Abstract
Mounting evidence supports the tenet that innate immune responses to luminal microbes participate in the development of gastrointestinal malignancies. The gastrointestinal tract is relatively unique in that it has evolved in the presence of diverse enteric microflora. Intestinal flora is required to develop a normal adaptive immune response in the periphery. With the characterization of the innate immune system, we have begun to understand the adaptations the intestine has made to the microbiota. The interaction between the microbiota and the intestinal mucosa through toll-like receptors (TLRs) is required to maintain intestinal homeostasis. In particular, intestinal epithelial cells and lamina propria mononuclear cells such as antigen presenting cells and T cells must respond to breaches in the mucosal barrier by activating TLR-dependent pathways that result in increased epithelial proliferation, wound healing, and recruitment of acute inflammatory cells. In the setting of chronic inflammation such as Helicobacter pylori (H. pylori) infection in the stomach or idiopathic inflammatory bowel disease (IBD), the process of repair may eventually result in carcinogenesis. The following review highlights human and animal data that support a role for innate immune responses and TLRs specifically in promoting gastrointestinal malignancies. Candidate pathways linking TLRs to gastrointestinal malignancies include activation of NF-κB and cyclooxygenase-2 (Cox-2). Studying the link between innate immune signaling and gastrointestinal malignancies offers the possibility to identify novel ways to both prevent and treat gastrointestinal cancer.
Introduction
The gastrointestinal tract (including the esophagus, stomach, small and large intestine) is a unique organ system in that it has a higher incidence of cancer development and cancer-related mortality than any other system in the body (Parkin et al., 1999). The human gastrointestinal tract contains 10–100 trillion microorganisms, increasing along the gastrointestinal tract from stomach (−103), jejunum (−104), ileum (105–108), to the colon (1010–1014). Therefore, the intestinal mucosa plays a crucial role in maintaining barrier function and protecting the host against bacterial invasion while permitting enteric bacteria to aid in nutrient metabolism. Approximately 300 to 400 square meters of the gastrointestinal mucosal surface area are in direct contact with potential pathogens and carcinogens. However, our commensal bacteria also help to maintain mucosal homeostasis. For example, germ-free mice demonstrate reduced intestinal epithelial cell proliferation when compared to colonized mice (Nowacki, 1993; Pull et al., 2005). The lining of the gastrointestinal tract is normally replaced every two to seven days (Brittan & Wright, 2002; Leblond, 1964; Potten et al., 1997). Since increased cell turnover is associated with tumorigenesis, an imbalance in epithelial cell proliferation and death in response to the microflora may lead to the higher incidence of malignancies within the gastrointestinal tract.
Epidemiological studies suggest that chronic inflammation plays a significant role in the development of gastrointestinal malignancies. A variety of chronic inflammatory conditions, e.g. Barrett’s esophagus, ulcerative colitis, and chronic gastritis caused by H. pylori infection, markedly elevate the risk of developing gastrointestinal malignancies. Therefore, focusing on the relationship between chronic inflammation and carcinogenesis may help to elucidate important mechanisms in the pathogenesis of gastrointestinal malignancies. For example, chronic inflammation caused by H. pylori infection is associated with a higher risk of developing gastric cancer and it is considered a carcinogen (Houghton & Wang, 2005; Peek & Blaser, 2002). A strong link between inflammation and cancer development is observed in IBD, which includes Crohn’s disease and ulcerative colitis (Itzkowitz & Yio, 2004). Importantly, the severity of inflammation correlates with the risk of colorectal cancer in patients with IBD (Rutter et al., 2004; Gupta et al., 2007). In the pathogenesis of IBD, commensal bacteria play a central role of the chronic intestinal inflammation. Initiation and perpetuation of intestinal inflammation has been thought to result from dysregulated immune responses to commensal bacteria in the genetically-susceptible host. The genetic polymorphisms predisposing to IBD are at least partly due to an abnormality of innate immune recognition of commensal bacteria. Therefore, innate immune abnormalities in response to specific or nonspecific luminal bacteria may be responsible for gastrointestinal malignancies induced by chronic gastrointestinal inflammation.
Given the relationship between inflammation and carcinogenesis, investigators have begun to address the role of TLRs and innate immune responses in inflammation-associated carcinogenesis in the gastrointestinal tract (Huang et al., 2005; Huang et al., 2007; Xiao et al., 2007; Fukata et al., 2007). Although the adaptive immune system prevents tumor growth through immunesurveillance, the innate immune system may promote tumor growth through inflammation-dependent and –independent mechanisms (Balkwill & Coussens, 2004; de Visser & Coussens, 2005). In this review, we will discuss the role of innate immune responses, specifically TLR signaling, in the pathogenesis of gastrointestinal malignancies.
TLR signaling in the normal and inflamed gastrointestinal mucosa
TLRs serve important immune and non-immune functions in the human intestine. There are several layers that compose the intestine from stomach to colon. Adjacent to the lumen, and thus the microflora, there is a single layer of intestinal epithelial cells (IEC) that prevent the passage of macromolecules and bacteria. Below this layer the lamina propria consists of a mixed infiltrate of macrophages, dendritic cells, lymphocytes (primarily IgA producing B cells), myofibroblasts, and occasional neutrophils. Although, the expression of TLRs in the gastrointestinal tract has been examined, expression, localization, and function of individual TLRs remain unclear (Figure 1). For example, mucosal expression of TLRs in the esophagus has not yet been elucidated. In the human stomach, TLR2, TLR4, TLR5, and TLR9 are known to be expressed by epithelial cells (Baoprasertkul et al., 2007; Schmausser et al., 2004; Schmausser et al., 2005). The small intestine (including duodenum, jejunum, and ileum) and the large intestine express most of the TLRs, but normally TLR signaling in IECs appears to be down-regulated (Abreu et al., 2005; Melmed et al., 2003; Otte et al., 2004). However, altered expression of TLRs in IEC has been reported in the setting of chronic inflammation. Increased expression of TLR4 and MD-2 has been demonstrated in H. pylori infected gastric epithelial cells from human biopsy specimens and a gastric cancer cell line (Ishihara et al., 2004). TLR4 is up-regulated in IBD, while the expression of TLR2 and TLR5 remains unchanged (Cario & Podolsky, 2000). Expression of TLR4 and TLR2 is also increased in lamina propria macrophages in IBD (Hausmann et al., 2002). We and others have demonstrated that inflammatory cytokines such as IFN-γ and TNF-α increase expression of TLR4 and MD-2 resulting in increased LPS responsiveness in human colonic epithelial cells (Abreu et al., 2001; Suzuki et al., 2003). Therefore, TLR signaling especially by TLR4 may be increased in the setting of chronic gastrointestinal inflammation.
Figure 1. Expression of TLRs by normal human gastrointestinal tract.
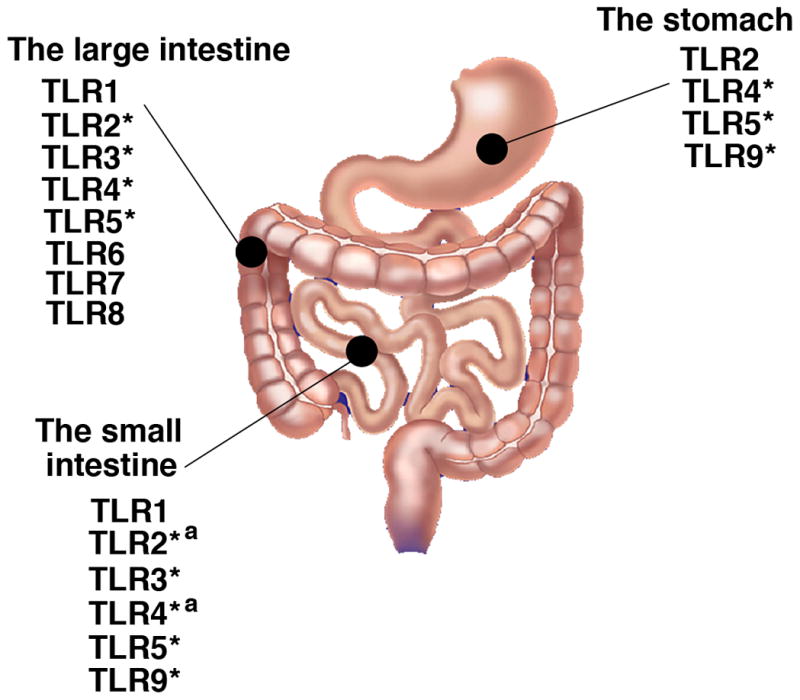
Most TLRs are expressed in the small intestine and the large intestine. Studies have mainly shown TLR mRNA expression (Abreu et al., 2001; Baoprasertkul et al., 2007; Du et al., 2000; Melmed et al., 2003; Otte et al., 2004; Rumio et al., 2004). * Protein expression has been confirmed (Cario & Podolsky, 2000; Furrie et al., 2005; Rock et al., 1998; Schmausser et al., 2005). In addition to IEC, lamina propria macrophages express TLR4 and TLR2 (Hausmann et al., 2002), and jejunal smooth muscle cells and myenteric prexus express TLR4 (Rumio et al., 2006). a denotes that the receptor has been shown to be functional in response to the appropriate ligand (Naik et al., 2001). There has been no data of TLR expression in the esophagus at present.
