Abstract
Cutaneous cholecalciferol synthesis has not been considered in making recommendations for vitamin D intake. Our objective was to model the effects of sun exposure, vitamin D intake, and skin reflectance (pigmentation) on serum 25-hydroxyvitamin D (25[OH]D) in young adults with a wide range of skin reflectance and sun exposure. Four cohorts of participants (n = 72 total) were studied for 7–8 wk in the fall, winter, spring, and summer in Davis, CA [38.5° N, 121.7° W, Elev. 49 ft (15 m)]. Skin reflectance was measured using a spectrophotometer, vitamin D intake using food records, and sun exposure using polysulfone dosimeter badges. A multiple regression model (R2 = 0.55; P < 0.0001) was developed and used to predict the serum 25(OH)D concentration for participants with low [median for African ancestry (AA)] and high [median for European ancestry (EA)] skin reflectance and with low [20th percentile, ∼20 min/d, ∼18% body surface area (BSA) exposed] and high (80th percentile, ∼90 min/d, ∼35% BSA exposed) sun exposure, assuming an intake of 200 iu/d (5 ug/d). Predicted serum 25(OH)D concentrations for AA individuals with low and high sun exposure in the winter were 24 and 42 nmol/L and in the summer were 40 and 60 nmol/L. Corresponding values for EA individuals were 35 and 60 nmol/L in the winter and in the summer were 58 and 85 nmol/L. To achieve 25(OH)D ≥75 nmol/L, we estimate that EA individuals with high sun exposure need 1300 iu/d vitamin D intake in the winter and AA individuals with low sun exposure need 2100–3100 iu/d year-round.
Introduction
Cholecalciferol (vitamin D3) is synthesized in the skin by exposure to UV light between 290 and 315 nm (1). Both cholecalciferol and ergocalciferol (vitamin D2, of plant origin) are also found in food. Vitamin D from either source is efficiently converted to 25-hydroxyvitamin D (25[OH]D)8 in the liver (2,3). Solar UV intensity is affected by latitude, altitude, time of day, and season (4,5), as well as by environmental factors, including air pollution, cloud cover, and natural ozone levels (6). Age, clothing type, and sunscreen also affect cutaneous vitamin D synthesis (7–9). Melanin, the principal skin pigment, reduces but does not block cholecalciferol synthesis. Thus, longer periods of sun exposure are required for equivalent vitamin D synthesis in people of African ancestry (AA) compared with those of European ancestry (EA) (9,10).
Although sunlight is known to stimulate cholecalciferol synthesis, few studies have used quantitative methods to assess the contribution of sun exposure to vitamin D status. The principal goal of the work presented here is to make such quantitative assessments in order to predict vitamin D status. We have assessed individual sun exposure using polysulfone (PS) dosimeter film badges and records of clothing use (11,12), skin pigmentation using reflectance spectrophotometry, and vitamin D intake using food records. This work was carried out as a university-based, year-long, longitudinal study of 72 healthy young adults with a wide range of skin pigmentation and sun exposure. Data were used to develop a multiple linear regression model to predict vitamin D status in individuals with high or low sun exposure as well as high or low skin reflectance (i.e. light or dark skin pigmentation, respectively). Using this model, we also estimated the additional vitamin D intake that would be needed to achieve target serum 25(OH)D concentrations of 50 and 75 nmol/L (13).
Materials and Methods
Participants.
Participants were recruited from the University of California, Davis (UCD) campus. Volunteers with high and low levels of skin pigmentation and outdoor activity were sought to achieve a wide range for both of these independent variables. Inclusion criteria were: BMI 18.5–30 kg/m2, 19–39 y of age, general good health, and a willingness follow the study protocol. Exclusion criteria included: planned travel outside the Davis area frequently during the study protocol (>2 wk total), daily sunscreen use on all or most exposed skin, use of tanning beds or high-dose vitamin D supplements (excluding RDA-level multivitamin supplements) within 2 mo, pregnancy, and presence of any disease, condition, or use of medication that might affect vitamin D metabolism, such as chronic kidney disease or liver disease. Oral contraceptive users were not excluded. The study was approved by the Institutional Review Board of UCD and written informed consent was obtained in accordance with the Helsinki Declaration of 1975 as revised in 1983.
Study design.
The study was an observational, prospective study conducted at the USDA Western Human Nutrition Research Center on the UCD campus and involved a total of 72 participants in 4 cohorts followed over 8-wk periods each academic quarter, except the fall quarter, when, for logistic reasons, the period was 7 wk. The fall cohort was followed from October 29 to December 15, 2006, the winter cohort from January 17 to March 16, 2007, the spring cohort from April 11 to June 8, 2007, and the summer cohort from July 18 to September 14, 2007. Participants attended an educational meeting the week prior to the study to learn how to keep food records, how to fill out the sun exposure questionnaires, and how to wear and maintain the PS dosimeter badges. Participants were asked to maintain their typical routines. Blood was drawn and skin reflectance measured at wk 0, 4, and 7 or 8. Participants who were identified as deficient (<37.5 nmol/L), using the mean serum 25(OH)D concentration, were provided with this information as well as diet counseling.
Sun exposure assessment.
Personal UV-B exposure was measured using PS dosimeter badges prepared by the laboratory of Prof. Kimlin. PS is a photosensitive polymer that is used as an objective way to measure broadband UV-B exposure and is sensitive to the same wavelengths that affect human skin (14). The PS badges are typically used to estimate the amount of erythemal UV-B exposure in terms of a minimal erythema dose for typical Caucasian skin, which is equivalent to 250 J/m2 of erythemal action spectrum-weighted UV energy (e.g. 1 minimal erythema dose causes slight pinkness to the skin). The error associated with the UV-B measurements using PS film was previously estimated to be ∼10% (15). Each PS film was mounted on a 3-cm × 3-cm white plastic holder with a central aperture measuring 1 cm2.
The PS badges were worn from 0700 to 1900 once per week on the same day by all participants on their right wrist by attaching it to a white sweat band. For each cohort, the PS badge days were chosen randomly with the restriction that each day of the week and weekend were included at least once. Participants kept daily logs recording all time spent outside from 0700 to 1900 that included specific information about time of day, location, outdoor activity, time in the direct sun or shade, sunscreen use, and clothing. The PS badge measurements were individually adjusted for body surface area (BSA) exposed to the sun (Table 1) based on daily clothing records kept by each participant. The daily PS badge measurement (J/m2) was multiplied by the mean m2 of exposed skin when participants were in the sun, resulting in an individual dose of sun exposure (J) received on that day.
TABLE 1.
