Abstract
This study examined the effects of an inhibitor of 20-hydroxyeicosatetraenoic acid (20-HETE) synthesis, N-(3-chloro-4-morpholin-4-yl)phenyl-N′-hydroxyimido formamide (TS-011), on infarct volume, volume at risk, cerebral blood flow (CBF), and levels of cytochrome P450 (CYP450) eicosanoids in the brain after transient occlusion of the middle cerebral artery (t-MCAO) in rats. TS-011 (0.1 mg/kg, iv) reduced cortical infarct volume by approximately 70% and total infarct volume by 55%. TS-011 had no effect on the volume at risk or CBF during or up to 30 mins after the ischemic period. TS-011 reduced the delayed fall in CBF seen 2 h after reperfusion. The levels of CYP450 eicosanoids were similar in the ischemic and contralateral hemispheres after t-MCAO. TS-011 reduced 20-HETE levels in cerebral tissue by 80% but had no effect on the levels of EETs. Administration of another 20-HETE inhibitor, HET0016 (0.01 to 1.0 mg/kg, iv) or a 20-HETE antagonist 20-hydroxyeicosa-6(Z),15(Z)-dienoic acid (10mg/kg, iv) also reduced infarct size. These results indicate that inhibitors of the synthesis or vasoconstrictor effects of 20-HETE reduce infarct size in rats after cerebral ischemia. The effects of TS-011 are not associated with changes in the area at risk or CBF and may be because of a potential protective effect in neurons subjected to ischemic stress.
Keywords: 20-HETE, ischemic stroke, cytochrome P450, cerebral blood flow, eicosanoids
Introduction
Recent studies have indicated that arachidonic acid (AA) is metabolized by cytochrome (CYP) P450 enzymes in cerebral arteries to 20-hydroxyeicosatetraenoic acid (20-HETE) (Harder et al, 1994) and that this compound is important in the regulation of cerebral vascular tone (Gebremedhin et al, 2000). 20- HETE is a potent constrictor of cerebral arteries that depolarizes vascular smooth muscle cells by decreasing the open-state probability of Ca2+-activated K+ channels (Gebremedhin et al, 1998), which increases Ca2+ influx through L-type Ca2+ channels (Lange et al, 1997). It also increases the production of reactive oxygen species (Cheng et al, 2008; Guo et al, 2007; Medhora et al, 2008), inhibits Na+, K+-ATPase activity (Cheng et al, 2008; Nowicki et al, 1997), and activates a number of the intracellular signaling pathways (Muthalif et al, 1998; Randriamboavonjy et al, 2003; Sun et al, 1999) involved in apoptosis and cell death.
Previous studies have indicated that 20-HETE is important in the development of cerebral vasospasm after subarachnoid hemorrhage as well as influencing infarct size after transient cerebral ischemia. In this regard, the levels of 20-HETE in cerebrospinal fluid increase after subarachnoid hemorrhage, and inhibition of the synthesis or vasoconstrictor actions of 20-HETE prevents the fall in cerebral blood flow(CBF) after subarachnoid hemorrhage in rats (Cambj-Sapunar et al, 2003; Kehl et al, 2002), and reverses delayed vasospasm in both rats and dogs (Hacein-Bey et al, 2006; Takeuchi et al, 2005). Plasma levels of 20-HETE increase in rats after transient occlusion of the middle cerebral artery (t-MCAO) (Omura et al, 2006) and blockade of the synthesis of 20-HETE with a highly selective, second-generation inhibitor, N-(3-chloro-4-morpholin-4-yl)phenyl-N′-hydroxyimido formamide (TS-011) (Miyata et al, 2005), markedly reduces infarct size in rats and improves neurologic outcome after MCAO (Omura et al, 2006; Tanaka et al, 2007). However, the mechanisms underlying the protective actions of TS-011 are unknown. It has been assumed because 20-HETE is a potent vasoconstrictor of cerebral arteries that blocking the synthesis of 20-HETE may increase the collateral blood flow to the ischemic penumbra during ischemia or alter cerebral hemodynamics after reperfusion. Conversely, 20-HETE activates a number of signaling pathways involved in apoptosis and cell death (Muthalif et al, 1998) so it is possible that inhibition of the synthesis or actions of 20-HETE with TS-011 might have a protective effect to oppose necrosis or apoptosis of neurons subjected to ischemic stress.
To explore these possibilities, the present study examined the effect of TS-011 on regional cerebral blood flow (rCBF), the profile of CYP450 eicosanoids in cerebral tissue, infarct size, and the volume at risk in rats subjected to t-MCAO. To ensure that the protective effects were related to blockade of the synthesis of 20-HETE rather than a nonspecific effect of TS-011, we also determined whether administration of a less selective and different inhibitor of the synthesis of 20-HETE, N-hydroxy- N-(4-butyl-2-methylphenyl)-formamidine (HET0016) (Miyata et al, 2001), or a chemically dissimilar 20-HETE antagonist, 20-hydroxyeicosa-6(Z),15(Z)- dienoic acid (6,15-20-HEDE) (Yu et al, 2003), has a similar effect to reduce infarct volume in rats subjected to t-MCAO.
Materials and methods
Experiments were performed on 180 male Wistar rats weighing between 270 and 310 g. The rats were housed in an AAALAC-accredited animal care facility at the Medical College of Wisconsin and they had free access to food and water throughout the study. All experimental procedures were approved by the Animal Care and Use Committees of the Medical College of Wisconsin and the Johns Hopkins University.
Middle Cerebral Artery Occlusion Model in Rats
Anesthesia was induced with 4% isoflurane and maintained with 1.5% isoflurane in a mixture of 30% oxygen and 70% nitrogen through an inhalation mask. Temperature was continuously monitored with a rectal probe and maintained at 37°C with a thermostatically regulated heated table. Catheters were placed in the femoral artery and vein for measurement of mean arterial pressure and infusion of drugs. Focal brain ischemia was induced using the intraluminal suture method as previously described (Nagasawa and Kogure, 1989; Omura et al, 2006). Briefly, the left common, internal and external carotid arteries were exposed through a midline incision of the neck. A 4-0 silicone-coated nylon suture was introduced into the internal artery and advanced to the origin of the left middle cerebral artery (MCA). The rats in different series of experiments were exposed to 60 to 120 mins of occlusion of the MCA to develop infarcts of varying severity (Nagasawa and Kogure, 1989) and the 4-0 silicone-coated nylon suture blocking the origin of the MCA was withdrawn to allow reperfusion. After 2 h of reperfusion, the rats were recovered from anesthesia. The effects of TS-011 on infarct size and rCBF were studied in two groups of animals. In one group, the rats received TS-011 (0.1 mg/kg, iv) or vehicle (11% sulfobutylether-7-β-cyclodextrin in a 250 mmol/L mannitol solution) 30 mins after the initiation of ischemia followed by a sustaining infusion (0.1 mg/kg per hour) for 120 min after ischemic period. In group 2, the rats were pretreated with TS-011 (0.1 mg/kg) or vehicle 30 mins before the initiation of ischemia period followed by a sustaining infusion (0.1 mg/kg per hour) throughout the ischemic period.