TLR4 is also important for healing of the injured intestinal epithelium (Fukata et al., 2005). We and others have described decreased epithelial cell proliferation in TLR4 or MyD88 deficient mice (Fukata et al., 2006; Fukata et al., 2005; Rakoff-Nahoum et al., 2004). We have also shown that TLR4 or MyD88 deficient mice have increased epithelial cell apoptosis after mucosal injury caused by dextran sulfate sodium (DSS) treatment (Fukata et al., 2006). As a mechanism, we have demonstrated that TLR4 signal induces Cox-2 and PGE2 in vitro and in vivo (Fukata et al., 2006). These data suggest a role for MyD88-dependent TLR4 signaling in regulation of IEC proliferation and apoptosis. Pull et al. have used MyD88−/− mice to create bone marrow chimeras (Pull et al., 2005). In response to injury, MyD88 expressing macrophages are required for IEC proliferation (Pull et al., 2005). In addition, MyD88-dependent signal is suggested to be involved in re-distribution of Cox-2 expressing stromal cells, which can modulate epithelial cell proliferation in response to mucosal injury (Brown et al., 2007). Because imbalance between epithelial cell proliferation and apoptosis is important for the process of inflammation-associated malignant transformation, TLR signaling via MyD88 may play a pivotal role in the malignant transformation in IEC (Figure 2).
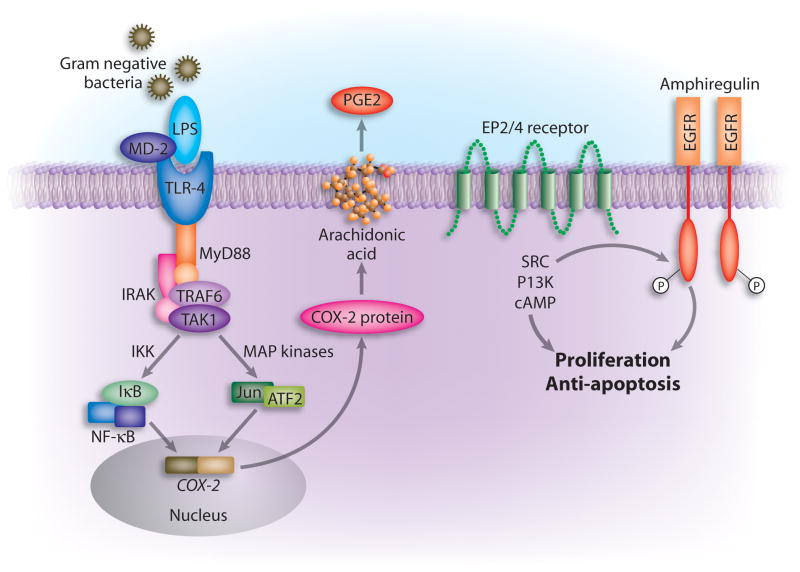
Figure 2. Model of TLR4-mediated Cox-2 regulation (Fukata et al., 2006).
In the setting of intestinal injury, LPS exposure of intestinal epithelial cells (possibly basolaterally) and lamina propria macrophages results in TLR4 activation and signaling via MyD88. This activates a variety of signaling pathways culminating in transcription factor translocation and engagement of the Cox-2 promoter. Cox-2 is transcribed and translated; it acts on arachidonic acid to generate PGG2 which is rapidly converted to PGH2 and then microsomal PG E synthase-1 converts it to PGE2. PGE2 through its receptors EP2 or EP4 can activate downstream signaling molecules such as the tyrosine kinase Src or the lipid kinase PI’3 kinase which can lead to transactivation of the EGF receptor. EGFR signaling is associated with proliferation and protection against apoptosis in intestinal epithelial cells. PGE2 produced by macrophages may also act in trans on intestinal epithelial cells. In the absence of TLR4 signaling, Cox-2 expression isgreatly decreased.
Role of TLR signaling in gastrointestinal carcinogenesis
Recognition of bacterial and/or viral products by TLR-expressing cells may induce an inflammatory response associated with tumor promotion (Coussens & Werb, 2002). Innate immune responses against luminal microbes may result in DNA damage, cell proliferation, and modulation of apoptosis. TLR signaling can play diverse roles with respect to tumorigenesis in the intestine. First, TLRs may serve as tumor promoters through its effects on the epithelium. This aspect of TLR signaling will be the principal focus of this review. TLRs may also support tumor growth through complex effects on the microenvironment. For example, concurrent bacterial infection can promote tumor growth in a model of subcutaneously implanted colorectal cancer cells in a TLR-dependent manner (Huang et al., 2007). Finally, TLR expression by colorectal tumor cells may facilitate evasion from immune surveillance in a mouse model of subcutaneous tumor xenograft (Huang et al., 2005). Taken together, TLR signaling in gastrointestinal tumor cells may be associated with subversion of host defense and progression of cancers. Because the density and diversity of commensal bacteria as well as the expression and function of TLRs differs throughout the gastrointestinal tract, the contribution of TLR signaling to carcinogenesis may differ according to location.
I) The upper gastrointestinal tract
In the esophagus, adenocarcinoma occurs in the setting of Barrett’s esophagus in which the lining squamous epithelium is replaced by small intestinal epithelium, a process termed intestinal metaplasia (Drewitz et al., 1997). The reason for intestinal metaplasia appears to be chronic reflux of gastric (generally acidic) contents into the lower esophagus (Fass et al., 2001). The process leading to intestinal metaplasia may be associated with chronic inflammation (Atherfold & Jankowski, 2006). The relationship between inflammation and tumorigenesis in esophageal adenocarcinoma remains controversial and it is fair to say that innate immune mechanisms have not been implicated thus far. It is also unclear whether the esophageal mucosa is normally exposed to bacterial antigens for protracted periods of time. Because of its motility, bacteria from the oral cavity and oropharynx may transit quickly and be destroyed by the gastric acid in the stomach. Exposure of the esophagus to PAMPs and bacteria has not been well studied.
The discovery of H. pylori in association with gastric cancer is the most direct proof that bacterial signaling and the response of the host can result in carcinogenesis (Peter & Beglinger, 2007). Inflammation is clearly a risk factor for the development of gastric cancer in response to H. pylori. A great deal of work has been done to identify bacterial factors important in H. pylori pathogenesis. Of these, expression of the cytotoxin-associated gene A (CagA) protein is thought to confer the greatest oncogenic risk. On the host side, TLR signaling may be quite important in H. pylori-associated gastric cancer. Chronic infection with H. pylori increases TLR4 and MD-2 expression in gastric epithelial cells, and recognition of H. pylori LPS augments NF-κB activation (Ishihara et al., 2004; Kawahara et al., 2001b; Schmausser et al., 2005). TLR4 is expressed at the apical and the basolateral pole of the normal gastric epithelial cells and in H. pylori gastritis, but the expression pattern appears to change to a diffuse and homogenous distribution within the cytoplasm accompanying cellular transformation to cancer (Schmausser et al., 2005). This alteration in expression can already be found during the stage of intestinal metaplasia in H. pylori infected subjects (Schmausser et al., 2005). Another study by the same authors has demonstrated that TLR4 is strongly expressed by tumor cells of H. pylori associated gastric carcinoma (Schmausser et al., 2005).
We have described that TLR4 signaling regulates colonic Cox-2 expression and PGE2 production (Fukata et al., 2006). Therefore, it is possible that the increased expression of Cox-2 and PGE2 seen in gastric cancer of H. pylori infected subjects may be related to TLR4 signaling in gastric epithelial cells (Fukumoto et al., 2003; Pomorski et al., 2001; Romano et al., 1998; Sawaoka et al., 1998; Sung et al., 2000). At this stage, the connection in the stomach between TLR4 and Cox-2 induction is speculative but highly likely. In deed, H. pylori LPS induces Cox-2 expression along with nuclear translocation of NF-κB p56 in the isolated guinea pig gastric epithelial cells (Kawahara et al., 2001a). In addition, other TLR signaling molecules, e.g., TLR2 and TLR9, have also been implicated in the up-regulation of Cox-2 through activation of Src and NF-κB in H. pylori infected mucosa (Chang et al., 2004).
Other inflammatory pathways lie downstream of pattern recognition receptors may play a role in H. pylori carcinogenesis. For example, H. pylori induces IL-8 secretion in a TLR2 dependent manner (Fischer et al., 2001; Mandell et al., 2004). In addition, CagA may enhance TLR2 signaling, because CagA+ H. pylori induces higher IL-8 secretion than CagA negative H. pylori in TLR2- and CD14-expressing HEK293 cells (Mandell et al., 2004).