Estimates of percent of BSA exposed based on the “rule of nines” and clothing worn1
| Body region2 | Clothing type | % BSA exposed |
|---|---|---|
| Head | Nothing | 4 |
| Beanie/bike helmet | 3 | |
| Baseball cap | 2 | |
| Cowboy hat | 1 | |
| Torso/arms | Nothing | 47 |
| Bikini top/sports bra | 42 | |
| Tank top | 18 | |
| Tee shirt | 10 | |
| Quarter length shirt | 3 | |
| Long sleeves | 0 | |
| Legs | Bikini bottom | 38 |
| Short shorts/ Skirt | 24 | |
| Knee-length shorts/skirt | 8 | |
| Full-length pants | 0 | |
| Feet | Nothing | 2 |
| Sandals | 1.5 | |
| Shoes | 0 |
From (15).
Hands contributed 4% of exposed BSA (0% if gloves were worn) and the neck 2% (0% if a scarf was worn).
To obtain the maximum possible UV-B exposures on days the sun badges were used by participants, a PS badge was also placed in a horizontal position in the direct sunlight (0700 to 1900) at the UCD Climate Station [Davis, CA, 38.5° N, 121.7° W, elev. 49 ft (15 m)] next to a UV-B Broadband Pyranometer (Yankee Environmental Systems) that measures global irradiance on a continual basis in the UV-B spectral range of 280–330 nm (16). The UV-B radiation detected by the instrument is converted to visible light and this signal is measured in W/m2 every 15 s and means are recorded every 3 min. We downloaded the erythemally weighted UV-B measurements from the USDA UV-B Network Web site (16) and converted the measurements to J/m2 to make them comparable to the PS badge measurements. UV-B exposure measurements (J/m2) taken using PS badges exposed in direct sun all day were compared with the pyranometer UV-B readings for the same day that participants wore the PS badges. These results were correlated (r = 0.91; P < 0.0001; data not shown), demonstrating the ability of the PS badge to accurately measure UV-B exposure.
Dietary assessment.
Vitamin D intake from food and supplements was determined using consecutive 4-d food records that were kept every other week for a total of 16 records/participant. Food records have previously been validated as an accurate tool to measure nutrient intake through observational studies (17). The 4-d food records included a weekend day to capture typical intake and days included rotated from Wednesday–Saturday to Sunday–Wednesday. The participants were trained in how to keep accurate food records by a registered dietitian. The food records were analyzed for vitamin D content using the Nutrition Data System for Research Program (2006, University of Minnesota, Minneapolis, MN) and a daily vitamin D intake in IU was determined (Vitamin D: 40 IU = 1 μg).
Skin reflectance assessment.
Skin reflectance (pigmentation) was measured using a Minolta 2500d spectrophotometer (Konica Minolta Sensing). Measurements were taken on the middle upper inner right arm, the dorsum of the right hand between the thumb and index finger, and the middle of the forehead at wk 0, 4, and 7 or 8. Each site was measured 3 times and the instrument was moved slightly over the region of interest to obtain a mean for the site. The measurements are expressed using the L* (lightness) value of the Commission International d'Eclairage system. The L* value ranges from 0 to 100, with 0 indicating no reflected light (pure black) and a value of 100 indicating 100% reflectance (pure white). The L* value is highly correlated to the Melanin Index (18) and the L* value was used in the multiple linear regression model.
Serum 25(OH)D.
Blood was drawn (week 0, 4, 7 or 8) after a 4 h fast from dietary fat. Serum was stored at −70°C until analysis. Serum 25(OH)D was analyzed by ultra-performance liquid chromatography-tandem MS (LC-MS) using a Diels-Alder derivative with 4-phenyl-1,2,4-triazoline-3,5-dione, as described (19,20), and by RIA (DiaSorin) according to the manufacturer's instructions with the following modification: the centrifugation following the precipitation step was performed at 3000 × g for 60 min at 10°C, rather than 1800 × g for 20 min at 20–25°C.
For the RIA procedure, the CV for duplicate samples measured on the same day was 5.0% (range, 0.04–10.0%) and for duplicate samples measured on different days was 11.6% (range, 7.2–16.0%). For the LC-MS procedure, the same-day CV was 3.2% (range, 1.6–4.8%), as previously reported (19). The USDA Western Human Nutrition Research Center participates in the Vitamin D External Quality Assessment Scheme (21) and calibration standards from this program analyzed during this period were all within acceptable limits (22).
Parathyroid hormone.
Intact serum parathyroid hormone (PTH) was measured using a chemiluminescent method (Immulite Siemens Medical Solutions Diagnostics) at wk 7 or 8.
Statistical methods.
SAS version 9.1.3 (StataCorp) was used to perform all statistical analyses. Continuous data were tested for normality using the Kolmogorov-Smirnov test and variables that were not normally distributed (P < 0.05) were transformed using Box-Cox transformations. ANOVA was performed for comparisons between cohorts and time-repeated were performed within cohorts. Duncan tests were performed for pairwise comparisons. If a variable could not be transformed to a normal distribution then a nonparametric test was performed. Variables are expressed as mean ± SD or as medians (range), depending on their distribution. Spearman correlation was used to examine associations between variables.
To predict serum 25(OH)D, multiple linear regression analysis was used with continuous variables for skin reflectance, sun exposure, and vitamin D intake, because they are known to influence status and dummy variables for cohort because of independent groups each season. Covariates, such as age, gender, BMI, oral contraceptive use, ethnicity, self-reported sun level (high or low), and athlete (yes or no) (athletes had high sun exposure) were explored in the model along with interactions, such as skin × sun, sun × diet, skin × diet, skin × sun × diet, and sun × each season. Pitman's test was used to determine whether a skin reflectance measurement from one site in the model was a significantly better predictor than another site and to determine whether J/m2 and joules were significantly different predictors from each other. All P-values < 0.05 were considered significant.
Results
Baseline characteristics.
The 72 study participants were of varied ancestry, most were female, and few were overweight (Table 2). When asked to categorize their habitual sun exposure as either high or low, 62% (45/72) said low and 38% (27/72) said high.
TABLE 2.
Baseline characteristics of study participants1
| Cohort | Fall | Winter | Spring | Summer | Total |
|---|---|---|---|---|---|
| n | |||||
| Participants | 17 | 17 | 20 | 18 | 72 |
| Ethnicity/ancestry23 | |||||
| European | 10 | 5 | 6 | 5 | 26 |
| Hispanic | 1 | 2 | 1 | 0 | 4 |
| North Asian | 1 | 3 | 2 | 6 | 12 |
| South Asian | 5 | 3 | 6 | 1 | 15 |
| African | 0 | 4 | 5 | 6 | 15 |
| Gender | |||||
| Female | 14 | 11 | 16 | 12 | 53 |
| Male | 3 | 6 | 4 | 6 | 19 |
| Age, y | 23 ± 3 | 27 ± 5 | 23 ± 4 | 22 ± 3 | 24 ± 4 |
| BMI ,4kg/m2 | 23 ± 2 | 24 ± 2 | 23 ± 2 | 22 ± 3 | 23 ± 3 |
Values are n or means ± SD.
Self-reported ethnicity/ancestry.