Monitoring of rCBF by Laser-Doppler Flowmetry
Blood flow in the cerebral cortex was continuously monitored during each experiment using laser-Doppler flowmetry. During surgery, the bone overlying the parietal cortex, 2mm posterior and 6mm lateral to the Bregma, was thinned until translucent using a dental drill and a 1mm laser-Doppler flow probe was placed over this region to monitor perfusion using a Perimed model 5000 flowmeter (Stockholm, Sweden).
In a separate series of experiments, rCBF was simultaneously monitored using four laser-Doppler probes placed 2mm posterior and 2, 6, and 10mm lateral from the Bregma; and 6mm posterior and 4mm lateral from the Bregma to better map potential changes in rCBF in the ischemic penumbra. At the end of each of these experiments, the location in which flow was monitored was confirmed by injecting a 2%solution of Evans blue into the cortical tissue under the probes.
Measurement of Infarct Volume in t-MCAO Rats
At 24 h after reperfusion, the rats were deeply anesthetized with 4% isoflurane and killed by decapitation. The brains were quickly removed and cut into 2-mm-thick coronal sections, and stained with 2% (2,3,5-triphenyltetrazolium chloride at 37°C for 30 mins in the dark. After staining, the slices were fixed with 10% buffered formalin solution (Bederson et al, 1986). Cortical and subcortical infarct volumes were measured using MetaMorph digital imaging software. Infarct volume was either expressed in absolute terms (cubic millimeters) or as a percent of area of the ischemic hemisphere.
Volume at Risk
This was determined in a subset of rats pretreated with vehicle or TS-011 as previously described (Goto et al, 2003). After occlusion of the MCA, the cerebral circulation was perfused with 30mL of a heparinized physiologic saline solution transcardially with the descending aorta tied off at pressure of 110mmHg followed by perfusion with 3mL of physiological saline solution stained with 2% Evans blue dye. The brains were then quickly removed and fixed in 10% formalin for 24 h. The brains were cut into 2mm coronal sections. Areas of pallor in contrast to well-perfused areas that are stained dark blue were measured using MetaMorph digital imaging software. Volume at risk was expressed in absolute terms (cubic millimeters).
Eicosanoid Levels in the Brain
Experiments were performed to measure both the free and esterified levels of eicosanoids in the ischemic and contralateral hemispheres using a liquid chromatography –mass spectrometry (LC/MS). The rats were pretreated with TS-011 (0.1 mg/kg, n = 12) or vehicle (n = 12) 30 mins before the initiation of the ischemic period followed by a sustaining infusion (0.1 mg/kg per hour) throughout the ischemic period. The rats were killed 30 mins or 24 h after MCAO and the brains were collected and divided into the ischemic and contralateral hemispheres. The cerebral hemispheres of the brain were collected separately. Free levels of CYP450 eicosanoids were measured after the brains were homogenized in 3mL of a 10 mmol/L potassium buffer (pH 7.7) and extracted twice with 6mL of ethyl acetate (Fisher Scientific, Pittsburgh, PA, USA). Esterified levels of eicosanoids were measured after performing a total lipid extraction (Rose and Oklander, 1965) followed by saponification and extraction of the fatty acids released. In these experiments, the hemispheres were powdered in liquid nitrogen and homogenized in 11mL of isopropanol containing 0.2 mmol/L butylated hydroxytoluene. Chloroform (7 mL) was added and the supernatant was separated. An aliquot of the extracted lipids (20%) was dried under nitrogen and then hydrolyzed by the addition of isopropanol (2 mL) and 1 N sodium hydroxide (2 mL) and heated to 52°C for 30 mins. The samples were acidified (pH 3.0) with 10 mol/L HCl and a 10 ng of an internal standard (d6-20- HETE) was added to each sample. The free fatty acids were then extracted twice with 5mL of hexane and the samples were dried under nitrogen and stored at −80°C until measurement by LC/MS.
LC/MS Analysis of Eicosanoids
Samples were reconstituted in 50 μL of 50% solution of methanol and water and the metabolites of AA were separated by high-pressure liquid chromatography on a Betabasic C18 column (150×2.1mm, 3 μm; Thermo Hypersil- Keystone, Bellefonte, PA, USA) at a flow rate of 0.2 mL/min using an isocratic elution with a 51:9:40:0.01 mixture of acetonitrile/methanol/water/acetic acid for 30 mins followed by a step gradient to 68:13:19:01 acetonitrile/methanol/water/acetic acid and water for 15 mins. The effluent was ionized using a negative ion electrospray (450°C, 4,500 V) with the collisional activated dissociation gas set at 7 L/min. All transitions had a scan time of 0.2 s and a unit resolution in both Q1 and Q3 set at 0.7±0.1 full-width at half maximum. The peaks eluting with a mass/charge ratio (m/z) of 319 > 301 (HETEs and EETs), 337 > 319 (DiHETEs), 319 > 245 (20-HETE), 325 > 251 (d6-20-HETE), 351 > 271 (prostaglandin D2 and E2), 353 > 309 (PGF2), 369 > 245 (PGF1α), and 369 > 195 (thromboxane A2) were monitored in the multiple reaction monitoring mode using a triple quadrupole mass spectrometer (ABI 3000; Applied Biosystems, Foster City, CA, USA). The ratio of ion abundances in the peaks of interest versus that seen in the internal standard was determined and compared with four-point individual standard curves generated for each analyte in each sample run over a range from 0.2 to 10 ng.
Statistical Analysis
Mean values±s.e.m. are presented. Regional cerebral blood flow was expressed as a percentage of the baseline value measured just before MCAO occlusion. The significance of differences in mean values between treatment groups was assessed using analysis of variance followed by Holm–Sidak test or an unpaired Student’s t-test when only two groups were compared. P < 0.05 was considered to be significant.
Results
Effect of TS-011 on Infarct Size and rCBF after t-MCAO in Rats
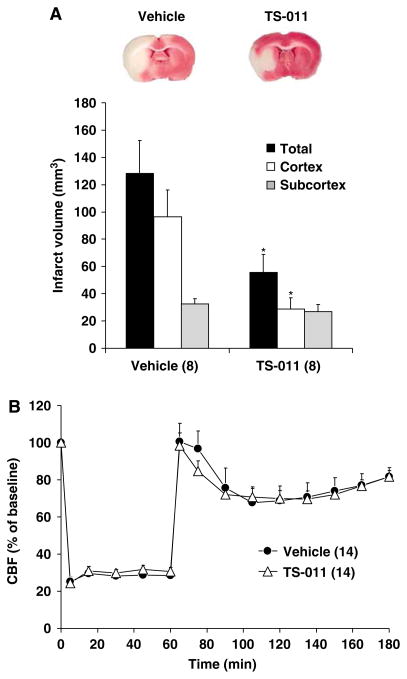
These animals were subjected to 60 mins of t-MCAO and TS-011 (0.1 mg/kg, iv) or vehicle were given 30 mins before reperfusion followed by a sustaining (0.1 mg/kg per hour) for a 120-min reperfusion period. TS-011 significantly reduced infarct volume in the cortex by 70%, but it had less effect on infarct size in the subcortical core. Total infarct volume was reduced by 55% (Figure 1A).
Figure 1.
