Abstract
Altered nutritional experiences such as undernutrition, overnutrition, and modified milk formula in the immediate postnatal life via the phenomenon of metabolic programming have been identified as one of the components in the etiology of metabolic syndrome. We have developed a rat model in which an altered dietary experience in the form of a high-carbohydrate (HC) milk formula in the immediate postnatal life of rat pups results in chronic hyperinsulinemia and adult-onset obesity in these rats. The HC dietary modification causes functional alterations in pancreatic islets and the hypothalamus during the period of the dietary modification. These early adaptations in islets (supporting hyperinsulinemia) and the hypothalamus (supporting hyperphagia and increased body weight gain) persist in the postweaning period despite withdrawal of the HC milk formula at the time of weaning. In female rat pups receiving the HC milk formula, metabolic programming effects translate into an adverse (hyperinsulinemic, hyperleptinemic, and obese) intrauterine environment during pregnancy, causing spontaneous transfer of the maternal phenotype to the progeny (generational effect). Our results suggest that alterations in feeding practices for babies (early introduction of cereals, fruits, etc.) and babies born to obese/hyperinsulinemic mothers may be contributing factors for the obesity epidemic prevalent in developed and developing countries.
Introduction
The incidence of obesity has reached epidemic proportions and is recognized as a major health concern worldwide. For example, in the United States, ∼66% of the adult population have been classified as overweight (BMI >25) (1). In addition, increased body weight gain is noted in a sizeable population of children belonging to all age groups (2). The presence of obesity significantly increases the risk for the development of several metabolic disorders such as type 2 diabetes, cardiovascular diseases, hypertension, hyperlipidemia, etc.
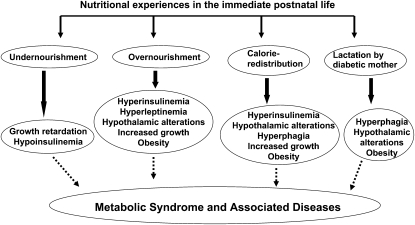
In recent decades, findings from retrospective human epidemiological studies and experimental animal models have strongly implicated altered nutritional experiences during early periods of life (fetal and immediate postnatal) as an important factor contributing to the development of the metabolic syndrome via the phenomenon of metabolic programming (3). Due to the overlap of the nutritional challenge with the critical window of the development of organs, functional alterations become permanent, resulting in a lasting imbalance in normal homeostatic metabolic mechanisms and resetting of physiological systems. Figure 1 indicates the consequences of altered nutritional experiences in the early postnatal period.
FIGURE 1 .
Metabolic programming effects due to altered nutritional experiences in the immediate postnatal life.
Metabolic programming in the immediate postnatal period
Several physiologic and metabolic mechanisms are not fully matured at birth and continue the process of maturation in the immediate postnatal period. In rodents, e.g., pancreatic islets and neurons continue to develop after birth (4,5). Thus, as was observed for the fetal period, the immediate postnatal period also becomes a critical window for development and, hence, alterations in the nutritional experience during this period function as independent cues for induction of metabolic programming effects.
One approach to altering nutrition in the suckling period has been to either increase or decrease litter size, resulting in changes in the quantity of milk available to new born pups (Fig. 1). Rat pups overnourished in the suckling period due to reduction in litter size [small litter (SL)4] demonstrated hyperinsulinemia, hyperleptinemia, and increased growth rate during not only the suckling period but also in the postweaning period (6). Significant changes in the hypothalamus of SL rats such as altered response of neurons in specific regulatory centers in the hypothalamus to insulin, leptin, and several neuropeptides (7) resulted in a strong predisposition for hyperphagia and increased body weight gain in SL rats. Rat pups raised in large litters demonstrate a decreased growth trajectory as well as reduced insulin levels (8).
The onset of hyperphagia, obesity, and other metabolic disorders in adulthood of pups born to normal rats but fostered by diabetic female rats underscores the recognition of the lactation period as being vulnerable for the establishment of metabolic programming effects due to altered nutritional experiences during this period (9). As observed for SL rats, structural and functional alterations in the hypothalamus [increased immunopositivity for neuropeptide Y, agouti-related polypeptide and galanin, and decreased immunopositivity for pro-opiomelanocortin and α-melanocortin-stimulating hormone in the arcuate nucleus (9)] supported such a phenotype. Further, rat pups that were malnourished only in the suckling period by being nursed by dams that were subject to a low-protein diet regimen displayed an altered feeding pattern as adults, lending support to the concept that the immediate postnatal life is vulnerable for metabolic programming effects (Fig. 1) (10).