Nationalities for European included Russian/Kazakhstan and American; Hispanic included South American and Mexican; North Asian included Chinese and Korean; South Asian included Indian, Vietnamese, Sri Lankan, Thai, Cambodian/Chinese/Malaysian, and Philipino; and African included American, African/Puerto Rican, African/European/Italian, and African/Mexican.
The mean of wk 0, 4, and 7 or 8 was used for each participant.
UV-B exposure.
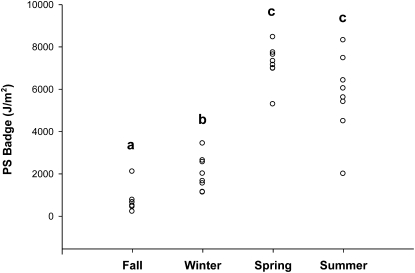
PS badges were placed in direct sun to measure maximum potential UV-B exposure intensity on the same day that badges were worn by participants to measure individual exposures (Fig. 1). The median exposure intensity for the fall cohort, 643 J/m2 (range: 213–2100 J/m2) was the lowest of the 4 cohorts. The winter median, 1830 J/m2 (range: 1124–3431 J/m2), was also relatively low but was nonetheless significantly greater than the fall median. The median exposure intensity in the spring cohort was 7230 J/m2 (range: 5282–8454 J/m2) and in the summer was 5818 J/m2 (range: 1995–8309 J/m2). The spring and summer exposure intensities did not differ from one another but were significantly greater than either fall or winter.
FIGURE 1 .
Maximum potential UV-B exposure measured by PS dosimeter badges exposed in the direct sun on a horizontal surface on the same day each week that participants wore personal dosimeter badges. One badge was exposed each day. Each point represents a single badge (n = 31). Study cohort dates: fall, 10/29–12/15; winter, 1/17–3/16; spring, 4/11–6/8; and summer, 7/18–9/14. Study cohorts without a common letter differ, P < 0.05.
Webb et al. (4) reported that a minimum exposure intensity of 200 J/m2 is needed to initiate cutaneous vitamin D synthesis. This level of exposure could theoretically have been reached on any of the days when exposure was measured in the present study. However, the exposure intensities reported here represent a 12-h period. The time needed to achieve 200 J/m2 would range from nearly a full day (e.g. on the day in December when the lowest level was measured, 213 J/m2) to <20 min on some days in the spring and summer cohorts.
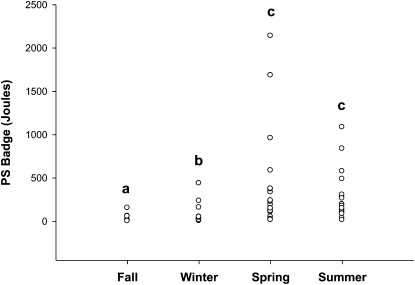
Individual sun exposure doses (determined for each participant from exposure intensity and amount of skin exposed to the sun) (Fig. 2) followed the same seasonal pattern as for exposure intensity (Fig. 1). The median for the fall cohort was 3 J (range: 1–154 J), the lowest of the 4 cohorts. The winter median of 26 J (range: 4–438 J) was significantly greater than the fall median. The spring median, 169 J (range: 16–2138 J), and the summer median, 163 J (range: 13–1085 J), were significantly greater than the medians for the other 2 cohorts but did not differ from one another.
FIGURE 2 .
Individual mean UV-B exposure measured by PS dosimeter badges worn by participants once a week for each cohort. Each point represents the mean dose for each participant over 7–8 wk, adjusted for BSA exposed to the sun (n = 72). Study cohort dates: fall, 10/29–12/15; winter, 1/17–3/16; spring, 4/11–6/8; and summer, 7/18–9/14. Study cohorts without a common letter differ, P < 0.05.
Sun exposure could vary by ancestry group due to differences in behavior. The median dose for participants of European (156 J), African (111 J), North Asian (76 J), and Hispanic (59 J) ancestry did not significantly differ from one another (adjusted for cohort). Doses for Hispanic and South Asian (16 J) participants did not differ from one another, but doses for South Asians were significantly less than doses for North Asian, African, and European participants (data not shown).
Dietary vitamin D intake.
Vitamin D intake did not vary by cohort and the overall median for all participants was 194 Iu/d (5 μg) (Table 3). Vitamin D intakes could differ between ancestry groups because of differences in food habits or supplement use. Median vitamin D intakes of European (236 iu) and South Asian (223 iu) participants were greater (P < 0.05) than the intakes of Hispanic (117 iu) participants but not of North Asian (184 iu) and African (148 iu) participants. Vitamin D intake was correlated with serum 25(OH)D (r = 0.27; P = 0.02; data not shown).
TABLE 3.
Serum 25(OH)D concentrations and vitamin D intakes for each cohort1
| Cohort |
||||
|---|---|---|---|---|
| Variable | Fall | Winter | Spring | Summer |
| 25(OH)D, nmol/L | ||||
| Wk 0 | 68 ± 27x | 45 ± 25 | 50 ± 27y | 64 ± 27 |
| Wk 4 | 63 ± 29y | 42 ± 23 | 47 ± 25y | 66 ± 30 |
| Wk 82 | 56 ± 31z | 44 ± 24 | 56 ± 23x | 61 ± 29 |
| Overall | 62 ± 28a | 44 ± 24b | 51 ± 24ab | 63 ± 28a |
| Vitamin D Intake,34IU/d | 220 (66–692) | 171 (36–621) | 181 (55–599) | 206 (50–219) |
Values are means ± SD or median (range), n = 72. Means in a row with superscripts a and b without a common letter differ, P < 0.05. Means in a column with superscripts x, y, and z without a common letter differ, P < 0.05.
Wk 7 for fall.
Includes supplements.
40 IU = 1 μg.
Skin reflectance.
Reflectance (L*) measured at all sites correlated with serum 25(OH)D. The forehead measurement had the highest correlation coefficient (r = 0.39; P = 0.0008), followed by the back-of-the-hand measurement (r = 0.38; P = 0.0011) and the inner-arm measurement (r = 0.29; P = 0.0136). The difference in reflectance between the inner arm and forehead measurements for an individual (a possible index of sun exposure) was not correlated with serum 25(OH)D (P > 0.05; data not shown).
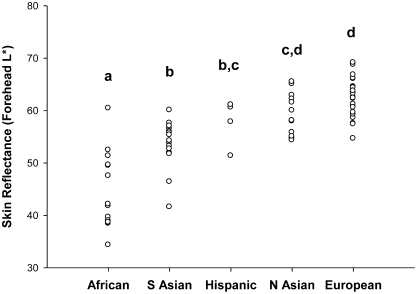
Skin reflectance varied by ancestry group (Fig. 3). Although the range of values within each group overlapped with all other groups, some significant differences were seen among group means. The mean forehead reflectance for African participants (44 ± 7 L* units) was significantly lower than for other ancestry groups. Mean reflectance for South Asian (54 ± 5 L* units) and Hispanic participants (58 ± 4 L* units) did not differ from one another nor did the mean for Hispanics differ from the North Asian mean (59 ± 4 L* units), which did not differ from the mean for European participants (63 ± 4 L* units).
