Abstract
Tea (Camellia sinensis, Theaceae) and tea polyphenols have been studied for the prevention of chronic diseases, including obesity. Obesity currently affects >20% of adults in the United States and is a risk factor for chronic diseases such as type II diabetes, cardiovascular disease, and cancer. Given this increasing public health concern, the use of dietary agents for the prevention of obesity would be of tremendous benefit. Whereas many laboratory studies have demonstrated the potential efficacy of green or black tea for the prevention of obesity, the underlying mechanisms remain unclear. The results of human intervention studies are mixed and the role of caffeine has not been clearly established. Finally, there is emerging evidence that high doses of tea polyphenols may have adverse side effects. Given that the results of scientific studies on dietary components, including tea polyphenols, are often translated into dietary supplements, understanding the potential toxicities of the tea polyphenols is critical to understanding their potential usefulness in preventing obesity. In this review, we will critically evaluate the evidence for the prevention of obesity by tea, discuss the relevance of proposed mechanisms in light of tea polyphenol bioavailability, and review the reports concerning the toxic effects of high doses of tea polyphenols and the implication that this has for the potential use of tea for the prevention of obesity. We hope that this review will expose areas for further study and encourage research on this important public health issue.
Introduction
Green tea (Camellia sinensis, Theaceae) is a popular beverage with worldwide consumption second only to water. The 3 main types of tea, green, black, and Oolong, which represent 20, 78, and 2% of world tea consumption, respectively, differ in terms of processing and chemical composition. In the processing of green tea, leaves are steamed or pan-fried to inhibit polyphenol oxidase activity. Chemically, green tea is characterized by the presence of large amounts polyphenols known as catechins (1). (-)-Epicatechin (EC),3 (-)-epigallocatechin (EGC), (-)-epicatechin-3-gallate (ECG), and (-)-epigallocatechin-3-gallate (EGCG) are the major green tea catechins (Fig. 1) (2). A typical cup of brewed green tea (2 g of leaves steeped in 200 mL of water) contains 240–320 mg of catechins, with EGCG accounting for 30–50% of that amount. In contrast, black tea is produced by crushing the tea leaves and allowing them to undergo an enzyme-mediated oxidation process known as fermentation. This process results in the conversion of the majority of catechins to theaflavins (TF) and thearubigins. The TF, which are benzotropolone-linked heterodimers of catechins, are responsible for the characteristic color and taste of black tea (Fig. 1). The thearubigins are oligomeric and polymeric polyphenolic compounds, which have only recently begun to be structurally characterized (3–5). Oolong tea is allowed to undergo partial oxidation, which results in the retention of higher levels of catechins than are found in black tea but also in the formation of unique dimeric and oligomeric polyphenols such as the theasinensins (Fig. 1) (6). Green, black, and Oolong teas also contain caffeine (∼2–5% by weight), theophylline, and theobromine.
FIGURE 1 .
Structures of representative tea polyphenols and caffeine.
Obesity is defined as a BMI ≥30 kg/m2. Between 1980 and 2007, the rate of obesity in the United States approximately doubled for the adult population (7). Similarly, there has been a dramatic increase in the rate of obesity in children. Obesity has been reported as a risk factor for a number of chronic diseases, including heart disease, liver disease, diabetes, cancer, arthritis, and others (8,9). For example, the EPIC study, a prospective study of 368,277 men and women in 9 European countries, found that waist circumference and waist:hip ratio were both positively correlated with increased risk of colon cancer in men and women (relative risk = 1.39–1.52) (10).
Finkelstein et al. (11) have reported that the public health impact of obesity in terms medical spending in the United States in 2006 was approximately $119 billion. Although surgical and pharmacological methods have been developed to treat obesity, these treatments can be costly and are not without potential adverse effects (12–14). The development of dietary agents for the prevention or treatment of obesity, and their use in combination with exercise and changes in energy consumption, could represent a cost-effective and safe means to deal with this growing public health crisis.
Tea and tea polyphenols have been extensively studied for their potential health beneficial effects. Epidemiological and laboratory studies have suggested that tea and tea polyphenols have preventive activity against a number of chronic diseases, including heart disease, neurodegenerative disease, cancer, diabetes, and obesity (15–18). Although laboratory studies using animal models have largely demonstrated obesity preventive effects of green tea, the effectiveness of Oolong and black teas has been less well studied. The effects of tea and tea polyphenols on obesity in humans have been suggested by epidemiological studies, but the number of controlled intervention studies is limited and the quality of the study designs is mixed. The roles of tea polyphenols and caffeine in the prevention of obesity by tea, and the interaction between these compounds, have not been fully elucidated. Finally, whereas a substantial collection of in vitro mechanistic studies have been reported, the relevance of these potential mechanisms have not been fully determined in vivo.
In this review, we summarize the literature on the potential efficacy of tea as an obesity-preventive agent. We critically analyze the conclusions of the reviewed studies, evaluate the proposed mechanisms of action, and suggest potential areas for future studies.
Laboratory Studies
Mechanistic studies using cell lines and purified enzymes have focused on the ability of tea polyphenols to inhibit differentiation or induce apoptosis in adipocyte-like cell lines, modulate transport mechanisms of carbohydrates and lipids, alter the expression of genes involved in energy metabolism, or directly inhibit key cellular enzymes [reviewed in (19,20)]. The potential relevance of these studies is unclear in most cases, although for some there is good correlation between mechanisms proposed in vitro and effects observed in vivo. For the purposes of this review, we have focused on mechanistic data generated from animal model studies (Fig. 2). If corresponding in vitro studies have been performed, we have included these for comparison. Mechanisms based solely on in vitro studies, and lacking correlative in vivo data, are beyond the scope of this review. Many animal studies have examined the effects of tea on obesity and related pathologies, including diabetes, hypercholesterolemia, and fatty liver disease [reviewed in (20,21)]. Most studies have used green tea, green tea extract (GTE), or pure EGCG in conjunction with both genetic and dietary models of obesity. Several key studies are reviewed in detail below.
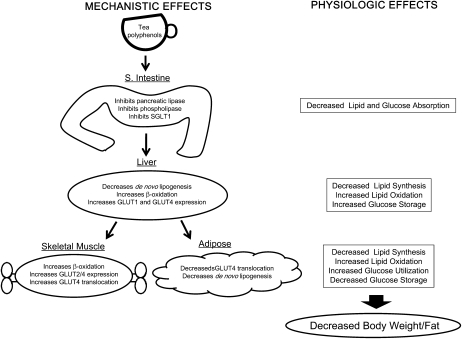
FIGURE 2 .
