Abstract
The concentration or threshold of 25-hydroxyvitamin D [25(OH)D] needed to maximally suppress intact serum parathyroid hormone (iPTH) has been suggested as a measure of optimal vitamin D status. Depending upon the definition of maximal suppression of iPTH and the 2-phase regression approach used, 2 distinct clusters for a single 25(OH)D threshold have been reported: 16–20 ng/mL (40–50 nmol/L) and 30–32 ng/mL (75–80 nmol/L). To rationalize the apparently disparate published results, we compared thresholds from several regression models including a 3-phase one to estimate simultaneously 2 thresholds before and after adjusting for possible confounding for age, BMI, glomerular filtration rate, dietary calcium, and season (April–September vs. October–March) within a single data set, i.e. data from the Tufts University Sites Testing Osteoporosis Prevention/Intervention Treatment study, consisting of 181 men and 206 women (total n = 387) ages 65–87 y. Plasma 25(OH)D and serum iPTH concentrations were (mean ± SD) 22.1 ± 7.44 ng/mL (55.25 ± 18.6 nmol/L) and 36.6 ± 16.03 pg/mL (3.88 ± 1.7 pmol/L), respectively. The 3-phase model identified 2 thresholds of 12 ng/mL (30 nmol/L) and 28 ng/mL (70 nmol/L); similar results were found from the 2-phase models evaluated, i.e. 13–20 and 27–30 ng/mL (32.5–50 and 67.5–75 nmol/L) and with previous results. Adjusting for confounding did not change the results substantially. Accordingly, the 3-phase model appears to be superior to the 2-phase approach, because it simultaneously estimates the 2 threshold clusters found from the 2-phase approaches along with estimating confidence limits. If replicated, it may be of both clinical and public health importance.
Introduction
The circulating blood concentration of 25-hydroxyvitamin D [25(OH)D]9 is currently considered to be the best measure of vitamin D status (1). In the absence of direct dose-response data between 25(OH)D and health outcomes, some have suggested using the concentration of 25(OH)D needed to maximally suppress intact serum parathyroid hormone (iPTH) as a possible approach for determining optimal vitamin D status, especially in persons over 60–65 y of age (2–4). For this population, maximal suppression of parathyroid hormone (PTH) is desired, because higher concentrations, even within the normal range, lead to bone resorption and bone loss (4,5).
Many investigators have reported the 25(OH)D concentration or threshold above which PTH was not further suppressed using a 2-phase regression approach. However, the threshold points identified have been strikingly varied, ranging from 10 to 50 ng/mL10 (6–11). Several factors could contribute to different threshold points, including differences in calcium intake and other population differences, differences in 25(OH)D or PTH assays, and/or differences related to the choice of statistical approach used. It is important to differentiate between biological and statistical sources of differences, because this may impact the approach to defining the threshold between 25(OH)D and PTH concentrations in men and women 60–65 y of age and older.
Several 2-phase regression approaches have been used to estimate the points of threshold in the association of 25(OH)D with PTH (4,5). In addition, a 3-phase approach has also been suggested (12). To date there has been no systematic evaluation, to our knowledge, of the impact that the choice of statistical approach has on the threshold point. The purpose of this analysis is, within the same data set, to estimate and compare thresholds estimated from several 2-and 3-phase models that use different assumptions about the shape of the underlying curves and the definition of maximal suppression of iPTH. The larger goal is to rationalize the apparently disparate threshold points that have been identified.
Materials and Methods
Study design.
This study is a reanalysis of baseline data from the 445 participants enrolled in the National Institutes on Aging STOP/IT or Sites Testing Osteoporosis Prevention/Intervention Treatment at Tufts University (10). Volunteers 65 y of age and older were recruited and subsequently enrolled into the study at an approximately even rate of 35 participants/mo over a 12.5-mo period. Prior to enrollment, it was established that all of the participants had normal liver and kidney function. The protocol for the Sites Testing Osteoporosis Prevention/Intervention Treatment Study was approved by the Human Investigation Review Committee at Tufts University and all volunteers gave written informed consent.
Measurements.
Medical history including prescription medicine usage was assessed using questionnaires. Dietary intake was assessed using a quantitative FFQ that included the use of food models to assess portion sizes. BMI was calculated as the ratio of measured weight in kilograms divided by measured height in squared meters.