The effect of TS-011 on infarct volume (A) and rCBF (B) in t-MCAO model of ischemic stroke. A representative example of a posterior view of a coronal section in a vehicle- and TS-011-pretreated rats is shown in the upper panel. TS-011 (0.1 mg/kg) or vehicle was administered 30 min before reperfusion and infused at a rate of 0.1 mg/kg per hour for the duration of experiment. Regional cerebral blood flow values at different time points are expressed as a percentage of the corresponding baseline value measured at time 0 (100%). *P<0.05 versus the corresponding value in the vehicle-treated group. There was no significant difference in relative CBF at any time between the groups. Numbers in parentheses indicate the number of animals studied per group.
The effect of TS-011 (0.1 mg/kg) on rCBF in these rats is presented in Figure 1B. Regional cerebral blood flow fell by 70% to 80% after occlusion of the MCA in both vehicle- and TS-011-treated rats. Regional cerebral blood briskly returned to control immediately after reperfusion, and then gradually declined by 20% to 30% over the next 30 mins after reperfusion. There were no significant differences in rCBF at any time during the experiment in the rats treated with vehicle or TS-011. Mean arterial pressure, temperature, arterial pH, pCO2, and pO2 were also stable and not different in TS-011 and vehicle-treated rats (Table 1) during these experiments.
Table 1.
Physiological parameters in TS-011- and vehicle-treated rats presented in Figure 1
| MAP (mm Hg) | pH | pO2 (mm Hg) | pCO2 (mm Hg) | |
|---|---|---|---|---|
| Pre-MCAO | ||||
| Vehicle (n = 8) | 100±2.78 | 7.40±0.01 | 171.1±5.24 | 43.5±1.46 |
| TS-011 (n = 8) | 108±4.49 | 7.42±0.01 | 162.4±5.16 | 40.9±1.36 |
| MCAO | ||||
| Vehicle (n = 8) | 97±3.65 | 7.39±0.01 | 172.4±3.66 | 44.2±1.19 |
| TS-011 (n = 8) | 103±3.10 | 7.40±0.01 | 164.9±6.65 | 42.1±1.06 |
| Reperfusion | ||||
| Vehicle (n = 8) | 97±3.65 | 7.38±0.01 | 159.3±4.74 | 44.5±1.06 |
| TS-011 (n = 8) | 104±3.32 | 7.40±0.01 | 168.1±3.52 | 43.4±1.17 |
MAP, mean arterial pressure; MCAO, occlusion of the middle cerebral artery.
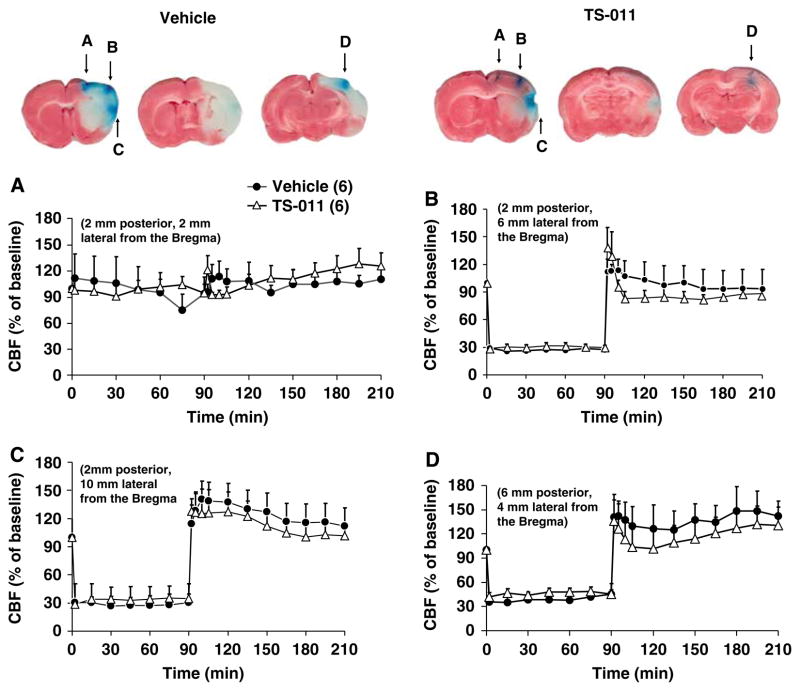
Effect of TS-011 on rCBF Monitored at Multiple Sites
Additional experiments were performed in which rCBF was monitored at multiple sites to better determine if TS-011 has any effect to improve rCBF in any region of the ischemic penumbra. The rats in this series of experiments were also subjected to a more severe, 90 min ischemia period and TS-011 or vehicle was injected 30 mins after occlusion of the MCA followed by a sustaining infusion for the 120 min duration of the reperfusion period. Blood flow fell by approximately 70% at sites B, C, and D during the ischemic period, but not in region A located 2mm posterior and 2mm lateral to the Bregma. No significant difference in rCBF was observed in rats treated with vehicle or TS-011 in any of these four cortical locations either during the ischemic or reperfusion periods (Figure 2A, B, C and D). Nevertheless, TS-011 still reduced infarct volume in the ischemic hemisphere by 70% even though no change in rCBF was detected in locations (as can be seen in Figure 2) in which flow was recorded and the cortical infarct in that area was prevented.
Figure 2.
The effect of TS-011 on rCBF monitored at multiple sites the ischemic hemisphere. TS-011 (0.1 mg/kg) or vehicle was injected 30 mins after occlusion of the MCA followed by an infusion (0.1 mg/kg per hour) for the duration of experiment. Cerebral blood flow was monitored simultaneously at four sites depicted in panels A, B, C and D (2mm posterior and 2 (site A), 6 (site B), and 10 (site C)mm lateral from the Bregma and 6mm posterior and 4mm lateral from the Bregma (site D). Sites stained in blue on the representative brain slices indicate the recording sites. Regional cerebral blood flow values at different time points are expressed as a percentage of the corresponding baseline value measured at time 0 (100%). Numbers in parentheses indicate the number of animals studied per group.