In addition to the TLRs, a study has found that nucleotide-binding oligomerization domain containing (Nod)1 senses the peptidoglycan of H. pylori and induces NF-κB-dependent IL-8 production (Viala et al., 2004). In fact, a significant increase in the bacterial load of H. pylori has been found in Nod1-deficient mice at 7 and 30 days after inoculation compared to WT mice (Viala et al., 2004). In Nod1-deficient mice, primary gastric epithelial cells produce lower amounts of the chemokine MIP-2 than WT mice in response to H. pylori challenge. Along the same lines, infection of MyD88-deficient mice with H. pylori has shown increased colonization and reduced mucosal inflammation and pro-inflammatory cytokine production in the stomach compared to WT mice (Rad et al., 2007). Therefore, TLR and Nod signaling may modulate the inflammatory effect of H. pylori and possibly influence development of gastric cancer.
In addition to adenocarcinoma of the stomach, H. pylori infection has been linked to the development of mucosa-associated lymphoid tissue (MALT)-type lymphoma in the stomach. Although, the role of TLRs in the pathogenesis of MALT lymphoma has not yet been examined, the immune response to chronic antigenic stimulation by H. pylori infection is thought to induce NF-κB activation resulting in the development of gastric MALT lymphoma (Farinha & Gascoyne, 2005; Stolte & Eidt, 1989; Wyatt & Rathbone, 1988). Recent data demonstrate that presentation of H. pylori by dendritic cells directs adaptive immune responses and requires MyD88-dependent signaling, since MyD88-deficient dendritic cells are profoundly impaired in this process (Rad et al., 2007). Thus, activation of TLR signaling by H. pylori may also be involved in the pathogenesis of MALT lymphoma.
The role of polymorphisms in TLRs has been addressed. First, patients with poorly-differentiated gastric adenocarcinomas are more likely to carry the germ-line Thr135Ala polymorphism in the leucine-rich repeat region of TLR4 (Ohara et al., 2006). This Thr135Ala polymorphism is present regardless of H. pylori association. TLR4 polymorphisms Asp299Gly may increase the risk of non-cardiac1 gastric cancer (Hold et al., 2007). In this study, 17% of Polish patients with gastric cancer had 1 or 2 TLR4 variant alleles vs. 8% of control subjects (OR=2.5, 95% CI, 1.6–4.0). Similarly, 15% percent of non-cardiac gastric cancer cases from a U.S. multi-center study carried at least 1 copy of the variant TLR4 allele, compared with only 8% of the control population (OR = 2.1, 95% CI, 1.1–4.2) (Gammon et al., 1997; Hold et al., 2007). Unfortunately these studies did not address the H. pylori association but the type of gastric cancers considered in these studies is more frequent with H. pylori. Therefore, TLR signaling both at a genetic level and genomic level may be involved in the development of gastric cancer.
II) The small intestine
The small intestine has a very low incidence of malignancy and represents less than five percent of all malignancies in the gastrointestinal tract (Haselkorn et al., 2005; Kelly & Bartram, 1993). Most small intestinal cancers arise from Crohn’s disease (Sigel et al., 1999). Because innate immune abnormalities are a central factor in the pathogenesis of Crohn’s disease, TLR signaling may be involved in Crohn’s disease-associated small intestinal cancer. At the present time, TLRs have not been examined in small bowel cancers.
III) The large intestine
The genetic mutations that occur in sporadic colorectal cancer have been well studied (Contasta et al., 2006; Frattini et al., 2004; Konishi et al., 1996). The sequence of gene mutations culminating in colon cancer has been elucidated by the work of Vogelstein and Kissler and others. In spite of the genetic model, chronic inflammation also plays a role in colorectal carcinogenesis. Epidemiologic studies demonstrate that increased C-reactive protein, a biomarker of inflammation, is associated with colon cancer and poor survival (Greten et al., 2004; Helzlsouer et al., 2006; Hope et al., 2005; Tlaskalova-Hogenova et al., 2004). Polymorphisms in cytokine genes, in particular IL-1 and IL-8, are associated with an increased risk of adenomatous polyps or cancer development (Gunter et al., 2006). An elevated serum concentration of IL-6 has correlated with a higher tumor stage and worse prognosis of colorectal cancer (Chung & Chang, 2003; De Vita et al., 2001; Kinoshita et al., 1999). These results suggest that inflammation can be an initiator as well as a promoter in colorectal carcinogenesis.
How can innate immune signaling be involved in sporadic colorectal carcinogenesis? The hypothesis that intestinal bacteria might play a key role in colorectal cancerogenesis dates back to 1971 (Hill et al., 1971). High concentrations of bacteria have been detected in biopsy specimens of 90% of colorectal cancers and 93% of adenomas in the large intestine, whereas no bacteria were found in the colonic mucosa of normal controls (Swidsinski et al., 1998). Several possibilities including carcinogenic bacterial enzymes and generation of reactive oxygen intermediates link intestinal bacteria to the development of colorectal cancer (Hope et al., 2005; Manju & Nalini, 2006). Recent data demonstrate that Enterococcus faecalis, a common gut commensal, can induce chromosomal instability through superoxide production (Wang & Huycke, 2007).
Recent advances in innate immunity may illuminate the mechanism underlying inflammation-induced colorectal carcinogenesis. A small Croatian study has shown that patients with colorectal cancer are more likely to carry a microsatellite GT polymorphism in the TLR2 gene as well as the Asp299Gly allele of the TLR4 gene (Boraska Jelavic et al., 2006). Blocking TLR4 signaling in colon cancer cells results in a reduction of tumor growth in a subcutaneously implanted mouse model (Huang et al., 2005). These results suggest that bacterial recognition system especially by TLRs may have a significant role in tumor progression.
More recently, involvement of TLR signaling through the adaptor molecule MyD88 in tumor growth and progression has been reported in ApcMin/+ mice model (Rakoff-Nahoum & Medzhitov, 2007). These mice carry a germline mutation in the tumor suppressor Apc gene, which is frequently mutated in sporadic and hereditary forms of human colorectal cancer. ApcMin/+ mice spontaneously develop multiple adenomas through gastrointestinal tract, especially the small intestine (Powell et al., 1992; Su et al., 1992). Although the overall incidence of intestinal tumors was similar to control ApcMin/+ mice, MyD88-deficiency in ApcMin/+ mice resulted in a significant decrease in visible tumors in the small and large intestine (Rakoff-Nahoum & Medzhitov, 2007). MyD88 deficient × ApcMin/+ mice demonstrated decreased expression of several growth modifier genes, including Cox-2 in the tumor tissue as compared to ApcMin/+ mice. The exact mechanism underlying MyD88-dependent tumorigenesis remains unclear. In particular, these results do not necessarily point to TLR-dependent signaling or to a role for small bowel bacteria since both are low under normal conditions (Abreu et al., 2001; Melmed et al., 2003). Since the ApcMin/+ mice demonstrate a lower incidence of tumor development in the germ-free state, there may be a role of TLR signaling in response to commensal bacteria in intestinal tumorigenesis (Dove et al., 1997).
Patients with IBD are at an increased risk of developing colorectal cancer (Choi & Zelig, 1994; Ekbom et al., 1990; Langholz et al., 1992). The risk of colorectal cancer in patients with IBD increases by 0.5–1.0% yearly beginning 8–10 years after diagnosis (Eaden et al., 2001; Gyde et al., 1988; Itzkowitz & Harpaz, 2004). Unlike sporadic colon cancers in which APC mutations occur early, APC mutations tend to be a late event. Recent data demonstrate that the severity of inflammation correlates with the risk of colitis-associated cancer (CAC) (Gupta et al., 2007; Rutter et al., 2004). Although the exact cause of the inflammation in IBD is still unknown, abnormal immune responses to luminal bacteria plays a crucial role in the pathogenesis of IBD. Mounting evidence supports a role for deranged innate immune abnormalities in IBD. Several polymorphisms associated with innate immune receptors have been reported as candidate genes in IBD. The first gene discovered associated with disease susceptibility in Crohn’s disease, NOD2/CARD15, encodes a protein that acts as an intracellular pattern recognition receptor (PRRs) for muramyl dipeptide (Chamaillard et al., 2003; Hugot et al., 2001). The Asp299Gly polymorphism of TLR4 is associated with both ulcerative colitis and Crohn’s disease (Brand et al., 2005; Franchimont et al., 2004; Gazouli et al., 2005; Oostenbrug et al., 2005; Ouburg et al., 2005; Torok et al., 2004b). Crohn’s disease has been associated with a TLR9 polymorphism, which is interesting given the animal data that TLR9 may be anti-inflammatory in certain contexts (Torok et al., 2004a). SNPs in TLR1, -2, and -6 have been examined in IBD. Although none of the SNPs was involved in disease susceptibility, all were associated with more colonic disease extent in ulcerative colitis and Crohn’s disease (Pierik et al., 2006). Although, these polymorphisms have not yet been associated with development of CAC, genotype-phenotype associations suggest a link between TLR polymorphisms and CAC. For instance, TLR1 R80T and TLR2 R753G SNP were associated with pancolitis in ulcerative colitis, and pancolitis is one of the risk factors for CAC (Itzkowitz & Harpaz, 2004; Pierik et al., 2006).