FIGURE 3 .
Forehead skin reflectance varies by ancestry. The L* scale for skin reflectance extends from 0 (black) to 100 (white). Each point represents the mean forehead skin reflectance for each participant, n = 72. Groups without a common letter differ, P < 0.05.
Vitamin D status.
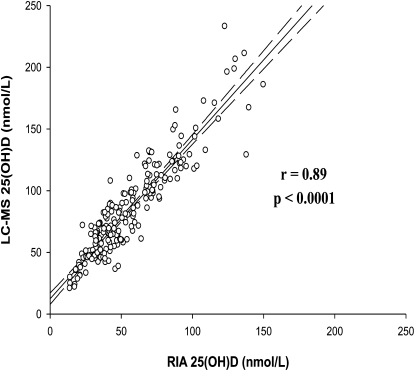
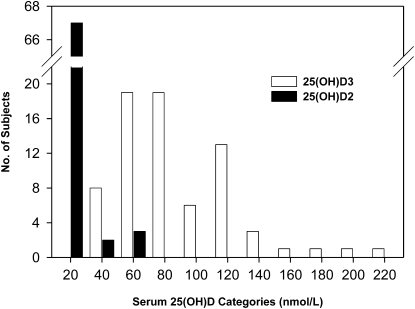
Serum 25(OH)D concentrations were measured both by RIA and LC-MS. These values were highly correlated (Fig. 4), as reported previously for data from the fall cohort only (19), although the LC-MS values were consistently greater than the RIA values. The LC-MS procedure distinguishes between 25(OH)D2 and 25(OH)D3 whereas the RIA does not. Most participants (93%; 67/72) had low mean serum 25(OH)D2 concentrations (<20 nmol/L for the mean of the 3 blood draws for each participant; Fig. 5). When 25(OH)D2 was expressed as a percent of total 25(OH)D, the median was 2.5% (25th/75th percentiles, 1.2/6.1%; range: 0.4–54%). For most participants (93%; 67/72), 25(OH)D2 contributed <20% of the total serum 25(OH)D concentration and in only 2 cases (2.8%; 2/72) was the proportion >50%. Thus, ergocalciferol was a minor contributor to vitamin D status for most study participants. The 25(OH)D concentrations from the RIA procedure were used in the analyses presented below, because this method is more widely used in population studies, thus making our results more directly comparable to other studies in the literature.
FIGURE 4 .
Association between the serum 25(OH)D concentrations measured by LC-MS and RIA for all samples from 72 participants, n = 216. Regression equation: LC-MS 25(OH)D0.1 = 1.02873 + (0.13195)(ln RIA 25(OH)D); R2 = 0.8351; P < 0.0001. Dashed lines = 95% CI.
FIGURE 5 .
Distribution of mean (baseline, 4 and 7 or 8 wk) serum 25(OH)D2 and 25(OH)D3 concentrations in the 72 study participants measured by LC-MS. The X-axis indicates 20 nmol/L increments from 0 to 220 nmol/L.
Because the cohorts in this study are made up of different groups of participants, differences in serum 25(OH)D among these cohorts cannot be attributed solely to seasonal differences in UV-B exposure. However, mean serum 25(OH)D in the fall cohort was significantly greater than the winter cohort but not significantly different from the spring and summer cohort (Table 3). Mean serum 25(OH)D in the winter cohort was significantly less than the fall and summer cohort but not the spring cohort (Table 3). Modest trends within cohorts were seen reflecting expected changes that would result from seasonal changes in UV-B exposure (Table 3). Mean serum 25(OH)D decreased significantly (by 18%) from the beginning to end of the fall cohort study period, whereas the mean increased significantly (by 12%) during the spring cohort study period. Significant differences were not seen within the winter and summer cohorts.
Serum 25(OH)D means were compared among different ancestry groups while adjusting for season. European participants had significantly greater serum 25(OH)D concentrations (78 ± 27 nmol/L) than Hispanic (52 ± 21 nmol/L), African (43 ± 17 nmol/L), South Asian (40 ± 13 nmol/L), and North Asian (40 ± 15 nmol/L) participants, who did not differ from one another.
PTH.
PTH secretion is inhibited by the active form of vitamin D; thus, high serum PTH can be associated with vitamin D deficiency (2). Serum PTH measured at wk 7 or 8 correlated negatively with serum 25(OH)D measured at the same time point (r = −0.23; P = 0.051; data not shown), suggesting that some participants in the study were vitamin D deficient.
Model predicting vitamin D status.
Single and multiple linear regression analysis was used to predict serum 25(OH)D using sun exposure, vitamin D intake, and skin reflectance. Vitamin D intake (including a variable for cohort) explained 21% of the variance in serum 25(OH)D (P = 0.011). Forehead skin reflectance was a better predictor of serum 25(OH)D than inner arm reflectance and marginally better than hand reflectance (data not shown), explaining 30% of the variance in serum 25(OH)D (P = 0.0002). UV-B dose (J) explained 42% of the variance in serum 25(OH)D (P < 0.0001). Together, UV-B dose, forehead skin reflectance, and dietary intake explained 55% of the variance in serum 25(OH)D (Table 4). When LC-MS data for total 25(OH)D were used rather than the RIA data, essentially the same results were seen for this analysis (data not shown). Sun exposure not adjusted for clothing (J/m2) was also a predictor when used in place of dose (J), although the R2 value was lower (R2 = 0.50; P < 0.0001; data not shown). Independently, BMI, age, gender, and sunscreen use (on PS badge days) were not significant predictors of vitamin D status in the full model (data not shown). Several interactions were tested in the model and none were significant (including sun × season, sun × skin reflectance, sun × diet, skin reflectance × diet, and skin reflectance × sun × diet). Sun exposure (J) had the greatest impact on serum 25(OH)D, followed by skin reflectance and vitamin D intake, as judged by the relative magnitude of the coefficients in the regression model (Table 4).
TABLE 4.
Multiple linear regression model using RIA data to predict serum 25(OH)D concentrations1
| RIA model | Estimate | SE | Estimate | P-value | R2 |
|---|---|---|---|---|---|
| Full model, n = 72 | 0.55 | ||||
| PS badge,2J | 1.12760 | 0.20888 | 0.67776 | <0.0001* | |
| Skin (forehead),3L* | 3.75x10−7 | 1.08x10−7 | 0.32280 | 0.0009* | |
| Vit D intake,46IU/d | 0.12436 | 0.06269 | 0.16904 | 0.0515 | |
| Cohort 1 (Fall) | Ref 5 | ||||
| Cohort 2 (Winter) | −0.57209 | 0.12627 | −0.5175 | <0.0001* | |
| Cohort 3 (Spring) | −0.60856 | 0.16080 | −0.5805 | 0.0003* | |
| Cohort 4 (Summer) | −0.37243 | 0.16190 | −0.3435 | 0.0246* |
Equation using RIA data: ln 25(OH)D = 1.57739 + (1.12760 × badge^0.1) + (0.0000003754065 × skin^3.4) + (0.12436 × ln diet) − (0.57209 × winter) − (0.60856 × spring) − (0.37243 × summer); (P < 0.0001)* *Significant P-value < 0.05.