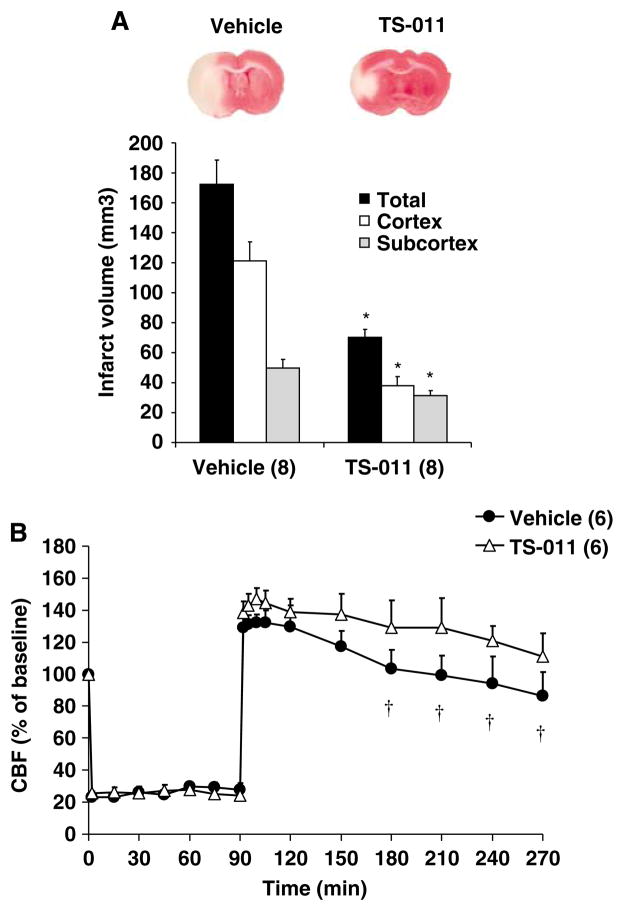
Effect of Pretreatment of Rats with TS-011 on Infarct Size and rCBF after Transient Occlusion of the MCA of Rats
We next examined whether pretreatment of the rats with TS-011 would alter the rCBF response during the ischemic period and/or enhance its protective actions on infarct volume after MCAO. The results of these experiments are presented in Figure 3A and B. Infarct volume was approximately 30% larger in the vehicle-treated rats in this group after 90 mins of ischemia and reperfusion than that seen after 60 mins of ischemia (Figure 1A). Pretreatment of rats with TS-011 reduced the total by 60%, cortical by 70%, and subcortical infarct volume by 35% (Figure 3A). There was no significant difference in the fall in rCBF seen during the ischemic period or the rCBF immediately after reperfusion in rats administered vehicle or TS-011. However, TS-011 did attenuate the delayed fall in rCBF seen 2 to 3 h after reperfusion and rCBF was higher at this time in rats treated with TS-011 than that seen in rats administered vehicle (Figure 3B).
Figure 3.
The effect of pretreatment of rats with TS-011 on infarct volume (A) and rCBF (B) after t-MCAO in rats. A representative example of a posterior view of a coronal section in a vehicle- and TS-011-pretreated rats is shown in the upper panel. TS-011 (0.1 mg/kg) or vehicle was injected 30 mins before t-MCAO followed by a continuous infusion (0.1 mg/kg per hour) during the ischemia period. Regional cerebral blood flow values at different time points are expressed as a percentage of the corresponding baseline value measured at time 0 (100%). *P<0.05 versus corresponding value measured in vehicle-treated group. †P<0.05 versus the rCBF immediately after reperfusion (90 mins). Numbers in parentheses indicate the number of animals studied per group.
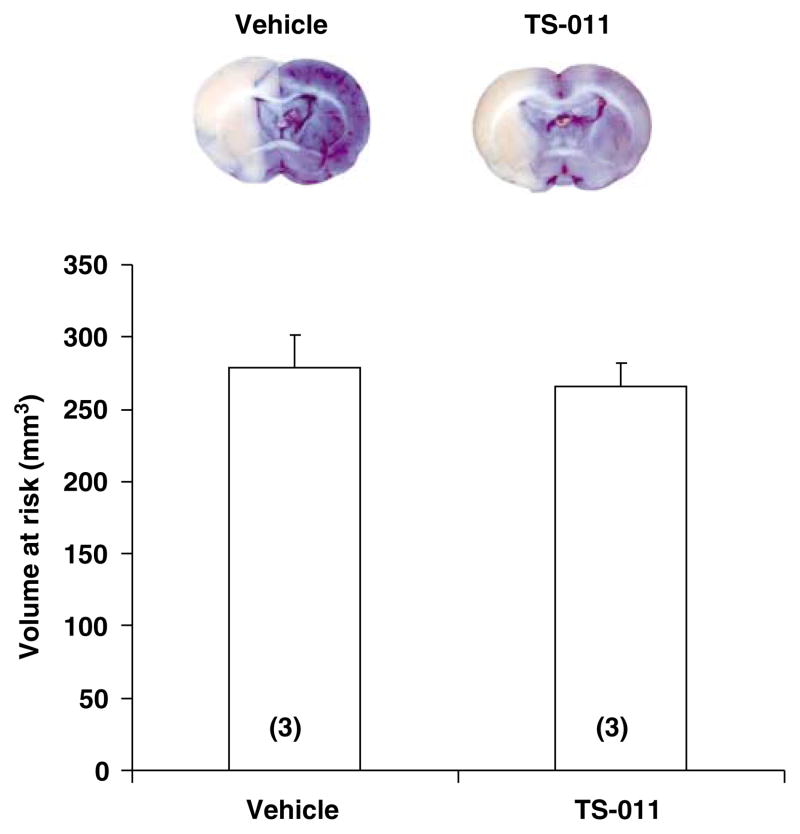
The volume at risk was also compared in a group of rats pretreated with vehicle or TS-011 (0.1 mg/kg, iv) followed by a continuous infusion of TS-011 (0.1 mg/kg per hour) or vehicle for the duration of experiment. The volume at risk was similar in vehicle- and TS-011-pretreated rats and averaged 278.2±23.32 versus 266.14±15.47mm3, respectively (Figure 4).
Figure 4.
The effect of pretreatment of rats with TS-011 on the volume at risk after t-MCAO. An example of a posterior view of a coronal section in vehicle- and TS-011-pretreated rats is presented in the upper panel. The volume at risk was determined from the area of pallor on coronal sections after vascular perfusion with Evans blue dye. TS-011 (0.1 mg/kg) or vehicle were injected 30 mins before MCAO followed by a continuous infusion of TS-011 (0.1 mg/kg per hour) or vehicle for the duration of experiment. Numbers in parentheses indicate the number of animals studied per group.
Eicosanoid Levels in the Brain after t-MCAO
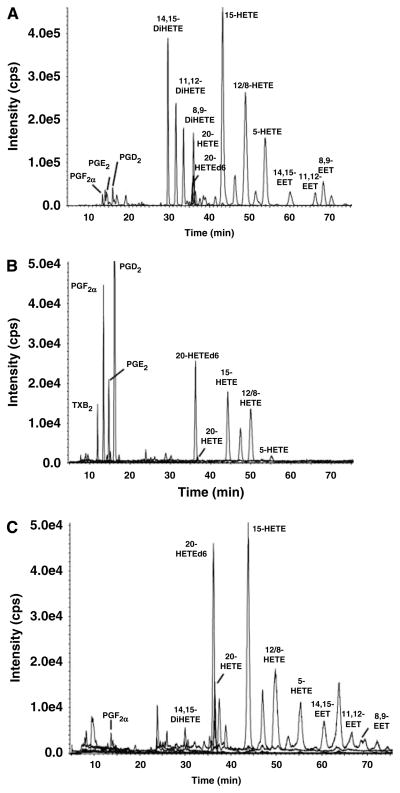
Representative chromatograms illustrating the eicosanoid profiles in the brain are presented in Figure 5. In panel A, microsomes prepared from the brain of an untreated rat were incubated at 37°C for 30 mins with a saturating concentration (40 μmol/L) of AA in the presence of 1mmol/L reduced nicotinamide adenine dinucleotide phosphate. The brain microsomes avidly produced 14,15-, 11,12-, and 8,9- DiHETEs; 20-HETE; 15-, 12-, and 5-HETEs along with lesser quantities of 14,15-, 11,12-, and 8,9-EETs. They also produced PGE2, PGF2α, and PGD2. A profile of free CYP450 eicosanoids detected after extraction of the brain with ethyl acetate is presented in Figure 5B. Under these conditions, we detected PGE2, PGF2á, PGD2, TxB2, 20-HETE, and 12-, 15- and 5-HETEs. The free levels of EETs and DiHETEs in the brain were very low and below the minimum detectable levels (< 10 pg/g).