Reported mechanism(s) by which tea polyphenols may modulate body weight and energy balance. Tea polyphenols have been shown to inhibit de novo lipogenesis, increase lipid oxidation, increase carbohydrate utilization, and decrease carbohydrate uptake. Target tissues include the small intestine, the liver, adipose tissue, and skeletal muscle. Abbreviations: S. intestine, small intestine.
Tea and dietary animal models of obesity
Bose et al. (22) reported that treatment with 7.0 μmol/g dietary EGCG for 15 wk reduced body weight gain (33–41%) in high-fat–fed male C57bl/6J mice compared with high-fat–fed controls. In addition, the EGCG-treated mice had significantly lower adipose tissue weight, fasting blood glucose, fasting plasma cholesterol, and plasma alanine aminotransferase levels. The EGCG-treated mice had higher fecal lipid concentrations compared with the high-fat–fed mice; there was a strong inverse correlation between fecal lipid content and body weight gain. This suggests that EGCG-mediated modulation of dietary fat could account for the decrease in body weight observed in the EGCG-treated mice (Fig. 2).
The same group reported that short-term (4 wk) dietary EGCG treatment (7.0 μmol/g) of obese C57bl/6J mice tended to reduce body weight gain compared with high-fat–fed controls. Although the decrease in body weight gain was not significant, there were significant reductions in mesenteric adipose tissue weight (36%) and fasting blood glucose (22%) in the EGCG-treated mice compared with high-fat–fed controls. This treatment regimen represents a more realistic obesity-related application of EGCG or green tea supplementation, because the most likely consumers of these products would have a preexisting weight problem. Given the small sample size (n = 10) and limited mechanistic data of this short-term study, further studies are warranted.
Wolfram et al. (23) demonstrated the effect of TEAVIGO (a proprietary GTE that contains 90% EGCG) on obesity. Treatment of high-fat/high-sucrose–fed C57bl/6J mice with 10 mg/g dietary TEAVIGO resulted in decreased body weight gain, fed-state plasma glucose, plasma triglycerides, and plasma leptin compared with high-fat/high-sucrose–fed controls. In the same study, the authors reported that 4-wk treatment with 10 mg/g dietary TEAVIGO could reduce body weight gain and body fat weight in obese Spague-Dawley rats (n = 8). Gene expression studies revealed that TEAVIGO treatment decreased adipose mRNA levels of fatty acid synthase (FAS) and acetyl CoA carboxylase-1, both key enzymes in de novo fatty acid synthesis. These observations suggest that tea polyphenols may modulate obesity by inhibiting de novo lipogenesis (Fig. 2).
In most obesity studies of tea in animals, the diets contain very high concentrations of fat (40–60%energy) that may not represent realistic consumption patterns. A recent study by Yuko et al. (24) is an exception. In this study, the effect of tea catechins (1 and 5 g/L in the drinking fluid) administered for 3 wk was determined in male Wistar rats fed a normal-fat diet (10%energy). The 0.5% catechin-treated group had significantly decreased body weight compared with the water-treated control. Both tea catechin groups had lower levels of serum cholesterol, serum triglycerides, and bile acids compared with the control; these values were dose dependent. Mesenteric and liver lipids were also dose dependently reduced compared with the water-treated group. By incorporating tea catechins into a normal diet, this group suggests that catechins can modulate lipid metabolism in nonobese subjects. This is a very important finding and should be confirmed with additional studies.
A more limited number of studies have examined the obesity-preventive effects of Oolong and black tea. Yang et al. (25) compared the effect of GTE, Oolong tea extract, and black tea extract (10 g/L in drinking fluid) in rats fed a high-sucrose diet. Both Oolong and black tea extract-treated rats, but not GTE-treated rats, had decreased body weight gains (28.8–35.0%) and feeding efficiency (19.8–32.2%) (27). In all of the tea-treated groups, plasma and liver triglycerides were reduced. It is interesting that GTE did not affect body weight gain in this study. The reasons for this are unclear but may be related to the polyphenol composition, particularities with the model, or some other unknown factors.
Tea and genetic animal models of obesity
The efficacy of GTE in the B6.V-Lepob/J (ob/ob) leptin-deficient mice has been examined (26). These mice were fed a high-fat diet supplemented with 0.5 mg/g dietary GTE (55% EGCG, 15% EGC, 21% ECG, and 8% EC; wt:wt) for 12 wk. GTE treatment resulted in a significant reduction in perirenal and total white adipose tissue weights compared with high-fat–fed control mice. Also, the GTE-treated mice had higher plasma HDL cholesterol and lower hepatic triglycerides. These effects on lipid homeostasis correlated with decreased adipose and hepatic expression of glucose-6-phosphate dehydrogenase and malic enzyme, 2 enzymes involved in fatty acid synthesis. Interestingly, there was no significant effect of GTE on body weight. This could be due to the very low dose of GTE used. Despite the lack of effect on body weight, the observed changes in lipid homeostasis are interesting.
Bruno et al. (27) compared the effects of treatment with diets containing 1 and 2% (wt:wt) GTE for 6 wk on ob/ob mice (n = 24) and C57bl/6J lean littermates. They found that lean and ob/ob mice treated with GTE weighed less than their respective non-GTE–treated controls. Furthermore, the GTE was more effective in the ob/ob mice than in the lean C57bl/6J mice. The obese mice weighed 23–25% less than controls, whereas the lean mice were only 11–20% less. In addition to reduced weight gain, this study found that GTE treatment reduced the extent of hepatic steatosis in ob/ob mice compared with ob/ob mice not treated with GTE. No dose-response relationship was observed for GTE, however, suggesting that 1% GTE may be the maximally effective dose. A dose-response study with lower doses of GTE would be interesting and useful for determining the minimum effective dose. This study did not state whether the GTE contained caffeine, an omission that limits interpretation of the results.
Interaction with exercise and tea in animal models of obesity
Exercise in combination with dietary changes to induce a negative energy balance has been the most common recommendation for weight loss. Few studies, however, have systematically examined the interaction between tea polyphenols and exercise. Murase et al. (28) have shown that mice consuming a 20%energy fat diet supplemented with 1.0–5.0 mg/g GTE and put on an exhaustive swimming regimen for 10 wk had increased swimming times compared with Balb/c mice that only had the swimming regimen. Mice treated with GTE also had decreased adipose tissue weight, increased oxygen consumption, and increased β-oxidation activity in muscle compared with mice given the swimming regimen only.