Several rodent models of CAC require commensal bacteria or specific bacteria (Helicobacter hepaticus) for initiation of colitis and development of dysplasia or cancer, suggesting an essential role for commensal bacteria in CAC (Itzkowitz & Yio, 2004; Maggio-Price et al., 2005; Sellon et al., 1998). Mice deficient in IL-2, IL-10, double knockouts for the T-cell receptor and p53, or tumor growth factor β1 and recombination activating gene (RAG)2 develop adenocarcinoma in the small and large intestine. In the absence of bacteria, these mouse models neither develop inflammation nor dysplasia or cancer (Engle et al., 2002; Kado et al., 2001; Schultz et al., 1999; Sellon et al., 1998). Given that Helicobacter hepaticus infection induces CAC in RAG2 deficient mice, these data support the premise that innate immune responses to commensal bacteria are sufficient to induce inflammation-associated gastrointestinal carcinogenesis (Erdman et al., 2003).
The other type of animal model of CAC uses a chemically induced colitis. Azoxymethane (AOM) is a colonic genotoxic carcinogen that has been extensively used in the investigation of colorectal carcinogenesis and enhances the incidence of dysplastic lesions in response to DSS-induced murine colitis (Paulsen et al., 2006; Suzuki et al., 2006; Suzuki et al., 2005). As with the genetic models of CAC, germ-free rats given AOM are protected from colonic dysplasia and cancer, suggesting a role of commensal bacteria in the chemically induced CAC model (Laqueur et al., 1981; Reddy et al., 1975). AOM intercalates in the DNA but reactive oxygen or nitrogen species resulting in oxidative DNA damage are necessary to induce dysplasia in C57BL/6 mice (Derdak et al., 2006; Rao et al., 2002).
TLR4 signaling may directly lead to the production of ROS/RONS (Asehnoune et al., 2004; Ishida et al., 2002; Matsuzawa et al., 2005; Park et al., 2004). To the extent that TLR4 signaling is necessary for neutrophil recruitment to the intestine (Fukata et al., 2005), TLR4 is indirectly responsible for ROS generation as well. ROS may also beintermediaries in TLR4-dependent activation of NF-κB (Asehnoune et al., 2004). Furthermore, we have currently described that TLR4-defficient mice exhibit a significantly decreased incidence and size of colorectal tumors in the AOM-DSS model compared to WT mice (Fukata et al., 2007). Mechanistically, we have shown that up-regulation of mucosal Cox-2 and amphiregulin expression downstream of TLR4 results in epidermal growth factor receptor phosphorylation and cell proliferation (Figure 3) (Fukata et al., 2006; Fukata et al., 2007).
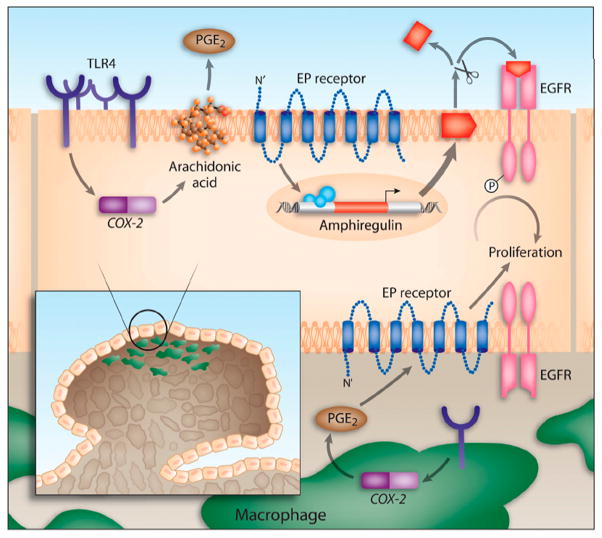
Figure 3. Model of TLR4-mediated colon carcinogenesis (Fukata et al., 2007).
TLR4 expression is increased in chronic intestinal inflammation. TLR4 signaling in response to LPS induces Cox-2 expression and PGE2 production. PGE2 through its receptors can act in a paracrine or autocrine fashion on colonocytes to stimulate the expression and release of amphiregulin, an EGFR ligand. EGFR signaling is associated with increased proliferation of colonocytes. Likewise, TLR4 expression in tumor-associated macrophages may also respond to LPS by inducing Cox-2 and PGE2, which may then act on the epithelium to stimulate proliferation of colonocytes.
Given our results in a CAC model, TLR signaling is clearly involved in the development of CAC. However, several TLR expressing cell types may contribute to the development of dysplasia or cancer. We have shown TLR signaling regulates Cox-2 expression in both IEC and lamina propria macrophages in the setting of colitis (Fukata et al., 2006; Fukata et al., 2005). Importantly, Cox-2 expression by macrophages infiltrated inside of the tumor has been thought to be an early event in colorectal carcinogenesis (Janne & Mayer, 2000). Karin and colleagues have generated mice with a conditional ablation of IKKβ in either IEC or myeloid cells and examined their effect on the development of CAC in the AOM-DSS model (Greten et al., 2004). The deletion of IKKβ in IEC results in a dramatic reduction in the number of adenomas, whereas deletion of IKKβ in myeloid cells has a modest effect on adenoma numbers but a greater effect on adenoma size. More importantly, deletion of IKKβ in IEC has no effect on the underlying inflammation. These data suggest that NF-κB may have differential roles in inflammation and carcinogenesis and these effects may be cell autonomous or through cell-cell interactions.
Given that an important endpoint of TLR signaling is NF-κB activation, TLRs may provide the input to NF-κB resulting in malignancy. The single immunoglobulin IL-1 receptor-related molecule (SIGIRR) acts as a negative regulator of TLR signaling. SIGIRR−/− animals demonstrate significantly greater inflammation and increased tumorigenesis following treatment with AOM-DSS (Garlanda et al., 2007; Xiao et al., 2007). Restitution of SIGIRR expression in the epithelium reduces tumors suggesting a role for epithelial TLR signaling in tumor development.
Concluding Remarks
Currently, safe and effective chemopreventive strategies for gastrointestinal malignancies are lacking. While Cox-2 inhibitors offered great promise for patients with previous adenomas, trials were discontinued due to the increase in thrombotic events (Arber et al., 2006; Bertagnolli et al., 2006). The prevalence of Helicobacter pylori infection is decreasing in the Western world but the incidence of inflammation-related gastric cancer remains high in the developing world. For patients with IBD, treatment has dramatically improved in the last ten years. 5-aminosalicylates have a modest chemopreventive benefit but some studies have not found any benefit (Bernstein et al., 2002). Targeted inhibition of specific TLR pathways may provide an effective strategy in preventing development of selected gastrointestinal malignancies. Because the gastrointestinal tract can be accessed without the need for systemic delivery, TLR antagonists may be developed to interrupt oncogenic pathways and delivered locally. Lastly, combination approaches may maximize the benefits of treatment while minimizing toxicity of individual compounds.
Footnotes
The cardia region of the stomach refers to the upper part of the stomach nearest the esophagus. Cancers of this region are rising but tend to occur in H. pylori negative individuals
Spechler SJ. (1999). The role of gastric carditis in metaplasia and neoplasia at the gastroesophageal junction. Gastroenterology 117: 218-28..