The high-carbohydrate rat model
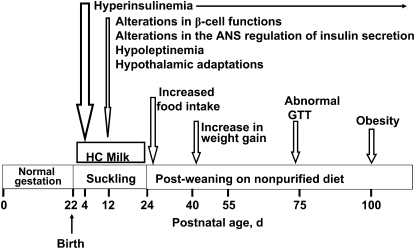
The long-term consequences of a high-carbohydrate (HC) dietary modification in newborn rats have been the focus of research in our laboratory (Fig. 2) (11–13). Our experimental approach enabled determination of the metabolic programming effects due to alterations in the quality of nutrition without changes in the total energy intake (energy redistribution) in the immediate postnatal period. This is in contrast to the SL rat model wherein newborn rat pups were overnourished due to reduction in litter size at birth. In the HC rat model, newborn rat pups are artificially raised on a HC milk formula from postnatal d 4 to 24 via i.g. cannulas. The energy composition of the HC milk formula was 56% carbohydrate, 20% fat, and 24% protein compared with 8% carbohydrate, 72% fat, and 24% protein in rat milk. HC rat pups are weaned on postnatal d 24 to a nonpurified diet. Rat pups nursed by their own dams [mother-fed (MF)] served as controls. Rat pups artificially reared on a high-fat milk formula, the macronutrient energy composition of which was similar to that of rat milk, served as experimental controls. These rats did not develop hyperinsulinemia or adult-onset obesity, indicating that the artificial rearing protocol per se did not induce any metabolic programming effects.
FIGURE 2 .
Immediate and long-term effects of artificial rearing on newborn rat pups given a HC milk formula.
Effects observed during the period of HC dietary modification.
Within 24 h after newborn rat pups were given the HC milk formula, their plasma insulin levels significantly increased and hyperinsulinemia persisted throughout the period of the HC dietary modification (12,13). Although plasma insulin levels were markedly higher in 12-d-old HC rats, their plasma glucose levels were normal. Additionally, the plasma leptin levels were significantly reduced in 12-d-old HC rats (14).
During the dietary modification in the preweaning period, several adaptations at the biochemical, molecular, and cellular levels in pancreatic islets of HC rats supported their hypersecretory capacity, including: 1) a marked increase in the sensitivity of insulin-secreting islet β-cells to a glucose stimulus; 2) increased low Km hexokinase activity; 3) increased activity of key enzymes involved in glucose metabolism; 4) the ability to secrete a moderate amount of insulin in the complete absence of glucose and under stringent calcium-deprived conditions; and 5) altered regulation of the autonomic nervous system control of insulin secretion via increased parasympathetic input and decreased sympathetic input. At the cellular level, more smaller-sized islets with increased insulin content were observed. At the molecular level, increased mRNA levels of the preproinsulin gene and some key transcription factors, such as pancreatic duodenal homeobox factor-1, involved in its expression and alterations in the expression of several clusters of genes involved in multiple cellular functions as indicated by gene array analyses were observed in islets isolated from 12 day-old HC rats (11,12).
Aberrations in the expression of hypothalamic neuropeptide genes involved in energy homeostasis were also evident in 12-d-old HC rats, suggesting that the HC dietary modulation in rat pups induced metabolic programming effects predisposing to the onset of hyperphagia and concomitant body weight gain in HC rats. Increases in the mRNA levels of orexigenic neuropeptides and decreases in the mRNA levels of anorexigenic neuropeptides were evident in the hypothalami of 12-d-old HC rats (14). Additionally, the mRNA levels of insulin receptor-β and the long form of the leptin receptor were also reduced in the hypothalami of 12-d-old HC rats (14).
Persistent effects observed in adulthood.
Although HC pups were weaned onto a nonpurified diet on postnatal d 24, hyperinsulinemia persisted in these rats. From the time of weaning, HC rats consumed increasing quantities of food, which translated into increased body weight gains such that by postnatal d 100, HC rats were significantly heavier compared with age-matched MF control rats (Fig. 2) (12,13).