In a second study, high-fat–fed C57bl/6J mice were treated with 5 mg/g dietary catechins, 30 min swimming 3 times/wk, or the combination for 15 wk (29). Body weight gain was reduced by 18% in the high-fat plus catechins group and 14% in the high-fat plus exercise group compared with the high-fat diet-treated mice not given catechins or exercise. The combination of high fat plus catechins and exercise group resulted in the greatest difference; body weight gain decreased by 33% compared with the high-fat–treated group. Mice on the catechins plus swimming regimen had higher muscular β-oxidation activity and higher lipid oxidation as determined by indirect calorimetry compared with the high fat and the high fat plus catechins group. Future studies should be conducted to determine the different dose-response relationships for tea catechins and exercise time, as well as the effect of different types of exercise (forced vs. voluntary, running wheel, etc.). These studies should also be extended to include obese as well as lean mice.
Related in vitro mechanistic studies
Tea and fat metabolism.
The in vivo effects of EGCG may be explained by underlying mechanisms suggested by in vitro studies of de novo lipogenesis, lipid absorption, and carbohydrate absorption and utilization (Fig. 2). Several recent in vitro studies have begun to examine the effects of tea polyphenols on FAS. Wang et al. (30,31) reported that EGCG and ECG, but not EC and EGC, inhibit FAS with inhibitory concentration 50% (IC50) = 52 and 42 μmol/L, respectively. Another study found that black tea extract inhibited FAS activity more potently than GTE, but the active components were not identified (32). The mechanisms of these inhibitory effects remain to be examined in more detail.
Two studies have reported that EGCG can inhibit pancreatic lipase activity, but the effective concentrations in these studies varied from 0.4 μmol/L to 1 mmol/L (6,33). The reasons for these large differences in effective concentration are unclear but could be related to differences in enzyme source or substrate used. Neither study presented clear dose-response curves for the inhibition. Both studies reported that the nongallated catechins (EC, catechins, and EGC) were much less effective inhibitors than the gallated catechins (ECG, EGCG, and gallocatechin-3-gallate). Nakai et al. (6) have reported that TF, TF-3-gallate (TF-3-G), and TF-3,3′-digallate potently inhibited pancreatic lipase activity (IC50 = 0.09–0.11 μmol/L). Interestingly, the gallated TF (IC50 = 0.09 and 0.11 μmol/L) and TF (IC50 = 0.10 μmol/L) did not differ. This suggests an alternative inhibitory mechanism or binding site for the TF compared with the catechins. This hypothesis should be tested using classical enzymology studies in coordination with in silico modeling techniques. Pancreatic phospholipase A2 (PLA2) has also been studied as a potential target for inhibition by tea catechins. The IC50 values for the inhibition of pancreatic PLA2 by catechin, EC, EGC, ECG, and EGCG were >6.0, >6.0, >6.0, 2.8, and 1.6 mmol/L, respectively (34). Although these inhibitory concentrations are very high, pancreatic PLA2 might still be a relevant target, because it is secreted into the intestinal lumen, where the concentrations of tea polyphenols are expected to be very high. Further, several studies in animal models have shown that treatment with tea preparations reduces dietary lipid absorption and postprandial lipid concentrations (22,33).
Tea and glucose uptake and utilization.
Several studies in dietary models of obesity have examined the role of green tea-mediated modulation of glucose uptake and disposition in obesity prevention. For example, treatment of normal-weight, male Wistar rats with green tea (composition and dose were not defined) for 3 wk resulted in a significant decrease in translocation of glucose transporter (GLU) 4 to the plasma membrane in adipose tissue and decreased glucose uptake by that tissue (35). Conversely, translocation of GLUT4 to the plasma membrane in skeletal muscle and glucose uptake were enhanced. Both of these findings indicated that treatment with green tea can increase carbohydrate catabolism. In a study of fructose-fed Wistar rats, Cao et al. (36) reported that treatment with 1 or 2 mg/g dietary GTE increased the mRNA expression of GLUT1 and GLUT4 in the liver and the mRNA expression of GLUT2 and GLUT4 in the skeletal muscle.
A role for inhibition of glucose uptake from the gut has been suggested by in vitro studies. Both EGCG and ECG have been shown to inhibit glucose transport by sodium-dependent glucose cotransporter 1 (SGLT1) when the transporter was expressed in Xenopus oocytes (37). The inhibitory constant for EGCG and ECG were 0.45 mmol/L. These concentrations are high, but because SGLT1 is expressed in the intestine, the levels may be achievable following oral administration of tea polyphenols. These findings confirmed earlier work showing that ECG is a competitive inhibitor of SGLT1, but the potential role of SGLT1 in vivo remains to be determined (38).
In studies of animal models of obesity and tea reported to date, the majority found no significant effect of tea or tea components on energy intake. Two exceptions have been reported. Yang et al. (25) reported a significant decrease (11.5%) in food intake by mice treated with Oolong tea extract compared with mice not treated with tea. Murase et al. (39) reported that 0.5% tea catechins reduced energy intake by 5.6%, although the trend was not significant. These results suggest that tea treatment reduces feeding efficiency rather than food intake.
The papers reviewed above demonstrate the potential efficacy of tea as an antiobesity agent. However, further studies are needed, particularly on the efficacy and mechanisms of action of black and Oolong teas and the interaction between caffeine and the tea polyphenols with regard to weight loss. A potential problem with the current body of literature is related to the amount of fat used in the experimental diets. The typical American diet contains 30%energy fat, whereas most of the reported animal studies used diets with 40–60%energy fat (40). Studies with a lower fat diet (30–40%energy) might produce data more relevant to the human situation. Similarly, weight loss regimens recommended to obese and overweight individuals often include reduced energy intake and exercise. Future studies should incorporate these factors in examining the efficacy of tea and tea polyphenols.
Human Studies
Epidemiological studies
A limited number of epidemiology studies have examined the impact of tea on body weight and other markers related to obesity. A 2003 cross-sectional epidemiological study of 1103 Taiwanese adults found that habitual tea drinkers (defined as someone who consumed tea at least once per week for 6 mo) who consumed tea for >10 y had lower percentage body fat (19.6% decrease) and waist:hip ratio (2.1% decrease) compared with nonhabitual consumers (41). In this study, green tea and Oolong tea were consumed more frequently than black tea (41.3 vs. 1.6%). A longitudinal analysis within The Netherlands Cohort study of 4280 adults found an inverse relationship between catechin consumption over the 14-y study period and BMI increase (42). The BMI increases for the lowest and highest quintiles of catechin consumption were 0.77 and 0.31 kg/m2, respectively.