Blood was drawn by venipuncture after an 8-h fast. Plasma samples were used to determine 25(OH)D concentrations using the method of Preece et al. (13). Subsequently, the 25(OH)D values were calibrated to those of the Diasorin based on a calibration study in which 40 samples were measured by both methods. Estimated Diasorin values (D) in ng/mL were calculated from the competitive binding protein values (C) in ng/mL as D = 5.742+0.538(C). Values of 25(OH)D were converted to units of nmol/L by multiplying by 2.5. iPTH from serum was measured with Allegro Intact PTH kits obtained from Nichols Institute. The inter-assay CV for iPTH was 6.6%. Serum creatinine was measured by colorimetry with a Cobas Fara centrifugal analyzer (Roche Instruments). Glomerular filtration rate (GFR) was calculated using the IDMS-Traceable Modification of Diet in Renal Disease Study equation (14). The equation is: GFR [mL/(min⋅1.73 m2)] = 175 ⋅ [serum creatinine (mg/dL)]−1.154 ⋅ (age)−0.203 ⋅ (0.742 if female) ⋅ (1.210 if Black). Serum normalized ionized calcium was measured with a Nova 7 analyzer (Nova Biomedical). All laboratory analyses were performed in the Tufts Nutrition Evaluation Laboratory in batches as the samples were collected.
Analytic sample.
We excluded 49 participants from the initial sample of 445 volunteers who reported taking anticonvulsants and thiazide diuretics. In addition, we excluded 8 participants who reported taking loop diuretics and 1 individual missing values for 25(OH)D and iPTH. The final sample size after all the exclusions was 387 and included 181 men and 206 women.
Statistical analysis.
The original data set in SPSS was converted to SAS 9 (SAS Institute) and STATA data sets using DBMS/Copy version 7 (Conceptual Software). Subsequent analyses in SAS and STATA 10 showed that there were no errors in the copies made from the original SPSS data set (data not shown). Means, SD, and Pearson correlation coefficients were calculated using SAS 9.1. Mean values for men and women were compared using a t test.
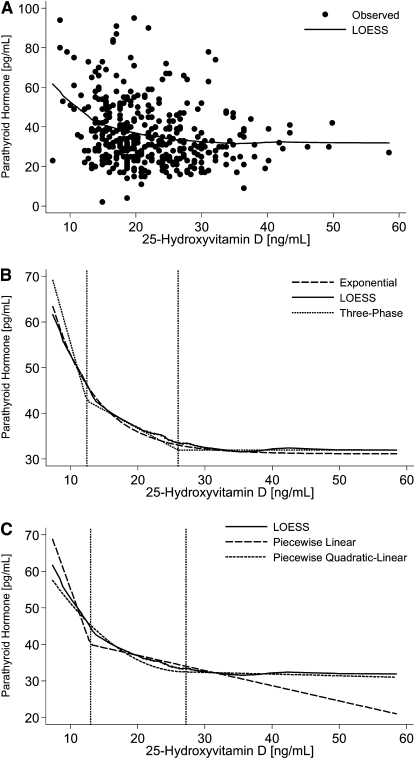
We began our preliminary analyses by fitting a locally weighted regression smoothing scatterplot (LOESS) line to the data as shown in Figure 1A (15). The LOESS procedure is a nonparametric method for fitting a smooth curve that best characterizes the relationship between 2 continuous variables, e.g. iPTH and 25(OH)D (16,17). The curvilinear shape of the LOESS line supported the fitting of the subsequently evaluated statistical models (Fig. 1A).
FIGURE 1 .
(A) Plot of original data with the LOESS model curve included. (B) Points of intersection or thresholds for the unadjusted 3-phase model compared with the LOESS and the unadjusted exponential model curves. (C) Comparison of the unadjusted regression lines from selected 2-phase models with the thresholds from the unadjusted 3-phase model. A more detailed explanation of the statistical methods is in Supplemental Appendix 1.