Several of the biochemical, molecular, and functional changes observed in islets of neonatal HC rats persisted in adult HC rats, suggesting permanency of the immediate effects on islet function to sustain hyperinsulinemia despite withdrawal of the HC milk formula at the time of weaning. The significant changes in islet functions in adult HC rats included: 1) a distinct leftward shift in the insulin secretory response to a glucose stimulus; 2) increased hexokinase activity; 3) increased β-cell mass; 4) increased mRNA levels of the preproinsulin gene and the transcription factor genes associated with preproinsulin gene expression; 5) alterations in the gene expression of several clusters of genes associated with various functions; and 6) altered autonomic activity, including increased parasympathetic and decreased sympathetic activities (11,12). Although random plasma glucose levels were normal in adult HC rats, in response to an oral glucose load they demonstrated an abnormal glucose tolerance on postnatal d 75 that worsened by postnatal d 270 (15).
Hypothalamic adaptations predisposing for hyperphagia in 12-d-old HC rats were also evident in the hypothalami of adult HC rats (14). Furthermore, epididymal adipose tissue mass was significantly increased in adult HC male rats. The lipogenic capacity of liver and adipose tissue was significantly higher in adult HC rats as indicated by increases in the activities of key lipogenic enzymes and in the in vitro synthesis of lipids (16).
Transgenerational effect.
The observation that offspring of HC female rats spontaneously acquired the HC phenotype of chronic hyperinsulinemia and adult-onset obesity without themselves having to undergo any dietary modification (17) indicates the establishment of a transgenerational effect in this HC rat model. The HC maternal environment during pregnancy was characterized by marked increases in plasma levels of insulin, leptin, proinflammatory markers, and markers of oxidative stress compared with MF female rats (14). The observation that 4-d-old MF (control) embryos developing in the HC maternal intrauterine environment demonstrated marked increases in body weight in adulthood supports the conclusion that fetal development in the adverse intrauterine environment in the HC female rat is a major contributor to the transgenerational effect observed in this model (12). Metabolic programming effects predisposing for the development of the HC phenotype were evident in term HC fetuses (18,19).
Possible mechanisms for the observed programming effects in the HC rat model
There is a paucity of information on the mechanisms supporting programming effects due to an altered nutritional experience in the immediate postnatal period. In the overnourished SL model, the presence of increased levels of insulin has been suggested to result in malprogramming of critical centers related to appetite regulation in the hypothalamus, resulting in hyperphagia, increased body weight gain, and other metabolic aberrations in later life (20). The precise mechanisms supporting this hypothesis have not been determined.
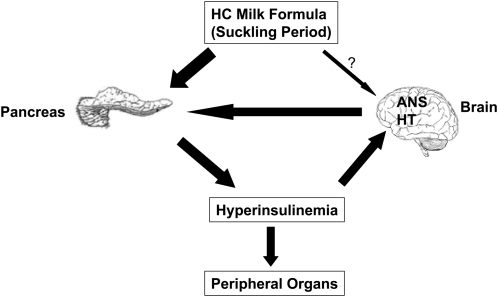
The immediate onset of hyperinsulinemia in HC rat pups indicates that pancreatic islets mount an appropriate response to the HC dietary intervention in these rat pups. This observation also suggests that islets may be one of the first targets responding to the HC milk formula (Fig. 2). The observed effects such as leftward shift in response to a glucose stimulus and increased glucose metabolism in HC islets are probably mechanisms supporting such an observation. However, the persistence of hyperinsulinemia in the postweaning period suggests that in addition to pancreatic islets, other organs may also be directly responding to the HC milk formula or to the primary metabolic responses to the HC milk formula, thereby establishing a cross-talk between several organs to support the permanent programming of hyperinsulinemia.
The hypothalamus is the primary center for energy homeostasis. The observed alterations in the mRNA levels of hypothalamic neuropeptides and receptors in 12-d-old HC rats favoring hyperphagia indicate that the hypothalamic energy circuitry is indeed responding to the HC dietary modification. It has been suggested that both insulin and leptin function as neurotrophic factors during early periods of development (21,22). Therefore, abnormal levels of these hormones during this critical period of development could malprogram vital centers involved in whole-body metabolic homeostasis in the brain. Based on these observations, it is tempting to postulate that the altered hormonal environment (hyperinsulinemia and hypoleptinemia) present in neonatal HC rats could be responsible for the malprogramming of the hypothalamic appetite regulatory mechanism. Increased levels of plasma insulin as well as altered levels of neuropeptides could also contribute to the modified regulation of the autonomic nervous system activity, resulting in increased parasympathetic and decreased sympathetic outflow that facilitate increased insulin secretion by HC islets. Therefore, it is possible that in neonatal HC rats, functional alterations at the level of pancreatic islets and the hypothalamus complement each other's functions to maintain increased levels of plasma insulin and to promote increased appetite, leading to adult-onset obesity (Fig. 3).