Human intervention studies on tea and weight gain
A larger number of intervention studies have been conducted on the effect of tea on markers of obesity [reviewed in (19,21)]. In 2005, Nagao et al. (43) examined the effect of supplementing Oolong tea with 2 different concentrations of GTE [containing either 22 mg (low) or 690 mg (high) catechins] on body weight and other markers in 35 healthy Japanese men. Following treatment with low or high catechin Oolong tea once daily for 12 wk, participants consuming high-catechin tea had lower body weight (2.4-kg decrease), BMI (0.8 kg/m2 decrease), waist circumference (3.4-cm decrease), and body fat mass (1.4-kg decrease) compared with baseline. These decreases were significantly greater in the high catechin tea-treated participants than in the low-catechin tea-treated participants.
In a follow-up study by the same group, 240 obese, Japanese participants were treated with a catechin-enriched green tea beverage (583 mg catechins) or a control green tea beverage (96 mg) once daily for 12 wk (44). Participants consuming the high-catechin beverage had a significant decrease in body weight (2.3% decrease), total fat area (4.9% decrease), and visceral fat (9.4% decrease) compared with baseline values. Percent body fat, waist and hip circumference, and LDL cholesterol were also significantly decreased compared with baseline. All of these decreases were greater in the high-catechin–treated group than in the low-catechin controls. Similar results were also reported in a study of Japanese participants with type II diabetes that were given 72.3 or 582.8 mg catechins in 1 can of green tea beverage per day (45). After the 12-wk period, the participants with 582.8 mg catechins had a significant decrease in waist circumference (3.67%) and systolic blood pressure (4.34%) from baseline values. In addition, there was a significant decrease in serum triglycerides (19.1%) and total cholesterol (2.4%) and an increase in insulin (37.0%) compared with the control group. In all 3 of these studies by Nagao et al. (43–45), the amount of caffeine in the tea beverages were held constant (21–23 mg) whereas the catechin concentration was varied. This strongly supports the hypothesis that catechins can affect markers of obesity.
The results of studies on tea and body weight and body fat have not been universally positive. For example, in a trial of 60 overweight or obese Thai subjects, ingestion of green tea capsules (250 mg each containing 33.6 mg of EGCG, 3 times/d) for 12 wk resulted in a significant decrease in body weight (3.9%) compared with baseline and placebo control, but there was no significant effect on BMI, waist:hip ratio, or percent body fat (46). A similar result was observed in a study of 46 overweight women in the Netherlands (47). Treatment with GTE (375 mg catechins) for 87 d in combination with reduced calorie diet resulted in no significant effect on BMI, waist:hip ratio, or fat mass. The doses of tea catechins used in these negative studies were somewhat lower than those used in the studies that reported positive doses. So the observed differences may be a dose effect and such an issue will be resolved only with carefully designed dose-response studies in a well-selected population.
Human intervention studies on tea and weight maintenance
Another critical question regarding the potential efficacy of tea as an antiobesity agent relates to its ability to aid in weight maintenance following weight loss. A recent meta-analysis of 11 studies on green tea and weight loss showed that habitual consumption of green tea had a beneficial effect on weight loss (mean weight loss of −1.3 kg) and aided weight maintenance following weight loss (48). The effects of green tea were modulated by chronic, high (>300 mg/d) intake of caffeine. Westererp-Plantenga et al. (49) reported that treatment of overweight or moderately obese subjects from the Netherlands (n = 76) with EGCG/caffeine capsules (0.55 mmol/0.77 mmol total daily dose) for 3 mo following weight loss resulted in further decrease in body weight and increased fat oxidation in people who habitually consumed <1.55 mmol caffeine/d. In individuals who chronically consumed >1.55 mmol caffeine/d, these weight maintenance effects were lost. This is somewhat counterintuitive given that increased caffeine consumption should facilitate weight loss and energy expenditure. The mechanism by which high chronic caffeine consumption affects weight maintenance by EGCG + caffeine is unclear but could be related to modulation of either EGCG or caffeine metabolism. The results of this study should be confirmed and the potential underlying mechanisms examined.
Human intervention studies on the interactions between tea and exercise
Maki et al. (50) examined the interaction between exercise and green tea consumption on weight loss in 107 overweight or obese subjects from the United States. Subjects consumed either a green tea beverage (625 mg catechins) or a placebo once per day in addition to 180 min exercise/wk. After 12 wk of treatment, there was a trend toward a greater decrease in body weight in the catechins group compared with the exercise-only control, but the effect was not significant. The catechin-treated group had a greater decrease from baseline in total abdominal fat (7.7 vs. 0.3% decrease) and serum triglycerides (11.2 vs. 1.9% decrease) than the exercise-only control. Given the combination effects of tea and exercise in animal models (see above), further studies on the interaction between tea and exercise in humans are warranted.
Other considerations
Effect of tea polyphenol bioavailability.
Our laboratory and others have extensively studied the biotransformation and bioavailability of the tea polyphenols (51–54). Typically, the plasma concentration of EGCG in human volunteers following consumption of a dose equivalent to 1 cup of green tea is ∼1 μmol/L (55,56). If pharmacological doses of EGCG (2.6 mmol) are administered to fasted volunteers, peak plasma concentrations reach 7.4 μmol/L (57). The plasma concentrations of TF following oral administration of black tea are much lower (<1 nmol/L) (58). These concentrations are well below those used in most of the in vitro mechanistic studies conducted, which typically use concentrations of tea polyphenols ranging from 10 to 1000 μmol/L (59–63). In the absence of supporting mechanistic data in animal model or human tissues, it is difficult to determine the relevance of some of the proposed mechanistic results. The effects of EGCG and other tea polyphenols on pancreatic lipase represent a possible exception to this trend. Studies have shown relatively high potency for inhibition of pure enzyme (IC50 for EGCG ∼0.5 μmol/L) and data from animal studies show that EGCG can increase fecal lipid and energy content (6,22,64). Future studies should focus on prioritizing potential targets derived from in vitro studies based on effective concentration and correlating these findings to mechanistic studies in animals.
Potential hepatotoxicity of high doses of green tea polyphenols.