Figure 5.
Representative LC/MS chromatograms illustrating the profile of CYP450 eicosanoids. A profile of eicosanoids formed by rat brain microsomes from an untreated rat incubated for 30 mins with AA (40 μmol/L) in the presence of 1 mmol/L nicotinamide adenine dinucleotide phosphate reduced (A). Free eicosanoid levels in the brain samples extracted with ethyl acetate (B), and esterified eicosanoid levels in the brain measured after total lipid extraction and saponification (C).
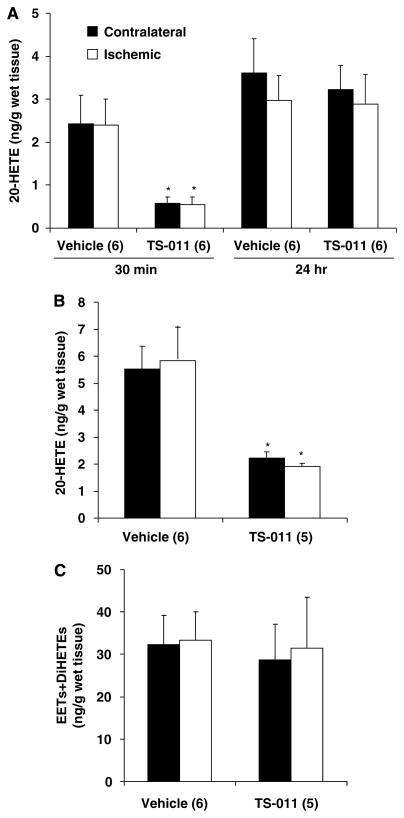
A comparison of the levels of free eicosanoids in the ischemic and contralateral hemispheres of rats pretreated with vehicle or TS-011 (0.1 mg/kg) is presented in Figure 6A. There was no significant difference in the levels of 20-HETE in the ischemic and contralateral hemispheres measured 30 mins or 24 h after reperfusion in the rats administered vehicle. Pretreatment of rats with TS-011 significantly reduced 20-HETE levels by more than 80% in both ischemic and contralateral hemispheres (Figure 6A). The levels of 20-HETE measured in both the ischemic and contralateral hemispheres returned to control 24 h later in the rats treated with TS-011. No significant difference in the levels of 12-, 15-, and 5-HETEs was seen in the ischemic or contralateral hemispheres, nor did TS-011 have any effect on the levels of these metabolites. Free levels of EETs and DiHETEs were not detectable in either the ischemic or contralateral hemisphere in these samples, suggesting that the free levels of these epoxygenase metabolites are normally below minimal detectable levels in cerebral tissue and do not increase in the brain after ischemia and reperfusion.
Figure 6.
The effect of pretreatment of TS-011 on brain levels of CYP450 eicosanoids after t-MCAO in rats. TS-011 (0.1 mg/kg) or vehicle was injected 30 mins before t-MCAO followed by a sustaining infusion (0.1 mg/kg per hour) during the ischemia period. Free (A) and esterified levels (B) of 20-HETE in the ischemic or contralateral hemispheres were determined by LC/MS 30 mins after reperfusion. Esterified levels of EETs and DiHETEs were also measured 30 mins following reperfusion after saponification of brain tissue (C). Free levels of 20-HETE were also measured 24 h after reperfusion (A). *P<0.05 versus the corresponding value measured in vehicle-treated group. Numbers in parentheses indicate the number of animals studied per group.
The total levels of CYP450 eicosanoids (free plus esterified) in the brain were measured after a total lipid extraction and saponification of the samples. A typical chromatogram obtained after saponification of cerebral tissue samples is presented in Figure 5C. 14,15-, 11,12-, and 8,9- EETs and DiHETEs, PGF2á, as well as 20-, 12-, 15- and 5-HETEs were all readily detected under these conditions.
20-HETE levels (Figure 6B) measured after saponification were 2- to 3-fold higher than the corresponding free levels measured in cerebral tissue after ethyl acetate extraction. There was no difference in 20-HETE levels in the ischemic and contralateral hemispheres. Pretreatment of rats with TS-011 reduced total 20-HETE levels by 70% (Figure 6B). EETs and DiHETEs in the saponified samples (Figure 6C) were much higher than the free levels seen in samples extracted with ethyl acetate. There was no difference in the levels of any of the regioisomers of EETs or DiHETEs measured in the ischemic and contralateral hemispheres. Moreover, the levels of EETs and DiHETEs in the brains of rats pretreated with TS-011 were not significantly different than those seen in rats administered vehicle (Figure 6C).
Effect of Another Inhibitor of the Synthesis of 20-HETE and a 20-HETE Antagonist on Infarct Size After Transient Occlusion of the MCAO in Rats
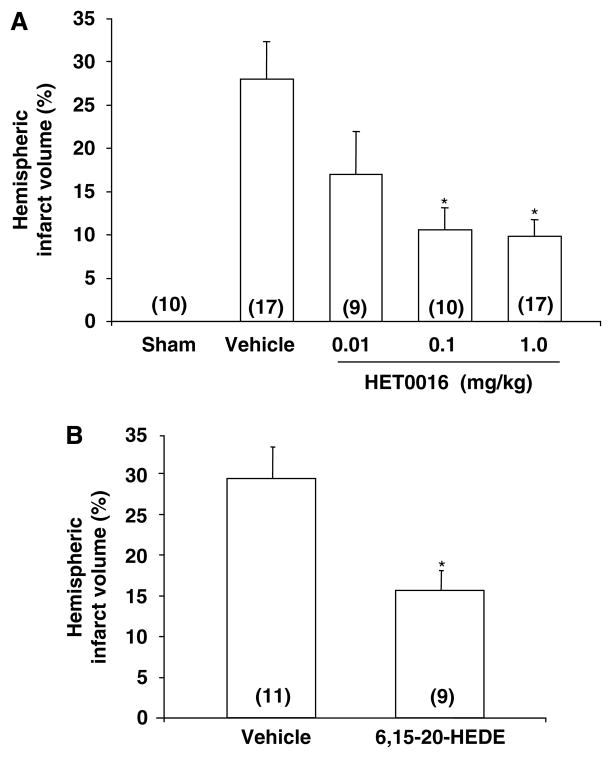
To exclude the possibility that the protective action of TS-011 on infarct size seen in the present study was because of an off-target effect unrelated to inhibition of the synthesis of 20-HETE, we studied the effects of a different 20-HETE synthesis inhibitor, HET0016, and a structurally dissimilar 20-HETE antagonist, 6,15-20-HEDE (10 mg/kg, iv), on infarct volume in rats subjected to t-MCAO. The results of these experiments are presented in Figure 7A and 7B. Rats were subjected to 60 mins of t-MCAO and treated with HET0016 (0.01, 0.1, and 1.0 mg/kg, iv) or vehicle (30% sulfobutylether-7-β-cyclodextrin in a 250 mmol/L mannitol solution) or 6,15-20-HEDE or its vehicle (0.1mL of a 0.1 mol/L NaPO4 buffer, pH 9.0) just before reperfusion. Administration of HET0016 at doses 0.1 and 1.0 mg/kg reduced infarct volume by 65% (Figure 7A). Administration of 6, 15-20-HEDE had a similar effect and reduced infarct size by 45% (Figure 7B).