FIGURE 3 .
Possible mechanisms involved in metabolic programming of the HC phenotype due to the HC dietary experience in neonatal rats.
The relevance of the HC rat model in the context of the human obesity epidemic
Dietary practices for infants have undergone vast changes in the past 50 y. Extrapolation of the results from the HC rat model to the human scenario suggests that feeding practices currently implemented for infants could be one of the contributing factors to the obesity epidemic. In this regard, it is interesting to note that over the past several decades a precipitous decline in breast-feeding rates has been reported. Concomitantly, there has been an increase in formula feeding coupled with early introduction of supplemental foods (carbohydrate-enriched) for babies. It is postulated that early introduction of carbohydrate-rich supplemental foods such as cereals, fruits, and fruit juices for infants will result in the exposure of the infant to increased carbohydrate during the critical period of organ development. Such a situation could have deleterious long-term consequences for these infants in terms of eventual development of obesity and metabolic syndrome, as observed in the HC rat model.
It has been shown that the consequences of metabolic programming effects due to early-life nutritional challenges can be exacerbated by a postnatal high-fat diet. For example, intrauterine growth-restricted rat fetuses developed marked obesity and other metabolic alterations compared with control rats fed a high-fat diet (23). Insulin resistance, puberty development, and fertility problems were evident in intrauterine growth-restricted rats exposed to a high-fat diet in the postnatal period (24). Along similar lines, it is possible that in infants exposed to increased carbohydrate-derived energy during infancy, consumption of a high-fat diet later in life could result in an augmentation of the early programming effects. Such a response could cause morbid obesity in such individuals.
In the context of the prevailing obesity epidemic, our results suggest that dietary practices for newborns should be perceived as a target period for implementing proper guidelines in the efforts to stem the tide of the obesity epidemic.
Acknowledgments
M.S. and M.S.P. wrote the paper. M.S.P. had primary responsibility for the final content. Both authors read and approved the final manuscript.
Presented as part of the symposium entitled “Nutritional Experiences in Early Life as Determinants of the Adult Metabolic Phenotype” at the Experimental Biology 2009 meeting, April 20, 2009, in New Orleans, LA. This symposium was sponsored by the ASN and supported by an unrestricted educational grant from the ASN Nutritional Sciences Council and Milk Specialties Global. The Guest Editor for this symposium publication was Marta Fiorotto. Guest Editor disclosure: no conflicts of interest.
Supported in part by NIH grant DK61518 (to M.S.P.).
Author disclosures: M. S. Patel and M. Srinivasan, no conflicts of interest.
Abbreviations used: HC, high carbohydrate; MF, mother-fed; SL, small litter.
References
- 1.Flegal KM, Graubard BI, Williamson DF, Gail MH. Excess deaths associated with underweight, overweight, and obesity. JAMA. 2005;293:1861–7. [DOI] [PubMed] [Google Scholar]
- 2.Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999–2004. JAMA. 2006;295:1549–55. [DOI] [PubMed] [Google Scholar]
- 3.Gluckman PD, Hanson MA, Beedle AS. Early life events and their consequences for later disease: a life history and evolutionary perspective. Am J Hum Biol. 2007;19:1–19. [DOI] [PubMed] [Google Scholar]
- 4.Kaung HL. Growth dynamics of pancreatic islet cell populations during fetal and neonatal development of the rat. Dev Dyn. 1994;200:163–75. [DOI] [PubMed] [Google Scholar]
- 5.Grove KL, Smith MS. Ontogeny of the hypothalamic neuropeptide Y system. Physiol Behav. 2003;79:47–63. [DOI] [PubMed] [Google Scholar]
- 6.Plagemann A. Perinatal nutrition and hormone-dependent programming of food intake. Horm Res. 2006;65 Suppl 3:83–9. [DOI] [PubMed] [Google Scholar]
- 7.Davidowa H, Plagemann A. Insulin resistance of hypothalamic arcuate neurons in neonatally overfed rats. Neuroreport. 2007;18:521–4. [DOI] [PubMed] [Google Scholar]
- 8.McCance RA. Food, growth, and time. Lancet. 1962;2:671–6. [DOI] [PubMed] [Google Scholar]
- 9.Fahrenkrog S, Harder T, Stolaczyk E, Melchior K, Franke K, Dudenhausen JW, Plagemann A. Cross-fostering to diabetic rat dams affects early development of mediobasal hypothalamic nuclei regulating food intake, body weight, and metabolism. J Nutr. 2004;134:648–54. [DOI] [PubMed] [Google Scholar]
- 10.Moura AS, Franco de Sa CC, Cruz HG, Costa CL. Malnutrition during lactation as a metabolic imprinting factor inducing the feeding pattern of offspring rats when adults. The role of insulin and leptin. Braz J Med Biol Res. 2002;35:617–22. [DOI] [PubMed] [Google Scholar]
- 11.Patel MS, Srinivasan M, Laychock SG. Metabolic programming: role of nutrition in the immediate postnatal life. J Inherit Metab Dis. 2009;32:218–28. [DOI] [PubMed] [Google Scholar]
- 12.Srinivasan M, Patel MS. Metabolic programming in the immediate postnatal period. Trends Endocrinol Metab. 2008;19:146–52. [DOI] [PubMed] [Google Scholar]
- 13.Patel MS, Srinivasan M. Metabolic programming as a consequence of the nutritional environment during fetal and the immediate postnatal periods. In: Hay WW, Thureen, PJ, editors. Neonatal nutrition and metabolism. Cambridge (UK): Cambridge University Press; 2006. p. 76–90.