The threshold values for 25(OH)D were calculated for both the 2-phase and 3-phase models using algorithms programmed into STATA 10.0 by one of the authors (R.A.D-A.) (18,19). This threshold point has been also referred to as the point of inflection, point of intersection, change-point, or “knot.” The 2-phase model is used to estimate a threshold that demarcates the point in the PTH-25(OH)D curve above which PTH is maximally suppressed. The 3-phase model is used to divide the curve into 3 parts with 2 thresholds or points of intersection. The 3 phases are: 1) phase 1, where PTH concentrations rapidly decline at a constant rate with increasing concentrations of 25(OH)D; 2) phase 2 where the rate of decrease in PTH concentrations with increasing concentrations of 25(OH)D changes to a slower and slower rate; and 3) phase 3 where PTH is maximally suppressed. The points of intersection or thresholds between or among phases were estimated using a grid search approach (18,19). The 95% confidence limits (CL) for the 2-phase and 3-phase models were calculated from bootstrap estimates based on 1000 replications (20,21). Bias corrected CL were calculated with Stata 10 (22,23) and corroborated using the Delta Method (results not shown). More details are available in Supplemental Appendix 1 and Supplemental Figure 1.
For the 2-phase model, maximal suppression of iPTH was defined in 2 ways. In the first way, a constraint was imposed that the slope of the line after the point of intersection must be zero. That is, after the threshold the change in iPTH per unit change in 25(OH)D was set at zero. In the other definition, no such constraint or restriction was imposed and the slope was allowed to be significantly different from zero.
The single point of inflection for the 2-phase models was estimated using the following statistical approaches where the constraint was imposed: piecewise linear, piecewise quadratic-linear, and exponential. Where the constraint was not imposed, the following statistical approaches were used: piecewise-linear, piecewise quadratic-linear, and piecewise quadratic-quadratic. The thresholds for the exponential model were approximated by calculating the point at which the change (Δ) in iPTH per unit change in 25(OH)D was approximately zero. Two values for ΔiPTH ≈ 0 were when ΔiPTH was 1/100th and 1/300th of the SD for iPTH, i.e. 0.15 pg/mL and 0.05 pg/mL, respectively. A 3rd approach using the exponential model to identify a threshold was also explored by applying the equation by Guillemant et al. (12). More details are available in Supplemental Appendix 1.
The thresholds were calculated for models unadjusted and adjusted for possible confounding. The potential confounders considered were: age, sex, BMI, GFR, ionized calcium, dietary calcium, and season [summer = April–September and winter = October–March]. In preliminary analyses of a linear model where iPTH was the dependent variable and 25(OH)D and 25(OH)D2 were forced into the model, we used a backward elimination procedure to identify the significant confounders of the relationship between iPTH and 25(OH)D as age, BMI, GFR, dietary calcium, and season.
Results
The age range for men and women was 65–87 y (Table 1). The sample was 97% White. Men had somewhat higher 25(OH)D values. Forty-one percent of the men (74 of 181) and 53% of the women (109 of 206) had their examinations in the summer (P < 0.05); significantly more men than women had their examination during the winter months when plasma 25(OH)D concentrations are at a low point. This helps to explain why season was found to be a significant confounder in preliminary analyses. In addition, men also had a somewhat higher GFR (P < 0.05) and slightly lower values for ionized serum calcium (P < 0.05).
TABLE 1.
Characteristics of the entire sample, men, and women1
| Characteristic | Total | Men | Women |
|---|---|---|---|
| n | 387 | 181 | 206 |
| Age, y | 70.8 ± 4.51 | 70.7 ± 4.64 | 70.8 ± 4.41 |
| Plasma 25(OH)D,2μg/L | 22.1 ± 7.44 | 23.7 ± 7.62 | 20.7 ± 6.99* |
| Intact serum PTH, pg/mL | 36.6 ± 16.03 | 35.3 ± 16.38 | 37.8 ± 15.66 |
| BMI, kg/m2 | 26.7 ± 4.07 | 26.9 ± 3.36 | 26.5 ± 4.60 |
| GFR, mL/(min⋅1.73m2) | 76.9 ± 14.9 | 79.2 ± 16.13 | 74.9 ± 13.49* |
| Dietary calcium, mg/d | 736 ± 352 | 721 ± 374 | 749 ± 331 |
| Serum ionized calcium,34mg/dL | 5.04 ± 0.15 | 5.02 ± 0.16 | 5.06 ± 0.15* |
Values are means ± SD. *Different from men, P < 0.05.
To convert to nmol/L, multiply μg/L by 2.5.
One man and 1 woman were missing data (n = 385).