Figure 7.
The effect of HET0016 (A) and 6,15-20-HEDE (B) on the infarct volume after t-MCAO. HET0016 (0.01 to 1.0 mg/kg, iv), the 20-HETE antagonist, 6,15-20-HEDE (10 mg/kg, iv), or vehicle were injected just before reperfusion. Infarct volumes are presented as a percent of total hemispheric volume. *P<0.05 versus the corresponding value measured in vehicle-treated rats. Numbers in parentheses indicate the number of animals studied per group.
Discussion
Recent studies have indicated that long-term blockade of the synthesis of 20-HETE with HET0016 (Poloyac et al, 2006) or acute blockade with TS-011 (Omura et al, 2006) reduces infarct size after t-MCAO, but its mechanism of action of these drugs remains to be determined. For example, it remains unknown whether the levels of 20-HETE increase in the brain either during or after MCAO, or whether the protective effect of 20-HETE inhibitors is related to recruitment of collateral flow to the ischemic penumbra or an effect on the survival of neurons subjected to ischemic stress.
The results of the present study confirm our previous report that administration of TS-011, which is the most potent and specific inhibitor of the synthesis of 20-HETE yet identified (Miyata et al, 2005), markedly reduces infarct size after t-MCAO. In the present study, TS-011 was effective in reducing infarct size in rats subjected to t-MCAO whether it was administered before or during the ischemic period. We also showed that effects of TS-011 were associated with a 70% to 80% reduction in 20-HETE levels in the brain and that it has no effect on the levels of EETs, DiHETEs, or 12-, 15- or 5-HETEs. These findings indicate that TS-011 crosses the blood–brain barrier and selectively inhibits the production of 20-HETE in the brain. They also indicate that the free levels of 20-HETE in the brain are dependent on continued synthesis, rather than determined by release of 20-HETE esterified in phospholipid pools.
We also found that the acute administration of HET0016, a less selective first-generation inhibitor of the synthesis of 20-HETE, had a similar effect as TS-011 to reduce infarct size after MCAO. This finding is consistent with the results of a recent study by Poloyac et al (2006). The differences between the two studies are that the minimal dose of HET0016 needed to reduce infarct size in the present study (0.1 mg/kg) was much lower than that used in the previous study and we gave the drug acutely during the ischemic period, whereas Poloyac et al (2006) pretreated the rats for several days before cerebral ischemia to ensure that the pools of 20-HETE in the brain were fully depleted.
We also found that 6,15-20-HEDE, which is a chemically dissimilar competitive antagonist of vasoconstrictive actions of 20-HETE (Yu et al, 2004), has a similar effect as both of the 20-HETE synthesis inhibitors to reduce infarct size after t-MCAO. Taken together, along with the current demonstration that TS-011 selectively reduces levels of 20-HETE in the brain, the beneficial effects of these three different compounds are because of their shared ability to block the synthesis and/or actions of 20-HETE rather than some common nonspecific effect of these three compounds.
Because 20-HETE is a potent endogenous constrictor of cerebral arteries and there is evidence that the release of fatty acids is elevated after ischemic stroke (Pilitsis et al, 2003), it has been assumed that the production and/or release of 20-HETE may be elevated after cerebral ischemia and that blocking the production of this compound may increase rCBF and oxygen delivery to the ischemic penumbra either during or after t-MCAO. However, the results of the present study indicate that the levels of 20-HETE in the ischemic hemisphere do not increase after t-MCAO and 30 mins of reperfusion. However, we cannot exclude the possibility that 20-HETE levels may increase markedly in some small compartment in the brain or at other time points such as during the ischemic period or later in the reperfusion period.
Other investigators have reported that administration of a soluble epoxide hydrolase inhibitor (Dorrance et al, 2005; Zhang et al, 2007) or following knockout of the sEH gene (Zhang et al, 2008) reduces infarct size after t-MCAO. These findings led to the suggestion that perhaps EETs may be released after cerebral ischemia and exert a protective role. The present results indicate that the brain avidly produces 14,15-, 11,12-, and 8,9-EETs and DiHETEs when incubated with AA; however, the endogenous free levels of these compounds in cerebral tissue are very low. These compounds were readily detectable after a total lipid extraction of the brain and saponification of the samples. This finding indicates that most of the EETs produced in the brain are esterified into membrane phospholipid pools. Moreover, we did not detect any change in the levels of esterified EETs or DiHETEs in the ischemic or contralateral hemispheres of the brain indicating that these compounds are probably not released in substantial amounts after t-MCAO.
In other experiments, we found that administration of TS-011 30 mins before reperfusion had no significant effect on rCBF either during or for 2 h after reperfusion. Additional experiments in which CBF was recorded at multiple sites also indicated that there were no regional differences in the recruitment of collateral flow in the penumbra in vehicle- or TS-011-treated animals at any point or time period during these experiments. The only hemodynamic effect we found in any of our studies was that pretreatment of rats with TS-011 attenuated the delayed postischemic fall in rCBF seen 2 to 3 h after reperfusion. This finding is consistent with a recent report of Poloyac et al, that pretreatment of rats with HET0016 had a similar effect to increase rCBF late in the postischemic period. However, it remains to be determined whether the small delayed fall in rCBF seen during the postischemic period contributes to further ischemic injury or if attenuation of this fall in flow can account for the reduction in infarct size seen in rats treated with 20-HETE inhibitors. In our view it seems more likely that the slightly greater perfusion seen in the rats treated with TS-011 in the postischemic period may simply reflect less tissue damage and a higher metabolic activity in the brain because of a neuroprotective action of TS-011. Moreover, the previous report by Omura et al (2006) showing that TS-011 is effective at reducing infarct size when administered up to 4 h after reperfusion further support our view that the protective effect of TS-011 in ischemic stroke is not because of recruitment of flow during the ischemic period or the small changes in rCBF found late in the postischemic period.
The mechanism of the neuroprotective effect of 20-HETE inhibitors and the 20-HETE antagonist remains to be determined. One possible mechanism is that 20-HETE inhibitors may act by preventing the inhibitory effects of 20-HETE on Na+, K+-ATPase activity, thereby preventing the secondary energy failure, cell swelling and death that typically occur 2 to 4 h after transient ischemia and reperfusion. Alternatively, 20-HETE is known to activate many downstream effectors pathways linked to apoptosis and cell death (Muthalif et al, 1998). 20-HETE has also been recently shown to promote cellular magnesium efflux (Ikari et al, 2004). Thus, inhibition of the formation of 20-HETE by TS-011 may increase intracellular magnesium concentration, thereby preserving ATP levels and/or opposing excitotoxic effects (Johnson and Ascher, 1990; McIntosh et al, 1988). Finally, there are several recent papers indicating that 20-HETE stimulates oxidative stress in cells through several mechanisms, including activation of reduced nicotinamide adenine dinucleotide phosphate oxidase, phosphorylation and uncoupling of NOS, and through a nicotinamide adenine dinucleotide phosphate oxidase -independent pathway, perhaps mitochondrial (Medhora et al, 2008). Thus, it remains possible that blockade of the formation of 20-HETE improves cell survival by reducing oxidative stress and its subsequent effects to increase apoptosis.