References
- Abreu MT, Fukata M, Arditi M. TLR signaling in the gut in health and disease. J Immunol. 2005;174:4453–60. doi: 10.4049/jimmunol.174.8.4453. [DOI] [PubMed] [Google Scholar]
- Abreu MT, Vora P, Faure E, Thomas LS, Arnold ET, Arditi M. Decreased expression of Toll-like receptor-4 and MD-2 correlates with intestinal epithelial cell protection against dysregulated proinflammatory gene expression in response to bacterial lipopolysaccharide. J Immunol. 2001;167:1609–16. doi: 10.4049/jimmunol.167.3.1609. [DOI] [PubMed] [Google Scholar]
- Arber N, Eagle CJ, Spicak J, Racz I, Dite P, Hajer J, et al. Celecoxib for the prevention of colorectal adenomatous polyps. N Engl J Med. 2006;355:885–95. doi: 10.1056/NEJMoa061652. [DOI] [PubMed] [Google Scholar]
- Asehnoune K, Strassheim D, Mitra S, Kim JY, Abraham E. Involvement of reactive oxygen species in Toll-like receptor 4-dependent activation of NF-kappa B. J Immunol. 2004;172:2522–9. doi: 10.4049/jimmunol.172.4.2522. [DOI] [PubMed] [Google Scholar]
- Atherfold PA, Jankowski JA. Molecular biology of Barrett’s cancer. Best Pract Res Clin Gastroenterol. 2006;20:813–27. doi: 10.1016/j.bpg.2006.04.003. [DOI] [PubMed] [Google Scholar]
- Balkwill F, Coussens LM. Cancer: an inflammatory link. Nature. 2004;431:405–6. doi: 10.1038/431405a. [DOI] [PubMed] [Google Scholar]
- Baoprasertkul P, Peatman E, Abernathy J, Liu Z. Structural characterisation and expression analysis of toll-like receptor 2 gene from catfish. Fish Shellfish Immunol. 2007;22:418–26. doi: 10.1016/j.fsi.2006.04.005. [DOI] [PubMed] [Google Scholar]
- Bernstein CN, Eaden J, Steinhart AH, Munkholm P, Gordon PH. Cancer prevention in inflammatory bowel disease and the chemoprophylactic potential of 5-aminosalicylic acid. Inflamm Bowel Dis. 2002;8:356–61. doi: 10.1097/00054725-200209000-00007. [DOI] [PubMed] [Google Scholar]
- Bertagnolli MM, Eagle CJ, Zauber AG, Redston M, Solomon SD, Kim K, et al. Celecoxib for the prevention of sporadic colorectal adenomas. N Engl J Med. 2006;355:873–84. doi: 10.1056/NEJMoa061355. [DOI] [PubMed] [Google Scholar]
- Boraska Jelavic T, Barisic M, Drmic Hofman I, Boraska V, Vrdoljak E, Peruzovic M, et al. Microsatelite GT polymorphism in the toll-like receptor 2 is associated with colorectal cancer. Clin Genet. 2006;70:156–60. doi: 10.1111/j.1399-0004.2006.00651.x. [DOI] [PubMed] [Google Scholar]
- Brand S, Staudinger T, Schnitzler F, Pfennig S, Hofbauer K, Dambacher J, et al. The role of Toll-like receptor 4 Asp299Gly and Thr399Ile polymorphisms and CARD15/NOD2 mutations in the susceptibility and phenotype of Crohn’s disease. Inflamm Bowel Dis. 2005;11:645–52. doi: 10.1097/01.mib.0000168372.94907.d2. [DOI] [PubMed] [Google Scholar]
- Brittan M, Wright NA. Gastrointestinal stem cells. J Pathol. 2002;197:492–509. doi: 10.1002/path.1155. [DOI] [PubMed] [Google Scholar]
- Brown SL, Riehl TE, Walker MR, Geske MJ, Doherty JM, Stenson WF, et al. Myd88-dependent positioning of Ptgs2-expressing stromal cells maintains colonic epithelial proliferation during injury. J Clin Invest. 2007;117:258–69. doi: 10.1172/JCI29159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cario E, Podolsky DK. Differential alteration in intestinal epithelial cell expression of toll-like receptor 3 (TLR3) and TLR4 in inflammatory bowel disease. Infect Immun. 2000;68:7010–7. doi: 10.1128/iai.68.12.7010-7017.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamaillard M, Girardin SE, Viala J, Philpott DJ. Nods, Nalps and Naip: intracellular regulators of bacterial-induced inflammation. Cell Microbiol. 2003;5:581–92. doi: 10.1046/j.1462-5822.2003.00304.x. [DOI] [PubMed] [Google Scholar]
- Chang YJ, Wu MS, Lin JT, Sheu BS, Muta T, Inoue H, et al. Induction of cyclooxygenase-2 overexpression in human gastric epithelial cells by Helicobacter pylori involves TLR2/TLR9 and c-Src-dependent nuclear factor-kappaB activation. Mol Pharmacol. 2004;66:1465–77. doi: 10.1124/mol.104.005199. [DOI] [PubMed] [Google Scholar]
- Choi PM, Zelig MP. Similarity of colorectal cancer in Crohn’s disease and ulcerative colitis: implications for carcinogenesis and prevention. Gut. 1994;35:950–4. doi: 10.1136/gut.35.7.950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung YC, Chang YF. Serum interleukin-6 levels reflect the disease status of colorectal cancer. J Surg Oncol. 2003;83:222–6. doi: 10.1002/jso.10269. [DOI] [PubMed] [Google Scholar]
- Contasta I, Pellegrini P, Berghella AM, Del Beato T, Adorno D. Colon cancer and gene alterations: their immunological implications and suggestions for prognostic indices and improvements in biotherapy. Cancer Biother Radiopharm. 2006;21:488–505. doi: 10.1089/cbr.2006.21.488. [DOI] [PubMed] [Google Scholar]
- Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–7. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Visser KE, Coussens LM. The interplay between innate and adaptive immunity regulates cancer development. Cancer Immunol Immunother. 2005;54:1143–52. doi: 10.1007/s00262-005-0702-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vita F, Romano C, Orditura M, Galizia G, Martinelli E, Lieto E, et al. Interleukin-6 serum level correlates with survival in advanced gastrointestinal cancer patients but is not an independent prognostic indicator. J Interferon Cytokine Res. 2001;21:45–52. doi: 10.1089/107999001459150. [DOI] [PubMed] [Google Scholar]
- Derdak Z, Fulop P, Sabo E, Tavares R, Berthiaume EP, Resnick MB, et al. Enhanced colon tumor induction in uncoupling protein-2 deficient mice is associated with NF-kappaB activation and oxidative stress. Carcinogenesis. 2006;27:956–61. doi: 10.1093/carcin/bgi335. [DOI] [PubMed] [Google Scholar]
- Dove WF, Clipson L, Gould KA, Luongo C, Marshall DJ, Moser AR, et al. Intestinal neoplasia in the ApcMin mouse: independence from the microbial and natural killer (beige locus) status. Cancer Res. 1997;57:812–4. [PubMed] [Google Scholar]
- Drewitz DJ, Sampliner RE, Garewal HS. The incidence of adenocarcinoma in Barrett’s esophagus: a prospective study of 170 patients followed 4.8 years. Am J Gastroenterol. 1997;92:212–5. [PubMed] [Google Scholar]
- Du X, Poltorak A, Wei Y, Beutler B. Three novel mammalian toll-like receptors: gene structure, expression, and evolution. Eur Cytokine Netw. 2000;11:362–71. [PubMed] [Google Scholar]
- Eaden JA, Abrams KR, Mayberry JF. The risk of colorectal cancer in ulcerative colitis: a meta-analysis. Gut. 2001;48:526–35. doi: 10.1136/gut.48.4.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekbom A, Helmick C, Zack M, Adami HO. Increased risk of large-bowel cancer in Crohn’s disease with colonic involvement. Lancet. 1990;336:357–9. doi: 10.1016/0140-6736(90)91889-i. [DOI] [PubMed] [Google Scholar]
- Engle SJ, Ormsby I, Pawlowski S, Boivin GP, Croft J, Balish E, et al. Elimination of colon cancer in germ-free transforming growth factor beta 1-deficient mice. Cancer Res. 2002;62:6362–6. [PubMed] [Google Scholar]
- Erdman SE, Poutahidis T, Tomczak M, Rogers AB, Cormier K, Plank B, et al. CD4+ CD25+ regulatory T lymphocytes inhibit microbially induced colon cancer in Rag2-deficient mice. Am J Pathol. 2003;162:691–702. doi: 10.1016/S0002-9440(10)63863-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farinha P, Gascoyne RD. Molecular pathogenesis of mucosa-associated lymphoid tissue lymphoma. J Clin Oncol. 2005;23:6370–8. doi: 10.1200/JCO.2005.05.011. [DOI] [PubMed] [Google Scholar]
- Fass R, Hell RW, Garewal HS, Martinez P, Pulliam G, Wendel C, et al. Correlation of oesophageal acid exposure with Barrett’s oesophagus length. Gut. 2001;48:310–3. doi: 10.1136/gut.48.3.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer W, Puls J, Buhrdorf R, Gebert B, Odenbreit S, Haas R. Systematic mutagenesis of the Helicobacter pylori cag pathogenicity island: essential genes for CagA translocation in host cells and induction of interleukin-8. Mol Microbiol. 2001;42:1337–48. doi: 10.1046/j.1365-2958.2001.02714.x. [DOI] [PubMed] [Google Scholar]