To convert to mmol/L, multiply mg/dL by 0.25.
Results from the various 2-phase approaches indicate that the estimated thresholds fall into 2 groups or clusters of values (Table 2). Estimated values tended to be in the 2 clusters of 13–20 ng/mL or 27–30 ng/mL in both unadjusted and adjusted models. The 1 exception was where the threshold was estimated to be ∼38 ng/mL 25(O)D from the exponential model assuming a Δ iPTH of 0.05 pg/mL. The calculated exponential model is:
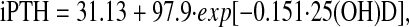 |
where 25(OH)D was in units of ng/mL. Using the equation by Guillemant et al. (10) of −3/C, the estimated threshold of 25(OH)D was 19.9 ng/mL.
TABLE 2.
Thresholds of plasma 25(OH)D for the maximal suppression of iPTH as estimated from the 2-phase model using selected statistical approaches1
| Point of inflection, ng/mL (95% CL) |
||
|---|---|---|
| Statistical approach | Unadjusted | Adjusted2 |
| Constraints: Δ PTH (pg/mL) per 1 ng/mL change in 25(OH)D = 0 | ||
| Piecewise linear | 20.8 (11.6–30.0) | 20.8 (11.8–30.0) |
| Piecewise quadratic-linear | 28.0 (13.2–39.8) | 29.0 (15.0–39.8) |
| Exponential3 | ||
| Δ= 0.05 | 37.7 (23.9–73.9) | 38.2 (24.2–88.5) |
| Δ= 0.15 | 30.4 (21.1–50.3) | 30.5 (21.2–56.9) |
| Constraints: none | ||
| Piecewise linear | 13.0 (10.0–28.4) | 18.6 (11.2–39.2) |
| Piecewise quadratic-linear | 27.2 (12.6–39.8) | 29.0 (12.8–39.8) |
| Piecewise quadratic-quadratic | 27.8 (12.6–39.8) | 28.8 (12.8–39.8) |
To convert to nmol/L, multiply ng/mL by 2.5.
Adjusted for age (y), BMI (kg/m2), GFR [mL/(min⋅1.73m2)], dietary calcium intake (mg/d), and season (April–September vs. October–March).
Model PTH = a + b(exp c[25(OH)D]) with the threshold calculated as the value of 25(OH)D where the difference between successive values of PTH was 0.05 pg/mL and 0.15 pg/mL, respectively.
Except for the case of the unconstrained piecewise linear or line-line model, adjusting for confounding had only a relatively minor effect on the estimates for the point of inflection. In that case, the unadjusted estimate of the threshold was 13.0 ng/mL 25(OH)D, whereas it was 18.6 ng/mL 25(OH)D after adjusting for confounding.
Estimated thresholds from the 3-phase model were consistent with the 2 clusters estimated from the 2-phase model (Table 3). The points of intersection between phases 1 and 2 were 12.4 ng/mL 25(OH)D for the unadjusted approach and 13.2 ng/mL 25(OH)D for the adjusted. Similarly, the point of intersection between phases 2 and 3 was estimated to be 26.0 ng/mL in the unadjusted model and a slightly higher value of ∼29 ng/mL after adjustment. Moreover, the 3 line segments from the 3-phase model had a shape that was almost identical to the shape of the LOESS and exponential model curves (Fig. 1B). Superimposing the points of intersection or threshold from the 3-phase model on top of the lines from the 2-phase model revealed that the threshold from the piecewise linear model corresponded to the threshold between phases 1 and 2, whereas the threshold for the piecewise quadratic-linear and exponential models correspond to the threshold between phases 2 and 3 of the 3-phase model (Fig. 1C).
TABLE 3.
Thresholds of plasma 25(OH)D for the maximal suppression of iPTH at the intersection of phases 1 and 2, and phases 2 and 3 as estimated from the 3-phase model1
| Point of inflection, μg/L (95% CL) |
||
|---|---|---|
| Thresholds | Unadjusted | Adjusted2 |
| Intersection of: | ||
| Phase 1 and Phase 2 | 12.4 (10.4–25.2) | 13.2 (10.4–28.0) |
| Phase 2 and Phase 3 | 26.0 (17.6–40.0) | 28.8 (18.8–40) |
To convert to nmol/L, multiply μg/L by 2.5.