One effect of the increasing number of reports describing the potential antiobesity and other beneficial effects of tea and tea polyphenols has been a proliferation of green tea-based dietary supplements. Sales of green tea-based dietary supplements in the US totaled (USD) 5.6 million in 2005, an increase of 94% from 2004 (65). Green tea supplements typically contain 0.4–8 mmol EGCG and recommend dosing of 1–2 capsules up to 3 times/d. This results in a total recommended dose that may be up to 5.2 mmol/d. Although there have been no reported adverse effects associated with green tea beverage consumption, green tea-based dietary supplements represent a different dosage form and have the potential to deliver a much higher dose of catechins than green tea beverages. Indeed, studies from our laboratory have shown that oral bolus dosing results in greatly increased peak plasma concentrations of EGCG compared with dietary administration of the same total daily dose [(66) and J. D. Lambert, unpublished results]. Treatment of mice with a single oral bolus dose of 1.1 mg/kg EGCG result in peak plasma concentrations of 2.0 μmol/L, whereas the same total daily dose given via the diet result in plasma concentrations of 0.5 μmol/L.
Since 1999, there have been 34 case studies linking consumption of green tea-based supplements to hepatotoxicity [reviewed in (67)]. In most cases, elevations in serum transaminase levels, as well as increased serum bilirubin, were observed. Histological examination revealed inflammatory, cholestatic, or necrotic liver damage depending on the subject. No clear determinants for the type of pathology observed have been reported. In ∼20% of these reported case studies, additional liver damage following rechallenge with the same preparation was observed. This suggests a causal relationship between hepatotoxicity and green tea.
Laboratory studies of green tea-derived preparations in rodents and dogs have generally supported the potential toxicity of those preparations at high doses (68,69). Oral administration of TEAVIGO or Polyphenon E (standardized tea polyphenol preparation containing 1.4 mmol/g EGCG, 0.16 mmol/g ECG, 0.10 mmol/g EGC, 0.31 mmol/g EC, and 6 mg/g caffeine) for 13 or 9 wk, respectively, to Beagle dogs resulted in dose-dependent toxicity and death (68). Vomiting and diarrhea were observed throughout both studies. In addition, 500 mg/kg, p.o. TEAVIGO caused proximal tubule necrosis and elevated serum bilirubin in all dogs treated. Most male dogs (2 of 3) had elevated serum aspartate aminotransferase levels. Female dogs (2 of 3), but not male dogs, had liver necrosis. Oral administration of 2000 mg/kg i.g. TEAVIGO to rats resulted in lethality in 80% of the animals treated (68). Histological analysis revealed hemorrhagic lesions in the stomach and intestine. Although the toxicity of orally administered EGCG or tea polyphenols has not been reported in mice, intraperitoneal administration of EGCG to CD-1 mice resulted in dose-dependent lethality beginning at 0.33 mmol/kg (69). Lethality was associated with increases in serum alanine aminotransferase levels, suggesting the involvement of hepatotoxicity.
These findings suggest that caution should be exercised in the use of green tea-based dietary supplements and that further studies are needed to determine the upper limit of safety for bolus dosing with tea polyphenols as well as the underlying mechanisms of toxicity. Further, no studies to our knowledge have been conducted to determine the upper limit of safety for TF or other higher molecular weight tea polyphenols. Such studies are also warranted.
In conclusion, laboratory studies in animals, a limited number of epidemiological studies, and small-scale human intervention studies support the hypothesis that tea and tea polyphenols have beneficial effects on weight gain, weight loss, and prevention of obesity. Although mechanistic studies have suggested that tea decreases lipid and carbohydrate absorption, increases lipid metabolism, inhibits de novo lipogenesis, and increases carbohydrate utilization, the relative importance of these mechanisms to human disease remains unclear. The majority of studies have been conducted using green tea, whereas a more limited number of have been conducted on black and Oolong teas. Given the high prevalence of black tea consumption (∼80% of worldwide tea consumption), further studies on the antiobesity effects of this beverage could be of great public health importance. The rapidly increasing incidence of obesity in the United States and the rest of the world makes it imperative to clearly understand the efficacy and mechanisms of action of potential preventive agents derived from the diet. Just as important, the therapeutic index of these agents must be established, not only when the compounds are delivered via the diet but also when (as they often are) converted to a bolus formulation (e.g. pill, capsule, tincture). Only with such a complete understanding can the potential benefits of dietary components with antiobesity effects, including tea, be realized.
Acknowledgments
K.A.G and J.D.L. performed literature search and wrote the manuscript. Both authors read and approved this manuscript.
Supported in part by NIH AT004678.
Author disclosures: K. A. Grove and J. D. Lambert, no conflicts of interest.
Abbreviations used: EC, (-)-epicatechin; ECG, (-)-epigcatechin-3-gallate; EGC, (-)-epigallocatechin; EGCG, (-)-epigallocatechin-3-gallate; FAS, fatty acid synthase; GLUT, glucose transporter; GTE, green tea extract; IC50, inhibitory concentration 50%; PLA2, phospholipase A2; SGLT1, sodium-dependent glucose transporter 1; TF, theaflavin; TF-3-G, theaflavin-3-gallate.