J, Mean joules per participant.
Mean forehead L* skin reflectance per participant.
IU/d, Daily dietary vitamin D, including supplements, per participant.
Ref, Reference dummy variable in the model.
40 IU = 1 μg.
Several covariates were significant predictors of serum 25(OH)D in the full model in combination with sun exposure, skin reflectance, and vitamin D intake. Including the variable “athlete” in the full model explained 59% of the variance in serum 25(OH)D; athlete was a positive predictor (P = 0.012). Adding ancestry/ethnicity explained 65% of variance in serum 25(OH)D, with North Asian being a negative predictor (P = 0.012). Oral contraceptive use was also a positive predictor, explaining 62% of the variance in 25(OH)D when added to the full model (P = 0.0011). Participants who used oral contraceptives did not have different sun exposure, vitamin D intake, or skin reflectance than other female participants (P > 0.05; data not shown). Oral contraceptive users had higher serum 25(OH)D concentrations (72 ± 21 nmol/L) than female nonusers (50 ± 27 nmol/L) (adjusted for season; P < 0.05), which is consistent with the literature (23).
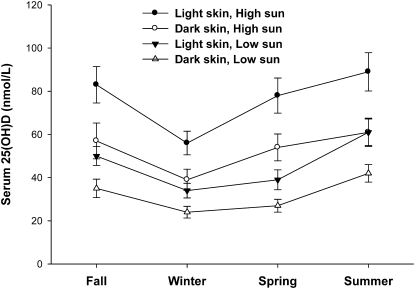
To illustrate the association of variation of sun exposure and skin reflectance with vitamin D status, the regression model was used to predict serum 25(OH)D in participants with low and high sun exposure and with low and high skin reflectance (4 groups). Low and high sun exposure were defined as the 20th and 80th percentiles of exposure for each cohort. These values were 2 and 56 J for fall, 7 and 158 J for winter, 24 and 958 J for spring, and 85 and 576 J for summer. Participants with low sun exposure on average spent 20 min/d in direct sun with ∼18% BSA exposed (i.e. face, neck, hands, and arms; as would be exposed when wearing long pants and a short-sleeve shirt), whereas participants with high sun exposure spent ∼90 min/d in direct sun with ∼35% BSA exposed (i.e. face, neck, hands, arms, and legs; as would be exposed when wearing a sleeveless top and shorts covering the thighs). The mean UV-B exposure in the low group for all seasons was 0.62 ± 0.56% of maximum possible exposure, whereas in the high group the mean exposure was 10.1 ± 2.2%. The mean for those with median exposure was 1.8 ± 1.0%. Low skin reflectance was defined as the median forehead reflectance for participants of AA (L* = 42) and high reflectance was defined as the median for participants of EA (L* = 63). Vitamin D intake was set at 200 iu (5 μg)/d, approximately the median for all participants. In these projections (Fig. 6), both sun exposure and skin reflectance influenced serum 25(OH)D during all seasons. The 2 low-sun groups had the lowest serum 25(OH)D concentrations each season (except summer), whereas low skin reflectance always produced a lower serum 25(OH)D than high skin reflectance.
FIGURE 6 .
Multiple, linear regression model prediction of serum 25(OH)D in 4 groups based on sun exposure [20th (low) or 80th (high) percentile for each season in Joules] and skin reflectance [median forehead reflectance for participants of African (dark skin) and European (light skin) ancestry]. Study cohort dates: fall, 10/29–12/15; winter, 1/17–3/16; spring, 4/11–6/8; and summer, 7/18–9/14. Vitamin D intake was set at 200 iu(5 μg)/d. The equation is shown in Table 4, footnote 1.
Vitamin D intake needed to maintain a healthy vitamin D status.
A shortcoming of current recommendations for vitamin D intake is that they are not individualized to reflect risk of deficiency, as could be determined by sun exposure and skin reflectance. To illustrate how recommendations might vary when these factors are considered, we estimated the amount of additional vitamin D intake that would be needed to achieve and maintain serum 25(OH)D at ≥50 nmol/L (Table 5) and ≥75 nmol/L (Table 6) using our model to estimate serum 25(OH)D (Fig. 6) and the response to vitamin D supplementation reported by Heaney et al. (24), 0.7 nmol/L for every additional 40 iu (1 μg) of vitamin D intake. Individuals with low skin reflectance and low sun exposure need the highest intakes to reach either 50 or 75 nmol/L, as expected. Participants of AA with low sun exposure would need from 650 to 1700 iu of supplemental vitamin D, depending on the season, to meet the 50-nmol/L threshold and 2100–3100 iu/d to meet the 75-nmol/L threshold. The corresponding values for participants of AA with high sun exposure are 0–850 iu/d for the 50-nmol/L threshold and 1000–2250 iu/d for the 75-nmol/L threshold. For participants of EA with high sun exposure, no additional vitamin D intake is needed to meet the 50-nmol/L threshold, whereas considerable supplemental intake, 1300 iu/d, is needed in the winter to meet the 75-nmol/L threshold. However, participants of EA with low sun exposure need considerable supplemental intake (850–1100 iu/d) to meet the 50-nmol/L threshold in the winter and spring and year-round intake ranging from 1000 to 2550 iu/d to meet the 75-nmol/L threshold.
TABLE 5.
Estimated vitamin D intake needed to achieve serum 25(OH)D 50 nmol/L in participants with median skin reflectance for African and European ancestries with low (20th percentile) and high (80th percentile) sun exposure for each season1–3
| AA |
EA |
|||
|---|---|---|---|---|
| Cohort | Low sun | High sun | Low sun | High sun |
| IU/d | ||||
| Fall | 1050 | 0 | 200 | 0 |
| Winter | 1700 | 850 | 1100 | 0 |
| Spring | 1500 | 0 | 850 | 0 |
| Summer | 650 | 0 | 0 | 0 |
Estimated using regression model (Table 4) to predict serum 25(OH)D based on skin reflectance, sun exposure and 200 iu/d current vitamin D intake, and using expected serum 25(OH)D response to additional vitamin D supplementation based on a dose-response study using 0.7 nmol/L for every additional 1 μg of vitamin D intake from supplements (24).
Values include the baseline intake of 200 iu/d.
40 iu = 1 μg.
TABLE 6.