Adjusted for age (y), BMI (kg/m2), GFR [mL/(min⋅1.73m2)], dietary calcium intake (mg/d), and season (April–September vs. October–March).
The Δ iPTH per Δ 25(OH)D for the 3 line segments or phases of the 3-phase model were for phases 1, 2, and 3: −5.219, −0.796, and 0.00007 pg/mL per ng/mL (−2.03, −0.313, and −0.002 pg/mL per nmol/L), respectively. The largest rate of decline in iPTH per unit decrease in 25(OH)D occurred during phase 1. The rate of decline in phase 2 was only ∼17% of the rate of decline during phase 1. In phase 3, no further declines in iPTH occurred with increasing concentrations of 25(OH)D.
Discussion
Within the broad range of 25(OH)D concentrations evaluated in this data set, the 3-phase model identified 2 threshold points, 1 at ∼12 ng/mL and 1 at ∼28 ng/mL. Although 2-phase models used previously, by definition, identified only 1 threshold point, the points identified have been strikingly close to either the lower or the higher of these 2 points (12 or 28 μg/L). These findings suggest that the statistical approach used accounts for a substantial proportion of the variability in the threshold points identified to date. As a result, we recommend use of the 3-phase approach, because it provides a more detailed description of the association of 25(OH)D with PTH. This approach should be useful in placing a new threshold into context within the literature with respect to whether it is the threshold point for the more rapid or the slower change in PTH. It is important to note that this study does not rule out the possibility that biological and environmental factors also affect the threshold point.
Several authors have questioned the use of iPTH to estimate optimal concentrations of 25(OH)D (4,5,17,24) and have proposed that history of falls, fracture risk, and chronic disease risk may be more appropriate clinical measures for determining optimal concentrations of vitamin D. We wholeheartedly agree about the importance of clinical endpoints in evaluating optimal 25(OH)D concentrations. However, we also think that an assessment of the maximal suppression of iPTH by 25(OH)D is a useful component in enhancing our understanding of that evaluation (25).
All of the linear regression approaches tend to draw a smooth line through the data. The objective is to develop a linear modeling approach that provides the advantages of the linear model but which closely approximates the shape of the LOESS curve (5,17,22). The lines from the piecewise quadratic-linear and piecewise quadratic-quadratic approaches closely approximate the shape of the LOESS curve; however, this approach allows only the estimation of the threshold where iPTH is maximally suppressed by 25(OH)D (Fig. 1A). The piecewise linear approach or the line-line approach, using the terminology of Aloia et al. (5), on the other hand, provides a good fit for only the first phase where there is a rapid increase in iPTH with decreasing concentrations of 25(OH)D. As a result, the different piecewise approaches appear to identify 2 separate thresholds or 2 clusters of 25(OH)D values located at the 2 points of intersection. The curve of the 3-phase approach not only provides an excellent approximation of the LOESS curve but it can be used also to estimate both thresholds simultaneously and evaluate the relative strengths or magnitude of the rate of change between and among those thresholds.
Two distinct clusters for 25(OH)D were also identified by Aloia et al. (5) and by Dawson-Hughes et al. (4). Based on a meta-analysis, Aloia et al. (5) reported clusters at 16–20 ng/mL and 30–32 ng/mL that are very similar to the 2 clusters found in this paper. In both of these papers, a 2-phase model was used to estimate the threshold for 25(OH)D. However, because different definitions for maximal suppression of iPTH were used in these papers, different conclusions were reached as to the threshold between iPTH and 25(OH)D. In the paper by Dawson-Hughes et al. (4), the authors define maximal suppression of iPTH by 25(OH)D as the concentration of 25(OH)D where iPTH will not decrease as a result of any further increases in 25(OH)D.
Aloia et al. (5) estimated the 25(OH)D threshold in the participants of a randomized clinical trial of 208 African American women 50–75 y of age. All participants had a total calcium intake of ∼1200–1500 mg/d. The women were randomly assigned to receive 20 μg/d (800 IU/d) of oral cholecalciferol or placebo. After 2 y, the cholecalciferol dose was raised to 50 μg/d (2000 IU/d) for the final study year. Among women who received 20 μg/d for the first year, iPTH dropped −13.4 pg/mL in those with baseline 25(OH)D <16.8 μg/L (42 nmol/L) but only −2.8 pg/mL in those with higher baseline 25(OH)D. Those results led Aloia et al. (5) to suggest that the threshold at 16.8 μg/L (42 nmol/L) was a better estimate of the concentration of 25(OH)D at the point of maximal suppression of iPTH. Interestingly, the results of Aloia et al. in Blacks were very similar to the present study, which used a sample that was 97% White.