- Franchimont D, Vermeire S, El Housni H, Pierik M, Van Steen K, Gustot T, et al. Deficient host-bacteria interactions in inflammatory bowel disease? The toll-like receptor (TLR)-4 Asp299gly polymorphism is associated with Crohn’s disease and ulcerative colitis. Gut. 2004;53:987–92. doi: 10.1136/gut.2003.030205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frattini M, Balestra D, Suardi S, Oggionni M, Alberici P, Radice P, et al. Different genetic features associated with colon and rectal carcinogenesis. Clin Cancer Res. 2004;10:4015–21. doi: 10.1158/1078-0432.CCR-04-0031. [DOI] [PubMed] [Google Scholar]
- Fukata M, Chen A, Klepper A, Krishnareddy S, Vamadevan AS, Thomas LS, et al. Cox-2 is regulated by Toll-like receptor-4 (TLR4) signaling: Role in proliferation and apoptosis in the intestine. Gastroenterology. 2006;131:862–77. doi: 10.1053/j.gastro.2006.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukata M, Michelsen KS, Eri R, Thomas LS, Hu B, Lukasek K, et al. Toll-like receptor-4 is required for intestinal response to epithelial injury and limiting bacterial translocation in a murine model of acute colitis. Am J Physiol Gastrointest Liver Physiol. 2005;288:G1055–65. doi: 10.1152/ajpgi.00328.2004. [DOI] [PubMed] [Google Scholar]
- Fukata M, Chen A, Vamadevan AS, Choen J, Breglio K, Krishnareddy S, et al. Toll-like receptor-4 (TLR4) promotes the development of colitis-associated colorectal tumors. Gastroenterology. 2007 doi: 10.1053/j.gastro.2007.09.008. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukumoto S, Ichihara T, Takada M, Kuroda Y. Expression of cyclooxygenases in Helicobacter pylori gastritis and residual gastritis after distal gastrectomy. World J Surg. 2003;27:145–8. doi: 10.1007/s00268-002-6460-z. [DOI] [PubMed] [Google Scholar]
- Furrie E, Macfarlane S, Thomson G, Macfarlane GT. Toll-like receptors-2, -3 and -4 expression patterns on human colon and their regulation by mucosal-associated bacteria. Immunology. 2005;115:565–74. doi: 10.1111/j.1365-2567.2005.02200.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gammon MD, Schoenberg JB, Ahsan H, Risch HA, Vaughan TL, Chow WH, et al. Tobacco, alcohol, and socioeconomic status and adenocarcinomas of the esophagus and gastric cardia. J Natl Cancer Inst. 1997;89:1277–84. doi: 10.1093/jnci/89.17.1277. [DOI] [PubMed] [Google Scholar]
- Garlanda C, Riva F, Veliz T, Polentarutti N, Pasqualini F, Radaelli E, et al. Increased susceptibility to colitis-associated cancer of mice lacking TIR8, an inhibitory member of the interleukin-1 receptor family. Cancer Res. 2007;67:6017–21. doi: 10.1158/0008-5472.CAN-07-0560. [DOI] [PubMed] [Google Scholar]
- Gazouli M, Mantzaris G, Kotsinas A, Zacharatos P, Papalambros E, Archimandritis A, et al. Association between polymorphisms in the Toll-like receptor 4, CD14, and CARD15/NOD2 and inflammatory bowel disease in the Greek population. World J Gastroenterol. 2005;11:681–5. doi: 10.3748/wjg.v11.i5.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greten FR, Eckmann L, Greten TF, Park JM, Li ZW, Egan LJ, et al. IKKbeta links inflammation and tumorigenesis in a mouse model of colitis-associated cancer. Cell. 2004;118:285–96. doi: 10.1016/j.cell.2004.07.013. [DOI] [PubMed] [Google Scholar]
- Gunter MJ, Canzian F, Landi S, Chanock SJ, Sinha R, Rothman N. Inflammation-related gene polymorphisms and colorectal adenoma. Cancer Epidemiol Biomarkers Prev. 2006;15:1126–31. doi: 10.1158/1055-9965.EPI-06-0042. [DOI] [PubMed] [Google Scholar]
- Gupta RB, Harpaz N, Itzkowitz S, Hossain S, Matula S, Kornbluth A, et al. Histologic inflammation is a risk factor for progression to colorectal neoplasia in ulcerative colitis: a cohort study. Gastroenterology. 2007 doi: 10.1053/j.gastro.2007.08.001. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyde SN, Prior P, Allan RN, Stevens A, Jewell DP, Truelove SC, et al. Colorectal cancer in ulcerative colitis: a cohort study of primary referrals from three centres. Gut. 1988;29:206–17. doi: 10.1136/gut.29.2.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haselkorn T, Whittemore AS, Lilienfeld DE. Incidence of small bowel cancer in the United States and worldwide: geographic, temporal, and racial differences. Cancer Causes Control. 2005;16:781–7. doi: 10.1007/s10552-005-3635-6. [DOI] [PubMed] [Google Scholar]
- Hausmann M, Kiessling S, Mestermann S, Webb G, Spottl T, Andus T, et al. Toll-like receptors 2 and 4 are up-regulated during intestinal inflammation. Gastroenterology. 2002;122:1987–2000. doi: 10.1053/gast.2002.33662. [DOI] [PubMed] [Google Scholar]
- Helzlsouer KJ, Erlinger TP, Platz EA. C-reactive protein levels and subsequent cancer outcomes: results from a prospective cohort study. Eur J Cancer. 2006;42:704–7. doi: 10.1016/j.ejca.2006.01.008. [DOI] [PubMed] [Google Scholar]
- Hill MJ, Drasar BS, Hawksworth G, Aries V, Crowther JS, Williams RE. Bacteria and aetiology of cancer of large bowel. Lancet. 1971;1:95–100. doi: 10.1016/s0140-6736(71)90837-3. [DOI] [PubMed] [Google Scholar]
- Hold GL, Rabkin CS, Chow WH, Smith MG, Gammon MD, Risch HA, et al. A functional polymorphism of toll-like receptor 4 gene increases risk of gastric carcinoma and its precursors. Gastroenterology. 2007;132:905–12. doi: 10.1053/j.gastro.2006.12.026. [DOI] [PubMed] [Google Scholar]
- Hope ME, Hold GL, Kain R, El-Omar EM. Sporadic colorectal cancer--role of the commensal microbiota. FEMS Microbiol Lett. 2005;244:1–7. doi: 10.1016/j.femsle.2005.01.029. [DOI] [PubMed] [Google Scholar]
- Houghton J, Wang TC. Helicobacter pylori and gastric cancer: a new paradigm for inflammation-associated epithelial cancers. Gastroenterology. 2005;128:1567–78. doi: 10.1053/j.gastro.2005.03.037. [DOI] [PubMed] [Google Scholar]
- Huang B, Zhao J, Li H, He KL, Chen Y, Chen SH, et al. Toll-like receptors on tumor cells facilitate evasion of immune surveillance. Cancer Res. 2005;65:5009–14. doi: 10.1158/0008-5472.CAN-05-0784. [DOI] [PubMed] [Google Scholar]
- Huang B, Zhao J, Shen S, Li H, He KL, Shen GX, et al. Listeria monocytogenes promotes tumor growth via tumor cell toll-like receptor 2 signaling. Cancer Res. 2007;67:4346–52. doi: 10.1158/0008-5472.CAN-06-4067. [DOI] [PubMed] [Google Scholar]
- Hugot JP, Chamaillard M, Zouali H, Lesage S, Cezard JP, Belaiche J, et al. Association of NOD2 leucine-rich repeat variants with susceptibility to Crohn’s disease. Nature. 2001;411:599–603. doi: 10.1038/35079107. [DOI] [PubMed] [Google Scholar]
- Ishida I, Kubo H, Suzuki S, Suzuki T, Akashi S, Inoue K, et al. Hypoxia diminishes toll-like receptor 4 expression through reactive oxygen species generated by mitochondria in endothelial cells. J Immunol. 2002;169:2069–75. doi: 10.4049/jimmunol.169.4.2069. [DOI] [PubMed] [Google Scholar]
- Ishihara S, Rumi MA, Kadowaki Y, Ortega-Cava CF, Yuki T, Yoshino N, et al. Essential role of MD-2 in TLR4-dependent signaling during Helicobacter pylori-associated gastritis. J Immunol. 2004;173:1406–16. doi: 10.4049/jimmunol.173.2.1406. [DOI] [PubMed] [Google Scholar]
- Itzkowitz SH, Harpaz N. Diagnosis and management of dysplasia in patients with inflammatory bowel diseases. Gastroenterology. 2004;126:1634–48. doi: 10.1053/j.gastro.2004.03.025. [DOI] [PubMed] [Google Scholar]
- Itzkowitz SH, Yio X. Inflammation and cancer IV. Colorectal cancer in inflammatory bowel disease: the role of inflammation. Am J Physiol Gastrointest Liver Physiol. 2004;287:G7–17. doi: 10.1152/ajpgi.00079.2004. [DOI] [PubMed] [Google Scholar]
- Janne PA, Mayer RJ. Chemoprevention of colorectal cancer. N Engl J Med. 2000;342:1960–8. doi: 10.1056/NEJM200006293422606. [DOI] [PubMed] [Google Scholar]
- Kado S, Uchida K, Funabashi H, Iwata S, Nagata Y, Ando M, et al. Intestinal microflora are necessary for development of spontaneous adenocarcinoma of the large intestine in T-cell receptor beta chain and p53 double-knockout mice. Cancer Res. 2001;61:2395–8. [PubMed] [Google Scholar]