References
- 1.Yang CS, Maliakal P, Meng X. Inhibition of carcinogenesis by tea. Annu Rev Pharmacol Toxicol. 2002;42:25–54. [DOI] [PubMed] [Google Scholar]
- 2.Balentine DA, Wiseman SA, Bouwens LC. The chemistry of tea flavonoids. Crit Rev Food Sci Nutr. 1997;37:693–704. [DOI] [PubMed] [Google Scholar]
- 3.Sang S, Tian S, Stark RE, Yang CS, Ho CT. New dibenzotropolone derivatives characterized from black tea using LC/MS/MS. Bioorg Med Chem. 2004;12:3009–17. [DOI] [PubMed] [Google Scholar]
- 4.Menet MC, Sang S, Yang CS, Ho CT, Rosen RT. Analysis of theaflavins and thearubigins from black tea extract by MALDI-TOF mass spectrometry. J Agric Food Chem. 2004;52:2455–61. [DOI] [PubMed] [Google Scholar]
- 5.Sang SM, Tian SY, Meng XF, Stark RE, Rosen RT, Yang CS, Ho CT. Theadibenzotropolone A, a new type pigment from enzymatic oxidation of (-)-epicatechin and (-)-epigallocatechin gallate and characterized from black tea using LC/MS/MS. Tetrahedron Lett. 2002;43:7129–33. [Google Scholar]
- 6.Nakai M, Fukui Y, Asami S, Toyoda-Ono Y, Iwashita T, Shibata H, Mitsunaga T, Hashimoto F, Kiso Y. Inhibitory effects of oolong tea polyphenols on pancreatic lipase in vitro. J Agric Food Chem. 2005;53:4593–8. [DOI] [PubMed] [Google Scholar]
- 7.Anonymous. State-specific prevalence of obesity among adults: United States, 2007. Atlanta: CDC; 2008. [PubMed]
- 8.Poirier P, Giles TD, Bray GA, Hong Y, Stern JS, Pi-Sunyer FX, Eckel RH. Obesity and cardiovascular disease: pathophysiology, evaluation, and effect of weight loss. Arterioscler Thromb Vasc Biol. 2006;26:968–76. [DOI] [PubMed] [Google Scholar]
- 9.Guh DP, Zhang W, Bansback N, Amarsi Z, Birmingham CL, Anis AH. The incidence of co-morbidities related to obesity and overweight: a systematic review and meta-analysis. BMC Public Health. 2009;9:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pischon T, Lahmann PH, Boeing H, Friedenreich C, Norat T, Tjonneland A, Halkjaer J, Overvad K, Clavel-Chapelon F, et al. Body size and risk of colon and rectal cancer in the European Prospective Investigation Into Cancer and Nutrition (EPIC). J Natl Cancer Inst. 2006;98:920–31. [DOI] [PubMed] [Google Scholar]
- 11.Finkelstein EA, Trogdon JG, Cohen JW, Dietz W. Annual medical spending attributable to obesity: payer- and service-specific estimates. Health Aff (Millwood). 2009;28:w822–w831. [DOI] [PubMed]
- 12.Pories WJ. Bariatric surgery: risks and rewards. J Clin Endocrinol Metab. 2008;93:S89–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Poitou Bernert C, Ciangura C, Coupaye M, Czernichow S, Bouillot JL, Basdevant A. Nutritional deficiency after gastric bypass: diagnosis, prevention and treatment. Diabetes Metab. 2007;33:13–24. [DOI] [PubMed] [Google Scholar]
- 14.Filippatos TD, Derdemezis CS, Gazi IF, Nakou ES, Mikhailidis DP, Elisaf MS. Orlistat-associated adverse effects and drug interactions: a critical review. Drug Saf. 2008;31:53–65. [DOI] [PubMed] [Google Scholar]
- 15.Lambert JD, Hong J, Yang GY, Liao J, Yang CS. Inhibition of carcinogenesis by polyphenols: evidence from laboratory investigations. Am J Clin Nutr. 2005;81:S284–91. [DOI] [PubMed] [Google Scholar]
- 16.Yang CS, Lambert JD, Ju J, Lu G, Sang S. Tea and cancer prevention: molecular mechanisms and human relevance. Toxicol Appl Pharmacol. 2007;224:265–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Higdon JV, Frei B. Tea catechins and polyphenols: health effects, metabolism, and antioxidant functions. Crit Rev Food Sci Nutr. 2003;43:89–143. [DOI] [PubMed] [Google Scholar]
- 18.Wolfram S, Wang Y, Thielecke F. Anti-obesity effects of green tea: from bedside to bench. Mol Nutr Food Res. 2006;50:176–87. [DOI] [PubMed] [Google Scholar]
- 19.Kao YH, Chang HH, Lee MJ, Chen CL. Tea, obesity, and diabetes. Mol Nutr Food Res. 2006;50:188–210. [DOI] [PubMed] [Google Scholar]
- 20.Moon HS, Lee HG, Choi YJ, Kim TG, Cho CS. Proposed mechanisms of (-)-epigallocatechin-3-gallate for anti-obesity. Chem Biol Interact. 2007;167:85–98. [DOI] [PubMed] [Google Scholar]
- 21.Thielecke F, Boschmann M. The potential role of green tea catechins in the prevention of the metabolic syndrome: a review. Phytochemistry. 2009;70:11–24. [DOI] [PubMed] [Google Scholar]
- 22.Bose M, Lambert JD, Ju J, Reuhl KR, Shapses SA, Yang CS. The major green tea polyphenol, (-)-epigallocatechin-3-gallate, inhibits obesity, metabolic syndrome, and fatty liver disease in high-fat-fed mice. J Nutr. 2008;138:1677–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wolfram S, Raederstorff D, Wang Y, Teixeira SR, Elste V, Weber P. TEAVIGO (epigallocatechin gallate) supplementation prevents obesity in rodents by reducing adipose tissue mass. Ann Nutr Metab. 2005;49:54–63. [DOI] [PubMed] [Google Scholar]
- 24.Ito Y, Ichikawa T, Morohoshi Y, Nakamura T, Saegusa Y, Ishihara K. Effect of tea catechins on body fat accumulation in rats fed a normal diet. Biomed Res. 2008;29:27–32. [DOI] [PubMed] [Google Scholar]
- 25.Yang M, Wang C, Chen H. Green, oolong and black tea extracts modulate lipid metabolism in hyperlipidemia rats fed high-sucrose diet. J Nutr Biochem. 2001;12:14–20. [DOI] [PubMed] [Google Scholar]
- 26.Kim HJ, Jeon SM, Lee MK, Jung UJ, Shin SK, Choi MS. Antilipogenic effect of green tea extract in C57BL/6J-Lep ob/ob mice. Phytother Res. 2009;23:467–71. [DOI] [PubMed] [Google Scholar]