In summary, the present study indicates that two different inhibitors of the synthesis of 20-HETE and a 20-HETE antagonist markedly reduce infarct volume after t-MCAO in rats suggesting that blockade of the synthesis and/or actions of this compound may have therapeutic potential in ischemic stroke. The beneficial effect of the inhibitor of the synthesis of 20-HETE to reduce infarct size is not associated with the area at risk or changes in rCBF during the ischemic period or the postischemic hyperemic period and may be because of a potential direct neuroprotective effect that will need to be confirmed and the mechanism elucidated in future studies.
Acknowledgments
We thank Averia Flasch and Lisa Henderson for technical assistance with LC/MS measurements.
This work was partially supported by Grants HL59996, HL29587, and NS 38684 from the National Institutes of Health.
Footnotes
Disclosure
Dr Miyata, Dr Omura, Dr Kawashima, and Dr Onishi are employees of Taisho Pharmaceutical Co (Saitama, Japan) that is evaluating TS-011 as a potential treatment for ischemic stroke.
References
- Bederson JB, Pitts LH, Germano SM, Nishimura MC, Davis RL, Bartkowski HM. Evaluation of 2,3,5- triphenyltetrazolium chloride as a stain for detection and quantification of experimental cerebral infarction in rats. Stroke. 1986;17:1304–8. doi: 10.1161/01.str.17.6.1304. [DOI] [PubMed] [Google Scholar]
- Cambj-Sapunar L, Yu M, Harder DR, Roman RJ. Contribution of 5-hydroxytryptamine1B receptors and 20-hydroxyeiscosatetraenoic acid to fall in cerebral blood flow after subarachnoid hemorrhage. Stroke. 2003;34:1269–75. doi: 10.1161/01.STR.0000065829.45234.69. [DOI] [PubMed] [Google Scholar]
- Cheng J, Ou JS, Singh H, Falck JR, Narsimhaswamy D, Pritchard KA, Jr, Schwartzman ML. 20-Hydroxyeicosatetraenoic acid causes endothelial dysfunction via eNOS uncoupling. Am J Physiol Heart Circ Physiol. 2008;294:H1018–26. doi: 10.1152/ajpheart.01172.2007. [DOI] [PubMed] [Google Scholar]
- Dorrance AM, Rupp N, Pollock DM, Newman JW, Hammock BD, Imig JD. An epoxide hydrolase inhibitor, 12-(3-adamantan-1-yl-ureido)dodecanoic acid (AUDA), reduces ischemic cerebral infarct size in stroke-prone spontaneously hypertensive rats. J Cardiovasc Pharmacol. 2005;46:842–8. doi: 10.1097/01.fjc.0000189600.74157.6d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebremedhin D, Lange AR, Lowry TF, Taheri MR, Birks EK, Hudetz AG, Narayanan J, Falck JR, Okamoto H, Roman RJ, Nithipatikom K, Campbell WB, Harder DR. Production of 20-HETE and its role in autoregulation of cerebral blood flow. Circ Res. 2000;87:60–5. doi: 10.1161/01.res.87.1.60. [DOI] [PubMed] [Google Scholar]
- Gebremedhin D, Lange AR, Narayanan J, Aebly MR, Jacobs ER, Harder DR. Cat cerebral arterial smooth muscle cells express cytochrome P450 4A2 enzyme and produce the vasoconstrictor 20-HETE which enhances L-type Ca2+ current. J Physiol. 1998;507(Part 3):771–81. doi: 10.1111/j.1469-7793.1998.771bs.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto S, Sampei K, Alkayed NJ, Dore S, Koehler RC. Characterization of a new double-filament model of focal cerebral ischemia in heme oxygenase-2-deficient mice. Am J Physiol Regul Integr Comp Physiol. 2003;285:R222–30. doi: 10.1152/ajpregu.00067.2003. [DOI] [PubMed] [Google Scholar]
- Guo AM, Arbab AS, Falck JR, Chen P, Edwards PA, Roman RJ, Scicli AG. Activation of vascular endothelial growth factor through reactive oxygen species mediates 20-hydroxyeicosatetraenoic acid-induced endothelial cell proliferation. J Pharmacol Exp Ther. 2007;321:18–27. doi: 10.1124/jpet.106.115360. [DOI] [PubMed] [Google Scholar]
- Hacein-Bey L, Harder DR, Meier HT, Varelas PN, Miyata N, Lauer KK, Cusick JF, Roman RJ. Reversal of delayed vasospasm by TS-011 in the dual hemorrhage dog model of subarachnoid hemorrhage. AJNR Am J Neuroradiol. 2006;27:1350–4. [PMC free article] [PubMed] [Google Scholar]
- Harder DR, Gebremedhin D, Narayanan J, Jefcoat C, Falck JR, Campbell WB, Roman R. Formation and action of a P-450 4A metabolite of arachidonic acid in cat cerebral microvessels. Am J Physiol. 1994;266:H2098–107. doi: 10.1152/ajpheart.1994.266.5.H2098. [DOI] [PubMed] [Google Scholar]
- Ikari A, Nakajima K, Suketa Y, Harada H, Takagi K. Activation of Na+-independent Mg2+ efflux by 20- hydroxyeicosatetraenoic acid in rat renal epithelial cells. Jpn J Physiol. 2004;54:415–9. doi: 10.2170/jjphysiol.54.415. [DOI] [PubMed] [Google Scholar]
- Johnson JW, Ascher P. Voltage-dependent block by intracellular Mg2+ of N-methyl-D-aspartate-activated channels. Biophys J. 1990;57:1085–90. doi: 10.1016/S0006-3495(90)82626-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehl F, Cambj-Sapunar L, Maier KG, Miyata N, Kametani S, Okamoto H, Hudetz AG, Schulte ML, Zagorac D, Harder DR, Roman RJ. 20-HETE contributes to the acute fall in cerebral blood flow after subarachnoid hemorrhage in the rat. Am J Physiol Heart Circ Physiol. 2002;282:H1556–65. doi: 10.1152/ajpheart.00924.2001. [DOI] [PubMed] [Google Scholar]
- Lange A, Gebremedhin D, Narayanan J, Harder D. 20- Hydroxyeicosatetraenoic acid-induced vasoconstriction and inhibition of potassium current in cerebral vascular smooth muscle is dependent on activation of protein kinase C. J Biol Chem. 1997;272:27345–52. doi: 10.1074/jbc.272.43.27345. [DOI] [PubMed] [Google Scholar]
- McIntosh TK, Faden AI, Yamakami I, Vink R. Magnesium deficiency exacerbates and pretreatment improves outcome following traumatic brain injury in rats: 31P magnetic resonance spectroscopy and behavioral studies. J Neurotrauma. 1988;5:17–31. doi: 10.1089/neu.1988.5.17. [DOI] [PubMed] [Google Scholar]