Estimated vitamin D intake needed to achieve serum 25(OH)D ≥75 nmol/L in participants with median skin reflectance for AA and EA with low (20th percentile) and high (80th percentile) sun exposure for each season12
| AA |
EA |
|||
|---|---|---|---|---|
| Cohort | Low sun | High sun | Low sun | High sun |
| IU/d | ||||
| Fall | 2500 | 1250 | 1650 | 0 |
| Winter | 3100 | 2250 | 2550 | 1300 |
| Spring | 2900 | 1400 | 2250 | 50 |
| Summer | 2100 | 1000 | 1000 | 0 |
40 iu = 1 μg.
Estimated as described in footnote to Table 5.
Discussion
Sun exposure adjusted for BSA exposed to the sun and skin reflectance were the principal predictors of vitamin D status in the present study. These associations are well recognized but have not, to our knowledge, been analyzed in a single, comprehensive model. For example, many studies have examined the threshold of UV-B exposure needed for dermal vitamin D synthesis (5,25–27) and others have used such data in conjunction with ambient UV-B exposure measurements to predict the impact of sun exposure on vitamin D status at different latitudes (6,28–31). Using individual measurements, several recent studies have also correlated outdoor activity with vitamin D status (32–34). Recent work by Armas et al. (35) has demonstrated a dose-response relationship between UV-B exposure from an artificial source and vitamin D status and that the relationship depends on the level of skin pigmentation assessed by skin reflectance. However, to our knowledge, the present study is the first to use a quantitative assessment of sun exposure, skin reflectance, and vitamin D intake in free-living participants to predict vitamin D status.
Participants in this study were college students recruited to represent a wide range of outdoor activity and it is reasonable to question whether their level of sun exposure is similar to other population groups. The median exposures for these participants in the fall, winter, spring, and summer were 0.5, 1.4, 2.3, and 2.8%, respectively, of maximum ambient exposure (overall median, 1.8%). These values are quite similar to population-based data from a previous study of U.S. adults (22–40 y of age) that found that mean exposures for women were 1.8, 1.7, 2.9, and 2.7% of maximum ambient exposure in fall, winter, spring, and summer, respectively (overall mean, 2.3%) and the corresponding values for men were 3.2, 2.2, 2.8, and 3.1% (overall mean, 2.8%) (36). Similar results were recently reported from Spain (37). These values represent typical exposure of indoor workers and do not include contributions from vacations (38,39). The 80th percentile for participants in the present study (10.1% of ambient) is similar to levels reported for outdoor workers (7–10%) in a recent review (40). Thus, sun exposure levels in the present study are similar to those reported for other adults in the US and Europe.
We compared the vitamin D status of participants in the present study to data for the U.S. population based on ancestry and ethnicity. The most recent NHANES (2000–2004) (41) reported mean serum 25(OH)D concentrations of 67, 40, and 54 nmol/L for non-Hispanic Whites, non-Hispanic Blacks, and Mexican-Americans from 20 to 59 y of age, respectively. In the present study, mean concentrations for European (nonathletes), African, and Hispanic participants were 66, 43, and 52 nmol/L, respectively. The present study used the same method of 25(OH)D analysis as the NHANES study. Thus, whereas season, latitude, age, diet, BMI, and personal UV-B exposure patterns significantly affect serum 25(OH)D, the vitamin D status of participants in the present study generally reflect expectations based on race and ethnicity for the U.S. population.
Dietary intake can be an important contributor to vitamin D status, but in our model intake was less significant as a predictor of status than either sun exposure or skin pigmentation. Other studies have also shown that vitamin D intake correlates poorly with serum 25(OH)D (42,43). On the other hand, in a recent study in the southwestern US, both sun exposure and vitamin D intake significantly predicted vitamin D status, although the effect was more pronounced in Whites than in Blacks or Hispanics (33). This may be because participants of EA typically consume higher levels of fortified dairy products and total vitamin D than other groups (44), as was seen in the present study.
The present study found that relatively high intakes of vitamin D would be needed to achieve serum 25(OH)D concentrations of 50 or 75 nmol/L for participants with low sun exposure. However, these results are relatively consistent with 2 recent studies that addressed this question with dose-response intervention trials. Cashman et al. (45) found that total intakes of 408 and 924 iu/d would be needed to maintain median serum 25(OH)D concentrations of 50 and 75 nmol/L, respectively, during the winter in Ireland and Northern Ireland. Using data from the present study, we estimate that an intake of 1100 and 2550 iu/d would be needed in the winter to maintain median serum 25(OH)D concentrations of 50 and 75 nmol/L, respectively, for participants of EA with low sun exposure, somewhat higher than expected based on the Irish study. This difference may be due to our use of a conservative estimate of the serum 25(OH)D response to supplementation, 0.7 nmol/L per μg (24), whereas the Irish study found a response of 2.0 nmol/L per μg which is consistent with the previous report that supplements <1400 iu/d (35 μg/d) have a greater relative effect on serum 25(OH)D than higher doses (46). In a second supplementation study conducted in New York (46), Black and White adults were recruited in winter and supplementation levels were adjusted at 8-wk intervals to achieve a serum 25(OH)D concentration of 75 nmol/L. Blacks had a mean total intake of 3916 iu/d and Whites had 3040 iu/d to achieve this level in 90% of participants. In the present study, we estimated that participants of AA with low sun exposure would need from 2100 to 3100 iu/d and participants of EA would need from 1000 to 2550 iu/d to reach 75 nmol/L, depending on the season.
Strengths of this study are that it involved longitudinal data collection from participants over each season pursuing their normal daily activities and included individuals with a wide range of skin reflectance and sun exposure behaviors. The study used objective, quantitative measures of sun exposure and skin reflectance, repeated measurement of dietary vitamin D intake, and careful assessment of vitamin D status using 2 independent methods. Some of the limitations of the study include a relatively small sample size (particularly for participants with low skin reflectance and high sun exposure), different groups of individuals each season, a limited range of dietary intake, and some imbalances in exposure across ancestry groups (e.g. all self-identified athletes were European and had the highest levels of sun exposure). In addition, age and BMI were not significant predictors of 25(OH)D, presumably because we recruited participants in a narrow age and BMI range. Other studies have shown that increasing age (47) and greater BMI (48) are associated with lower serum 25(OH)D.
In summary, we have found that participants with high skin reflectance and high sun exposure are at low risk of vitamin D insufficiency and need a supplemental intake of 1300 iu/d only in the winter, whereas participants with low skin reflectance and low sun exposure need supplemental intake from 2100 to 3100 iu/d year-round to maintain a target serum level of 75 nmol/L. The methods used here could be applied in future population-based studies to confirm these observations and to determine whether it is feasible to develop recommendations for vitamin D intake based on these personal characteristics.
Acknowledgments
L.M.H. and C.B.S. designed research, analyzed data, wrote the paper, and had primary responsibility for final content; L.M.H conducted research; P.A.A, B.D.H., and M.G.K. provided essential reagents and analyzed data; J.R.S. provided access to USDA monitoring station, and L.R.W. provided RIA analysis of 25(OH)D. All authors read and approved the final manuscript.
