BA, Overbaugh J, et al. Short-term effect of zidovudine on plasma and genital human immunodeficiency virus type 1 and viral turnover in these compartments. J Virol. 2003;77:7702–7705. doi: 10.1128/JVI.77.13.7702-7705.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.John GC, Nduati RW, Mbori-Ngacha DA, et al. Correlates of mother-to-child human immunodeficiency virus type 1 (HIV-1) transmission: association with maternal plasma HIV-1 RNA load, genital HIV-1 DNA shedding, and breast infections. J Infect Dis. 2001;183:206–212. doi: 10.1086/317918. [DOI] [PubMed] [Google Scholar]