- Kawahara T, Kuwano Y, Teshima-Kondo S, Kawai T, Nikawa T, Kishi K, et al. Toll-like receptor 4 regulates gastric pit cell responses to Helicobacter pylori infection. J Med Invest. 2001a;48:190–7. [PubMed] [Google Scholar]
- Kawahara T, Teshima S, Oka A, Sugiyama T, Kishi K, Rokutan K. Type I Helicobacter pylori lipopolysaccharide stimulates toll-like receptor 4 and activates mitogen oxidase 1 in gastric pit cells. Infect Immun. 2001b;69:4382–9. doi: 10.1128/IAI.69.7.4382-4389.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly IM, Bartram CI. Pseudotumoral appearance of small bowel strictureplasty for Crohn’s disease. Abdom Imaging. 1993;18:366–8. doi: 10.1007/BF00201784. [DOI] [PubMed] [Google Scholar]
- Kinoshita T, Ito H, Miki C. Serum interleukin-6 level reflects the tumor proliferative activity in patients with colorectal carcinoma. Cancer. 1999;85:2526–31. doi: 10.1002/(sici)1097-0142(19990615)85:12<2526::aid-cncr6>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Konishi M, Kikuchi-Yanoshita R, Tanaka K, Muraoka M, Onda A, Okumura Y, et al. Molecular nature of colon tumors in hereditary nonpolyposis colon cancer, familial polyposis, and sporadic colon cancer. Gastroenterology. 1996;111:307–17. doi: 10.1053/gast.1996.v111.pm8690195. [DOI] [PubMed] [Google Scholar]
- Langholz E, Munkholm P, Davidsen M, Binder V. Colorectal cancer risk and mortality in patients with ulcerative colitis. Gastroenterology. 1992;103:1444–51. doi: 10.1016/0016-5085(92)91163-x. [DOI] [PubMed] [Google Scholar]
- Laqueur GL, Matsumoto H, Yamamoto RS. Comparison of the carcinogenicity of methylazoxymethanol-beta-D-glucosiduronic acid in conventional and germfree Sprague-Dawley rats. J Natl Cancer Inst. 1981;67:1053–5. [PubMed] [Google Scholar]
- Leblond CP. Classification Of Cell Populations On The Basis Of Their Proliferative Behavior. Natl Cancer Inst Monogr. 1964;14:119–50. [PubMed] [Google Scholar]
- Maggio-Price L, Bielefeldt-Ohmann H, Treuting P, Iritani BM, Zeng W, Nicks A, et al. Dual infection with Helicobacter bilis and Helicobacter hepaticus in p-glycoprotein-deficient mdr1a−/− mice results in colitis that progresses to dysplasia. Am J Pathol. 2005;166:1793–806. doi: 10.1016/S0002-9440(10)62489-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandell L, Moran AP, Cocchiarella A, Houghton J, Taylor N, Fox JG, et al. Intact gram-negative Helicobacter pylori, Helicobacter felis, and Helicobacter hepaticus bacteria activate innate immunity via toll-like receptor 2 but not toll-like receptor 4. Infect Immun. 2004;72:6446–54. doi: 10.1128/IAI.72.11.6446-6454.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manju V, Nalini N. Effect of ginger on bacterial enzymes in 1,2-dimethylhydrazine induced experimental colon carcinogenesis. Eur J Cancer Prev. 2006;15:377–83. doi: 10.1097/00008469-200610000-00001. [DOI] [PubMed] [Google Scholar]
- Matsuzawa A, Saegusa K, Noguchi T, Sadamitsu C, Nishitoh H, Nagai S, et al. ROS-dependent activation of the TRAF6-ASK1-p38 pathway is selectively required for TLR4-mediated innate immunity. Nat Immunol. 2005;6:587–92. doi: 10.1038/ni1200. [DOI] [PubMed] [Google Scholar]
- Melmed G, Thomas LS, Lee N, Tesfay SY, Lukasek K, Michelsen KS, et al. Human intestinal epithelial cells are broadly unresponsive to Toll-like receptor 2-dependent bacterial ligands: implications for host-microbial interactions in the gut. J Immunol. 2003;170:1406–15. doi: 10.4049/jimmunol.170.3.1406. [DOI] [PubMed] [Google Scholar]
- Naik S, Kelly EJ, Meijer L, Pettersson S, Sanderson IR. Absence of Toll-like receptor 4 explains endotoxin hyporesponsiveness in human intestinal epithelium. J Pediatr Gastroenterol Nutr. 2001;32:449–53. doi: 10.1097/00005176-200104000-00011. [DOI] [PubMed] [Google Scholar]
- Nowacki MR. Cell proliferation in colonic crypts of germ-free and conventional mice--preliminary report. Folia Histochem Cytobiol. 1993;31:77–81. [PubMed] [Google Scholar]
- Ohara T, Morishita T, Suzuki H, Hibi T. Heterozygous Thr 135 Ala polymorphism at leucine-rich repeat (LRR) in genomic DNA of toll-like receptor 4 in patients with poorly-differentiated gastric adenocarcinomas. Int J Mol Med. 2006;18:59–63. [PubMed] [Google Scholar]
- Oostenbrug LE, Drenth JP, de Jong DJ, Nolte IM, Oosterom E, van Dullemen HM, et al. Association between Toll-like receptor 4 and inflammatory bowel disease. Inflamm Bowel Dis. 2005;11:567–75. doi: 10.1097/01.mib.0000161305.81198.0f. [DOI] [PubMed] [Google Scholar]
- Otte JM, Cario E, Podolsky DK. Mechanisms of cross hyporesponsiveness to Toll-like receptor bacterial ligands in intestinal epithelial cells. Gastroenterology. 2004;126:1054–70. doi: 10.1053/j.gastro.2004.01.007. [DOI] [PubMed] [Google Scholar]
- Ouburg S, Mallant-Hent R, Crusius JB, van Bodegraven AA, Mulder CJ, Linskens R, et al. The toll-like receptor 4 (TLR4) Asp299Gly polymorphism is associated with colonic localisation of Crohn’s disease without a major role for the Saccharomyces cerevisiae mannan-LBP-CD14-TLR4 pathway. Gut. 2005;54:439–40. doi: 10.1136/gut.2004.051383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park HS, Jung HY, Park EY, Kim J, Lee WJ, Bae YS. Cutting edge: direct interaction of TLR4 with NAD(P)H oxidase 4 isozyme is essential for lipopolysaccharide-induced production of reactive oxygen species and activation of NF-kappa B. J Immunol. 2004;173:3589–93. doi: 10.4049/jimmunol.173.6.3589. [DOI] [PubMed] [Google Scholar]
- Parkin DM, Pisani P, Ferlay J. Global cancer statistics. CA Cancer J Clin. 1999;49:33–64. 1. doi: 10.3322/canjclin.49.1.33. [DOI] [PubMed] [Google Scholar]
- Paulsen JE, Knutsen H, Olstorn HB, Loberg EM, Alexander J. Identification of flat dysplastic aberrant crypt foci in the colon of azoxymethane-treated A/J mice. Int J Cancer. 2006;118:540–6. doi: 10.1002/ijc.21416. [DOI] [PubMed] [Google Scholar]
- Peek RM, Jr, Blaser MJ. Helicobacter pylori and gastrointestinal tract adenocarcinomas. Nat Rev Cancer. 2002;2:28–37. doi: 10.1038/nrc703. [DOI] [PubMed] [Google Scholar]
- Peter S, Beglinger C. Helicobacter pylori and gastric cancer: the causal relationship. Digestion. 2007;75:25–35. doi: 10.1159/000101564. [DOI] [PubMed] [Google Scholar]
- Pierik M, Joossens S, Van Steen K, Van Schuerbeek N, Vlietinck R, Rutgeerts P, et al. Toll-like receptor-1, -2, and -6 polymorphisms influence disease extension in inflammatory bowel diseases. Inflamm Bowel Dis. 2006;12:1–8. doi: 10.1097/01.mib.0000195389.11645.ab. [DOI] [PubMed] [Google Scholar]
- Pomorski T, Meyer TF, Naumann M. Helicobacter pylori-induced prostaglandin E(2) synthesis involves activation of cytosolic phospholipase A(2) in epithelial cells. J Biol Chem. 2001;276:804–10. doi: 10.1074/jbc.M003819200. [DOI] [PubMed] [Google Scholar]
- Potten CS, Booth C, Pritchard DM. The intestinal epithelial stem cell: the mucosal governor. Int J Exp Pathol. 1997;78:219–43. doi: 10.1046/j.1365-2613.1997.280362.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell SM, Zilz N, Beazer-Barclay Y, Bryan TM, Hamilton SR, Thibodeau SN, et al. APC mutations occur early during colorectal tumorigenesis. Nature. 1992;359:235–7. doi: 10.1038/359235a0. [DOI] [PubMed] [Google Scholar]
- Pull SL, Doherty JM, Mills JC, Gordon JI, Stappenbeck TS. Activated macrophages are an adaptive element of the colonic epithelial progenitor niche necessary for regenerative responses to injury. Proc Natl Acad Sci U S A. 2005;102:99–104. doi: 10.1073/pnas.0405979102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rad R, Brenner L, Krug A, Voland P, Mages J, Lang R, et al. Toll-like receptor-dependent activation of antigen-presenting cells affects adaptive immunity to Helicobacter pylori. Gastroenterology. 2007;133:150–163. e3. doi: 10.1053/j.gastro.2007.04.071. [DOI] [PubMed] [Google Scholar]