- 27.Bruno RS, Dugan CE, Smyth JA, DiNatale DA, Koo SI. Green tea extract protects leptin-deficient, spontaenously obese mice from hepatic steatosis and injury. J Nutr. 2008;138:323–31. [DOI] [PubMed] [Google Scholar]
- 28.Murase T, Haramizu S, Shimotoyodome A, Nagasawa A, Tokimitsu I. Green tea extract improves endurance capacity and increases muscle lipid oxidation in mice. Am J Physiol Regul Integr Comp Physiol. 2005;288:708–15. [DOI] [PubMed]
- 29.Murase T, Haramizu S, Shimotoyodome A, Tokimitsu I. Reduction of diet-induced obesity by a combination of tea-catechin intake and regular swimming. Int J Obes (Lond). 2006;30:561–8. [DOI] [PubMed] [Google Scholar]
- 30.Wang X, Song KS, Guo QX, Tian WX. The galloyl moiety of green tea catechins is the critical structural feature to inhibit fatty-acid synthase. Biochem Pharmacol. 2003;66:2039–47. [DOI] [PubMed] [Google Scholar]
- 31.Wang X, Tian W. Green tea epigallocatechin gallate: a natural inhibitor of fatty-acid synthase. Biochem Biophys Res Commun. 2001;288:1200–6. [DOI] [PubMed] [Google Scholar]
- 32.Du YT, Wang X, Wu XD, Tian WX. Keemun black tea extract contains potent fatty acid synthase inhibitors and reduces food intake and body weight of rats via oral administration. J Enzyme Inhib Med Chem. 2005;20:349–56. [DOI] [PubMed] [Google Scholar]
- 33.Ikeda I, Tsuda K, Suzuki Y, Kobayashi M, Unno T, Tomoyori H, Goto H, Kawata Y, Imaizumi K, et al. Tea catechins with a galloyl moiety suppress postprandial hypertriacylglycerolemia by delaying lymphatic transport of dietary fat in rats. J Nutr. 2005;135:155–9. [DOI] [PubMed] [Google Scholar]
- 34.Wang S, Noh SK, Koo SI. Green tea catechins inhibit pancreatic phospholipase A (2) and intestinal absorption of lipids in ovariectomized rats. J Nutr Biochem. 2006;17:492–8. [DOI] [PubMed] [Google Scholar]
- 35.Ashida H, Furuyashiki T, Nagayasu H, Bessho H, Sakakibara H, Hashimoto T, Kanazawa K. Anti-obesity actions of green tea: possible involvements in modulation of the glucose uptake system and suppression of the adipogenesis-related transcription factors. Biofactors. 2004;22:135–40. [DOI] [PubMed] [Google Scholar]
- 36.Cao H, Hininger-Favier I, Kelly MA, Benaraba R, Dawson HD, Coves S, Roussel AM, Anderson RA. Green tea polyphenol extract regulates the expression of genes involved in glucose uptake and insulin signaling in rats fed a high fructose diet. J Agric Food Chem. 2007;55:6372–8. [DOI] [PubMed] [Google Scholar]
- 37.Hossain SJ, Kato H, Aoshima H, Yokoyama T, Yamada M, Hara Y. Polyphenol-induced inhibition of the response of na (+)/glucose cotransporter expressed in Xenopus oocytes. J Agric Food Chem. 2002;50:5215–9. [DOI] [PubMed] [Google Scholar]
- 38.Shimizu M, Kobayashi Y, Suzuki M, Satsu H, Miyamoto Y. Regulation of intestinal glucose transport by tea catechins. Biofactors. 2000;13:61–5. [DOI] [PubMed] [Google Scholar]
- 39.Murase T, Nagasawa A, Suzuki J, Hase T, Tokimitsu I. Beneficial effects of tea catechins on diet-induced obesity: stimulation of lipid catabolism in the liver. Int J Obes Relat Metab Disord. 2002;26:1459–64. [DOI] [PubMed] [Google Scholar]
- 40.Newmark HL, Yang K, Lipkin M, Kopelovich L, Liu Y, Fan K, Shinozaki HA. Western-style diet induces benign and malignant neoplasms in the colon of normal C57Bl/6 mice. Carcinogenesis. 2001;22:1871–5. [DOI] [PubMed] [Google Scholar]
- 41.Wu CH, Lu FH, Chang CS, Chang TC, Wang RH, Chang CJ. Relationship among habitual tea consumption, percent body fat, and body fat distribution. Obes Res. 2003;11:1088–95. [DOI] [PubMed] [Google Scholar]
- 42.Hughes LA, Arts IC, Ambergen T, Brants HA, Dagnelie PC, Goldbohm RA, van den Brandt PA, Weijenberg MP. Higher dietary flavone, flavonol, and catechin intakes are associated with less of an increase in BMI over time in women: a longitudinal analysis from the Netherlands Cohort Study. Am J Clin Nutr. 2008;88:1341–52. [DOI] [PubMed] [Google Scholar]
- 43.Nagao T, Komine Y, Soga S, Meguro S, Hase T, Tanaka Y, Tokimitsu I. Ingestion of a tea rich in catechins leads to a reduction in body fat and malondialdehyde-modified LDL in men. Am J Clin Nutr. 2005;81:122–9. [DOI] [PubMed] [Google Scholar]
- 44.Nagao T, Hase T, Tokimitsu I. A green tea extract high in catechins reduces body fat and cardiovascular risks in humans. Obesity (Silver Spring). 2007;15:1473–83. [DOI] [PubMed] [Google Scholar]
- 45.Nagao T, Meguro S, Hase T, Otsuka K, Komikado M, Tokimitsu I, Yamamoto T, Yamamoto K. A catechin-rich beverage improves obesity and blood glucose control in patients with type 2 diabetes. Obesity (Silver Spring). 2009;17:310–7. [DOI] [PubMed] [Google Scholar]
- 46.Auvichayapat P, Prapochanung M, Tunkamnerdthai O, Sripanidkulchai BO, Auvichayapat N, Thinkhamrop B, Kunhasura S, Wongpratoom S, Sinawat S, et al. Effectiveness of green tea on weight reduction in obese Thais: a randomized, controlled trial. Physiol Behav. 2008;93:486–91. [DOI] [PubMed] [Google Scholar]
- 47.Diepvens K, Kovacs EM, Nijs IM, Vogels N, Westerterp-Plantenga MS. Effect of green tea on resting energy expenditure and substrate oxidation during weight loss in overweight females. Br J Nutr. 2005;94:1026–34. [DOI] [PubMed] [Google Scholar]
- 48.Hursel R, Viechtbauer W, Westerterp-Plantenga MS. The effects of green tea on weight loss and weight maintenance: a meta-analysis. Int J Obes (Lond). 2009;33:956–61. [DOI] [PubMed] [Google Scholar]