There are several advantages to the approaches we have used in this study. The 3-phase model represents a systematic approach to the estimation of multiple change point parameters, along with assessment of the amount of uncertainty via estimation of SE and CL. Other models including fractional polynomials and cubic splines may be potential alternatives to the 3-phase model. However, these models might be monotonic, making it difficult to identify thresholds and change points.
Although the 3-phase model approach appears to be an improvement over the 2-phase model for estimating the threshold for 25(OH)D at the point of maximal suppression of iPTH, validation of the model will require replication in other data sets. The impact that different serum 25(OH)D and iPTH assay methods might have on the estimation of threshold points needs to be explored. Also, it remains to be seen if the 3-phase model will be appropriate for other life-stage and racial/ethnic groups. If replicated in other data sets for this age group, we think that the approach may be of both clinical and public health importance.
Supplementary Material
Acknowledgments
R.A.D-A., B.D-H., C.T.S., E.A.Y., A.C.L., V.L.B., A.L.C., and M.F.P. designed research; B.D-H. and S.S.H. provided essential materials; R.A.D-A., C.T.S., and G.C. performed statistical analysis; R.A.D-A., B.D-H., C.T.S., E.A.Y., A.C.L., S.S.H., A.L.C., and M.F.P. wrote the paper; and R.A.D-A., B.D-H., C.T.S., E.A.Y., S.S.H., and M.F.P. had primary responsibility for the final content. All authors read and approved the final manuscript.
Supported by a grant from the National Institute on Aging, NIH, Bethesda, MD, grant number AG10353, and by an Office of Dietary Supplements administrative supplement to NIH grant number 5R37 HL045508-17. The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the CDC, the NIH, or the US Department of Health and Human Services.
Author disclosures: R. A. Durazo-Arvizu, B. Dawson-Hughes, C. T. Sempos, E. A. Yetley, A. C. Looker, G. Cao, S. S. Harris, V. L. Burt, A. L. Carriquiry, and M. F. Picciano, no conflicts of interest.
Supplemental Figure 1 and Supplemental Appendix 1 are available with the online posting of this paper at jn.nutrition.org.
Abbreviations used: GFR, glomerular filtration rate; LOESS, locally weighted regression smoothing scatterplot; PTH, parathyroid hormone; iPTH, intact parathyroid hormone; 25(OH)D, 25-hydroxyvitamin D.
Conversion: nmol/L = 2.5 ⋅ ng/mL.
References
- 1.Food and Nutrition Board, Institute of Medicine. Dietary reference intakes for calcium, magnesium, phosphorus, vitamin D, and fluoride: dietary reference intakes for calcium, magnesium, phosphorus, vitamin D, and fluoride. Washington, DC: National Academy Press; 1997. p. 250–87.