- 14.Srinivasan M, Mitrani P, Sadhanandan G, Dodds C, Shbeir-ElDika S, Thamotharan S, Ghanim H, Dandona P, Devaskar SU, et al. A high-carbohydrate diet in the immediate postnatal life of rats induces adaptations predisposing to adult-onset obesity. J Endocrinol. 2008;197:565–74. [DOI] [PubMed] [Google Scholar]
- 15.Vadlamudi S, Hiremagalur BK, Tao L, Kalhan SC, Kalaria RN, Kaung HL, Patel MS. Long-term effects on pancreatic function of feeding a HC formula to rats during the preweaning period. Am J Physiol. 1993;265:E565–71. [DOI] [PubMed] [Google Scholar]
- 16.Hiremagalur BK, Vadlamudi S, Johanning GL, Patel MS. Long-term effects of feeding high carbohydrate diet in pre-weaning period by gastrostomy: a new rat model for obesity. Int J Obes Relat Metab Disord. 1993;17:495–502. [PubMed] [Google Scholar]
- 17.Vadlamudi S, Kalhan SC, Patel MS. Persistence of metabolic consequences in the progeny of rats fed a HC formula in their early postnatal life. Am J Physiol. 1995;269:E731–8. [DOI] [PubMed] [Google Scholar]
- 18.Srinivasan M, Dodds C, Ghanim H, Gao T, Ross PJ, Browne RW, Dandona P, Patel MS. Maternal obesity and fetal programming: effects of a high carbohydrate nutritional modification in the immediate postnatal life of female rats. Am J Physiol Endocrinol Metab. 2008;295:E895–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Srinivasan M, Aalinkeel R, Song F, Mitrani P, Pandya JD, Strutt B, Hill DJ, Patel MS. Maternal hyperinsulinemia predisposes rat fetuses for hyperinsulinemia, and adult-onset obesity and maternal mild food restriction reverses this phenotype. Am J Physiol Endocrinol Metab. 2006;290:E129–34. [DOI] [PubMed] [Google Scholar]
- 20.Plagemann A. Perinatal programming and functional teratogenesis: impact on body weight regulation and obesity. Physiol Behav. 2005;86:661–8. [DOI] [PubMed] [Google Scholar]
- 21.Bouret SG, Simerly RB. Minireview: leptin and development of hypothalamic feeding circuits. Endocrinology. 2004;145:2621–6. [DOI] [PubMed] [Google Scholar]
- 22.Plagemann A. “Fetal programming” and ‘functional teratogenesis’: on epigenetic mechanisms and prevention of perinatally acquired lasting health risks. J Perinat. Med. 2004;32:297–305. [DOI] [PubMed] [Google Scholar]
- 23.Desai M, Babu J, Ross MG. Programmed metabolic syndrome:prenatal undernutrition and postweaning overnutrition. Am J Physiol Regul Integr Comp Physiol. 2007;293:R2306–14. [DOI] [PubMed] [Google Scholar]
- 24.Xu W, Zhu MW, Lin JF. Fetal growth retardation and postnatal high fat diet on the development of insulin resistance and fertility: experiments with rats. Zhonghua Yi Xue Za Zhi. 2007;87:1633–6. [PubMed] [Google Scholar]