- Medhora M, Chen Y, Gruenloh S, Harland D, Bodiga S, Zielonka J, Gebremedhin D, Gao Y, Falck JR, Anjaiah S, Jacobs ER. 20-HETE increases superoxide production and activates NAPDH oxidase in pulmonary artery endothelial cells. Am J Physiol Lung Cell Mol Physiol. 2008;294:L902–11. doi: 10.1152/ajplung.00278.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyata N, Seki T, Tanaka Y, Omura T, Taniguchi K, Doi M, Bandou K, Kametani S, Sato M, Okuyama S, Cambj-Sapunar L, Harder DR, Roman RJ. Beneficial effects of a new 20-hydroxyeicosatetraenoic acid synthesis inhibitor, TS-011 [N-(3-chloro-4-morpholin-4-yl) phenyl-N′-hydroxyimido formamide], on hemorrhagic and ischemic stroke. J Pharmacol Exp Ther. 2005;314:77–85. doi: 10.1124/jpet.105.083964. [DOI] [PubMed] [Google Scholar]
- Miyata N, Taniguchi K, Seki T, Ishimoto T, Sato-Watanabe M, Yasuda Y, Doi M, Kametani S, Tomishima Y, Ueki T, Sato M, Kameo K. HET0016, a potent and selective inhibitor of 20-HETE synthesizing enzyme. Br J Pharmacol. 2001;133:325–9. doi: 10.1038/sj.bjp.0704101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthalif MM, Benter IF, Karzoun N, Fatima S, Harper J, Uddin MR, Malik KU. 20-Hydroxyeicosatetraenoic acid mediates calcium/calmodulin-dependent protein kinase II-induced mitogen-activated protein kinase activation in vascular smooth muscle cells. Proc Natl Acad Sci USA. 1998;95:12701–6. doi: 10.1073/pnas.95.21.12701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagasawa H, Kogure K. Correlation between cerebral blood flow and histologic changes in a new rat model of middle cerebral artery occlusion. Stroke. 1989;20:1037–43. doi: 10.1161/01.str.20.8.1037. [DOI] [PubMed] [Google Scholar]
- Nowicki S, Chen SL, Aizman O, Cheng XJ, Li D, Nowicki C, Nairn A, Greengard P, Aperia A. 20-Hydroxyeicosa-tetraenoic acid (20 HETE) activates protein kinase C. Role in regulation of rat renal Na+, K+-ATPase. J Clin Invest. 1997;99:1224–30. doi: 10.1172/JCI119279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omura T, Tanaka Y, Miyata N, Koizumi C, Sakurai T, Fukasawa M, Hachiuma K, Minagawa T, Susumu T, Yoshida S, Nakaike S, Okuyama S, Harder DR, Roman RJ. Effect of a new inhibitor of the synthesis of 20- HETE on cerebral ischemia reperfusion injury. Stroke. 2006;37:1307–13. doi: 10.1161/01.STR.0000217398.37075.07. [DOI] [PubMed] [Google Scholar]
- Pilitsis JG, Coplin WM, O’Regan MH, Wellwood JM, Diaz FG, Fairfax MR, Michael DB, Phillis JW. Measurement of free fatty acids in cerebrospinal fluid from patients with hemorrhagic and ischemic stroke. Brain Res. 2003;985:198–201. doi: 10.1016/s0006-8993(03)03044-0. [DOI] [PubMed] [Google Scholar]
- Poloyac SM, Zhang Y, Bies RR, Kochanek PM, Graham SH. Protective effect of the 20-HETE inhibitor HET0016 on brain damage after temporary focal ischemia. J Cereb Blood Flow Metab. 2006;26:1551–61. doi: 10.1038/sj.jcbfm.9600309. [DOI] [PubMed] [Google Scholar]
- Randriamboavonjy V, Busse R, Fleming I. 20-HETEinduced contraction of small coronary arteries depends on the activation of Rho-kinase. Hypertension. 2003;41:801–6. doi: 10.1161/01.HYP.0000047240.33861.6B. [DOI] [PubMed] [Google Scholar]
- Rose HG, Oklander M. Improved procedure for the extraction of lipids from human erythrocytes. J Lipid Res. 1965;6:428–31. [PubMed] [Google Scholar]
- Sun CW, Falck JR, Harder DR, Roman RJ. Role of tyrosine kinase and PKC in the vasoconstrictor response to 20-HETE in renal arterioles. Hypertension. 1999;33:414–8. doi: 10.1161/01.hyp.33.1.414. [DOI] [PubMed] [Google Scholar]
- Takeuchi K, Renic M, Bohman QC, Harder DR, Miyata N, Roman RJ. Reversal of delayed vasospasm by an inhibitor of the synthesis of 20-HETE. Am J Physiol Heart Circ Physiol. 2005;289:H2203–11. doi: 10.1152/ajpheart.00556.2005. [DOI] [PubMed] [Google Scholar]
- Tanaka Y, Omura T, Fukasawa M, Horiuchi N, Miyata N, Minagawa T, Yoshida S, Nakaike S. Continuous inhibition of 20-HETE synthesis by TS-011 improves neurological and functional outcomes after transient focal cerebral ischemia in rats. Neurosci Res. 2007;59:475–80. doi: 10.1016/j.neures.2007.08.018. [DOI] [PubMed] [Google Scholar]
- Yu M, Alonso-Galicia M, Sun CW, Roman RJ, Ono N, Hirano H, Ishimoto T, Reddy YK, Katipally KR, Reddy KM, Gopal VR, Yu J, Takhi M, Falck JR. 20-Hydroxyeicosatetraenoic acid (20-HETE): structural determinants for renal vasoconstriction. Bioorg Med Chem. 2003;11:2803–21. doi: 10.1016/s0968-0896(03)00192-5. [DOI] [PubMed] [Google Scholar]
- Yu M, Cambj-Sapunar L, Kehl F, Maier KG, Takeuchi K, Miyata N, Ishimoto T, Reddy LM, Falck JR, Gebremedhin D, Harder DR, Roman RJ. Effects of a 20-HETE antagonist and agonists on cerebral vascular tone. Eur J Pharmacol. 2004;486:297–306. doi: 10.1016/j.ejphar.2004.01.009. [DOI] [PubMed] [Google Scholar]
- Zhang W, Koerner IP, Noppens R, Grafe M, Tsai HJ, Morisseau C, Luria A, Hammock BD, Falck JR, Alkayed NJ. Soluble epoxide hydrolase: a novel therapeutic target in stroke. J Cereb Blood Flow Metab. 2007;27:1931–40. doi: 10.1038/sj.jcbfm.9600494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Otsuka T, Sugo N, Ardeshiri A, Alhadid YK, Iliff JJ, DeBarber AE, Koop DR, Alkayed NJ. Soluble epoxide hydrolase gene deletion is protective against experimental cerebral ischemia. Stroke. 2008;39:2073–8. doi: 10.1161/STROKEAHA.107.508325. [DOI] [PMC free article] [PubMed] [Google Scholar]