- 49.Westerterp-Plantenga MS, Lejeune MP, Kovacs EM. Body weight loss and weight maintenance in relation to habitual caffeine intake and green tea supplementation. Obes Res. 2005;13:1195–204. [DOI] [PubMed] [Google Scholar]
- 50.Maki KC, Reeves MS, Farmer M, Yasunaga K, Matsuo N, Katsuragi Y, Komikado M, Tokimitsu I, Wilder D, et al. Green tea catechin consumption enhances exercise-induced abdominal fat loss in overweight and obese adults. J Nutr. 2009;139:264–70. [DOI] [PubMed] [Google Scholar]
- 51.Kohri T, Nanjo F, Suzuki M, Seto R, Matsumoto N, Yamakawa M, Hojo H, Hara Y, Desai D, et al. Synthesis of (-)-[4–3H]epigallocatechin gallate and its metabolic fate in rats after intravenous administration. J Agric Food Chem. 2001;49:1042–8. [DOI] [PubMed] [Google Scholar]
- 52.Chow HH, Cai Y, Hakim IA, Crowell JA, Shahi F, Brooks CA, Dorr RT, Hara Y, Alberts DS. Pharmacokinetics and safety of green tea polyphenols after multiple-dose administration of epigallocatechin gallate and polyphenon E in healthy individuals. Clin Cancer Res. 2003;9:3312–9. [PubMed] [Google Scholar]
- 53.Lambert JD, Yang CS. Cancer chemopreventive activity and bioavailability of tea and tea polyphenols. Mutat Res. 2003;523–524:201–8. [DOI] [PubMed] [Google Scholar]
- 54.Lambert JD, Sang S, Yang CS. Biotransformation of green tea polyphenols and the biological activities of those metabolites. Mol Pharm. 2007;4:819–25. [DOI] [PubMed] [Google Scholar]
- 55.Chow HH, Cai Y, Alberts DS, Hakim I, Dorr R, Shahi F, Crowell JA, Yang CS, Hara Y. Phase I pharmacokinetic study of tea polyphenols following single-dose administration of epigallocatechin gallate and polyphenon E. Cancer Epidemiol Biomarkers Prev. 2001;10:53–8. [PubMed] [Google Scholar]
- 56.Lee MJ, Maliakal P, Chen L, Meng X, Bondoc FY, Prabhu S, Lambert G, Mohr S, Yang CS. Pharmacokinetics of tea catechins after ingestion of green tea and (-)-epigallocatechin-3-gallate by humans: formation of different metabolites and individual variability. Cancer Epidemiol Biomarkers Prev. 2002;11:1025–32. [PubMed] [Google Scholar]
- 57.Chow HH, Hakim IA, Vining DR, Crowell JA, Ranger-Moore J, Chew WM, Celaya CA, Rodney SR, Hara Y, et al. Effects of dosing condition on the oral bioavailability of green tea catechins after single-dose administration of Polyphenon E in healthy individuals. Clin Cancer Res. 2005;11:4627–33. [DOI] [PubMed] [Google Scholar]
- 58.Mulder TP, van Platerink CJ, Wijnand Schuyl PJ, van Amelsvoort JM. Analysis of theaflavins in biological fluids using liquid chromatography-electrospray mass spectrometry. J Chromatogr B Biomed Sci Appl. 2001;760:271–9. [DOI] [PubMed] [Google Scholar]
- 59.Ku HC, Chang HH, Liu HC, Hsiao CH, Lee MJ, Hu YJ, Hung PF, Liu CW, Kao YH. Green tea (-)-epigallocatechin gallate inhibits insulin stimulation of 3T3–L1 preadipocyte mitogenesis via the 67-kDa laminin receptor pathway. Am J Physiol Cell Physiol. 2009;297:C121–32. [DOI] [PubMed] [Google Scholar]
- 60.Wang CT, Chang HH, Hsiao CH, Lee MJ, Ku HC, Hu YJ, Kao YH. The effects of green tea (-)-epigallocatechin-3-gallate on reactive oxygen species in 3T3–L1 preadipocytes and adipocytes depend on the glutathione and 67 kDa laminin receptor pathways. Mol Nutr Food Res. 2009;53:349–60. [DOI] [PubMed] [Google Scholar]
- 61.Liu HS, Chen YH, Hung PF, Kao YH. Inhibitory effect of green tea (-)-epigallocatechin gallate on resistin gene expression in 3T3–L1 adipocytes depends on the ERK pathway. Am J Physiol Endocrinol Metab. 2006;290:E273–81. [DOI] [PubMed] [Google Scholar]
- 62.Wu BT, Hung PF, Chen HC, Huang RN, Chang HH, Kao YH. The apoptotic effect of green tea (-)-epigallocatechin gallate on 3T3–L1 preadipocytes depends on the Cdk2 pathway. J Agric Food Chem. 2005;53:5695–701. [DOI] [PubMed] [Google Scholar]
- 63.Cho SY, Park PJ, Shin HJ, Kim YK, Shin DW, Shin ES, Lee HH, Lee BG, Baik JH, Lee TR. (-)-Catechin suppresses expression of Kruppel-like factor 7 and increases expression and secretion of adiponectin protein in 3T3–L1 cells. Am J Physiol Endocrinol Metab. 2007;292:E1166–72. [DOI] [PubMed] [Google Scholar]
- 64.Yang TT, Koo MW. Chinese green tea lowers cholesterol level through an increase in fecal lipid excretion. Life Sci. 2000;66:411–23. [DOI] [PubMed] [Google Scholar]
- 65.Blumenthal M, Ferrier GKL, To CC. Total sales of herbal supplements in United States show steady growth sales in mass market channel show continued decline. HerbalGram. 2006;71:64–6. [Google Scholar]
- 66.Lambert JD, Lee MJ, Diamond L, Ju J, Hong J, Bose M, Newmark HL, Yang CS. Dose-dependent levels of epigallocatechin-3-gallate in human colon cancer cells and mouse plasma and tissues. Drug Metab Dispos. 2006;34:8–11. [DOI] [PubMed] [Google Scholar]
- 67.Mazzanti G, Menniti-Ippolito F, Moro PA, Cassetti F, Raschetti R, Santuccio C, Mastrangelo S. Hepatotoxicity from green tea: a review of the literature and two unpublished cases. Eur J Clin Pharmacol. 2009;65:331–41. [DOI] [PubMed] [Google Scholar]
- 68.Isbrucker RA, Edwards JA, Wolz E, Davidovich A, Bausch J. Safety studies on epigallocatechin gallate (EGCG) preparations. Part 2. Dermal, acute and short-term toxicity studies. Food Chem Toxicol. 2006;44:636–50. [DOI] [PubMed] [Google Scholar]
- 69.Galati G, Lin A, Sultan AM, O'Brien PJ. Cellular and in vivo hepatotoxicity caused by green tea phenolic acids and catechins. Free Radic Biol Med. 2006;40:570–80. [DOI] [PubMed] [Google Scholar]