Supported by NIH grant P60MD0222 (to L.M.H. and C.B.S.), USDA CRIS project no. 5306-51530-006-00D, a Bristol-Myers Squibb Foundation, Inc. Freedom to Discover Grant, and a grant from the Gustavus and Louise Pfeiffer Research Foundation. P.A.A. and B.D.H. are supported by the National Institute of Environmental Health Sciences (NIEHS) Center for Children's Environmental Health and Disease Prevention P01 ES011269, the U.S. Environmental Protection Agency (EPA) Center for Children's Environmental Health and Disease Prevention R833292 and R829388, the NIEHS/EPA Children's Center, the California Dairy Research Foundation grant 07 HAB-01-NH, the NIEHS Advanced Training in Environmental Toxicology grant T32 ES007059, the Autism Speaks grant 4933 (analytical work), and the NIEHS Superfund grant P42 ES004699 (analytical work). M.G.K. is supported through a Queensland Cancer Council Senior Research Fellowship.
Author disclosures: L. M. Hall, M. G. Kimlin, P. A. Aronov, B. D. Hammock, J. R. Slusser, L. R. Woodhouse, and C. B. Stephensen, no conflicts of interest.
Abbreviations used: 25(OH)D, 25-hydroxyvitamin D; AA, African ancestry; BSA, body surface area; EA, European ancestry; L*, lightness scale; LC-MS, liquid chromatography-tandem MS; PS, polysulfone; PTH, parathyroid hormone; UCD, University of California, Davis.
References
- 1.MacLaughlin JA, Anderson RR, Holick MF. Spectral character of sunlight modulates photosynthesis of previtamin D3 and its photoisomers in human skin. Science. 1982;216:1001–3. [DOI] [PubMed] [Google Scholar]
- 2.Feldman D, Pike JW, Glorieux FH. Vitamin D. 1st vol, 2nd ed. Burlington (MA): Elsevier Academic Press; 2005.
- 3.Heaney RP, Armas LA, Shary JR, Bell NH, Binkley N, Hollis BW. 25-Hydroxylation of vitamin D3: relation to circulating vitamin D3 under various input conditions. Am J Clin Nutr. 2008;87:1738–42. [DOI] [PubMed] [Google Scholar]
- 4.Holick MF. Photosynthesis of vitamin D in the skin: effect of environmental and life-style variables. Fed Proc. 1987;46:1876–82. [PubMed] [Google Scholar]
- 5.Webb AR, Kline L, Holick MF. Influence of season and latitude on the cutaneous synthesis of vitamin D3: exposure to winter sunlight in Boston and Edmonton will not promote vitamin D3 synthesis in human skin. J Clin Endocrinol Metab. 1988;67:373–8. [DOI] [PubMed] [Google Scholar]
- 6.Engelsen O, Brustad M, Aksnes L, Lund E. Daily duration of vitamin D synthesis in human skin with relation to latitude, total ozone, altitude, ground cover, aerosols and cloud thickness. Photochem Photobiol. 2005;81:1287–90. [DOI] [PubMed] [Google Scholar]
- 7.Matsuoka LY, Wortsman J, Dannenberg MJ, Hollis BW, Lu Z, Holick MF. Clothing prevents ultraviolet-B radiation-dependent photosynthesis of vitamin D3. J Clin Endocrinol Metab. 1992;75:1099–103. [DOI] [PubMed] [Google Scholar]
- 8.Matsuoka LY, Ide L, Wortsman J, MacLaughlin JA, Holick MF. Sunscreens suppress cutaneous vitamin D3 synthesis. J Clin Endocrinol Metab. 1987;64:1165–8. [DOI] [PubMed] [Google Scholar]
- 9.Clemens TL, Adams JS, Henderson SL, Holick MF. Increased skin pigment reduces the capacity of skin to synthesise vitamin D3. Lancet. 1982;1:74–6. [DOI] [PubMed] [Google Scholar]
- 10.Holick MF, MacLaughlin JA, Doppelt SH. Regulation of cutaneous previtamin D3 photosynthesis in man: skin pigment is not an essential regulator. Science. 1981;211:590–3. [DOI] [PubMed] [Google Scholar]
- 11.Herlihy E, Gies PH, Roy CR, Jones M. Personal dosimetry of solar UV radiation for different outdoor activities. Photochem Photobiol. 1994;60:288–94. [DOI] [PubMed] [Google Scholar]
- 12.Chodick G, Kleinerman RA, Linet MS, Fears T, Kwok RK, Kimlin MG, Alexander BH, Freedman DM. Agreement between diary records of time spent outdoors and personal ultraviolet radiation dose measurements. Photochem Photobiol. 2008;84:713–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bischoff-Ferrari HA, Giovannucci E, Willett WC, Dietrich T, Dawson-Hughes B. Estimation of optimal serum concentrations of 25-hydroxyvitamin D for multiple health outcomes. Am J Clin Nutr. 2006;84:18–28. [DOI] [PubMed] [Google Scholar]
- 14.Kimlin MG, Parisi AV, Wong JC. Quantification of personal solar UV exposure of outdoor workers, indoor workers and adolescents at two locations in Southeast Queensland. Photodermatol Photoimmunol Photomed. 1998;14:7–11. [DOI] [PubMed] [Google Scholar]
- 15.Parisi A, Wong CF. A dosimeter technique for the measurement of ultraviolet exposure to plants. Photochem Photobiol. 1994;60:470–4. [Google Scholar]
- 16.Colorado State University. UV-B Monitoring and Research Program [cited 2007 Sep 24]. Available from: http://uvb.nrel.colostate.edu/UVB/index.jsf.
- 17.Karvetti RL, Knuts LR. Validity of the estimated food diary: comparison of 2-day recorded and observed food and nutrient intakes. J Am Diet Assoc. 1992;92:580–4. [PubMed] [Google Scholar]
- 18.Shriver MD, Parra EJ. Comparison of narrow-band reflectance spectroscopy and tristimulus colorimetry for measurements of skin and hair color in persons of different biological ancestry. Am J Phys Anthropol. 2000;112:17–27. [DOI] [PubMed] [Google Scholar]
- 19.Aronov PA, Hall LM, Dettmer K, Stephensen CB, Hammock BD. Metabolic profiling of major vitamin D metabolites using Diels-Alder derivatization and ultra-performance liquid chromatography-tandem mass spectrometry. Anal Bioanal Chem. 2008;391:1917–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vreeken RJ, Honing M, van Baar BL, Ghijsen RT, de Jong GJ, Brinkman UA. On-line post-column Diels-Alder derivatization for the determination of vitamin D3 and its metabolites by liquid chromatography/thermospray mass spectrometry. Biol Mass Spectrom. 1993;22:621–32. [DOI] [PubMed] [Google Scholar]
- 21.Endocrine Laboratory, Charing Cross Hospital, Londan, UK. Vitamin D External Quality Assessment Scheme (DEQAS) [cited 2007 Oct 1]. Available from: http://www.deqas.org/.