- Rakoff-Nahoum S, Medzhitov R. Regulation of spontaneous intestinal tumorigenesis through the adaptor protein MyD88. Science. 2007;317:124–7. doi: 10.1126/science.1140488. [DOI] [PubMed] [Google Scholar]
- Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S, Medzhitov R. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell. 2004;118:229–41. doi: 10.1016/j.cell.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Rao CV, Indranie C, Simi B, Manning PT, Connor JR, Reddy BS. Chemopreventive properties of a selective inducible nitric oxide synthase inhibitor in colon carcinogenesis, administered alone or in combination with celecoxib, a selective cyclooxygenase-2 inhibitor. Cancer Res. 2002;62:165–70. [PubMed] [Google Scholar]
- Reddy BS, Narisawa T, Wright P, Vukusich D, Weisburger JH, Wynder EL. Colon carcinogenesis with azoxymethane and dimethylhydrazine in germ-free rats. Cancer Res. 1975;35:287–90. [PubMed] [Google Scholar]
- Rock FL, Hardiman G, Timans JC, Kastelein RA, Bazan JF. A family of human receptors structurally related to Drosophila Toll. Proc Natl Acad Sci U S A. 1998;95:588–93. doi: 10.1073/pnas.95.2.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romano M, Ricci V, Memoli A, Tuccillo C, Di Popolo A, Sommi P, et al. Helicobacter pylori up-regulates cyclooxygenase-2 mRNA expression and prostaglandin E2 synthesis in MKN 28 gastric mucosal cells in vitro. J Biol Chem. 1998;273:28560–3. doi: 10.1074/jbc.273.44.28560. [DOI] [PubMed] [Google Scholar]
- Rumio C, Besusso D, Arnaboldi F, Palazzo M, Selleri S, Gariboldi S, et al. Activation of smooth muscle and myenteric plexus cells of jejunum via Toll-like receptor 4. J Cell Physiol. 2006;208:47–54. doi: 10.1002/jcp.20632. [DOI] [PubMed] [Google Scholar]
- Rumio C, Besusso D, Palazzo M, Selleri S, Sfondrini L, Dubini F, et al. Degranulation of paneth cells via toll-like receptor 9. Am J Pathol. 2004;165:373–81. doi: 10.1016/S0002-9440(10)63304-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutter M, Saunders B, Wilkinson K, Rumbles S, Schofield G, Kamm M, et al. Severity of inflammation is a risk factor for colorectal neoplasia in ulcerative colitis. Gastroenterology. 2004;126:451–9. doi: 10.1053/j.gastro.2003.11.010. [DOI] [PubMed] [Google Scholar]
- Sawaoka H, Kawano S, Tsuji S, Tsuji M, Sun W, Gunawan ES, et al. Helicobacter pylori infection induces cyclooxygenase-2 expression in human gastric mucosa. Prostaglandins Leukot Essent Fatty Acids. 1998;59:313–6. doi: 10.1016/s0952-3278(98)90079-5. [DOI] [PubMed] [Google Scholar]
- Schmausser B, Andrulis M, Endrich S, Lee SK, Josenhans C, Muller-Hermelink HK, et al. Expression and subcellular distribution of toll-like receptors TLR4, TLR5 and TLR9 on the gastric epithelium in Helicobacter pylori infection. Clin Exp Immunol. 2004;136:521–6. doi: 10.1111/j.1365-2249.2004.02464.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmausser B, Andrulis M, Endrich S, Muller-Hermelink HK, Eck M. Toll-like receptors TLR4, TLR5 and TLR9 on gastric carcinoma cells: an implication for interaction with Helicobacter pylori. Int J Med Microbiol. 2005;295:179–85. doi: 10.1016/j.ijmm.2005.02.009. [DOI] [PubMed] [Google Scholar]
- Schultz M, Tonkonogy SL, Sellon RK, Veltkamp C, Godfrey VL, Kwon J, et al. IL-2-deficient mice raised under germfree conditions develop delayed mild focal intestinal inflammation. Am J Physiol. 1999;276:G1461–72. doi: 10.1152/ajpgi.1999.276.6.G1461. [DOI] [PubMed] [Google Scholar]
- Sellon RK, Tonkonogy S, Schultz M, Dieleman LA, Grenther W, Balish E, et al. Resident enteric bacteria are necessary for development of spontaneous colitis and immune system activation in interleukin-10-deficient mice. Infect Immun. 1998;66:5224–31. doi: 10.1128/iai.66.11.5224-5231.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigel JE, Petras RE, Lashner BA, Fazio VW, Goldblum JR. Intestinal adenocarcinoma in Crohn’s disease: a report of 30 cases with a focus on coexisting dysplasia. Am J Surg Pathol. 1999;23:651–5. doi: 10.1097/00000478-199906000-00003. [DOI] [PubMed] [Google Scholar]
- Spechler SJ. The role of gastric carditis in metaplasia and neoplasia at the gastroesophageal junction. Gastroenterology. 1999;117:218–28. doi: 10.1016/s0016-5085(99)70571-8. [DOI] [PubMed] [Google Scholar]
- Stolte M, Eidt S. Lymphoid follicles in antral mucosa: immune response to Campylobacter pylori? J Clin Pathol. 1989;42:1269–71. doi: 10.1136/jcp.42.12.1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su LK, Kinzler KW, Vogelstein B, Preisinger AC, Moser AR, Luongo C, et al. Multiple intestinal neoplasia caused by a mutation in the murine homolog of the APC gene. Science. 1992;256:668–70. doi: 10.1126/science.1350108. [DOI] [PubMed] [Google Scholar]
- Sung JJ, Leung WK, Go MY, To KF, Cheng AS, Ng EK, et al. Cyclooxygenase-2 expression in Helicobacter pylori-associated premalignant and malignant gastric lesions. Am J Pathol. 2000;157:729–35. doi: 10.1016/S0002-9440(10)64586-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki M, Hisamatsu T, Podolsky DK. Gamma interferon augments the intracellular pathway for lipopolysaccharide (LPS) recognition in human intestinal epithelial cells through coordinated up-regulation of LPS uptake and expression of the intracellular Toll-like receptor 4-MD-2 complex. Infect Immun. 2003;71:3503–11. doi: 10.1128/IAI.71.6.3503-3511.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki R, Kohno H, Sugie S, Nakagama H, Tanaka T. Strain differences in the susceptibility to azoxymethane and dextran sodium sulfate-induced colon carcinogenesis in mice. Carcinogenesis. 2006;27:162–9. doi: 10.1093/carcin/bgi205. [DOI] [PubMed] [Google Scholar]
- Suzuki R, Kohno H, Sugie S, Tanaka T. Dose-dependent promoting effect of dextran sodium sulfate on mouse colon carcinogenesis initiated with azoxymethane. Histol Histopathol. 2005;20:483–92. doi: 10.14670/HH-20.483. [DOI] [PubMed] [Google Scholar]
- Swidsinski A, Khilkin M, Kerjaschki D, Schreiber S, Ortner M, Weber J, et al. Association between intraepithelial Escherichia coli and colorectal cancer. Gastroenterology. 1998;115:281–6. doi: 10.1016/s0016-5085(98)70194-5. [DOI] [PubMed] [Google Scholar]
- Tlaskalova-Hogenova H, Stepankova R, Hudcovic T, Tuckova L, Cukrowska B, Lodinova-Zadnikova R, et al. Commensal bacteria (normal microflora), mucosal immunity and chronic inflammatory and autoimmune diseases. Immunol Lett. 2004;93:97–108. doi: 10.1016/j.imlet.2004.02.005. [DOI] [PubMed] [Google Scholar]
- Torok HP, Glas J, Tonenchi L, Bruennler G, Folwaczny M, Folwaczny C. Crohn’s disease is associated with a toll-like receptor-9 polymorphism. Gastroenterology. 2004a;127:365–6. doi: 10.1053/j.gastro.2004.05.051. [DOI] [PubMed] [Google Scholar]
- Torok HP, Glas J, Tonenchi L, Mussack T, Folwaczny C. Polymorphisms of the lipopolysaccharide-signaling complex in inflammatory bowel disease: association of a mutation in the Toll-like receptor 4 gene with ulcerative colitis. Clin Immunol. 2004b;112:85–91. doi: 10.1016/j.clim.2004.03.002. [DOI] [PubMed] [Google Scholar]
- Viala J, Chaput C, Boneca IG, Cardona A, Girardin SE, Moran AP, et al. Nod1 responds to peptidoglycan delivered by the Helicobacter pylori cag pathogenicity island. Nat Immunol. 2004;5:1166–74. doi: 10.1038/ni1131. [DOI] [PubMed] [Google Scholar]
- Wang X, Huycke MM. Extracellular superoxide production by Enterococcus faecalis promotes chromosomal instability in mammalian cells. Gastroenterology. 2007;132:551–61. doi: 10.1053/j.gastro.2006.11.040. [DOI] [PubMed] [Google Scholar]
- Wyatt JI, Rathbone BJ. Immune response of the gastric mucosa to Campylobacter pylori. Scand J Gastroenterol Suppl. 1988;142:44–9. [PubMed] [Google Scholar]
- Xiao H, Gulen MF, Qin J, Yao J, Bulek K, Kish D, et al. The Toll-interleukin-1 receptor member SIGIRR regulates colonic epithelial homeostasis, inflammation, and tumorigenesis. Immunity. 2007;26:461–75. doi: 10.1016/j.immuni.2007.02.012. [DOI] [PubMed] [Google Scholar]