- 2.Brannon PM, Yetley EA, Bailey RL, Picciano MF. Overview of the conference “Vitamin D and Health in the 21st Century: an Update.” Am J Clin Nutr. 2008;88 Suppl:S483–90. [DOI] [PubMed] [Google Scholar]
- 3.Brannon PM, Yetley EA, Bailey RL, Picciano MF. Summary of roundtable discussion on vitamin D research needs. Am J Clin Nutr. 2008;88 Suppl:S587–92. [DOI] [PubMed] [Google Scholar]
- 4.Dawson-Hughes B, Heaney RP, Holick MF, Lips P, Meunier PJ, Vieth R. Estimates of optimal vitamin D status. Osteoporos Int. 2005;16:713–6. [DOI] [PubMed] [Google Scholar]
- 5.Aloia JF, Talwar SA, Pollack S, Feuerma M, Yeh JK. Optimal vitamin D status and serum parathyroid hormone concentrations in African American women. Am J Clin Nutr. 2006;84:602–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gannage-Yared MH, Chemali R, Yaacoub N, Halaby G. Hypovitaminosis D in a sunny country: relation to lifestyle and bone markers. J Bone Miner Res. 2000;15:1856–61. [DOI] [PubMed] [Google Scholar]
- 7.Thomas MK, Lloyd-Jones DM, Thadhani RI, Shaw AC, Deraska DJ, Kitch BT, Vamvakas EC, Dick IM, Prince RL, et al. Hypovitaminosis D in medical inpatients. N Engl J Med. 1998;338:777–83. [DOI] [PubMed] [Google Scholar]
- 8.Malabanan A, Veronikis IE, Holick MF. Redefining vitamin D insufficiency. Lancet. 1998;351:805–6. [DOI] [PubMed] [Google Scholar]
- 9.Chapuy MC, Schott AM, Garnero P, Hans D, Delmas PD, Meunier PJ. Healthy elderly French women living at home have secondary hyperparathyroidism and high bone turnover in winter. EPIDOS Study Group. J Clin Endocrinol Metab. 1996;81:1129–33. [DOI] [PubMed] [Google Scholar]
- 10.Dawson-Hughes B, Harris SS, Dallal GE. Plasma calcidiol, season, and serum parathyroid hormone concentrations in healthy elderly men and women. Am J Clin Nutr. 1997;65:67–71. [DOI] [PubMed] [Google Scholar]
- 11.Kinyamu HK, Gallagher JC, Rafferty KA, Balhorn KE. Dietary calcium and vitamin D intake in elderly women: effect on serum parathyroid hormone and vitamin D metabolites. Am J Clin Nutr. 1998;67:342–8. [DOI] [PubMed] [Google Scholar]
- 12.Guillemant J, Taupin P, Le HT, Taright N, Allemandou A, Péres G, Guillemant S. Vitamin D status during puberty in French healthy male adolescents. Osteoporos Int. 1999;10:222–5. [DOI] [PubMed] [Google Scholar]
- 13.Preece MA, O'Riordan JL, Lawson DE, Kodicek E. A competitive protein-binding assay for 25-hydroxycholecalciferol and 25-hydroxyergocalciferol in serum. Clin Chim Acta. 1974;54:235–42. [DOI] [PubMed] [Google Scholar]
- 14.Levey AS, Coresh J, Greene T, Stevens LA, Zhang Y, Hendriksen S, Kusek JW, Van Lente F. Using standardized serum creatinine values in the Modification of Diet in Renal Disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145:247–54. [DOI] [PubMed] [Google Scholar]
- 15.Cleveland WS. Robust locally weighted regression and smoothing scatterplots. J Am Stat Assoc. 1979;74:829–36. [Google Scholar]
- 16.Cleveland WS, Devlin ST. Locally weighted regression: an approach to regression analysis by local fitting. J Am Stat Assoc. 1988;83:596–610. [Google Scholar]
- 17.Vieth R, El-Hajj Fuleihan G. There is no lower threshold level for parathyroid hormone as 25-hydroxyvitamin D concentrations increase. J Endocrinol Invest. 2005;28:183–6. [DOI] [PubMed] [Google Scholar]
- 18.Goetghebeur EJT, Pocock SJ. Detection and estimation of J-shaped risk-response relationship. J R Stat Soc [Ser A]. 1995;158:107–21. [Google Scholar]
- 19.Lerman PM. Fitting segmented regression models by grid search. Appl Stat. 1980;29:77–84. [Google Scholar]
- 20.Hinkley DV. Inference in two-phase regression. J Am Stat Assoc. 1971;66:736–43. [Google Scholar]
- 21.Efron B, Tibshirani R. Bootstrap methods for standard errors, confidence intervals, and other measures of statistical accuracy. Stat Sci. 1986;1:54–75. [Google Scholar]
- 22.Efron B, Tibshirani RJ. An introduction to the bootstrap. monographs on statistics and applied probability. New York: Chapman & Hall; 1993.
- 23.StataCorp. Stata 10 base reference manual. College Station (TX): Stata Press; 2007.
- 24.Heaney RP. Serum 25-hydroxyvitamin D and parathyroid hormone exhibit threshold behavior. J Endocrinol Invest. 2005;28:180–2. [DOI] [PubMed] [Google Scholar]
- 25.Holick MF. Too little vitamin D in premenopausal women: why should we care? Am J Clin Nutr. 2002;76:3–4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.