- 22.Carter GD, Carter CR, Gunter E, Jones J, Jones G, Makin HL, Sufi S. Measurement of Vitamin D metabolites: an international perspective on methodology and clinical interpretation. J Steroid Biochem Mol Biol 2004;89–90:467–71. [DOI] [PubMed] [Google Scholar]
- 23.Harris SS, Dawson-Hughes B. The association of oral contraceptive use with plasma 25-hydroxyvitamin D levels. J Am Coll Nutr. 1998;17:282–4. [DOI] [PubMed] [Google Scholar]
- 24.Heaney RP, Davies KM, Chen TC, Holick MF, Barger-Lux MJ. Human serum 25-hydroxycholecalciferol response to extended oral dosing with cholecalciferol. Am J Clin Nutr. 2003;77:204–10. [DOI] [PubMed] [Google Scholar]
- 25.Matsuoka LY, Wortsman J, Haddad JG, Hollis BW. In vivo threshold for cutaneous synthesis of vitamin D3. J Lab Clin Med. 1989;114:301–5. [PubMed] [Google Scholar]
- 26.Edvardsen K, Brustad M, Engelsen O, Aksnes L. The solar UV radiation level needed for cutaneous production of vitamin D3 in the face. A study conducted among subjects living at a high latitude (68 degrees N). Photochem Photobiol Sci. 2007;6:57–62. [DOI] [PubMed] [Google Scholar]
- 27.Brustad M, Edvardsen K, Wilsgaard T, Engelsen O, Aksnes L, Lund E. Seasonality of UV-radiation and vitamin D status at 69 degrees north. Photochem Photobiol Sci. 2007;6:903–8. [DOI] [PubMed] [Google Scholar]
- 28.Kimlin MG, Downs NJ, Parisi AV. Comparison of human facial UV exposure at high and low latitudes and the potential impact on dermal vitamin D production. Photochem Photobiol Sci. 2003;2:370–5. [DOI] [PubMed] [Google Scholar]
- 29.Kimlin MG, Schallhorn KA. Estimations of the human 'vitamin D' UV exposure in the USA. Photochem Photobiol Sci. 2004;3:1067–70. [DOI] [PubMed] [Google Scholar]
- 30.Webb AR, Engelsen O. Calculated ultraviolet exposure levels for a healthy vitamin D status. Photochem Photobiol. 2006;82:1697–703. [DOI] [PubMed] [Google Scholar]
- 31.Livesey J, Elder P, Ellis MJ, Florkowski C, McKenzie R, Liley B. Response to the letter by Bolland et al on defining vitamin D deficiency. N Z Med J. 2007;120:U2815. [PubMed] [Google Scholar]
- 32.Scragg R, Camargo CA Jr. Frequency of leisure-time physical activity and serum 25-hydroxyvitamin D levels in the US population: results from the Third National Health and Nutrition Examination Survey. Am J Epidemiol. 2008;168:577–86, discussion 587–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jacobs ET, Alberts DS, Foote JA, Green SB, Hollis BW, Yu Z, Martinez ME. Vitamin D insufficiency in southern Arizona. Am J Clin Nutr. 2008;87:608–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sahota H, Barnett H, Lesosky M, Raboud JM, Vieth R, Knight JA. Association of vitamin D related information from a telephone interview with 25-hydroxyvitamin D. Cancer Epidemiol Biomarkers Prev. 2008;17:232–8. [DOI] [PubMed] [Google Scholar]
- 35.Armas LA, Dowell S, Akhter M, Duthuluru S, Huerter C, Hollis BW, Lund R, Heaney RP. Ultraviolet-B radiation increases serum 25-hydroxyvitamin D levels: the effect of UVB dose and skin color. J Am Acad Dermatol. 2007;57:588–93. [DOI] [PubMed] [Google Scholar]
- 36.Godar DE, Wengraitis SP, Shreffler J, Sliney DH. UV doses of Americans. Photochem Photobiol. 2001;73:621–9. [DOI] [PubMed] [Google Scholar]
- 37.Gurrea G, Canada J. Study of UV radiation dose received by the Spanish population. Photochem Photobiol. 2007;83:1364–70. [DOI] [PubMed] [Google Scholar]
- 38.Diffey B. A behavioral model for estimating population exposure to solar ultraviolet radiation. Photochem Photobiol. 2008;84:371–5. [DOI] [PubMed] [Google Scholar]
- 39.Thieden E, Philipsen PA, Wulf HC. Ultraviolet radiation exposure pattern in winter compared with summer based on time-stamped personal dosimeter readings. Br J Dermatol. 2006;154:133–8. [DOI] [PubMed] [Google Scholar]
- 40.Godar DE. UV doses worldwide. Photochem Photobiol. 2005;81:736–49. [DOI] [PubMed] [Google Scholar]
- 41.Looker AC, Pfeiffer CM, Lacher DA, Schleicher RL, Picciano MF, Yetley EA. Serum 25-hydroxyvitamin D status of the US population: 1988–1994 compared with 2000–2004. Am J Clin Nutr. 2008;88:1519–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Takeuchi A, Okano T, Ishida Y, Kobayashi T. Effects of dietary vitamin D intake on plasma levels of parathyroid hormone and vitamin D metabolites in healthy Japanese. Miner Electrolyte Metab. 1995;21:217–22. [PubMed] [Google Scholar]
- 43.Thomas MK, Lloyd-Jones DM, Thadhani RI, Shaw AC, Deraska DJ, Kitch BT, Vamvakas EC, Dick IM, Prince RL, et al. Hypovitaminosis D in medical inpatients. N Engl J Med. 1998;338:777–83. [DOI] [PubMed] [Google Scholar]
- 44.Calvo MS, Whiting SJ, Barton CN. Vitamin D intake: a global perspective of current status. J Nutr. 2005;135:310–6. [DOI] [PubMed] [Google Scholar]
- 45.Cashman KD, Hill TR, Lucey AJ, Taylor N, Seamans KM, Muldowney S, Fitzgerald AP, Flynn A, Barnes MS, et al. Estimation of the dietary requirement for vitamin D in healthy adults. Am J Clin Nutr. 2008;88:1535–42. [DOI] [PubMed] [Google Scholar]
- 46.Aloia JF, Patel M, Dimaano R, Li-Ng M, Talwar SA, Mikhail M, Pollack S, Yeh JK. Vitamin D intake to attain a desired serum 25-hydroxyvitamin D concentration. Am J Clin Nutr. 2008;87:1952–8. [DOI] [PubMed] [Google Scholar]
- 47.MacLaughlin J, Holick MF. Aging decreases the capacity of human skin to produce vitamin D3. J Clin Invest. 1985;76:1536–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wortsman J, Matsuoka LY, Chen TC, Lu Z, Holick MF. Decreased bioavailability of vitamin D in obesity. Am J Clin Nutr. 2000;72:690–3. [DOI] [PubMed] [Google Scholar]